Effect of Filter Medium on Water Quality during Passive Biofilter Activation in a Recirculating Aquaculture System for Oncorhynchus mykiss
Abstract
Highlights
- The high specific surface area of the biofilter media has a positive effect on nitrification.
- The smooth surface of the biofilter medium reduces the efficiency of nitrification.
- Passive biofilter activation can be used in salmonid RAS.
- P and C concentrations do not limit nitrogen transformation processes.
Abstract
1. Introduction
2. Materials and Methods
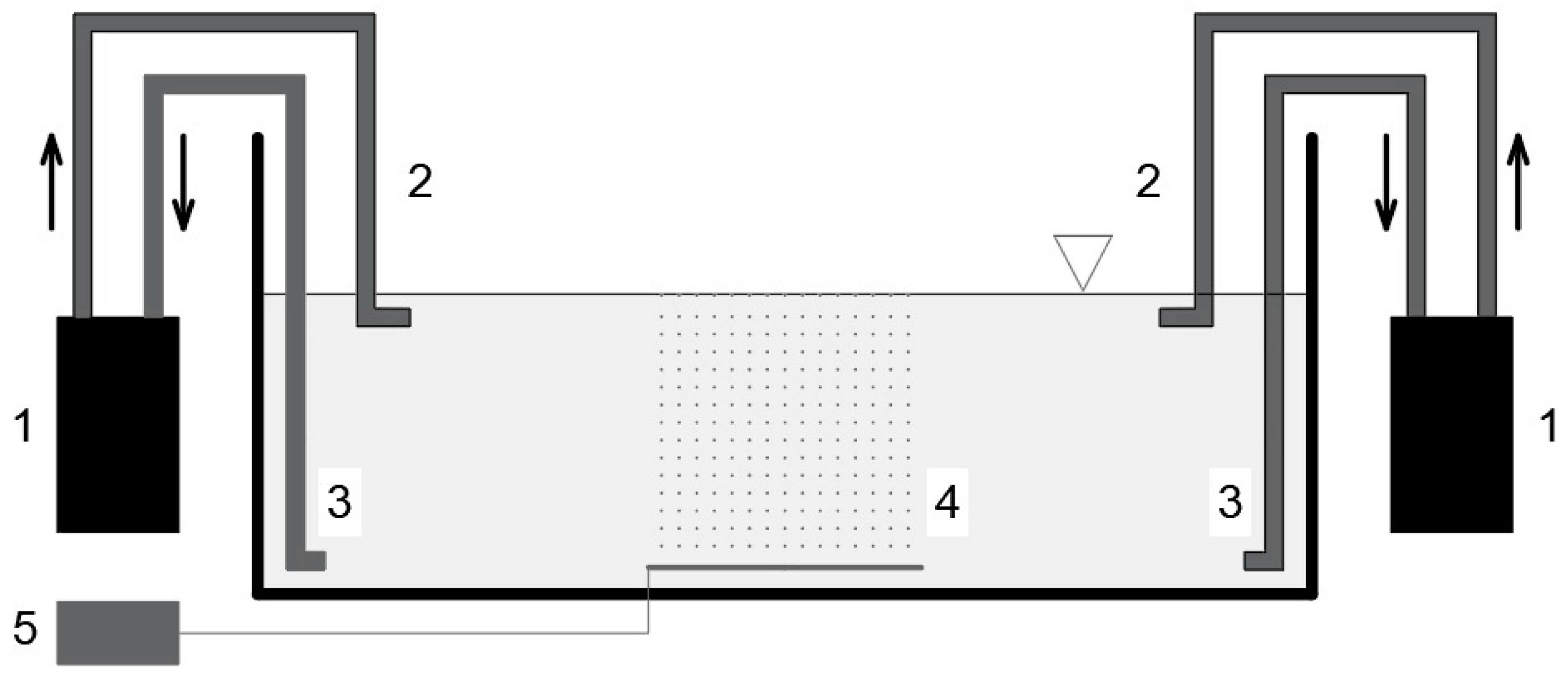
2.1. Recirculating System
2.2. Hydrochemical Parameters
2.3. Statistical Analysis
3. Results and Discussion
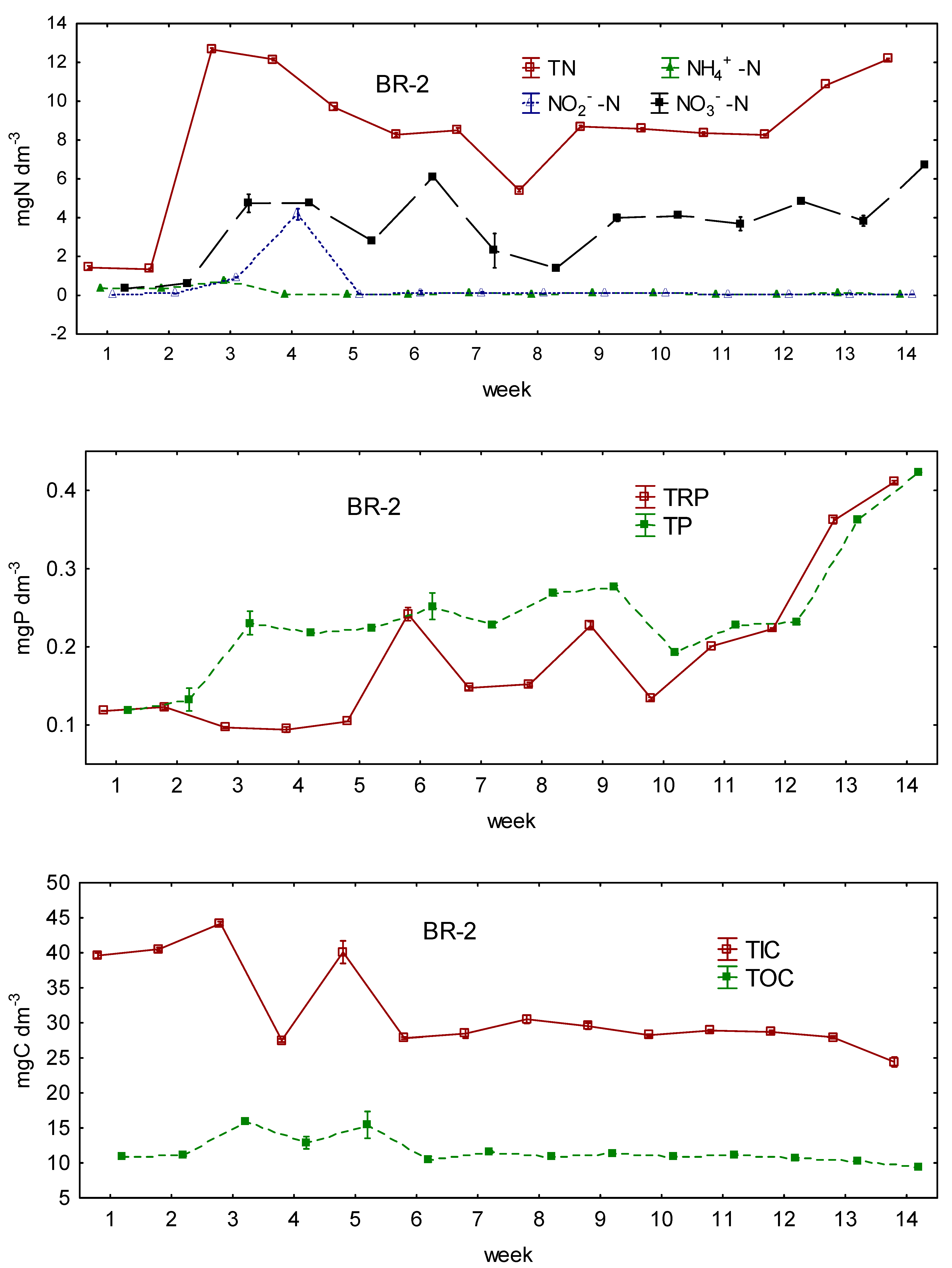
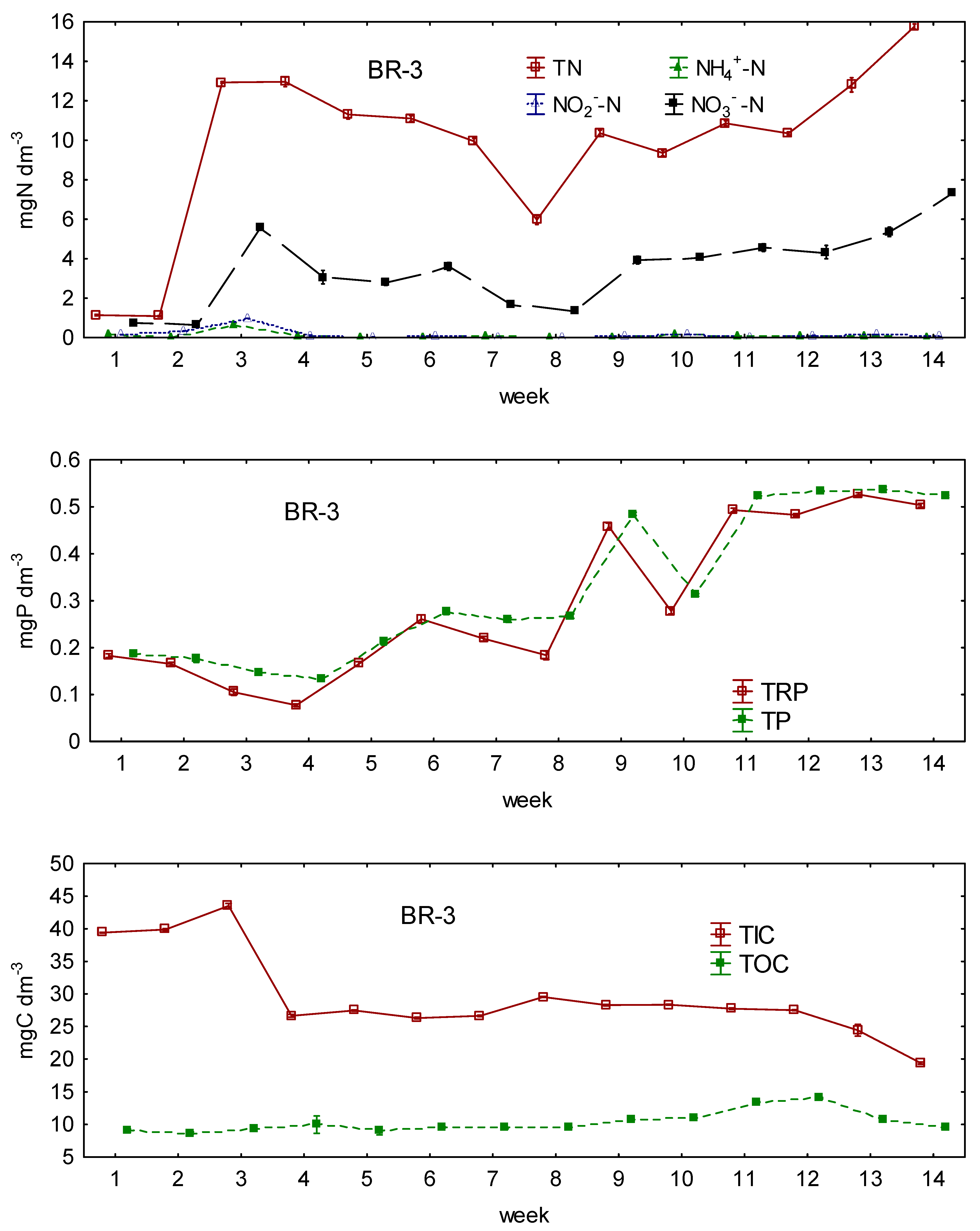
3.1. Changes in the Concentrations of N, P, and C during the Experiment
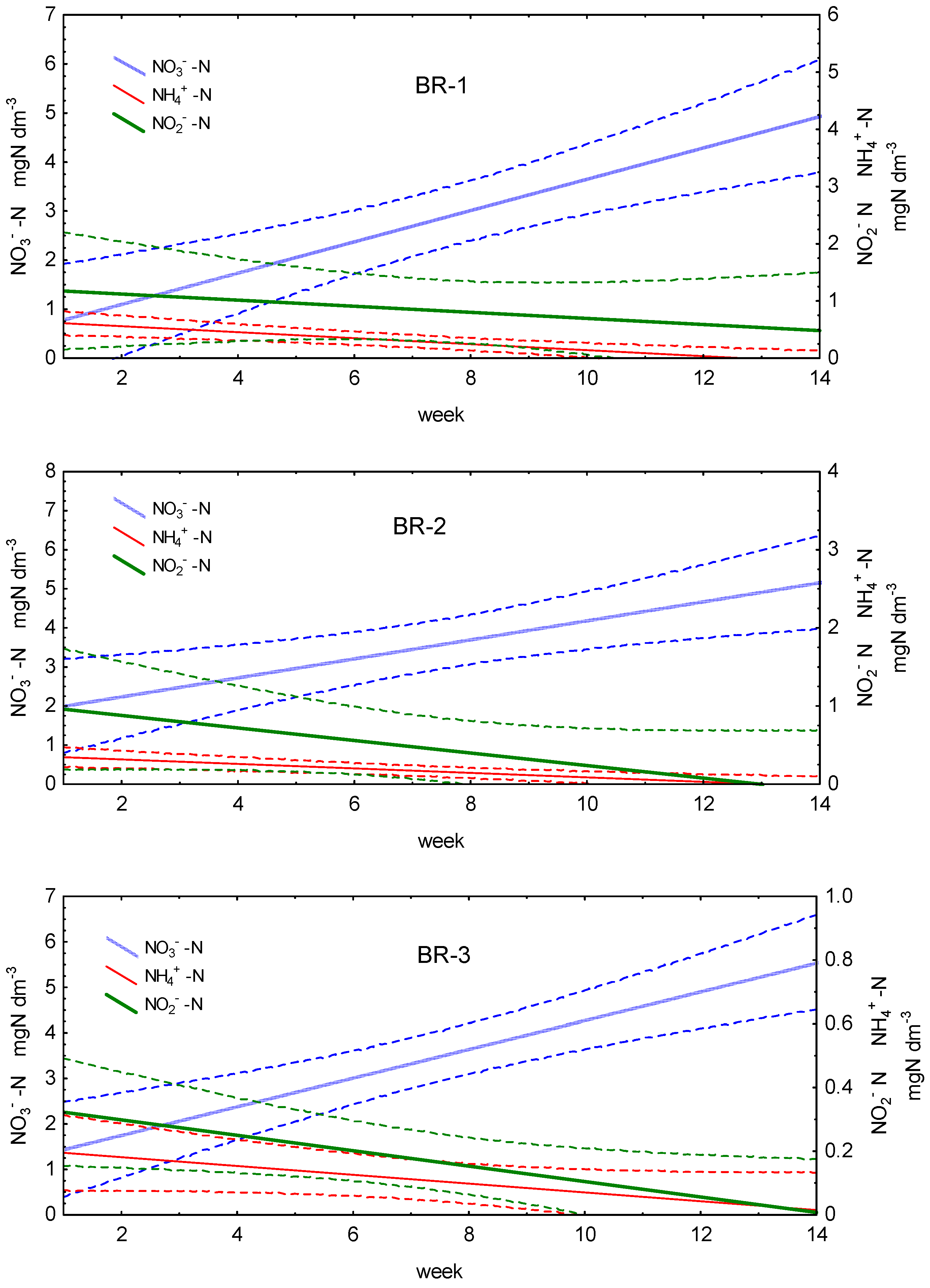
3.2. Differences in N, P, and C Conversion between Bioreactors
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| Bioreactor | NO2−-N | NO3−-N | NH4+-N | TN | TRP | TP | TIC | TOC | |
|---|---|---|---|---|---|---|---|---|---|
| mg N dm−3 | mg P dm−3 | mg C dm−3 | |||||||
| BR-1 | inflow | 0.811 | 2.857 | 0.276 | 7.777 | 0.213 | 0.252 | 32.2 | 11.2 |
| ±1.401 | ±2.065 | ±0.363 | ±3.624 | ±0.128 | ±0.117 | ±6.2 | ±1.4 | ||
| outflow | 0.845 | 2.850 | 0.266 | 7.803 | 0.214 | 0.251 | 32.5 | 11.0 | |
| ±1.42 | ±2.072 | ±0.347 | ±3.554 | ±0.130 | ±0.119 | ±6.0 | ±1.2 | ||
| F | 1.024 | 1.006 | 1.112 | 1.039 | 1.037 | 1.030 | 1.052 | 1.397 | |
| P | 0.949 | 0.999 | 0.936 | 0.984 | 0.990 | 0.991 | 0.902 | 0.720 | |
| BR-2 | inflow | 0.432 | 3.563 | 0.157 | 8.350 | 0.186 | 0.241 | 32.1 | 11.8 |
| ±1.042 | ±1.854 | ±0.195 | ±3.531 | ±0.097 | ±0.079 | ±6.3 | ±2.2 | ||
| outflow | 0.445 | 3.578 | 0.159 | 8.315 | 0.190 | 0.250 | 31.7 | 11.4 | |
