Hydrogen Yield from CO2 Reforming of Methane: Impact of La2O3 Doping on Supported Ni Catalysts
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Catalyst Preparation
2.3. Catalyst Characterization and Activity
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Q.; Chen, X.; Jha, A.N.; Rogers, H. Natural gas from shale formation—The evolution, evidences and challenges of shale gas revolution in United States. Renew. Sustain. Energy Rev. 2014, 30, 1–28. [Google Scholar] [CrossRef]
- Montgomery, C.T.; Smith, M.B. Hydraulic Fracturing: History of an Enduring Technology. J. Pet. Technol. 2010, 62, 26–40. [Google Scholar] [CrossRef]
- Themelis, N.J.; Ulloa, P.A. Methane generation in landfills. Renew. Energy 2007, 32, 1243–1257. [Google Scholar] [CrossRef]
- Izquierdo, U.; Barrio, V.; Requies, J.; Cambra, J.; Güemez, M.; Arias, P. Tri-reforming: A new biogas process for synthesis gas and hydrogen production. Int. J. Hydrog. Energy 2013, 38, 7623–7631. [Google Scholar] [CrossRef]
- Al-Fatesh, A.S.; Kumar, R.; Kasim, S.O.; Ibrahim, A.A.; Fakeeha, A.H.; Abasaeed, A.E.; Alrasheed, R.; Bagabas, A.; Chaudhary, M.L.; Frusteri, F.; et al. The effect of modifier identity on the performance of Ni-based catalyst supported on γ-Al2O3 in dry reforming of methane. Catal. Today 2020, 348, 236–242. [Google Scholar] [CrossRef]
- Ibrahim, A.A.; Al-Fatesh, A.S.; Khan, W.U.; Kasim, S.O.; Abasaeed, A.E.; Fakeeha, A.H.; Bonura, G.; Frusteri, F. Enhanced coke suppression by using phosphate-zirconia supported nickel catalysts under dry methane reforming conditions. Int. J. Hydrog. Energy 2019, 44, 27784–27794. [Google Scholar] [CrossRef]
- Al-Fatesh, A.S.; Abu-Dahrieh, J.K.; Atia, H.; Armbruster, U.; Ibrahim, A.A.; Khan, W.U.; Abasaeed, A.E.; Fakeeha, A.H. Effect of pre-treatment and calcination temperature on Al2O3-ZrO2 supported Ni-Co catalysts for dry reforming of methane. Int. J. Hydrog. Energy 2019, 44, 21546–21558. [Google Scholar] [CrossRef]
- Zhang, S.; Muratsugu, S.; Ishiguro, N.; Tada, M. Ceria-Doped Ni/SBA-16 Catalysts for Dry Reforming of Methane. ACS Catal. 2013, 3, 1855–1864. [Google Scholar] [CrossRef]
