Effects of Oxygen and Steam Equivalence Ratios on Updraft Gasification of Biomass
Abstract
1. Introduction
- (1)
- Reworking and integrating available experimental data obtained from different feedstock at similar gasification conditions;
- (2)
- To draw generalized correlation relationships between Equivalent Ratios and plant performances for gasification of generic lignocellulosic biomass. These relationships appear not sufficiently investigated, especially for auto thermal fixed bed gasifiers.
2. Materials and Methods
2.1. Feedstock
2.2. Description of the Plant and Procedures
2.3. Chemical Analysis
3. Results and Discussion
3.1. Effects of Different Values of ER(H2O) on Syngas Quality and Yield
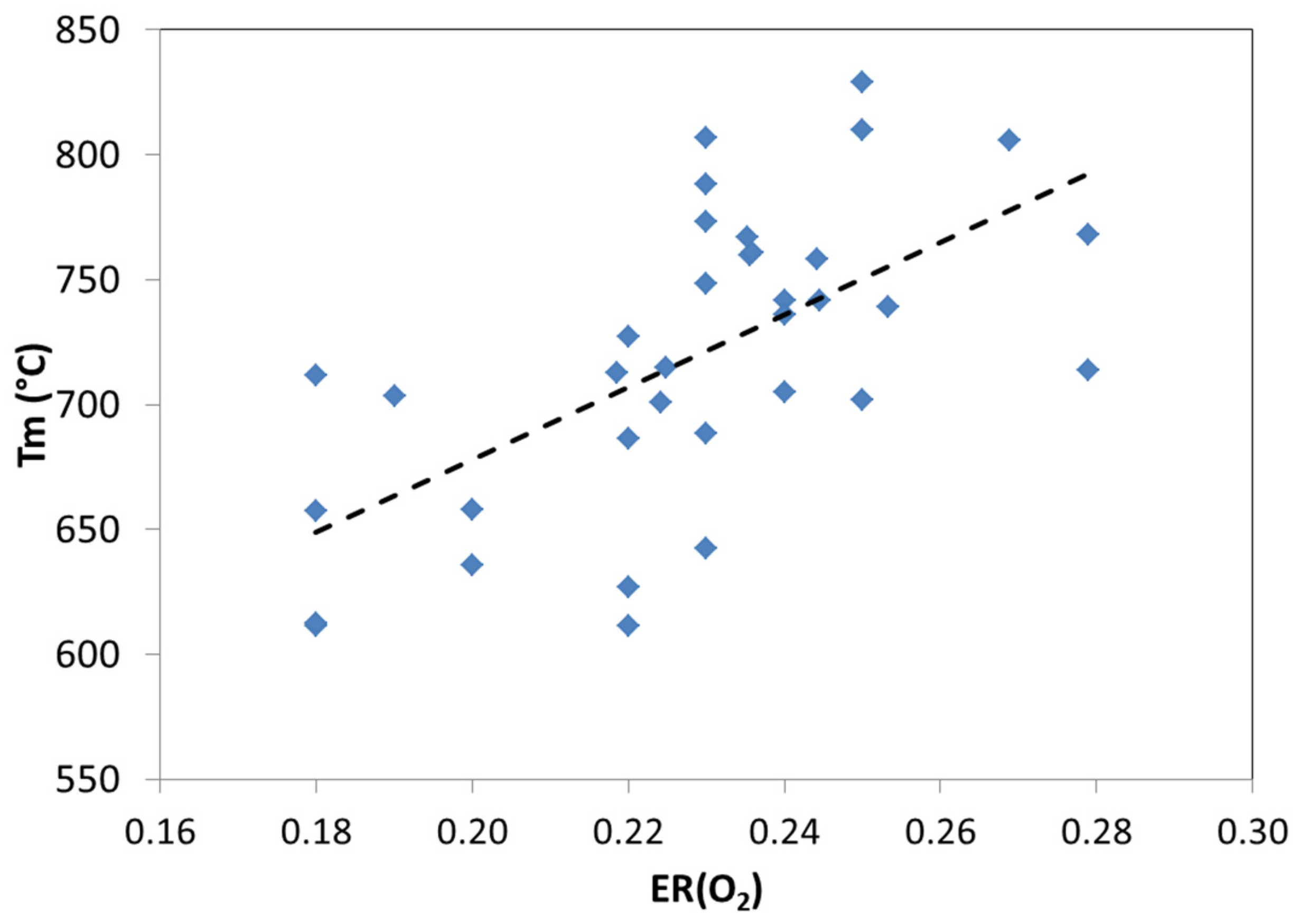
3.2. Effects of Different Values of ER(O2) on Syngas Quality and Yield
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Segurado, R.; Pereira, S.; Correia, D.; Costa, M. Techno-economic analysis of a trigeneration system based on biomass gasification. Renew. Sustain. Energy Rev. 2019, 103, 501–514. [Google Scholar] [CrossRef]
- Kozlov, A.N.; Tomin, N.V.; Sidorov, D.N.; Lora, E.E.S.; Kurbatsky, V.G. Optimal Operation Control of PV-Biomass Gasifier-Diesel-Hybrid Systems Using Reinforcement Learning Techniques. Energies 2020, 13, 2632. [Google Scholar] [CrossRef]
- Kozlov, A.; Marchenko, O.; Solomin, S. The Modern State of Wood Biomass Gasification Technologies and Their Economic Efficiency. Energy Procedia 2019, 158, 1004–1008. Available online: http://creativecommons.org/licenses/by-nc-nd/4.0 (accessed on 24 April 2021). [CrossRef]
- Di Blasi, C. Modeling wood gasification in a countercurrent fixed-bed reactor. AIChE J. 2004, 50, 2306–2319. [Google Scholar] [CrossRef]
- Mandl, C.; Obernberger, I.; Biedermann, F. Modelling of an updraft fixed-bed gasifier operated with softwood pellets. Fuel 2010, 89, 3795–3806. [Google Scholar] [CrossRef]
- Ferreira, S.; Monteiro, E.; Brito, P.; Vilarinho, C. A Holistic Review on Biomass Gasification Modified Equilibrium Models. Energies 2019, 12, 160. [Google Scholar] [CrossRef]
- Nowicki, L.; Siuta, D.; Markowski, M. Pyrolysis of Rapeseed Oil Press Cake and Steam Gasification of Solid Residues. Energies 2020, 13, 4472. [Google Scholar] [CrossRef]
- Nowicki, L.; Siuta, D.; Markowski, M. Carbon Dioxide Gasification Kinetics of Char from Rapeseed Oil Press Cake. Energies 2020, 13, 2318. [Google Scholar] [CrossRef]
