Studying the Complexity of Biomass Derived Biofuels
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Instruments and Methods
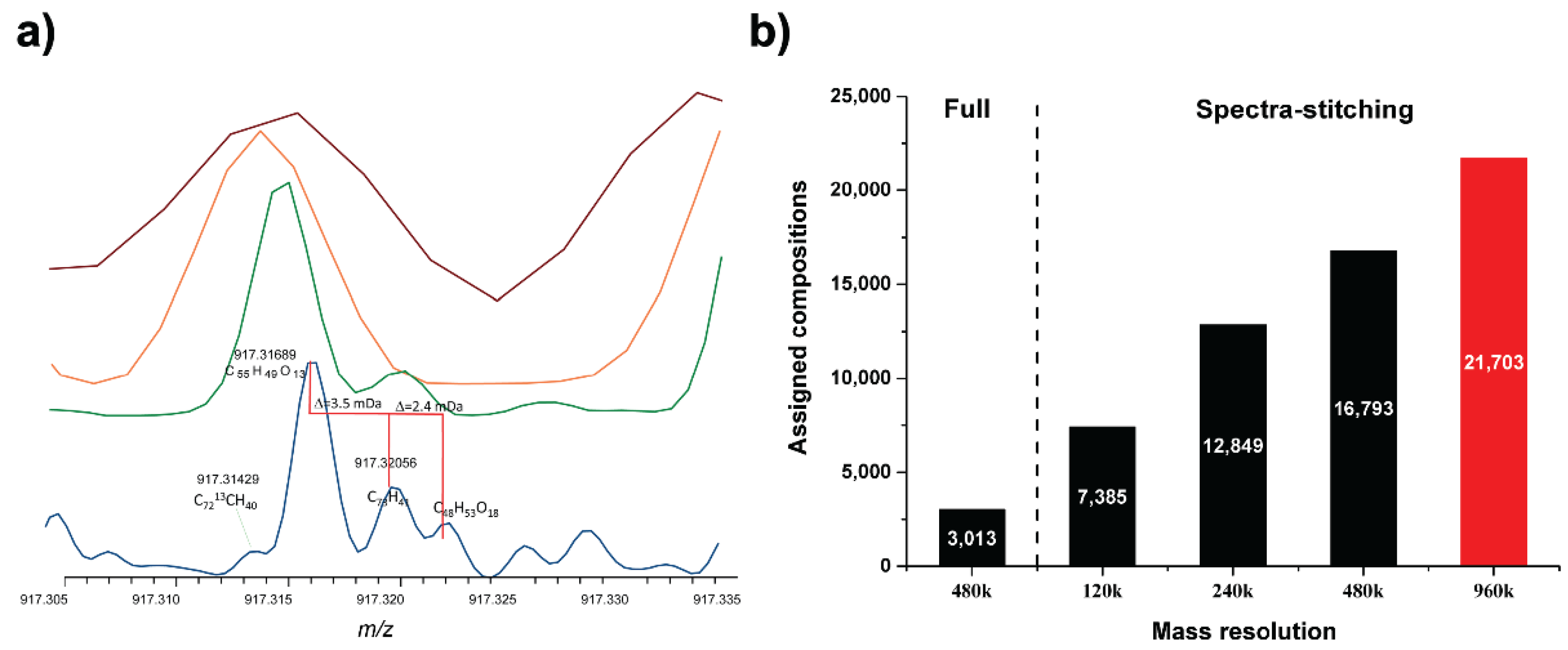
2.3. Data Analysis
3. Results
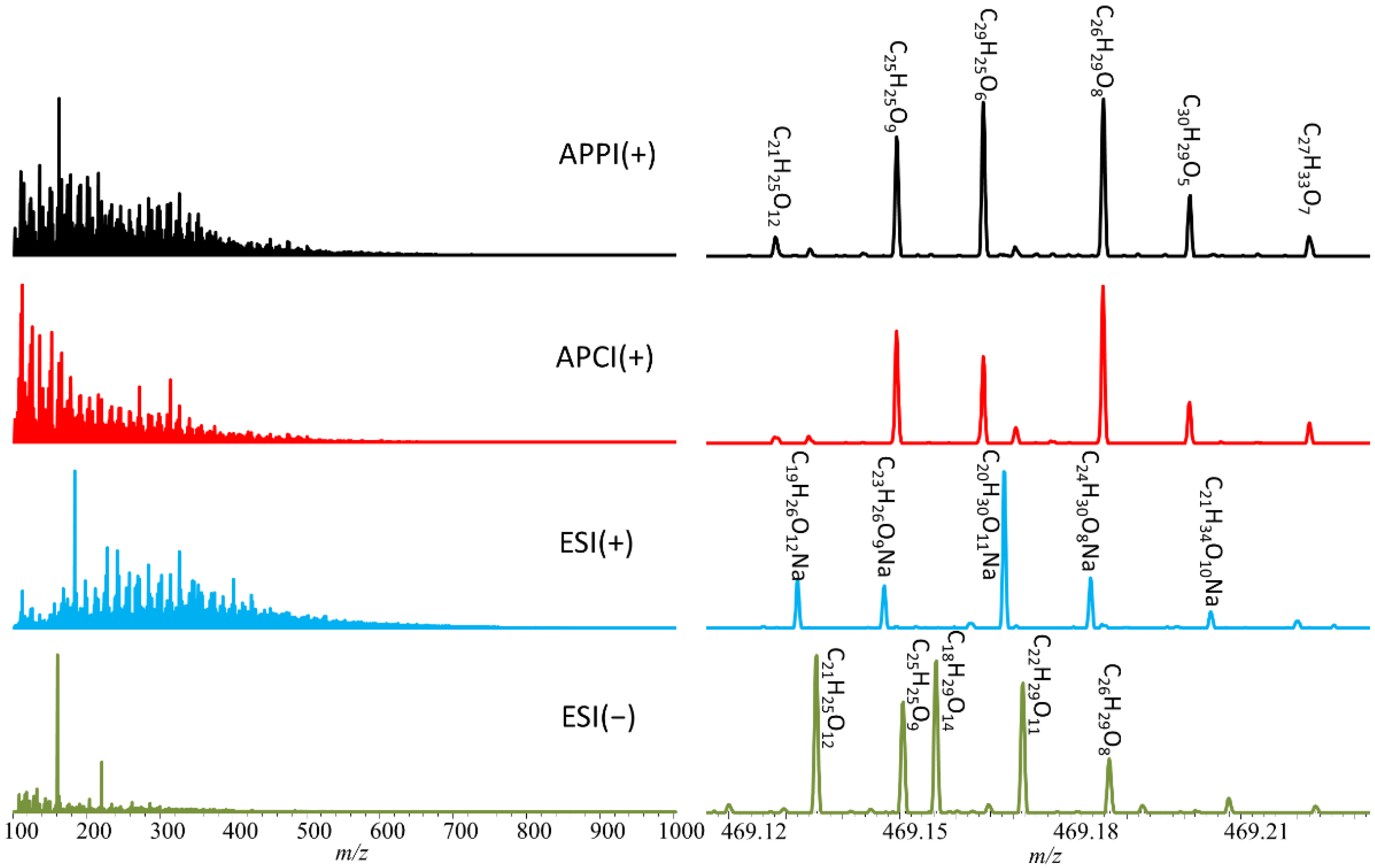
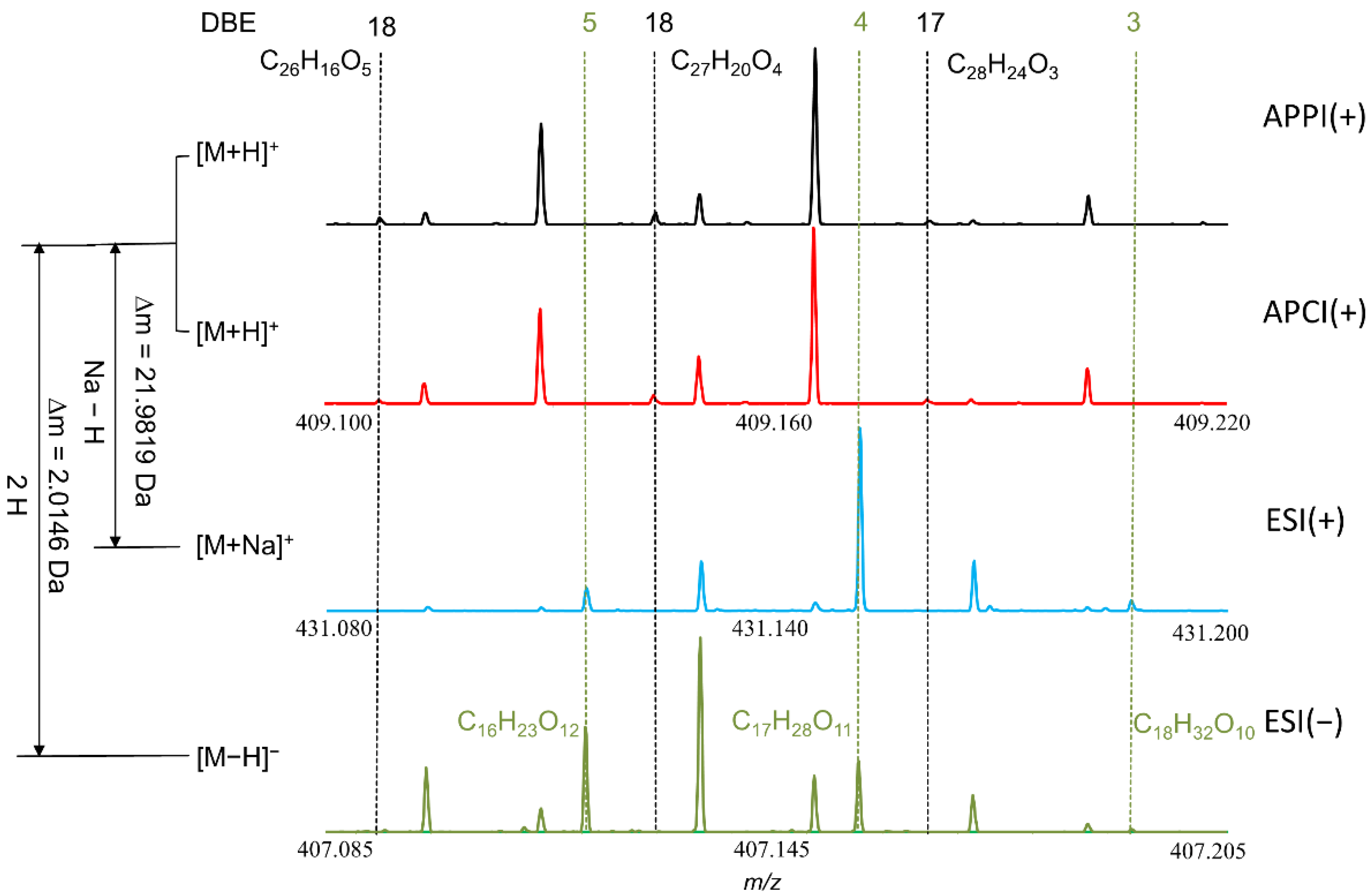
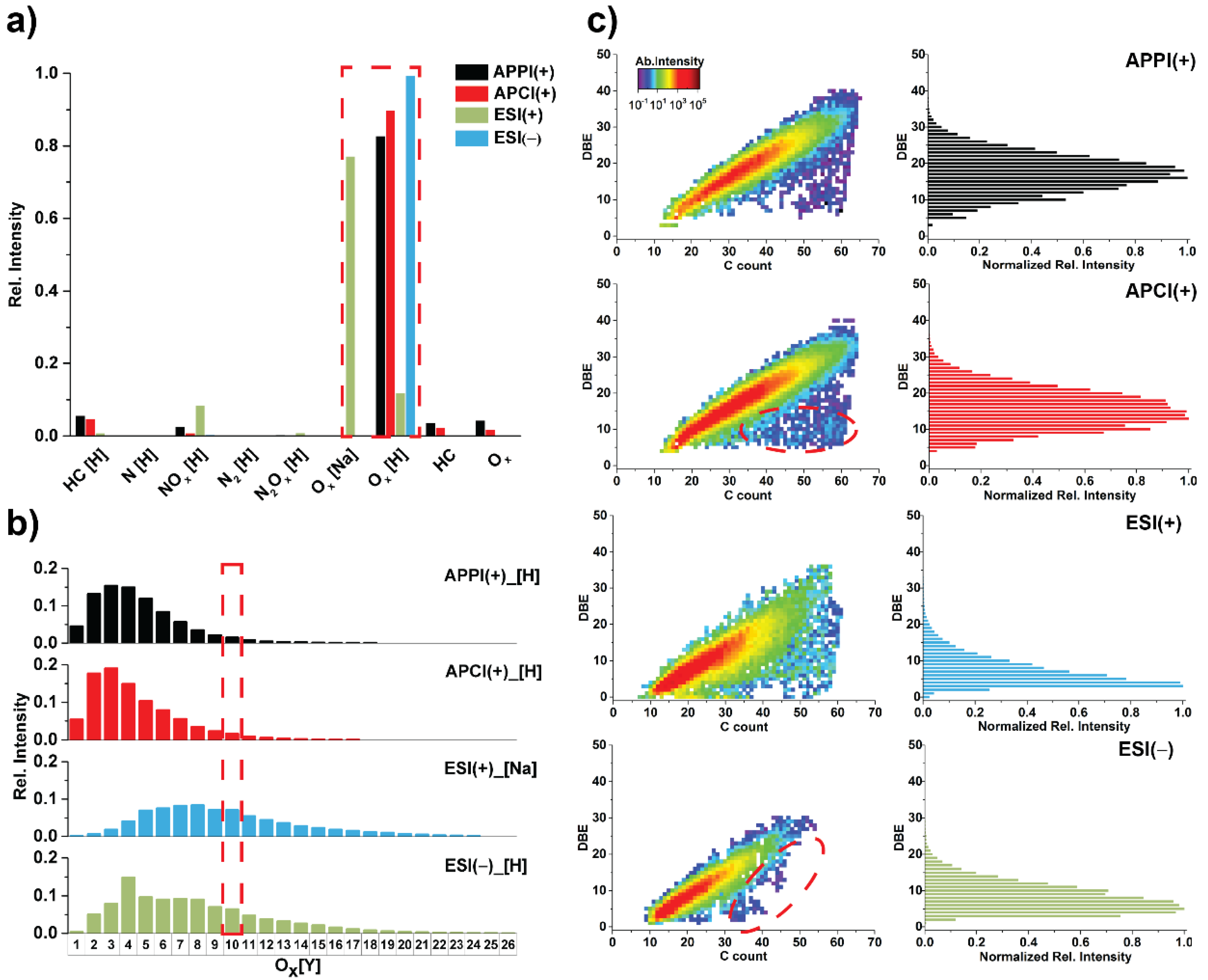
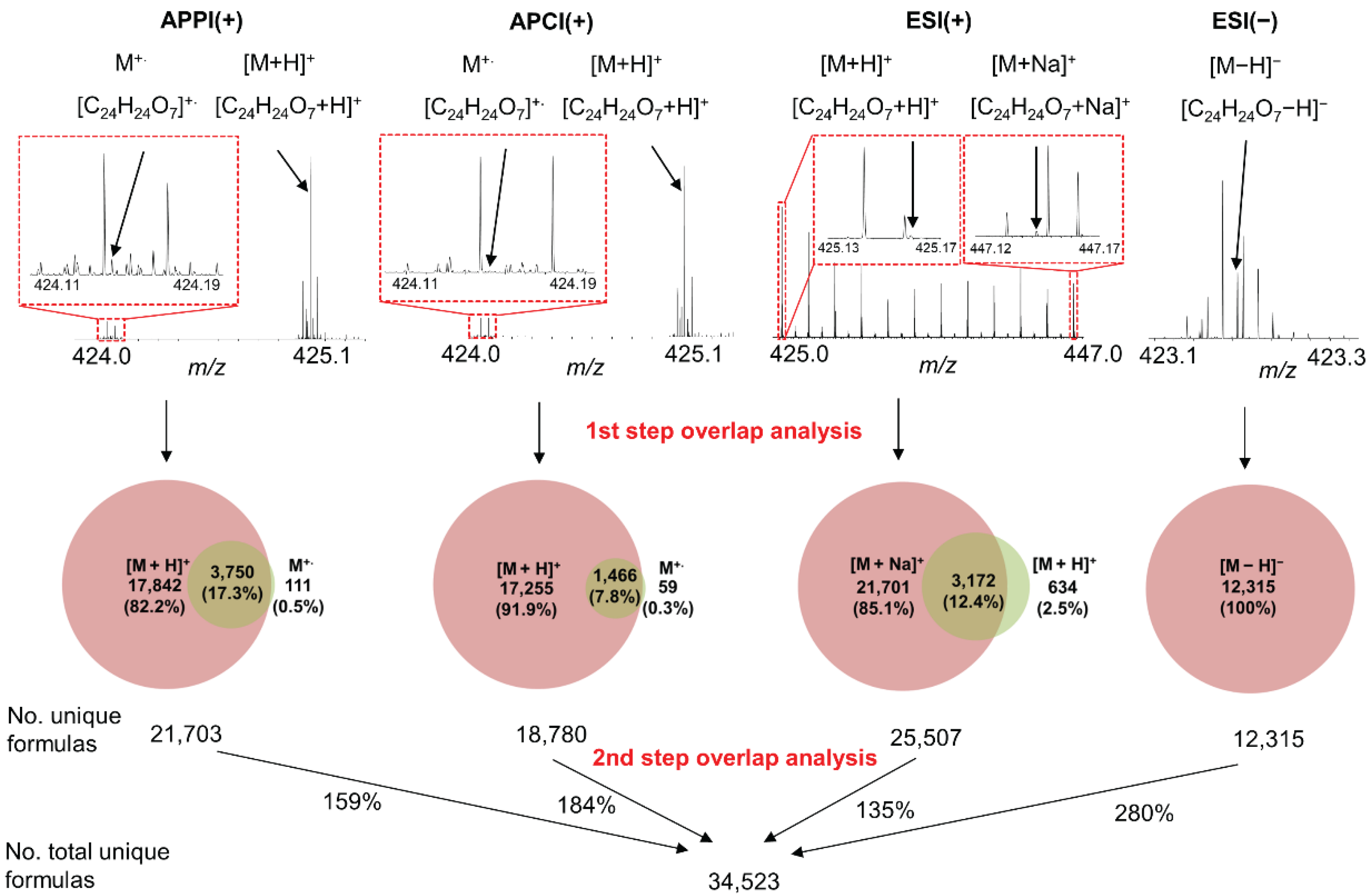
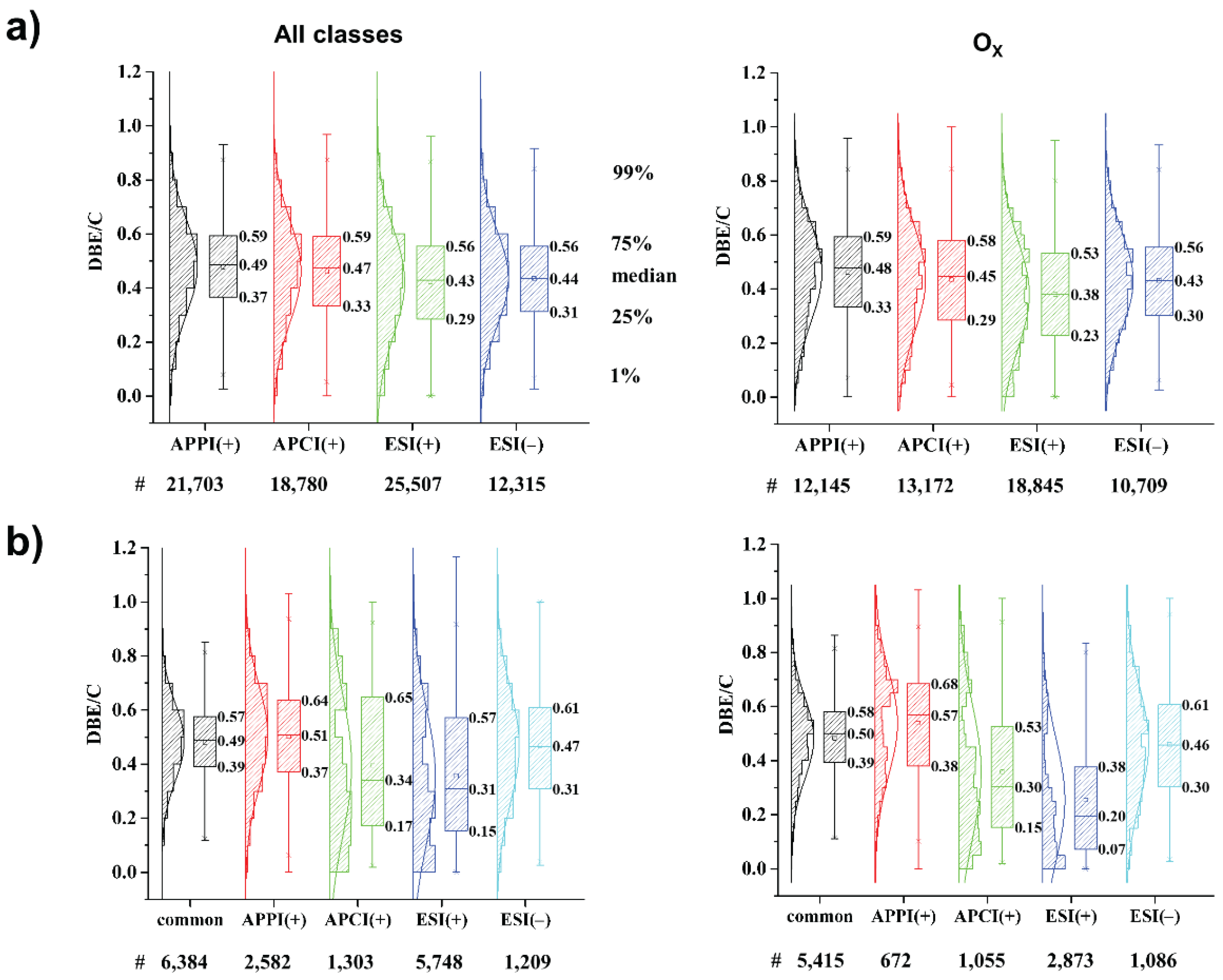
3.1. Ionization Effects
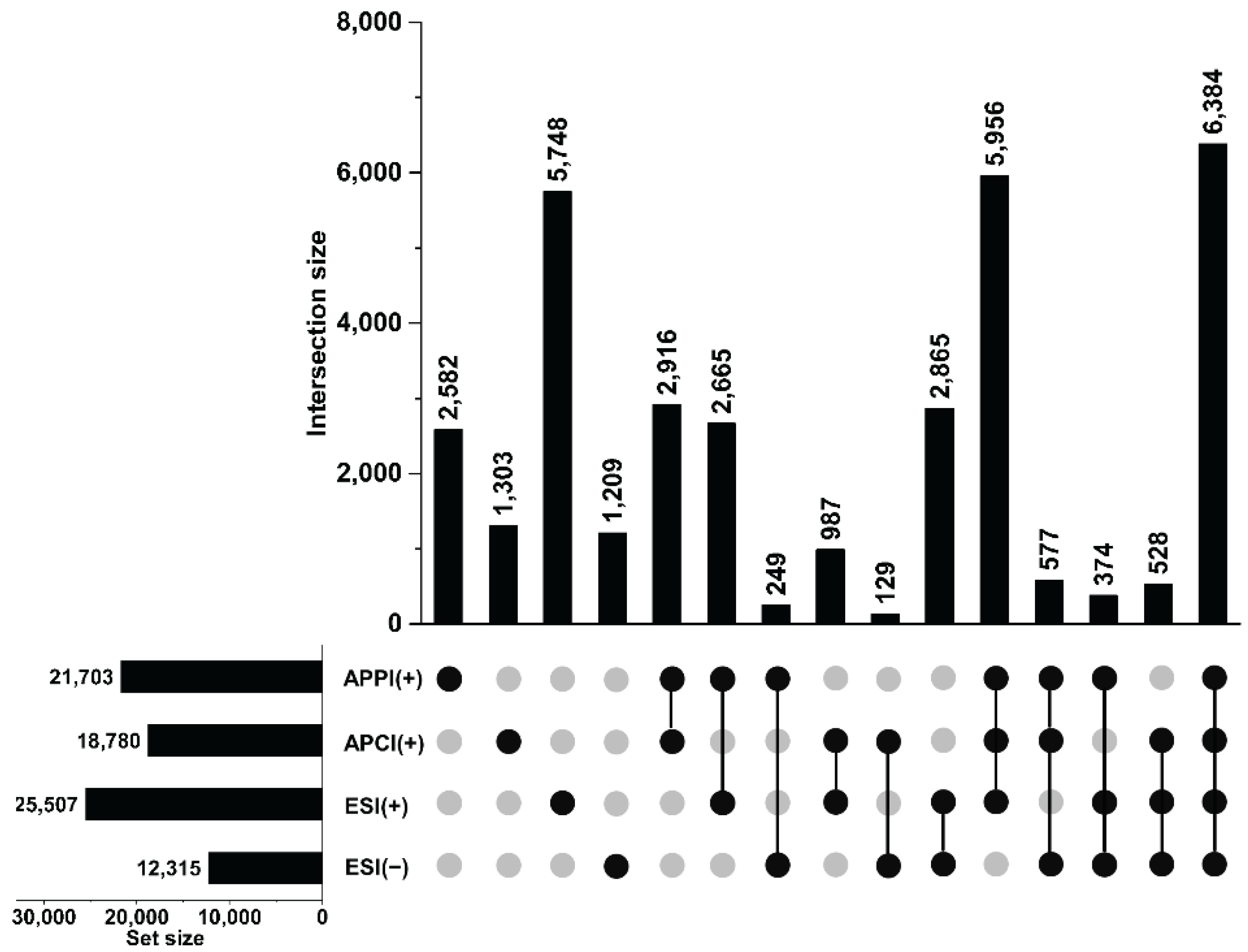
3.2. Total Unique Compositions with Complementary Ionization Techniques
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vispute, T.P.; Zhang, H.; Sanna, A.; Xiao, R.; Huber, G.W. Renewable chemical commodity feedstocks from integrated catalytic processing of pyrolysis oils. Science 2010, 330, 1222–1227. [Google Scholar] [CrossRef]
- Liu, C.; Wang, H.; Karim, A.M.; Sun, J.; Wang, Y. Catalytic fast pyrolysis of lignocellulosic biomass. Chem. Soc. Rev. 2014, 43, 7594–7623. [Google Scholar] [CrossRef]
- Talmadge, M.S.; Baldwin, R.M.; Biddy, M.J.; McCormick, R.L.; Beckham, G.T.; Ferguson, G.A.; Czernik, S.; Magrini-Bair, K.A.; Foust, T.D.; Metelski, P.D.; et al. A perspective on oxygenated species in the refinery integration of pyrolysis oil. Green Chem. 2014, 16, 407–453. [Google Scholar] [CrossRef]
- Bridgwater, A.; Peacocke, G. Fast pyrolysis processes for biomass. Renew. Sustain. Energy Rev. 2000, 4, 1–73. [Google Scholar] [CrossRef]
- Rinaldi, R.; Schüth, F. Design of solid catalysts for the conversion of biomass. Energy Environ. Sci. 2009, 2, 610. [Google Scholar] [CrossRef]