- Boyano, A.; Morosuk, T.; Blanco-Marigorta, A.; Tsatsaronis, G. Conventional and advanced exergoenvironmental analysis of a steam methane reforming reactor for hydrogen production. J. Clean. Prod. 2012, 20, 152–160. [Google Scholar] [CrossRef]
- Kim, A.R.; Lee, H.Y.; Cho, J.M.; Choi, J.-H.; Bae, J.W. Ni/M-Al2O3 (M=Sm, Ce or Mg) for combined steam and CO2 reforming of CH4 from coke oven gas. J. CO2 Util. 2017, 21, 211–218. [Google Scholar] [CrossRef]
- Lougou, B.G.; Shuai, Y.; Chaffa, G.; Xing, H.; Tan, H.; Du, H. Analysis of CO2 utilization into synthesis gas based on solar thermochemical CH4-reforming. J. Energy Chem. 2019, 28, 61–72. [Google Scholar] [CrossRef]
- Wu, Z.; Yang, B.; Miao, S.; Liu, W.; Xie, J.; Lee, S.; Pellin, M.J.; Xiao, D.; Su, D.; Ma, D. Lattice Strained Ni-Co alloy as a High-Performance Catalyst for Catalytic Dry Reforming of Methane. ACS Catal. 2019, 9, 2693–2700. [Google Scholar] [CrossRef]
- Arora, S.; Prasad, R. An overview on dry reforming of methane: Strategies to reduce carbonaceous deactivation of catalysts. RSC Adv. 2016, 6, 108668–108688. [Google Scholar] [CrossRef]
- Varga, E.; Pusztai, P.; Steinrück, H.-P.; Óvári, L.; Papp, C.; Kónya, Z.; Oszkó, A.; Erdőhelyi, A.; Kiss, J. Probing the interaction of Rh, Co and bimetallic Rh–Co nanoparticles with the CeO2 support: Catalytic materials for alternative energy generation. Phys. Chem. Chem. Phys. 2015, 17, 27154–27166. [Google Scholar] [CrossRef]
- Pakhare, D.; Spivey, J. A review of dry (CO2) reforming of methane over noble metal catalysts. Chem. Soc. Rev. 2014, 43, 7813–7837. [Google Scholar] [CrossRef] [PubMed]
- Damyanova, S.; Shtereva, I.; Pawelec, B.; Mihaylov, L.; Fierro, J.L.G. Characterization of none and yttrium-modified Ni-based catalysts for dry reforming of methane. Appl. Catal. B Environ. 2020, 278, 119335. [Google Scholar] [CrossRef]
- Jalali, R.; Rezaei, M.; Nematollahi, B.; Baghalha, M. Preparation of Ni/MeAl2O4-MgAl2O4 (Me=Fe, Co, Ni, Cu, Zn, Mg) nanocatalysts for the syngas production via combined dry reforming and partial oxidation of methane. Renew. Energy 2020, 149, 1053–1067. [Google Scholar] [CrossRef]
