Abstract
Recently, there has been a growing interest in the use of new types of cheaper raw materials for biodiesel production. There are many prospects for microalgae, which do not compete for land with conventional biodiesel raw materials, are characterized by rapid reproduction and high biomass accumulation, and under certain conditions, some are able to accumulate a large amount of oil. A number of studies have been conducted on the extraction of oil from microalgae cells and transesterification with various acyl receptors. This paper provides an overview of the results of research on microalgal biomass preparation and oil extraction. Indicators of the quality of the oil are presented and its suitability for biodiesel synthesis is analyzed. The homogeneous and heterogeneous catalysts used for oil transesterification are described and the optimal conditions of the process when using various alcohols as acyl receptors are presented. Much attention is paid to the parameters affecting the transesterification efficiency and biodiesel yield. The physical and chemical, and operational and environmental properties of biodiesel obtained from algae oil are analyzed. The evaluation of the economic efficiency of biodiesel synthesis is also presented.
1. Introduction
Recently, interest in the use of microalgae biomass for various purposes has been steadily increasing. This interest relates to the properties of microalgae, such as the high rate of accumulation of biomass, providing possibilities to accumulate oil and other compounds including lipids, carbohydrates, pigments, etc., that can be used in the food, pharmaceutical, and biofuel sectors. Microalgae can be cultivated on non-arable land and in areas unsuitable for conventional agriculture, and they can be cultivated with water unsuitable for the irrigation of conventional plants, including wastewater [1].
Due to the high lipid content of certain microalgal species grown under appropriate conditions, there is increasing interest in the use of microalgae oil for biodiesel production, replacing the vegetable oil (rapeseed, sunflower) commonly used for biodiesel synthesis [2].
However, the use of microalgae in biofuel production is still problematic due to the high material and energy costs for microalgae cultivation and processing, and so far, biodiesel produced from microalgae biomass is still unable to compete with mineral fuels. As a result, intensive research is being carried out to develop less energy-intensive microalgae cultivation and processing technologies.
The purpose of this review is to explore the options of using microalgae biomass for biodiesel fuel synthesis. In this paper a brief overview of microalgae biomass preparation, oil extraction and transesterification technologies are given. Much attention is paid to the parameters affecting transesterification efficiency and biodiesel yield. Physical and chemical, and operational and environmental properties of biodiesel obtained from algae oil are analyzed. The evaluation of the economic efficiency of biodiesel synthesis is also presented.
2. Preparation of Microalgae Biomass and Oil Extraction
To prepare microalgae oil from biomass for the production of biofuels, it is necessary to extract the oil from microalgae cells, which have strong cell walls composed of polysaccharides, glycoproteins, proteoglycans, and other compounds that are resistant to chemical and mechanical effects. For the oil to be completely extracted, it is necessary to break down the cell walls; various mechanical or non-mechanical methods are used to destroy them. Non-mechanical cell lysis methods use chemicals or enzymatic preparations and osmotic shock. Various chemicals have the ability to break down the walls of microalgae cells. Their degradation efficiency depends on the type of microalgae, which determines the composition of the cell walls. Chemicals used include antibiotics, detergents, solvents, acids, and alkalis. Acid treatment has been extensively studied using various types of algae; it is effective at high temperatures (approximately 160 °C) [3]. Alkali treatment is also performed at a high temperature (120 °C), which is required for protein denaturation. By combining chemical and mechanical treatment, the process temperature may be lowered. Enzymatic cell lysis produces higher levels of oil from microalgae than mechanical or chemical methods. Zheng et al. [4] obtained an 8.1-fold higher oil content from microalgae Chlorella biomass after cellulase treatment of the biomass, a 7.46-fold higher oil content from biomass after treatment with the enzyme preparation Lysozyme, and a 2.37-fold higher amount when biomass was treated with Snailase than that with no usage of enzymes. However, enzymatic treatment requires a longer duration, the application of such a process is expensive, and not all enzymes are suitable for microbial cell lysis. To increase the efficiency of the process, it is proposed to use mixtures of immobilized enzymes and to combine the enzymatic cell disruption method with mechanical disruption [5].
Mechanical methods include milling in a loaded mill, pressing, high-pressure homogenization, autoclaving, lyophilization, and sonication and microwave treatment of cells [6]. Some methods of cell disruption, such as milling, sonication, or high-pressure homogenization, require a certain amount of water, so this process is often performed before drying [6].
The efficiency of high-pressure homogenization depends directly on the pressure and the treatment time or the number of cycles. The specific energy consumption of this process depends on the concentration of microalgae in the suspension, the type of microalgae and their growing conditions [5,7]. Although high-pressure homogenization is one of the most suitable methods on an industrial scale, it has a number of drawbacks, especially in the use of low-concentration microalgae suspensions, due to increasing energy consumption. High-speed homogenization is an effective method of disrupting cell walls. It is very simple, with a short process time and the possibility to use relatively high concentrations of suspensions, thereby reducing the cost of dewatering and related additional operations. However, this process is highly energy intensive and involves protein denaturation; therefore, it is rarely used in microalgae processing plants [8].
Recently, the possibilities of microalgal cell disruption by ultrasonication have been increasingly investigated. Some researchers claim that ultrasonication is the most effective cell wall disruption technology compared to milling, autoclaving, microwave processing, and ultrasonication [9]. Skorupskaite et al. [10] found that in the treatment of Ankistrodesmus fusiformis with ultrasonication, the cell disruption efficiency is approximately 40% higher than that using the ultrahomogenization method. Halim et al. found that ultrasonication improves oil extraction from microalgae Chlorella sp. cells by 7-fold [11]. A 5.11-fold higher oil yield from ultrasound-treated Chlorella vulgaris microalgae cells than from untreated cells was found by Zheng et al. [2], and 2.625-fold higher yields were obtained by Prabakaran and Ravindran [12]. These authors report a 2.57-fold increase in the oil yield from the microalga Nostoc sp. and a 3.63-fold higher oil yield from the microalga Tolypothrix sp. biomass after sonication compared to that with the untreated biomass. However, ultrasonic applications also have certain drawbacks. One of the main issues is that for some species of microalgae, this method achieves low disruption effectiveness, and in addition, ultrasonication generates excess heat. Therefore, to reduce energy consumption and increase the efficiency of cell lysis, the possibility of combining ultrasound with various solvent systems or other disruption methods is being investigated.
Microwave treatment is an even more efficient method of cell lysis than ultrasonication and bead milling [12]. It is often used in conjunction with solvent extraction, thereby increasing the yield of oil and reducing the amount of solvent required as well as the duration of the process. Table 1 shows the application efficiency of microwave treatment in terms of oil yield from microalgae cells. The data provided show that the use of ultrasound for the treatment of microalgae biomass before the oil extraction can increase the lipid yield of microalgae Chlorella sp. biomass by up to 3.8 times and increase the yield of microalgae oil from Scenedesmus obliguus biomass by up to 77%. These algae are mainly used for the synthesis of biodiesel due to their high oil yield.

Table 1.
Influence of microwave treatment on the oil yield for individual species of microalgae [8].
However, microwave-assisted (extraction) processes also have certain drawbacks: only polar solvents can be used, free radicals are formed due to high temperatures, and chemical conversion takes place.
3. Extraction of Microalgae Oil
Three main technologies are used to extract microalgae oil: mechanical pressing, extraction with organic solvents, and supercritical fluid extraction [15].
3.1. Mechanical Oil Pressing
The simplest way to extract the oil is by mechanical pressing, but with this method approximately 25–30% of the oil remains in the microalgae cake (Table 2). For the purposes of this method, the usual equipment used for the extraction of oilseed oil-mechanical presses-can be used. Dried raw material is supplied to the presses, which is sometimes heated before the oil is extracted. The process and equipment are simple, but there is a significant loss of oil, since it is impossible to extract the entire lick in the microalgae cells by pressing (Table 2).

Table 2.
Oil extraction methods.
3.2. Oil Extraction with Organic Solvents
The use of organic solvents is more efficient for oil extraction. Polar and non-polar organic solvents, such as methanol, isopropanol, benzene, cyclohexane, hexane, acetone, and chloroform, are used for this purpose.
A non-polar solvent, hexane, is often used, and in the laboratory, the oil is extracted with a mixture of polar and non-polar solvents, chloroform and methanol. Thus, polar and non-polar lipids are extracted from microalgae, and the total lipid yield is higher than that from extraction with polar solvents alone.
According to the studies of Halim et al. [16], by using a mixture of hexane and isopropanol to extract lipids from the microalgae Chlorococcum sp., the extraction efficiency increases 3-fold compared with that from extraction using only hexane [16]. Other researchers have found that the usage of a mixture of chloroform and methanol more than doubles the lipid extraction yield compared with extraction with hexane [17]. The solvent extraction method allows the extraction of up to 96–98% of oil from microalgae cells.
3.3. Supercritical Fluid Extraction
The supercritical fluid extraction method, which can also be used for the extraction of microalgae oil, has an even higher oil extraction efficiency [18,19]. CO2 is used as the oil solvent for supercritical extraction, and it is liquefied under high pressure and temperature. This method can extract up to 100% of the oil from microalgae cells, and the extracted oil is of high purity [20]. This method is environmentally friendly and does not use any chemical solvents, and CO2 is non-flammable, inexpensive, selective, environmentally friendly, and safe. The process takes place in an oxygen-free environment, so the oil is protected from oxidation. CO2 extracts non-polar lipids, so polar solvents, such as ethanol or acetyl chloride, are sometimes used in conjunction with carbon dioxide. The crushing of algae cells using the techniques described above is used to increase the mass transfer and solvent access to the microalgae particles in order to increase the lipid yield [20].
4. Algal Oil Content and Fatty Acid Composition
Microalgae cells contain proteins, carbohydrates, lipids, and other components. The lipid content is important for biodiesel production and depends on the type of microalgae and their cultivation conditions [21]. Table 3 provides a summary of lipid levels in algal biomass, indicating that microalgae cells can contain up to 61.8% lipids. The data provided show that some species of microalgae accumulate very small amounts of oil. Between 1.9 and 10.1% of oil is found in their biomass. Up to 50% of the oil by weight is found in the larger part of the cells of algal species and only a small amount of the microalgae species is able to accumulate more than 50% of the oil in their cells. In the production of biodiesel, those microalgae with higher oil content in their cells are promising. The prospects for Chlorella vulgaris and Scenedesmus obligus use in the synthesis of biodiesel have recently been investigated. Even more promising in this respect are Tribonema minus and Desmodesmus sp. F 2. microalgae species. The data in the table show that more than 60% of the oil is found in their cells.

Table 3.
Lipid content in microalgal biomass.
Microalgae produce neutral lipids consisting of mono-, di-, and triglycerides and sterols and polar lipids consisting of phospholipids and glycolipids. The ratio of neutral to polar lipids differs and depends on the type of microalgae and the growing conditions (duration, growth medium, illumination, and temperature). The results of some studies suggest that higher levels of lipids in microalgal cells accumulate in the culture medium in the presence of salt (seawater) [38].
Liu et al. found that in the presence of iron trichloride in the culture medium, the microalga Chlorella vulgaris accumulates 3–7-fold higher lipid levels [39]. Many authors have observed that microalgae accumulate oil more intensively under nitrogen starvation conditions, and the lipid content under these conditions can be increased up to 2.8-fold in the case of Chlorella vulgaris [40]. Under these conditions, B. braunii IPE 001 accumulated 64.3% lipids [41]. It was determined that microalgal Nannochloropsis sp. biomass cultivated under nitrogen starvation conditions in seawater accumulated 59.9% lipids [42]. According to some studies, more oil accumulates in algae cells when the culture medium contains reduced amounts of phosphorus and sulfur compounds [41].
Neutral lipids are used in the production of biodiesel, so varying the growing conditions aims to extract more of them. Neutral lipid levels have been observed to be higher in the later stationary phase of microalgal growth. In addition, different fatty acid compositions of microalgal oil are found at different phases of microalgae cultivation [43]. Table 4 shows the fatty acid composition of different species of microalgal oil. The main fatty acids in microalgae oil contain 12 to 18 carbon atoms. These are stearic, palmitic, palmitoleic, and linolenic acids. Compared to vegetable rapeseed oil, which is mainly used for biodiesel production in Europe, microalgae oil has a higher content of saturated and monounsaturated fatty acids and a lower content of polyunsaturated fatty acids. Polar lipids—free fatty acids—make up a significant part of microalgae lipids. Their content varies greatly depending on the type of microalgae and the growing conditions, and the acidity of the oil can range from 0.2 to 84% [44]. This acidity poses problems for the direct use of microalgae oils in conventional biodiesel synthesis using alkaline catalysts. If the acidity of the oil is greater than 2% during alkaline catalysis, the reaction of free fatty acids with alkali produces soaps, making it difficult to separate the biodiesel from the reaction product and simultaneously reducing the yield.

Table 4.
Fatty acid composition of microalgae oil.
5. Transesterification
Due to the high viscosity and density, the direct use of oil in a diesel engine is difficult. To improve the performance characteristics, an oil transesterification process is applied. This process involves the conversion of triglycerides, which contain three fatty acid molecules, first to diglycerides, then to monoglycerides, and finally to the trihydroxyl alcohol glycerol and shorter three fatty acid ester molecules. The separation of glycerol gives a product with a lower viscosity and density, namely, biodiesel, which can be used directly in a diesel engine. To complete the transesterification reaction, three molecules of alcohol are required per molecule of triglycerides to give one molecule of glycerol and three molecules of fatty acid methyl esters (Figure 1). Because the reaction is reversible, it is recommended to use a super-stoichiometric amount of alcohol to shift the equilibrium and increase the fatty acid ester yield [51,52,53,54].
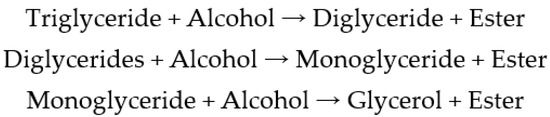
Figure 1.
Oil transesterification reaction.
The transesterification reaction takes place slowly, so catalysts and higher temperatures are used to accelerate it. The parameters of the transesterification process, the chosen catalyst and its amount, depend on the kind and quality of the raw materials.
5.1. Catalysts Used for Oil Transesterification
Catalysts are used to accelerate the transesterification reaction. Depending on the aggregate state of the catalysts, the process is divided into homogeneous and heterogeneous catalysis. The advantages and disadvantages of homogeneous and heterogeneous catalysis in the process of transesterification of algal oil are presented in Table 5. Homogeneous catalysts are divided into alkaline and acidic. In industrial biodiesel production, the transesterification process using homogeneous alkaline catalysts is most commonly used [54]. When homogeneous catalysts are used, the reaction proceeds rapidly because the reactants and the catalyst are in the same physical state. However, homogeneous alkaline catalysis is only suitable when high-quality fatty feedstocks with a low content of free fatty acids and moisture are used. If free fatty acid-rich feedstocks are used, the free fatty acids are esterified with alcohol using acid catalysts prior to the conventional transesterification procedure [55,56].
In the case of heterogeneous catalysis, the catalysts are solids that are insoluble in oil and alcohol. Heterogeneous catalysts are easily separated from the reaction mixture to give pure (98%) salt-free glycerol [57]. The heterogeneous catalysis process can be applied to lower-quality feedstocks with higher amounts of moisture and free fatty acids, and the catalysts can be reused. However, when applying heterogeneous catalysis, a lower ester yield is obtained than when applying homogeneous catalysts, and the process is slower. Table 6 presents the data obtained by scientists on the application of homogeneous catalysis in the synthesis of biodiesel from algal oil.