| ±1.157 | ±1.990 | ±0.224 | ±3.512 | ±0.099 | ±0.078 | ±6.3 | ±1.7 | ||
| F | 1.231 | 1.152 | 1.318 | 1.010 | 1.023 | 1.028 | 1.014 | 1.688 | |
| P | 0.976 | 0.983 | 0.987 | 0.994 | 0.931 | 0.941 | 0.887 | 0.569 | |
| BR-3 | inflow | 0.168 | 3.574 | 0.103 | 9.749 | 0.292 | 0.326 | 29.6 | 10.5 |
| ±0.249 | ±1.956 | ±0.155 | ±4.261 | ±0.163 | ±0.158 | ±6.6 | ±1.6 | ||
| outflow | 0.162 | 3.418 | 0.107 | 9.673 | 0.294 | 0.327 | 29.6 | 10.1 | |
| ±0.252 | ±1.928 | ±0.185 | ±4.260 | ±0.164 | ±0.158 | ±6.6 | ±1.7 | ||
| F | 1.016 | 1.029 | 1.416 | 1.000 | 1.011 | 1.000 | 1.004 | 1.017 | |
| P | 0.946 | 0.834 | 0.949 | 0.963 | 0.964 | 0.995 | 0.965 | 0.606 | |

References
- FAO. The State of World Fisheries and Aquaculture 2020: Sustainability in Action; FAO: Rome, Italy, 2020. [Google Scholar] [CrossRef]
- Sirakov, I. Flesh quality in rainbow trout (Oncorhynchus mykiss W.) and brown trout (Salmo trutta m. fario L.) cultivated in recirculation aquaculture system. Int. J. Curr. Microbiol. Appl. Sci. 2015, 4, 50–57. [Google Scholar]
- Wang, C.; Li, Z.; Wang, T.; Xu, X.; Zhang, X.; Li, D. Intelligent fish farm—The future of aquaculture. Aquac. Int. 2021, 29, 2681–2711. [Google Scholar] [CrossRef]
- Minaz, M.; Kubilay, A. Operating parameters affecting biofloc technology: Carbon source, carbon/nitrogen ratio, feeding regime, stocking density, salinity, aeration, and microbial community manipulation. Aquac. Int. 2021, 29, 1121–1140. [Google Scholar] [CrossRef]
- Zou, Y.; Hu, Z.; Zhang, J.; Xie, H.; Guimbaud, C.; Fang, Y. Effects of pH on nitrogen transformations in media-based aquaponics. Bioresour. Technol. 2016, 210, 81–87. [Google Scholar] [CrossRef]