- Liu, C.-J.; Ye, J.; Jiang, J.; Pan, Y. Progresses in the Preparation of Coke Resistant Ni-based Catalyst for Steam and CO2 Reforming of Methane. ChemCatChem 2011, 3, 529–541. [Google Scholar] [CrossRef]
- Wang, N.; Shen, K.; Yu, X.; Qian, W.; Chu, W. Preparation and characterization of a plasma treated NiMgSBA-15 catalyst for methane reforming with CO2 to produce syngas. Catal. Sci. Technol. 2013, 3, 2278–2287. [Google Scholar] [CrossRef]
- Bradford, M.C.; Vannice, M.A. The role of metal–support interactions in CO2 reforming of CH4. Catal. Today 1999, 50, 87–96. [Google Scholar] [CrossRef]
- Tang, M.; Liu, K.; Roddick, D.M.; Fan, M. Enhanced lattice oxygen reactivity over Fe2O3/Al2O3 redox catalyst for chemical-looping dry (CO2) reforming of CH4: Synergistic La-Ce effect. J. Catal. 2018, 368, 38–52. [Google Scholar] [CrossRef]
- Faria, E.; Neto, R.; Colman, R.; Noronha, F. Hydrogen production through CO2 reforming of methane over Ni/CeZrO2/Al2O3 catalysts. Catal. Today 2014, 228, 138–144. [Google Scholar] [CrossRef]
- Stagg-Williams, S.M.; Noronha, F.B.; Fendley, G.; Resasco, D.E. CO2 Reforming of CH4 over Pt/ZrO2 Catalysts Promoted with La and Ce Oxides. J. Catal. 2000, 194, 240–249. [Google Scholar] [CrossRef]
- Li, Z.-X.; Shi, F.-B.; Li, L.-L.; Zhang, T.; Yan, C.-H. A facile route to ordered mesoporous-alumina-supported catalysts, and their catalytic activities for CO oxidation. Phys. Chem. Chem. Phys. 2010, 13, 2488–2491. [Google Scholar] [CrossRef] [PubMed]
- Dehimi, L.; Benguerba, Y.; Virginie, M.; Hijazi, H. Microkinetic modelling of methane dry reforming over Ni/Al2O3 catalyst. Int. J. Hydrog. Energy 2017, 42, 18930–18940. [Google Scholar] [CrossRef]
- Lašič Jurković, D.; Liu, J.-L.; Pohar, A.; Likozar, B. Methane Dry Reforming over Ni/Al2O3 Catalyst in Spark Plasma Reactor: Linking Computational Fluid Dynamics (CFD) with Reaction Kinetic Modelling. Catal. Today 2021, 362, 11–21. [Google Scholar] [CrossRef]
- Bian, Z.; Zhong, W.; Yu, Y.; Wang, Z.; Jiang, B.; Kawi, S. Dry reforming of methane on Ni/mesoporous-Al2O3 catalysts: Effect of calcination temperature. Int. J. Hydrog. Energy 2021. [Google Scholar] [CrossRef]
- Newnham, J.; Mantri, K.; Amin, M.H.; Tardio, J.; Bhargava, S.K. Highly stable and active Ni-mesoporous alumina catalysts for dry reforming of methane. Int. J. Hydrog. Energy 2012, 37, 1454–1464. [Google Scholar] [CrossRef]
