Hydrogen Purification Performance Optimization of Vacuum Pressure Swing Adsorption on Different Activated Carbons
Abstract
1. Introduction
2. Heat and Mass Transfer and Adsorption Model
3. Model Parameters and Validation
3.1. Adsorption Isotherms of AC5-KS and R2030
3.2. Breakthrough Curves Validation of AC5-KS and R2030 Adsorption Beds
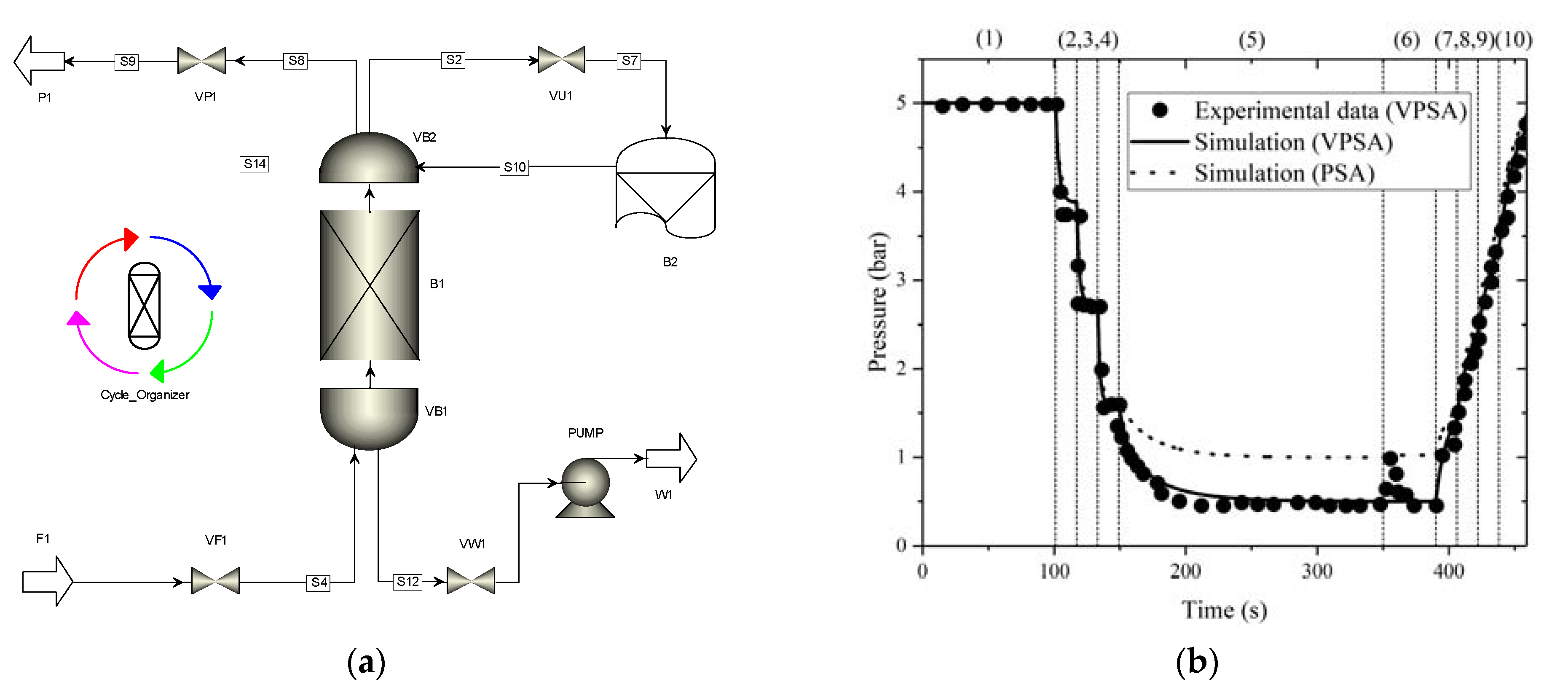
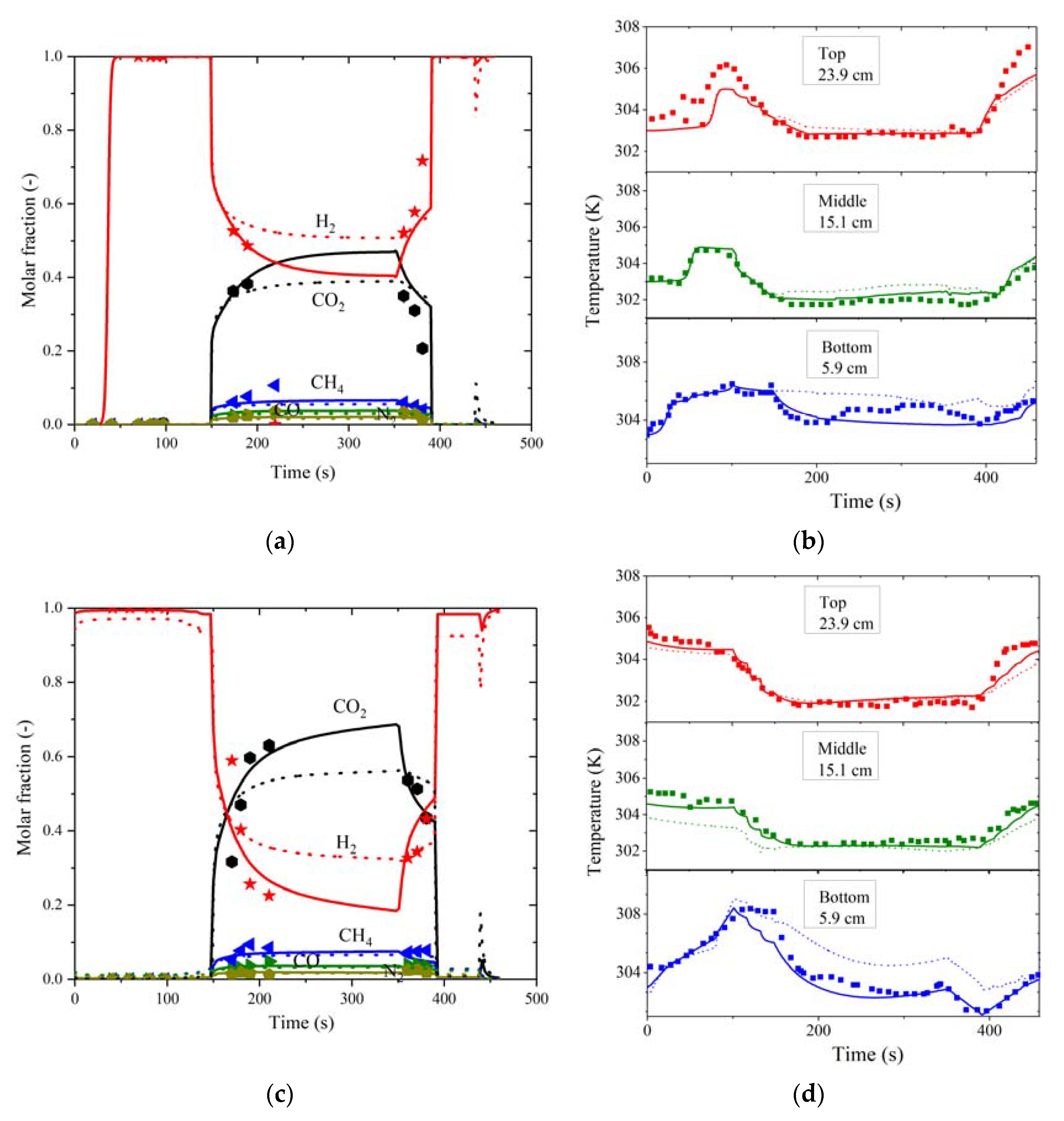
3.3. VPSA and PSA Cycle Validation and Comparison in AC5-KS
4. Parametric Study and Optimization of Hydrogen Purification
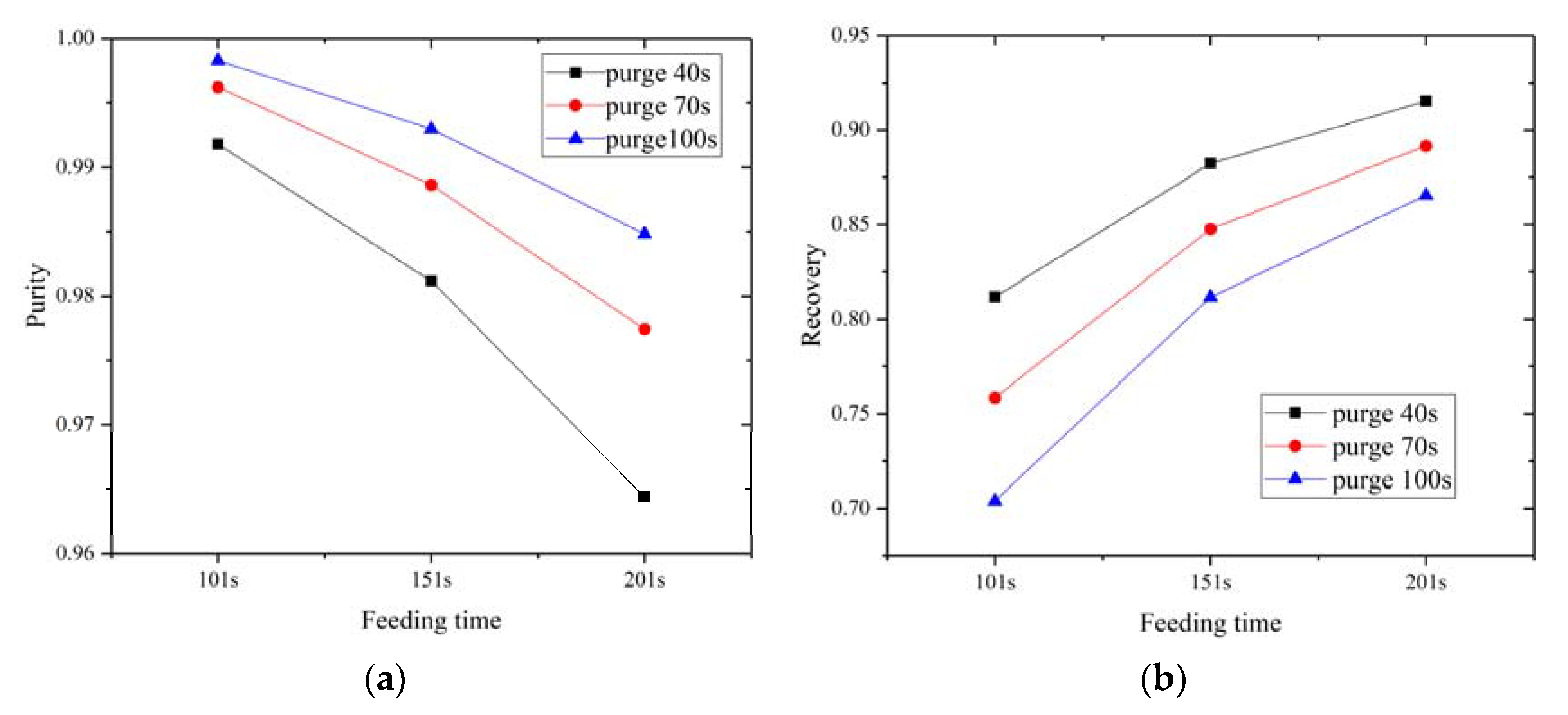
4.1. Parametric Study of Hydrogen Purification
4.2. Optimization of Hydrogen Purification Performance Based on Interior Point Method
5. Conclusions
- AC5-KS had greater adsorption capability than R2030 when purifying hydrogen from multi-component gas (H2/CO2/CH4/CO/N2 = 0.79/0.17/0.021/0.012/0.007). AC5-KS could adsorb 17.80–25.75% more CO2 than R2030 when the adsorption pressure was 5 bar and the temperature was 303–323 K.
