Use of High-Frequency Ultrasound Waves for Boiler Water Demineralization/Desalination Treatment
Abstract
1. Introduction
2. Materials and Methods
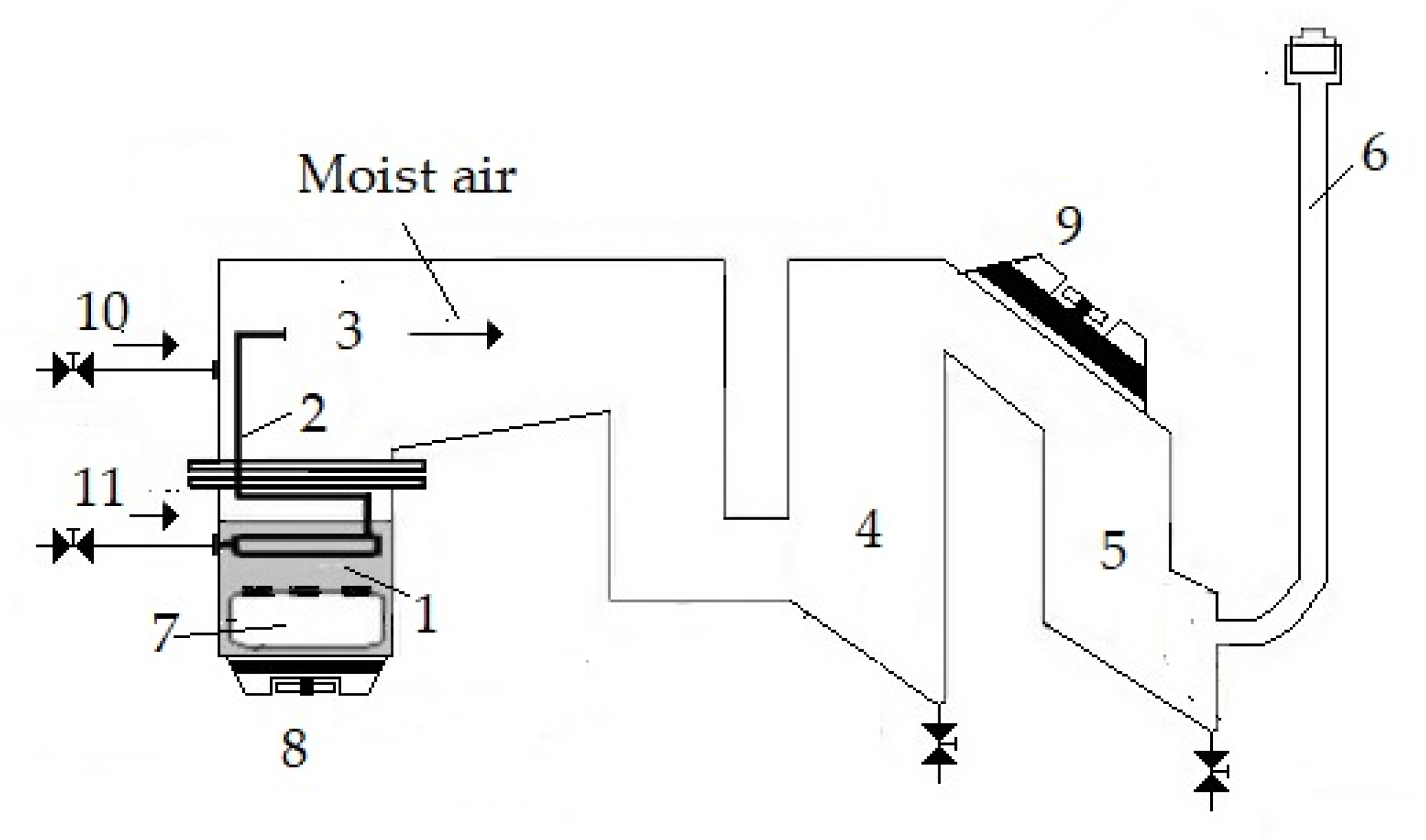
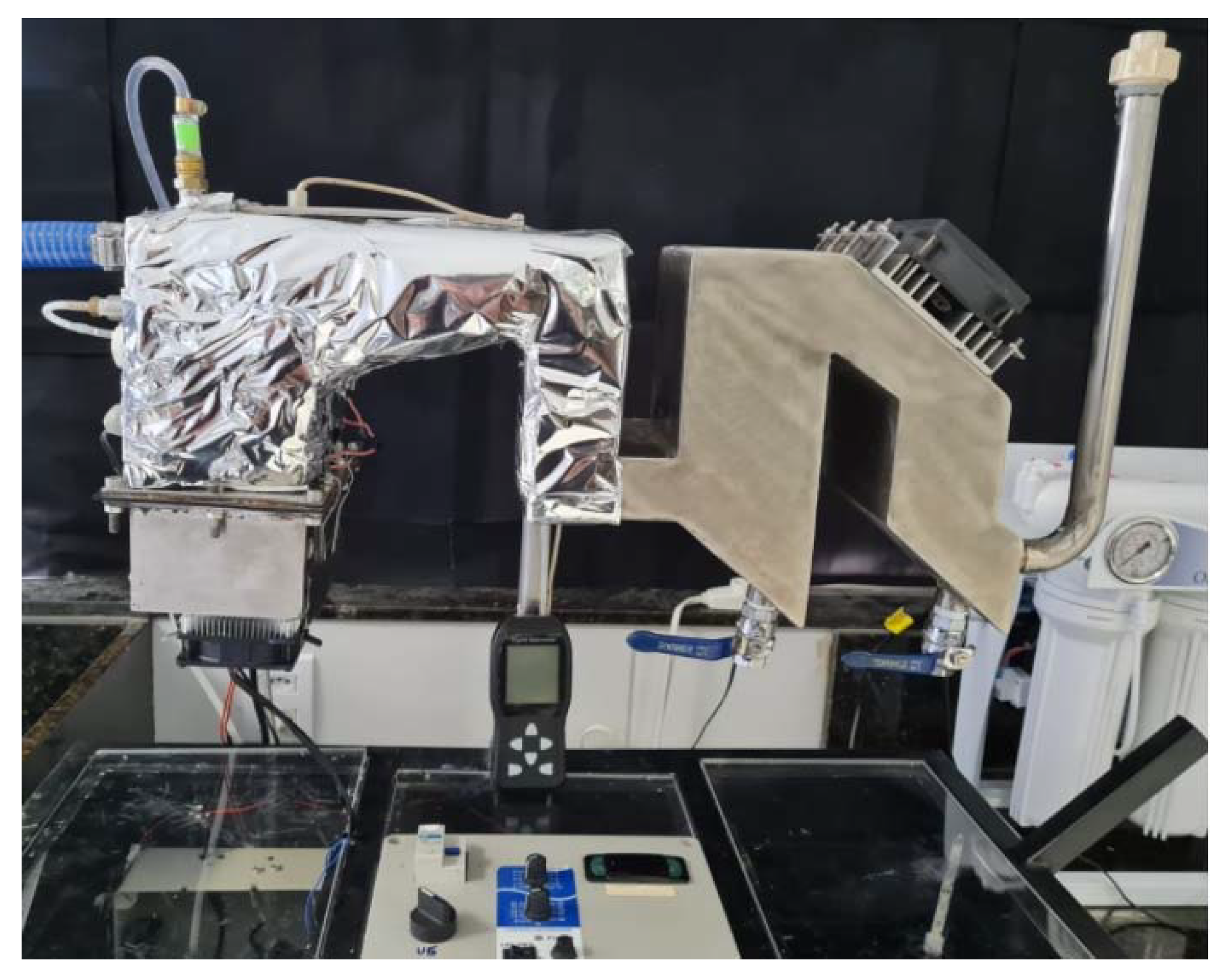
2.1. Experimental Setup
2.2. Analysis of Psychrometric Conditions
- —mass flow rate of atomized water, kg of water/s;
- —inlet mass flow rate of dry air, kg of dry air/s;
- —absolute humidity at point 2, kg of water/kg of dry air;
- —absolute humidity at point 3, kg of water/kg of dry air.
- t—dry-bulb temperature, °C;
- p—total pressure, kPa.
2.3. Mass and Energy Balances
- —mass flow rate of dry air at point 2, kg/s;
- —mass flow rate of water collected in chamber 4, kg of water/s;
- —mass flow rate of water collected in chamber 5, kg of water/s;
- —mass flow rate of dry air at point 5, kg of dry air/s;
- —absolute humidity at point 5, kg of water/kg of dry air.
- —air specific enthalpy of moist air at point 2, kJ/kg;
- —specific enthalpy of ultrasonically atomized water, kJ/kg;
- —specific heat capacity of water in the liquid phase in the atomization chamber, kJ/(kg·K);
- —temperature of the atomization chamber, K;
- —ambient temperature, K;
- —specific enthalpy of condensed water at point 4, kJ/kg;
- —specific enthalpy of condensed water at point 5, kJ/kg;
- —specific enthalpy of moist air at point 5, kJ/kg.
2.4. Principal Component Analysis (PCA)