- Montoya, J.; Romero-Pascual, E.; Gimon, C.; Del Angel, P.; Monzón, A. Methane reforming with CO2 over Ni/ZrO2–CeO2 catalysts prepared by sol–gel. Catal. Today 2000, 63, 71–85. [Google Scholar] [CrossRef]
- Li, W.; Zhao, Z.; Jiao, Y. Dry reforming of methane towards CO-rich hydrogen production over robust supported Ni catalyst on hierarchically structured monoclinic zirconia nanosheets. Int. J. Hydrog. Energy 2016, 41, 17907–17921. [Google Scholar] [CrossRef]
- Jang, J.T.; Yoon, K.J.; Bae, J.W.; Han, G.Y. Cyclic production of syngas and hydrogen through methane-reforming and water-splitting by using ceria–zirconia solid solutions in a solar volumetric receiver–reactor. Sol. Energy 2014, 109, 70–81. [Google Scholar] [CrossRef]
- Hu, X.; Jia, X.; Zhang, X.; Liu, Y.; Liu, C.-J. Improvement in the activity of Ni/ZrO2 by cold plasma decomposition for dry reforming of methane. Catal. Commun. 2019, 128, 105720. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, J.; Zhou, Z.; Chen, S.; Zhang, T.; Song, F.; Zhang, Q.; Tsubaki, N.; Tan, Y.; Han, Y. Effects of the surface adsorbed oxygen species tuned by rare-earth metal doping on dry reforming of methane over Ni/ZrO2 catalyst. Appl. Catal. B Environ. 2020, 264, 118522. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, B.; Zhu, C.; Tian, Y. Preparation of La2NiO4/ZSM-5 catalyst and catalytic performance in CO2/CH4 reforming to syngas. Appl. Catal. A Gen. 2005, 292, 138–143. [Google Scholar] [CrossRef]
- Horváth, A.; Stefler, G.; Geszti, O.; Kienneman, A.; Pietraszek, A.; Guczi, L. Methane dry reforming with CO2 on CeZr-oxide supported Ni, NiRh and NiCo catalysts prepared by sol–gel technique: Relationship between activity and coke formation. Catal. Today 2011, 169, 102–111. [Google Scholar] [CrossRef]
- Tran, N.T.; Van Le, Q.; Van Cuong, N.; Nguyen, T.D.; Phuc, N.H.H.; Phuong, P.T.; Monir, M.U.; Aziz, A.A.; Truong, Q.D.; Abidin, S.Z.; et al. La-doped cobalt supported on mesoporous alumina catalysts for improved methane dry reforming and coke mitigation. J. Energy Inst. 2020, 93, 1571–1580. [Google Scholar] [CrossRef]