- The hydrogen purification performance of VPSA was better than that of PSA. Therefore, the parameters of the VPSA system for hydrogen purification was further studied and optimized.
- Results show that feeding time has a negative effect on hydrogen purity and has a positive effect on hydrogen recovery, while purging time has a positive effect on hydrogen purity and a negative effect on hydrogen recovery.
- The performance of hydrogen purification in the VPSA process was optimized by the interior point method combined with an ANN model. The optimal results show that the maximum hydrogen recovery reached 88.65% when feeding time was set to 223 s and purging time was set to 96 s, and the maximum hydrogen purity reached 99.33% when feeding time was set to 100 s and purging time was set to 45 s.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
| ap | specific surface area of adsorbent, m2/m3 |
| bi | Langmuir constant, 1/bar |
| ci | molar concentration of component i, mol/m3 |
| cg | molar concentration of mixture, mol/m3 |
| Cpai | specific heat capacity of adsorbed phase, J/mol/K |
| Cpg | specific gas phase heat capacity at constant pressure, J/mol/K |
| cps | specific heat capacity of adsorbent, J/kg/K |
| cpw | specific heat capacity of column wall, J/kg/K |
| DL | axial dispersion coefficient, W/m2/K |
| hgs | heat transfer coefficient between gas and solid phase, W/m2/K |
| hin | heat transfer coefficient between gas phase and bed wall, W/m2/K |
| hout | heat transfer coefficient between wall and environment, W/m2/K |
| ∆Hi | heat of adsorption of component i, J/mol |
| k1i | parameter of nis in Equation (6), mol/kg |
| k2i | parameter of nis in Equation (6), mol/kg/K |
| k3i | parameter of bi in Equation (6), 1/bar |
| k4i | parameter of bi in Equation (6), K |
| KLg | axial gas phase thermal conductivity, W/m/K |
| KLs | axial solid phase thermal conductivity, W/m/K |
| Kw | axial thermal conductivity for wall, W/m/K |
| M | molecular weight of mixture, kg/mol |
| Mi | molecular weight of component i, kg/mol |
| ni* | equilibrium adsorption amount, mol/kg |
| ni | dynamic adsorption amount of ith component, mol/kg |
| nis | saturation adsorption amount of ith component, mol/kg |
| p | pressure, bar |
| R | universal gas constant, 8.314 J/mol/K |
| Rin | inner radius of adsorption column, m |
| Rout | outer radius of adsorption column, m |
| Rp | particle radius, m |
| t | time, s |
| T | temperature of adsorption bed, K |
| Tg | gas phase temperature, K |
| Ts | solid phase temperature, K |
| Tw | wall temperature, K |
| Tf | ambient temperature, K |
| wi | mass transfer coefficient of component i, 1/s |
| yi | molar fraction of component i in gas phase |
| z | axial position in adsorbent bed, m |
| Greek symbols | |
| εb | bed porosity |
| νg | superficial velocity, m/s |
| μ | dynamic viscosity, N∙s/m2 |
| ρg | gas phase density, kg/m3 |
| ρp | adsorbent density, kg/m3 |