2.5. Data Collection and Preparation
- Xi—standardized value;
- xi—individual observation;
- —arithmetic mean;
- S—standard deviation.
2.6. Statistical Analysis of Data
3. Results and Discussion
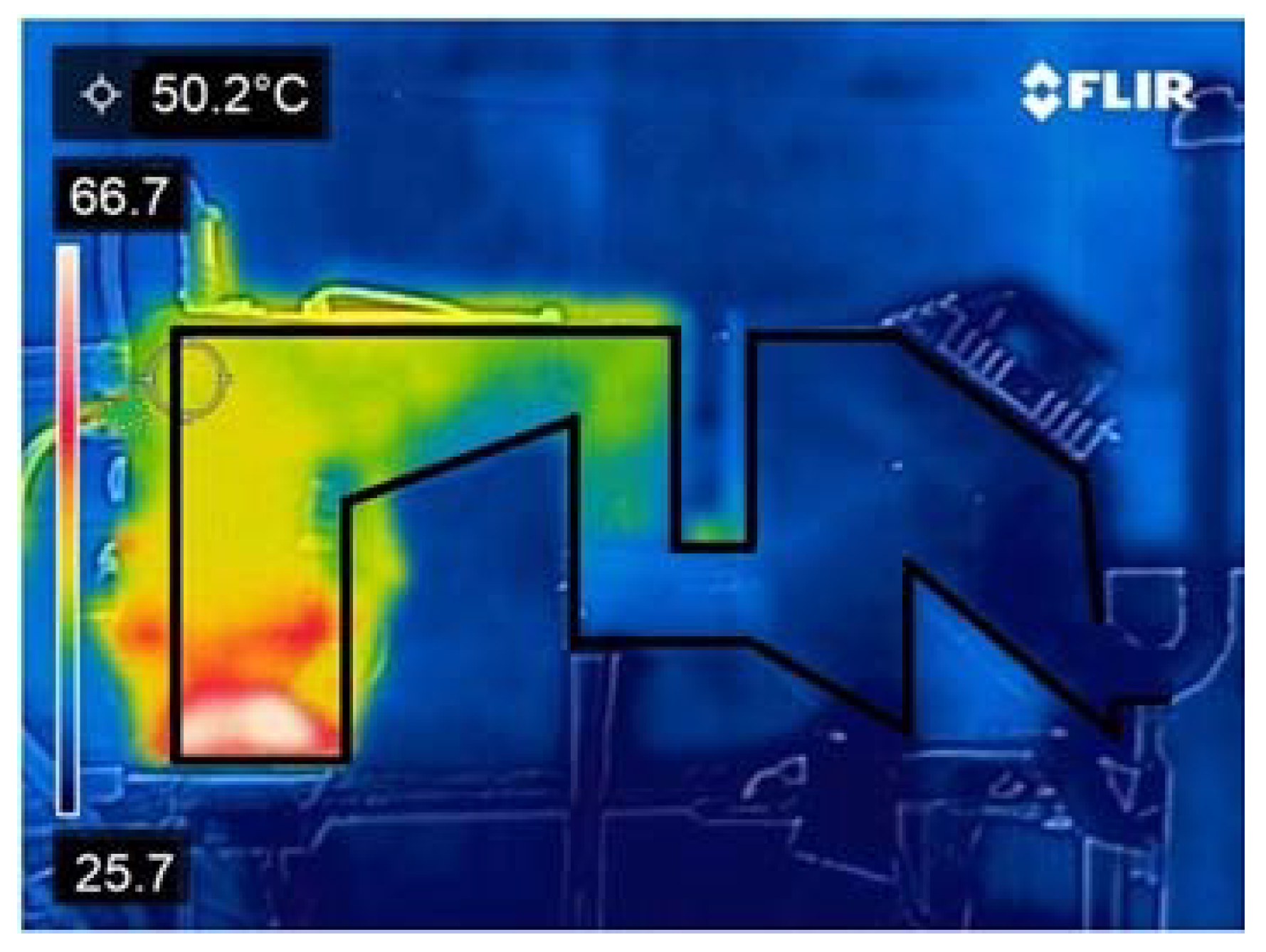
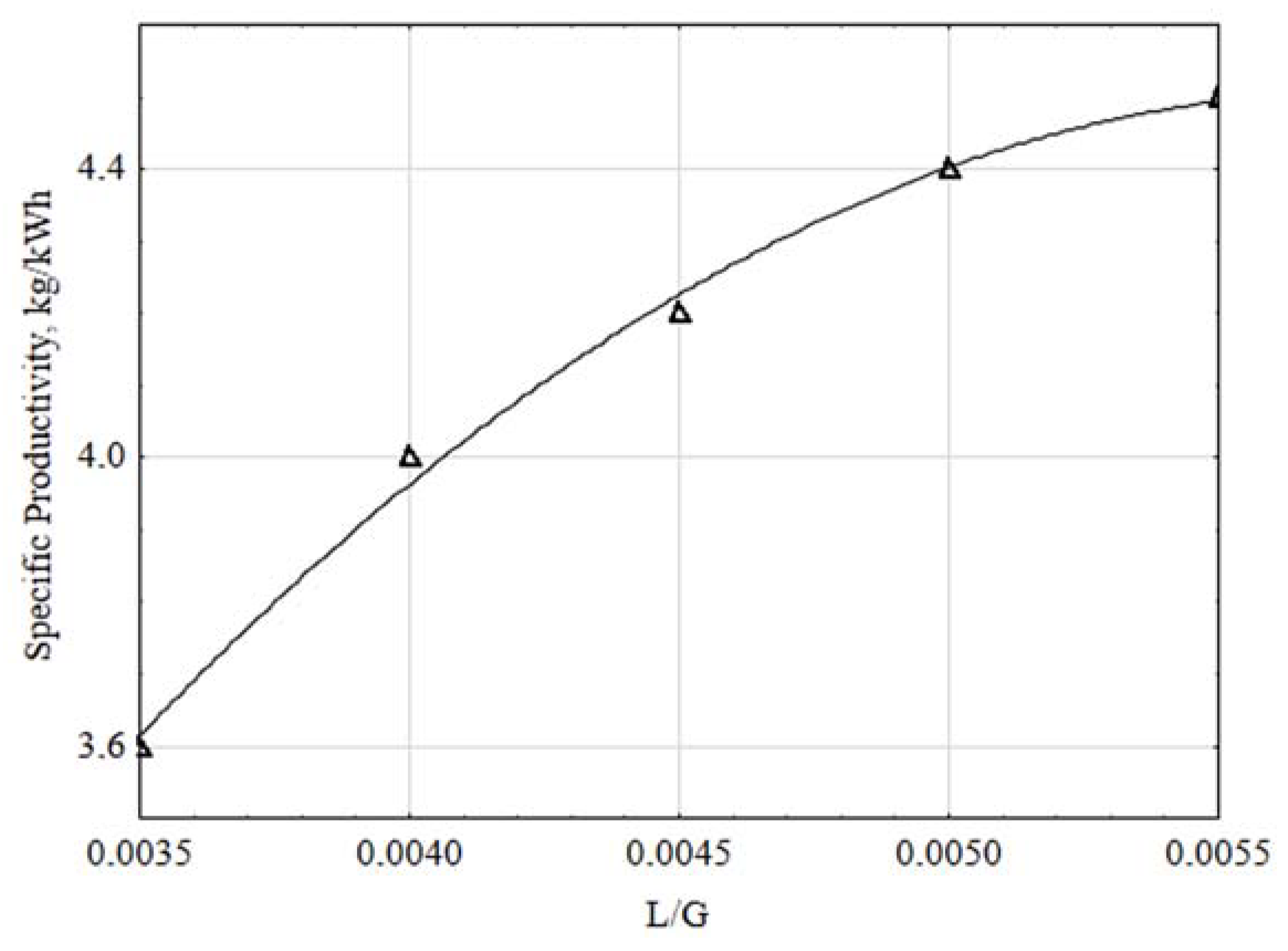
3.1. Bench Prototype Thermal Profile
3.2. Results of Principal Component Analysis
3.2.1. Eigenvalue
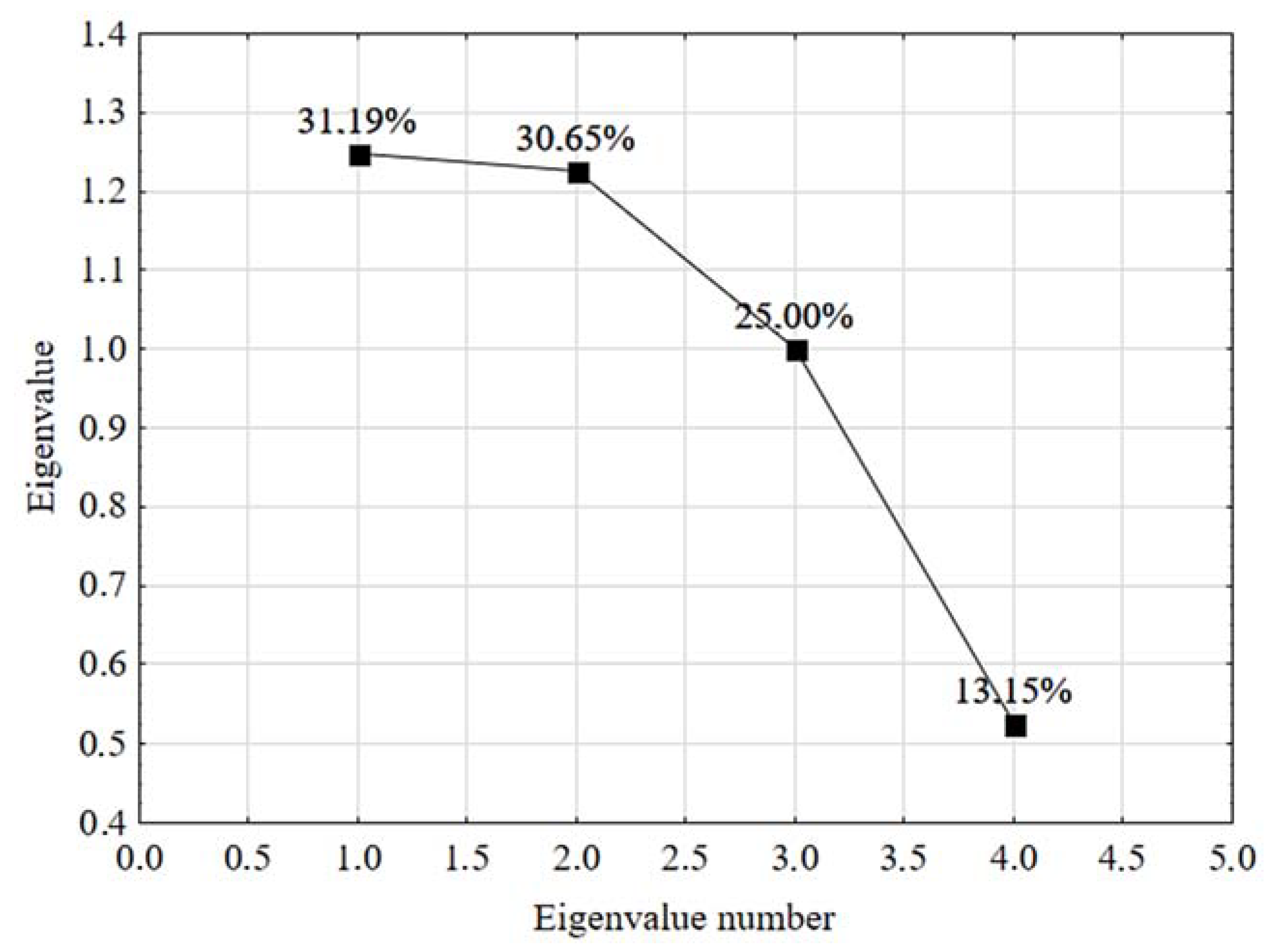
3.2.2. Scree Plot Graph
3.2.3. Statistical Importance of Variables
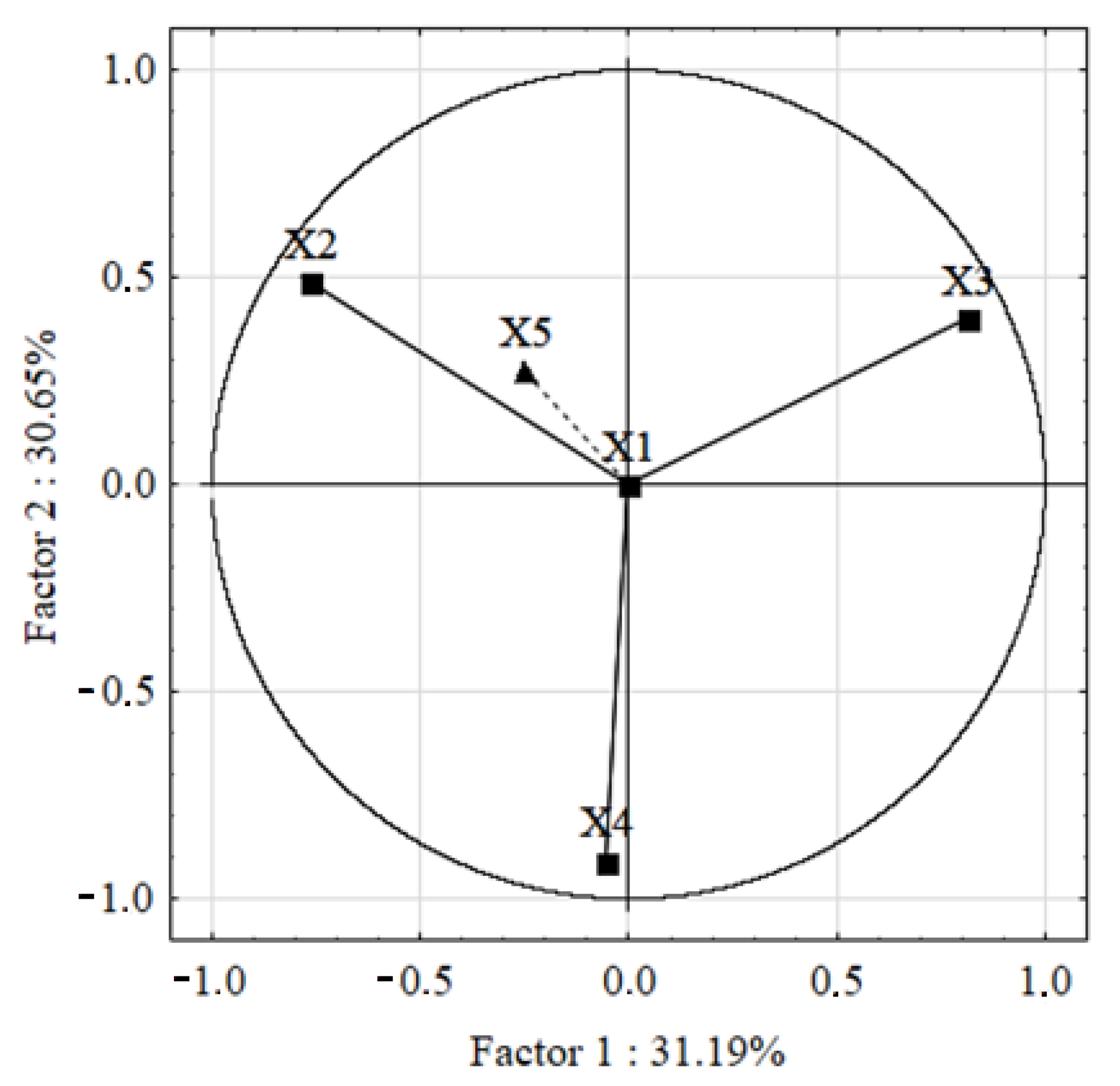
3.2.4. Hypersphere Graphs and Loading of Variables
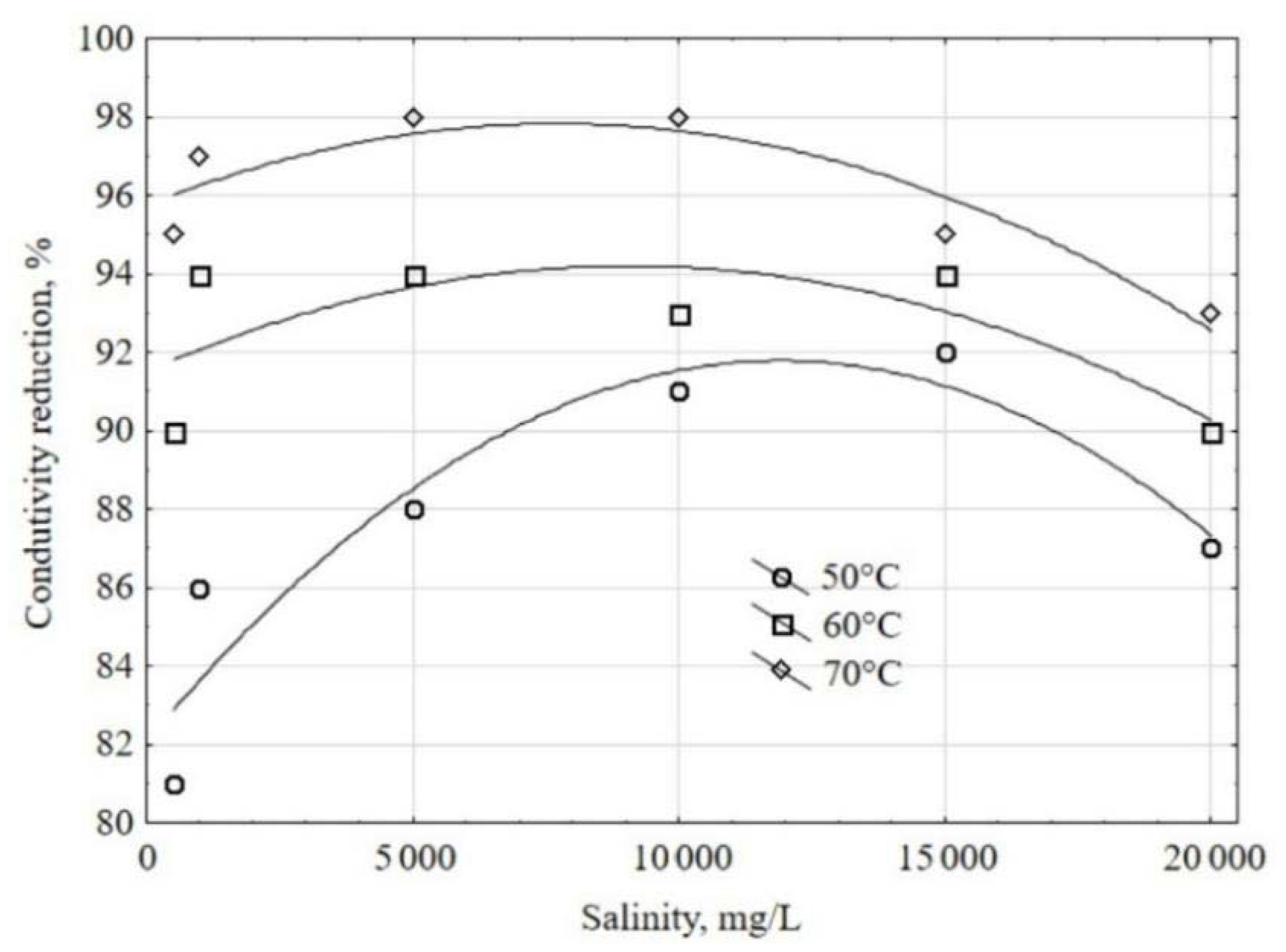
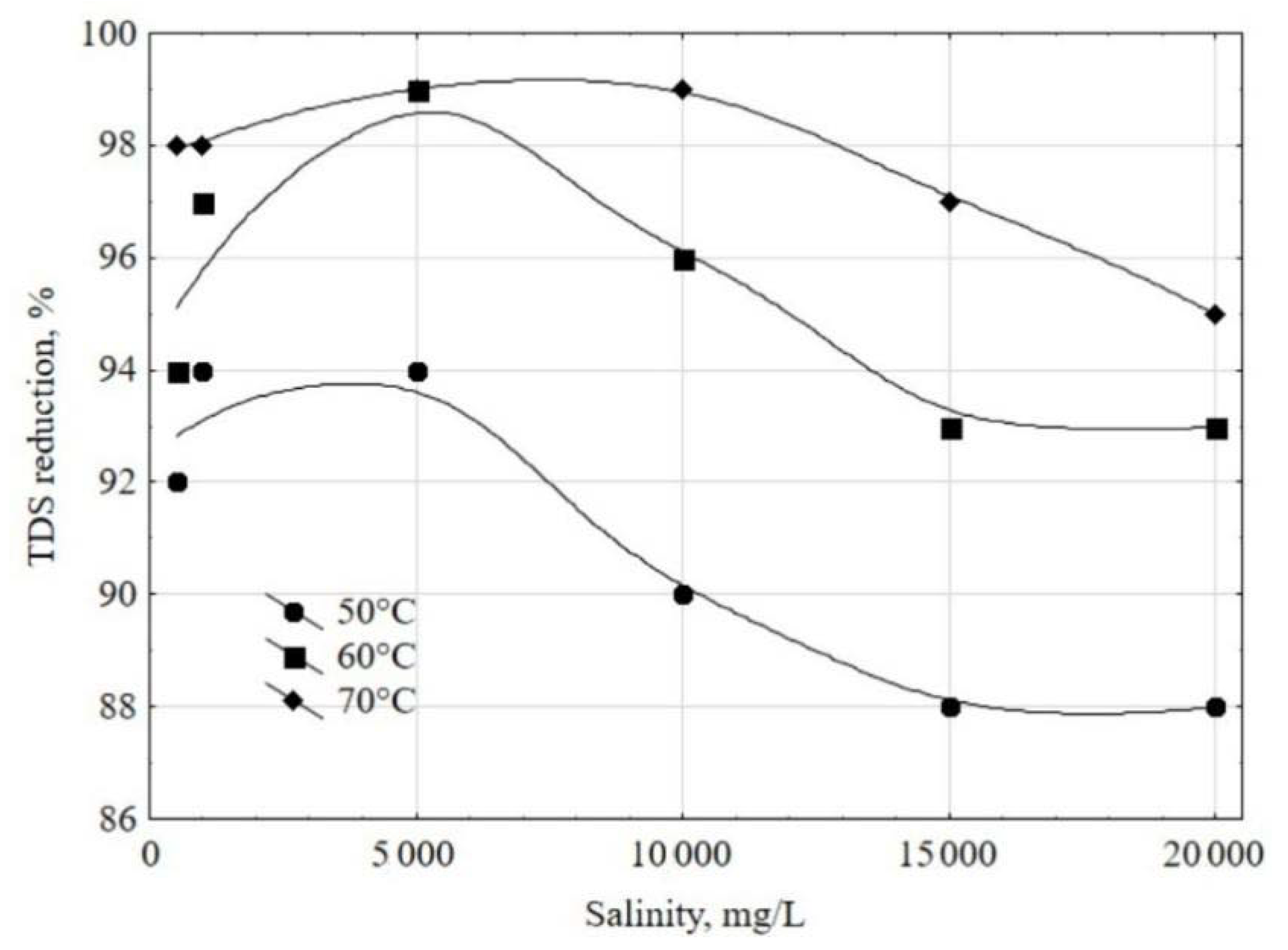
3.3. Tests with Feed Waters with Different Salinities
- Mass air flow rate: 25 kg/h;
- Air temperature at the mixing point with atomized water: 70 °C;
- Feed water conductivity: 5000 μS/cm;
- Pressure inside the prototype: 18 cm H2O.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Larsen, M.A.D.; Petrovic, S.; Engström, R.E.; Drews, M.; Liersch, M.; Karlsson, K.B.; Howells, M. Challenges of data availability: Analysing the water-energy nexus in electricity Generation. Energy Strategy Rev. 2019, 26, 100426. [Google Scholar] [CrossRef]
- Martínez Moya, S.; Boluda Botella, N. Review of techniques to reduce and prevent carbonate scale. Prospecting in water treatment by magnetism and electromagnetism. Water 2021, 13, 2365. [Google Scholar] [CrossRef]