- Liu, H.; Hadjltaief, H.B.; Benzina, M.; Gálvez, M.E.; Da Costa, P. Natural clay based nickel catalysts for dry reforming of methane: On the effect of support promotion (La, Al, Mn). Int. J. Hydrog. Energy 2019, 44, 246–255. [Google Scholar] [CrossRef]
- Yabe, T.; Mitarai, K.; Oshima, K.; Ogo, S.; Sekine, Y. Low-temperature dry reforming of methane to produce syngas in an electric field over La-doped Ni/ZrO2 catalysts. Fuel Process. Technol. 2017, 158, 96–103. [Google Scholar] [CrossRef]
- Al-Fatesh, A.S.; Arafat, Y.; Ibrahim, A.A.; Kasim, S.O.; Alharthi, A.; Fakeeha, A.H.; Abasaeed, A.E.; Bonura, G.; Frusteri, F. Catalytic Behaviour of Ce-Doped Ni Systems Supported on Stabilized Zirconia under Dry Reforming Conditions. Catalysts 2019, 9, 473. [Google Scholar] [CrossRef]
- Mandal, S.; Santra, C.; Kumar, R.; Pramanik, M.; Rahman, S.; Bhaumik, A.; Maity, S.; Sen, D.; Chowdhury, B. Niobium doped hexagonal mesoporous silica (HMS-X) catalyst for vapor phase Beckmann rearrangement reaction. RSC Adv. 2014, 4, 845–854. [Google Scholar] [CrossRef]
- Rahman, S.; Santra, C.; Kumar, R.; Bahadur, J.; Sultana, A.; Schweins, R.; Sen, D.; Maity, S.; Mazumdar, S.; Chowdhury, B. Highly active Ga promoted Co-HMS-X catalyst towards styrene epoxidation reaction using molecular O2. Appl. Catal. A Gen. 2014, 482, 61–68. [Google Scholar] [CrossRef]
- Jiang, X.-Y.; Zhou, R.-X.; Pan, P.; Zhu, B.; Yuan, X.-X.; Zheng, X.-M. Effect of the addition of La2O3 on TPR and TPD of CuOγ-Al2O3 catalysts. Appl. Catal. A Gen. 1997, 150, 131–141. [Google Scholar] [CrossRef]
- Mo, W.; Ma, F.; Ma, Y.; Fan, X. The optimization of Ni–Al2O3 catalyst with the addition of La2O3 for CO2–CH4 reforming to produce syngas. Int. J. Hydrog. Energy 2019, 44, 24510–24524. [Google Scholar] [CrossRef]
- Goula, M.; Charisiou, N.; Siakavelas, G.; Tzounis, L.; Tsiaoussis, I.; Panagiotopoulou, P.; Goula, G.; Yentekakis, I. Syngas production via the biogas dry reforming reaction over Ni supported on zirconia modified with CeO2 or La2O3 catalysts. Int. J. Hydrog. Energy 2017, 42, 13724–13740. [Google Scholar] [CrossRef]