Table 6.
Effectiveness of homogeneous catalyst application for transesterification of microalgae oil.

Table 5.
Characteristics of different types of catalysts of transesterification of algal oil.
Table 5.
Characteristics of different types of catalysts of transesterification of algal oil.
| Catalysis Type | Catalyst | Advantages | Disadvantages | References |
|---|---|---|---|---|
| Homogeneous chemical alkaline catalysis | NaOH, KOH, NaOCH3, KOCH3. | Low process temperature and pressure High ester yield Fast process | Quality raw materials High purification costs of products (esters and glycerol) The catalyst is not reused | [58,59,60,61,62,63] |
| Homogeneous chemical acid catalysis | H2SO4, H3PO4, HCl, HNO3. | Raw materials with a higher content of free fatty acids and moisture | Catalysts cause corrosion of the equipment used High purification costs of products (esters and glycerol) The catalyst is not reused | [59,61,64,65] |
| Heterogeneous chemical catalysis | Metal oxides (Mg, Ca, Al, Fe), Silicates Carbonate, Ion exchange | Raw materials with a higher content of free fatty acids Easy catalyst separation The catalyst is reused and can be regenerated | Usually lower ester yields than during homogeneous catalysis A long process | [66,67,68,69,70,71] |
| Heterogeneous biocatalysis | Enzyme | Raw materials with a higher content of free fatty acids and moisture Low process temperature Easy catalyst separation The catalyst is reused and can be regenerated Environmentally friendly process | Sensitivity of the catalyst to higher temperatures Sensitivity of the catalyst to alcohols and process products A long process An expensive process | [72,73,74,75,76,77,78] |
Using homogeneous alkaline catalysis in the process of algal oil transesterification, the amount of catalyst ranged from 0.3% to 3.5%. For the transesterification of algal oil, as with other oils, NaOH is the most commonly used catalyst. Renita et al. obtained a 95% yield of Caulerpa peltata algal oil methyl ester using 0.3% NaOH [58]. Using a higher amount, 0.8% NaOH, the yield of Chlorella protothecoides oil methyl esters was 98.76% [60]. A further increase in the sodium hydroxide content to 3.499 w% for the transesterification of algae (Hydrodictyon) oil (from the Ganga Canal) with methanol resulted in an 87.421% yield of esters [59].
Chen et al. investigated the effect of different alkaline (NaOH, KOH, KOCH3) catalysts on the effectiveness of transesterification of previously esterified Scenedesmus sp. oil. The yield of methyl esters using NaOH, KOH, and KOCH3 was 88.3%, 91.6%, and 87.6%, respectively [63]. A maximum yield of 100% algal (from the outlet channel of the Pondy Reservoir, Chenna) oil ethyl ester was obtained using 1.25% KOH in 360 min at a high molar ratio of ethanol to oil (250:75) [62].
Chamola et al. studied water algae (Hydrodictyon) collected from the Ganga Canal, whose oils were esterified and transesterified with methanol using an acidic and alkaline catalyst. The highest yield of esters was 89.583% at a H2SO4 concentration of 3.361% for 60.443 min at 50 °C with a methanol to oil molar ratio of 8:1. Under the same conditions, an ester yield of 87.421% was obtained when an alkaline catalyst NaOH was used [59]. Sumprasit et al. also obtained better results using an acid catalyst than an alkaline or combined (acid + alkaline) catalyst. The conversion of Spirulina platensis microalgae oil under the same process conditions was 79.45% at a H2SO4 concentration of 1 g/g oil, and the ester yield was 32.0945% using 0.05 g/g NaOH and 46.59% using 0.1 g/g H2SO4 + 0.05 g/g NaOH. The highest conversion was achieved in 180 min at 60 °C and 5 mL/g methanol. Under the same conditions, the usage of HCl, HNO3, and H3PO4 resulted in significantly lower conversions: the obtained ester yields were 58.6%, 40.3%, and 33.6%, respectively [61].
Kim et al. performed simultaneous extraction and esterification studies of Nannochloropsis gaditana (80% moisture) biomass using the acid catalysts HCl (35%) and H2SO4 (98%) and found that application of HCl results in a 10% higher ester yield; therefore, the assumption was made that the effectiveness of algae cell disruption by using HCl is better than that of H2SO4; therefore, the yields of both the released oil and formed esters are higher [79].
In the preparation of methyl esters from high-acidity (8.5, 23, and 96% of free fatty acids) Scenedesmus sp., a two-step process was applied to maximize the product yield. In the first stage, free fatty acids were esterified with sulfuric acid (3.3%), and in the second stage, a 100% biodiesel yield was obtained in 30 min using 2% of an alkaline catalyst (KOH) [63]. Sumprasit et al. tried to apply a two-stage process for the transesterification of Spirulina platensis oil, but obtained a low product yield of 46.59%; they also found that esterification with an acid catalyst was more appropriate in this case, achieving a 79.45% product yield in the same amount of time [61].
For simple separation and reusability, heterogeneous catalysts are also used for the transesterification of algal oil (Table 7). Metal oxides are often used as heterogeneous catalysts. Pure CaO and MgO were used in the transesterification of Nannochloropsis oculata oil with methanol, but they were not active as catalysts. Similar results were obtained by other researchers [69], who found that the transesterification of Chlorella vulgaris oil using CaO resulted in only a 30% yield of esters. In the study of mixed oxides as catalysts, CaO/Al2O3 showed higher activity when 80% CaO was deposited on Al2O3 than with 50% CaO on Al2O3. An ester yield of 97.5% was obtained with a catalyst content of 2%, a process time of 4 h and a molar ratio of methanol to oil of 30:1. In contrast, Umdu et al., using a 6:1 molar ratio of alcohol to oil under the same conditions, reported only a 23% ester yield [70].

Table 7.
Summary of studies of different heterogeneous catalyst types.
Siva and Marimuthu (2015) obtained a yield of 96.3% algal methyl esters using 1.25% nano-CaO obtained from eggshells as a catalyst, but these data contradict Umdu et al. [70] and Narula et al. [69], who argued that CaO is ineffective as a catalyst. Teo et al. reported a high (90%) yield of Nannochloropsis oculata methyl esters using 1.25% calcined sand dollar (CSD) as the catalyst. This catalyst was prepared by washing sand dollars with chlorine bleach solution and water and heating at 800 °C for 6 h to decompose calcium and magnesium carbonates to oxides [71].
Ionic resins can be successfully used as catalysts in the production of biodiesel from microalgae oil, which has a higher content of free fatty acids. The use of three ion-exchange resins (Amberlite-15, designed as A-15, as well as CT-275 and CT-269), KSF clay, and silica-alumina in the transesterification of Nannochloropsis gaditana oil with 22% FFA with methanol was investigated. Using Amberlite-15, CT-275, and CT-269, the yield of methyl esters was above 90%. Using KFC clay as a catalyst, only a 67% yield of esters was obtained, and the lowest yield was 18.2% using silica-alumina. It was assumed that low acidity catalysts affect the process less effectively than acidic catalysts when a high acidity feedstock is used [67].
Carrero et al. investigated the use of zeolites as catalysts in the production of methyl esters of Nannochlorops is microalgae oil. Hierarchical ZSM-5 (h-ZSM-5) and beta (h-beta) zeolites were prepared from organofunctionalized seeds. H-Beta zeolite had the best activity, but a low ester content of 26% was obtained at a methanol to oil molar ratio of 100:1 at 115 °C for 4 h [81]. McNeff et al. investigated the application of metal oxide-based catalysts in the oil transesterification process at high temperatures (300–450 °C) and pressure (2500 psi). Base-modified titania was found to be a suitable catalyst for the transesterification of algal oil: a 90.2% ester yield was achieved [66].
As biocatalysts, lipases are attractive heterogeneous catalysts for the synthesis of biodiesel because they can simultaneously esterify free fatty acids and transesterify triglycerides, so they can be used for the production of biodiesel from oil with a higher content of free fatty acids. Using enzymatic catalysts, it is possible to transesterify moisture-containing feedstocks [52,82]. However, lipases are sensitive to higher temperatures, chemicals (alcohols, especially methanol), and solution media (pH); therefore, it is important to select the optimal process conditions to obtain the maximum product yield without inactivating lipases. Lipases immobilized on solid supports are commonly used in the biodiesel production process.
Bayramoglu et al. found that the application of immobilized lipase from C. rugosa on biosilica-g-p S for transesterification of quadricauda oil resulted in a 96.4% biodiesel yield. It was stated that the catalytic efficiency of immobilized lipase is 10% higher than that of non-immobilized lipase [73]. A similar 95% Chlorella vulgaris conversion rate was achieved using ethanol and methanol as transesterification agents and biocatalysts from Rhizomucor miehei lipase (Lipase GH2). However, achieving the same conversion rate with ethanol requires a 3.5-fold higher lipase content than that with methanol [75]. A slightly higher yield of 96.9 w% Chlorella sp. ethyl esters was achieved using 10% Lipolase 100 L and an ethanol to oil molar ratio of 3:1 at 30 °C for 26 h [72]. Raoufi et al. used 7% (w/v) lipase from Pseudomonas aeruginosa for Spirulina platensis oil transesterification and obtained an 87.6% methyl ester yield [76]. Immobilized Candida antarctica lipase has been successfully used in the transesterification of several types of algal oil with methanol. At an enzyme content of 7%, a methanol to oil molar ratio of 3:1, 40 °C temperature, and a 48 h duration, an ester yield of 88.5%, 87%, and 89% was obtained using Gracilaria edulis oil, Ulva lactuca oil, and Enteromorpha compressa oil, respectively. A small ± 2% difference in biodiesel yield is obtained using different algal oils [78]. Equal amounts (12.5%) of whole cell (Rhizopus oryzae 262) and purified lipase (Rhizopus oryzae 262) were used to transesterify the marine macroalgae Enteromorpha compressa oil with methanol. A higher yield of 92% was achieved in a shorter time (24 h) using purified lipase, and the application of whole cell catalyst yielded 83% esters after 48 h [77].
It is important that catalysts should be catalytically effective for more than one use, as their reusability reduces the cost of biodiesel. Researchers have found that chemical/natural heterogeneous catalysts can be used for 2–50 cycles (Table 8), depending on the type of catalyst.

Table 8.
Reuse of heterogeneous catalysts in biodiesel synthesis.
Nanocrystalline calcium oxide without additional preparation for reuse can be used for 3–8 cycles, depending on the type of oil/fat. This material is inactivated after three cycles of transesterification of poultry fat and after eight cycles of soybean oil [83]. Liu et al. state that CaO can be used up to 20 times for the transesterification of soybean oil and that its activity is only slightly reduced [39]. Granados et al. found that the yield of methyl esters of sunflower oil decreased from 94 to 81% using CaO as a catalyst for eight cycles [84].
Yoosuk et al. investigated the use of CaO for the transesterification of palm oil and found that it can be used five times without additional preparation because both fresh and used catalysts have the same morphological structure [85]. It has been found that calcium methoxide (Ca(OCH3)2) can be used up to 20 times as a catalyst for the transesterification of soybean oil [102].
KOH/NaX can be reused as a catalyst in the synthesis of soybean oil fatty acid methyl esters only after further processing. Prior to reuse, the catalyst was washed with cyclohexane, calcined at 398 K for 2 h, impregnated with 5% KOH solution and found to be effective after such treatment [86]. After filtration and drying at 100 °C, the catalyst Mg2CoAl can be used for seven cycles [87]. A Na/SiO2 catalyst can be successfully used for five cycles for the transesterification of Jatropha curcus oil with methanol. However, after each cycle, the catalyst must be regenerated by washing with methanol and drying at 100 °C for 2 h [88].
Sendzikiene et al. investigated the possibilities of using dolomite as a catalyst in the process of transesterification of sunflower oil. It was found that biodiesel meeting the requirements of the standard is obtained using dolomite for two cycles, and after the third cycle, a high degree of transesterification is achieved (95.72%), but the product does not meet the requirements of the standard for ester content (96.5%) [90]. Similar results were obtained by Ilgen, who found that dolomite can be used as a catalyst for three cycles, as the degree of transesterification decreases by only 1.5%, but the maximum degree of transesterification of the resulting biodiesel was 91.78%. Dolomite in biodiesel synthesis can be used in more cycles, but it needs to be further processed [89]. Ngamcharussrivichi et al. successfully used dolomite seven times, but the catalyst was centrifuged and washed with methanol after each reaction [91]. Wei et al. used eggshells as a catalyst in biodiesel production and found that the material could be used 13 times without loss of activity, but was inactivated after more than 17 reuses [92]. Starch-derived solid acid catalysts can be successfully used in the synthesis of up to 50 cycles of biodiesel from waste oil without further treatment [94].
Biocatalysts are expensive, so their stability and activity during repeated experiments are of great importance [103]. Studies have shown that lipases can be used for 1 to 100 cycles, depending on their type, process conditions, and the type and amount of reagents involved in the reaction [95,98,100]. Methanol was found to inactivate lipases. Some authors found that the activity of Novozyme 435 decreases with each cycle [95,98]. This effect is explained not only by the inactivation of lipases by methanol, but also by the absorption of glycerol on the surface of the biocatalyst [97]. In contrast, Nguyen et al. showed that in the process of insect fat transesterification with methanol, Novozyme 435 does not lose its activity for 20 cycles [104]. To avoid inactivation by methanol, some authors have recommended the insertion of methanol into reaction media in a few stages. Watanabe et al. applied three-step methanolysis of soybean oil and found that the activity of Novozyme 435 was not reduced by using it for 25 cycles [97]. According to Shimada et al. [98,99], when three-step methanolysis is used, the enzyme does not lose its activity for 50–52 cycles, and in two-step methanolysis [98], a 95% conversion of vegetable oil is achieved using lipase for 70 cycles.
Although ethanol is less harmful to lipases than methanol, it depends on the type of biocatalyst. In the transesterification of fish oils with ethanol, Novozyme 435 loses 16% activity after 10 cycles, and Lipozyme RM IM and Lipozyme TL IM are not suitable for reuse, as they lose 75% activity after the first cycle and even 90% after the second cycle [100]. Studies have shown that the supply of ethanol in stages also increases the possibilities of using the enzyme. Novozyme 435 does not lose activity for 37 cycles during the two-step ethanolysis process and for 54 cycles during the three-step ethanolysis process [98]. Novozyme 435 can be used for five cycles without losing activity in the ultrasonic wave environment by transesterifying soybean oil with methanol [105].
Lipases from C. rugosa can be used for six cycles, and activity remains at 76% of the baseline [73]. A lipase from P. aeruginosa can be successfully used for algal oil transesterification with methanol for 10 cycles [76]. Aguieiras et al. investigated the possibilities of reusing Novozyme 435, Lipozyme RM IM, and Lipozyme TL IM immobilized lipases in the process of transesterification of soybean oil with ethanol. The biocatalysts were washed with various solvents before reuse: ethanol, butanol, and hexane. Hexane was found to inactivate Novozyme 435, and ethanol inactivated Lipozyme TL IM [106].
5.2. Alcohols and Other Acyl Receptors Used for Transesterification of Microalgal Oil
Although methanol, ethanol, propanol, butanol, and other higher alcohols can be used for transesterification, methanol and ethanol are most commonly used. Methanol is widely known for its low cost and physical and chemical merits, and the advantage of ethanol over methanol is that it is produced from biomass, making it renewable and less hazardous to the environment.