- Zhu, Z.; Rao, R.; Zhao, Z.; Chen, J.; Jiang, W.; Bi, F.; Yang, Y.; Zhang, X. Research progress on removal of phthalates pollutants from environment. J. Mol. Liq. 2022, 355, 118930. [Google Scholar] [CrossRef]
- Gong, Y.; Wang, Y.; Lin, N.; Wang, R.; Wang, M.; Zhang, X. Iron-based materials for simultaneous removal of heavy metal(loid)s and emerging organic contaminants from the aquatic environment: Recent advances and perspectives. Environ. Pollut. 2022, 299, 118871. [Google Scholar] [CrossRef]
- Li, X.; Jie, B.; Lin, X.; Deng, Z.; Qian, J.; Yang, Y.; Zhang, X. Application of sulfate radicals-based advanced oxidation technology in degradation of trace organic contaminants (TrOCs): Recent advances and prospects. J. Environ. Manag. 2022, 308, 114664. [Google Scholar] [CrossRef]
- Čuda, P.; Pospíšil, P.; Tenglerová, J. Reverse osmosis in water treatment for boilers. Desalination 2006, 198, 41–46. [Google Scholar] [CrossRef]
- Asadi, N.; Soleimanimehr, H.; Alinia-ziazi, A. An investigation on boiler feed water treatment using reverse osmosis and ion exchange by WAVE software. J. Appl. Res. Water Wastewater 2021, 8, 124–128. [Google Scholar] [CrossRef]
- Fetyan, N.A.H.; Attia, T.M.S. Water purification using ultrasound waves: Application and challenges. Arab. J. Basic Appl. Sci. 2020, 27, 194–207. [Google Scholar] [CrossRef]