- Sharifzadeh, M.; Sadeqzadeh, M.; Guo, M.; Borhani, T.N.; Murthy Konda, N.V.S.N.; Garcia, M.C.; Wang, L.; Hallett, J.; Shah, N. The multi-scale challenges of biomass fast pyrolysis and bio-oil upgrading: Review of the state of art and future research directions. Prog. Energy Combust. Sci. 2019, 71, 1–80. [Google Scholar] [CrossRef]
- Czernik, S.; Bridgwater, A. Overview of applications of biomass fast pyrolysis oil. Energy Fuels 2004, 18, 590–598. [Google Scholar] [CrossRef]
- Cao, Z.; Xu, Y.; Lyu, P.; Dierks, M.; Morales-García, Á.; Schrader, W.; Nachtigall, P.; Schüth, F. Flexibilization of Biorefineries: Tuning Lignin Hydrogenation by Hydrogen Partial Pressure. ChemSusChem 2021, 14, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Biswas, B.; Pandey, N.; Bisht, Y.; Singh, R.; Kumar, J.; Bhaskar, T. Pyrolysis of agricultural biomass residues: Comparative study of corn cob, wheat straw, rice straw and rice husk. Bioresour. Technol. 2017, 237, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Pu, Y.Q.; Cao, S.L.; Ragauskas, A.J. Application of quantitative P-31 NMR in biomass lignin and biofuel precursors characterization. Energy Environ. Sci. 2011, 4, 3154–3166. [Google Scholar] [CrossRef]
- Ben, H.X.; Ragauskas, A.J. NMR Characterization of Pyrolysis Oils from Kraft Lignin. Energy Fuels 2011, 25, 2322–2332. [Google Scholar] [CrossRef]
- Hawkes, J.A.; D’Andrilli, J.; Agar, J.N.; Barrow, M.P.; Berg, S.M.; Catalán, N.; Chen, H.; Chu, R.K.; Cole, R.B.; Dittmar, T.; et al. An international laboratory comparison of dissolved organic matter composition by high resolution mass spectrometry: Are we getting the same answer? Limnol. Oceanogr. Methods 2020, 18, 235–258. [Google Scholar] [CrossRef]
