Fractionation of Lignocellulosic Fibrous Straw Digestate by Combined Hydrothermal and Enzymatic Treatment
Abstract
:1. Introduction
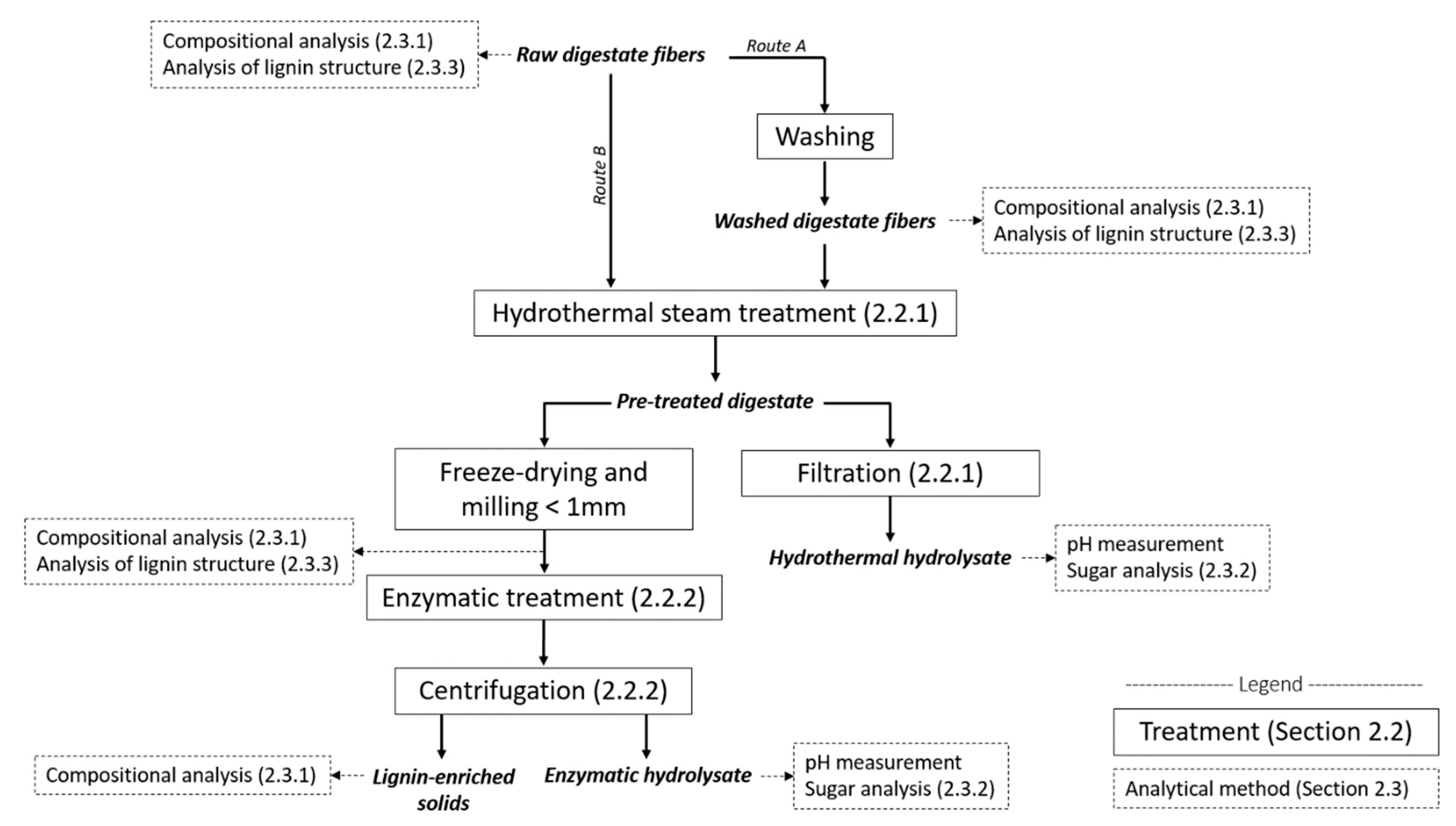
2. Materials and Methods
2.1. Materials
2.2. Treatments
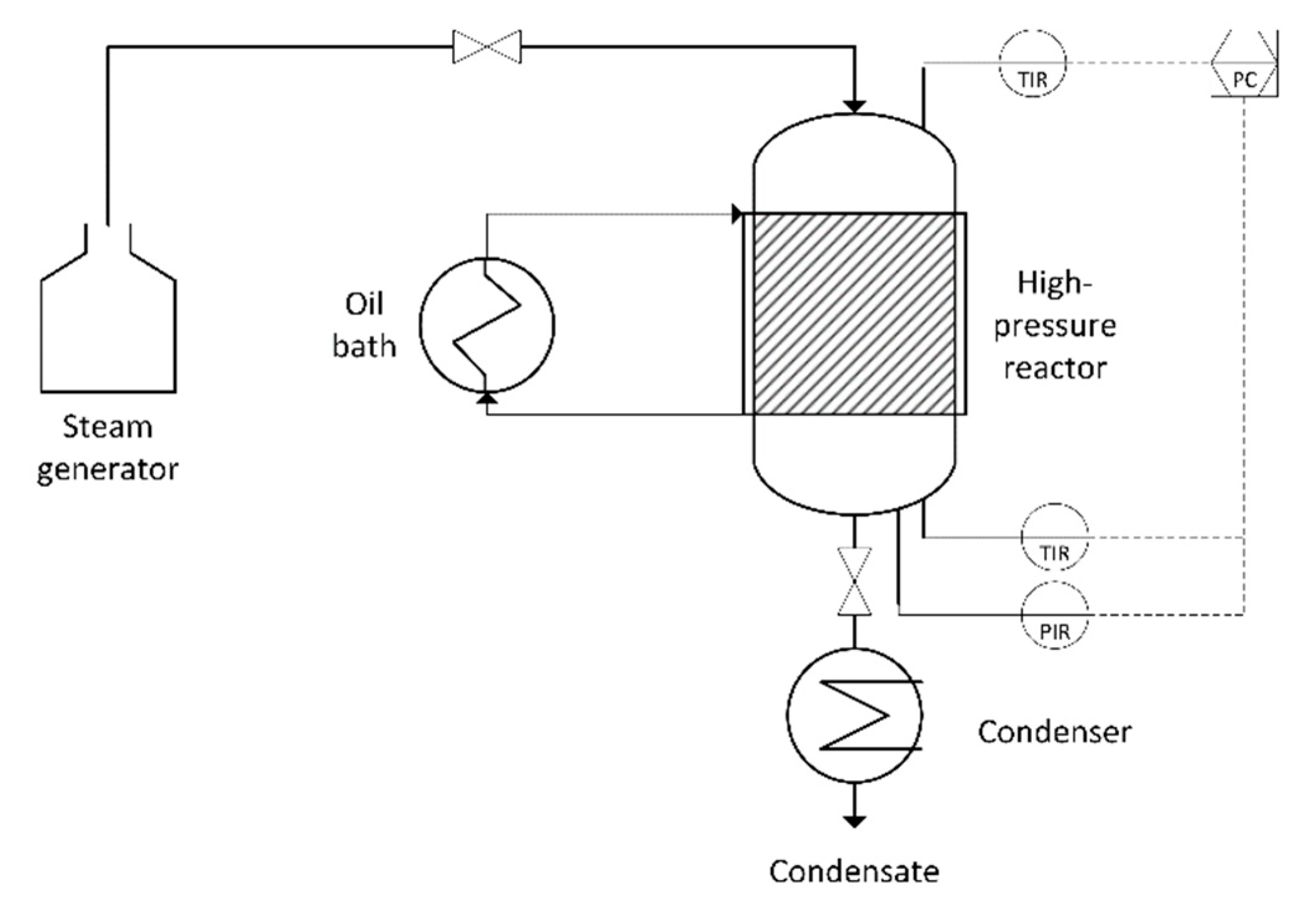
2.2.1. Hydrothermal Steam Treatment
2.2.2. Enzymatic Treatment
2.3. Analytical Methods
2.3.1. Compositional Analysis
2.3.2. Sugar Analysis (Monomers/Oligomers)
2.3.3. Analysis of Lignin Structure
2.3.4. Statistical Analysis
3. Results
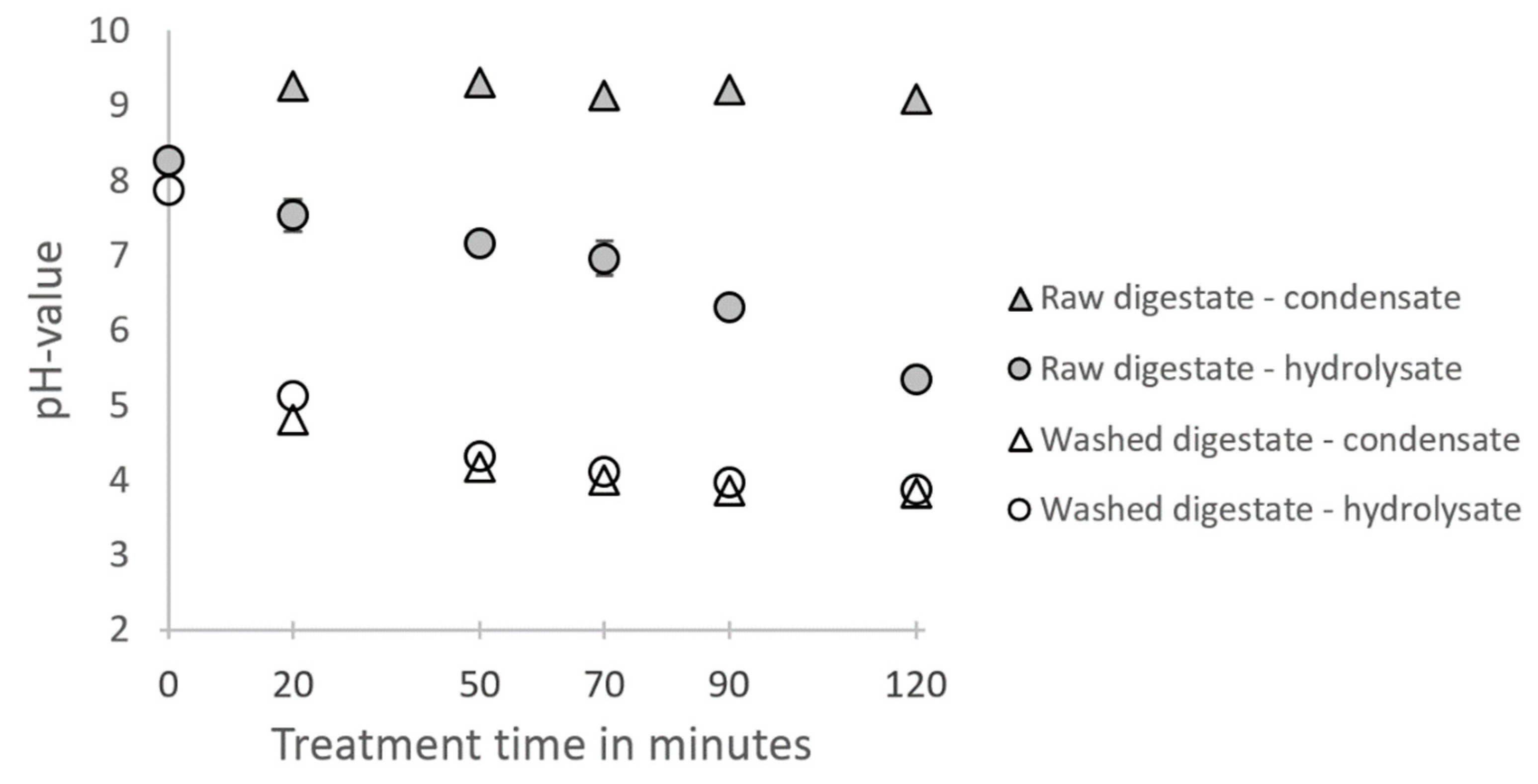
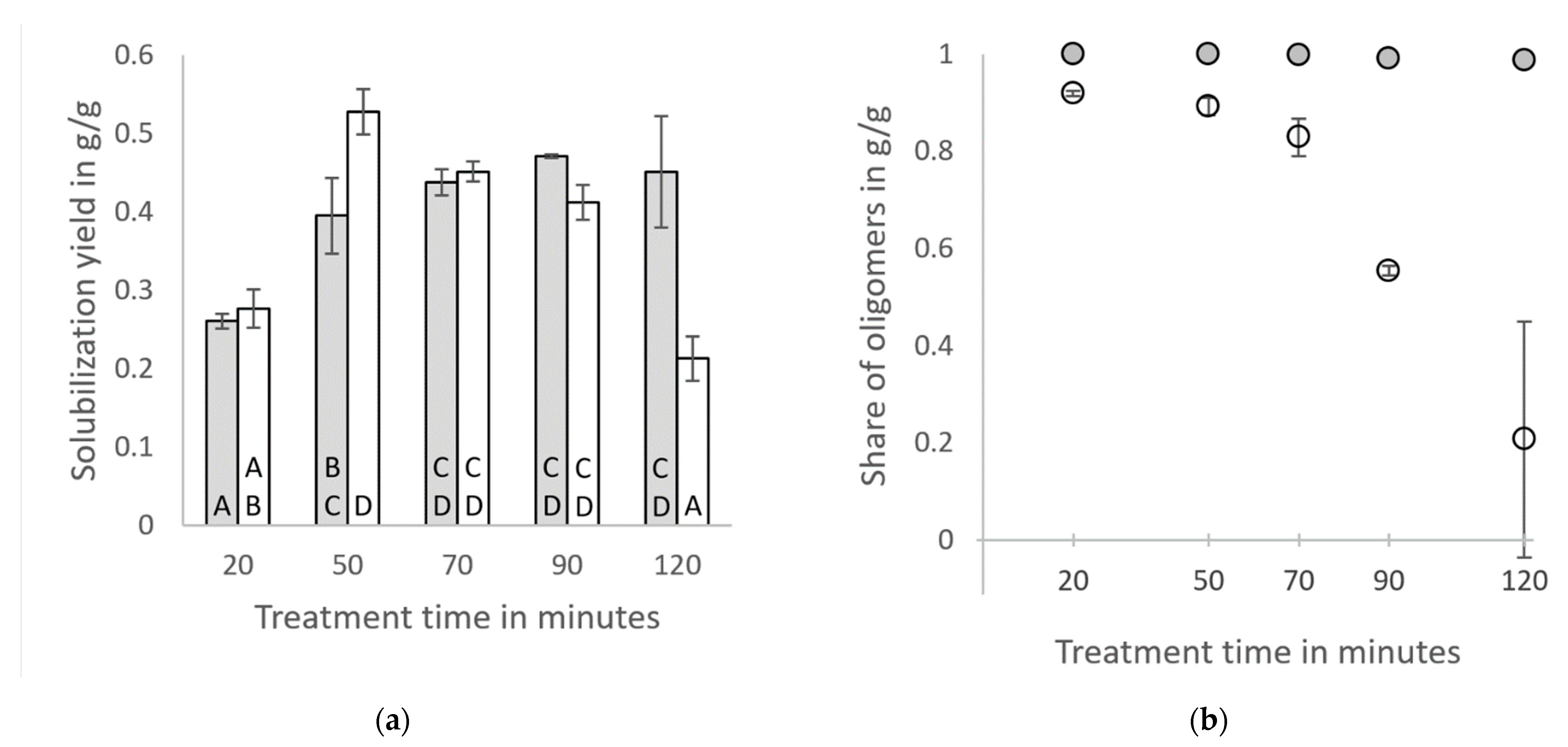
3.1. Solubilization of Hemicellulose by Hydrothermal Steam Treatment
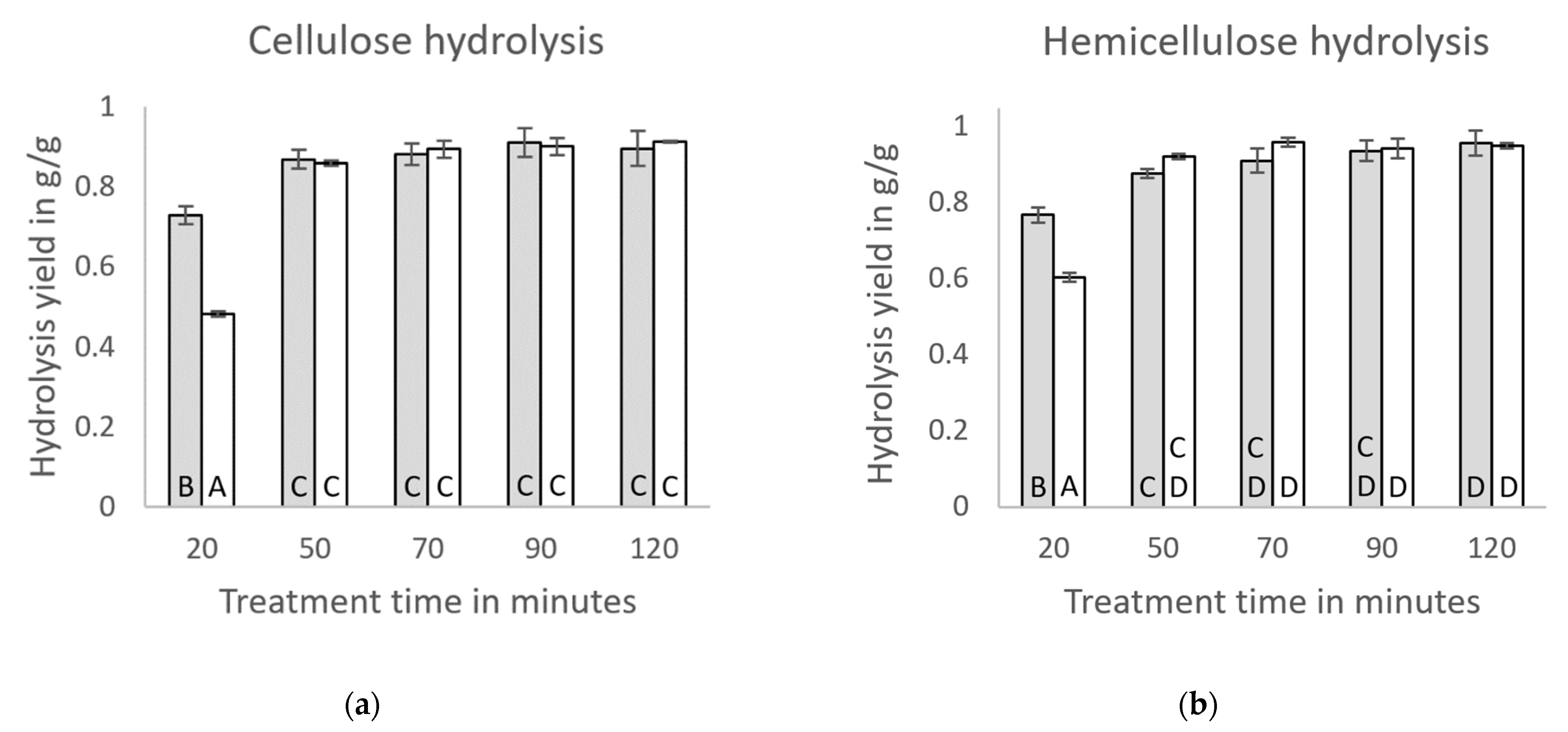
3.2. Hydrolysis of Cellulose by Enzymatic Treatment