- Chong, M.N.; Jin, B.; Chow, C.W.K.; Saint, C. Recent developments in photocatalytic water treatment technology: A review. Water Res. 2010, 44, 2997–3027. [Google Scholar] [CrossRef]
- Wang, Z.H.; Liu, X.-Y.; Zhang, H.-Q.; Wang, Y.; Xu, Y.-F.; Peng, B.-L.; Liu, Y. Modeling of kinetic characteristics of alkaline-surfactant-polymer-strengthened foams decay under ultrasonic standing wave. Pet. Sci. 2022, in press. [CrossRef]
- Huggi, M.; Mise, S.R. Optimized ANN model for ultrasonication wastewater treatment process. Int. J. Adv. Res. Eng. Technol. 2019, 10, 94–102. [Google Scholar] [CrossRef]
- Stefan, A.; Balan, G. The chemistry of the raw water treated by air-jet ultrasound generator. Rom. J. Tech. Sci. Appl. Mech. 2011, 56, 85–92. [Google Scholar]
- Leong, T.; Juliano, P.; Knoerzer, K. Advances in ultrasonic and megasonic processing of foods. Food Eng. Rev. 2017, 9, 237–256. [Google Scholar] [CrossRef]
- Chu, C.-L.; Lu, T.-Y.; Fuh, Y.-K. The suitability of ultrasonic and megasonic cleaning of nanoscale patterns in ammonia hydroxide solutions for particle removal and feature damage. Semicond. Sci. Technol. 2020, 35, 45001. [Google Scholar] [CrossRef]
- Entezari, M.H.; Tahmasbi, M. Water softening by combination of ultrasound and ion Exchange. Ultrason. Sonochem. 2009, 16, 356–360. [Google Scholar] [CrossRef]
- Hiratsuka, A.; Pathak, D. Application of ultrasonic waves for the improvement of water treatment. J. Water Resour. Prot. 2013, 5, 604–610. [Google Scholar] [CrossRef][Green Version]