- Hertkorn, N.; Frommberger, M.; Witt, M.; Koch, B.P.; Schmitt-Kopplin, P.; Perdue, E.M. Natural Organic Matter and the Event Horizon of Mass Spectrometry. Anal. Chem. 2008, 80, 8908–8919. [Google Scholar] [CrossRef] [PubMed]
- Xian, F.; Hendrickson, C.L.; Marshall, A.G. High resolution mass spectrometry. Anal. Chem. 2012, 84, 708–719. [Google Scholar] [CrossRef] [PubMed]
- Headley, J.V.; Barrow, M.P.; Peru, K.M.; Fahlman, B.; Frank, R.A.; Bickerton, G.; McMaster, M.E.; Parrott, J.; Hewitt, L.M. Preliminary fingerprinting of Athabasca oil sands polar organics in environmental samples using electrospray ionization Fourier transform ion cyclotron resonance mass spectrometry. Rapid Commun. Mass Spectrom. 2011, 25, 1899–1909. [Google Scholar] [CrossRef]
- Aebersold, R.; Mann, M. Mass spectrometry-based proteomics. Nature 2003, 422, 198–207. [Google Scholar] [CrossRef]
- Palacio Lozano, D.C.; Gavard, R.; Arenas-Diaz, J.P.; Thomas, M.J.; Stranz, D.D.; Mejía-Ospino, E.; Guzman, A.; Spencer, S.E.F.; Rossell, D.; Barrow, M.P. Pushing the analytical limits: New insights into complex mixtures using mass spectra segments of constant ultrahigh resolving power. Chem. Sci. 2019, 6966–6978. [Google Scholar] [CrossRef]