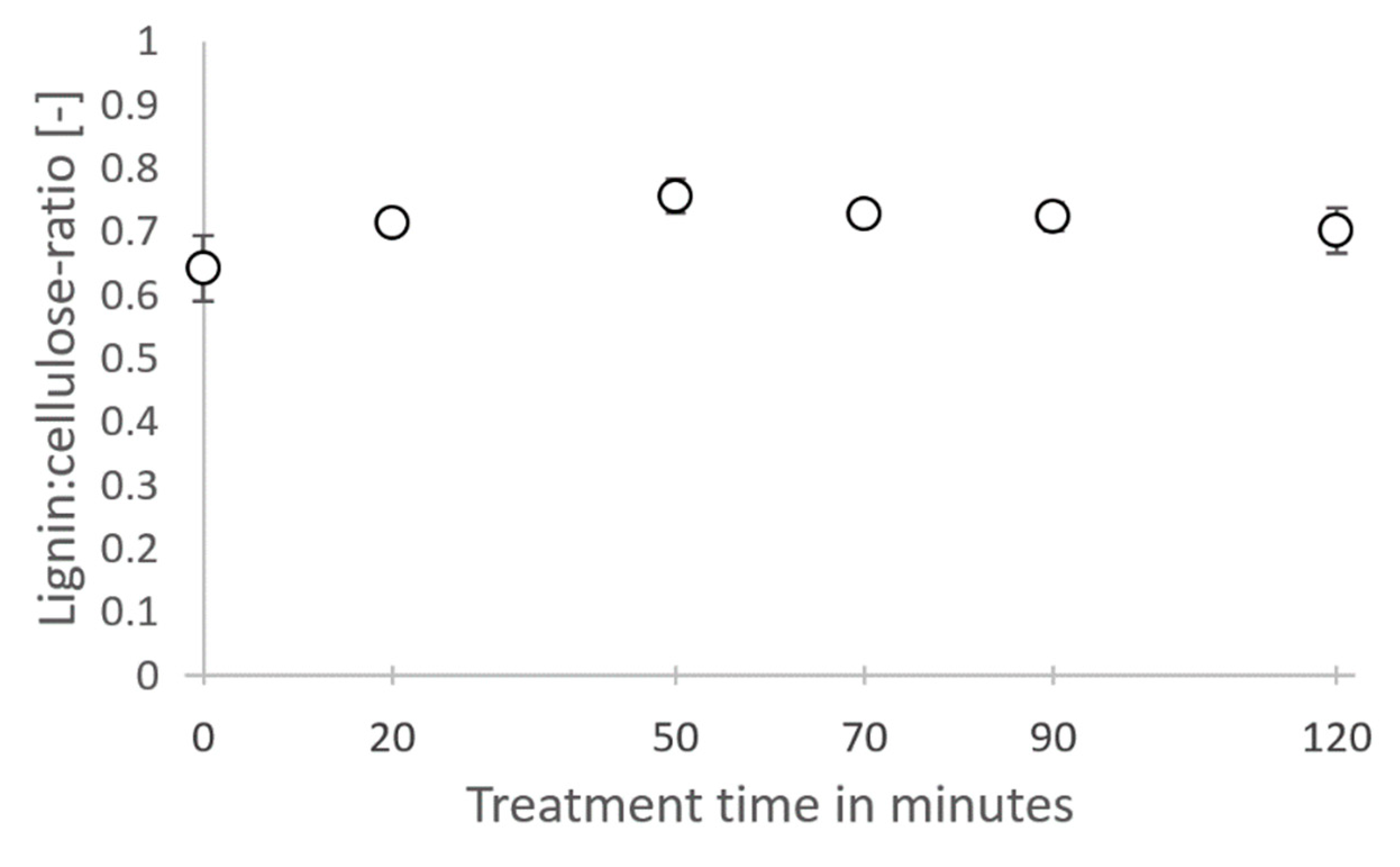
3.3. Structure of Lignin after Hydrothermal Steam Treatment
4. Discussion
4.1. Solubilization of Hemicellulose by Hydrothermal Steam Treatment
4.2. Hydrolysis of Cellulose by Enzymatic Treatment
4.3. Structure of Lignin after Hydrothermal Steam Treatment
5. Conclusions
- About half of the hemicellulose contained in the digestate can be solubilized and recovered in the hydrolysate after a hydrothermal steam treatment at 180 °C for at least 50 min. During the hydrothermal treatment of the raw digestate, much higher pH-values are observed than during the treatment of the washed digestate, most likely due to the contained ammonia in the raw digestate. Higher pH values prevent the hydrolysis and further degradation of xylans for long treatment times. However, if xylo-oligosaccharides are a targeted product, it has to be considered that the hydrolysates from the raw digestate are very complex mixtures, making a separation challenging.
- Cellulose mainly stays with the solid fibers and can be enzymatically hydrolyzed to glucose with a yield of around 90% after a hydrothermal steam treatment at 180 °C for 50 min for both the raw and the washed digestate. Furthermore, the remaining hemicellulose can be enzymatically hydrolyzed nearly completely at these conditions. For shorter treatment times, a higher enzymatic degradability of carbohydrates is observed for the raw digestate than for the washed digestate.