- Tourab, A.E.; Blanco-Marigorta, A.M.; Elharidi, A.M.; Suárez-López, M.J. A novel humidification technique used in water desalination systems based on the humidification–dehumidification process: Experimentally and theoretically. Water 2020, 12, 2264. [Google Scholar] [CrossRef]
- El-Maghlany, W.M.; Tourab, A.E.; Hegazy, A.H.; Teamah, M.A.; Hanafy, A.A. Experimental study on productivity intensification of HDH desalination unit utilizing two-stage dehumidification. Desalin. Water Treat. 2018, 107, 28–40. [Google Scholar] [CrossRef]
- Hosseingholilou, B.; Banakar, A.; Mostafaei, M. Design and evaluation of a novel ultrasonic desalination system by response surface methodology. Desalin. Water Treat. 2019, 164, 263–275. [Google Scholar] [CrossRef]
- Niam, A.G.; Sucahyo, L. Ultrasonic atomizer application for Low Cost Aeroponic Chambers (LCAC): A review. IOP Conf. Ser. Earth Environ. Sci. 2020, 542, 12034. [Google Scholar] [CrossRef]
- Odu, S.O.; Van Der Ham, A.G.J.; Metz, S.; Kersten, S.R.A. Design of a process for supercritical water desalination with zero liquid discharge. Ind. Eng. Chem. Res. 2015, 54, 5527–5535. [Google Scholar] [CrossRef]
- Choong, W.H.; Nasip, M.N.B. Ultrasonic atomiser system performance characterisation study for water purification system development. Trans. Sci. Technol. 2021, 8, 239–244. [Google Scholar]