- Zhu, S.-M.; Deng, Y.-L.; Ruan, Y.-J.; Guo, X.S.; Shi, M.M.; Shen, J.Z. Biological denitrification using poly(butylene succinate) as carbon source and biofilm carrier for recirculating aquaculture system effluent treatment. Bioresour. Technol. 2015, 192, 603–610. [Google Scholar] [CrossRef]
- Ridha, M.T.; Cruz, E.M. Effect of biofilter media on water quality and biological performance of the Nile tilapia Oreochromis niloticus L. reared in a simple recirculating system. Aquac. Eng. 2001, 24, 157–166. [Google Scholar] [CrossRef]
- Boaventura, T.P.; Miranda-Filho, K.C.; Oréfice, R.L.; Luz, R.K. Influence of porosity of low-density polyethylene media on the maturation process of biofilters used in recirculating aquaculture systems. Aquac. Int. 2018, 26, 1035–1049. [Google Scholar] [CrossRef]
- DeLong, D.P.; Losardo, T.M. How to Start a Biofilter; United States Department of Agriculture Southern Regional Aquaculture Center: Stoneville, MS, USA, 2012; p. 4. [Google Scholar]
- Navada, S.; Vadstein, O.; Spanu, C.; Mikkelsen, Ø.; Kolarevic, J. Biofilms remember: Osmotic stress priming as a microbial management strategy for improving salinity acclimation in nitrifying biofilms. Water Res. 2020, 176, 115732. [Google Scholar] [CrossRef]
- Li, C.; Liang, J.; Lin, X.; Xu, H.; Tadda, M.A.; Lan, L. Fast start-up strategies of MBBR for mariculture wastewater treatment. J. Environ. Manage. 2019, 248, 109267. [Google Scholar] [CrossRef]
- APHA. Standard Methods for Examination of Water and Wastewater, 20th ed.; American Public Health Association: Washington, DC, USA, 1999; p. 1325. ISBN 978-0875532356. [Google Scholar]
- Dias, J.; Bellingham, M.; Hassan, J.; Barrett, M.; Stephenson, T.; Soares, A. Influence of carrier media physical properties on start-up of moving attached growth systems. Bioresour. Technol. 2018, 266, 463–471. [Google Scholar] [CrossRef]
