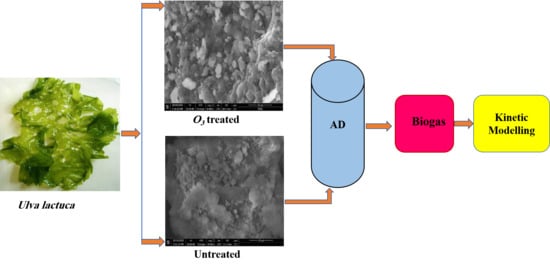
Enhancement of Biogas Production from Macroalgae Ulva latuca via Ozonation Pretreatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of Green Algae Ulva lactuca
2.2. Chemical Analysis of Algae Powder
2.3. Ozonation Pretreatment of Ulva lactuca
2.4. Inoculum and Substrates Preparation
2.4.1. Source of Manure
2.4.2. Source of Activated Sludge
2.5. Biogas Tests
2.6. Characterization and Measurement
2.7. Kinetics Study and Statistical Analysis
3. Results and Discussion
3.1. Characterization for Ulva lactuca
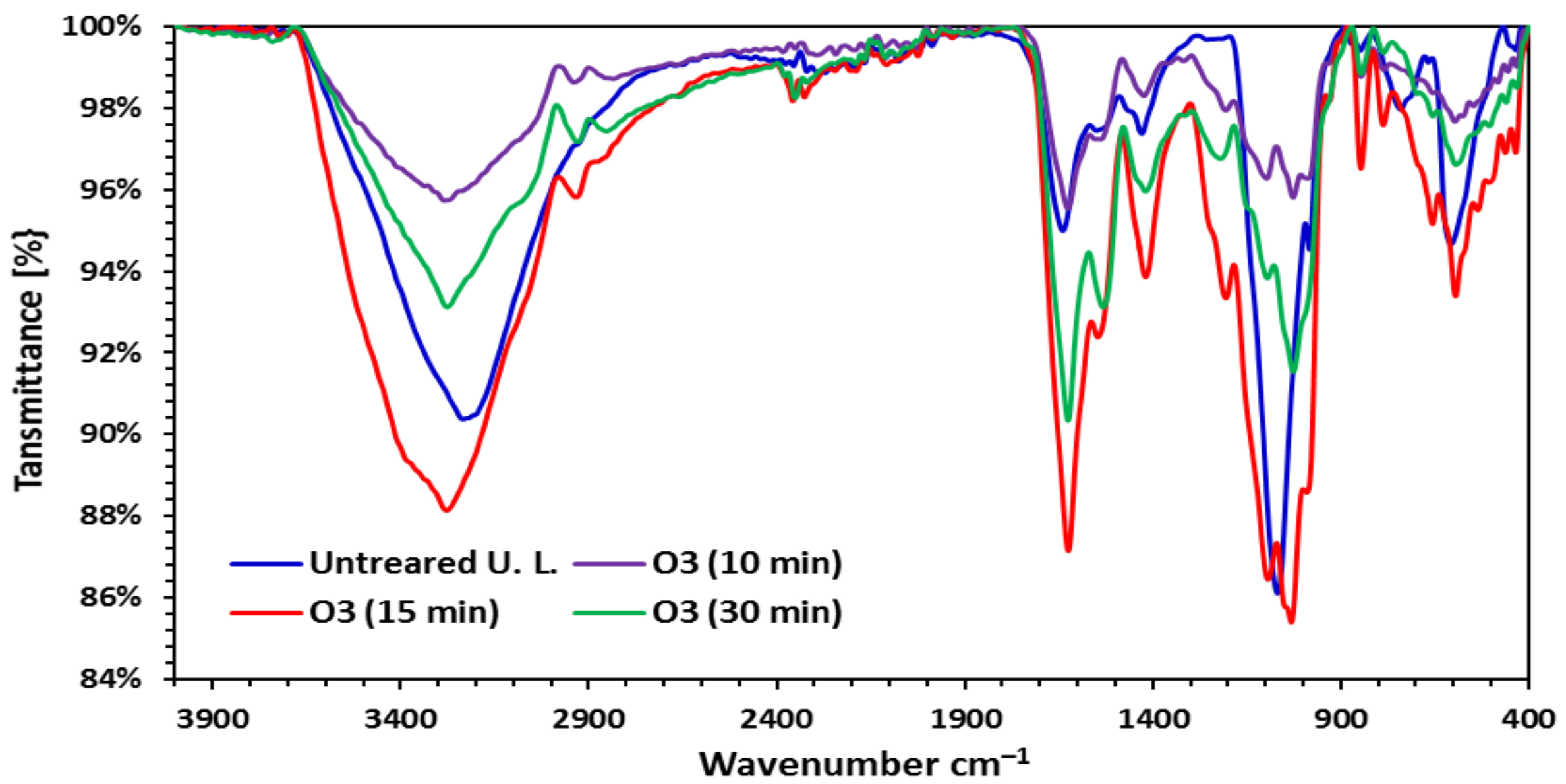
