Generic and Open-Source Exergy Analysis—Extending the Simulation Framework TESPy
Abstract
:1. Introduction
1.1. Simulation-Based Thermodynamic Analysis
- A validated model can be used to answer what-if questions (e.g., in parameter and sensitivity analyses and process design studies) about the behavior of a real-world system [7] and thus to improve understanding of its operation.
- Computers accelerate the speed, increase the quantity, and can improve the quality of calculations [8].
- An extensive, disruptive, or expensive operation of a real-world system can be avoided. Furthermore, scale-ups or novel concepts or components can be examined before they are realized.
- During the cost- and time-limited design cycle of a product, virtual prototypes based on modeling and simulation can be used [9].
1.2. Motivation of the Approach
| Solver | Features | Exergy Analysis | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| NameSource | eq.-Orient. † | seq.-mod. | Part-Load | Properties | Flowsheeting | Notes, Remarks | |||||
| Proprietary, closed-source (with license fee or free of charge) | |||||||||||
| Aspen Plus a | ✓ | ✓ | ✓ | ✓ | ✓ | focus on chemical engineering | |||||
| ExerCom b | ✓ | ✓ | exergy routine [51] for ASPEN | ||||||||
| Chemcad c | ✓ | ✓ | ✓ | ✓ | focus on chemical engineering | ||||||
| Cycle Tempo d | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | exergy analysis, for systems with | |||
| Ebsilon e | ✓ | ✓ | ✓ | ✓ | ✓ | focus on power plants | |||||
| EES f | ✓ | ✓ | heat transfer and property library | ||||||||
| TAESS g | ✓h | Excel exergy cost [52] calc., EES exampl. | |||||||||
| IPSEpro i | ✓ | ✓ | ✓ | ✓ | conceived for thermal power plants | ||||||
| KPRO j | ✓ | ✓ | ✓ | ✓ | closed-loop calc., no iteration control req. | ||||||
| Modelon k | ✓ | ✓ | ✓ | ✓ | steady-state and transient simulation | ||||||
| PEPSE l | n/a | n/a | ✓ | ✓ | ✓ | steady-state energy balance software | |||||
| PPSD m | ✓ | ✓ | ✓ | ✓ | focus on steam generators/boilers | ||||||
| ProSimPlus n | ✓ | ✓ | ✓ | ✓ | steady-state simulation | ||||||
| Thermoflow o | ✓ | stand-alone mod. for spec. plant categ. | |||||||||
| TRNSYS p | n/a | n/a | ✓ | ✓ | ✓ | focus on transient simulation | |||||
| ValiEnergy q | n/a | n/a | ✓ | ✓ | ✓ | Energy performance monitoring sys. | |||||
| Free and open-source (free of charge) | |||||||||||