- Umeki, K.; Namioka, T.; Yoshikawa, K. Analysis of an updraft biomass gasifier with high temperature steam using a numerical model. Appl. Energy 2012, 90, 38–45. [Google Scholar] [CrossRef]
- Kardaś, D.; Kluska, J.; Kazimierski, P. The course and effects of syngas production from beechwood and RDF in updraft reactor in the light of experimental tests and numerical calculations. Therm. Sci. Eng. Prog. 2018, 8, 136–144. [Google Scholar] [CrossRef]
- Barman, N.S.; Ghosh, S.; De, S. Gasification of biomass in a fixed bed downdraft gasifier—A realistic model including tar. Bioresour. Technol. 2012, 107, 505–511. [Google Scholar] [CrossRef]
- Manera, C.; Perondi, D.; Barcellos, T.; & Godinho, M. CO2 gasification of elephant grass: Effect of Ni/mayenite catalyst on dry reforming of tar. Biomass Bioenergy 2020, 143, 105829. [Google Scholar] [CrossRef]
- Ismail, T.M.; El-Salam, M.A. Parametric studies on biomass gasification process on updraft gasifier high temperature air gasification. Appl. Therm. Eng. 2017, 112, 1460–1473. [Google Scholar] [CrossRef]
- James, A.M.; Yuan, W.; Boyette, M.D.; Wang, D. The effect of air flow rate and biomass type on the performance of an updraft biomass gasifier. BioResources 2015, 10, 3615–3624. [Google Scholar] [CrossRef]
- Kihedu, J.H.; Yoshiie, R.; Naruse, I. Performance indicators for air and air–steam auto-thermal updraft gasification of biomass in packed bed reactor. Fuel Process. Technol. 2016, 141, 93–98. [Google Scholar] [CrossRef]
- Oveisi, E.; Sokhansanj, S.; Lau, A.; Lim, J.; Bi, X.; Preto, F.; Mui, C. Characterization of Recycled Wood Chips, Syngas Yield, and Tar Formation in an Industrial Updraft Gasifier. Environ. 2018, 5, 84. [Google Scholar] [CrossRef]
- Vidian, F.; Surjosatyo, A.; Nugroho, Y.S. Thermodynamic Model for Updraft Gasifier with External Recirculation of Pyrolysis Gas. J. Combust. 2016, 2016, 1–6. [Google Scholar] [CrossRef]
- Paulauskas, R.; Zakarauskas, K.; Striūgas, N. An Intensification of Biomass and Waste Char Gasification in a Gasifier. Energies 2021, 14, 1983. [Google Scholar] [CrossRef]
- Cerone, N.; Zimbardi, F.; Villone, A.; Strjiugas, N.; Kiyikci, E.G. Gasification of Wood and Torrefied Wood with Air, Oxygen, and Steam in a Fixed-Bed Pilot Plant. Energy Fuels 2016, 30, 4034–4043. [Google Scholar] [CrossRef]
- Cerone, N.; Zimbardi, F.; Contuzzi, L.; Alvino, E.; Carnevale, M.O.; Valerio, V. Updraft Gasification at Pilot Scale of Hydrolytic Lignin Residue. Energy Fuels 2014, 28, 3948–3956. [Google Scholar] [CrossRef]