- A solid enriched in lignin remains after both treatment steps. The lignin is partially degraded due to depolymerization and repolymerization reactions, which is indicated by a reduced content of ether and ester bonds. The reduced ether content as well as the high ash content should be considered if a catalytic depolymerization is targeted.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Details about the Methodology
Appendix A.1. Severity Factor of Hydrothermal Treatment
Appendix A.2. Hemicellulose Solubilization after Hydrothermal Treatment
Appendix A.3. Hydrolysis Yields after Enzymatic Treatment
Appendix A.4. Thioacidolysis
Appendix A.5. Content of Ester-Bound Phenolic Acids
Appendix A.6. Considerations and Calculations on the Linkages in Lignin
Appendix A.6.1. Estimation of the Content of Ether Linkages from the Results of Thioacidolysis
Appendix A.6.2. Estimation of the Content in Ester Linkages with P-Coumaric Acid from the Results of Saponification
Appendix B. Kinetic Modeling of Linkage Degradation

| Sum of Squared Errors | |||
|---|---|---|---|
| [s−1] | [s−1] | [mole%2] | |
| and both as free variables | 1.1 × 10−4 | 1.0 × 10−4 | 14.9 |
| free, fixed- > very low | 1.5 × 10−4 | 1.7 × 10−7 | 20.4 |
| free, fixed- > very high | 1.1 × 10−4 | 1.7 × 10−3 | 14.9 |
Appendix C. Additional Experimental Results
Appendix C.1. Indications for Occurring Repolymerization Reactions in Lignin during Hydrothermal Treatment
| p-Hydroxy-Benzoic Acid | Vanillic Acid | Vanillin | pCA | FA | ||
|---|---|---|---|---|---|---|
| Concentration in hydrolysate | [mg/L] | 6.5 | 6.6 | 19.4 | 53.0 | 15.0 |
| Released amount in hydrolysate per 100 g digestate | [mg/100 g] | 2.8 | 2.8 | 8.3 | 22.8 | 6.4 |
| Released amount in hydrolysate per 100 g digestate expected a | [mg/100 g] | 383.0 | 55.6 | |||
| Recovery | [%] | 5.9 | 11.6 |
Appendix C.2. Composition of Solid Residues after Enzymatic Hydrolysis
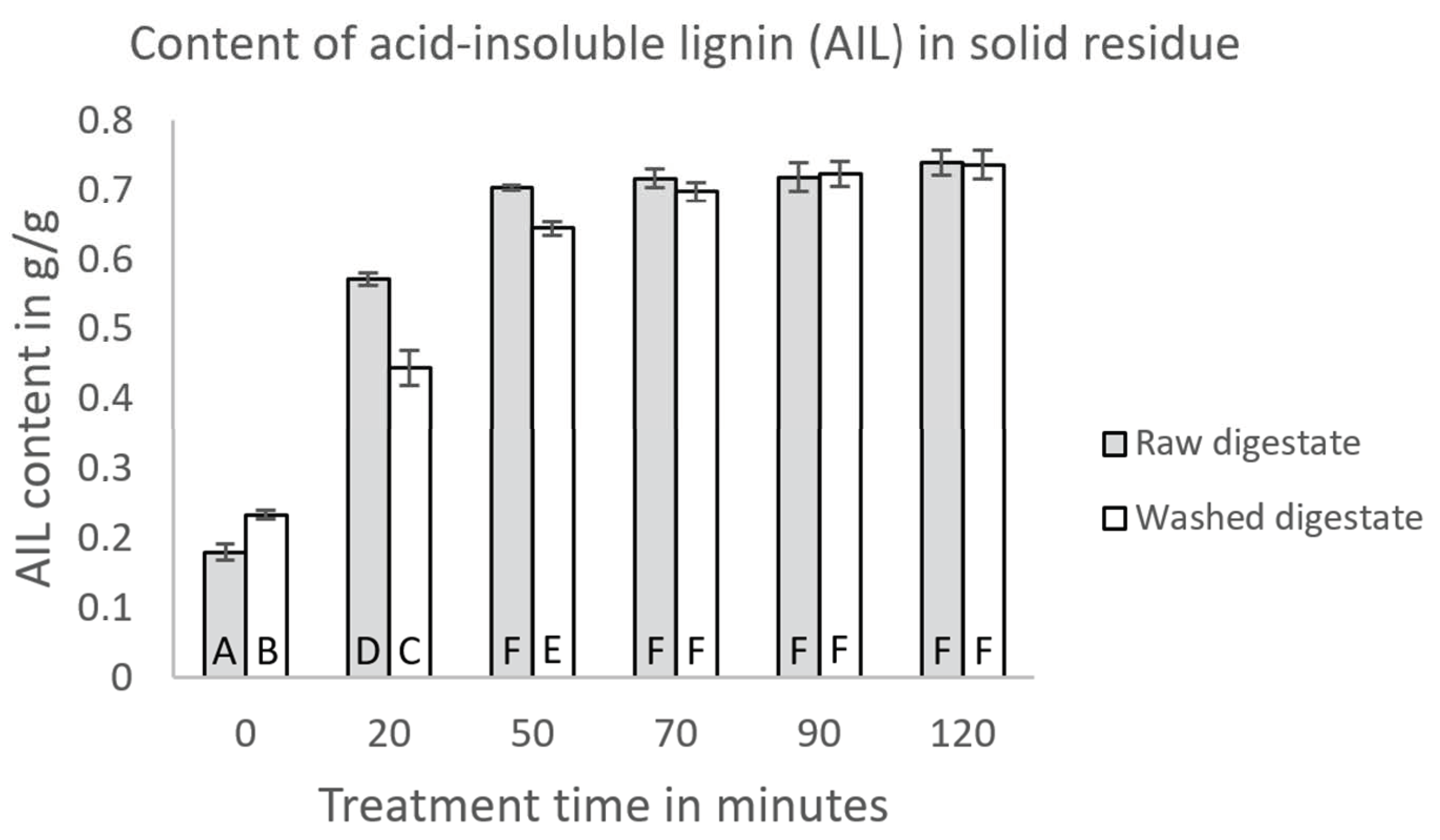
| Initial Substrate | Hydrothermal Treatment Time | AIL Content | Protein Content | Ash Content |
|---|---|---|---|---|
| Washed digestate | 20 | 44.4 | 7.5 | 4.1 |
| Washed digestate | 70 | 69.7 | 10.0 | 10.0 |
| Washed digestate | 120 | 73.6 | 10.3 | 8.5 |
| Raw digestate | 120 | 73.9 | 10.9 | 7.7 |
Appendix C.3. Results from Lignin Analysis
| (a) | Washed Digestate | |||
| Analysis of Ether Bonds | Analysis of Ester Bonds | |||
| Treatment Time | Released Monomers by Thioacidolysis | Resulting Ether Bond Content | Released Monomers by Saponification | Resulting Ester Bond Content |
| [min] | [µmole/gLignin] | [mole%] | [mg/gLignin] | [mole%] |
| 0 | 620 ± 52 | 34.8 ± 1.5 | 31.5 ± 1.7 | 3.7 ± 0.2 |
| 20 | 409 ± 82 | 28.2 ± 2.8 | 25.2 ± 1.4 | 3.0 ± 0.2 |
| 50 | 296 ± 6 | 24.0 ± 0.3 | 18.5 ± 1.8 | 2.2 ± 0.2 |
| 70 | 207 ± 5 | 20.0 ± 0.3 | 14.9 ± 0.9 | 1.8 ± 0.1 |
| 90 | 190 ± 19 | 19.2 ± 1.0 | 14.4 ± 0.7 | 1.7 ± 0.1 |
| 120 | 167 ± 2 | 18.0 ± 0.1 | 12.1 ± 1.0 | 1.4 ± 0.1 |
| (b) | Raw Digestate | |||
| Analysis of Ether Bonds | Analysis of Ester Bonds | |||
| Treatment Time | Released Monomers by Thioacidolysis | Resulting Ether Bond Content | Released Monomers by Saponification | Resulting Ester Bond Content |
| [min] | [µmole/gLignin] | [mole%] | [mg/gLignin] | [mole%] |
| 0 | 639 | 35.5 | 36.2 | 4.3 |
| 20 | 307 | 24.5 | 20.3 | 2.4 |
| 50 | 183 | 18.9 | 11.0 | 1.3 |
| 70 | 168 | 18.1 | 8.8 | 1.1 |
| 90 | 175 | 18.4 | 8.9 | 1.1 |
| 120 | 159 | 17.6 | 7.0 | 0.8 |
References
- Andersen, L.; Lamp, A.; Dieckmann, C.; Baetge, S.; Schmidt, L.-M.; Kaltschmitt, M. Biogas plants as key units of biorefinery concepts: Options and their assessment. J. Biotechnol. 2018, 283, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Sayara, T.; Sánchez, A. A Review on Anaerobic Digestion of Lignocellulosic Wastes: Pretreatments and Operational Conditions. Appl. Sci. 2019, 9, 4655. [Google Scholar] [CrossRef]
- Xu, N.; Liu, S.; Xin, F.; Zhou, J.; Jia, H.; Xu, J.; Jiang, M.; Dong, W. Biomethane Production from Lignocellulose: Biomass Recalcitrance and Its Impacts on Anaerobic Digestion. Front. Bioeng. Biotechnol. 2019, 7, 191. [Google Scholar] [CrossRef] [PubMed]
- Chandler, J.; Jewell, W. Predicting Methane Fermentation Biodegradability: Final Report; Solar Energy Research Institute: Golden, CO, USA, 1980. [Google Scholar]
- Triolo, J.M.; Sommer, S.G.; Møller, H.B.; Weisbjerg, M.R.; Jiang, X.Y. A new algorithm to characterize biodegradability of biomass during anaerobic digestion: Influence of lignin concentration on methane production potential. Bioresour. Technol. 2011, 102, 9395–9402. [Google Scholar] [CrossRef]
- Khan, M.U.; Ahring, B.K. Lignin degradation under anaerobic digestion: Influence of lignin modifications—A review. Biomass Bioenergy 2019, 128, 105325. [Google Scholar] [CrossRef]
- Li, W.; Khalid, H.; Zhu, Z.; Zhang, R.; Liu, G.; Chen, C.; Thorin, E. Methane production through anaerobic digestion: Participation and digestion characteristics of cellulose, hemicellulose and lignin. Appl. Energy 2018, 226, 1219–1228. [Google Scholar] [CrossRef]
- Santi, G.; Proietti, S.; Moscatello, S.; Stefanoni, W.; Battistelli, A. Anaerobic digestion of corn silage on a commercial scale: Differential utilization of its chemical constituents and characterization of the solid digestate. Biomass Bioenergy 2015, 83, 17–22. [Google Scholar] [CrossRef]
- Tambone, F.; Genevini, P.; D’Imporzano, G.; Adani, F. Assessing amendment properties of digestate by studying the organic matter composition and the degree of biological stability during the anaerobic digestion of the organic fraction of MSW. Bioresour. Technol. 2009, 100, 3140–3142. [Google Scholar] [CrossRef]
- Kumar, P.; Barrett, D.M.; Delwiche, M.J.; Stroeve, P. Methods for Pretreatment of Lignocellulosic Biomass for Efficient Hydrolysis and Biofuel Production. Ind. Eng. Chem. Res. 2009, 48, 3713–3729. [Google Scholar] [CrossRef]