| COCO r | ✓ | ✓ | ✓ | CAPE-OPEN compliant chem. flowsh. | |||||||
| DWSIM s | ✓ | ✓ | ✓ | CAPE-OPEN compliant chem. flowsh. | |||||||
| ThermoCycle t | ✓ | ✓ | ✓ | thermal systems library for Modelica | |||||||
2. Methodology
2.1. Simulation of Thermal Conversion Processes
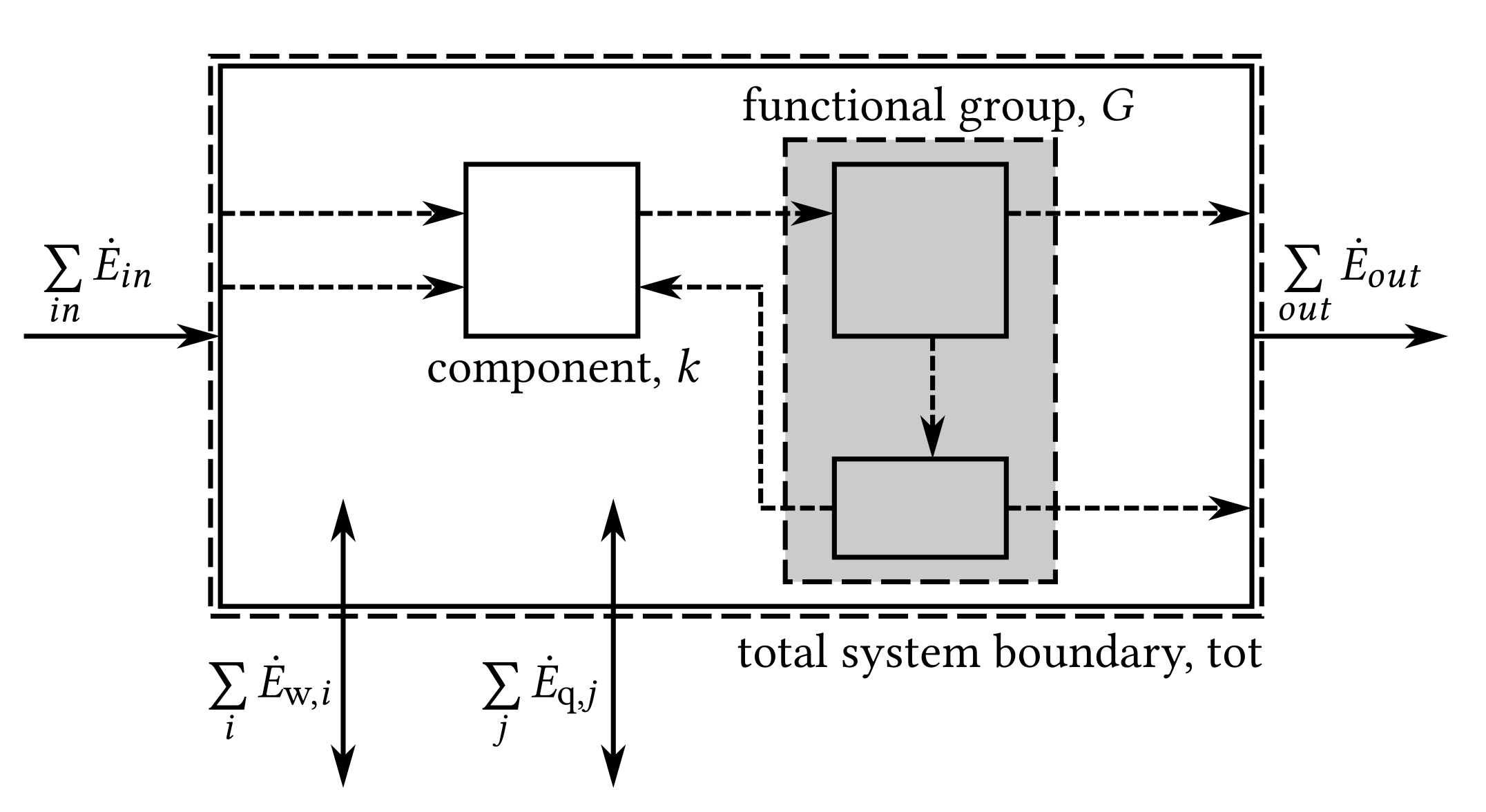
2.2. Exergy Analysis
2.3. Component-Based Thermodynamic Model
2.3.1. Turbomachinery
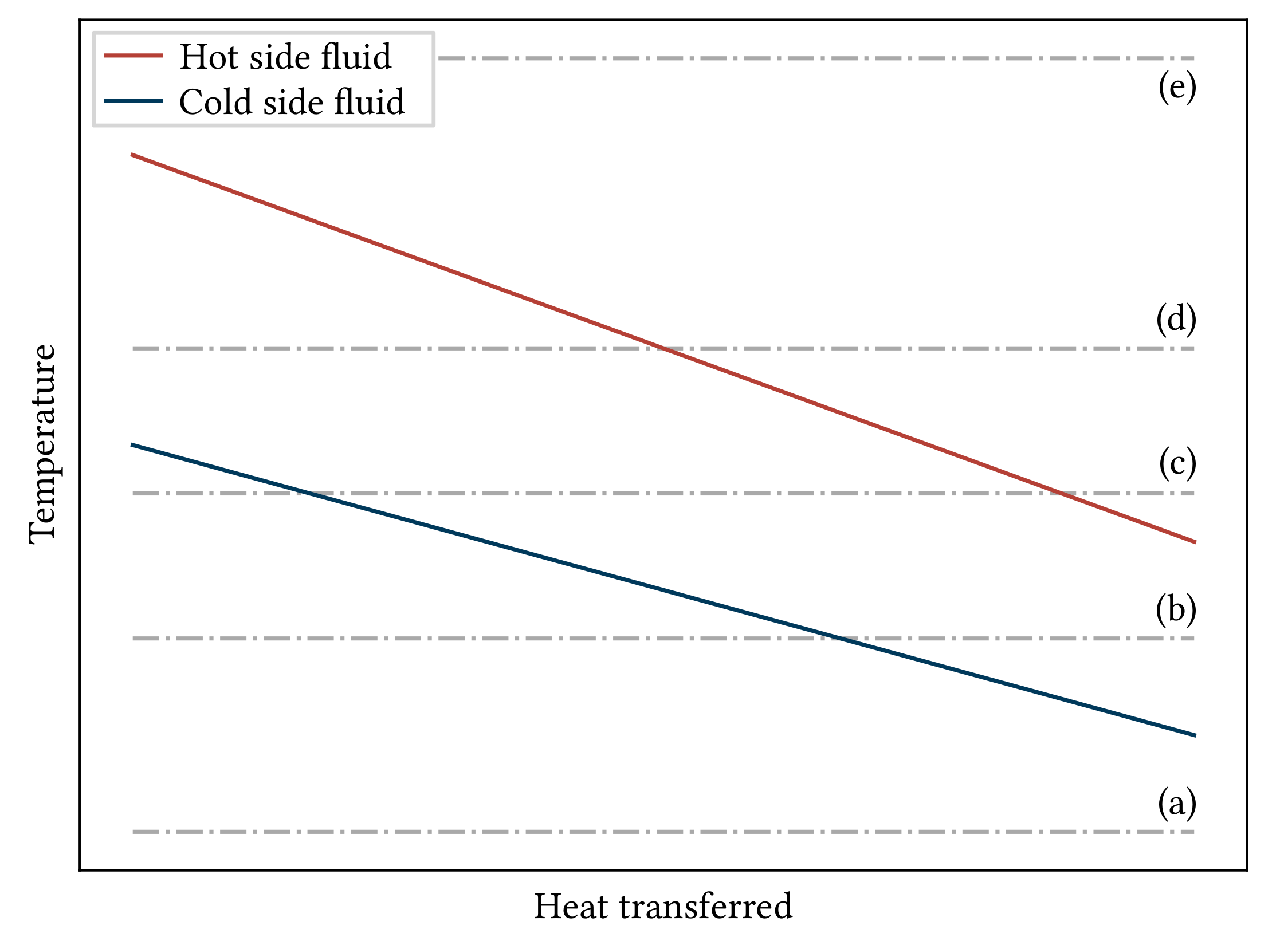
2.3.2. Heat Exchangers
2.3.3. Energy Balance Closing Components
2.3.4. Merge Points
2.3.5. Steam Drum
2.3.6. Motors and Generators
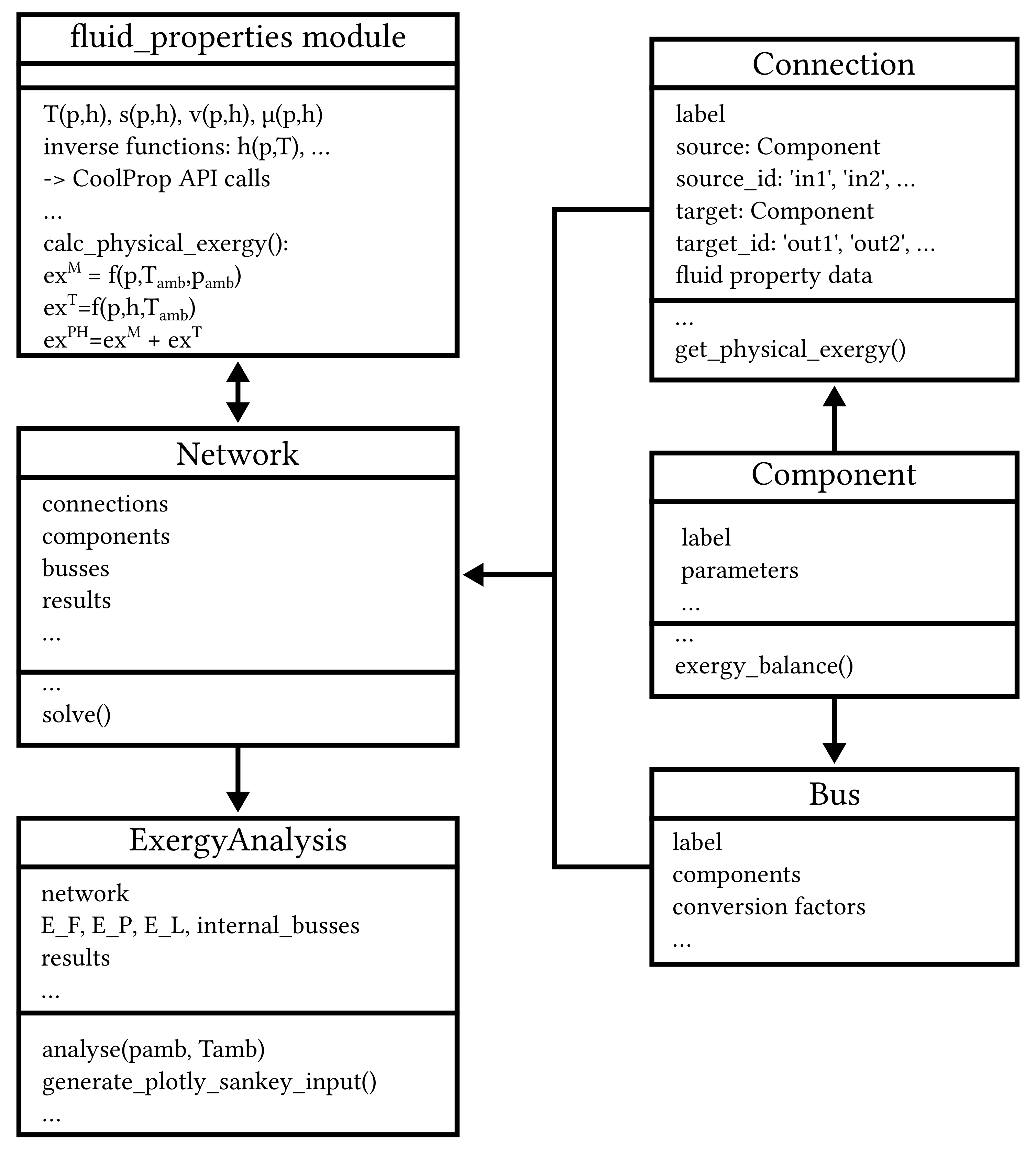
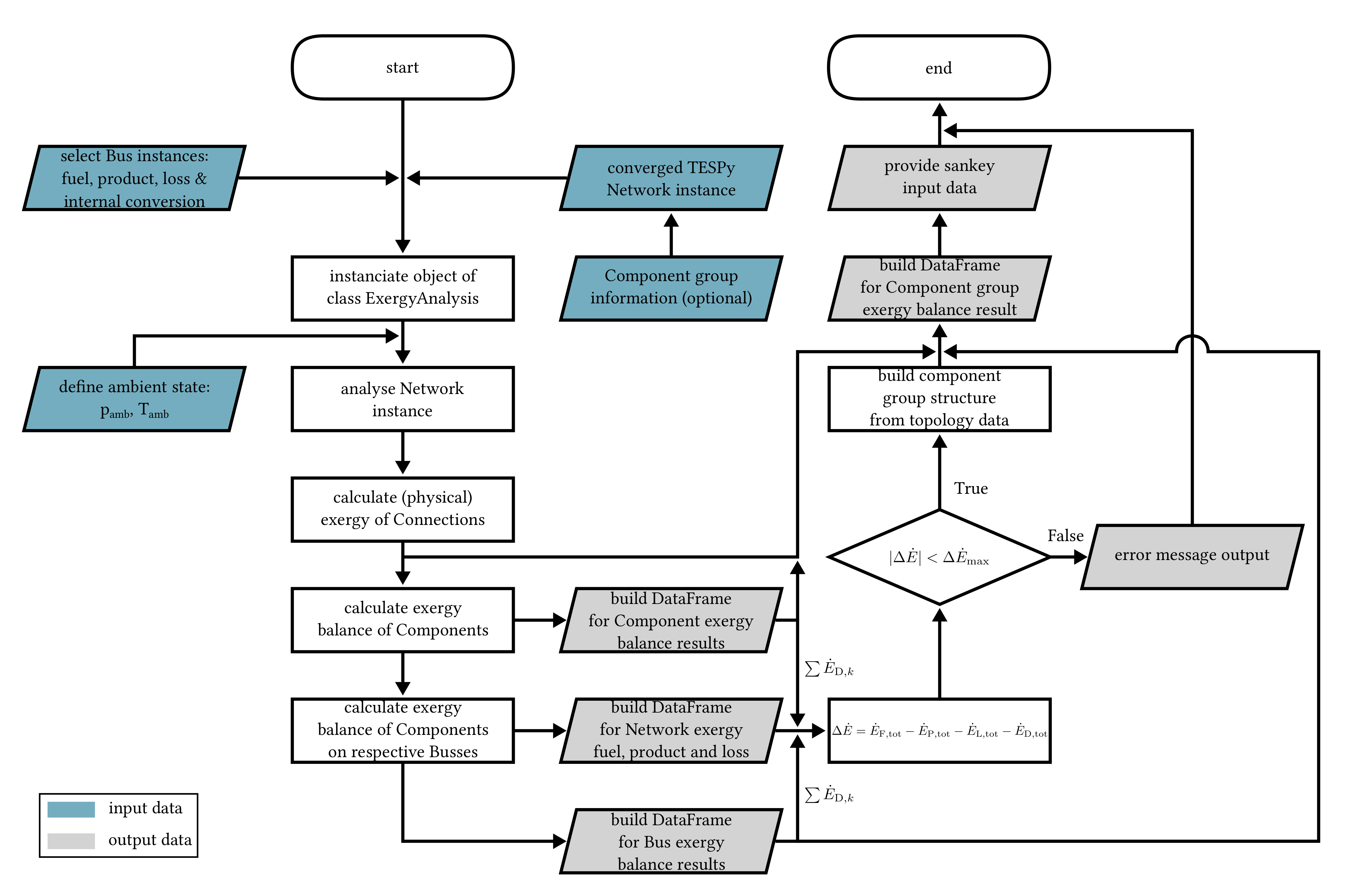
3. Implementation of the Exergy Analysis in TESPy
4. Example Applications
- The so-called “Solar Energy Generating System VI” (SEGS) [72], see Section 4.1
- Supercritical CO2 power cycle [73], see Section 4.2
- Refrigeration machine using air as working fluid [74], see Section 4.3
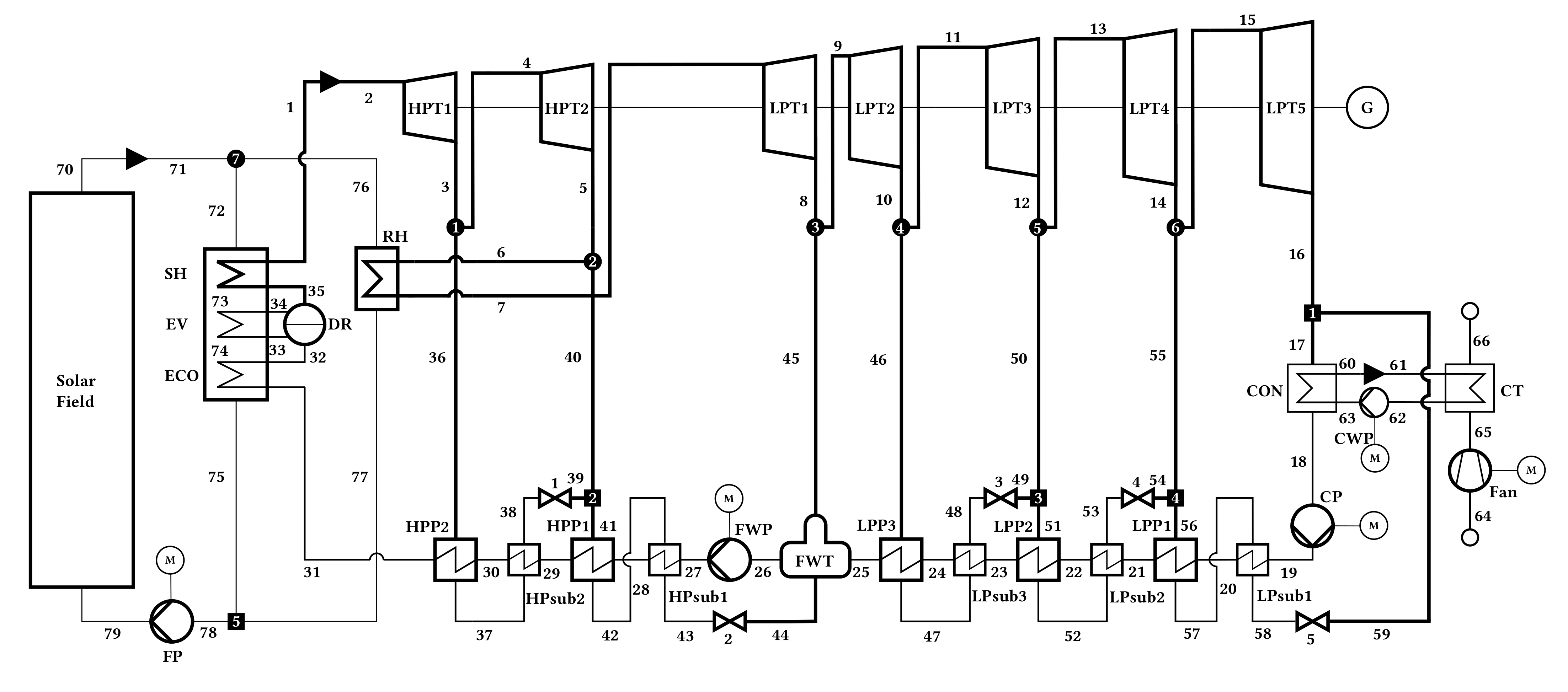
4.1. Solar Energy Generating System
4.1.1. Process Simulation and Validation
| Parameter | Symbol | Unit | Value |
|---|---|---|---|
| HTF temperature | 390 | ||
| HTF mass flow ratio | - | 0.13 | |