- Cerone, N.; Zimbardi, F.; Contuzzi, L.; Prestipino, M.; Carnevale, M.O.; Valerio, V. Air-steam and oxy-steam gasification of hydrolytic residues from biorefinery. Fuel Process. Technol. 2017, 167, 451–461. [Google Scholar] [CrossRef]
- Cerone, N.; Zimbardi, F. Gasification of Agroresidues for Syngas Production. Energies 2018, 11, 1280. [Google Scholar] [CrossRef]
- Liakakou, E.; Vreugdenhil, B.; Cerone, N.; Zimbardi, F.; Pinto, F.; André, R.; Marques, P.; Mata, R.; Girio, F. Gasification of lignin-rich residues for the production of biofuels via syngas fermentation: Comparison of gasification technologies. Fuel 2019, 251, 580–592. [Google Scholar] [CrossRef]
- Sansaniwal, S.; Rosen, M.; Tyagi, S. Global challenges in the sustainable development of biomass gasification: An overview. Renew. Sustain. Energy Rev. 2017, 80, 23–43. [Google Scholar] [CrossRef]
- Ahmad, A.A.; Zawawi, N.A.; Kasim, F.H.; Inayat, A.; Khasri, A. Assessing the gasification performance of biomass: A review on biomass gasification process conditions, optimization and economic evaluation. Renew. Sustain. Energy Rev. 2016, 53, 1333–1347. [Google Scholar] [CrossRef]
- Sikarwar, V.S.; Zhao, M.; Fennell, P.S.; Shah, N.; Anthony, E.J. Progress in biofuel production from gasification. Prog. Energy Combust. Sci. 2017, 61, 189–248. [Google Scholar] [CrossRef]
- Saravanakumar, A.; Haridasan, T.; Reed, T.B.; Bai, R.K. Experimental investigation and modelling study of long stick wood gasification in a top lit updraft fixed bed gasifier. Fuel 2007, 86, 2846–2856. [Google Scholar] [CrossRef]
- Arena, U. Process and technological aspects of municipal solid waste gasification. A review. Waste Manag. 2012, 32, 625–639. [Google Scholar] [CrossRef] [PubMed]
- Narváez, I.; Orío, A.; Aznar, M.P.; Corella, J. Biomass Gasification with Air in an Atmospheric Bubbling Fluidized Bed. Effect of Six Operational Variables on the Quality of the Produced Raw Gas. Ind. Eng. Chem. Res. 1996, 35, 2110–2120. [Google Scholar] [CrossRef]
- Seggiani, M.; Vitolo, S.; Puccini, M.; Bellini, A. Cogasification of sewage sludge in an updraft gasifier. Fuel 2012, 93, 486–491. [Google Scholar] [CrossRef]
- Wang, L.; Weller, C.L.; Jones, D.D.; Hanna, M.A. Contemporary issues in thermal gasification of biomass and its application to electricity and fuel production. Biomass Bioenergy 2008, 32, 573–581. [Google Scholar] [CrossRef]
- Czernik, S.; Bridgwater, A.V. Overview of Applications of Biomass Fast Pyrolysis Oil. Energy Fuels 2004, 18, 590–598. [Google Scholar] [CrossRef]