| ρs | solid phase density, kg/m3 |
| ρw | wall density, kg/m3 |
References
- Winter, C.J. Hydrogen energy—Abundant, efficient, clean: A debate over the energy-system-of-change. Int. J. Hydrogen Energy 2009, 34, S1–S52. [Google Scholar] [CrossRef]
- Yáñez, M.; Relvas, F.; Ortiz, A.; Gorri, D.; Mendes, A.; Ortiz, I. PSA purification of waste hydrogen from ammonia plants to fuel cell grade. Sep. Purif. Technol. 2020, 240, 116334. [Google Scholar] [CrossRef]
- Golmakani, A.; Nabavi, S.A.; Manovi, V. Effect of impurities on ultra-pure hydrogen production by pressure vacuum swing adsorption. J. Ind. Eng. Chem. 2020, 82, 278–289. [Google Scholar] [CrossRef]
- da Silva Veras, T.; Mozer, T.S.; da Costa Rubim Messeder dos Santos, D.; da Silva César, A. Hydrogen: Trends, production and characterization of the main process worldwide. Int. J. Hydrogen Energy 2017, 42, 2018–2033. [Google Scholar] [CrossRef]
- Elam, C.C.; Evans, R.J. Overview of hydrogen production. Prepr. Pap. Am. Chem. Soc. Div. Fuel Chem. 2004, 49, 481–482. [Google Scholar]
- Sircar, S.; Golden, T.C. Purification of hydrogen by pressure swing adsorption. Sep. Sci. Technol. 2000, 35, 667–687. [Google Scholar] [CrossRef]
- Song, C.F.; Liu, Q.L.; Ji, N.; Kansha, Y.; Tsutsumi, A. Optimization of steam methane reforming coupled with pressure swing. Appl. Energy 2015, 154, 392–401. [Google Scholar] [CrossRef]
- Casas, N.; Schell, J.; Pini, R.; Mazzotti, M. Fixed bed adsorption of CO2/H2 mixtures on activated carbon: Experiments and modeling. Adsorption 2012, 18, 143–161. [Google Scholar] [CrossRef]
- Yang, J.Y.; Lee, C.H. Separation of hydrogen mixtures by a two-bed pressure swing adsorption process using zeolite 5A. Ind. Eng. Chem. Res. 1997, 36, 2789–2798. [Google Scholar] [CrossRef]
- Park, J.Y.; Yang, S.I.; Choi, D.Y. Adsorption dynamics of H2/CO2, H2/CO and H2/CH4 mixtures in Li-X Zeolite bed. Korean Chem. Eng. Res. 2008, 5, 783–791. [Google Scholar]
- You, Y.W.; Lee, D.G.; Kim, K.H.; Oh, M.; Lee, C.H. Layered two- and four-bed PSA processes for H2 recovery from coal gas. Chem. Eng. Sci. 2012, 68, 413–423. [Google Scholar]
- Olajossy, A.; Gawdzik, A.; Budner, Z.; Dula, J. Methane separation from coal mine methane gas by vacuum pressure swing adsorption. Chem. Eng. Res. Des. 2003, 81, 474–482. [Google Scholar] [CrossRef]
- Yang, R.T.; Doong, S.J. Gas separation by pressure swing adsorption: A pore-diffusion model for bulk separation. AlChE J. 1985, 31, 1829–1842. [Google Scholar] [CrossRef]
- Malek, A.; Farooq, S. Study of a six-bed pressure swing adsorption process. AlChE J. 1997, 43, 2509–2523. [Google Scholar] [CrossRef]
- Jiang, L.; Fox, V.G.; Biegler, L.T. Simulation and optimal design of multiple-bed pressure swing adsorption systems. AlChE J. 2004, 50, 2904–2917. [Google Scholar] [CrossRef]
- Casas, N.; Schell, J.; Joss, L.; Mazzotti, M. A parametric study of a PSA process for pre-combustion CO2 capture. Sep. Purif. Technol. 2013, 104, 183–192. [Google Scholar] [CrossRef]