| Steam temperatures | , | 371, 371 | |
| Steam pressures | , | 100, 17.1 | |
| Steam mass flow | 38.97 | ||
| Condensation pressure | bar | 0.08 | |
| Pumps, efficiencies | , | % | 70.0, 95.0 |
| Generator, efficiency | % | 97.0 | |
| Air fan, efficiency | % | 60.0 | |
| Preheaters, upper temperature difference | 5 | ||
| Subcoolers, lower temperature difference | 10 |
4.1.2. Results of the Exergy Analysis
4.2. Supercritical Carbon Dioxide Power Cycle
4.2.1. Process Simulation and Validation
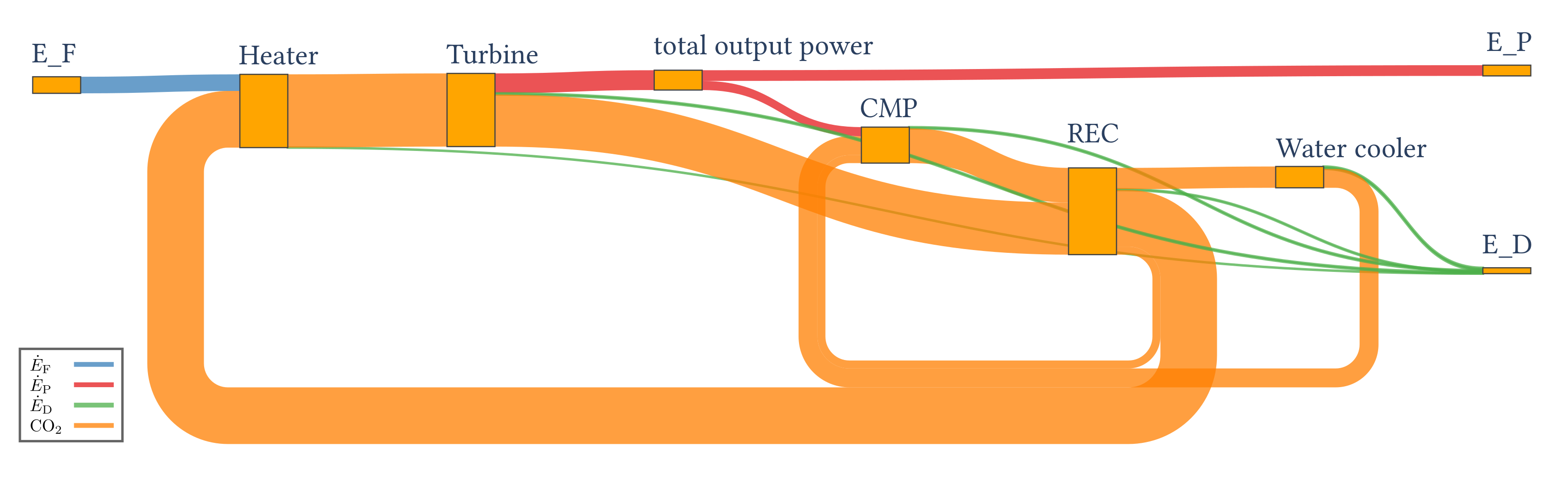
4.2.2. Results of the Exergy Analysis
4.3. Air Refrigeration Cycle
4.3.1. Process Simulation and Validation
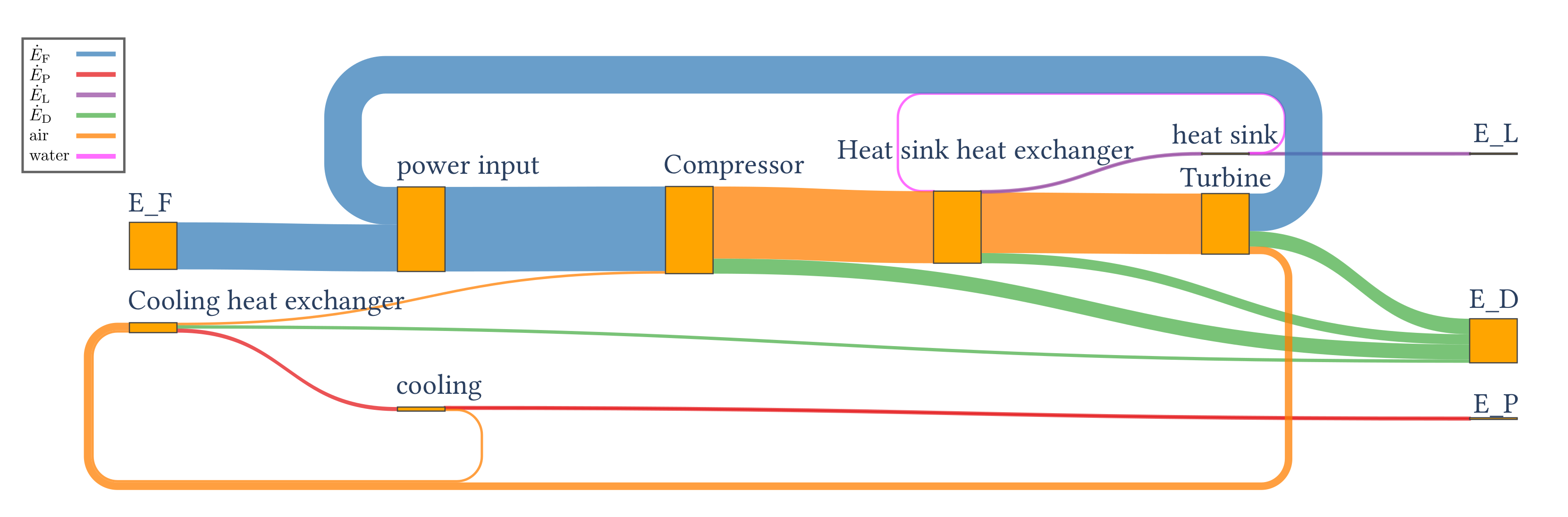
4.3.2. Results of the Exergy Analysis
5. Conclusions
- A complete set of exergy balance equations, including definitions of fuel and product exergy, for the most important components in thermal conversion processes is implemented.
- The paper presents a generic and reproducible workflow. It allows researchers to perform exergy analyses based on any thermodynamic application modeled by the software.
- The thermodynamic models and the results of the exergy analysis have been validated based on published research.