- Lindfors, C.; Paasikallio, V.; Kuoppala, E.; Reinikainen, M.; Oasmaa, A.; Solantausta, Y. Co-processing of Dry Bio-oil, Catalytic Pyrolysis Oil, and Hydrotreated Bio-oil in a Micro Activity Test Unit. Energy Fuels 2015, 29, 3707–3714. [Google Scholar] [CrossRef]
- Kumar, A.; Jones, D.D.; Hanna, M.A. Thermochemical Biomass Gasification: A Review of the Current Status of the Technology. Energies 2009, 2, 556–581. [Google Scholar] [CrossRef]
- Di Blasi, C.; Signorelli, A.G.; Portoricco, G. Countercurrent Fixed-Bed Gasification of Biomass at Laboratory Scale. Ind. Eng. Chem. Res. 1999, 38, 2571–2581. [Google Scholar] [CrossRef]
- Gao, N.; Li, A.; Quan, C.; Gao, F. Hydrogen-rich gas production from biomass steam gasification in an updraft fixed-bed gasifier combined with a porous ceramic reformer. Int. J. Hydrogen Energy 2008, 33, 5430–5438. [Google Scholar] [CrossRef]
- Hashim, S.; Mohamed, A.; Bhatia, S. Oxygen separation from air using ceramic-based membrane technology for sustainable fuel production and power generation. Renew. Sustain. Energy Rev. 2011, 15, 1284–1293. [Google Scholar] [CrossRef]
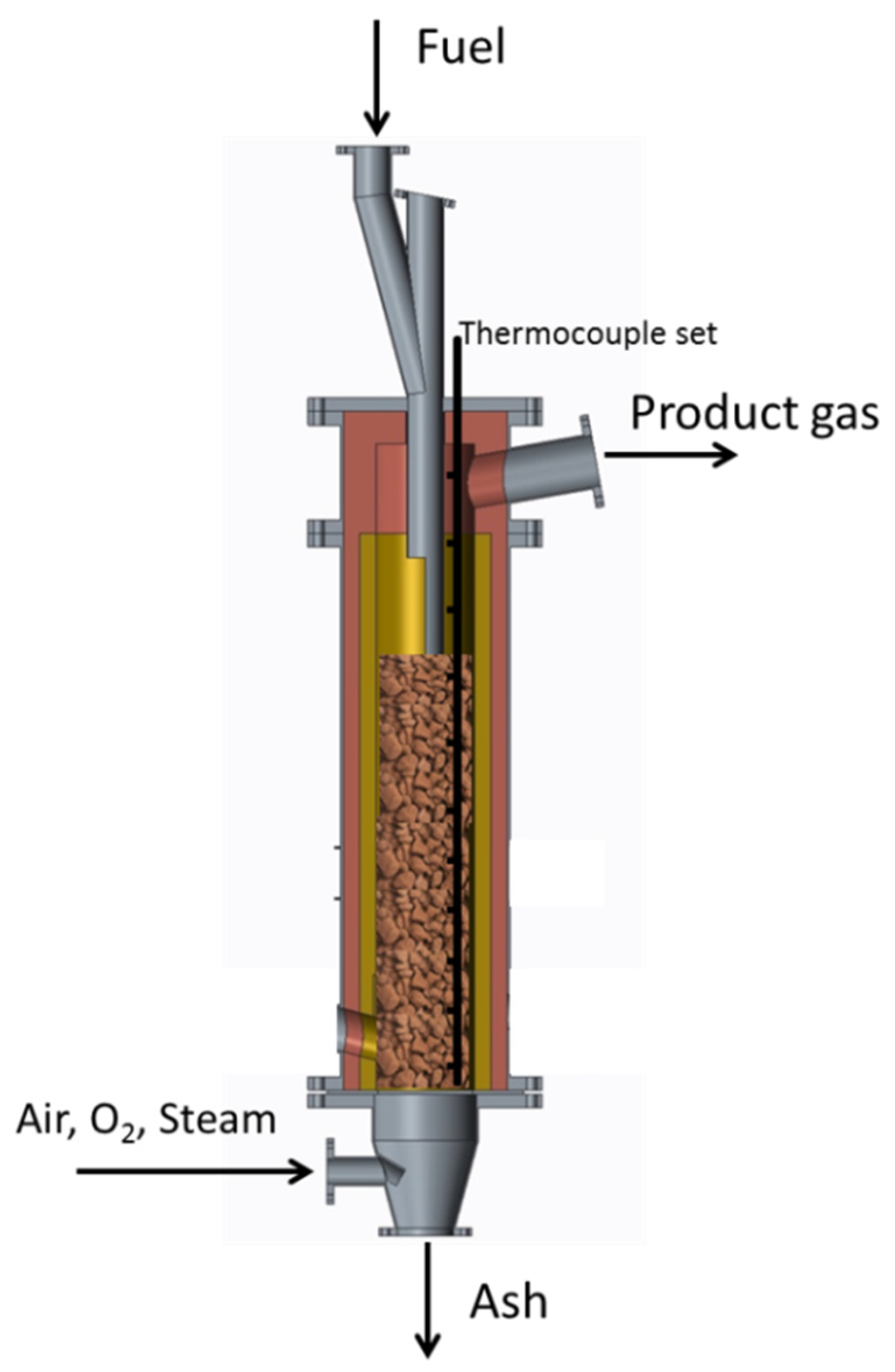






| Biomass | C% | H% | N% | O% * |
|---|---|---|---|---|
| Wood Eucalyptus (WE) | 48.1 | 6.3 | 0.3 | 41.7 |
| Torriefied Eucalyptus (TE) | 51.7 | 5.9 | 0.3 | 37.6 |
| Torriefied Spruce (TS) | 54.8 | 6.3 | 1.0 | 37.5 |
| Hydrolytic Lignin (L) | 49.5 | 6.06 | 1.2 | 34.0 |
| Almond shells (A) | 47.9 | 6.3 | 0.36 | 45.4 |
| Hazelnut shells (N) | 50.5 | 6.64 | 1.7 | 40.0 |
| Biomass | Hexosans% | Pentosans% | Lignin (Klason)% | Lignin (Klason) ac. sol.% |
|---|---|---|---|---|
| Wood Eucalyptus (WE) | 37.5 | 28.3 | 28.0 | nd |
| Torriefied Eucalyptus (TE) | 32.9 | 9.96 | 46.6 | nd |
| Torriefied Spruce (TS) | 44.6 | 1.14 | 44.8 | nd |
| Hydrolytic Lignin (L) | 36.7 | 4.0 | 47.5 | 2.45 |
| Almond shells (A) | 31.2 | 28.0 | 30.2 | 1.98 |
| Hazelnut shells (N) | 22.2 | 12.2 | 40.9 | 1.30 |
| Biomass | Density (kg/m3) | Moisture% | Volatile% | Fix Carbon% | Ash% | LHV (MJ/kg) | HHV (MJ/kg) |
|---|---|---|---|---|---|---|---|
| Wood Eucalyptus (WE) | 188 | 13.9 | 88 | 8.4 | 3.6 | 16.8 | 18.2 |
| Torriefied Eucalyptus (TE) | 274 | 6.1 | 79.7 | 15.1 | 5.2 | 18.8 | 20.2 |
| Torriefied Spruce (TS) | 173 | 6.5 | 76.6 | 23 | 0.4 | 18.9 | 20.6 |
| Hydrolytic Lignin (L) | 372 | 5.8 | 66.1 | 23.1 | 10.8 | 18.1 | 18.9 |
| Almond shells (A) | 417 | 11.8 | 80.6 | 18.2 | 1.2 | 18.1 | 19.5 |
| Hazelnut shells (N) | 299 | 5.0 | 78.0 | 20.9 | 1.1 | 17.8 | 19.4 |