- Qasem, N.A.A.; Ben-Mansour, R. Energy and productivity efficient vacuum pressure swing adsorption process to separate CO2 from CO2/N2 mixture using Mg-MOF-74: A CFD simulation. Appl. Energy 2018, 209, 190–202. [Google Scholar] [CrossRef]
- Moon, D.K.; Lee, D.G.; Lee, C.H. H2 pressure swing adsorption for high pressure syngas from an integrated gasification combined cycle with a carbon capture process. Appl. Energy 2016, 183, 760–774. [Google Scholar] [CrossRef]
- Brea, P.; Delgado, J.A.; Águeda, V.I.; Uguina, M.A. Comparison between MOF UTSA-16 and BPL activated carbon in hydrogen purification by PSA. Chem. Eng. J. 2018, 355, 279–289. [Google Scholar] [CrossRef]
- Delgado, J.A.; Águeda, V.I.; Uguina, M.A.; Brea, P.; Grande, C.A. Comparison and evaluation of agglomerated MOFs in biohydrogen purification by means of pressure swing adsorption (PSA). Chem. Eng. J. 2017, 326, 117–129. [Google Scholar] [CrossRef]
- Lopes, F.V.S.; Grande, C.A.; Alírio, E.R. Fast-cycling VPSA for hydrogen purification. Fuel 2012, 93, 510–523. [Google Scholar] [CrossRef]
- Xiao, J.S.; Li, R.P.; Bénard, P.; Chahine, R. Heat and Mass Transfer Model of Multicomponent Adsorption System for Hydrogen Purification. Int. J. Hydrogen Energy 2015, 40, 4794–4803. [Google Scholar] [CrossRef]
- Xiao, J.S.; Peng, Y.Z.; Bénard, P.; Chahine, R. Thermal Effects on Breakthrough Curves of Pressure Swing Adsorption for Hydrogen Purification. Int. J. Hydrogen Energy 2016, 41, 8236–8245. [Google Scholar] [CrossRef]
- Xiao, J.S.; Fang, L.; Bénard, P.; Chahine, R. Parametric study of pressure swing adsorption cycle for hydrogen purification using Cu-BTC. Int. J. Hydrogen Energy 2018, 43, 13962–13974. [Google Scholar] [CrossRef]
- Mondal, M.; Datta, A. Energy transfer in hydrogen separation from syngas using pressure swing adsorption (PSA) process: A thermodynamic model. Int. J. Energy Res. 2016, 41, 448–458. [Google Scholar] [CrossRef]
- Choi, W.K.; Kwon, T.I.; Yeo, Y.K.; Lee, H.; Song, H.K.; Na, B.K. Optimal Operation of the Pressure Swing Adsorption (PSA) Process for CO2 Recover. Korean J. Chem. Eng. 2003, 20, 617–623. [Google Scholar] [CrossRef]
- Huang, Q.L.; Malekian, A.; Eić, M. Optimization of PSA process for producing enriched hydrogen from plasma reactor gas. Chem. Eng. Sci. 2008, 62, 22–31. [Google Scholar] [CrossRef]
- Oliveira, L.M.; Koivisto, H.; Iwakiri, I.G.; Loureiro, J.M.; Ribeiro, A.M.; Nogueira, I.B. Modelling of a Pressure Swing Adsorption Unit by Deep Learning and Artificial Intelligence Tools. Chem. Eng. Sci. 2020, 224, 115801. [Google Scholar] [CrossRef]
- Yu, X.; Shen, Y.; Guan, Z.; Zhang, D.; Tang, Z.; Li, W. Multi-objective optimization of ANN-based PSA model for hydrogen purification from steam-methane reforming gas. Int. J. Hydrogen Energy 2021, 46, 11740–11755. [Google Scholar] [CrossRef]