3.1.1. Fourier Transform Infrared Spectra (FTIR)
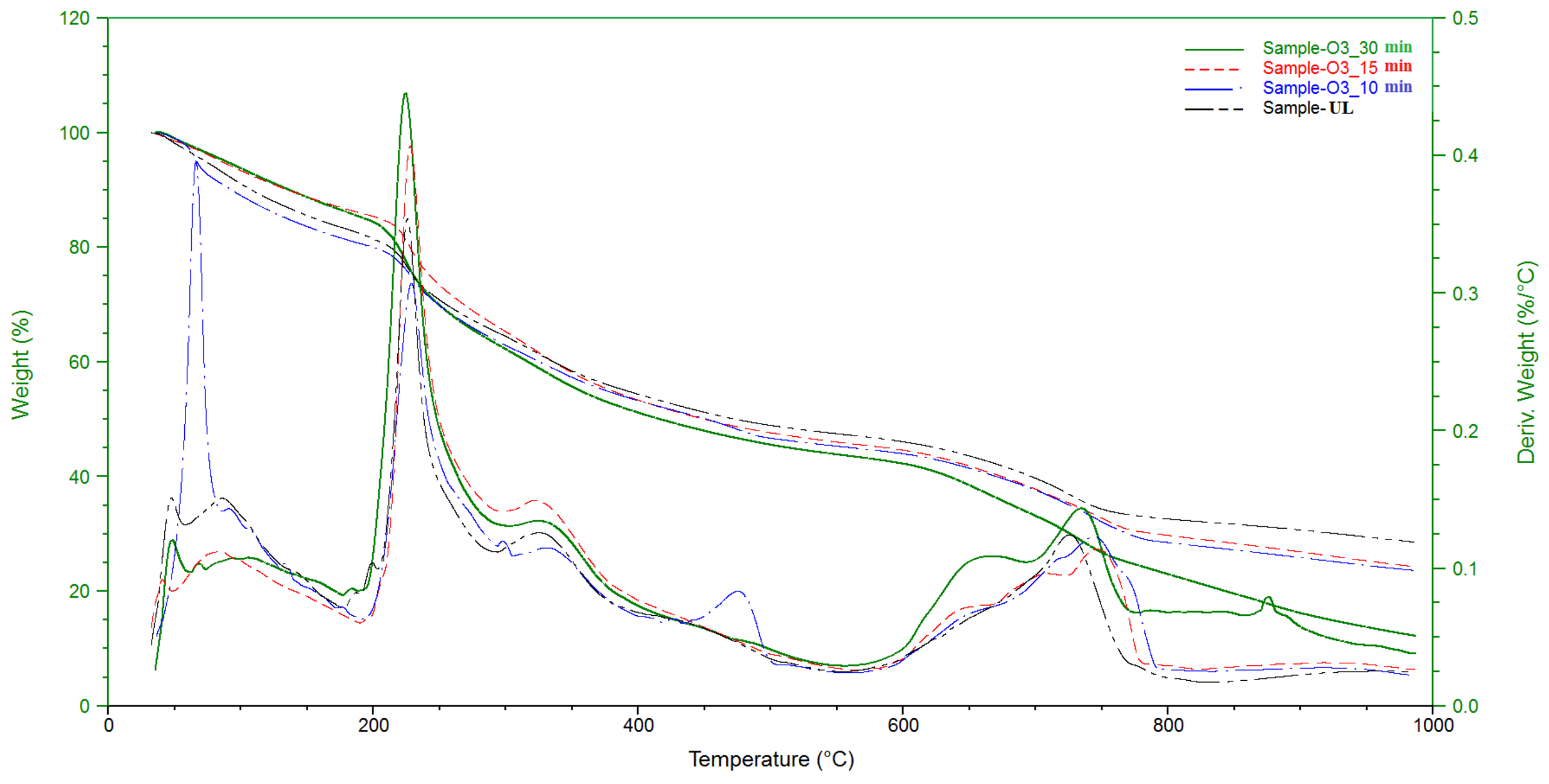
3.1.2. Thermal Analysis (TGA)
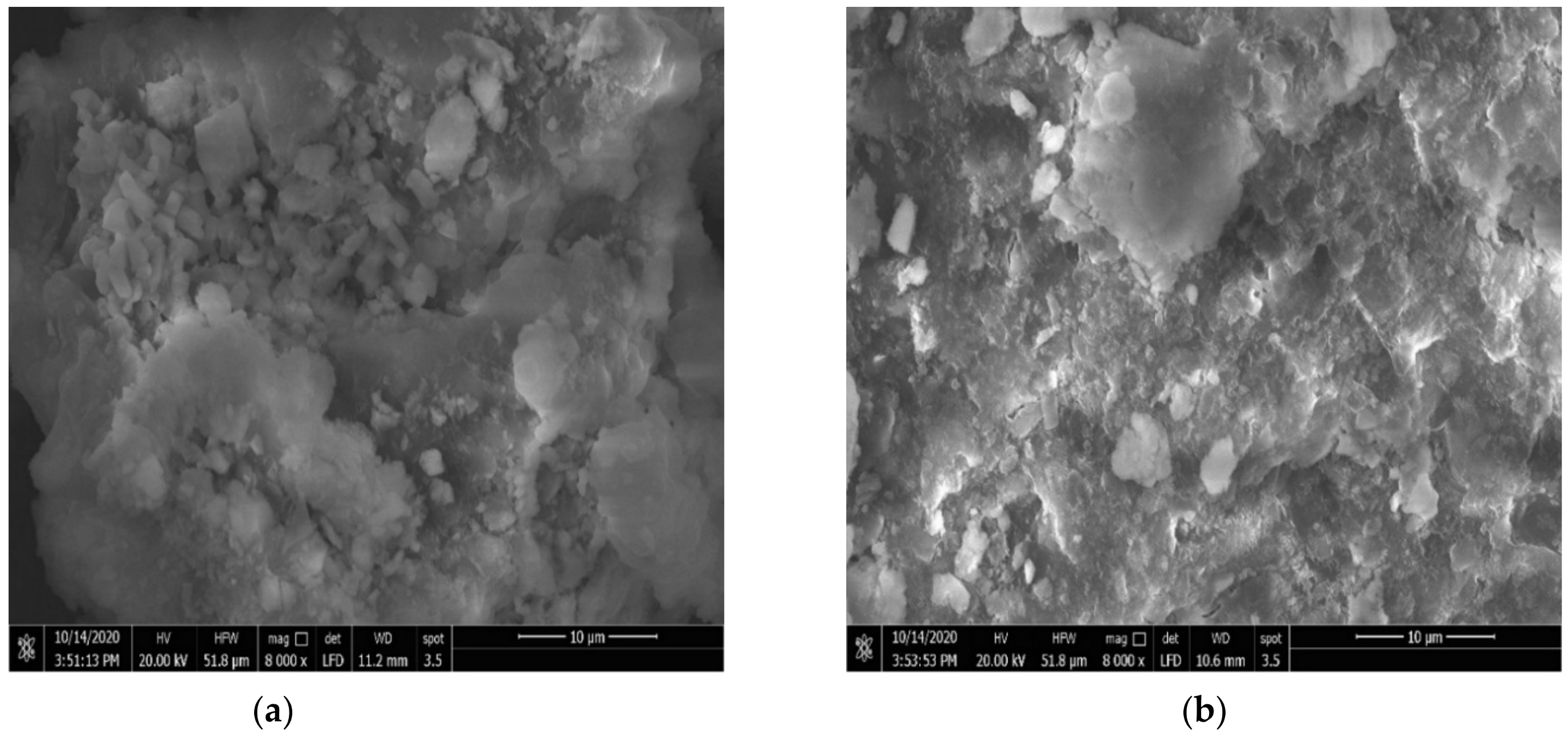