| Biomass | O2 for Combustion kg/kg | Air for Combustion kg/kg | H2O for Oxidation kg/kg |
|---|---|---|---|
| Wood Eucalyptus (WE) | 1.12 | 4.86 | 0.502 |
| Torriefied Eucalyptus (TE) | 1.25 | 5.42 | 0.721 |
| Torriefied Spruce (TS) | 1.59 | 6.87 | 0.80 |
| Hydrolytic Lignin (L) | 1.47 | 6.38 | 1.11 |
| Almond shells (A) | 1.33 | 5.75 | 0.93 |
| Hazelnut shells (N) | 1.40 | 6.08 | 1.06 |
| Phase | Reaction | Stoichiometry | Enthalpy |
|---|---|---|---|
| Pyrolysis | CHxOy ↔ H2 + CO + CH4 + CO2 + H2O + Tar + Cgraphite | ||
| Reduction | Bouduard reaction | C(graphite) + CO2 ↔ 2CO | ∆H = 172.6 kJ/mol |
| Water gas reaction | C(grafite) + H2O ↔ CO + H2 | ∆H = 131.4 kJ/mol | |
| Water Gas Shift | CO + H2O ↔ CO2 + H2 | ∆H = −41.2 kJ/mol | |
| C-Methanation | C + 2H2 ↔ CH4 | ∆H = −78.84 kJ/mol | |
| Steam reforming | CH4 + H2O ↔ CO2 + 3H2 | ∆H = 206 kJ/mol | |
| CO-Methanation | CO + 3 H2 ↔ CH4 + H2O | ∆H = −206 kJ/mol | |
| CO2-Methanation | CO2 + 4 H2 ↔ CH4 + 2 H2O | ∆H = −165 kJ/mol | |
| Oxydation | Combustion | C(graphite) + O2 ↔ CO2 | ∆H = −393.5 kJ/mol |
| Partial combustion | C(graphite) + ½ O2 ↔ CO | ∆H = −110.5 kJ/mol | |
| Tar decomposition | Reforming | CnHx + mH2O ↔ nCO +(m + x/2)H2 | |
| Thermal cracking | CnHx ↔ nC + (x/2)H2 | for n = 1, ∆H = 74.9 kJ/mol | |
| Experiment Code a | Biomass | Oxidant | Feeding Rate kg (dry)/h | Particle Residence Time, h | Air kg/h | O2 kg/h | ER (O2) kg/kg | Steam kg/h | ER (H2O) kg/kg | Average T in Bed, °C | Gas Residence Time b, s |
|---|---|---|---|---|---|---|---|---|---|---|---|
| WE1(25/24) | Eucal. wood | Air and steam | 15.0 | 0.89 | 18.0 | 0.0 | 0.25 | 3.5 | 0.24 | 702 | 5.51 |
| WE2(24/31) | Eucal. wood | Oxygen and steam | 15.0 | 0.89 | 0.0 | 4.0 | 0.24 | 4.5 | 0.31 | 742 | 10.4 |
| WE3(24/40) | Eucal. wood | Oxygen and steam | 15.0 | 0.89 | 0.0 | 4.0 | 0.24 | 5.8 | 0.40 | 736 | 9.34 |
| TE1(24/17) | Torrefied Eucal. | Air and steam | 18.8 | 1.09 | 24.0 | 0.0 | 0.24 | 3.7 | 0.17 | 705 | 3.93 |
| TE2(23/19) | Torrefied Eucal. | Oxygen and steam | 18.8 | 1.09 | 0.0 | 5.5 | 0.23 | 4.0 | 0.19 | 688 | 8.73 |
| TE3(23/24) | Torrefied Eucal. | Oxygen and steam | 18.8 | 1.09 | 0.0 | 5.5 | 0.23 | 5.0 | 0.24 | 642 | 8.22 |
| TS1(23) | Torrefied Spruce | Air | 9.4 | 1.43 | 14.0 | 0.0 | 0.23 | 0.0 | 0.00 | 788 | 7.92 |
| TS2(23/22) | Torrefied Spruce | Air and steam | 9.4 | 1.43 | 14.0 | 0.0 | 0.23 | 2.5 | 0.22 | 807 | 6.00 |
| TS3(23/26) | Torrefied Spruce | Air and steam | 9.4 | 1.43 | 14.0 | 0.0 | 0.23 | 3.0 | 0.26 | 773 | 6.55 |