- Mankar, A.R.; Pandey, A.; Modak, A.; Pant, K.K. Pretreatment of lignocellulosic biomass: A review on recent advances. Bioresour. Technol. 2021, 334, 125235. [Google Scholar] [CrossRef]
- Schutyser, W.; Renders, T.; van den Bosch, S.; Koelewijn, S.-F.; Beckham, G.T.; Sels, B.F. Chemicals from lignin: An interplay of lignocellulose fractionation, depolymerisation, and upgrading. Chem. Soc. Rev. 2018, 47, 852–908. [Google Scholar] [CrossRef] [PubMed]
- Garrote, G.; Domínguez, H.; Parajó, J.C. Hydrothermal processing of lignocellulosic materials. Holz Roh Werkst. 1999, 57, 191–202. [Google Scholar] [CrossRef]
- Jeong, S.-Y.; Lee, J.-W. Hydrothermal Treatment. In Pretreatment of Biomass; Elsevier: Amsterdam, The Netherlands, 2015; pp. 61–74. ISBN 9780128000809. [Google Scholar]
- Heitz, M.; Carrasco, F.; Rubio, M.; Chauvette, G.; Chornet, E.; Jaulin, L.; Overend, R.P. Generalized correlations for the aqueous liquefaction of lignocellulosics. Can. J. Chem. Eng. 1986, 64, 647–650. [Google Scholar] [CrossRef]
- Dogaris, I.; Karapati, S.; Mamma, D.; Kalogeris, E.; Kekos, D. Hydrothermal processing and enzymatic hydrolysis of sorghum bagasse for fermentable carbohydrates production. Bioresour. Technol. 2009, 100, 6543–6549. [Google Scholar] [CrossRef] [PubMed]
- Scherzinger, M.; Kaltschmitt, M. Thermal pre-treatment options to enhance anaerobic digestibility—A review. Renew. Sustain. Energy Rev. 2021, 137, 110627. [Google Scholar] [CrossRef]
- Yelle, D.J.; Kaparaju, P.; Hunt, C.G.; Hirth, K.; Kim, H.; Ralph, J.; Felby, C. Two-Dimensional NMR Evidence for Cleavage of Lignin and Xylan Substituents in Wheat Straw Through Hydrothermal Pretreatment and Enzymatic Hydrolysis. Bioenergy Res. 2013, 6, 211–221. [Google Scholar] [CrossRef]
- Trajano, H.L.; Engle, N.L.; Foston, M.; Ragauskas, A.J.; Tschaplinski, T.J.; Wyman, C.E. The fate of lignin during hydrothermal pretreatment. Biotechnol. Biofuels 2013, 6, 110. [Google Scholar] [CrossRef]
- Reynolds, W.; Kirsch, C.; Smirnova, I. Thermal-Enzymatic Hydrolysis of Wheat Straw in a Single High Pressure Fixed Bed. Chem. Ing. Tech. 2015, 87, 1305–1312. [Google Scholar] [CrossRef]
- Li, J.; Henriksson, G.; Gellerstedt, G. Lignin depolymerization/repolymerization and its critical role for delignification of aspen wood by steam explosion. Bioresour. Technol. 2007, 98, 3061–3068. [Google Scholar] [CrossRef]
- Li, J.; Gellerstedt, G.; Toven, K. Steam explosion lignins; their extraction, structure and potential as feedstock for biodiesel and chemicals. Bioresour. Technol. 2009, 100, 2556–2561. [Google Scholar] [CrossRef]
- Scherzinger, M.; Kulbeik, T.; Kaltschmitt, M. Autoclave pre-treatment of green wastes—Effects of temperature, residence time and rotation speed on fuel properties. Fuel 2020, 273, 117796. [Google Scholar] [CrossRef]
- Kulbeik, T.; Scherzinger, M.; Höfer, I.; Kaltschmitt, M. Autoclave pre-treatment of foliage—Effects of temperature, residence time and water content on solid biofuel properties. Renew. Energy 2021, 171, 275–286. [Google Scholar] [CrossRef]
- Aragón-Briceño, C.; Ross, A.B.; Camargo-Valero, M.A. Evaluation and comparison of product yields and bio-methane potential in sewage digestate following hydrothermal treatment. Appl. Energy 2017, 208, 1357–1369. [Google Scholar] [CrossRef]
- Ekpo, U.; Ross, A.B.; Camargo-Valero, M.A.; Williams, P.T. A comparison of product yields and inorganic content in process streams following thermal hydrolysis and hydrothermal processing of microalgae, manure and digestate. Bioresour. Technol. 2016, 200, 951–960. [Google Scholar] [CrossRef] [PubMed]
- Wilk, M.; Śliz, M.; Gajek, M. The effects of hydrothermal carbonization operating parameters on high-value hydrochar derived from beet pulp. Renew. Energy 2021, 177, 216–228. [Google Scholar] [CrossRef]
- Ding, L.; Cheng, J.; Qiao, D.; Yue, L.; Li, Y.-Y.; Zhou, J.; Cen, K. Investigating hydrothermal pretreatment of food waste for two-stage fermentative hydrogen and methane co-production. Bioresour. Technol. 2017, 241, 491–499. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, F.; Shen, X.; Yi, W.; Li, Z.; Li, Y.; Tian, C. Comparison study on fuel properties of hydrochars produced from corn stalk and corn stalk digestate. Energy 2018, 165, 527–536. [Google Scholar] [CrossRef]
- Andersen, L.F.; Parsin, S.; Lüdtke, O.; Kaltschmitt, M. Biogas production from straw—The challenge feedstock pretreatment. Biomass Conv. Bioref. 2022, 12, 379–402. [Google Scholar] [CrossRef]
- Zieliński, M.; Kisielewska, M.; Dudek, M.; Rusanowska, P.; Nowicka, A.; Krzemieniewski, M.; Kazimierowicz, J.; Dębowski, M. Comparison of microwave thermohydrolysis and liquid hot water pretreatment of energy crop Sida hermaphrodita for enhanced methane production. Biomass Bioenergy 2019, 128, 105324. [Google Scholar] [CrossRef]
- Kabel, M.; Carvalheiro, F.; Garrote, G.; Avgerinos, E.; Koukios, E.; Parajó, J.C.; Gírio, F.; Schols, H.; Voragen, A. Hydrothermally treated xylan rich by-products yield different classes of xylo-oligosaccharides. Carbohydr. Polym. 2002, 50, 47–56. [Google Scholar] [CrossRef]
- Conrad, M.; Smirnova, I. Two-Step Autohydrolysis Pretreatment: Towards High Selective Full Fractionation of Wheat Straw. Chem. Ing. Tech. 2020, 92, 1723–1732. [Google Scholar] [CrossRef]
- Sun, X.F.; Xu, F.; Sun, R.C.; Wang, Y.X.; Fowler, P.; Baird, M.S. Characteristics of degraded lignins obtained from steam exploded wheat straw. Polym. Degrad. Stab. 2004, 86, 245–256. [Google Scholar] [CrossRef]
- Ambye-Jensen, M.; Thomsen, S.T.; Kádár, Z.; Meyer, A.S. Ensiling of wheat straw decreases the required temperature in hydrothermal pretreatment. Biotechnol. Biofuels 2013, 6, 116. [Google Scholar] [CrossRef] [PubMed]
- DIN EN ISO 18122. Biogene Festbrennstoffe—Bestimmung des Aschegehaltes (ISO/DIS 18122:2021); Deutsche und Englische Fassung prEN ISO 18122:2021. Beuth Verlag GmbH: Berlin, Germany, 2021.