- Masser, M.P.; Rakocy, J.; Losardo, T.M. Recirculating Aquaculture Tank Production Systems; United States Department of Agriculture Southern Regional Aquaculture Center: Stoneville, MS, USA, 1999; p. 10. [Google Scholar]
- Avnimelech, Y.; Ritvo, G. Shrimp and fish pond soils: Processes and management. Aquaculture 2003, 220, 549–567. [Google Scholar] [CrossRef]
- Schneider, O.; Sereti, V.; Eding, E.H.; Verreth, J.A.J. Molasses as C source for heterotrophic bacteria production on solid fish waste. Aquaculture 2006, 261, 1239–1248. [Google Scholar] [CrossRef]
- Ruiz, P.; Vidal, J.M.; Sepulveda, D.; Torres, C.; Villouta, G.; Carrasco, C.; Aguilera, F.; Ruiz-Tagle, N.; Urrutia, H. Overview and future perspectives of nitrifying bacteria on biofilters for recirculating aquaculture systems. Rev. Aquac. 2020, 12, 1478–1494. [Google Scholar] [CrossRef]
- Sikora, M.; Nowosad, J.; Biegaj, M.; Kucharczyk, D.; Dębowski, M. The possibility of application of agglomerate elastomers (EPP) as media for biological bed in aquaculture. Aquac. Res. 2018, 49, 2988–2994. [Google Scholar] [CrossRef]
- Owatari, M.S.; Jesus, G.F.A.; de Melo Filho, M.E.S.; Lapa, K.R.; Martins, M.L.; Mouriño, J.L.P. Synthetic fibre as biological support in freshwater recirculating aquaculture systems (RAS). Aquac. Eng. 2018, 82, 56–62. [Google Scholar] [CrossRef]
- Żarski, D.; Kucharczyk, D.; Targońska, K.; Chyła, B.; Dobrołowicz, A. Dynamics of changes in nitrogen and phosphorus compounds during intensive rearing of ide, Leuciscus idus (L.), in a recirculating system. Arch. Polish Fish. 2008, 16, 459–467. [Google Scholar] [CrossRef]