- Rüger, C.P.; Miersch, T.; Schwemer, T.; Sklorz, M.; Zimmermann, R. Hyphenation of Thermal Analysis to Ultrahigh-Resolution Mass Spectrometry (Fourier Transform Ion Cyclotron Resonance Mass Spectrometry) Using Atmospheric Pressure Chemical Ionization For Studying Composition and Thermal Degradation of Complex Materials. Anal. Chem. 2015, 87, 6493–6499. [Google Scholar] [CrossRef]
- Hockaday, W.C.; Purcell, J.M.; Marshall, A.G.; Baldock, J.A.; Hatcher, P.G. Electrospray and photoionization mass spectrometry for the characterization of organic matter in natural waters: A qualitative assessment. Limnol. Oceanogr. Methods 2009, 7, 81–95. [Google Scholar] [CrossRef]
- Wei, Y.; Lei, H.; Wang, L.; Zhu, L.; Zhang, X.; Liu, Y.; Chen, S.; Ahring, B. Liquid–Liquid Extraction of Biomass Pyrolysis Bio-oil. Energy Fuels 2014, 28, 1207–1212. [Google Scholar] [CrossRef]
- Kanaujia, P.K.; Sharma, Y.; Agrawal, U.; Garg, M. Analytical approaches to characterizing pyrolysis oil from biomass. TrAC Trends Anal. Chem. 2013, 42, 125–136. [Google Scholar] [CrossRef]
- Putman, J.C.; Moulian, R.; Barrere-Mangote, C.; Rodgers, R.P.; Bouyssiere, B.; Giusti, P.; Marshall, A.G. Probing Aggregation Tendencies in Asphaltenes by Gel Permeation Chromatography. Part 1: Online Inductively Coupled Plasma Mass Spectrometry and Offline Fourier Transform Ion Cyclotron Resonance Mass Spectrometry. Energy Fuels 2020, 34, 8308–8315. [Google Scholar] [CrossRef]
- Vetere, A.; Profrock, D.; Schrader, W. Quantitative and Qualitative Analysis of Three Classes of Sulfur Compounds in Crude Oil. Angew. Chem. Int. Edit. 2017, 56, 10933–10937. [Google Scholar] [CrossRef]