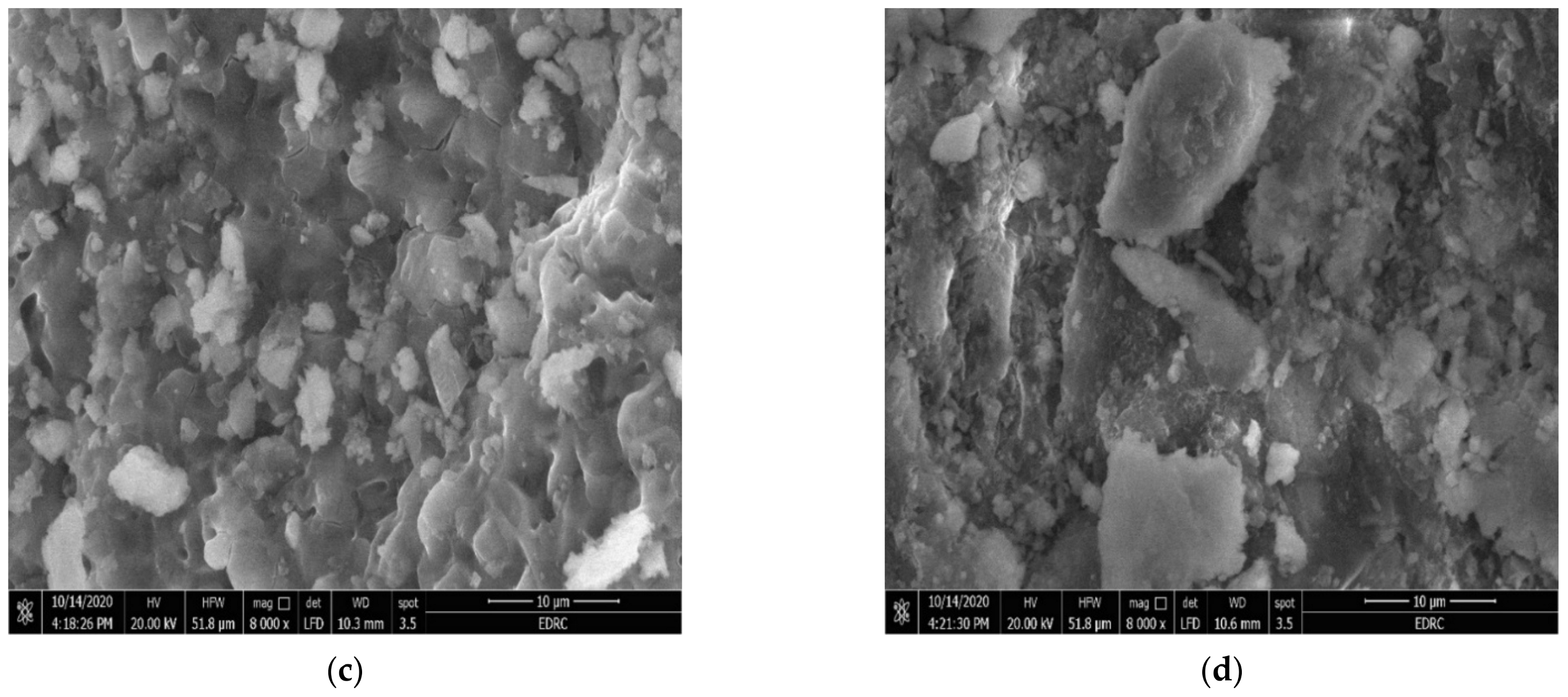
3.1.3. Scanning Electron Microscopy (SEM)
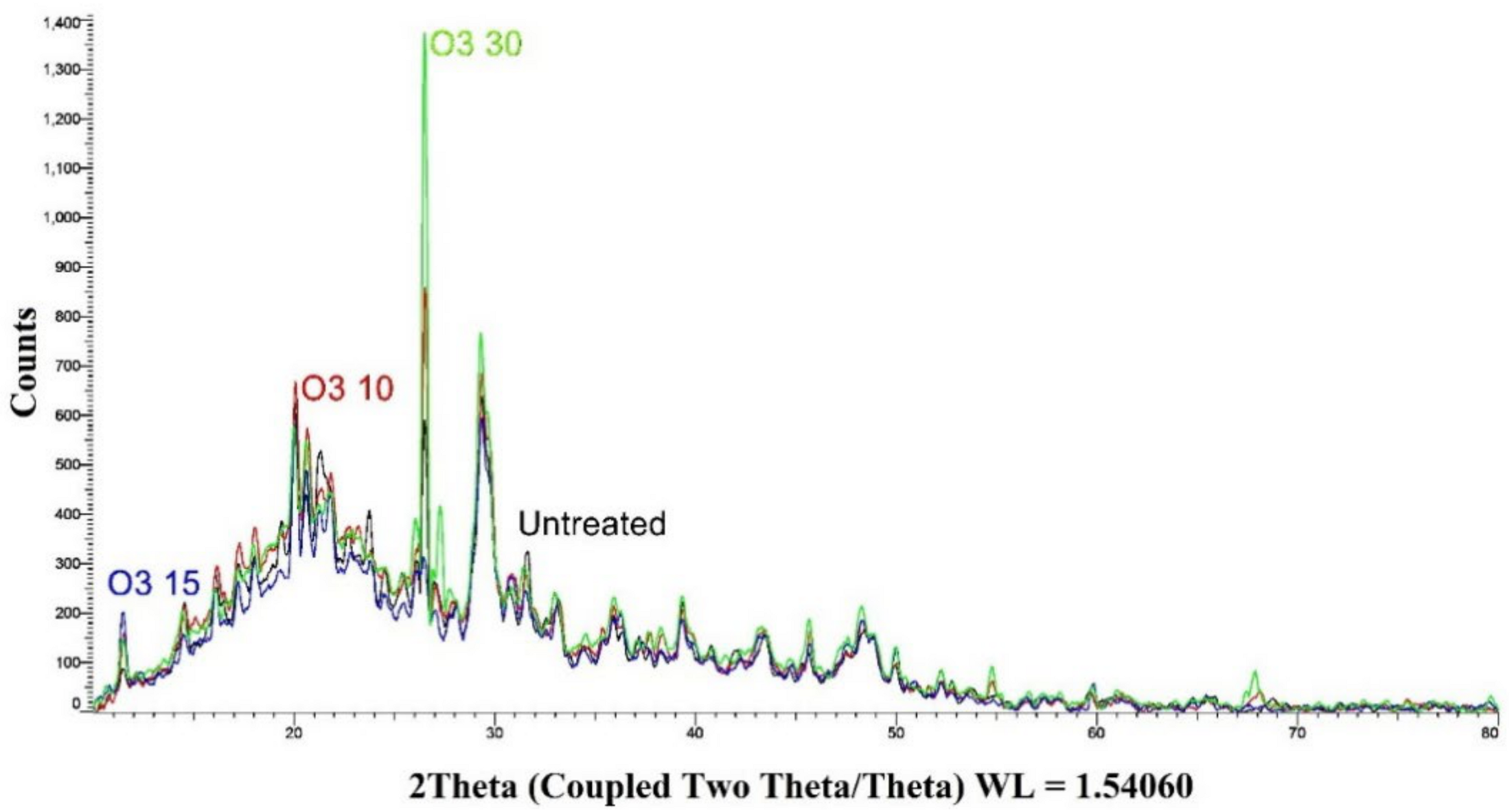