According to stoichiometry, three moles of alcohol are required to fully convert one mole of triglyceride to the corresponding esters. However, the reaction is reversible, and a super-stoichiometric amount of alcohol is used to shift the equilibrium towards the reaction products.
In conventional biodiesel synthesis using methanol, the ratio of alcohol to oil is 6:1. Because microalgae oil often has a high content of free fatty acids, the synthesis of biodiesel from such oil is subjected to homogeneous acid catalysis in the first stage. This process is relatively slow and is carried out at higher temperatures using more alcohol than in alkaline catalysis. Rahman et al. found that the optimal ratio of methanol to oil for the free fatty acid esterification of the microalgae Spirulina maxima is 12:1, followed by 9:1 for subsequent transesterification [107].
An even higher molar ratio of methanol to oil of 56:1 in the production of biodiesel from Chlorella protothecoides oil was used by Chen et al. [63]; however, Mao and Wu (2006) indicate that with a relatively high ratio of methanol to oil of 45:1, only a 68% yield of biodiesel is achieved. Controversial results were obtained by Mandotra et al. [65]. By using a 6:1 molar ratio of methanol to oil for Scenedesmus abiindans microalgae oil transesterification applying acid catalysis, the authors obtained a 90.6% methyl ester yield [65]. Table 9 shows the data on the amount of alcohol used for esterification and transesterification of microalgae oil with methanol and the obtained ester yield.

Table 9.
Molar ratio of alcohol to oil and yield of esters obtained.
A large excess of methanol is not possible with enzymatic transesterification, as methanol inactivates the biocatalyst lipases. When using methanol in enzymatic processes, it is recommended to take a molar ratio of alcohol to oil of not more than 3:1 and to supply it to the reaction medium in stages. Even when using ethanol instead of methanol, it is proposed to use a maximum molar ratio of ethanol to oil of 3:1 [72].
Higher alcohols, such as propanol, isopropanol, and butanol, are less inactivating towards enzymatic preparations, but little research has been done on the transesterification of microalgae oil. To avoid the inactivation of enzymes, it is proposed to replace the conventional alcohols used in biodiesel synthesis with other acyl receptors, such as methyl or ethyl acetates. Enzymatic preparations are less sensitive to these compounds, the glycerol phase does not form, and the resulting triacetyl glycerol can be used directly as a fuel in a mixture with fatty acid esters. To increase the efficiency of transesterification, it is possible to carry out the process under supercritical conditions, i.e., at elevated pressure and temperature.
Methanol and ethanol were used for the transesterification of algal oil under supercritical conditions. Srivastava et al. used a 23.4:1 molar ratio of methanol to oil for Chlorella CG12 oil transesterification and obtained an ester yield of 98.12% [109]. The results of the transesterification studies of Chlorella protothecoides oil showed that at the optimal molar ratio of methanol to oil of 19:1, a 90.8% yield of esters was obtained, and at the optimal ratio of ethanol to oil of 33:1, a 87.8% yield was achieved [110]. Methyl acetate can also be used for transesterification under supercritical conditions. The process of Nannochloropsis salina sp. oil extraction and transesterification with supercritical methyl acetate at a molar ratio of 40:1 gives an ester yield of 80% [111]. Reddy et al. investigated the process of biodiesel synthesis under supercritical conditions using moist microalgal biomass with ethanol for transesterification. It was determined that a maximum ester yield of ∼67% could be obtained at a molar ratio of 9:1 (v/w) of ethanol to wet microalgae biomass [112].
To increase the efficiency of the process and reduce the cost of biodiesel, the so-called in situ method of simultaneous oil extraction from microalgae biomass and transesterification is being intensively studied. However, in this case, achieving a high ester yield requires a significantly higher amount of alcohol than that in the conventional transesterification process. Qian et al. found that a 98% yield of esters could be obtained by alkaline catalysis using a molar ratio of 180:1 methanol to oil over 3 h at 40 °C [113]. Lower yields are reported by Abo El-Enin et al., who used a 720:1 molar ratio of methanol to oil, but achieved only a 90% yield of esters by performing the process at higher temperatures [114]. Zhang et al. found that at a molar ratio of methanol to oil of 360:1, a yield of only 90.4% methyl esters can be obtained [115,116]. In the transesterification of Nannochloropsis sp., increasing the molar ratio of methanol to oil from 200:1 to 400:1 increased the yield of esters from 56.1% to 79.87% [117]. Using Chlorella sp. for in situ transesterification, the optimal ratio of methanol to oil found was 315:1, and an even higher increase in the alcohol content did not yield better results. In situ transesterification studies of Chlorella microalgae were performed using a molar ratio of methanol to oil of 105–524. The ester yields were found to increase with increasing molar ratio of methanol to oil to 315. An even higher increase in the methanol content had no positive effect [118]. Velasquez-Orta et al. investigated the influence of the molar ratio on the yield of Chlorella vulgaris esters by varying the molar ratio of methanol to oil from 300:1 to 800:1 and found that the highest yield was obtained at 600:1 [119]. Ethanol requires a higher molar ratio of alcohol to oil than methanol.
5.3. Use of Solvents to Increase the Effectiveness of Transesterification
The use of solvents significantly increases the efficiency of heterogeneous catalysis, especially enzymatic catalysis. The solvents dissolve the glycerol formed during the process, preventing it from coating the surface of the catalysts, which would otherwise reduce the catalytic efficiency. Solvents also increase the alcohol and oil intersolubility, making the process more efficient.
Hexane, acetone, butanol, and ionic liquids can be used as solvents. High efficiency was demonstrated by t-butanol used in the enzymatic transesterification of microalgae Chlorella vulgaris oil with methanol. Using an oil and butanol ratio of 1:1, the yield of esters was greater than 97% [120]. Similar results were obtained for transesterification using Nanochloropsis oceganica IMETI microalgae oil. Wang et al. obtained a 99.1% yield of esters in the enzymatic process using t-butanol as a solvent [121]. Li et al. reported a 98.2% yield of methyl esters using hexane [122]. Lai et al. achieved a 90.7% methyl ester yield using the ionic liquid [BMIm] [PF6] [123]. An even lower yield was obtained using dimethyl carbonate as the solvent.
Solvents are also often used in the simultaneous algae oil extraction and transesterification process in situ, thereby eliminating the separate procedure of oil extraction. Additional solvents used in the in situ process make the extraction of oil faster and more efficient, and they increase the solubility between alcohol and oil. As solvents, n-hexane [124], methylene dichloride [125], chloroform [126], and others (Table 10) were used.

Table 10.
Summary of solvents studied for in situ transesterification.
Im et al. investigated the use of various solvents (n-hexane, carbon tetrachloride, toluene, benzene, 1,2-dichloroethane, chloroform) in the transesterification of N. oceanica algae biomass with methanol. Under the same process, the maximum ester yield was 90.6%, which was obtained using chloroform, and the yields were 69.3%, 61.9%, 61.5%, 50.4%, and 33.7% when benzene, dichloroethane, toluene, carbon tetrachloride, and n-hexane were used, respectively. These results demonstrate the importance of solvent use and the selection of the appropriate solvent for process efficiency [126]. Karimi et al. [126] and Kim et al. [79] obtained very similar results of algae biomass in situ transesterification with methanol using chloroform as solvent.
In the transesterification of Nannochloropsis gaditana (wet) with methanol using chloroform, the yield of esters was 90%, while that using hexane under the same conditions was only 70%. Cheng et al., using chloroform as a solvent, obtained a slightly lower yield of 84.6% Chlorella pyrenoidosa methyl esters [128].
Kim et al. [130] found that dichloroethane was the most efficient solvent for the transesterification of Nannochloropsis gaditana with ethanol without a catalyst, resulting in an ester yield of 91.85%. The use of other solvents, such as dichloromethane and chloroform, gives a lower yield of ethyl esters. Even 97.3% and 98.28% ester yields were obtained using hexane as the solvent for transesterification of Chlorella vulgaris ESP-31 and Scenedesmus obliquus biomass with methanol, respectively [124,125]. A slightly lower yield of 90.9% methyl esters of Nannochloropsis sp. was obtained using the same solvent [117].
Li et al. used methylene dichloride as a solvent for the transesterification of Nannochloropsis sp. biomass with methanol. Low yields of esters were obtained, but it was shown that the amount of solvent also had a significant effect on the yield. The process conditions were the same, but by using a 2:1 (v/v) mixture of methanol and methylene dichloride, the yield of esters was 2.6%, and when the ratio was increased to 3:1 (v/v), the yield reached 28% [125].
Studies have been performed on microalgae Chlorella sp. to use mineral diesel as a solvent for the transesterification of oil via an in situ process. The optimal conditions of transesterification in diesel fuel media were as follows: 4.54:1 molar ratio of ethanol to oil, 13.26% of the biocatalyst Lipozyme RM IM, and a duration of 13 h. The obtained product contained 7% biodiesel in mixture with fossil diesel fuel and met the requirements of the mineral diesel standard EN 590. The degree of microalgae oil transesterification was 98% [131].
5.4. Influence of Temperature on Transesterification Efficiency
Temperature is an important factor in determining the rate of reactions. At higher temperatures, transesterification reactions occur more rapidly because they are endothermic, the oil–alcohol mixture becomes more homogeneous and the viscosity of the reaction mixture decreases [133]. Temperatures close to the boiling points of alcohols are most commonly used in the transesterification process. However, to reduce energy consumption, it is appropriate to carry out the process at the lowest possible temperatures. For the study of homogeneous biodiesel synthesis, the optimal process temperature ranges from 30 to 100 °C. Acid esterification at 50–60 °C yields approximately 79–89% ester [59,61]. Miao and Wu studied the process of acid esterification of Chlorella protothecoides oil with methanol, increasing the temperature from 30 to 90 °C. It was found that the most efficient synthesis takes place at 50 °C, and further increasing the temperature decreases the yield of biodiesel. However, at 30 °C and 50 °C, low product yields of 56% and 58%, respectively, were obtained [64]. A higher yield for transesterification of Scenedesmus abundant oil was achieved only at 100 °C [65].
A high yield of 98.78% Chlorella protothecoides methyl esters was obtained during alkaline transesterification at 65 °C [60]. A similar yield of 95% esters was obtained by Renita et al. at a process temperature of 60 °C [58]. The two-stage process and the same temperature gave a maximum yield of 100% methyl esters produced from the high acidity Scenedesmus sp. oil [63].
During heterogeneous synthesis using CaO-MgO and CaO/Al2O3 mixtures as catalysts, the optimum process temperature is 50–60 °C [68,69,70,71,80]. The yield of micro Chlorella vulgaris methyl esters was 88.89% at 50 °C [69]. Umdu et al. obtained a 97.5% yield of methyl esters of Nannochloropsis oculata oil at the same process temperature and catalyst CaO/Al2O3 [70]. A similar yield of methyl esters (96.3%) was achieved by scientists by transesterifying algal (from the coastal area near Chennai, India) oil at 60 °C using nano-CaO as a catalyst [68]. Using calcined sand dollar (CaO-MgO) for the transesterification of algae (from the Palar River near Chengalpet), a yield of methyl esters of 90% was reported upon transesterification at 60 °C [71].
Siva and Marimuthu (2015) investigated the influence of temperature on the process of transesterification of algae (from the Palar River near Chengalpet) oil with methanol using a catalyst from eggshell (nano-CaO). The temperature was varied from 40 °C to 70 °C, and at the optimal process temperature of 55 °C, the yield of esters was 96.3%.
Using the ion-exchange resins Amberlite-15, CT-275, and CT-269, the yield of methyl esters is greater than 90% only when the process is performed at 100 °C [67]. A slightly higher temperature of 115 °C was used for the transesterification of Nannochloropsis microalgae oil using the catalyst h-beta zeolite, but the yield of esters was low, reaching only 26% [81].
Using enzymes as a catalyst, the optimum process temperature is 30–40 °C. At higher temperatures, denaturation of proteins occurs, and the catalytic activity of lipases decrease [72,73,74,75,76]. Bayramoglu et al. analyzed the catalytic activity of unimmobilized and immobilized lipase from C. rugosa at a temperature range of 20–60 °C and found that the maximum activity of unimmobilized lipase and immobilized lipase is reached at 35 °C and 40 °C, respectively [73].
The optimal temperature determined in the methanolysis process of Spirulina platensis oil using immobilized lipase from Pseudomonas aeruginosa was also 40 °C [76]. Bharathiraja et al. studied the transesterification processes of Gracilaria edulis, Ulva lactuca, and Enteromorpha compressa oils and selected the optimal temperature of 40 °C, with 88.5%, 87%, and 89% oil conversion yields, respectively [78]. Using Rhizomucor miehei lipase, the highest conversion rate was achieved at 30 °C: the Chlorella vulgaris oil methyl ester yield reached 87%, and the ethyl ester yield reached 86%. Lower yields were obtained at both lower and higher temperatures [75].
Makareviciene et al. confirmed that the optimal temperature for the enzymatic synthesis of Chlorella sp. oil ethyl esters using Lipolase 100 L as the catalyst is 30 °C, with a 96.9% oil conversion yield [72]. The same optimal process temperature was determined for transesterification with algal oil obtained from marine macroalgae Enteromorpha compressa, both using whole cells (Rhizopus oryzae 262) and purified lipase as catalysts [77].
High temperatures are applied in a supercritical non-catalytic process. Chlorella protothecoides oil was esterified with supercritical methanol and ethanol to give ester yields of 90.8% and 87.8%, respectively. Supercritical methanol and ethanol gave higher yields at 320 °C and 340 °C, respectively [110]. Hegel et al. reported that the optimal temperature for transesterification of H. coffeaeformis oil with supercritical ethanol was 305 °C [134]. Patil et al. applied supercritical methyl acetate (glycerine-free) technology and determined that the optimal process temperature of 310 °C achieved the maximum ester yield [135].
McNeff et al. found that by increasing the temperature from 322 to 434 °C, the yield of biodiesel increases from 15.5 to 87.3 w% during the transesterification of soybean oil with methanol using base-modified titania. The optimum determined transesterification temperature of algae oil was 348 °C, and a base-modified titania catalyst conversion of 90.2 w% was achieved [66].
Jazzar et al. investigated the influence of lower temperatures (245–290 °C) on the transesterification of dry and wet (80% humidity) Nannochloropsis gaditana microalgae biomass with supercritical methanol [136]. The obtained data show that the maximum yield of 0.46–0.48 g fatty acid methyl esters/g biomass can be achieved at 255–265 °C in 50 min, taking a ratio of 10:1 mL of methanol to dry microalgae. The results obtained also showed that the moisture content did not significantly affect the biodiesel yield, as the biodiesel yield obtained from dry algae was only 2–9% higher than that from wet algae.
High temperatures accelerate the reactions but also lead to thermal decomposition of the esters. According to the obtained results, esters decompose at temperatures above 350 °C [137], but this is strongly influenced by the fatty acid composition of biodiesel. Saturated fatty acids are more thermally stable. Patil et al. (2017) investigated the thermal decomposition of biodiesel produced from the algae Nannochloropsis salina in a nitrogen environment. A decomposition of 10% was determined at 205 °C, 50% of esters degraded at 300 °C, and 90% fatty acid methyl esters decomposed at 475 °C.