- Van Wyk, S.; van der Ham, A.G.J.; Kersten, S.R.A. Potential of supercritical water desalination (SCWD) as zero liquid discharge (ZLD) technology. Desalination 2020, 495, 114593. [Google Scholar] [CrossRef]
- Abdelaziz, G.B.; El-Said, E.M.S.; Dahab, M.A.; Omara, M.A.; Sharshir, S.W. Hybrid solar desalination systems review. Energy Sources A Recovery Util. Environ. Eff. 2021, 1–31. [Google Scholar] [CrossRef]
- Giwa, A.; Akther, N.; Al Housani, A.; Haris, S.; Hasan, S.W. Recent advances in humidification dehumidification (HDH) desalination processes: Improved designs and productivity. Renew. Sustain. Energy Rev. 2016, 57, 929–944. [Google Scholar] [CrossRef]
- Beltrán-Prieto, J.C.; Beltrán-Prieto, L.A. Estimation of psychrometric parameters of vapor water mixtures in air. Appl. Eng. Educ. 2016, 24, 39–43. [Google Scholar] [CrossRef]
- Devres, Y.O. Psychrometric properties of humid air: Calculation procedures. Appl. Energy. 1994, 48, 1–18. [Google Scholar] [CrossRef]
- Garg, K.; Das, S.K.; Tyagi, H. Thermal design of a humidification-dehumidification desalination cycle consisting of packed-bed humidifier and finned-tube dehumidifier. Int. J. Heat Mass Transf. 2022, 183, 122153. [Google Scholar] [CrossRef]
- Kabeel, A.E.; Hamed, M.H.; Omara, Z.M.; Sharshir, S.W. Water desalination using a humidification-dehumidification technique—A detailed review. Nat. Resour. 2013, 4, 286–305. [Google Scholar] [CrossRef]