| TS4(25/20) | Torrefied Spruce | Oxygen and steam | 12.2 | 1.10 | 0.0 | 4.0 | 0.25 | 3.0 | 0.20 | 829 | 12.4 |
| TS5(25/27) | Torrefied Spruce | Oxygen and steam | 12.2 | 1.10 | 0.0 | 4.0 | 0.25 | 4.0 | 0.27 | 810 | 10.7 |
| LA1(22/41) | Lignin | Air and steam | 18.8 | 1.66 | 26.5 | 0.0 | 0.22 | 8.5 | 0.41 | 611 | 3.2 |
| LA2(20/25) | Lignin | Air and steam | 14.7 | 2.12 | 19 | 0.0 | 0.20 | 4.0 | 0.25 | 658 | 3.5 |
| LAS(22) | Lignin | Air | 18.0 | 1.73 | 25.5 | 0.0 | 0.22 | 0 | 0 | 727 | 3.3 |
| LO1(22/30) | Lignin | Oxygen and steam | 17.0 | 1.83 | 0.0 | 5.5 | 0.22 | 5.5 | 0.30 | 627 | 6.4 |
| LO2(20/25) | Lignin | Oxygen and steam | 17.0 | 1.83 | 0.0 | 4.0 | 0.20 | 4.5 | 0.25 | 636 | 6.9 |
| LOS1(22) | Lignin | Oxygen | 17.0 | 1.83 | 0.0 | 5.5 | 0.22 | 0 | 0 | 686 | 7.9 |
| LO3(18/5) | Lignin | Oxygen and steam | 17.0 | 1.83 | 0.0 | 4.5 | 0.18 | 1.0 | 0.05 | 612 | 7.2 |
| LOS2(18) | Lignin | Oxygen | 17.0 | 1.83 | 0.0 | 4.5 | 0.18 | 0 | 0 | 712 | 7.7 |
| LO4(18/25) | Lignin | Oxygen and steam | 17.0 | 1.83 | 0.0 | 4.5 | 0.18 | 4.5 | 0.25 | 613 | 7.3 |
| LO5(18/13) | Lignin | Oxygen and steam | 17 | 1.8 | 0 | 4.5 | 0.18 | 2.5 | 0.13 | 657 | 7.1 |
| NA1(19/28) | Hazelnut shells | Air and steam | 16.3 | 1.52 | 18.9 | 0.0 | 0.19 | 4.80 | 0.28 | 703 | 4.72 |
| NA2(24/22) | Hazelnut shells | Air and steam | 20.4 | 1.22 | 29.3 | 0.0 | 0.24 | 4.80 | 0.22 | 760 | 3.31 |
| NA3(22/18) | Hazelnut shells | Air and steam | 20.4 | 1.22 | 27.1 | 0.0 | 0.22 | 4.00 | 0.18 | 713 | 3.60 |
| NO1(28/23) | Hazelnut shells | Oxygen and steam | 20.4 | 1.22 | 0.0 | 8.0 | 0.28 | 5.00 | 0.23 | 768 | 6.86 |
| NO2(28/28) | Hazelnut shells | Oxygen and steam | 20.4 | 1.22 | 0.0 | 8.0 | 0.28 | 6.00 | 0.28 | 714 | 6.67 |
| MAS1(24) | Almond shells | Air | 12.4 | 2.81 | 16.7 | 0.0 | 0.24 | 0.00 | 0.00 | 767 | 6.16 |
| MAS2(24) | Almond shells | Air | 21.2 | 1.64 | 28.8 | 0.0 | 0.24 | 0.00 | 0.00 | 761 | 3.59 |
| MA1(22/24) | Almond shells | Air and steam | 22.4 | 1.57 | 28.9 | 0.0 | 0.22 | 5.00 | 0.24 | 701 | 3.12 |
| MA2(24/28) | Almond shells | Air and steam | 21.2 | 1.57 | 29.8 | 0.0 | 0.24 | 5.50 | 0.28 | 741 | 2.82 |
| MA3(22/19) | Almond shells | Air and steam | 22.4 | 1.54 | 29.0 | 0.0 | 0.22 | 4.00 | 0.19 | 715 | 3.24 |
| MA4(24/25) | Almond shells | Air and steam | 22.1 | 1.57 | 31.0 | 0.0 | 0.24 | 5.20 | 0.25 | 758 | 2.74 |
| MA5(25/30) | Almond shells | Air and steam | 21.6 | 1.48 | 31.5 | 0.0 | 0.25 | 6.00 | 0.30 | 739 | 2.68 |
| MO1(23/28) | Almond shells | Oxygen and steam | 21.2 | 1.64 | 0.0 | 6.5 | 0.23 | 5.50 | 0.28 | 748 | 6.50 |