- Guricza, L.M.; Schrader, W. Argentation chromatography coupled to ultrahigh-resolution mass spectrometry for the separation of a heavy crude oil. J. Chromatogr. A 2017, 1484, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Beccaria, M.; Siqueira, A.L.M.; Maniquet, A.; Giusti, P.; Piparo, M.; Stefanuto, P.H.; Focant, J.F. Advanced mono- and multi-dimensional gas chromatography-mass spectrometry techniques for oxygen-containing compound characterization in biomass and biofuel samples. J. Sep. Sci. 2020, 44, 115–134. [Google Scholar] [CrossRef]
- Zhao, C.; Lercher, J.A. Upgrading pyrolysis oil over Ni/HZSM-5 by cascade reactions. Angew. Chem. Int. Ed. Engl. 2012, 51, 5935–5940. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Kou, Y.; Lemonidou, A.A.; Li, X.; Lercher, J.A. Highly Selective Catalytic Conversion of Phenolic Bio-Oil to Alkanes. Angew. Chem. Int. Ed. Engl. 2009, 121, 4047–4050. [Google Scholar] [CrossRef]
- Hertzog, J.; Carre, V.; Le Brech, Y.; Dufour, A.; Aubriet, F. Toward Controlled Ionization Conditions for ESI-FT-ICR-MS Analysis of Bio-Oils from Lignocellulosic Material. Energy Fuels 2016, 30, 5729–5739. [Google Scholar] [CrossRef]
- Miettinen, I.; Mäkinen, M.; Vilppo, T.; Jänis, J. Compositional Characterization of Phase-Separated Pine Wood Slow Pyrolysis Oil by Negative-Ion Electrospray Ionization Fourier Transform Ion Cyclotron Resonance Mass Spectrometry. Energy Fuels 2015, 29, 1758–1765. [Google Scholar] [CrossRef]
- Kekäläinen, T.; Venäläinen, T.; Jänis, J. Characterization of Birch Wood Pyrolysis Oils by Ultrahigh-Resolution Fourier Transform Ion Cyclotron Resonance Mass Spectrometry: Insights into Thermochemical Conversion. Energy Fuels 2014, 28, 4596–4602. [Google Scholar] [CrossRef]