- Zhang, J.; Gao, Y.; Jia, X.; Wang, J.; Chen, Z.; Xu, Y. Oxygen vacancy-rich mesoporous ZrO2 with remarkably enhanced visible-light photocatalytic performance. Sol. Energy Mater. Sol. Cells 2018, 182, 113–120. [Google Scholar] [CrossRef]
- Titus, J.; Roussière, T.; Wasserschaff, G.; Schunk, S.; Milanov, A.; Schwab, E.; Wagner, G.; Oeckler, O.; Gläser, R. Dry reforming of methane with carbon dioxide over NiO–MgO–ZrO2. Catal. Today 2016, 270, 68–75. [Google Scholar] [CrossRef]
- Alipour, Z.; Rezaei, M.; Meshkani, F. Effects of support modifiers on the catalytic performance of Ni/Al2O3 catalyst in CO2 reforming of methane. Fuel 2014, 129, 197–203. [Google Scholar] [CrossRef]
- Mierczynski, P.; Mierczynska, A.; Ciesielski, R.; Mosinska, M.; Nowosielska, M.; Czylkowska, A.; Maniukiewicz, W.; Szynkowska, M.I.; Vasilev, K. High Active and Selective Ni/CeO2–Al2O3 and Pd–Ni/CeO2–Al2O3 Catalysts for Oxy-Steam Reforming of Methanol. Catalysts 2018, 8, 380. [Google Scholar] [CrossRef]
- Li, K.; Pei, C.; Li, X.; Chen, S.; Zhang, X.; Liu, R.; Gong, J. Dry reforming of methane over La2O2CO3-modified Ni/Al2O3 catalysts with moderate metal support interaction. Appl. Catal. B Environ. 2020, 264, 118448. [Google Scholar] [CrossRef]
- Khani, Y.; Bahadoran, F.; Shariatinia, Z.; Varmazyari, M.; Safari, N. Synthesis of highly efficient and stable Ni/CexZr1-xGdxO4 and Ni/X-Al2O3 (X = Ce, Zr, Gd, Ce-Zr-Gd) nanocatalysts applied in methane reforming reactions. Ceram. Int. 2020, 46, 25122–25135. [Google Scholar] [CrossRef]
- Lim, Z.-Y.; Tu, J.; Xu, Y.; Chen, B. Ni@ZrO2 yolk-shell catalyst for CO2 methane reforming: Effect of Ni@SiO2 size as the hard-template. J. Colloid Interface Sci. 2021, 590, 641–651. [Google Scholar] [CrossRef]
- Aghaali, M.H.; Firoozi, S. Enhancing the catalytic performance of Co substituted NiAl2O4 spinel by ultrasonic spray pyrolysis method for steam and dry reforming of methane. Int. J. Hydrog. Energy 2021, 46, 357–373. [Google Scholar] [CrossRef]
- Medeiros, R.L.; Macedo, H.P.; Melo, V.R.; Oliveira, Â.A.S.; Barros, J.M.; Melo, M.A.; Melo, D.M. Ni supported on Fe-doped MgAl2O4 for dry reforming of methane: Use of factorial design to optimize H2 yield. Int. J. Hydrog. Energy 2016, 41, 14047–14057. [Google Scholar] [CrossRef]