5.5. Influence of Process Duration on the Effectiveness of Transesterification
An important parameter for the effective transesterification of the oil is the reaction time. The optimal duration of transesterification with methanol was found to be 50–90 min using NaOH as a catalyst. A yield of 98.76% of Chlorella protothecoides oil methyl esters was obtained in 52 min [60]. Response surface methodology was applied to optimize the acid and alkaline transesterification processes of algae (Hydrodictyon) oil, and the optimal process duration was found to be 73.637 min using an alkaline catalyst and 60.443 min using an acid catalyst, giving product yields of 87.421 w% and 89.583 w%, respectively [59].
Renita et al. reported a 90% yield of Caulerpa peltata algal oil methyl esters obtained in 90 min [58]. Because the ethanolysis process is slower, freshwater algae oil triglycerides were transesterified with ethanol in 360 min [62]. Other researchers stated that the free fatty acid esterification process takes longer than transesterification [61,64,65]. High acidity oil is transesterified using a two-stage process, and the maximum Scenedesmus sp. oil conversion is obtained at 180 min [63]. Longer process times are required for heterogeneous catalysis or simultaneous oil extraction and transesterification processes in situ. In the transesterification of algal oil using heterogeneous chemical catalysts, the process time is 3–4 h, giving a transesterification yield of 89.89–97.5% [67,68,69,70,71,80]. In heterogeneous synthesis using biocatalysts, the duration is very important, and the yield of esters is proportional to the duration of the process. Using lipase from Pseudomonas aeruginosa in the synthesis of biodiesel from Spirulina wider oil, with a process duration of 24 h, a biodiesel yield of 64.1% was reported, and after 48 h, the yield was increased to 87.6% [76]. Bharathiraja et al. also found that yields of 88.5%, 87%, and 89% of Gracilaria edulis, Ulva lactuca, and Enteromorpha compressa fatty acid methyl esters were obtained with a process duration of 48 h [78].
For some researchers, shorter reaction times were sufficient for high biodiesel yields. Makareviciene et al. [72] reported that a 96.9% yield of Chlorella sp. fatty acid ethyl esters was reached in 26 h, and a similar yield of esters of 95–96.4% was reached in 24 h by other researchers [73,74,75].
The duration of the simultaneous oil extraction and transesterification (in situ) process is long because two processes take place simultaneously: oil extraction and transesterification. Using the in situ process, Ehimem et al. [118] achieved a yield of 91.3% esters after 8 h (catalyst H2SO4), Velasquez-Orta et al. [119] achieved a 90–92% yield of Chlorella sp. oil methyl esters after 19 h (catalysts CH3ONa and H2SO4), and Tran et al. [124] obtained a 97.3% yield of methyl esters of Chlorella vulgaris (ESP-31) only after 48 h of reaction. Macías-Sánchez et al. (2015) investigated the influence of in situ process time and temperature on the yield of Nannochloropsis gaditana oil methyl esters and found that a 100% ester yield could be achieved at 80 °C in 300 min, at 90 °C in 180 min, and at 100 °C in 105 min.
Nan et al. found that the transesterification of Chlorella protothecoides oil under supercritical conditions gave a yield of 90.8% after 31 min and 87.8% with ethanol after 35 min [110].
6. Properties of Algal Biodiesel
To use biofuels made from algae oil in diesel engines, their quality must meet the requirements of the standard for biodiesel fuel. The most commonly used biodiesel quality standards are EN 14214, which is used in Europe, and ASTM D6751, which is used in the U.S.A. Table 11 shows the quality indicators of algal oil esters and compares them with the requirements of the European standard EN 14214.
As seen from the presented data, the quality indicators of algal oil esters meet most of the requirements of the standard EN 14214. The ester content is an important indicator of the successful conversion of oil to biodiesel. The content of all esters analyzed was greater than 90%, but not all met the minimum ester content of 96.5% required by the standard.
The content of mono-, di-, and triglycerides indicates whether all glycerides have been converted to esters and glycerol. According to these indicators, all algae oil esters meet the requirements of the standard.
Linolenic acid is an undesirable polyunsaturated fatty acid, as it accelerates oxidation; therefore, its content is limited to biodiesel, and according to the requirements of the standard EN 14214, its content cannot exceed 12%. Researchers studying the linolenic acid content, iodine number, and water content in biodiesel found that esters meet the requirements of the standard according to these indicators [72,138].
Viscosity is the most important parameter of all fuels. It affects fuel injection efficiency, especially at low temperatures, where an increase in viscosity affects the fuel flow. At low temperatures, biodiesel becomes very viscous or even solidifies. According to the standard, the permissible kinematic viscosity limits are 3.5–5.0 mm2/s. The viscosity of the analyzed biodiesel samples at 40 °C was 3.74–5.76 mm2/s, and not all biofuels met the requirements of the standard.
The viscosity of Chlorella protothecoides methylesters was 5.2 mm2/s [64]. Chen et al. studied three different types of methyl esters (Scenedesmus sp. methyl ester, Nannochloropsis sp. methyl ester, and Dinoflagellate methyl ester) produced under the same conditions, and Nannochloropsis sp. methyl ester did not meet the requirements, with a kinematic viscosity of 5.76 mm2/s [63].
Density is an important characteristic of diesel fuel. Density, like viscosity, depends on the composition of fatty acids and the purity of the product. According to the requirements of the standard EN 14214, the density should be measured at a temperature of 15 °C and should be in the range of 860–900 kg/m3. The density of the analyzed algae esters at 15 °C ranged from 883.6 to 893.6 kg/m3 and met the requirements of the standard.
According to the requirements of the standard EN 14214, the maximum permissible acid number of biodiesel fuel is 0.50 mg KOH/g. Using biodiesel with a higher free fatty acid content can cause corrosion in the engine. According to published data, the acid number of biodiesel produced from algae oil varied from 0.37 to 0.52 mg KOH/g [62,63,64,72,138,139].

Table 11.
Properties of biodiesel from different algae oils (UNE-EN 14214).
Table 11.
Properties of biodiesel from different algae oils (UNE-EN 14214).
| Property | Test | Limits | Units | Algae Methylester [139] | Chlorella sp. Methylester [138] | Chlorella protothecoides Methylester [64] | Chlorella sp. Ethylester [72] | Algal Ethylester [62] | Scenedesmus sp. Methyl Ester [63] | Nannochloropsis sp. Methyl Ester [63] | Dinoflagellate Methyl Ester [63] |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ester Content | EN 14103 | 96.5 min | % (m/m) | 98.7 | 97.42 | 96.9 | 91.0 | 92.2 | 96.9 | ||
| Density, 15 °C | EN ISO 3675 EN ISO 12185 | 860–900 | kg/m3 | 883.6 | 864 | 893.6 | 801 | 852 | 854 | 878 | |
| Kinematic viscosity, 40 °C | EN ISO 3104 | 3.5–5.0 | mm2/s | 4.55 | 4.73 | 5.2 | 4.86 | 4.15 | 5.76 | 3.74 | |
| Flash point | EN ISO 2719 EN ISO 3679 | 101 min | °C | 140 | 179 | 115 | 98 | ||||
| Cetane Number | EN ISO 5165 | 51 min | – | 59 | 52 | 52 | |||||
| Copper Strip Corrosion | EN ISO 2160 | Class 1 | rating | 2.1 | 1 | ||||||
| Oxidation Stability | EN 14112 | 8 min | hours | 2.3 | 6.76 | 6.76 | 5.42 | 1.93 | 1.02 | ||
| Acid Value | EN 14112 | 0.50 max. | mg KOH/g | 0.32 | 0.37 | 0.374 | 0.28 | 0.52 | 0.46 | 0.44 | |
| Iodine value | EN 14111 | 120 max. | g J2/100 g | 97.12 | 97.12 | ||||||
| Linolenic acid methyl ester content | EN 14103 | 12 max. | % (mass) | 6.85 | 8.92 | ||||||
| Free Glycerol | EN 14105 EN 14106 | 0.02 max. | % (m/m) | 0.002 | |||||||
| Total Glycerol | EN 14105 | 0.25 max. | % (m/m) | 0.013 | |||||||
| Monoglyceride content | EN 14105 | 0.8 max. | % (m/m) | n.d | 0.792 | 0.792 | |||||
| Diglyceride content | EN 14105 | 0.20 max. | % (m/m) | 0.122 | 0.152 | ||||||
| Triglyceride content | EN 14105 | 0.20 max. | % (m/m) | 0.197 | 0.158 | ||||||
| Water content | EN ISO 12937 | 500 max. | ppm | 190 | 220 | 250 | 200 | ||||
| Sulfur Content | EN ISO 20846 | 10 max. | mg/kg | 6.70 | 8.1 | 8.1 | |||||
| Phosphorus Content | EN 14107 | 4 max. | Ppm | 3 | |||||||
| CFPP | EN 116 | Report | °C | −14 | −11 | −10 |
Oxidation stability is a very important property that demonstrates the ability of a fuel to retain its required properties during storage and transportation. Stored biodiesel is easily oxidized upon contact with atmospheric oxygen. During oxidation, free fatty acids, peroxides, hydroperoxides, aldehydes, and polymers are formed, which impair engine performance, especially that of the engine fuel injection system [140]. It was determined that esters obtained from various algal oils have low oxidation stability (1.02–6.76 h). This indicator is expressed in terms of the induction period (IP), which, according to the requirements of the biodiesel standard, must be at least 8 h at 110 °C. The oxidation stability of fatty acid esters can be increased by using natural and synthetic antioxidants [141].
The cetane number of biodiesel must be at least 51, and the cetane number of algal oil esters analyzed by scientists ranges from 52 to 59 [62,72].
When evaluating the quality of biodiesel, it is very important to also evaluate the low-temperature properties. According to the standard EN 14214, the main characteristic of low-temperature properties is the cold filter plugging point (CFPP). Different requirements for the CFPP value vary from country to country depending on climatic conditions. Additionally, a different CFPP value is set for different seasons, i.e., summer, winter, and the transitional period. It was found that the CFPP of Chlorella sp. ethylester is minus 10 °C [72], of Chlorella protothecoides methylester is minus 11 °C [64], and of algae methylester is minus 14 °C [139]. The low-temperature properties of biofuels can be improved by mixing them with products with better properties or by adding additives (depressants).
7. Engine Performance and Exhaust Gas Emissions Operating with Algal Biodiesel and Their Blends with Mineral Diesel Fuel
With the growing interest in the use of microalgae for biofuel production, the performance and environmental properties of biodiesel derived from microalgae oil are being compared with those of conventional biodiesel and mineral diesel.
The use of microalgal biodiesel diesel engines has been found to increase brake-specific fuel consumption in the same way as conventional biodiesel compared to mineral diesel. Rajak et al. found a 6.4% higher specific fuel consumption using Spirulina microalgae biodiesel than mineral diesel. This result is explained by the lower calorific value and lower heating value of microalgae oil methyl esters than mineral diesel, so more fuel is needed to obtain the same power output. Algae biodiesel made from microalgae Chlorella vulgaris oil exhibited up to 5.2% lower brake thermal efficiency than mineral diesel [142]. This result is explained by the poorer combustion properties and higher viscosity of microalgae biodiesel than mineral fuels. Microalgae biodiesel has a 14% lower peak pressure value than mineral diesel. This value is also associated with a higher biodiesel viscosity and lower calorific value than mineral diesel [143].
The performance of a diesel engine is characterized by the cylinder pressure and crank angle. The cylinder pressure using microalgae biodiesel is significantly lower than that using mineral diesel. This difference is explained by the higher viscosity and lower volatility of biodiesel and the associated heavier fuel–air mixture mixing and atomization efficiency.
With the use of microalgae biodiesel, an early start of combustion and an early pressure rise are observed due to the shorter ignition delay period and the higher cetane number and oxygen content of the microalgae biodiesel than mineral diesel. Higher cetane numbers and oxygen content result in advancement in rapid combustion during the premixed combustion phase, and thus higher peak pressure using biodiesel than mineral diesel [144].
Researchers found that the application of algae biodiesel increases combustion noise, decreases engine torque output, and slightly increases heat release compared to using mineral diesel. The use of such biodiesel is proposed to solve the problems of increasing combustion noise and decreasing output torque by retarding the injection timing [145].
Ignition delay is one of the combustion characteristics that indicates the time interval between injection and combustion start. The ignition delay period was found to be lower with microalgae biodiesel than with mineral diesel, which is also related to fuel viscosity. A lower calorific value of biodiesel also results in a lower cumulative heat release and duration of combustion, while a higher oxygen content in biodiesel results in a higher in-cylinder temperature [144].
A number of studies have been conducted to evaluate the exhaust emissions of engines operated with algae oil methyl esters compared to mineral diesel. This interest is explained by the higher oxygen content of biodiesel than of mineral diesel, which results in better fuel combustion; the fuel–air mixture reduces the CO content in exhaust gases by full combustion. Significant reductions in unburned hydrocarbons were also observed, which was associated with a higher cetane number of biodiesel than of mineral diesel, resulting in decreases in ignition delay. Some authors attribute the decrease in HC emissions to a lower carbon and hydrogen content in biodiesel or the presence of unsaturated fatty acids in the methyl esters.
For nitrogen oxide emissions, the results are contradictory. Some authors point out that these emissions are lower than those with mineral diesel, while others have observed an increase. Higher NOx concentrations in exhaust gases are explained by the higher density of biodiesel in relation to increased injection pressures causing excess fuel delivery. In addition, the oxygen in biodiesel results in higher combustion temperatures and allows nitrogen to react with oxygen to increase NOx concentrations during combustion. Some authors cite the presence of unsaturated fatty acids in biodiesel as one of the reasons for the increase in NOx concentration [144].
The use of microalgae biodiesel in a diesel engine reduces the smoke opacity of the exhaust gases. Smoke is produced by an incomplete combustion of fuels, especially aromatic hydrocarbons. The lower smoke opacity of the engine when running on biodiesel is explained by the absence of aromatic hydrocarbons and sulphur compounds in the fuel, as well as their better combustion due to the oxygen in the biodiesel. Table 12 provides a summary of the research results obtained by various authors investigating the performance properties of microalgae biodiesel and the concentrations of harmful components in exhaust gases in comparison with the use of mineral diesel.
The study of unregulated emissions showed that the use of Chlorella emersonii biodiesel and its blends with mineral diesel has higher acetone and formaldehyde emissions than the use of mineral diesel, the toluene emissions are lower, and the formaldehyde emissions are load dependent [146]. The use of blends of microalgae biodiesel and mineral diesel has the same impact on biodiesel emissions as pure biodiesel. Venu et al. investigated engine emissions when mixtures of methyl esters of Chlorella emersonii with mineral diesel at 10%, 20%, 30%, and 40% by volume were used; they found that by using a mixture of 20% methyl esters with mineral diesel, the concentrations of carbon monoxide and hydrocarbons were lowest, and minimal smoke opacity was registered. However, the presence of biodiesel in blends slightly increases the NOx emissions. The incorporation of algae oil methyl esters into mineral diesel reduced unregulated emissions, such as toluene and acetaldehyde. Therefore, the authors, summarizing the obtained results, propose to use the B20 mixture as a potential alternative fuel, the use of which generates the least harmful emissions and has better engine performance [146].

Table 12.
Engine emissions using microalgae biodiesel compared to mineral diesel.
Table 12.