- Brecht, D.; Uteschil, F.; Schmitz, O.J. Development of a fast-switching dual (ESI/APCI) ionization source for liquid chromatography/mass spectrometry. Rapid Commun. Mass Spectrom. 2020, 34. [Google Scholar] [CrossRef] [PubMed]
- Lenzen, C.; Winterfeld, G.A.; Schmitz, O.J. Comparison of piracetam measured with HPLC-DAD, HPLC-ESI-MS, DIP-APCI-MS, and a newly developed and optimized DIP-ESI-MS. Anal. Bioanal. Chem. 2016, 408, 4103–4110. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.K.; Andersson, J.T.; Schrader, W. Characterization of Supercomplex Crude Oil Mixtures: What Is Really in There? Angew. Chem. Int. Ed. 2009, 48, 1788–1791. [Google Scholar] [CrossRef] [PubMed]
- Akalın, M.K.; Karagöz, S. Analytical pyrolysis of biomass using gas chromatography coupled to mass spectrometry. TrAC Trends Anal. Chem. 2014, 61, 11–16. [Google Scholar] [CrossRef]
- Marshall, A.G.; Hendrickson, C.L.; Jackson, G.S. Fourier transform ion cyclotron resonance mass spectrometry: A primer. Mass Spectrom. Rev. 1998, 17, 1–35. [Google Scholar] [CrossRef]
- Hu, Q.; Noll, R.J.; Li, H.; Makarov, A.; Hardman, M.; Graham Cooks, R. The Orbitrap: A new mass spectrometer. J. Mass Spectrom. 2005, 40, 430–443. [Google Scholar] [CrossRef]
- Staš, M.; Chudoba, J.; Kubička, D.; Pospíšil, M. Chemical characterization of pyrolysis bio-oil: Application of Orbitrap mass spectrometry. Energy Fuels 2015, 29, 3233–3240. [Google Scholar] [CrossRef]