- Lavoie, J.-M. Review on dry reforming of methane, a potentially more environmentally-friendly approach to the increasing natural gas exploitation. Front. Chem. 2014, 2, 81. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Q.; Liu, Y.; Zhang, Y. Dry reforming of methane over Ni/MgO-Al2O3 catalysts prepared by two-step hydrothermal method. Appl. Surf. Sci. 2016, 389, 25–33. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, B.; Qin, L.; Wang, Y.; Yu, F.; Han, J. Non-thermal plasma-enhanced dry reforming of methane and CO2 over Ce-promoted Ni/C catalysts. Mol. Catal. 2020, 485, 110821. [Google Scholar] [CrossRef]
- Zheng, X.; Li, Y.; Zhang, L.; Shen, L.; Xiao, Y.; Zhang, Y.; Au, C.; Jiang, L. Insight into the effect of morphology on catalytic performance of porous CeO2 nanocrystals for H2S selective oxidation. Appl. Catal. B Environ. 2019, 252, 98–110. [Google Scholar] [CrossRef]
- Lei, H.; Zhang, P. Preparation of alumina/silica core-shell abrasives and their CMP behavior. Appl. Surf. Sci. 2007, 253, 8754–8761. [Google Scholar] [CrossRef]
- Sanchez, E.A.; Comelli, R.A. Hydrogen by glycerol steam reforming on a nickel–alumina catalyst: Deactivation processes and regeneration. Int. J. Hydrog. Energy 2012, 37, 14740–14746. [Google Scholar] [CrossRef]
- Goharshadi, E.K.; Hadadian, M. Effect of calcination temperature on structural, vibrational, optical, and rheological properties of zirconia nanoparticles. Ceram. Int. 2012, 38, 1771–1777. [Google Scholar] [CrossRef]
- Heshmatpour, F.; Aghakhanpour, R.B. Synthesis and characterization of superfine pure tetragonal nanocrystalline sulfated zirconia powder by a non-alkoxide sol–gel route. Adv. Powder Technol. 2012, 23, 80–87. [Google Scholar] [CrossRef]
- Kim, S.; Crandall, B.S.; Lance, M.J.; Cordonnier, N.; Lauterbach, J.; Sasmaz, E. Activity and stability of NiCe@SiO multi–yolk–shell nanotube catalyst for tri-reforming of methane. Appl. Catal. B Environ. 2019, 259, 118037. [Google Scholar] [CrossRef]

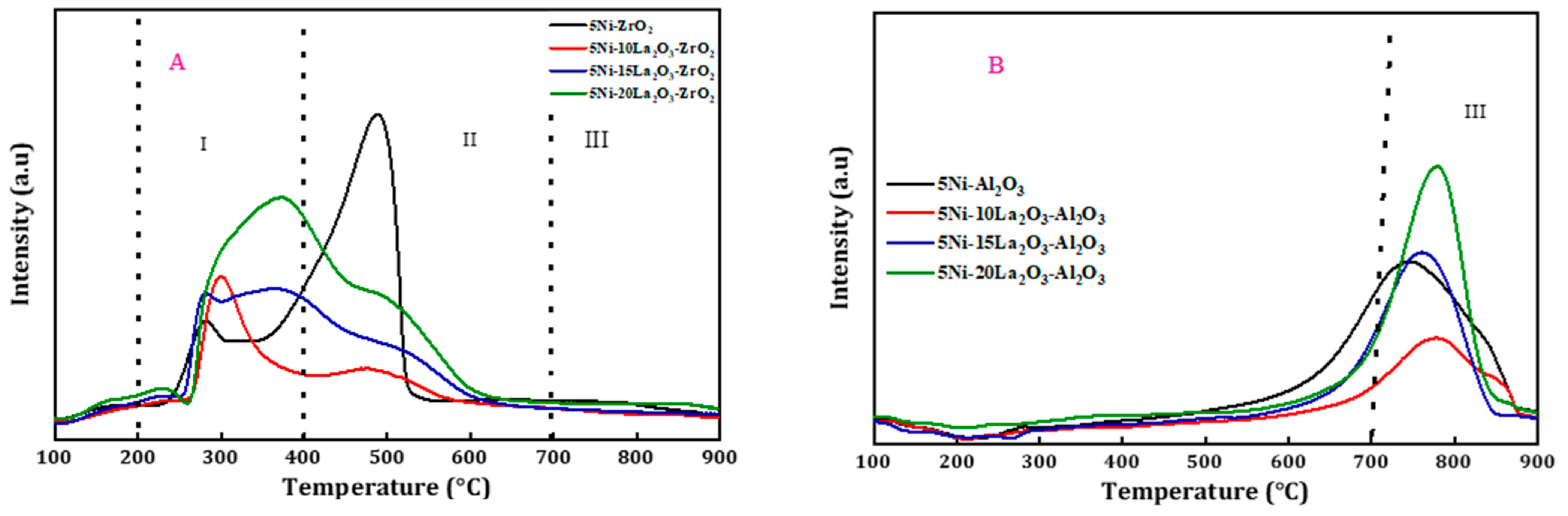
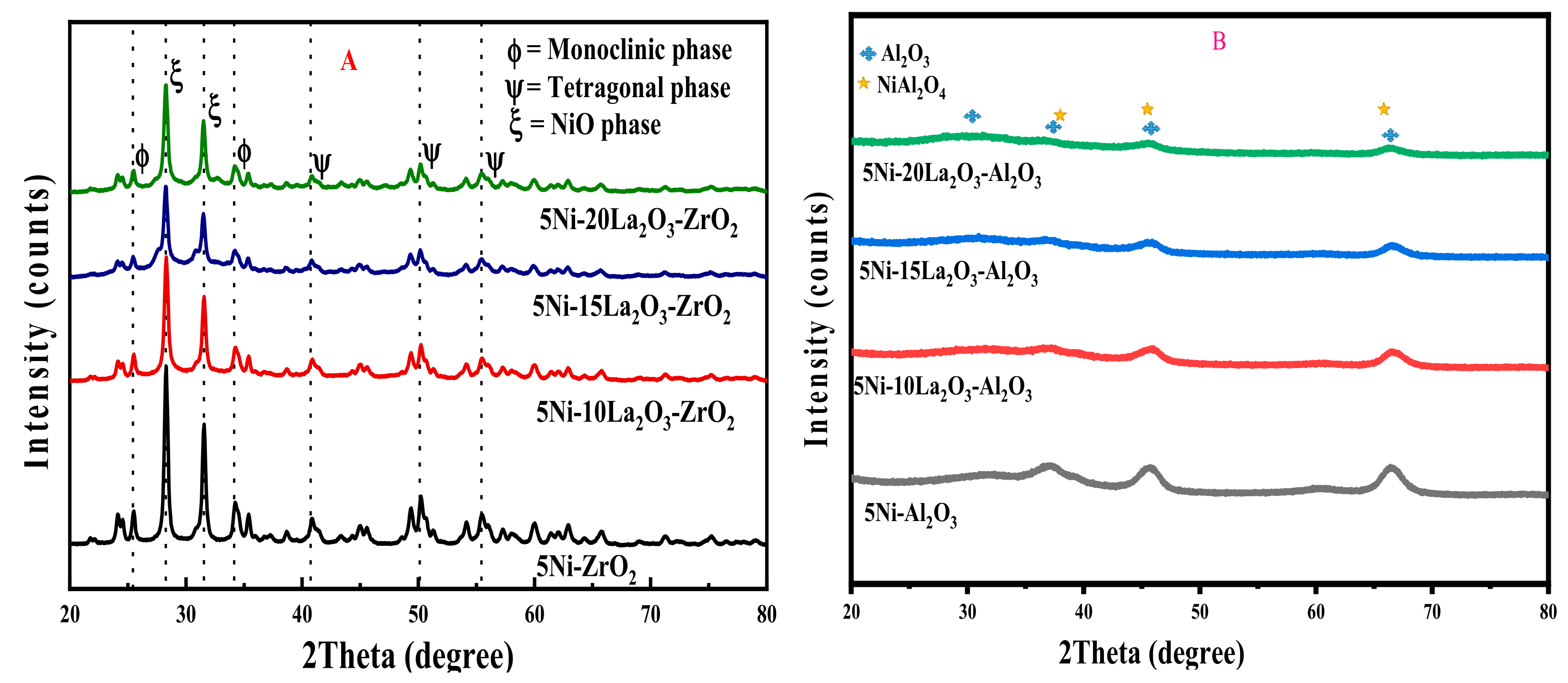
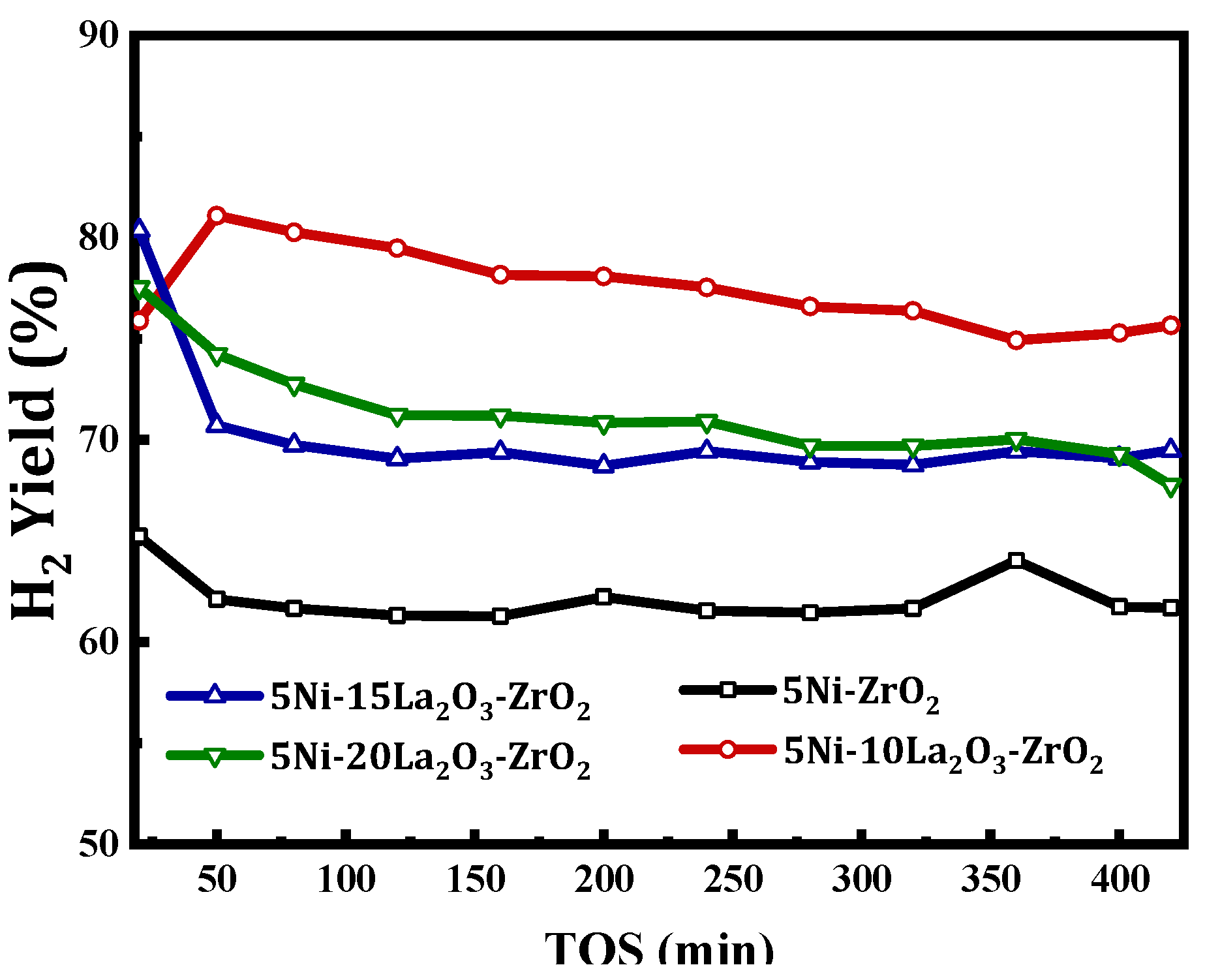

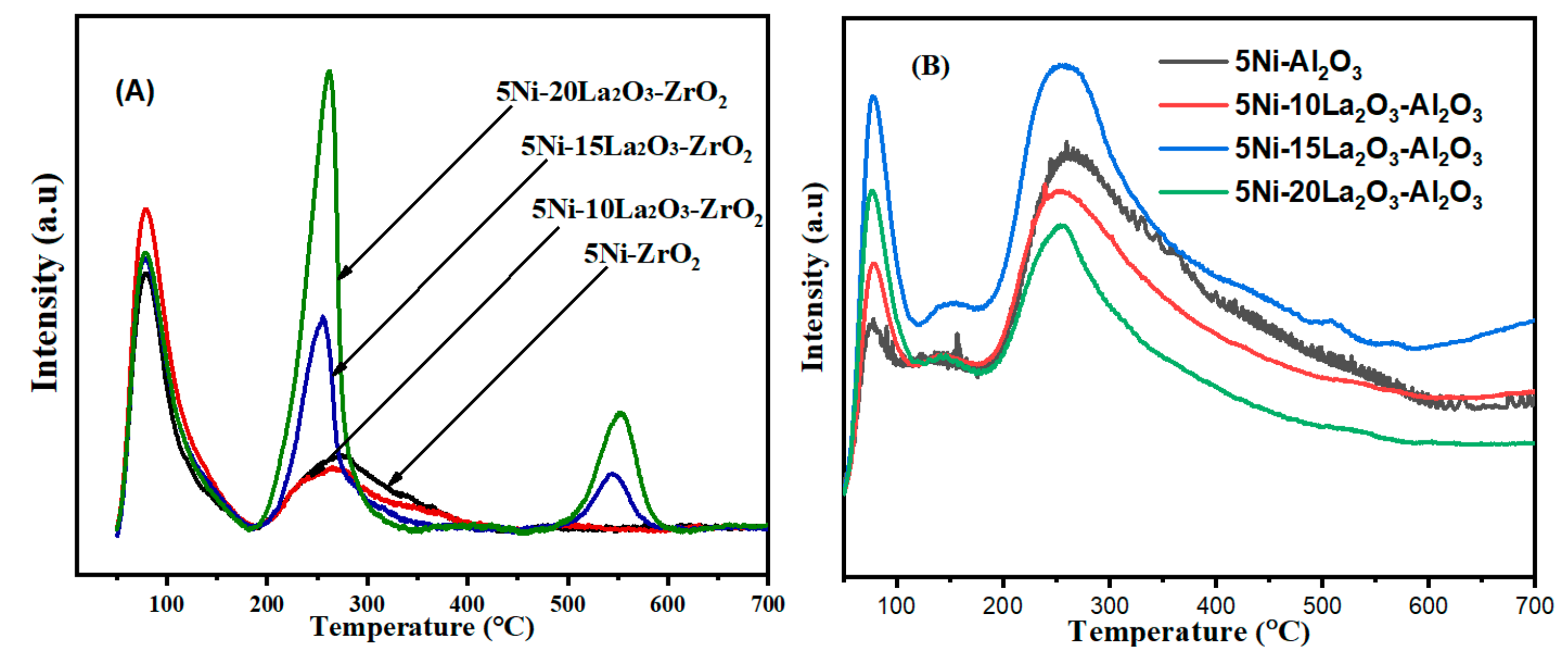

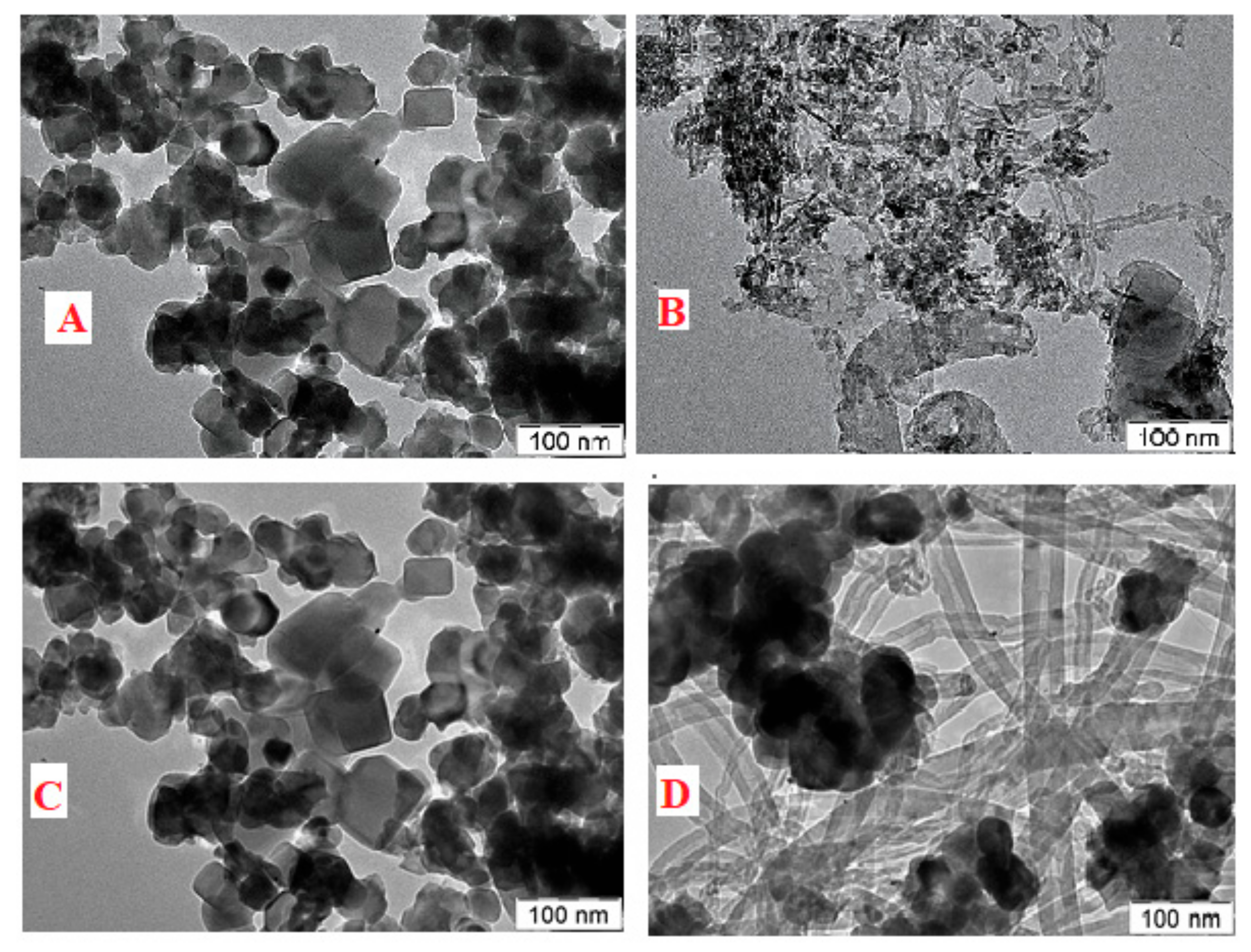