Engine emissions using microalgae biodiesel compared to mineral diesel.
| Microalgae | CO | CH | NOx | Smoke Opacity | Reference |
|---|---|---|---|---|---|
| Chlorella vulgaris | Lower | Lower by 6 percent at any load | Lower by 10 ppm at 1.19 kW, 8 ppm at 2.31 kW and 7 ppm at 4.53 and 5.15 kW brake power load | Lower | [143] |
| Chlorella emersonii | Lower by app. 10% | Lower by 58 ppm | Higher | Lower up to 40% | [146] |
| Lower | Lower by 79% | Higher by 7% | [147] | ||
| Spirulina platensis | 61% lower at full load | 10% lower | Higher by 21% lower at full load | Lower by 14% | [144] |
| Spirulina | Lower by 6.1% | Particulate matter lower by 60% | [142] |
Similar results were obtained by Rajak et al. after testing a diesel engine using Spirulina microalgae methyl ester 20, 40, 60, and 80% blends with mineral diesel. The lowest concentrations of harmful components and cylinder pressures, brake thermal efficiency (BTE), exhaust temperature, and maximum rise pressure rates compared to those with diesel fuel were determined using a mixture of 20% microalgae biodiesel and 80% mineral diesel. The incorporation of biodiesel into mineral diesel resulted in 4.9% lower NOx emissions, particulate matter emissions were reduced by 20.7%, and smoke opacity was reduced by 5.4%, but an increase in CO2 emissions was observed at all loads [148].
Nautiyal et al. performed engine tests using 10% and 20% algae biodiesel blends with mineral diesel and found that higher amounts of biodiesel in the blend significantly reduced the CO and HC emissions. Like other researchers, they found a slight increase in NOx emissions, which is more pronounced with higher levels of biodiesel in the blend [144].
The opposite results were obtained by Mahendran et al. after analysing exhaust gas emissions when the engine was running on 5, 15, and 25% microalgae biodiesel blends with mineral diesel. The authors point out that working with a 25% biodiesel blend with mineral diesel reduces NOx emissions, while the HC emissions are similar to those when working with mineral diesel [149]. Slightly different results were obtained by Piloto-Rodrígueza et al., who state that the concentrations of harmful components in exhaust gases when the engine is working with a 20% biodiesel blend are similar to those when working with pure mineral diesel [150].
Makareviciene et al. investigated the exhaust emissions of a diesel engine while operating with a 30:70 v/v mixture of microalgae Chlorella sp. biodiesel and mineral diesel and found that, compared to the use of pure mineral diesel, the smokiness of exhaust gases decreased by 10–75%, the HC concentrations decreased by 5–25%, and CO concentrations decreased by approximately 10% depending on the operating mode. The NOx concentrations were at the same level as that with mineral diesel [60].
8. Economic Indicators of Biodiesel Production from Microalgae Oil
It is important to evaluate not only the properties of biodiesel and its impact on the environment but also its production cost. Most studies show that it is currently not viable to produce biodiesel from algae oil on an industrial scale.
Chen et al. state that several strategies can be used to reduce the cost of biodiesel from microalgae: production of other valuable products (protein and vitamins) with microalgae biodiesel; simultaneous cultivation of fish and other water organisms; and microalgae cultivation and application in other technological operations: wastewater treatment, biogas production, and biogas upgrading [151].
Studies have shown that 71 g of biodiesel and 446 L of biogas can be produced from 1 kg of microalgae biomass [61]. Economic analysis shows that the production of biodiesel and biogas from biomass can reduce the cost of biodiesel by 35%. It is less expensive to grow algae in open ponds than in photo-bioreactors. It has been found that microalgae, which improve water quality, can also grow in ponds where shrimp are farmed [152]. However, co-cultivation of shrimps, crayfish, fish, and microalgae reduces their productivity because they are used for food [153]. It is also important to investigate whether toxic compounds excreted by microalgae do not harm other aquatic populations [154].
Chisti estimated that 1 kg of algal biomass costs USD 0.47–0.6 at a bioreactor capacity of 10,000 t/m. When calculating the price of oil, it was assumed that in algal biomass—with 30% oil, in which case the price of oil is USD 1.4–1.8/L, for comparison—palm oil cost approximately USD 0.52/L, and the price of mineral diesel ranged from USD 0.66 to 0.79/L [21]. The price of mineral diesel consists of 20% taxes, 52% crude oil, 19% refining expenses, and 9% distribution and marketing. If the average price of mineral diesel before taxes is USD 0.49/L, it is 35% less expensive than biodiesel from palm oil (USD 0.66/L), which is the least expensive oil from which biodiesel can be produced. The price of oil conversion into biofuels is USD 0.14/L. For biodiesel to compete with mineral diesel, the price of the oil must not exceed USD 0.48/L, and it must not be taxed. It is therefore necessary to reduce the cost of obtaining algal oil, which can be achieved by increasing the amount of oil in the biomass. The cost of producing biodiesel from algal oil can be reduced by improving the capabilities of microalgae through genetic engineering and advances in the engineering of photo-bioreactors [21].
The extraction of oil from algae accounts for the largest share of costs. The higher the amount of oil extracted, the lower the price of biodiesel [155,156]. Brownbridge et al. evaluated the economic and technical indicators of the use of algae in biodiesel production and found that at 100 thousand tons of biodiesel per year, the cost of biodiesel production at the plant will be 0.8–1.6 pounds/kg [156]. Kumar and Singh obtained very similar results with the same production capacity at a cost of USD 1.11–2.22/kg [157]. Due to the high cost of biodiesel, there is a need to produce not only biofuels but also other value-added products from algae [156]. Williams and Laurens [158], Goh et al. [159], and Farieda et al. [160] also agree that producing biodiesel alone from algal oil is not economically viable.
Earlier, researchers estimated the lowest cost of microalgae biodiesel at USD 18.35/gallon and sales at USD 21.11/gallon. These estimates prove that at such prices, biodiesel from microalgae cannot compete with diesel from oil, whose average selling price in 2013 amounted to USD 8.32/gallon in Italy and USD 3.91/gallon in the U.S. [161]. A study by Gallagher showed that the cost-effectiveness of using algae in biodiesel production would depend on state subsidies and the price of oil, which should be higher than USD 100/barrel [162].
Sawaengsak et al. [163] agreed with Gallagher [162] that if the price of oil was USD 100/barrel, biodiesel from algae oil could be competitive. He and colleagues investigated possible ways to make biodiesel from Chlorella sp. algae profitable and found that even in the production of high value-added omega-3 fatty acids, industrial biodiesel production is not profitable. In this study, the price of biodiesel from microalgae was approximately 68 THB/L (or equal to approximately USD 2.2 USD/L) when algae were grown on raceway ponds. Biodiesel from algal oil could be competitive even with a high price of mineral oil, as another option, to produce animal feed from biomass because Chlorella sp. has high levels of protein, carbohydrates, lipids, trace elements, vitamins, and carotenoids; thus increasing biodiesel production revenues. To reduce the costs of growing microalgae, it is appropriate to use greenhouse gases and wastewater in their cultivation while simultaneously treating the wastewater [164,165]. The property of algae to treat wastewater containing metal compounds can be applied in the treatment of industrial wastewater. When microalgae Botryococcus sp. were used for domestic wastewater treatment, the removal of nitrogen was 64.5%, of phosphorus was 89.8%, and of total organic carbon was 67.9%, and the fat accumulation reached 61.7% m/m dry weight [166]. Botryococcus braunii microalgae have been found to remove 100% phosphorus and 65% nitrogen compounds during wastewater cultivation [167].
Researchers have estimated the cost of the in situ process to produce biodiesel from algal biomass. In the production of biodiesel under laboratory conditions, it was found that more than 80% of the total costs are borne by the prices of raw materials (alcohol, solvent, catalyst) [168]. It is very important to recycle excess alcohol and solvents and return them to the process. It has been found that approximately 90% of alcohol and solvents can be recycled. Recycling of alcohol and solvents can reduce the cost of the product by 67.64–88.01% [168,169,170], which is USD 3.59–19.70 per kg of biodiesel.
Macías-Sánchez et al. state that the cost can be significantly reduced by replacing the expensive (USD 1.65/kg) catalyst with acetyl chloride sulfuric acid (USD 0.07/kg) [169]. Other raw materials, such as ethanol, are three times more expensive than methanol, and chloroform is twice as expensive as hexane. Replacing chloroform with hexane reduced the price of biodiesel from USD 19.7 to USD 7.66, i.e., 61.12% [168].
The researchers who studied the supercritical biodiesel production process estimated the net energy ratio (NER). The process is economically viable if the NER is more than 1, and it has been found to be an economically viable approach due to the use of wet microalgae (Nannochloropsis gaditana), for which drying and oil extraction would account for a large part of the cost [136]. Huesemann and Benemann state that an NER > 1 can only be obtained when microalgae are grown in raceway ponds [171]. Lardon et al. investigated the process of biodiesel production from wet and dry microalgae and found that for 1 kg of biodiesel produced, the total energy consumption was 106.4 MJ when dry microalgae biomass was used and 41.4 MJ for wet microalgae biomass [172]. The NER value was 3.54 when wet microalgae was used and 0.97 for dry microalgae.
9. Conclusions and Future Trends
This chapter reports on recent advances in exploring the potential use of microalgae oil in biodiesel synthesis. The use of microalgal biomass for biodiesel production has certain specificities. Microalgae cells are surrounded by solid cell walls, which must be broken down in order to extract the oil, so mechanical and non-mechanical methods of cell breakdown are proposed before oil extraction. Recently, much attention has been paid by researchers to methods such as ultrasonication and microwave treatment methods, which are characterized by high efficiency.
These methods can be used in combination with oil extraction and transesterification. Microalgae oils contains higher levels of saturated fatty acids than vegetable oils and are often high in free fatty acids; therefore making them unsuitable for direct transesterification with alcohols using alkaline catalysts. Prior to transesterification of such an oil, it is necessary to apply the free fatty acid esterification procedure using mineral and organic acids as catalysts.
Homogeneous catalysts, such as potassium and sodium hydroxide and sulfuric and hydrochloric acids, are used for transesterification of the oil. Their content is similar to that for the transesterification of conventional feedstocks, and high yields of esters are obtained by using 0.3 to 3.5% catalyst. Among the heterogeneous catalysts, metal oxides and their mixtures are the most commonly used. Higher algal oil ester yields were obtained with CaO/Al2O3, a catalyst, while zeolites and ion exchange resins did not show high efficiency in the transesterification of microalgal oil.
Enzymatic preparations as heterogeneous catalysts are of increasing interest to scientists. These preparations are particularly suitable for the synthesis of biodiesel using oils with higher free fatty acid content, as they simultaneously catalyze the processes of esterification of free acids and transesterification of triglycerides. However, the optimal amount of these catalysts is relatively high, and high yields of esters were obtained using 7–10% lipases. Enzymes as catalysts are expensive, so opportunities are being sought to reuse them and to regenerate them effectively when they lose their effectiveness. Methanol is most commonly used as an acyl receptor in the production of biodiesel. The use of other acyl receptors for transesterification is also being investigated under laboratory conditions. The amount of alcohol in the reaction medium is one of the most important factors in obtaining a high yield of esters. A super-stoichiometric alcohol content is used to transesterify algal oil. High yields of methyl/ethyl esters of microalgae oil were obtained using a molar ratio of alcohol to oil of 9:1 to 33:1. An even higher molar ratio (up to 800:1) was used in the in situ process. The use of solvents significantly increases the efficiency of heterogeneous and enzymatic transesterification. The possibilities of using hexane, carbon tetrachloride, toluene, benzene, 1,2-dichloroethane, and chloroform were investigated, and it was found that the selection of a suitable solvent is of great importance for the process efficiency. Temperature also has a significant effect on the efficiency of the transesterification process. During alkaline and acid catalysis, the optimum temperature is close to the boiling point of the alcohols. The temperature of enzymatic catalysis is lower and ranges from 30–50 °C. High temperatures (255–475 °C) are used in supercritical non-catalytic processes. The optimal process time using homogeneous alkaline and acid catalysts is 50–90 min, while when using heterogeneous catalysts, the duration is longer and reaches 24 h.
The performance characteristics of microalgae biodiesel are close to those of conventional biodiesel. The analysis of gas emissions showed that compared to diesel fuel, the mixture containing 20% microalgae biodiesel and 80% mineral diesel exhibited the lowest concentrations of harmful components in exhaust gases, cylinder pressures, brake thermal efficiency (BTE), exhaust temperature, and maximum rise pressure rates.
The analysis of the economic indicators of biodiesel production from algae biomass shows that biodiesel cannot currently compete with mineral diesel due to higher material and energy costs and, at the same time, the higher cost of biodiesel. Several scenarios are proposed to address this issue: production of other valuable products with microalgae biodiesel; simultaneous cultivation of fish, turtles, and shrimp; and microalgae cultivation and application in other technological operations, such as wastewater treatment, biogas production, and biogas upgrading. This should be the focus of research in the coming years.