- Staš, M.; Chudoba, J.; Auersvald, M.; Kubička, D.; Conrad, S.; Schulzke, T.; Pospíšil, M. Application of orbitrap mass spectrometry for analysis of model bio-oil compounds and fast pyrolysis bio-oils from different biomass sources. J. Anal. Appl. Pyrolysis 2017, 124, 230–238. [Google Scholar] [CrossRef]
- Nunes, V.O.; Silva, R.V.S.; Romeiro, G.A.; Azevedo, D.A. The speciation of the organic compounds of slow pyrolysis bio-oils from Brazilian tropical seed cake fruits using high-resolution techniques: GC × GC-TOFMS and ESI(±)-Orbitrap HRMS. Microchem. J. 2020, 153, 104514. [Google Scholar] [CrossRef]
- Silva, R.V.S.; Pereira, V.B.; Stelzer, K.T.; Almeida, T.A.; Romeiro, G.A.; Azevedo, D.A. Comprehensive study of the liquid products from slow pyrolysis of crambe seeds: Bio-oil and organic compounds of the aqueous phase. Biomass Bioenergy 2019, 123, 78–88. [Google Scholar] [CrossRef]
- Gaspar, A.; Schrader, W. Expanding the data depth for the analysis of complex crude oil samples by Fourier transform ion cyclotron resonance mass spectrometry using the spectral stitching method. Rapid Commun. Mass Spectrom. 2012, 26, 1047–1052. [Google Scholar] [CrossRef]
- Oliveros, J.C. (2007–2015) Venny. An Interactive Tool for Comparing Lists with Venn’s Diagrams. Available online: https://bioinfogp.cnb.csic.es/tools/venny/index.html (accessed on 7 April 2021).
- Littlefield, K.; Monroe, M. Venn Diagram Plotter; PNNL: Richland, WA, USA, 2008. [Google Scholar]
- Vetere, A.; Schrader, W. Mass Spectrometric Coverage of Complex Mixtures: Exploring the Carbon Space of Crude Oil. ChemistrySelect 2017, 2, 849–853. [Google Scholar] [CrossRef]