- Due to the fully integrated solution, changes in topology or parameter specifications of an existing model do not require changes in the exergy analysis setup.
- Updating and evaluating exergy analyses of published research is possible.
- Analysis of processes with conversion of matter, such as gas turbine power plants or power-to-gas facilities.
- Provision of exergoeconomical analysis tools.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| Abbreviations | |
| API | Application programming interface |
| CMP | Compressors |
| CON | Condenser |
| CSP | Concentrating solar power |
| CP | Condensate pump |
| CT | Cooling tower |
| CW | Cooling water |
| CWP | Cooling water pump |
| ECO | Economizer |
| EO | Equation-oriented |
| EV | Evaporator |
| DR | Drum |
| FP | Field pump |
| FWP | Feed water pump |
| FWT | Feed water tank |
| HEOS | Helmholtz equation of state |
| HPP | High-pressure preheater |
| HPT | High-pressure turbine |
| HTF | Heat-transfer fluid |
| IAPWS | International Association for the Properties of Water and Steam |
| LPP | Low-pressure preheater |
| LPT | Low-pressure turbine |
| NIST | National Institute of Standards and Technology |
| REC | Recuperators |
| RH | Reheater |
| sCO2 | supercritical CO2 |
| SH | Superheater |
| SEGS | Solar Energy Generating System |
| SG | Steam generator |
| SM | Sequential-modular |
| sub | Subcooler |
| TESPy | Thermal Engineering Systems in Python |
| Latin symbols | |
| Mass flow, kg/s | |
| c | Flow velocity, m/s |
| Exergy flow, W | |
| g | Gravity, m/s² |
| h | Specific enthalpy, J/kg |
| p | Pressure, bar |
| Heat flow, W | |
| s | Specific Entropy, J/kgK |
| T | Temperature, |
| Power, W | |
| x | Steam mass fraction, - |
| y | Exergy destruction ratio, - |
| z | Height, m |
| Greek symbols | |
| Energetic efficiency | |
| Difference | |
| Exergetic efficiency | |
| Subscripts and superscripts | |
| 0 | Reference state |
| ap | Approach point |
| CH | Chemical |
| cmp | Compressor |
| D | Destruction |
| el | Electrical |
| F | Fuel |
| g | gth component of functional group |
| gen | Generator |
| G | Functional group |
| in | Inlet |
| k | kth component |
| KN | Kinetic |
| L | Loss |
| m | Mechanical |
| M | Mechanical |
| min | Minimum |
| mot | Motor |
| out | Outlet |
| P | Product |
| PH | Physical |
| PT | Potential |
| s | Isentropic |
| sat | At saturation |
| T | Thermal |
| tot | Total |
| tur | Turbine |
References
- McLeod, J. Simulation Is Wha-a-At. Simulation 1963, 1, 5–6. [Google Scholar] [CrossRef]
- Youle, P. H3. Simulation techniques in chemical reaction engineering. Chem. Eng. Sci. 1961, 14, 252–257. [Google Scholar] [CrossRef]
- Shubik, M. Simulation of the industry and the firm. Am. Econ. Rev. 1960, 50, 908–919. [Google Scholar]
- Motard, R.L.; Shacham, M.; Rosen, E.M. Steady state chemical process simulation. AIChE J. 1975, 21, 417–436. [Google Scholar] [CrossRef]
- Marquardt, W. Von der Prozeßsimulation zur Lebenszyklusmodellierung. Chem.-Ing.-Tech. 1999, 71, 1119–1137. (In German) [Google Scholar] [CrossRef]
- Walter, H.; Epple, B. (Eds.) Numerical Simulation of Power Plants and Firing Systems; Springer: Vienna, Austria, 2017. [Google Scholar] [CrossRef]
- Banks, J.; Carson, J.S.; Nelson, B.L.; Nicol, D.M. Discrete-Event System Simulation; Pearson: Upper Saddle River, NJ, USA, 2010. [Google Scholar]
- Westerberg, A.W.; Hutchison, H.P.; Motard, R.L.; Winter, P. Process Flowsheeting; Cambridge University Press: Cambridge, UK, 1979. [Google Scholar]
- Sinha, R.; Paredis, C.J.J.; Liang, V.C.; Khosla, P.K. Modeling and simulation methods for design of engineering systems. J. Comput. Inf. Sci. Eng. 2001, 1, 84–91. [Google Scholar] [CrossRef]
- Takamatsu, T. The nature and role of process systems engineering. Comput. Chem. Eng. 1983, 7, 203–218. [Google Scholar] [CrossRef]
- Zeigler, B.P. Object-Oriented Simulation with Hierarchical, Modular Models; Academic Press: San Diego, CA, USA, 1990. [Google Scholar]
- Marquardt, W. Towards a process modeling methodology. In Methods of Model Based Process Control; Berber, R., Ed.; Kluwer: Dordrecht, The Netherlands, 1995; pp. 3–40. [Google Scholar] [CrossRef]
- Schuler, H. (Ed.) Prozeßsimulation; VCH: Weinheim, Germany, 1995; (In German). [Google Scholar] [CrossRef]
- Stamatelopoulos, G.N. Berechnung und Optimierung von Kraftwerkskreisläufen; Fortschritt-Berichte 340, Reihe 6; VDI Verlag: Düsseldorf, Germany, 1996. (In German) [Google Scholar]
- Zoder, M.; Balke, J.; Hofmann, M.; Tsatsaronis, G. Simulation and exergy analysis of energy conversion processes using a free and open-source framework—Python based object-oriented programming for gas- and steam turbine cycles. Energies 2018, 11, 2609. [Google Scholar] [CrossRef] [Green Version]
- Miller, K.W.; Voas, J.; Costello, T. Free and Open Source Software. IT Prof. 2010, 12, 14–16. [Google Scholar] [CrossRef]
- Feller, J.; Fitzgerald, B.; Hissam, S.A.; Lakhani, K.R. (Eds.) Perspectives on Free and Open Source Software; MIT Press: Cambridge, MA, USA, 2005. [Google Scholar]
- Crowston, K.; Wei, K.; Howison, J.; Wiggins, A. Free/Libre open-source software development: What we know and what we do not know. ACM Comput. Surv. 2012, 44, 7:1–7:35. [Google Scholar] [CrossRef]
- Tsatsaronis, G. Thermoeconomic analysis and optimization of energy systems. Prog. Energ. Combust. 1993, 19, 227–257. [Google Scholar] [CrossRef]
- Giglmayr, I.E. Modellierung von Heiz- und Heizkraftwerken; Fortschritt-Berichte 470, Reihe 6; VDI Verlag: Düsseldorf, Germany, 2001. (In German) [Google Scholar]
- Martelli, E.; Alobaid, F.; Elsido, C. Design optimization and dynamic simulation of steam cycle power plants: A review. Front. Energy Res. 2021, 9, 676969. [Google Scholar] [CrossRef]
- Asprion, N.; Rumpf, B.; Gritsch, A. Work flow in process development for energy efficient processes. Appl. Therm. Eng. 2011, 31, 2067–2072. [Google Scholar] [CrossRef]
- Asprion, N.; Mollner, S.; Poth, N.; Rumpf, B. Energy Management in Chemical Industry. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Weinheim, Germany, 2010; pp. 501–520. [Google Scholar] [CrossRef]
- Morris, D.R.; Szargut, J. Standard chemical exergy of some elements and compounds on the planet earth. Energy 1986, 11, 733–755. [Google Scholar] [CrossRef]
- Szargut, J.; Morris, D.; Steward, F. Exergy Analysis of Thermal, Chemical, and Metallurgical Processes; Hemisphere: New York, NY, USA, 1988. [Google Scholar]
- Szargut, J. Chemical exergies of the elements. Appl. Energ. 1989, 32, 269–286. [Google Scholar] [CrossRef]
- Szargut, J.; Valero, A.; Stanek, W.; Valero, A. Towards an International Reference Environment of Chemical Exergy. In Proceedings of the ECOS, Trondheim, Norway, 20–22 June 2005; pp. 409–417. [Google Scholar]