- Wilhelm, L.R. Numerical calculation of psychrometric properties in SI units. Trans. ASAE 1976, 19, 318–321. [Google Scholar] [CrossRef]
- Chen, Q.; Akhtar, F.H.; Burhan, M.; Kumja, M.; Ng, K.C. A novel zero-liquid discharge desalination system based on the humidification-dehumidification process: A preliminary study. Water Res. 2021, 207, 117794. [Google Scholar] [CrossRef]
- Groenewald, J.W.D.; Nelson, L.R.; Hundermark, R.J.; Phage, K.; Sakaran, R.L.; van Rooyen, Q.; Cizek, A. Furnace integrity monitoring using principal component analysis: An industrial case study. J. S. Afr. Inst. Min. Metal. 2018, 118, 345–352. [Google Scholar] [CrossRef]
- Teng, S.Y.; How, B.S.; Leong, W.D.; Teoh, J.H.; Siang Cheah, A.C.; Motavasel, Z.; Lam, H.L. Principal component analysis-aided statistical process optimisation (PASPO) for process improvement in industrial refineries. J. Clean. Prod. 2019, 225, 359–375. [Google Scholar] [CrossRef]
- Jolliffe, I.T.; Cadima, J. Principal component analysis: A review and recent developments. Phil. Trans. R. Soc. A 2016, 374, 20150202. [Google Scholar] [CrossRef]
- Bro, R.; Smilde, A.K. Principal component analysis. Anal. Methods 2014, 6, 2812–2831. [Google Scholar] [CrossRef]
- Husson, F.; Lê, S.; Pagès, J. Exploratory Multivariate Analysis by Example Using R, 2nd ed.; Chapman; Hall/CRC: Boca Raton, FL, USA, 2017; Available online: http://factominer.free.fr/bookV2/index.html (accessed on 17 July 2021).