- Diedrich, H.; Stahl, A.; Frerichs, H. NCHS-Elementaranalyse: M02.001. 02., Hamburg. 2019. Available online: https://www.tuhh.de/zentrallabor/methoden/ac-methoden/m02001.html (accessed on 22 April 2021).
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass: Laboratory Analytical Procedure (LAP) NREL/TP-510-42618. 2012. Available online: https://www.nrel.gov/docs/gen/fy13/42618.pdf (accessed on 10 August 2021).
- Sluiter, A.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D. Determination of Extractives in Biomass: Laboratory Analytical Procedure (LAP) NREL/TP-510-42620. 2005. Available online: https://www.nrel.gov/docs/gen/fy08/42620.pdf (accessed on 10 August 2021).
- Scholz, M.; Stahl, A. Titrimetrische Bestimmung von Ammonium-Stickstoff. M02.014. 01., Hamburg. 2016. Available online: https://www.tuhh.de/zentrallabor/methoden/ac-methoden/m02014.html (accessed on 22 April 2021).
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D. Determination of Sugars, Byproducts, and Degradation Products in Liquid Fraction Process Samples: Laboratory Analytical Procedure (LAP) NREL/TP-510-42623. 2006. Available online: https://www.nrel.gov/docs/gen/fy08/42623.pdf (accessed on 14 February 2022).
- Rolando, C.; Monties, B.; Lapierre, C. Thioacidolysis. In Methods in Lignin Chemistry; Timell, T.E., Lin, S.Y., Dence, C.W., Eds.; Springer: Berlin/Heidelberg, Germany, 1992; pp. 334–349. ISBN 978-3-642-74067-1. [Google Scholar]
- Yue, F.; Lu, F.; Sun, R.-C.; Ralph, J. Syntheses of lignin-derived thioacidolysis monomers and their uses as quantitation standards. J. Agric. Food Chem. 2012, 60, 922–928. [Google Scholar] [CrossRef] [PubMed]
- Linh, T.N.; Fujita, H.; Sakoda, A. Release kinetics of esterified p-coumaric acid and ferulic acid from rice straw in mild alkaline solution. Bioresour. Technol. 2017, 232, 192–203. [Google Scholar] [CrossRef] [PubMed]
- Galkin, M.V.; Samec, J.S.M. Lignin Valorization through Catalytic Lignocellulose Fractionation: A Fundamental Platform for the Future Biorefinery. ChemSusChem 2016, 9, 1544–1558. [Google Scholar] [CrossRef] [PubMed]
- Galkin, M.V.; Smit, A.T.; Subbotina, E.; Artemenko, K.A.; Bergquist, J.; Huijgen, W.J.J.; Samec, J.S.M. Hydrogen-free catalytic fractionation of woody biomass. ChemSusChem 2016, 9, 3280–3287. [Google Scholar] [CrossRef] [PubMed]
- Yan, N.; Zhao, C.; Dyson, P.J.; Wang, C.; Liu, L.; Kou, Y. Selective degradation of wood lignin over noble-metal catalysts in a two-step process. ChemSusChem 2008, 1, 626–629. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Wang, F.; Cai, J.; Wang, Y.; Zhang, J.; Yu, W.; Xu, J. Lignin depolymerization (LDP) in alcohol over nickel-based catalysts via a fragmentation–hydrogenolysis process. Energy Environ. Sci. 2013, 6, 994. [Google Scholar] [CrossRef]
- Feghali, E.; Carrot, G.; Thuéry, P.; Genre, C.; Cantat, T. Convergent reductive depolymerization of wood lignin to isolated phenol derivatives by metal-free catalytic hydrosilylation. Energy Environ. Sci. 2015, 8, 2734–2743. [Google Scholar] [CrossRef]
- Eckstein, P.P. Angewandte Statistik Mit SPSS: Praktische Einführung für Wirtschaftswissenschaftler; 3., vollst. überarb. und erw. Aufl.; Gabler: Wiesbaden, Germany, 2000; ISBN 3-409-32232-9. [Google Scholar]
- Conrad, M.; Smirnova, I. Counter-Current Suspension Extraction Process of Lignocellulose in Biorefineries to Reach Low Water Consumption, High Extraction Yields, and Extract Concentrations. Processes 2021, 9, 1585. [Google Scholar] [CrossRef]
- Zhao, C.; Shao, Q.; Chundawat, S.P.S. Recent advances on ammonia-based pretreatments of lignocellulosic biomass. Bioresour. Technol. 2020, 298, 122446. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Lee, Y.Y.; Kim, T.H. A review on alkaline pretreatment technology for bioconversion of lignocellulosic biomass. Bioresour. Technol. 2016, 199, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Overend, R.P.; Chornet, E. Fractionation of lignocellulosics by steam-aqueous pretreatments. Phil. Trans. R. Soc. Lond. A 1987, 321, 523–536. [Google Scholar] [CrossRef]
- Moniz, P.; Pereira, H.; Quilhó, T.; Carvalheiro, F. Characterisation and hydrothermal processing of corn straw towards the selective fractionation of hemicelluloses. Ind. Crops Prod. 2013, 50, 145–153. [Google Scholar] [CrossRef]
- Conrad, M.; Häring, H.; Smirnova, I. Design of an industrial autohydrolysis pretreatment plant for annual lignocellulose. Biomass Conv. Bioref. 2019, 11, 2293–2310. [Google Scholar] [CrossRef]
- Van Zandvoort, I.; Wang, Y.; Rasrendra, C.B.; van Eck, E.R.H.; Bruijnincx, P.C.A.; Heeres, H.J.; Weckhuysen, B.M. Formation, molecular structure, and morphology of humins in biomass conversion: Influence of feedstock and processing conditions. ChemSusChem 2013, 6, 1745–1758. [Google Scholar] [CrossRef]
- Li, H.-Q.; Jiang, W.; Jia, J.-X.; Xu, J. pH pre-corrected liquid hot water pretreatment on corn stover with high hemicellulose recovery and low inhibitors formation. Bioresour. Technol. 2014, 153, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wu, H.; Shakeel, U.; Wang, C.; Yan, T.; Xu, X.; Xu, J. Synergistic effect of acidity balance and hydrothermal pretreatment severity on alkali extraction of hemicelluloses from corn stalk. Biomass Conv. Bioref. 2022, 12, 459–468. [Google Scholar] [CrossRef]
- Wang, Z.-W.; Zhu, M.-Q.; Li, M.-F.; Wei, Q.; Sun, R.-C. Effects of hydrothermal treatment on enhancing enzymatic hydrolysis of rapeseed straw. Renew. Energy 2019, 134, 446–452. [Google Scholar] [CrossRef]
- Thomsen, M.H.; Thygesen, A.; Thomsen, A.B. Hydrothermal treatment of wheat straw at pilot plant scale using a three-step reactor system aiming at high hemicellulose recovery, high cellulose digestibility and low lignin hydrolysis. Bioresour. Technol. 2008, 99, 4221–4228. [Google Scholar] [CrossRef]
- Xin, D.; Yang, Z.; Liu, F.; Xu, X.; Zhang, J. Comparison of aqueous ammonia and dilute acid pretreatment of bamboo fractions: Structure properties and enzymatic hydrolysis. Bioresour. Technol. 2015, 175, 529–536. [Google Scholar] [CrossRef]
- Imman, S.; Arnthong, J.; Burapatana, V.; Champreda, V.; Laosiripojana, N. Influence of alkaline catalyst addition on compressed liquid hot water pretreatment of rice straw. Chem. Eng. J. 2015, 278, 85–91. [Google Scholar] [CrossRef]
- Abu-Omar, M.M.; Barta, K.; Beckham, G.T.; Luterbacher, J.S.; Ralph, J.; Rinaldi, R.; Román-Leshkov, Y.; Samec, J.S.M.; Sels, B.F.; Wang, F. Guidelines for performing lignin-first biorefining. Energy Environ. Sci. 2021, 14, 262–292. [Google Scholar] [CrossRef]
- Bouxin, F.P.; McVeigh, A.; Tran, F.; Westwood, N.J.; Jarvis, M.C.; Jackson, S.D. Catalytic depolymerisation of isolated lignins to fine chemicals using a Pt/alumina catalyst: Part 1—Impact of the lignin structure. Green Chem. 2015, 17, 1235–1242. [Google Scholar] [CrossRef]
- Van den Bosch, S.; Renders, T.; Kennis, S.; Koelewijn, S.-F.; van den Bossche, G.; Vangeel, T.; Deneyer, A.; Depuydt, D.; Courtin, C.M.; Thevelein, J.M.; et al. Integrating lignin valorization and bio-ethanol production: On the role of Ni-Al2O3 catalyst pellets during lignin-first fractionation. Green Chem. 2017, 19, 3313–3326. [Google Scholar] [CrossRef]