- Szargut, J. Egzergia; Slesian University of Technology: Gliwice, Poland, 2007. (In Polish) [Google Scholar]
- Ahrendts, J. Die Exergie Chemisch Reaktionsfähiger Systeme; VDI-Forsch.-Heft 579; VDI Verlag: Düsseldorf, Germany, 1977. (In German) [Google Scholar]
- Ahrendts, J. Reference states. Energy 1980, 5, 666–677. [Google Scholar] [CrossRef]
- Kameyama, H.; Yoshida, K.; Yamauchi, S.; Fueki, K. Evaluation of reference exergies for the elements. Appl. Energ. 1982, 11, 69–83. [Google Scholar] [CrossRef]
- Van Gool, W. Thermodynamics of chemical references for exergy analysis. Energ. Convers. Manage. 1998, 39, 1719–1728. [Google Scholar] [CrossRef]
- Diederichsen, C. Referenzumgebungen zur Berechnung der Chemischen Exergie; Fortschritt-Berichte 50, Reihe 19; VDI Verlag: Düsseldorf, Germany, 1991. (In German) [Google Scholar]
- Shieh, J.H.; Fan, L.T. Estimation of Energy (Enthalpy) and Exergy (Availability) Contents in Structurally Complicated Materials. Energy Sources 1982, 6, 1–46. [Google Scholar] [CrossRef]
- Stepanov, V.S. Chemical Energies and Exergies of Fuels, 2nd ed.; Nauka: Novosibirsk, Russia, 1990. (In Russian) [Google Scholar]
- Stepanov, V.S. Chemical energies and exergies of fuels. Energy 1995, 20, 235–242. [Google Scholar] [CrossRef]
- Fratzscher, W.; Michalek, K.; Szargut, J. Unterschiedliche Bezugssysteme zur Berechnung der chemischen Exergie. Chem. Tech. 1989, 41, 321–327. (In German) [Google Scholar]
- Rosen, M.A.; Scott, D.S. The enhancement of a process simulator for complete energy-exergy analysis. In Analysis of Energy Systems—Design and Operation; American Society of Mechanical Engineers: New York, NY, USA, 1985; pp. 71–80. [Google Scholar]
- Munsch, M.; Mohr, T.; Futterer, E. Exergetische Analyse und Bewertung verfahrenstechnischer Prozesse mit einem Flow-Sheeting-Programm. Chem.-Ing.-Tech. 1990, 62, 995–1002. (In German) [Google Scholar] [CrossRef]
- Futterer, E.; Gruhn, G.; Munsch, M.; Mohr, T. Rechnergestützte exergetische Optimierung verfahrenstechnischer Prozesse. Chem.-Ing.-Tech. 1991, 63, 204–212. (In German) [Google Scholar] [CrossRef]
- Yang, Y.; Yang, J.; Zhu, X.; Ling, W. Enhancement and application of a flowsheeting simulator for second law analysis. In Proceedings of the ECOS, Zaragoza, Spain, 15–18 June 1992; pp. 85–91. [Google Scholar]
- Riedl, K. Exergetische und Exergoökonomische Bewertung von Verfahren der Energie- und Stoffwandlung. Ph.D. Thesis, Martin-Luther-Universität Halle-Wittenberg, Halle, Germany, 2006. (In German). [Google Scholar]
- Querol, E.; Gonzalez-Regueral, B.; Ramos, A.; Perez-Benedito, J. Novel application for exergy and thermoeconomic analysis of processes simulated with Aspen Plus®. Energy 2011, 36, 964–974. [Google Scholar] [CrossRef]
- Frenzel, P.E. Bewertung von Syntheserouten auf Basis von Exergiebilanzen. Ph.D. Thesis, RWTH, Aachen, Germany, 2014. (In German). [Google Scholar]
- Abdollahi-Demneh, F.; Moosavian, M.A.; Omidkhah, M.R.; Bahmanyar, H. Calculating exergy in flowsheeting simulators: A HYSYS implementation. Energy 2011, 36, 5320–5327. [Google Scholar] [CrossRef]
- Ghannadzadeh, A.; Thery-Hetreux, R.; Baudouin, O.; Baudet, P.; Floquet, P.; Joulia, X. General methodology for exergy balance in ProSimPlus® process simulator. Energy 2012, 44, 38–59. [Google Scholar] [CrossRef] [Green Version]
- Montelongo-Luna, J.M.; Svrcek, W.Y.; Young, B.R. An exergy calculator tool for process simulation. Asia-Pac. J. Chem. Eng. 2007, 2, 431–437. [Google Scholar] [CrossRef]
- Zargarzadeh, M.; Karimi, I.A.; Alfadala, H. Olexan: A Tool for Online Exergy Analysis. In Proceedings of the 17th European Symposium on Computer Aided Process Engineering (ESCAPE17), Bucharest, Romania, 27–30 May 2007. [Google Scholar]
- Zhao, P. A Computer Program for the Exergoeconomic Analysis of Energy Conversion Plants. Ph.D. Thesis, Technische Universtät Berlin, Berlin, Germany, 2015. [Google Scholar] [CrossRef]
- Eisermann, W.; Hasberg, W.; Tsatsaronis, G. THESIS—Ein Rechenprogramm zur Simulation und Entwicklung von Energieumwandlungsanlagen. Brennst.-Wärme-Kraft 1984, 36, 45–52. (In German) [Google Scholar]
- Hinderink, A.; Kerkhof, F.; Lie, A.; De Swaan Arons, J.; Van Der Kooi, H. Exergy analysis with a flowsheeting simulator—I. Theory; calculating exergies of material streams. Chem. Eng. Sci. 1996, 51, 4693–4700. [Google Scholar] [CrossRef]
- Lozano, M.; Valero, A. Theory of the exergetic cost. Energy 1993, 18, 939–960. [Google Scholar] [CrossRef]
- Biegler, L.T. Simultaneous Modular Simulation and Optimization; Technical Report; Carnegie Mellon University, Department of Chemical Engineering: Pittsburgh, PA, USA, 1983. [Google Scholar]
- Shacham, M.; Macchieto, S.; Stutzman, L.; Babcock, P. Equation oriented approach to process flowsheeting. Comput. Chem. Eng. 1982, 6, 79–95. [Google Scholar] [CrossRef]
- Rosen, E.M. Steady State Chemical Process Simulation: A State-of-the-Art Review. In Computer Applications to Chemical Engineering; Squires, R.G., Reklaitis, G.V., Eds.; ACS: Washington, DC, USA, 1980; pp. 3–36. [Google Scholar] [CrossRef] [Green Version]
- Auzinger, D.; Peer, L.; Wacker, H. Steady State Simulation of Chemical Plants—Flowsheeting. In Proceedings of the Second European Symposium on Mathematics in Industry; Neunzert, H., Ed.; Teubner: Stuttgart, Germany, 1988; pp. 285–300. [Google Scholar] [CrossRef]
- Dimian, A.C.; Bildea, C.S.; Kiss, A.A. Steady state flowsheeting. In Computer Aided Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2016; pp. 73–125. [Google Scholar] [CrossRef]
- Fratzscher, W.; Brodjanskij, V.M.; Michalek, K. Exergie; VEB Deutscher Verlag für Grundstoffindustrie: Leipzig, Germany, 1986. (In German) [Google Scholar]
- Bejan, A.; Tsatsaronis, G.; Moran, M. Thermal Design and Optimization; J. Wiley: New York, NY, USA, 1996. [Google Scholar]
- Morosuk, T.; Tsatsaronis, G. Splitting physical exergy. Energy 2019, 167, 698–707. [Google Scholar] [CrossRef]
- Bošnjaković, F. Kampf den Nichtumkehrbarkeiten! Arch. Wärmewirtsch. 1938, 19, 1–2. (In German) [Google Scholar]
- Lazzaretto, A.; Tsatsaronis, G. SPECO: A systematic and general methodology for calculating efficiencies and costs in thermal systems. Energy 2006, 31, 1257–1289. [Google Scholar] [CrossRef]
- Tsatsaronis, G. Combination of Exergetic and Economic Analysis in Energy Conversion Processes. In Proceedings of the European Congress on Economics and Management of Energy in Industry, Algarve, Portugal, 2–5 April 1984; Pergamon: Oxford, UK, 1984; pp. 151–157. [Google Scholar]
- Witte, F. Read the Docs—Thermal Engineering Systems in Python (TESPy); 2022. Retrieved: 8 April 2022. Available online: https://tespy.readthedocs.io/ (accessed on 8 April 2022).