Author Contributions
Conceptualization, V.M. and E.S.; software, V.M. and E.S.; validation, V.M. and E.S.; formal analysis and investigation, V.M. and E.S.; data curation, V.M. and E.S.; writing—original draft preparation, V.M. and E.S.; writing—review and editing, V.M. and E.S.; visualization, V.M. and E.S.; supervision, V.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Khan, M.I.; Shin, J.H.; Kim, J.D. The promising future of microalgae: Current status, challenges, and optimization of a sustainable and renewable industry for biofuels, feed, and other products. Microb. Cell Factories 2018, 17, 36. [Google Scholar] [CrossRef] [PubMed]
- Júnior, W.G.M.; Gorgich, M.; Corrêa, P.; Martins, A.A.; Mata, T.; Caetano, N.S. Microalgae for biotechnological applications: Cultivation, harvesting and biomass processing. Aquaculture 2020, 528, 735562. [Google Scholar] [CrossRef]
- Harun, R.; Danquah, M.K. Influence of acid pre-treatment on microalgal biomass for bioethanol production. Process Biochem. 2011, 46, 304–319. [Google Scholar] [CrossRef]
- Zheng, H.; Yin, J.; Gao, Z.; Huang, H.; Ji, X.-J.; Dou, C. Disruption of Chlorella vulgaris Cells for the Release of Biodiesel-Producing Lipids: A Comparison of Grinding, Ultrasonication, Bead Milling, Enzymatic Lysis, and Microwaves. Appl. Biochem. Biotechnol. 2011, 164, 1215–1224. [Google Scholar] [CrossRef]
- Wang, D.; Li, Y.; Hu, X.; Su, W.; Zhong, M. Combined enzymatic and mechanical cell disruption and lipid extraction of green alga Neochloris oleabubdans. Int. J. Mol. Sci. 2015, 16, 7707–7722. [Google Scholar] [CrossRef] [PubMed]
- Halim, R.; Danquah, M.K.; Webley, P.A. Extraction of oil from microalgae for biodiesel production: A review. Biotechnol. Adv. 2012, 30, 709–732. [Google Scholar] [CrossRef]
- Grimi, N.; Dubois, A.; Marchal, L.; Jubeau, S.; Lebovka, N.; Vorobiev, E. Selective extraction from microalgae Nannochloropsis sp. using different methods of cell disruption. Bioresour. Technol. 2014, 153, 254–259. [Google Scholar] [CrossRef]
- Günerken, E.; D’Hondt, E.; Eppink, M.; Garcia-Gonzalez, L.; Elst, K.; Wijffels, R. Cell disruption for microalgae biorefineries. Biotechnol. Adv. 2015, 33, 243–260. [Google Scholar] [CrossRef]
- Mubarak, M.; Shaija, A.; Suchithra, T. Ultrasonication: An effective pre-treatment method for extracting lipid from Salvinia molesta for biodiesel production. Resour. Technol. 2016, 2, 126–132. [Google Scholar] [CrossRef] [Green Version]
- Skorupskaite, V.; Makareviciene, V.; Ubartas, M.; Karosiene, J.; Gumbyte, M. Green algae Ankistrodesmus fusiformis cell disruption using different modes. Biomass-Bioenergy 2017, 107, 311–316. [Google Scholar] [CrossRef]
- Halim, R.; Rupasinghe, T.W.; Tull, D.; Webley, P. Mechanical cell disruption for lipid extraction from microalgal biomass. Bioresour. Technol. 2013, 140, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Prabakaran, P.; Ravindran, A. A comparative study on effective cell disruption methods for lipid extraction from microalgae. Lett. Appl. Microbiol. 2011, 53, 150–154. [Google Scholar] [CrossRef]
- Balasubramanian, S.; Allen, J.D.; Kanitkar, A.; Boldor, D. Oil extraction from Scenedesmus obliquus using a continuous microwave system—design, optimization, and quality characterization. Bioresour Technol. 2011, 102, 3396–3403. [Google Scholar] [CrossRef]
- Sheng, J.; Vannela, R.; Rittmann, B.E. Evaluation of cell-disruption effects of pulsedelectric-field treatment of Synechocystis PCC 6803. Environ. Sci. Technol. 2011, 45, 3795–3802. [Google Scholar] [CrossRef] [PubMed]
- Lavenburg, V.M.; Rosentrater, K.A.; Jung, S. Extraction Methods of Oils and Phytochemicals from Seeds and Their Environmental and Economic Impacts. Processes 2021, 9, 1839. [Google Scholar] [CrossRef]
- Halim, R.; Gladman, B.; Danquah, M.; Webley, P. Oil extraction from microalgae for biodiesel production. Bioresour. Technol. 2011, 102, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, A.; Patil, C.; Moholkar, V.S. Mechanistic Assessment of Microalgal Lipid Extraction. Ind. Eng. Chem. Res. 2010, 49, 2979–2985. [Google Scholar] [CrossRef]
- Aresta, M.; DiBenedetto, A.; Carone, M.; Colonna, T.; Fragale, C. Production of biodiesel from macroalgae by supercritical CO2 extraction and thermochemical liquefaction. Environ. Chem. Lett. 2005, 3, 136–139. [Google Scholar] [CrossRef]
- Demirbas, A.; Demirbas, F.M. Importance of algae oil as a source of biodiesel. Energ. Convers Manag. 2011, 52, 163–170. [Google Scholar] [CrossRef]
- Patil, P.D.; Dandamudi, K.P.R.; Wang, J.; Deng, Q.; Deng, S. Extraction of bio-oils from algae with supercritical carbon dioxide and co-solvents. J. Supercrit. Fluids 2018, 135, 60–68. [Google Scholar] [CrossRef]
- Chisti, Y. Biodiesel from microalgae. Biotechnol. Adv. 2007, 25, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Sajjadi, B.; Chen, W.-Y.; Raman, A.A.A.; Ibrahim, S. Microalgae lipid and biomass for biofuel production: A comprehensive review on lipid enhancement strategies and their effects on fatty acid composition. Renew. Sustain. Energy Rev. 2018, 97, 200–232. [Google Scholar] [CrossRef]
- Sachdeva, N.; Gupta, R.P.; Mathur, A.S.; Tuli, D.K. Enhanced lipid production in thermo-tolerant mutants of Chlorella pyrenoidosa NCIM 2738. Bioresour. Technol. 2016, 221, 576–587. [Google Scholar] [CrossRef] [PubMed]
- Bordoloi, N.; Narzari, R.; Sut, D.; Saikia, R.; Chutia, R.S.; Kataki, R. Characterization of bio-oil and its sub-fractions from pyrolysis of Scenedesmus dimorphus. Renew. Energy 2016, 98, 245–253. [Google Scholar] [CrossRef]
- Rodolfi, L.; Chini Zittelli, G.; Bassi, N.; Padovani, G.; Biondi, N.; Bonini, G.; Tredici, M.R. Microalgae for oil: Strain selection, induction of lipid synthesis and outdoor mass cultivation in a low-cost photobioreactor. Biotechnol. Bioeng. 2009, 102, 100–112. [Google Scholar] [CrossRef] [PubMed]
- Calixto, C.D.; Santana, J.K.D.S.; Tibúrcio, V.P.; Pontes, L.D.F.B.L.D.; Sassi, C.F.D.C.; da Conceição, M.M.; Sassi, R. Productivity and fuel quality parameters of lipids obtained from 12 species of microalgae from the northeastern region of Brazil. Renew. Energy 2018, 115, 1144–1152. [Google Scholar] [CrossRef]
- Chua, E.T.; Schenk, P.M. A biorefinery for Nannochloropsis: Induction, harvesting, and extraction of EPA-rich oil and high-value protein. Bioresour. Technol. 2017, 244, 1416–1424. [Google Scholar] [CrossRef] [Green Version]
- Sinha, S.K.; Gupta, A.; Bharalee, R. Production of biodiesel from freshwater microalgae and evaluation of fuel properties based on fatty acid methyl ester profile. Biofuels 2016, 7, 69–78. [Google Scholar] [CrossRef]
- Khatoon, H.; Rahman, N.A.; Banerjee, S.; Harun, N.; Suleiman, S.S.; Zakaria, N.H.; Lananan, F.; Hamid, S.H.A.; Endut, A. Effects of different salinities and pH on the growth and proximate composition of Nannochloropsis sp. and Tetraselmis sp. isolated from South China Sea cultured under control and natural condition. Int. Biodeterior. Biodegrad. 2014, 95, 11–18. [Google Scholar] [CrossRef]
- Wen, X.; Geng, Y.; Li, Y. Enhanced lipid production in Chlorella pyrenoidosa by continuous culture. Bioresour. Technol. 2014, 161, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, M.; Van Hille, R.P.; Harrison, S.T.L. Lipid productivity, settling potential and fatty acid profile of 11 microalgal species grown under nitrogen replete and limited conditions. J. Appl. Phycol. 2011, 24, 989–1001. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, D.; Ding, K.; Che, R.; Xu, J.-W.; Zhao, P.; Li, T.; Ma, H.; Yu, X. Production of biomass and lipids by the oleaginous microalgae Monoraphidium sp. QLY-1 through heterotrophic cultivation and photo-chemical modulator induction. Bioresour. Technol. 2016, 211, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.-Y.; Liu, B.-F.; Kong, F.; Zhao, L.; Xie, G.-J.; Ren, N.-Q. Enhanced lipid accumulation of green microalga Scenedesmus sp. by metal ions and EDTA addition. Bioresour. Technol. 2014, 169, 763–767. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.R.; Deviram, G.; Mathimani, T.; Duc, P.A.; Pugazhendhi, A. Microalgae as rich source of polyunsaturated fatty acids. Biocatal. Agric. Biotechnol. 2019, 17, 583–588. [Google Scholar] [CrossRef]
- Li, Y.; Han, F.; Xu, H.; Mu, J.; Chen, D.; Feng, B.; Zeng, H. Potential lipid accumulation and growth characteristic of the green alga Chlorella with combination cultivation mode of nitrogen (N) and phosphorus (P). Bioresour. Technol. 2014, 174, 24–32. [Google Scholar] [CrossRef]
- Hui, W.; Wenjun, Z.; Wentao, C.; Lili, G.; Tianzhong, L. Strategy study on enhancing lipid productivity of filamentous oleaginous microalgae Tribonema. Bioresour. Technol. 2016, 218, 161–166. [Google Scholar] [CrossRef]
- Ho, S.-H.; Chang, J.-S.; Lai, Y.-Y.; Chen, C.-N.N. Achieving high lipid productivity of a thermotolerant microalga Desmodesmus sp. F2 by optimizing environmental factors and nutrient conditions. Bioresour. Technol. 2014, 156, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Takagi, M.; Yoshida, K.T. Effect of salt concentration on intracellular accumulation of lipids and triacylglyceride in marine microalgae Dunaliella cells. J. Biosci. Bioeng. 2006, 101, 223–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; He, H.; Wang, Y.; Zhu, S.; Piao, X. Transesterification of soybean oil to biodiesel using CaO as a solid base catalyst. Fuel 2008, 87, 216–221. [Google Scholar] [CrossRef]
- Converti, A.; Casazza, A.A.; Ortiz, E.Y.; Perego, P.; Del Borghi, M. Effect of temperature and nitrogen concentration on the growth and lipid content of Nannochloropsis oculata and Chlorella vulgaris for biodiesel production. Chem. Eng. Process. Process Intensif. 2009, 48, 1146–1151. [Google Scholar] [CrossRef]
- Tasić, M.B.; Pinto, L.F.R.; Klein, B.C.; Veljković, V.; Filho, R.M. Botryococcus braunii for biodiesel production. Renew. Sustain. Energy Rev. 2016, 64, 260–270. [Google Scholar] [CrossRef]
- Jiang, L.; Luo, S.; Fan, X.; Yang, Z.; Guo, R. Biomass and lipid production of marine microalgae using municipal wastewater and high concentration of CO2. Appl. Energy 2011, 88, 3336–3341. [Google Scholar] [CrossRef]
- Deshmukh, S.; Kumar, R.; Bala, K. Microalgae biodiesel: A review on oil extraction, fatty acid composition, properties and effect on engine performance and emissions. Fuel Process. Technol. 2019, 191, 232–247. [Google Scholar] [CrossRef]
- Krohn, B.J.; McNeff, C.V.; Yan, B.; Nowlan, D. Production of algae-based biodiesel using the continuous catalytic Mcgyan (R) process. Bioresour Technol. 2011, 102, 94–100. [Google Scholar] [CrossRef]
- Najafabadi, H.A.; Malekzadeh, M.; Jalilian, F.; Vossoughi, M.; Pazuki, G. Effect of various carbon sources on biomass and lipid production of Chlorella vulgaris during nutrient sufficient and nitrogen starvation conditions. Bioresour. Technol. 2015, 180, 311–317. [Google Scholar] [CrossRef]
- Andruleviciute, V.; Makareviciene, V.; Skorupskaite, V.; Gumbyte, M. Biomass and oil content of Chlorella sp., Haematococcus sp., Nannochloris sp. and Scenedesmus sp. under mixotrophic growth conditions in the presence of technical glycerol. J. Appl. Phycol. 2013, 26, 83–90. [Google Scholar] [CrossRef]
- Cheirsilp, B.; Torpee, S. Enhanced growth and lipid production of microalgae under mixotrophic culture condition: Effect of light intensity, glucose concentration and fed-batch cultivation. Bioresour. Technol. 2012, 110, 510–516. [Google Scholar] [CrossRef]
- El Baky, H.A.; El-Baroty, G.S.; Bouaid, A.; Martinez, M.; Aracil, J. Enhancement of lipid accumulation in Scenedesmus obliquus by Optimizing CO2 and Fe3+ levels for biodiesel production. Bioresour. Technol. 2012, 119, 429–432. [Google Scholar] [CrossRef] [PubMed]
- Schenk, P.M.; Thomas-Hall, S.R.; Stephens, E.; Marx, U.C.; Mussgnug, J.H.; Posten, C.; Kruse, O.; Hankamer, B. Second generation biofuels: High-efficiency microalgae for biodiesel production. BioEnergy Res. 2008, 1, 20–43. [Google Scholar] [CrossRef]
- Thapa, H.R.; Naik, M.; Okada, S.; Takada, K.; Molnar, I.; Xu, Y.; Devarenne, T. A squalene synthase-like enzyme initiates production of tetraterpenoid hydrocarbons in Botryococcus braunii Race L. Nat. Commun. 2016, 7, 11198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanli, H.; Canakci, M. Effects of Different Alcohol and Catalyst Usage on Biodiesel Production from Different Vegetable Oils. Energy Fuels 2008, 22, 2713–2719. [Google Scholar] [CrossRef]
- Sendzikiene, E.; Sinkuniene, D.; Kazanceva, I.; Kazancev, K. Optimization of low quality rapeseed oil transesterification with butanol by applying the response surface methodology. Renew. Energy 2016, 87, 266–272. [Google Scholar] [CrossRef]
- Dwivedi, G.; Sharma, M.R. Application of Box-Bchnkcn design in optimization of biodiesel yield from pongamia oil and its stability analysis. Fuel 2015, 145, 256–262. [Google Scholar] [CrossRef]
- Fukuda, H.; Kondo, A.; Noda, H. Biodiesel Fuel Production by Transesterification of Oils. J. Biosci. Bioeng. 2001, 92, 405–416. [Google Scholar] [CrossRef]
- Sendzikiene, E.; Makareviciene, V.; Janulis, P.; Kitrys, S. Kinetics of free fatty acids esterification with methanol in the production of biodiesel fuel. Eur. J. Lipid Sci. Technol. 2004, 106, 831–836. [Google Scholar] [CrossRef]
- Asadi, M.; Hooper, J.F.; Lupton, D.W. Biodiesel synthesis using integrated acid and base catalysis in continuous flow. Tetrahedron 2016, 72, 3729–3733. [Google Scholar] [CrossRef]