- Da Costa Sousa, L.; Jin, M.; Chundawat, S.P.S.; Bokade, V.; Tang, X.; Azarpira, A.; Lu, F.; Avci, U.; Humpula, J.; Uppugundla, N.; et al. Next-generation ammonia pretreatment enhances cellulosic biofuel production. Energy Environ. Sci. 2016, 9, 1215–1223. [Google Scholar] [CrossRef]
- Gosselink, R.J.; van Dam, J.E.; Jong, E.; de Scott, E.L.; Sanders, J.P.; Li, J.; Gellerstedt, G. Fractionation, analysis, and PCA modeling of properties of four technical lignins for prediction of their application potential in binders. Holzforschung 2010, 64, 193–200. [Google Scholar] [CrossRef]
- Pasco, M.F.; Suckling, I.D. Lignin Removal During Kraft Pulping. An Investigation by Thioacidolysis. Holzforschung 1994, 48, 504–508. [Google Scholar] [CrossRef]
- Constant, S.; Basset, C.; Dumas, C.; Di Renzo, F.; Robitzer, M.; Barakat, A.; Quignard, F. Reactive organosolv lignin extraction from wheat straw: Influence of Lewis acid catalysts on structural and chemical properties of lignins. Ind. Crops Prod. 2015, 65, 180–189. [Google Scholar] [CrossRef]
- Heikkinen, H.; Elder, T.; Maaheimo, H.; Rovio, S.; Rahikainen, J.; Kruus, K.; Tamminen, T. Impact of steam explosion on the wheat straw lignin structure studied by solution-state nuclear magnetic resonance and density functional methods. J. Agric. Food Chem. 2014, 62, 10437–10444. [Google Scholar] [CrossRef]
- Lancefield, C.S.; Ojo, O.S.; Tran, F.; Westwood, N.J. Isolation of Functionalized Phenolic Monomers through Selective Oxidation and C–O Bond Cleavage of the β-O-4 Linkages in Lignin. Angew. Chem. 2015, 127, 260–264. [Google Scholar] [CrossRef]
- Grabber, J.H.; Quideau, S.; Ralph, J. p-coumaroylated syringyl units in maize lignin: Implications for β-ether cleavage by thioacidolysis. Phytochemistry 1996, 43, 1189–1194. [Google Scholar] [CrossRef]
- Durot, N.; Gaudard, F.; Kurek, B. The unmasking of lignin structures in wheat straw by alkali. Phytochemistry 2003, 63, 617–623. [Google Scholar] [CrossRef]
- Del Río, J.C.; Rencoret, J.; Prinsen, P.; Martínez, Á.T.; Ralph, J.; Gutiérrez, A. Structural characterization of wheat straw lignin as revealed by analytical pyrolysis, 2D-NMR, and reductive cleavage methods. J. Agric. Food Chem. 2012, 60, 5922–5935. [Google Scholar] [CrossRef] [PubMed]
- Pan, G.X.; Bolton, J.L.; Leary, G.J. Determination of Ferulic and p -Coumaric Acids in Wheat Straw and the Amounts Released by Mild Acid and Alkaline Peroxide Treatment. J. Agric. Food Chem. 1998, 46, 5283–5288. [Google Scholar] [CrossRef]
- Bardet, M.; Robert, D.R.; Lundquist, K. On the reactions and degradation of the lignin during steam hydrolysis of aspen wood. Sven. Papp. 1985, 88, r61–r67. [Google Scholar]
- Pielhop, T.; Reinhard, C.; Hecht, C.; Del Bene, L.; Studer, M.H.; Rudolf von Rohr, P. Application potential of a carbocation scavenger in autohydrolysis and dilute acid pretreatment to overcome high softwood recalcitrance. Biomass Bioenergy 2017, 105, 164–173. [Google Scholar] [CrossRef]
- Lan, W.; Luterbacher, J.S. Preventing Lignin Condensation to Facilitate Aromatic Monomer Production. Chimia 2019, 73, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, H.; Sun, S.; Cao, X.; Sun, R. Effect of hydrothermal pretreatment on the structural changes of alkaline ethanol lignin from wheat straw. Sci. Rep. 2016, 6, 39354. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Collins, S.R.A.; Elliston, A.; Wellner, N.; Dicks, J.; Roberts, I.N.; Waldron, K.W. Release of cell wall phenolic esters during hydrothermal pretreatment of rice husk and rice straw. Biotechnol. Biofuels 2018, 11, 162. [Google Scholar] [CrossRef] [PubMed]
- Wörmeyer, K.; Ingram, T.; Saake, B.; Brunner, G.; Smirnova, I. Comparison of different pretreatment methods for lignocellulosic materials. Part II: Influence of pretreatment on the properties of rye straw lignin. Bioresour. Technol. 2011, 102, 4157–4164. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, H.A.; Conrad, M.; Sun, S.-N.; Sanchez, A.; Rocha, G.J.M.; Romaní, A.; Castro, E.; Torres, A.; Rodríguez-Jasso, R.M.; Andrade, L.P.; et al. Engineering aspects of hydrothermal pretreatment: From batch to continuous operation, scale-up and pilot reactor under biorefinery concept. Bioresour. Technol. 2020, 299, 122685. [Google Scholar] [CrossRef] [PubMed]
- Nimz, H.; Das, K.; Minemura, N. Niedermolekulare Spaltprodukte des Lignins, 1. Der Abbau des Buchenlignins mit Thioessigsäure. Chem. Ber. 1971, 104, 1871–1876. [Google Scholar] [CrossRef]
- Del Río, J.C.; Lino, A.G.; Colodette, J.L.; Lima, C.F.; Gutiérrez, A.; Martínez, Á.T.; Lu, F.; Ralph, J.; Rencoret, J. Differences in the chemical structure of the lignins from sugarcane bagasse and straw. Biomass Bioenergy 2015, 81, 322–338. [Google Scholar] [CrossRef]
- Ralph, J.; Hatfield, R.D.; Quideau, S.; Helm, R.F.; Grabber, J.H.; Jung, H.-J.G. Pathway of p-Coumaric Acid Incorporation into Maize Lignin As Revealed by NMR. J. Am. Chem. Soc. 1994, 116, 9448–9456. [Google Scholar] [CrossRef]
- Crestini, C.; Argyropoulos, D.S. Structural Analysis of Wheat Straw Lignin by Quantitative 31 P and 2D NMR Spectroscopy. The Occurrence of Ester Bonds and α-O-4 Substructures. J. Agric. Food Chem. 1997, 45, 1212–1219. [Google Scholar] [CrossRef]
- Grabber, J.H. How Do Lignin Composition, Structure, and Cross-Linking Affect Degradability? A Review of Cell Wall Model Studies. Crop Sci. 2005, 45, 820–831. [Google Scholar] [CrossRef]
- Atsushi, K.; Azuma, J.; Koshijima, T. Lignin-Carbohydrate Complexes and Phenolic Acids in Bagasse. Holzforschung 1984, 38, 141–149. [Google Scholar] [CrossRef]
- Tuyet Lam, T.B.; Iiyama, K.; Stone, B.A. Cinnamic acid bridges between cell wall polymers in wheat and phalaris internodes. Phytochemistry 1992, 31, 1179–1183. [Google Scholar] [CrossRef]
- Ralph, J. Hydroxycinnamates in lignification. Phytochem. Rev. 2010, 9, 65–83. [Google Scholar] [CrossRef]
- Rinaldi, R.; Jastrzebski, R.; Clough, M.T.; Ralph, J.; Kennema, M.; Bruijnincx, P.C.A.; Weckhuysen, B.M. Paving the Way for Lignin Valorisation: Recent Advances in Bioengineering, Biorefining and Catalysis. Angew. Chem. Int. Ed. Engl. 2016, 55, 8164–8215. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.K.; Ximenes, E.; Kim, Y.; Ladisch, M.R. Adsorption of enzyme onto lignins of liquid hot water pretreated hardwoods. Biotechnol. Bioeng. 2015, 112, 447–456. [Google Scholar] [CrossRef] [PubMed]
- MacAskill, J.J.; Suckling, I.D.; Lloyd, J.A.; Manley-Harris, M. How well do isolated lignins mimic the inhibitory behaviour of cell wall lignins during enzymatic hydrolysis of hydrothermally treated softwood? Biomass Conv. Bioref. 2021. [Google Scholar] [CrossRef]
 ) and a dashed line to fit the data for raw digestate (
) and a dashed line to fit the data for raw digestate (  ) and the resulting degradation rate constants are given below the diagrams. For the washed digestate, measurements were performed in duplicates from samples of one hydrothermally treated sample (error bars indicating the standard deviation). For the raw digestate, measurements were performed as single determinations.