- Tsatsaronis, G. Energietechnik I; Class Notes; Technische Universität Berlin: Berlin, Germany, 2021. (In German) [Google Scholar]
- Witte, F.; Tuschy, I. TESPy: Thermal Engineering Systems in Python. J. Open Source Softw. 2020, 5, 2178. [Google Scholar] [CrossRef]
- Sweigart, A. Automate the Boring Stuff with Python, 2nd ed.; No Starch Press: San Francisco, CA, USA, 2016. [Google Scholar]
- Bell, I.H.; Wronski, J.; Quoilin, S.; Lemort, V. Pure and Pseudo-pure Fluid Thermophysical Property Evaluation and the Open-Source Thermophysical Property Library CoolProp. Ind. Eng. Chem. Res. 2014, 53, 2498–2508. [Google Scholar] [CrossRef] [Green Version]
- McKinney, W. Data Structures for Statistical Computing in Python. In Proceedings of the 9th Python in Science Conference, Austin, TX, USA, 28 June–3 July 2010; van der Walt, S., Millman, J., Eds.; 2010; pp. 56–61. [Google Scholar] [CrossRef] [Green Version]
- Grassmann, P. Die Exergie und das Flussbild der technisch nutzbaren Leistung. Allg. Wärmetechn. 1959, 9, 79–86. (In German) [Google Scholar]
- Plotly Technologies Inc. Plotly Python Open Source Graphing Library. 2021. Available online: https://plotly.com/python/sankey-diagram/ (accessed on 8 April 2022).
- Witte, F.; Meier, J.; Tuschy, I.; Hofmann, M. Solar Energy Generating System (SEGS) Model in TESPy, v1.4. 2022. [CrossRef]
- Witte, F.; Meier, J.; Tuschy, I.; Hofmann, M. Supercritical CO2 Power Cycle Model in TESPy, v1.4. 2022. [CrossRef]
- Witte, F.; Meier, J.; Tuschy, I.; Hofmann, M. Refrigeration Machine Model in TESPy, v1.3. 2022. [CrossRef]
- Penkuhn, M.; Tsatsaronis, G. Exergoeconomic analyses of different sCO2 cycle configurations. In Proceedings of the 6th International Symposium—Supercritical CO2 Power Cycles, Pittsburgh, PA, USA, 27–29 March 2018; pp. 1–17. [Google Scholar]
- Morosuk, T.; Tsatsaronis, G. Advanced Exergoeconomic Analysis of a Refrigeration Machine: Part 1—Methodology and First Evaluation. In Proceedings of the ASME 2011 International Mechanical Engineering Congress & Exposition, Denver, CO, USA, 11–17 November 2011; pp. 47–56. [Google Scholar] [CrossRef]
- Witte, F. TESPy: Thermal Engineering Systems in Python, v0.6.0. 2022. [CrossRef]
- Kearney, D.; Miller, C.E. Technical Evaluation of Project Feasibilty for SEGS VI; Draft Report; Luz Partnership Management: Westwood, CA, USA, 1988. [Google Scholar]
- Lippke, F. Simulation of the Part-Load Behavior of a 30 MWe SEGS Plant; Technical Report SAND-95-1293; Sandia National Lab.: Albuquerque, NM, USA, 1995. [Google Scholar] [CrossRef] [Green Version]
- Therminol VP–1 Heat Transfer Fluid. Data Sheet, Eastman Chemical Company, 2019. Retrieved: 2 November 2020. Available online: https://www.therminol.com/product/71093459 (accessed on 8 April 2022).
- Patnode, A.M. Simulation and Performance Evaluation of Parabolic Trough Solar Power Plants. Master’s Thesis, University of Wisconsin-Madison, WI, USA, 2006. [Google Scholar]
- Manzolini, G.; Giostri, A.; Saccilotto, C.; Silva, P.; Macchi, E. Development of an innovative code for the design of thermodynamic solar power plants. Part A: Code description and test case. Renew. Energy 2011, 36, 1993–2003. [Google Scholar] [CrossRef]
- Chen, C.; Witte, F.; Tuschy, I.; Kolditz, O.; Shao, H. Parametric optimization and comparative study of an organic Rankine cycle power plant for two-phase geothermal sources. Energy 2022, 252, 123910. [Google Scholar] [CrossRef]
- Vilar-Dias, J.L.; Galindo, M.A.S.; Lima-Neto, F.B. Cultural Weight-Based Fish School Search: A Flexible Optimization Algorithm For Engineering. In Proceedings of the 2021 IEEE Congress on Evolutionary Computation (CEC), Kraków, Poland, 28 June–1 July 2021; IEEE: Piscataway, NJ, USA, 2021. [Google Scholar] [CrossRef]
- Witte, F.; Meier, J.; Tuschy, I.; Hofmann, M. Solar Energy Generating System (SEGS) Model in TESPy, v1.4; 2022. Retrieved: 8 April 2022. Available online: https://github.com/fwitte/SEGS_exergy (accessed on 7 April 2022).
- Witte, F.; Meier, J.; Tuschy, I.; Hofmann, M. Supercritical CO2 Power Cycle Model in TESPy, v1.4; 2022. Retrieved: 8 April 2022. Available online: https://github.com/fwitte/sCO2_exergy (accessed on 8 April 2022).
- Witte, F.; Meier, J.; Tuschy, I.; Hofmann, M. Refrigeration Machine Model in TESPy, v1.3; 2022. Retrieved: 8 April 2022. Available online: https://github.com/fwitte/refrigeration_cycle_exergy (accessed on 8 April 2022).