- Pulkkinen, J.T.; Eriksson-Kallio, A.M.; Aalto, S.L.; Tiirola, M.; Koskela, J.; Kiuru, T.; Vielma, J. The effects of different combinations of fixed and moving bed bioreactors on rainbow trout (Oncorhynchus mykiss) growth and health, water quality and nitrification in recirculating aquaculture systems. Aquac. Eng. 2019, 85, 98–105. [Google Scholar] [CrossRef]
- Fernandes, P.M.; Pedersen, L.; Pedersen, P.B. Influence of fixed and moving bed biofilters on micro particle dynamics in a recirculating aquaculture system. Aquac. Eng. 2017, 78, 32–41. [Google Scholar] [CrossRef]
- Pulkkinen, J.T.; Kiuru, T.; Aalto, S.L.; Koskela, J.; Vielma, J. Startup and effects of relative water renewal rate on water quality and growth of rainbow trout (Oncorhynchus mykiss) in a unique RAS research platform. Aquac. Eng. 2018, 82, 38–45. [Google Scholar] [CrossRef]
- Shitu, A.; Zhu, S.; Qi, W.; Abubakar, M.A.; Liu, D. Performance of novel sponge biocarrier in MBBR treating recirculating aquaculture systems wastewater: Microbial community and kinetic study. J. Environ. Manag. 2020, 275, 111264. [Google Scholar] [CrossRef]
- Fu, H.; Wang, J.; Ren, H.; Ding, L. Acceleration of start-up of moving bed biofilm reactor at low temperature by adding specialized quorum sensing bacteria. Bioresour. Technol. 2022, 358, 127249. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, K.; Zimmermann, J.; Meyer, S.; Schulz, C. Start-up of recirculating aquaculture systems: How do water exchange rates influence pikeperch (Sander lucioperca) and water composition? Aquac. Eng. 2018, 83, 151–159. [Google Scholar] [CrossRef]
- Davidson, J.; Good, C.; Welsh, C.; Summerfelt, S.T. Comparing the effects of high vs. low nitrate on the health, performance, and welfare of juvenile rainbow trout Oncorhynchus mykiss within water recirculating aquaculture systems. Aquac. Eng. 2014, 59, 30–40. [Google Scholar] [CrossRef]