| MAO(27/23) | Almond shells | Enriched air and steam | 21.2 | 1.64 | 9.1 | 5.5 | 0.27 | 4.50 | 0.23 | 806 | 4.63 |
| Experiment Code | H2 g/kg | CO g/kg | CO2 g/kg | CnHm g/kg | Syngas a STP m3/kg | LHW MJ/m3 | Density kg/STPm3 | CGE% | Net CGE% | CLE% | Net CLE% | Plant Power b kWth |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WE1(25/24) | 24.3 | 565 | 318 | 19.1 | 1.96 | 5.79 | 1.12 | 57 | 55 | 29 | 28 | 47.3 |
| WE2(24/31) | 35.6 | 684 | 335 | 18.5 | 1.25 | 10.4 | 0.96 | 72 | 69 | 16 | 15 | 53.9 |
| WE3(24/40) | 29.9 | 481 | 449 | 28.7 | 1.23 | 9.89 | 1.02 | 59 | 56 | 27 | 26 | 50.6 |
| TE1(24/17) | 37.5 | 648 | 533 | 31.3 | 2.19 | 5.75 | 1.11 | 67 | 66 | 14 | 14 | 65.9 |
| TE2(23/19) | 40.3 | 892 | 326 | 31.9 | 1.3 | 11.1 | 0.95 | 82 | 80 | 4 | 3 | 75.7 |
| TE3(23/24) | 37.4 | 658 | 579 | 62.1 | 1.34 | 10.6 | 1.02 | 76 | 73 | 5 | 5 | 74.3 |
| TS1(23) | 20.2 | 650 | 436 | 74.0 | 2.09 | 5.35 | 1.19 | 67 | 67 | 4 | 4 | 29.1 |
| TS2(23/22) | 52.4 | 628 | 786 | 28.6 | 2.79 | 4.42 | 1.12 | 74 | 72 | 7 | 7 | 32.0 |
| TS3(23/26) | 51.5 | 576 | 854 | 54.1 | 2.62 | 4.84 | 1.12 | 78 | 75 | 4 | 4 | 32.9 |
| TS4(25/20) | 46.7 | 614 | 693 | 75.7 | 1.42 | 10.5 | 0.99 | 82 | 80 | 6 | 6 | 50.6 |
| TS5(25/27) | 51.3 | 671 | 555 | 58.8 | 1.5 | 10.7 | 0.92 | 84 | 81 | 11 | 11 | 54.1 |
| LA1(22/41) | 35 | 402 | 629 | 32.2 | 2.17 | 4.54 | 1.06 | 0.54 | 0.51 | 0.20 | 0.19 | 51 |
| LA2(20/25) | 32 | 372 | 657 | 33.3 | 2.32 | 4.00 | 1.09 | 0.51 | 0.48 | 0.21 | 0.20 | 38 |
| LAS(22) | 18 | 578 | 341 | 43.5 | 2.00 | 5.08 | 1.10 | 0.56 | 0.56 | 0.19 | 0.19 | 51 |
| LO1(22/30) | 49 | 330 | 868 | 65.4 | 1.54 | 8.13 | 0.95 | 0.69 | 0.66 | 0.11 | 0.10 | 59 |
| LO2(20/25) | 45 | 377 | 778 | 53.1 | 1.40 | 8.53 | 0.94 | 0.66 | 0.63 | 0.13 | 0.12 | 56 |
| LOS1(22) | 26 | 761 | 366 | 19.4 | 1.21 | 9.82 | 0.98 | 0.65 | 0.65 | 0.09 | 0.09 | 56 |
| LO3(18/5) | 45 | 550 | 607 | 38.0 | 1.43 | 8.97 | 0.91 | 0.71 | 0.70 | 0.11 | 0.10 | 61 |
| LOS2(18) | 31 | 629 | 510 | 38.6 | 1.25 | 9.56 | 0.99 | 0.64 | 0.64 | 0.09 | 0.09 | 55 |
| LO4(18/25) | 44 | 450 | 709 | 60.1 | 1.40 | 9.22 | 0.92 | 0.71 | 0.68 | 0.10 | 0.10 | 61 |
| LO5(18/13) | 35 | 842 | 187 | 28.7 | 1.30 | 10.9 | 0.9 | 0.78 | 0.77 | 0.10 | 0.09 | 67 |
| NA1(19/28) | 24.8 | 564 | 403 | 55.08 | 1.77 | 6.43 | 1.14 | 0.64 | 0.61 | 0.19 | 0.18 | 52 |
| NA2(24/22) | 25.8 | 673 | 342 | 38.66 | 1.88 | 6.37 | 0.95 | 0.66 | 0.64 | 0.25 | 0.24 | 68 |
| NA3(22/18) | 29.4 | 699 | 265 | 24.03 | 2.01 | 5.90 | 1.09 | 0.66 | 0.64 | 0.23 | 0.22 | 67 |