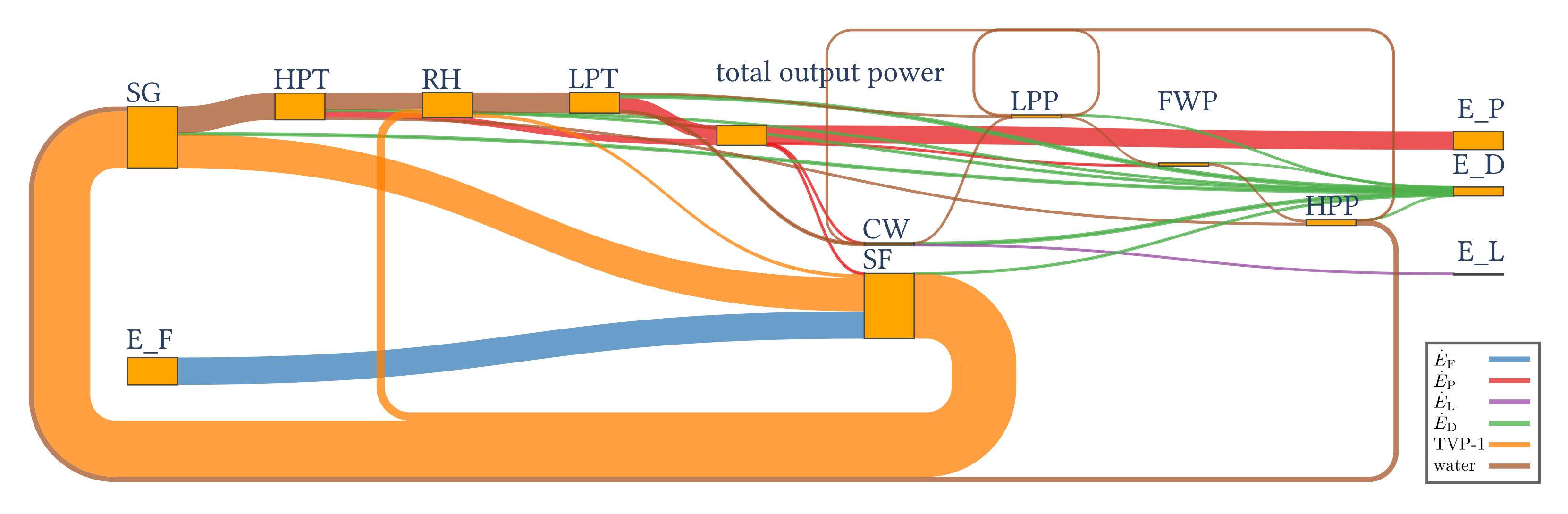


| 47.63 | 31.77 | 15.37 | 0.49 |
| Functional Group G | (%) | (%) | |||
|---|---|---|---|---|---|
| Low-pressure turbine (LPT) | 36.01 | 31.94 | 4.07 | 8.6 | 26.5 |
| Cooling water system (CW) | 4.09 | 0.59 | 3.50 | 7.3 | 22.8 |
| Steam generator (SG) | 107.46 | 104.94 | 2.52 | 5.3 | 16.4 |
| High-pressure turbine (HPT) | 46.66 | 45.06 | 1.60 | 3.4 | 10.4 |
| Reheater (RH) | 43.64 | 42.41 | 1.23 | 2.6 | 8.0 |
| Solar field (SF) | 114.09 | 112.89 | 1.20 | 2.5 | 7.8 |
| Low-pressure preheater (LPP) | 5.25 | 4.51 | 0.74 | 1.6 | 4.8 |
| High-pressure preheater (HPP) | 10.13 | 9.79 | 0.33 | 0.7 | 2.2 |
| Feedwater pump (FWP) | 5.26 | 5.07 | 0.18 | 0.4 | 1.2 |
| Parameter | Symbol | Unit | Value |
|---|---|---|---|
| Turbine inlet temperature | 600 | ||
| Turbine inlet pressure | 250 | ||
| Compressor inlet pressures | , | 75, 75 | |
| Compressors, efficiencies | , | % | 85.0, 95.1 |
| Turbine, efficiencies | , | % | 90.0, 98.0 |
| Recuperators, lower temperature difference | 5 |
| 154.93 | 100.00 | 54.93 | 0.00 |
| Component k | (%) | (%) | (%) | |||
|---|---|---|---|---|---|---|
| Compressor 1 | 47.49 | 40.20 | 7.29 | 84.6 | 4.7 | 13.3 |
| Compressor 2 | 37.58 | 32.81 | 4.76 | 87.3 | 3.1 | 8.7 |
| Heater | 154.93 | 154.09 | 0.84 | 99.5 | 0.5 | 1.5 |
| Recuperator 1 | 73.81 | 69.93 | 3.87 | 94.8 | 2.5 | 7.1 |
| Recuperator 2 | 139.19 | 135.43 | 3.76 | 97.3 | 2.4 | 6.8 |
| Turbine | 197.19 | 185.07 | 12.12 | 93.9 | 7.8 | 22.1 |
| Water cooler | 22.28 | nan | 22.28 | nan | 14.4 | 40.6 |
| Parameter | Symbol | Unit | Value |
|---|---|---|---|
| Compressor inlet temperature | −30 | ||
| Compressor inlet pressure | 1 | ||
| Turbine inlet temperature | 35 | ||
| Turbine inlet pressure | 5 | ||
| Ambient air temperatures | , | 25, 40 | |
| Refrigeration temperatures | , | −10, −20 | |
| Compressor/turbine efficiencies | , | % | 80.0, 96.2 |
| 439.80 | 15.51 | 412.22 | 12.07 |
| Component k | (%) | (%) | (%) | |||
|---|---|---|---|---|---|---|
| Compressor | 815.29 | 674.08 | 141.21 | 82.7 | 32.1 | 34.3 |
| Cooling heat exchanger | 46.30 | 15.51 | 30.79 | 33.5 | 7.0 | 7.5 |
| Heat sink heat exchanger | 107.31 | 12.07 | 95.24 | 11.2 | 21.7 | 23.1 |
| Turbine | 549.60 | 404.62 | 144.98 | 73.6 | 33.0 | 35.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Witte, F.; Hofmann, M.; Meier, J.; Tuschy, I.; Tsatsaronis, G. Generic and Open-Source Exergy Analysis—Extending the Simulation Framework TESPy. Energies 2022, 15, 4087. https://doi.org/10.3390/en15114087
Witte F, Hofmann M, Meier J, Tuschy I, Tsatsaronis G. Generic and Open-Source Exergy Analysis—Extending the Simulation Framework TESPy. Energies. 2022; 15(11):4087. https://doi.org/10.3390/en15114087
Chicago/Turabian StyleWitte, Francesco, Mathias Hofmann, Julius Meier, Ilja Tuschy, and George Tsatsaronis. 2022. "Generic and Open-Source Exergy Analysis—Extending the Simulation Framework TESPy" Energies 15, no. 11: 4087. https://doi.org/10.3390/en15114087
APA StyleWitte, F., Hofmann, M., Meier, J., Tuschy, I., & Tsatsaronis, G. (2022). Generic and Open-Source Exergy Analysis—Extending the Simulation Framework TESPy. Energies, 15(11), 4087. https://doi.org/10.3390/en15114087