- Bournay, L.; Casanave, D.; Delfort, B.; Hillion, G.; Chodorge, J. New heterogeneous process for biodiesel production: A way to improve the quality and the value of the crude glycerin produced by biodiesel plants. Catal. Today 2005, 106, 190–192. [Google Scholar] [CrossRef]
- Renita, A.A.; Amarnath, J.D.; Sivasubramanian, S. A study on the optimization of algae biodiesel reaction parameters using response surface methodology. Int. J. Chem. Eng. Appl. 2012, 3, 311–314. [Google Scholar]
- Chamola, R.; Khan, M.F.; Raj, A.; Verma, M.; Jain, S. Response surface methodology based optimization of in situ transesterification of dry algae with methanol, H2SO4 and NaOH. Fuel 2018, 239, 511–520. [Google Scholar] [CrossRef]
- Makareviciene, V.; Skorupskaite, V.; Levisauskas, D.; Andruleviciute, V.; Kazancev, K. The Optimization of Biodiesel Fuel Production from Microalgae Oil Using Response Surface Methodology. Int. J. Green Energy 2013, 11, 527–541. [Google Scholar] [CrossRef]
- Sumprasit, N.; Wagle, N.; Glanpracha, N.; Annachhatre, A.P. Biodiesel and biogas recovery from Spirulina platensis. Int. Biodeterior. Biodegrad. 2017, 119, 196–204. [Google Scholar] [CrossRef]
- Vijayaraghavan, K.; Hemanathan, K. Biodiesel Production from Freshwater Algae. Energy Fuels 2009, 23, 5448–5453. [Google Scholar] [CrossRef]
- Chen, L.; Liu, T.; Zhang, W.; Chen, X.; Wang, J. Biodiesel production from algae oil high in free fatty acids by two-step catalytic conversion. Bioresour. Technol. 2012, 111, 208–214. [Google Scholar] [CrossRef]
- Miao, X.; Wu, Q. Biodiesel production from heterotrophic microalgal oil. Bioresour. Technol. 2006, 97, 841–846. [Google Scholar] [CrossRef]
- Mandotra, S.; Kumar, P.; Suseela, M.; Ramteke, P. Fresh water green microalga Scenedesmus abundans: A potential feedstock for high quality biodiesel production. Bioresour. Technol. 2014, 156, 42–47. [Google Scholar] [CrossRef] [PubMed]
- McNeff, C.V.; McNeff, L.C.; Yan, B.; Nowlan, D.T.; Rasmussen, M.; Gyberg, A.E.; Krohn, B.J.; Fedie, R.L.; Hoye, T.R. A continuous catalytic system for biodiesel production. Appl. Catal. A Gen. 2008, 343, 39–48. [Google Scholar] [CrossRef]
- Carrero, A.; Vicente, G.; Rodriguez, R.; Peso, G.L.; del Santos, C. Synthesis of fatty acids methyl esters (FAMEs) from Nannochiorvpsis gadinnui microalga using heterogeneous acid catalysts. Biochem. Eng. J. 2015, 97, 119–124. [Google Scholar] [CrossRef]
- Manikandan, G.; Rajasekaran, R. Transesterification of algal oil using nano CaO catalyst. Int. J. Chem. Sci. 2013, 11, 591–597. [Google Scholar]
- Narula, V.; Khan, M.F.; Negi, A.; Kalra, S.; Thakur, A.; Jain, S. Low temperature optimization of biodiesel production from algal oil using CaO and CaO/Al2O3 as catalyst by the application of response surface methodology. Energy 2017, 140, 879–884. [Google Scholar] [CrossRef]
- Umdu, E.S.; Tuncer, M.; Seker, E. Transesterification of Nannochloropsis oculata microalga’s lipid to biodiesel on Al2O3 supported CaO and MgO catalysts. Bioresour. Technol. 2009, 100, 2828–2831. [Google Scholar] [CrossRef] [Green Version]
- Teo, S.H.; Islam, A.; Ng, F.L.; Taufiq-Yap, Y.H. Biodiesel synthesis from photoautotrophic cultivated oleaginous microalgae using sand dollar catalyst. RSC Adv. 2015, 5, 47140–47152. [Google Scholar] [CrossRef]
- Makareviciene, V.; Gumbyte, M.; Skorupskaite, V.; Sendzikiene, E. Biodiesel fuel production by enzymatic microalgae oil transesterification with ethanol. J. Renew. Sustain. Energy 2017, 9, 023101. [Google Scholar] [CrossRef]
- Bayramoglu, G.; Akbulut, A.; Ozalp, V.C.; Arica, M.Y. Imobilized lipase on micro-porous biosilica for enzymatic transesterification of algal oil. Chem. Eng. Res. Des. 2015, 95, 12–21. [Google Scholar] [CrossRef]
- Huang, J.; Xia, J.; Jiang, W.; Li, Y.; Li, J. Biodiesel production from microalgae oil catalyzed by a recombinant lipase. Bioresour. Technol. 2015, 180, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Bi, J.; Wang, J.; Ouyang, J.; Xiao, Y.; Hao, H.; Bao, Y.; Wang, Y.; Yin, Q. Liquid–liquid equilibrium of binary and ternary systems composed by palm oil or palm oil fractions with methanol/ethanol and water. Fluid Phase Equilibria 2015, 404, 17–25. [Google Scholar] [CrossRef]
- Raoufi, Z.; Gargari, S.L.M. Biodiesel production from microalgae oil by lipase from Pseudomonas aeruginosa displayed on yeast cell surface. Biochem. Eng. J. 2018, 140, 1–8. [Google Scholar] [CrossRef]
- Bharathiraja, B.; Kumar, R.R.; Chakravarthy, M.; Yogendran, D.; Jayamuthunagai, J.; Kumar, R.P.; Yuvaraj, D.; Palani, S. Analysis of reaction parameters for the transesterification of algal oil from Enteromorpha compressa using purified lipase and whole cell biocatalyst. J. Chem. Pharm. Res. 2015, 7, 395–410. [Google Scholar]
- Bharathiraja, B.; Kumar, R.R.; PraveenKumar, R.; Chakravarthy, M.; Yogendran, D.; Jayamuthunagai, J. Biodiesel production from different algal oil using immobilized pure lipase and tailor made rPichia pastoris with Cal A and Cal B genes. Bioresour. Technol. 2016, 213, 69–78. [Google Scholar] [CrossRef]
- Kim, B.; Im, H.; Lee, J.W. In situ transesterification of highly wet microalgae using hydrochloric acid. Bioresour. Technol. 2015, 185, 421–425. [Google Scholar] [CrossRef]
- Silva, S.; Mariuthu, C. Production of biodiesel by transesterification of algae oil with an assistance of nano-CaO catalyst derived from egg shell. Int. J. Chem. Technol. Res. 2015, 7, 2112–2116. [Google Scholar]
- Carrero, A.; Vicente, G.; Rodríguez, R.; Linares, M.; del Peso, G. Hierarchical zeolites as catalysts for biodiesel production from Nannochloropsis microalga oil. Catal. Today 2011, 167, 148–153. [Google Scholar] [CrossRef]
- Ching-Velasquez, J.; Fernández-Lafuente, R.; Rodrigues, R.C.; Plata, V.; Rosales-Quintero, A.; Torrestiana-Sánchez, B.; Tacias-Pascacio, V.G. Production and characterization of biodiesel from oil of fish waste by enzymatic catalysis. Renew. Energy 2020, 153, 1346–1354. [Google Scholar] [CrossRef]
- Reddy, C.R.V.; Oshel, A.R.; Verkade, J.G. Room-Temperature Conversion of Soybean Oil and Poultry Fat to Biodiesel Catalyzed by Nanocrystalline Calcium Oxides. Energy Fuels 2006, 20, 1310–1314. [Google Scholar] [CrossRef]
- Granados, M.L.; Poves, M.D.Z.; Alonso, D.M.; Mariscal, R.; Galisteo, F.C.; Moreno-Tost, R.; Santamaría, J.; Fierro, J.L.G. Biodiesel from sunflower oil by using activated calcium oxide. Appl. Catal. B Environ. 2007, 73, 317–326. [Google Scholar] [CrossRef]
- Yoosuk, B.; Udomsap, P.; Puttasawat, B.; Krasae, P. Modification of calcite by hydration–dehydration method for heterogeneous biodiesel production process: The effects of water on properties and activity. Chem. Eng. J. 2010, 162, 135–141. [Google Scholar] [CrossRef]
- Xie, W.; Huang, X.; Li, H. Soybean oil methyl esters preparation using NaX zeolites loaded with KOH as a heterogeneous catalyst. Bioresour. Technol. 2007, 98, 936–939. [Google Scholar] [CrossRef] [PubMed]
- Li, E.; Xu, Z.P.; Rudolph, V. MgCoAl-LDH derived heterogeneous catalysts for the ethanol transesterification of canola oil to biodiesel. Appl. Catal. B Env. 2009, 88, 42–49. [Google Scholar] [CrossRef]
- Kumar, D.; Kumar, G.P.; Singh, C. Ultrasonic-assisted transesterification of Jatropha curcus oil using solid catalyst, Na/SiO2. Ultrason. Sonochem. 2010, 17, 839–844. [Google Scholar] [CrossRef]
- Ilgen, O. Dolomite as a heterogeneous catalyst for transesterification of canola oil. Fuel Process. Technol. 2010, 92, 452–455. [Google Scholar] [CrossRef]
- Sendžikienė, E.; Makarevičienė, V.; Kazancev, K. Application of dolomite as a heterogeneous catalyst of biodiesel synthesis. Transport 2018, 33, 1155–1161. [Google Scholar] [CrossRef] [Green Version]
- Ngamcharussrivichai, C.; Nunthasanti, P.; Tanachai, S.; Bunyakiat, K. Biodiesel production through transesterification over natural calciums. Fuel Process. Technol. 2010, 91, 1409–1415. [Google Scholar] [CrossRef]
- Wei, Z.; Xu, C.; Li, B. Application of waste eggshell as low-cost solid catalyst for biodiesel production. Bioresour. Technol. 2009, 100, 2883–2885. [Google Scholar] [CrossRef] [PubMed]
- Gupta, J.; Agarwal, M. Preparation and characterization of CaO nanoparticle for biodiesel production. AIP Conf. Proc. 2016, 1724, 020066. [Google Scholar]
- Lou, W.-Y.; Zong, M.-H.; Duan, Z.-Q. Efficient production of biodiesel from high free fatty acid-containing waste oils using various carbohydrate-derived solid acid catalysts. Bioresour. Technol. 2008, 99, 8752–8758. [Google Scholar] [CrossRef]
- Du, W.; Xu, Y.; Liu, D.; Zeng, J. Comparative study on lipase-catalyzed transformation of soybean oil for biodiesel production with different acyl acceptors. J. Mol. Catal. B Enzym. 2004, 30, 125–129. [Google Scholar] [CrossRef]
- Nguyen, H.C.; Liang, S.-H.; Doan, T.T.; Su, C.-H.; Yang, P.-C. Lipase-catalyzed synthesis of biodiesel from black soldier fly (Hermetica illucens): Optimization by using response surface methodology. Energy Convers. Manag. 2017, 145, 335–342. [Google Scholar] [CrossRef]
- Watanabe, Y.; Shimada, Y.; Sugihara, A.; Tominaga, Y. Conversion of degummed soybean oil to biodiesel fuel with immobilized Candida antarctica lipase. J. Mol. Catal. B Enzym. 2002, 17, 151–155. [Google Scholar] [CrossRef]
- Shimada, Y.; Watanabe, Y.; Sugihara, A.; Tominaga, Y. Enzymatic alcoholysis for biodiesel fuel production and application of the reaction to oil processing. J. Mol. Catal. B Enzym. 2002, 17, 133–142. [Google Scholar] [CrossRef]
- Shimada, Y.; Watanabe, Y.; Samukawa, T.; Sugihara, A.; Noda, H.; Fukuda, H.; Tominaga, Y. Conversion of vegetable oil to biodiesel using immobilized Candida antarctica lipase. J. Am. Oil Chem. Soc. 1999, 76, 789–793. [Google Scholar] [CrossRef]
- Marín-Suárez, M.; Méndez-Mateos, D.; Guadix, A.; Guadix, E.M. Reuse of immobilized lipases in the transesterification of waste fish oil for the production of biodiesel. Renew. Energy 2019, 140, 1–8. [Google Scholar] [CrossRef]
- Surendhiran, D.; Vijay, M.; Sirajunnisa, A.R. Biodiesel production from marine microagla Chlorella salina using whole cell yeast immobilized on sugarcane bagasse. J. Env. Chem. Eng. 2014, 2, 1294–1300. [Google Scholar] [CrossRef]
- Liu, X.; Piao, X.; Wang, Y.; Zhu, S. Calcium Ethoxide as a Solid Base Catalyst for the Transesterification of Soybean Oil to Biodiesel. Energy Fuels 2008, 22, 1313–1317. [Google Scholar] [CrossRef]
- Ramachandran, K.; Suganya, T.; Gandhi, N.N.; Renganathan, S. Recent developments for biodiesel production by ultrasonic assist transesterification using different heterogeneous catalyst: A review. Renew. Sustain. Energy Rev. 2013, 22, 410–418. [Google Scholar] [CrossRef]
- Nguyen, H.C.; Liang, S.-H.; Chen, S.-S.; Su, C.-H.; Lin, J.-H.; Chien, C.-C. Enzymatic production of biodiesel from insect fat using methyl acetate as an acyl acceptor: Optimization by using response surface methodology. Energy Convers. Manag. 2018, 158, 168–175. [Google Scholar] [CrossRef]
- Yu, D.; Tian, L.; Wu, H.; Wang, S.; Wang, Y.; Ma, D.; Fang, X. Ultrasonic irradiation with vibration for biodiesel production from soybean oil by Novozym 435. Process Biochem. 2010, 45, 519–525. [Google Scholar] [CrossRef]
- Aguieiras, E.C.G.; Ribeiro, D.S.; Couteiro, P.P.; Bastos, C.M.B.; de Queiroz, D.S.; Parreira, J.M.; Langone, M.A.P. Investigation of the Reuse of Immobilized Lipases in Biodiesel Synthesis: Influence of Different Solvents in Lipase Activity. Appl. Biochem. Biotechnol. 2016, 179, 485–496. [Google Scholar] [CrossRef]
- Rahman, M.; Aziz, M.; Al-Khulaidi, R.A.; Sakib, N.; Islam, M. Biodiesel production from microalgae Spirulina maxima by two step process: Optimization of process variable. J. Radiat. Res. Appl. Sci. 2017, 10, 140–147. [Google Scholar] [CrossRef] [Green Version]
- Ashokkumar, V.; Agila, E.; Salam, Z.; Ponraj, M.; Din, M.F.M.; Ani, F.N. A study on large scale cultivation of Microcystis aeruginosa under open raceway pond at semi-continuous mode for biodiesel production. Bioresour. Technol. 2014, 172, 186–193. [Google Scholar] [CrossRef]
- Srivastava, G.; Paul, A.K.; Goud, V.V. Optimization of non-catalytic transesterification of microalgae oil to biodiesel under supercritical methanol condition. Energy Convers. Manag. 2018, 156, 269–278. [Google Scholar] [CrossRef]
- Nan, Y.; Liu, J.; Lin, R.; Tavlarides, L.L. Production of biodiesel from microalgae oil (Chlorella protothecoides) by non-catalytic transesterification in supercritical methanol and ethanol: Process optimization. J. Supercrit. Fluids 2015, 97, 174–182. [Google Scholar] [CrossRef]
- Lee, J.-S.; Saka, S. Biodiesel production by heterogeneous catalysts and supercritical technologies. Bioresour. Technol. 2010, 101, 7191–7200. [Google Scholar] [CrossRef]
- Reddy, H.K.; Muppaneni, T.; Patil, P.; Ponnusamy, S.; Cooke, P.; Schaub, T.; Deng, S. Direct conversion of wet algae to crude biodiesel under supercritical ethanol conditions. Fuel 2014, 115, 720–726. [Google Scholar] [CrossRef]
- Qian, J.; Yang, Q.; Sun, F.; He, M.; Chen, Q.; Yun, Z.; Qin, L. Cogeneration of biodiesel and nontoxic rapeseed meal from rapeseed through in-situ alkaline transesterification. Bioresour. Technol. 2012, 128, 8–13. [Google Scholar] [CrossRef]
- Abo El-Enin, S.A.; Attia, N.K.; El-Ibiari, N.N.; El-Diwani, G.I.; El-Khatib, K.M. In-situ transesterification of rapeseed and cost indicators for biodiesel production. Renew. Sustain. Energy Rev. 2013, 18, 471–477. [Google Scholar] [CrossRef]