) and the resulting degradation rate constants are given below the diagrams. For the washed digestate, measurements were performed in duplicates from samples of one hydrothermally treated sample (error bars indicating the standard deviation). For the raw digestate, measurements were performed as single determinations.
 ) and a dashed line to fit the data for raw digestate (
) and a dashed line to fit the data for raw digestate (  ) and the resulting degradation rate constants are given below the diagrams. For the washed digestate, measurements were performed in duplicates from samples of one hydrothermally treated sample (error bars indicating the standard deviation). For the raw digestate, measurements were performed as single determinations.
) and the resulting degradation rate constants are given below the diagrams. For the washed digestate, measurements were performed in duplicates from samples of one hydrothermally treated sample (error bars indicating the standard deviation). For the raw digestate, measurements were performed as single determinations.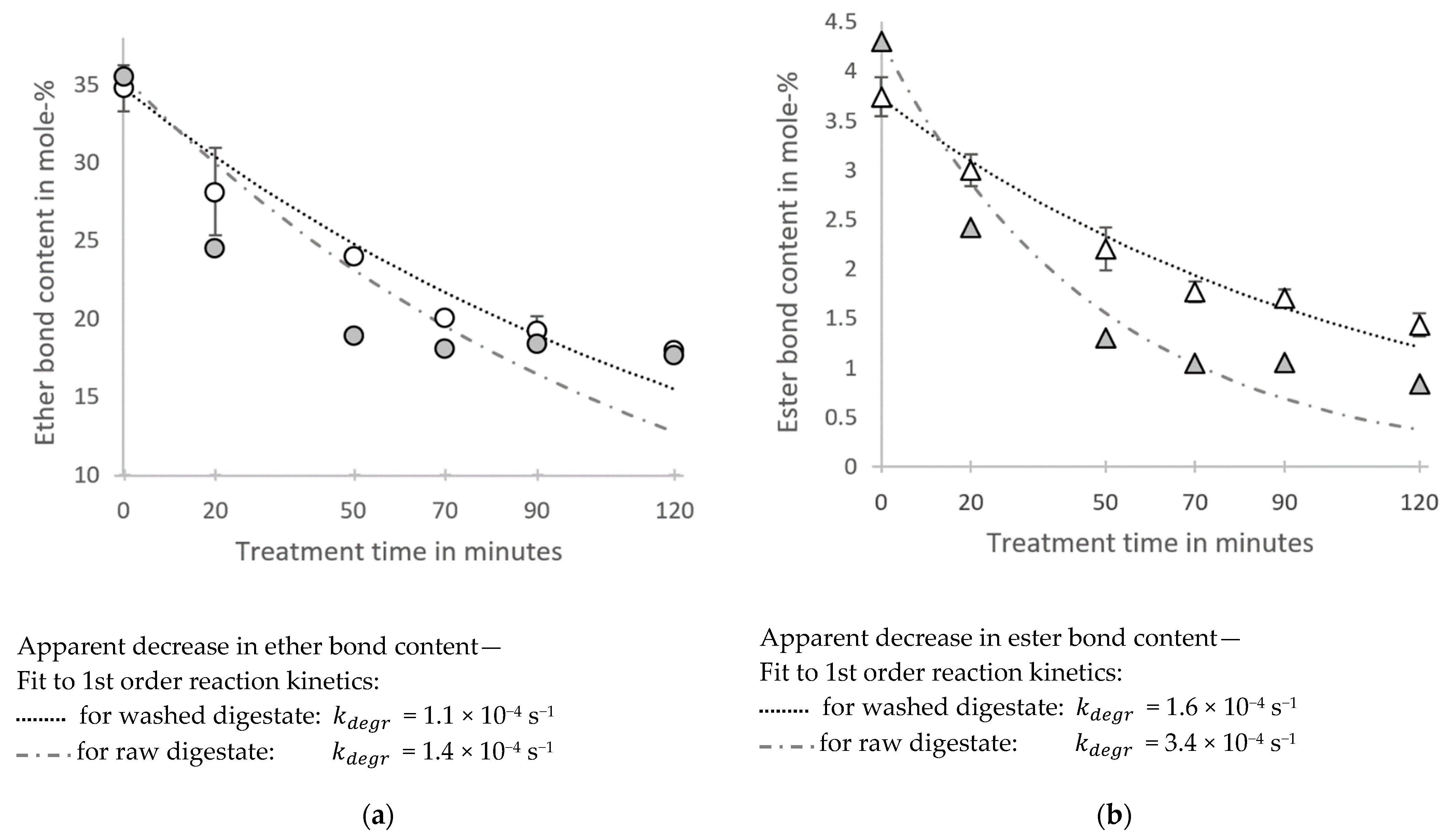

| Raw Solid Digestate | Washed Solid Digestate | |
|---|---|---|
| In wt% (Based on Dry Mass) | ||
| Cellulose | 30.6 ± 1.2 | 37.5 ± 1.7 |
| Hemicellulose | 20.2 ± 1.0 | 25.7 ± 2.1 |
| Lignin | 17.9 ± 0.3 | 23.3 ± 0.7 |
| Total amount extractives * | 19.3 ± 1.2 | 4.5 ± 1.4 |
| Type of Biomass | Wheat Straw | Barley Straw | Maize Straw | Rice Straw | Straw Digestate |
|---|---|---|---|---|---|
| Lignin to holocellulose ratio | 0.29 | 0.26 | 0.29 | 0.21 | 0.42 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Steinbrecher, T.; Bonk, F.; Scherzinger, M.; Lüdtke, O.; Kaltschmitt, M. Fractionation of Lignocellulosic Fibrous Straw Digestate by Combined Hydrothermal and Enzymatic Treatment. Energies 2022, 15, 6111. https://doi.org/10.3390/en15176111
Steinbrecher T, Bonk F, Scherzinger M, Lüdtke O, Kaltschmitt M. Fractionation of Lignocellulosic Fibrous Straw Digestate by Combined Hydrothermal and Enzymatic Treatment. Energies. 2022; 15(17):6111. https://doi.org/10.3390/en15176111
Chicago/Turabian StyleSteinbrecher, Timo, Fabian Bonk, Marvin Scherzinger, Oliver Lüdtke, and Martin Kaltschmitt. 2022. "Fractionation of Lignocellulosic Fibrous Straw Digestate by Combined Hydrothermal and Enzymatic Treatment" Energies 15, no. 17: 6111. https://doi.org/10.3390/en15176111
APA StyleSteinbrecher, T., Bonk, F., Scherzinger, M., Lüdtke, O., & Kaltschmitt, M. (2022). Fractionation of Lignocellulosic Fibrous Straw Digestate by Combined Hydrothermal and Enzymatic Treatment. Energies, 15(17), 6111. https://doi.org/10.3390/en15176111