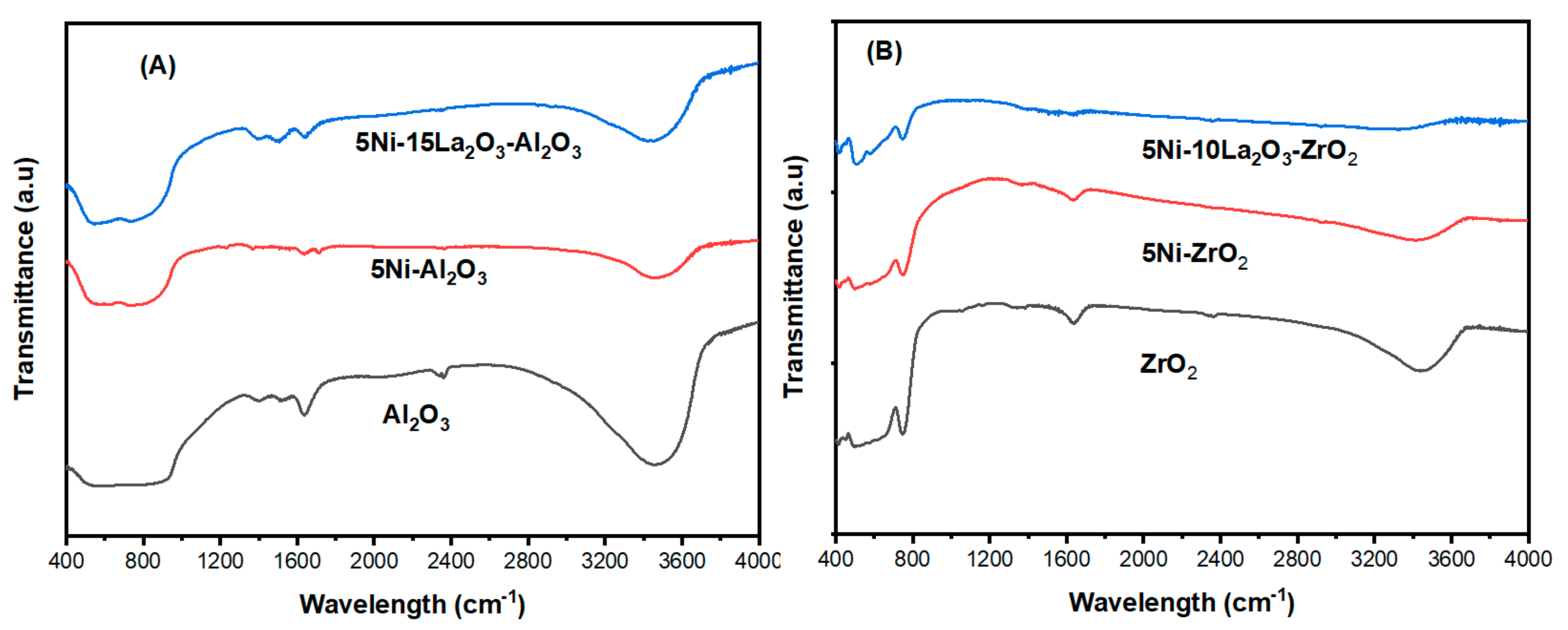

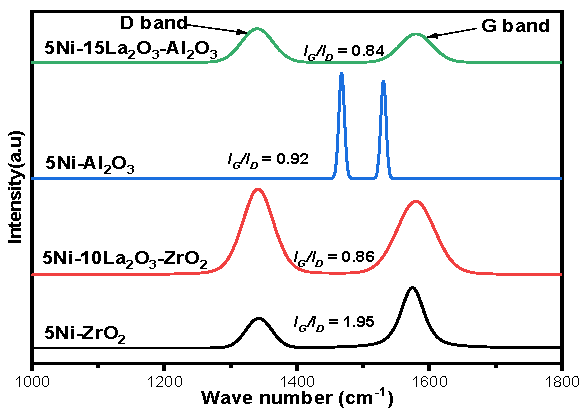
| Catalyst | Feed (CH4:CO2: Inert) | Reaction Temperature (°C) | GHSV (mL/(g·h) | Yield (%) | Ref. |
|---|---|---|---|---|---|
| Ni/Zr-γAl2O3 | 1:1:3 | 750 | 54,000 | 63 | [50] |
| Ni@ZrO2-SiZr-7.7 | 1:1:1 | 800 | 72,000 | 81 | [51] |
| 0.8% Ni+0.2% Co-MgAl2O4 | 1:1:0.5 | 700 | 54,000 | 51 | [52] |
| 10Ni+1%Fe-MgAl2O4 | 1:1:1 | 750 | 30,000 | 78 | [53] |
| 5Ni-10La2O3-ZrO2 | 1:1:0.33 | 700 | 42,000 | 80 | This work |
| 5Ni-15La2O3-Al2O3 | 1:1:0.33 | 700 | 42,000 | 84 | This work |
| Catalyst | Weak-Basicity (µmol/g) | Medium-Basicity (µmol/g) | Strong-Basicity (µmol/g) | Total-Basicity (µmol/g) |
|---|---|---|---|---|
| 5Ni-ZrO2 a | 0.52 | 0.48 | 0.00 | 1 |
| 5Ni-10La2O3-ZrO2 | 0.66 | 0.29 | 0.00 | 0.95 |
| 5Ni-15La2O3-ZrO2 | 0.63 | 0.49 | 0.12 | 1.24 |
| 5Ni-20La2O3-ZrO2 | 0.52 | 0.94 | 0.27 | 1.73 |
| 5Ni-Al2O3 a | 0.44 | 0.38 | 0.18 | 1 |
| 5Ni-10La2O3-Al2O3 | 0.42 | 0.11 | 0.00 | 0.53 |
| 5Ni-15La2O3-Al2O3 | 0.32 | 0.10 | 0.00 | 0.42 |
| 5Ni-20La2O3-Al2O3 | 0.35 | 0.06 | 0.00 | 0.41 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abasaeed, A.; Kasim, S.; Khan, W.; Sofiu, M.; Ibrahim, A.; Fakeeha, A.; Al-Fatesh, A. Hydrogen Yield from CO2 Reforming of Methane: Impact of La2O3 Doping on Supported Ni Catalysts. Energies 2021, 14, 2412. https://doi.org/10.3390/en14092412
Abasaeed A, Kasim S, Khan W, Sofiu M, Ibrahim A, Fakeeha A, Al-Fatesh A. Hydrogen Yield from CO2 Reforming of Methane: Impact of La2O3 Doping on Supported Ni Catalysts. Energies. 2021; 14(9):2412. https://doi.org/10.3390/en14092412
Chicago/Turabian StyleAbasaeed, Ahmed, Samsudeen Kasim, Wasim Khan, Mahmud Sofiu, Ahmed Ibrahim, Anis Fakeeha, and Ahmed Al-Fatesh. 2021. "Hydrogen Yield from CO2 Reforming of Methane: Impact of La2O3 Doping on Supported Ni Catalysts" Energies 14, no. 9: 2412. https://doi.org/10.3390/en14092412
APA StyleAbasaeed, A., Kasim, S., Khan, W., Sofiu, M., Ibrahim, A., Fakeeha, A., & Al-Fatesh, A. (2021). Hydrogen Yield from CO2 Reforming of Methane: Impact of La2O3 Doping on Supported Ni Catalysts. Energies, 14(9), 2412. https://doi.org/10.3390/en14092412