- Zhang, J.; Cui, C.; Chen, H.; Liu, J. The Completion of Esterification of Free Fatty Acids inZanthoxylum BungeanumSeed Oil with Ethanol. Int. J. Green Energy 2014, 11, 822–832. [Google Scholar] [CrossRef]
- Zhang, X.; Yan, S.; Tyagi, R.D.; Surampalli, R.Y.; Valéro, J.R. Ultrasonication aided in-situ transesterification of microbial lipids to biodiesel. Bioresour. Technol. 2014, 169, 175–180. [Google Scholar] [CrossRef]
- Dianursanti; Religia, P.; Wijanarko, A. Utilization of n-Hexane as Co-solvent to Increase Biodiesel Yield on Direct Transesterification Reaction from Marine Microalgae. Procedia Environ. Sci. 2015, 23, 412–420. [Google Scholar] [CrossRef] [Green Version]
- Ehimen, E.A.; Sun, Z.; Carrington, G.C. Use of Ultrasound and Co-Solvents to Improve the In-Situ Transesterification of Microalgae Biomass. Procedia Environ. Sci. 2012, 15, 47–55. [Google Scholar] [CrossRef] [Green Version]
- Velasquez-Orta, S.; Lee, J.; Harvey, A. Alkaline in situ transesterification of Chlorella vulgaris. Fuel 2011, 94, 544–550. [Google Scholar] [CrossRef]
- Wu, S.; Song, L.; Sommerfeld, M.; Hu, Q.; Chen, W. Optimization of an effective method for the conversion of crude algal lipids into biodiesel. Fuel 2017, 197, 467–473. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, J.; Gerken, H.; Zhang, C.; Hu, Q.; Li, Y. Highly-efficient enzymatic conversion of crude algal oils into biodiesel. Bioresour. Technol. 2014, 172, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xu, H.; Wu, Q. Large-scale biodiesel production from microalgaChlorella protothecoides through heterotrophic cultivation in bioreactors. Biotechnol. Bioeng. 2007, 98, 764–771. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.-Q.; Hu, Z.-L.; Wang, P.-W.; Yang, Z. Enzymatic production of microalgal biodiesel in ionic liquid [BMIm][PF6]. Fuel 2011, 95, 329–333. [Google Scholar] [CrossRef]
- Tran, D.-T.; Yeh, K.-L.; Chen, C.-L.; Chang, J.-S. Enzymatic transesterification of microalgal oil from Chlorella vulgaris ESP-31 for biodiesel synthesis using immobilized Burkholderia lipase. Bioresour. Technol. 2012, 108, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lian, S.; Tong, D.; Song, R.; Yang, W.; Fan, Y.; Qing, R.; Hu, C. One-step production of biodiesel from Nannochloropsis sp. on solid base Mg–Zr catalyst. Appl. Energy 2011, 88, 3313–3317. [Google Scholar] [CrossRef]
- Im, H.; Lee, H.; Park, M.S.; Yang, J.-W.; Lee, J.W. Concurrent extraction and reaction for the production of biodiesel from wet microalgae. Bioresour. Technol. 2013, 152, 534–537. [Google Scholar] [CrossRef] [PubMed]
- Karimi, M. Exergy-based optimization of direct conversion of microalgae biomass to biodiesel. J. Clean. Prod. 2017, 141, 50–55. [Google Scholar] [CrossRef]
- Cheng, J.; Qiu, Y.; Zhang, J.; Huang, R.; Yang, W.; Fan, Z. Conversion of lipids from wet microalgae into biodiesel using sulfonated graphene oxide catalysts. Bioresour. Technol. 2017, 244, 569–574. [Google Scholar] [CrossRef]
- Guldhe, A.; Moura, C.V.; Singh, P.; Rawat, I.; Moura, E.M.; Sharma, Y.; Bux, F. Conversion of microalgal lipids to biodiesel using chromium-aluminium mixed oxide as a heterogeneous solid acid catalyst. Renew. Energy 2017, 105, 175–182. [Google Scholar] [CrossRef]
- Kim, B.; Park, J.; Son, J.; Lee, J.W. Catalyst-free production of alkyl esters from microalgae via combined wet in situ transesterification and hydrothermal liquefaction (iTHL). Bioresour. Technol. 2017, 244, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Gumbytė, M.; Makareviciene, V.; Skorupskaite, V.; Sendzikiene, E.; Kondratavicius, M. Enzymatic microalgae oil transesterification with ethanol in mineral diesel fuel media. J. Renew. Sustain. Energy 2018, 10, 013105. [Google Scholar] [CrossRef]
- Makareviciene, V.; Gumbyte, M.; Sendzikiene, E. Simultaneous extraction of microalgae Ankistrodesmus sp. oil and enzymatic transesterification with ethanol in the mineral diesel medium. Food Bioprod. Process. 2019, 116, 89–97. [Google Scholar] [CrossRef]
- Anitescu, G.; Deshpande, A.; Tavlarides, L.L. Integrated Technology for Supercritical Biodiesel Production and Power Cogeneration. Energy Fuels 2008, 22, 1391–1399. [Google Scholar] [CrossRef]
- Hegel, P.E.; Martín, L.A.; Popovich, C.A.; Damiani, C.; Leonardi, P.I. Biodiesel production from Halamphora coffeaeformis microalga oil by supercritical ethanol transesterification. Chem. Eng. Process. Process Intensif. 2019, 145, 107670. [Google Scholar] [CrossRef]
- Patil, P.D.; Reddy, H.; Muppaneni, T.; Deng, S. Biodiesel fuel production from algal lipids using supercritical methyl acetate (glycerin-free) technology. Fuel 2017, 195, 201–207. [Google Scholar] [CrossRef] [Green Version]
- Jazzar, S.; Olivares-Carrillo, P.; Rios, A.P.D.L.; Marzouki, M.N.; Fernandez, F.G.A.; Sevilla, J.M.F.; Molina-Grima, E.; Smaali, I.; Quesada-Medina, J. Direct supercritical methanolysis of wet and dry unwashed marine microalgae (Nannochloropsis gaditana) to biodiesel. Appl. Energy 2015, 148, 210–219. [Google Scholar] [CrossRef]
- Imahara, H.; Minami, E.; Hari, S.; Saka, S. Thermal stability of biodiesel in supercritical methanol. Fuel 2008, 87, 1–6. [Google Scholar] [CrossRef]
- Makarevičienė, V.; Lebedevas, S.; Rapalis, P.; Gumbyte, M.; Skorupskaite, V.; Žaglinskis, J. Performance and emission characteristics of diesel fuel containing microalgae oil methyl esters. Fuel 2014, 120, 233–239. [Google Scholar] [CrossRef]
- Yaşar, F. Comparision of fuel properties of biodiesel fuels produced from different oils to determine the most suitable feedstock type. Fuel 2019, 264, 116817. [Google Scholar] [CrossRef]
- Pullen, J.; Saeed, K. An overview of biodiesel oxidation stability. Renew. Sustain. Energy Rev. 2012, 16, 5924–5950. [Google Scholar] [CrossRef]
- Zaleckas, E.; Makarevičienė, V.; Sendžikienė, E. Possibilities of using camelina sativa oil for producing biodiesel fuel. Transport 2012, 27, 60–66. [Google Scholar] [CrossRef]
- Rajak, U.; Nashine, P.; Verma, T.N.; Pugazhendhi, A. Performance, combustion and emission analysis of microalgae Spirulina in a common rail direct injection diesel engine. Fuel 2019, 255, 115855. [Google Scholar] [CrossRef]
- Satputaley, S.; Zodpe, D.; Deshpande, N. Performance, Combustion and Exhaust Emissions Analysis of a Diesel Engine Fuelled with Algae Oil and Algae Biodiesel. Mater. Today Proc. 2018, 5, 23022–23032. [Google Scholar] [CrossRef]
- Nautiyal, P.; Subramanian, K.; Dastidar, M.; Kumar, A. Experimental assessment of performance, combustion and emissions of a compression ignition engine fuelled with Spirulina platensis biodiesel. Energy 2019, 193, 116861. [Google Scholar] [CrossRef]
- Haik, Y.; Selim, M.Y.E.; Abdulrehman, T. The decrease in the engine compression ratio resulted in a decrease in the combustion noise. Energy 2011, 36, 1827–1835. [Google Scholar] [CrossRef]
- Venu, H.; Raju, V.D.; Subramani, L.; Appavu, P. Experimental assessment on the regulated and unregulated emissions of DI diesel engine fuelled with Chlorella emersonii methyl ester (CEME). Renew. Energy 2019, 151, 88–102. [Google Scholar] [CrossRef]
- Ospina, G.; Selim, M.Y.E.; Al Omari, S.A.B.; Ali, M.I.H.; Husien, M.M. Engine roughness and exhaust emissions of a diesel engine fuelled with three biofuels. Renew. Energy 2019, 134, 1465–1472. [Google Scholar] [CrossRef]
- Rajak, U.; Nashine, P.; Verma, T.N. Effect of spirulina microalgae biodiesel enriched with diesel fuel on performance and emission characteristics of CI engine. Fuel 2020, 268, 117305. [Google Scholar] [CrossRef]
- Mahendran, J.; Saravanan, K.; Ragulnath, D. Performance and emission characteristics of algae derived biodiesel processes. Mater. Today Proc. 2020, 21, 268–271. [Google Scholar] [CrossRef]
- Piloto-Rodrígueza, R.; Sánchez-Borrotoa, Y.; Melo-Espinosaa, E.A.; Verhelst, S. Assessment of diesel engine performance when fueled with biodiesel from algae and microalgae: An overview. Renew. Sustain. Energy Rev. 2017, 69, 833–842. [Google Scholar] [CrossRef]
- Chen, J.; Li, J.; Dong, W.; Zhang, X.; Tyagi, R.D.; Drogui, P.; Surampalli, R.Y. The potential of microalgae in biodiesel production. Renew. Sustain. Energy Rev. 2018, 90, 336–346. [Google Scholar] [CrossRef]
- Ge, H.; Li, J.; Chang, Z.; Chen, P.; Shen, M.; Zhao, F. Effect of microalgae with semicontinuous harvesting on water quality and zootechnical performance of white shrimp reared in the zero water exchange system. Aquac. Eng. 2016, 72–73, 70–76. [Google Scholar] [CrossRef]
- Tibbetts, S.M.; Yasumaru, F.; Lemos, D. In vitro prediction of digestible protein content of marine microalgae (Nannochloropsis granulata) meals for Pacific white shrimp (Litopenaeus vannamei) and rainbow trout (Oncorhynchus mykiss). Algal Res. 2017, 21, 76–80. [Google Scholar] [CrossRef] [Green Version]
- Qian, Z.J.; Kang, K.H.; Ryu, B. Chapter 35—Microalgae-derived toxic compounds. In Handbook of Marine Microalgae; Kim, S.-K., Ed.; Academic Press: Boston, MA, USA, 2015; pp. 527–537. [Google Scholar]
- Torres, C.M.; Ríos, S.D.; Torras, C.; Salvadó, J.; Mateo-Sanz, J.M.; Jiménez, L. Microalgae-based biodiesel: A multicriteria analysis of the production process using realistic scenarios. Bioresour. Technol. 2013, 147, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Brownbridge, G.; Azadi, P.; Smallbone, A.; Bhave, A.; Taylor, B.; Kraft, M. The future viability of algae-derived biodiesel under economic and technical uncertainties. Bioresour. Technol. 2014, 151, 166–173. [Google Scholar] [CrossRef]
- Kumar, D.; Singh, B. Algal biorefinery: An integrated approach for sustainable biodiesel production. Biomass-Bioenergy 2019, 131, 105398. [Google Scholar] [CrossRef]
- Williams, P.J.L.B.; Laurens, L.M. Microalgae as biodiesel & biomass feedstocks: Review & analysis of the biochemistry, energetics & economics. Energy Environ. Sci. 2010, 3, 554–590. [Google Scholar]
- Goh, B.H.H.; Ong, H.C.; Cheah, M.Y.; Chen, W.-H.; Yu, K.L.; Mahlia, T.M.I. Sustainability of direct biodiesel synthesis from microalgae biomass: A critical review. Renew. Sustain. Energy Rev. 2019, 107, 59–74. [Google Scholar] [CrossRef]
- Ferreira, G.F.; Pinto, L.F.R.; Maciel Filho, R.; Fregolente, L.V. A review on lipid production from microalgae: Association between cultivation using waste streams and fatty acid profiles. Renew. Sustain. Energy Rev. 2019, 109, 448–466. [Google Scholar] [CrossRef]
- Tercero, E.A.R.; Domenicali, G.; Bertucco, A. Autotrophic production of biodiesel from microalgae: An updated process and economic analysis. Energy 2014, 76, 807–815. [Google Scholar] [CrossRef]
- Gallagher, B.J. The economics of producing biodiesel from algae. Renew. Energy 2011, 36, 158–162. [Google Scholar] [CrossRef]
- Sawaengsak, W.; Silalertruksa, T.; Bangviwat, A.; Gheewala, S. Life cycle cost of biodiesel production from microalgae in Thailand. Energy Sustain. Dev. 2014, 18, 67–74. [Google Scholar] [CrossRef]
- Faried, M.; Samer, M.; Abdelsalam, E.M.; Yousef, R.; Attia, Y.; Ali, A.S. Biodiesel production from microalgae: Processes, technologies and recent advancements. Renew. Sustain. Energy Rev. 2017, 79, 893–913. [Google Scholar] [CrossRef]
- Rashid, N.; Rehman, M.S.U.; Sadiq, M.; Mahmood, T.; Han, J.-I. Current status, issues and developments in microalgae derived biodiesel production. Renew. Sustain. Energy Rev. 2014, 40, 760–778. [Google Scholar] [CrossRef]
- Shen, L.; Ndayambaje, J.D.; Murwanashyaka, T.; Cui, W.; Manirafasha, E.; Chen, C.; Wang, Y.; Lu, Y. Assessment upon heterotrophic microalgae screened from wastewater microbiota for concurrent pollutants removal and biofuel production. Bioresour. Technol. 2017, 245, 386–393. [Google Scholar] [CrossRef]
- Rinna, F.; Buono, S.; Cabanelas, I.T.D.; Nascimento, I.A.; Sansone, G.; Barone, C.M.A. Wastewater treatment by microalgae can generate high quality biodiesel feedstock. J. Water Process Eng. 2017, 18, 144–149. [Google Scholar] [CrossRef]
- Kim, B.; Chang, Y.K.; Lee, J.W. Efficient solvothermal wet in situ transesterification of Nannochloropsis gaditana for biodiesel production. Bioprocess Biosyst. Eng. 2017, 40, 723–730. [Google Scholar] [CrossRef] [PubMed]
- Macías-Sánchez, M.D.; Robles-Medina, A.; Hita-Peña, E.; Jiménez-Callejón, M.J.; Estéban-Cerdán, L.; González-Moreno, P.A.; Molina-Grima, E. Biodiesel production from wet microalgal biomass by direct transesterification. Fuel 2015, 150, 14–20. [Google Scholar] [CrossRef]
- Sitthithanaboon, W.; Reddy, H.K.; Muppaneni, T.; Ponnusamy, S.; Punsuvon, V.; Holguim, F.; Dungan, B.; Deng, S. Single-step conversion of wet Nannochloropsis gaditana to biodiesel under subcritical methanol conditions. Fuel 2015, 147, 253–259. [Google Scholar] [CrossRef] [Green Version]
- Huesemann, M.H.; Benemann, J.R. Biofuels from microalgae: Review of products, process and potential, with special focus on Dunaliella sp. In The Alga Dunaliella: Biodiversity, Physiology, Genomics and Biotechnology; Ben-Amotz, A., Polle, J.E.W., Subba Rao, D.V., Eds.; Science Publishers: Enfield, NH, USA, 2009; pp. 445–474. [Google Scholar]
- Lardon, L.; Hélias, A.; Sialve, B.; Steyer, J.-P.; Bernard, O. Life-Cycle Assessment of Biodiesel Production from Microalgae. Environ. Sci. Technol. 2009, 43, 6475–6481. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).