- Stavrakidis-Zachou, O.; Ernst, A.; Steinbach, C.; Wagner, K.; Waller, U. Development of denitrification in semi-automated moving bed biofilm reactors operated in a marine recirculating aquaculture system. Aquac. Int. 2019, 27, 1485–1501. [Google Scholar] [CrossRef]
- He, Q.; Zhang, D.; Main, K.; Feng, C.; Ergas, S.J. Biological denitrification in marine aquaculture systems: A multiple electron donor microcosm study. Bioresour. Technol. 2018, 263, 340–349. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Shi, C.; Yu, Y.; Ruan, Y.; Kong, D.; Lv, X.; Xu, P.; Awasthi, M.K.; Dong, M. Interrelationships between tetracyclines and nitrogen cycling processes mediated by microorganisms: A review. Bioresour. Technol. 2021, 319, 124036. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.; Ngo, H.H.; Guo, W.; Johnston, A.; Listowski, A. Effects of sponge size and type on the performance of an up-flow sponge bioreactor in primary treated sewage effluent treatment. Bioresour. Technol. 2010, 101, 1416–1420. [Google Scholar] [CrossRef]
- Massoompour, A.R.; Borghei, S.M.; Raie, M. Enhancement of biological nitrogen removal performance using novel carriers based on the recycling of waste materials. Water Res. 2020, 170, 115340. [Google Scholar] [CrossRef]
- Chen, X.; Kong, L.; Wang, X.; Tian, S.; Xiong, Y. Accelerated start-up of moving bed biofilm reactor by using a novel suspended carrier with porous surface. Bioprocess Biosyst. Eng. 2015, 38, 273–285. [Google Scholar] [CrossRef]
- Bonisławska, M.; Nędzarek, A.; Rybczyk, A. Assessment of the use of precipitating agents and ceramic membranes for treatment of effluents with high concentrations of nitrogen and phosphorus from recirculating aquaculture systems. Aquac. Res. 2019, 50, 1248–1256. [Google Scholar] [CrossRef]