- Gaspar, A.; Zellermann, E.; Lababidi, S.; Reece, J.; Schrader, W. Impact of different ionization methods on the molecular assignments of asphaltenes by FT-ICR mass spectrometry. Anal. Chem. 2012, 84, 5257–5267. [Google Scholar] [CrossRef] [PubMed]
- Ware, R.L.; Rowland, S.M.; Rodgers, R.P.; Marshall, A.G. Advanced Chemical Characterization of Pyrolysis Oils from Landfill Waste, Recycled Plastics, and Forestry Residue. Energy Fuels 2017. [Google Scholar] [CrossRef]
| Median/Mean | APPI(+) | APCI(+) | ESI(+) | ESI(−) |
|---|---|---|---|---|
| All assigned compositions | 0.21/0.24 | 0.20/0.23 | 0.26/0.29 | 0.37/0.40 |
| Ox | 0.19/0.22 | 0.19/0.22 | 0.29/0.33 ([M+Na]+) 0.23/0.28 ([M+H]+) 0.29/0.33 (total) | 0.35/0.39 |
| NOx | 0.26/0.30 | 0.24/0.27 | 0.25/0.26 | 0.43/0.45 |
| N2Ox | 0.24/0.26 | 0.23/0.25 | 0.24/0.27 | 0.33/0.42 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Schrader, W. Studying the Complexity of Biomass Derived Biofuels. Energies 2021, 14, 2032. https://doi.org/10.3390/en14082032
Xu Y, Schrader W. Studying the Complexity of Biomass Derived Biofuels. Energies. 2021; 14(8):2032. https://doi.org/10.3390/en14082032
Chicago/Turabian StyleXu, Yun, and Wolfgang Schrader. 2021. "Studying the Complexity of Biomass Derived Biofuels" Energies 14, no. 8: 2032. https://doi.org/10.3390/en14082032
APA StyleXu, Y., & Schrader, W. (2021). Studying the Complexity of Biomass Derived Biofuels. Energies, 14(8), 2032. https://doi.org/10.3390/en14082032