- Lopes, F.V.S.; Grande, C.A.; Ribeiro, A.M.; Oliveira, E.L.G.; Loureiro, J.M.; Rodrigues, A.E. Enhancing capacity of activated carbons for hydrogen production. Int. Eng. Chem. Res. 2009, 48, 3978–3990. [Google Scholar] [CrossRef]
- Tao, W.; Ma, S.; Xiao, J.S.; Bénard, P.; Chahine, R. Simulation and optimization for hydrogen purification performance of vacuum pressure swing adsorption. Energy Procedia 2019, 158, 1917–1923. [Google Scholar] [CrossRef]
- Hermes, R.; Amaro, G.; Frederico, W. Methane/nitrogen separation through pressure swing adsorption process from nitrogen-rich streams. Chem. Eng. Process. 2016, 103, 70–79. [Google Scholar]
- Park, Y.; Moon, D.K.; Kim, Y.H.; Ahn, H.; Lee, C.H. Adsorption isotherms of CO2, CO, N2, CH4, Ar and H2 on activated carbon and zeolite LiX up to 1.0MPa. Adsorption 2014, 20, 631–647. [Google Scholar] [CrossRef]
- Lopes, F.V.S.; Grande, C.A.; Rodrigues, A.E. New Pressure Swing Adsorption Cycles for Hydrogen Purification from Steam Reforming Off-Gases; University of Porto: Porto, Portugal, 1999. [Google Scholar]
- Lopes, F.V.S.; Grande, C.A.; Rodrigues, A.E. Activated carbon for hydrogen purification by pressure swing adsorption: Multicomponent breakthrough curves and PSA performance. Chem. Eng. Sci. 2011, 66, 303–317. [Google Scholar] [CrossRef]
- Xiao, J.S.; Li, C.L.; Fang, L.; Böwer, P.; Wark, M.; Bénard, P.; Chahine, R. Machine learning–based optimization for hydrogen purification performance of layered bed pressure swing adsorption. Int. J. Energy Res. 2020, 44, 4475–4492. [Google Scholar] [CrossRef]
- Ito, Y. Approximation of continuous functions on Rd by linear combinations of shifted rotations of a sigmoid function with and without scaling. Neural Netw. 1992, 5, 105–115. [Google Scholar] [CrossRef]
- Ma, S.; Tong, L.; Ye, F.; Xiao, J.S.; Bénard, P.; Chahine, R. Hydrogen purification layered bed optimization based on artificial neural network prediction of breakthrough curves. Int. J. Hydrogen Energy 2019, 44, 5324–5333. [Google Scholar] [CrossRef]
- Ye, F.; Ma, S.; Tong, L.; Xiao, J.S.; Bénard, P.; Chahine, R. Artificial neural network based optimization for hydrogen purification performance of pressure swing adsorption. Int. J. Hydrogen Energy 2019, 44, 5334–5344. [Google Scholar] [CrossRef]



| Components | k1i (mol/kg) | k2i × 102 (mol/kg/K) | k3i × 105 (1/bar) | k4i (K) | ∆Hi (kJ/mol) |
|---|---|---|---|---|---|
| AC5-KS | - | - | - | - | - |
| CO | 5.416 | −0.4595 | 5.94 | 2359 | 23.23 |
| CO2 | 13.78 | −1.635 | 4.733 | 2735 | 28.02 |
| H2 | −7.892 | 3.259 | 0.9998 | 2300 | 7.26 |
| N2 | 0.4764 | 1.085 | 2.098 | 2560 | 18.27 |
| CH4 | 6.217 | −0.4504 | 1.88 | 2931 | 23.18 |
| R2030 | - | - | - | - | - |
| CO | 6.225 | −1.27 | 35.66 | 2142 | 19.100 |
| CO2 | 13.94 | −2.316 | 35.46 | 2195 | 27.870 |
| H2 | −6.792 | 2.551 | 1.083 | 2438 | 3.192 |
| N2 | 0.000005328 | 0.9999 | 1.048 | 2800 | 11.834 |
| CH4 | 6.973 | −0.7784 | 21.86 | 2132 | 17.652 |