- Santorio, S.; del Rio, A.V.; Amorim, C.L.; Arregui, L.; Castro, P.M.L.; Mosquera-Corral, A. Pilot-scale continuous flow granular reactor for the treatment of extremely low-strength recirculating aquaculture system wastewater. J. Environ. Chem. Eng. 2022, 10, 107247. [Google Scholar] [CrossRef]
- Rojas-Tirado, P.; Bovbjerg, P.; Vadstein, O.; Pedersen, L. Microbial dynamics in RAS water: Effects of adding acetate as a biodegradable carbon-source. Aquac. Eng. 2019, 84, 106–116. [Google Scholar] [CrossRef]
| RK Plast | Mutag-BioChip30 | LevaPor | ||
|---|---|---|---|---|
| BR-1 | BR-2 | BR-3 | ||
| Shape | saddle | round chips | cube | |
| Size | mm | 30 × 15 | 30 × 1.1 | 20 × 20 × 7 |
| Weight | kg m−3 | 145 | 165 | 26–28 |
| Surface (SSA) | m2 m−3 | 700 | 5500 | 2700 |
| Composition | Polypropylene | Polyethylene | Polyurethane + activated carbon | |
 |  |  | ||
| Bioreactor | |||||
|---|---|---|---|---|---|
| BR-1 | BR-2 | BR-3 | |||
| NO2−-N | mg N dm−3 | range | 0.009–5.198 | 0.012–4.382 | 0.016–0.982 |
| mean | 0.828 b | 0.439 ab | 0.165 a | ||
| median | 0.128 | 0.086 | 0.076 | ||
| SD | 1.382 | 1.081 | 0.246 | ||
| NO3−-N | mg N dm−3 | range | 0.198–6.521 | 0.335–6.778 | 0.600–7.326 |
| mean | 2.851 a | 3.585 a | 3.496 a | ||
| median | 2.754 | 3.985 | 3.774 | ||
| SD | 2.030 | 1.887 | 1.908 | ||
| NH4+-N | mg N dm−3 | range | 0.024–1.290 | 0.026–0.834 | 0.020–0.728 |
| mean | 0.271 b | 0.158 ab | 0.105 a | ||
| median | 0.084 | 0.075 | 0.048 | ||
| SD | 0.350 | 0.206 | 0.168 | ||
| TN | mg N dm−3 | range | 1.230–12.749 | 1.302–12.716 | 1.067–15.859 |
| mean | 7.790 a | 8.310 a | 9.711 a | ||
| median | 8.367 | 8.562 | 10.617 | ||
| SD | 3.523 | 3.456 | 4.182 | ||
| TRP | mg P dm−3 | range | 0.072–0.533 | 0.092–0.412 | 0.075–0.529 |
| mean | 0.187 a | 0.188 a | 0.293 b | ||