| NO1(28/23) | 36.1 | 744 | 352 | 32.96 | 1.23 | 11.0 | 0.92 | 0.76 | 0.73 | 0.17 | 0.17 | 77 |
| NO2(28/28) | 39.7 | 675 | 257 | 27.78 | 1.22 | 10.6 | 1.10 | 0.73 | 0.70 | 0.19 | 0.18 | 74 |
| MAS1(24) | 15.3 | 635 | 451 | 40.4 | 1.78 | 5.76 | 0.98 | 0.57 | 0.57 | 0.11 | 0.11 | 35 |
| MAS2(24) | 18.1 | 615 | 360 | 33.9 | 1.79 | 5.62 | 1.11 | 0.56 | 0.56 | 0.13 | 0.13 | 59 |
| MA1(22/24) | 27.6 | 508 | 306 | 22.2 | 1.71 | 5.59 | 1.19 | 0.53 | 0.51 | 0.27 | 0.27 | 59 |
| MA2(24/28) | 38.5 | 548 | 213 | 28.6 | 2.09 | 5.56 | 1.05 | 0.64 | 0.62 | 0.13 | 0.13 | 68 |
| MA3(22/19) | 25.9 | 577 | 371 | 23.8 | 1.61 | 6.32 | 1.18 | 0.56 | 0.55 | 0.28 | 0.27 | 63 |
| MA4(24/25) | 35.9 | 630 | 643 | 34.0 | 2.03 | 6.10 | 1.11 | 0.69 | 0.66 | 0.15 | 0.14 | 76 |
| MA5(25/30) | 40.0 | 564 | 273 | 25.6 | 2.10 | 5.62 | 1.09 | 0.65 | 0.63 | 0.11 | 0.11 | 71 |
| MO1(23/28) | 35.5 | 580 | 479 | 36.8 | 1.15 | 10.41 | 1.10 | 0.66 | 0.64 | 0.20 | 0.19 | 70 |
| MAO(27/23) | 25.7 | 704 | 595 | 31.3 | 1.39 | 8.46 | 1.10 | 0.65 | 0.63 | 0.19 | 0.18 | 69 |
| ER (O2) | ER (H2O) | H2 g/kg | CO g/kg | CO2 g/kg | Syngas m3/kg | H2/CO | |
|---|---|---|---|---|---|---|---|
| TS1 | 0.23 | 0 | 20 | 650 | 436 | 2.09 | 0.44 |
| TS3 | 0.23 | 0.26 | 52 | 576 | 854 | 2.62 | 1.25 |
| LAS(22) | 0.22 | 0 | 18 | 578 | 341 | 2.00 | 0.43 |
| LA1(22/41) | 0.22 | 0.41 | 35 | 402 | 629 | 2.17 | 1.21 |
| LOS1(22) | 0.22 | 0 | 26 | 761 | 366 | 1.21 | 0.49 |
| LO1(22/30) | 0.22 | 0.30 | 49 | 330 | 868 | 1.54 | 2.08 |
| LOS2(18) | 0.18 | 0 | 31 | 629 | 510 | 1.25 | 0.66 |
| LO4(18/25) | 0.18 | 0.25 | 44 | 450 | 709 | 1.40 | 1.38 |
| MAS1(24) | 0.24 | 0 | 15 | 635 | 451 | 1.78 | 0.34 |
| MA4(24/25) | 0.24 | 0.25 | 36 | 630 | 643 | 2.03 | 0.80 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cerone, N.; Zimbardi, F. Effects of Oxygen and Steam Equivalence Ratios on Updraft Gasification of Biomass. Energies 2021, 14, 2675. https://doi.org/10.3390/en14092675
Cerone N, Zimbardi F. Effects of Oxygen and Steam Equivalence Ratios on Updraft Gasification of Biomass. Energies. 2021; 14(9):2675. https://doi.org/10.3390/en14092675
Chicago/Turabian StyleCerone, Nadia, and Francesco Zimbardi. 2021. "Effects of Oxygen and Steam Equivalence Ratios on Updraft Gasification of Biomass" Energies 14, no. 9: 2675. https://doi.org/10.3390/en14092675
APA StyleCerone, N., & Zimbardi, F. (2021). Effects of Oxygen and Steam Equivalence Ratios on Updraft Gasification of Biomass. Energies, 14(9), 2675. https://doi.org/10.3390/en14092675