- Kaiser, H.F. The application of electronic computers to factor analysis. Educ. Psychol. Meas. 1960, 20, 141–151. [Google Scholar] [CrossRef]
- Ahmadvand, S.; Abbasi, B.; Azarfar, B.; Elhashimi, M.; Zhang, X.; Abbasi, B. Looking beyond energy efficiency: An applied review of water desalination technologies and an introduction to capillary-driven desalination. Water 2019, 11, 696. [Google Scholar] [CrossRef]
- Yang, Z.; Lian, Z.; Tao, R.; Zhong, H.K. Experimental study on the performance of the internally-heated ultrasonic atomization liquid desiccant regeneration system. Appl. Therm. Eng. 2019, 163, 114211. [Google Scholar] [CrossRef]
- Kashyap, V.; Al-Bayati, A.; Sajadi, S.M.; Irajizad, P.; Wang, S.H.; Ghasemi, H. A flexible anti-clogging graphite film for scalable solar desalination by heat localization. J. Mater. Chem. A 2017, 5, 15227–15234. [Google Scholar] [CrossRef]
- Zhang, X.; Kan, W.; Jiang, H.; Chen, Y.; Cheng, T.; Jiang, H.; Hu, X. Capillary-driven low grade heat desalination. Desalination 2017, 410, 10–18. [Google Scholar] [CrossRef]


| Measuring Instrument | Accuracy | Range |
|---|---|---|
| Oscilloscope | 10–300 MHz | |
| Voltmeter | 45–260 V | |
| Digital Wattmeter | 0.5–22,000 W | |
| Thermohygrometer | ||
| Temperature | −20–70 °C | |
| Relativity Humidity | 0–100% | |
| Multi-Digital Camera | °C | −20 to 400 °C |
| E-Type Thermocouple | −270–870 °C |
| Run | X1 (L/h) | X2 (μS/cm) | X3 (K) | X4 (cm H2O) | X5 (%) |
|---|---|---|---|---|---|
| 1 | 0.000 | −0.467 | −0.471 | −0.438 | −0.336 |
| 2 | 2.000 | −0.467 | −0.471 | −0.438 | −1.497 |
| 3 | −2.000 | −0.467 | −0.471 | −0.438 | −1.265 |
| 4 | 0.000 | 0.884 | −0.471 | −0.438 | 0.361 |
| 5 | 0.000 | 2.386 | −0.471 | −0.438 | 1.755 |
| 6 | 0.000 | −0.467 | 0.943 | −0.438 | −0.103 |
| 7 | 0.000 | −0.467 | 2.357 | −0.438 | 0.826 |
| 8 | 0.000 | −0.467 | −0.471 | 0.548 | −0.103 |
| 9 | 0.000 | −0.467 | −0.471 | 2.521 | 0.361 |
| Principal Component | Eigenvalue | Total Variance (%) | Cumulative Eigenvalue | Cumulative Total Variance (%) |
|---|---|---|---|---|
| 1 | 1.247791 | 31.19477 | 1.247791 | 31.1948 |
| 2 | 1.226021 | 30.65052 | 2.473812 | 61.8453 |
| 3 | 1.000000 | 25.00000 | 3.473812 | 86.8453 |
| 4 | 0.526188 | 13.15471 | 4.000000 | 100.0000 |
| PC1 | PC2 | PC3 | |
|---|---|---|---|
| X1 | 0.000000 | 0.000000 | 1.000000 |
| X2 | −0.763880 | 0.488767 | 0.000000 |
| X3 | 0.813313 | 0.399881 | 0.000000 |
| X4 | −0.052919 | −0.909518 | 0.000000 |
| Control Parameter | Recommended Value for the Pressure Range of 10–20 kgf/cm2 | Experimental Results | |
|---|---|---|---|
| Raw Water | Treated Water | ||
| pH | 10.5–11.5 | 8.49 ± 0.05 | 7.75 ± 0.01 |
| Conductivity (μS/cm) | <4000 | 616 ± 6 | 59.60 ± 0.04 |
| Alkalinity (mg/L CaCO3) | <800 | 80 ± 1 | 15.00 ± 0.01 |
| Alkalinity as NaOH (mg/L CaCO3) | 150–350 | - | - |
| Chloride (mg/L Cl) | <400 | 130 ± 2 | 15.00 ± 0.01 |
| Total hardness (mg/L CaCO3) | <2.0 | 135 ± 2 | 1.00 ± 0.01 |
| Total iron (mg/L Fe) | <5.0 | - | - |
| Phosphate (mg/L PO43−) | 30–50 | 6.21 ± 0.01 | 1.61 ± 0.01 |
| Sulphite (mg/L SO32−) | 30–50 | 5.00 ± 0.01 | 10.00 ± 0.01 |
| Silica (mg/L Si) | <150 | - | - |
| Total dissolved solids (mg/L) | <300 | 273 ± 3 | 35.28 ± 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brandão, Y.F.F.; dos Santos, L.B.; de Araújo, G.P.; Pedrosa Júnior, L.P.; da Costa Neto, B.F.; da Silva, R.d.C.F.S.; Benachour, M.; Converti, A.; Sarubbo, L.A.; dos Santos, V.A. Use of High-Frequency Ultrasound Waves for Boiler Water Demineralization/Desalination Treatment. Energies 2022, 15, 4431. https://doi.org/10.3390/en15124431
Brandão YFF, dos Santos LB, de Araújo GP, Pedrosa Júnior LP, da Costa Neto BF, da Silva RdCFS, Benachour M, Converti A, Sarubbo LA, dos Santos VA. Use of High-Frequency Ultrasound Waves for Boiler Water Demineralization/Desalination Treatment. Energies. 2022; 15(12):4431. https://doi.org/10.3390/en15124431
Chicago/Turabian StyleBrandão, Yago Fraga Ferreira, Leonardo Bandeira dos Santos, Gleice Paula de Araújo, Leonildo Pereira Pedrosa Júnior, Benjamim Francisco da Costa Neto, Rita de Cássia Freire Soares da Silva, Mohand Benachour, Attilio Converti, Leonie Asfora Sarubbo, and Valdemir Alexandre dos Santos. 2022. "Use of High-Frequency Ultrasound Waves for Boiler Water Demineralization/Desalination Treatment" Energies 15, no. 12: 4431. https://doi.org/10.3390/en15124431
APA StyleBrandão, Y. F. F., dos Santos, L. B., de Araújo, G. P., Pedrosa Júnior, L. P., da Costa Neto, B. F., da Silva, R. d. C. F. S., Benachour, M., Converti, A., Sarubbo, L. A., & dos Santos, V. A. (2022). Use of High-Frequency Ultrasound Waves for Boiler Water Demineralization/Desalination Treatment. Energies, 15(12), 4431. https://doi.org/10.3390/en15124431