| median | 0.214 | 0.150 | 0.239 | ||
| SD | 0.127 | 0.096 | 0.1560 | ||
| TP | mg P dm−3 | range | 0.102–0.548 | 0.119–0.423 | 0.132–0.541 |
| mean | 0.251 a | 0.242 a | 0.326 b | ||
| median | 0.232 | 0.229 | 0.272 | ||
| SD | 0.116 | 0.077 | 0.152 | ||
| TIC | mg C dm−3 | range | 23.7–45.2 | 23.9–44.4 | 19.3–43.8 |
| mean | 32.3 a | 31.9 a | 29.6 a | ||
| median | 30.0 | 28.9 | 27.6 | ||
| SD | 6.0 | 6.3 | 6.5 | ||
| TOC | mg C dm−3 | range | 9.5–14.6 | 9.2–18.8 | 8.6–14.1 |
| mean | 11.1 b | 11.6 b | 10.3 a | ||
| median | 11.0 | 11.0 | 9.6 | ||
| SD | 1.2 | 2.1 | 1.6 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nędzarek, A.; Bonisławska, M.; Tórz, A.; Tański, A.; Formicki, K. Effect of Filter Medium on Water Quality during Passive Biofilter Activation in a Recirculating Aquaculture System for Oncorhynchus mykiss. Energies 2022, 15, 6890. https://doi.org/10.3390/en15196890
Nędzarek A, Bonisławska M, Tórz A, Tański A, Formicki K. Effect of Filter Medium on Water Quality during Passive Biofilter Activation in a Recirculating Aquaculture System for Oncorhynchus mykiss. Energies. 2022; 15(19):6890. https://doi.org/10.3390/en15196890
Chicago/Turabian StyleNędzarek, Arkadiusz, Małgorzata Bonisławska, Agnieszka Tórz, Adam Tański, and Krzysztof Formicki. 2022. "Effect of Filter Medium on Water Quality during Passive Biofilter Activation in a Recirculating Aquaculture System for Oncorhynchus mykiss" Energies 15, no. 19: 6890. https://doi.org/10.3390/en15196890
APA StyleNędzarek, A., Bonisławska, M., Tórz, A., Tański, A., & Formicki, K. (2022). Effect of Filter Medium on Water Quality during Passive Biofilter Activation in a Recirculating Aquaculture System for Oncorhynchus mykiss. Energies, 15(19), 6890. https://doi.org/10.3390/en15196890