| Adsorbents | AC5-KS | R2030 | Adsorption Bed | Value |
|---|---|---|---|---|
| Adsorbent density, ρp (kg/m3) | 786 | 874 | Internal bed radius, Rin (m) | 0.021 |
| Solid density, ρs (kg/m3) | 2110 | 2162 | Bed length, L (m) | 0.2456 |
| Particle porosity, εp | 0.627 | 0.60 | Bed porosity, εb | 0.404 |
| Particle specific heat, cps (J/kg/K) | 1046 | 880 | Wall density, ρw (kg/m3) | 7870 |
| Particle radius, Rp (m) | 0.00145 | 0.00117 | Wall specific heat, cpw (J/kg/K) | 142 |
| Gas phase thermal conductivity, KLg (W/m/K) | 0.1083 | 0.1083 | Wall thermal conductivity, Kw (W/m/K) | 400.9 |
| Solid phase thermal conductivity, KLs (W/m/K) | 20.58 | 20.58 | Heat transfer coefficient, hin (W/m2/K) | 55 |
| Heat transfer coefficient, hgs (W/m2/K) | 130 | 130 | Heat transfer coefficient, hout (W/m2/K) | 50 |
| Mixture Composition | Adsorption Pressure (bar) | Feed Temperature (K) | Feed Flow Rate (298 K, 1 bar) (m3s−1) | Initial Gas | Ambient Temperature (K) |
|---|---|---|---|---|---|
| N2/CO/CO2/CH4/H2 = 0.007/0.012/0.021/0.23/0.73 | 5 | 323 | 5 × 10−5 | He | 303 |
| Cycle | A0 | A1 | A2 | A3 | A4 | B0 | B1 | B2 | B3 | B4 | C0 | C1 | C2 | C3 | C4 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| tFeed (s) | 101 | 101 | 101 | 101 | 101 | 151 | 151 | 151 | 151 | 151 | 201 | 201 | 201 | 201 | 201 |
| tPurge (s) | 40 | 70 | 100 | 130 | 160 | 40 | 70 | 100 | 130 | 160 | 40 | 70 | 100 | 130 | 160 |
| Feed temperature: 303 K, Ambient temperature: 303 K, Flow rate: 5 × 10−5 m3/s (298 K 1 bar) | |||||||||||||||
| phigh = 5 bar, plow = 0.5 bar, pinter = 3.875 bar, tED1 = tED2 = tED3 = tEP3 = tEP2 = tEP1 = 16 s, tBlow down = 201 s, tPress = 21 s | |||||||||||||||
| Optimization Target | Optimal Parameters | ANN Prediction | Aspen Confirmation |
|---|---|---|---|
| Max | 223, 96 | 0.9800, 0.8865 | 0.9791, 0.8844 |
| Max | 100, 45 | 0.9933, 0.8000 | 0.9929, 0.8009 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, J.; Mei, A.; Tao, W.; Ma, S.; Bénard, P.; Chahine, R. Hydrogen Purification Performance Optimization of Vacuum Pressure Swing Adsorption on Different Activated Carbons. Energies 2021, 14, 2450. https://doi.org/10.3390/en14092450
Xiao J, Mei A, Tao W, Ma S, Bénard P, Chahine R. Hydrogen Purification Performance Optimization of Vacuum Pressure Swing Adsorption on Different Activated Carbons. Energies. 2021; 14(9):2450. https://doi.org/10.3390/en14092450
Chicago/Turabian StyleXiao, Jinsheng, Ang Mei, Wei Tao, Shuo Ma, Pierre Bénard, and Richard Chahine. 2021. "Hydrogen Purification Performance Optimization of Vacuum Pressure Swing Adsorption on Different Activated Carbons" Energies 14, no. 9: 2450. https://doi.org/10.3390/en14092450
APA StyleXiao, J., Mei, A., Tao, W., Ma, S., Bénard, P., & Chahine, R. (2021). Hydrogen Purification Performance Optimization of Vacuum Pressure Swing Adsorption on Different Activated Carbons. Energies, 14(9), 2450. https://doi.org/10.3390/en14092450