3.1.4. X-ray Diffraction (XRD)
3.2. Chemical Compositions of Ulva lactuca
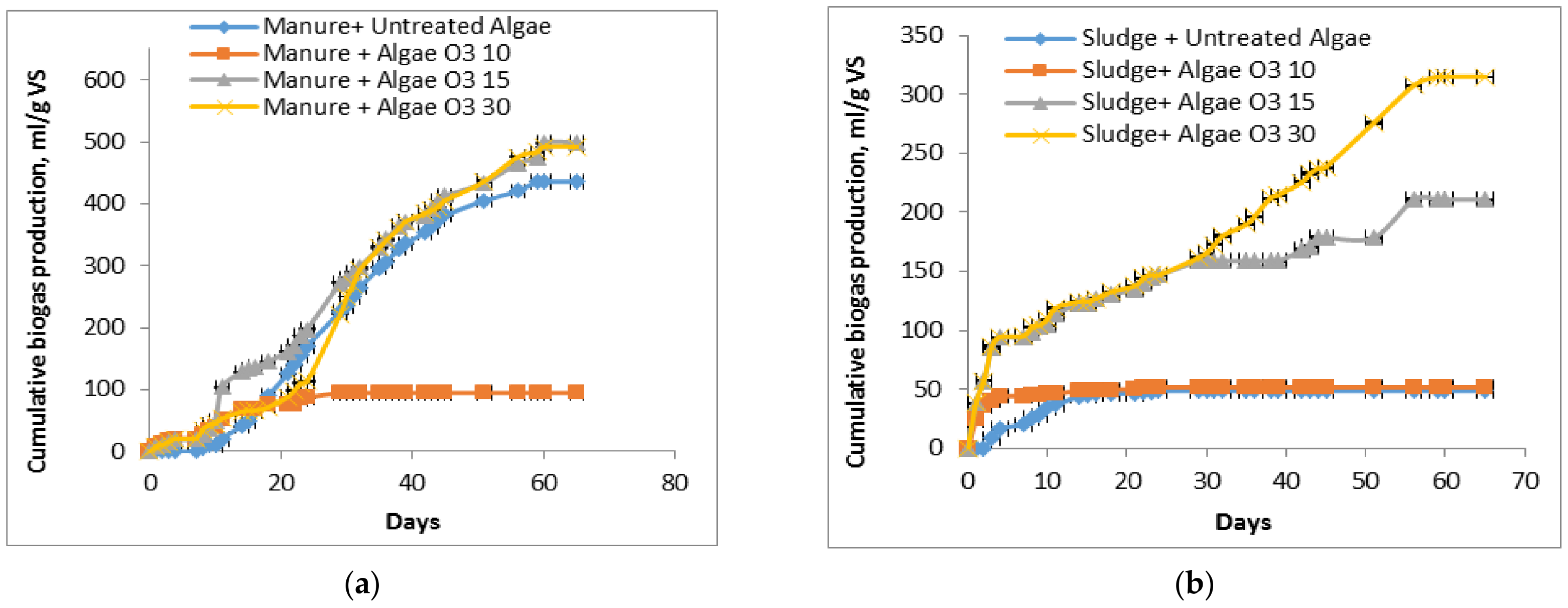
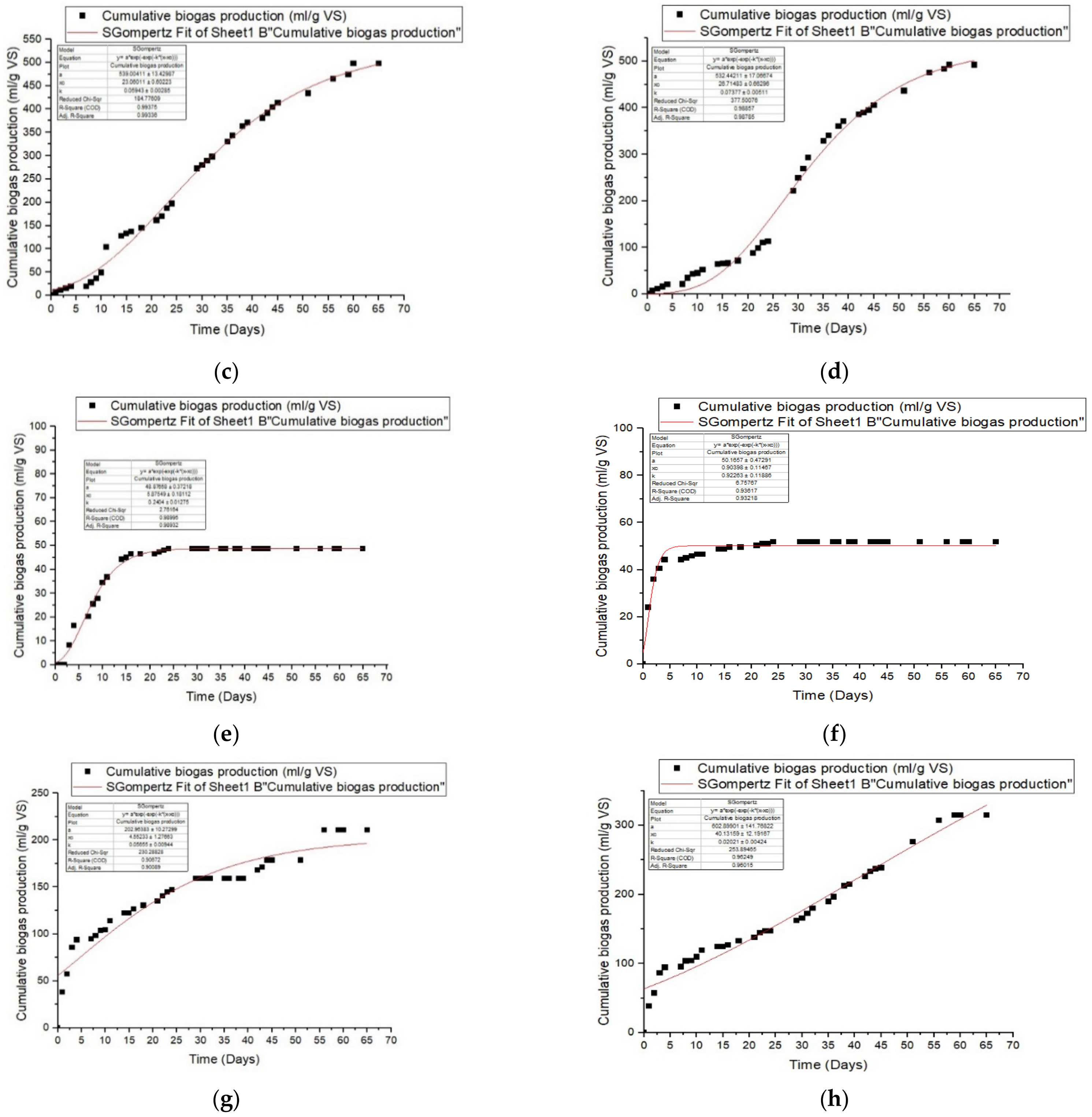
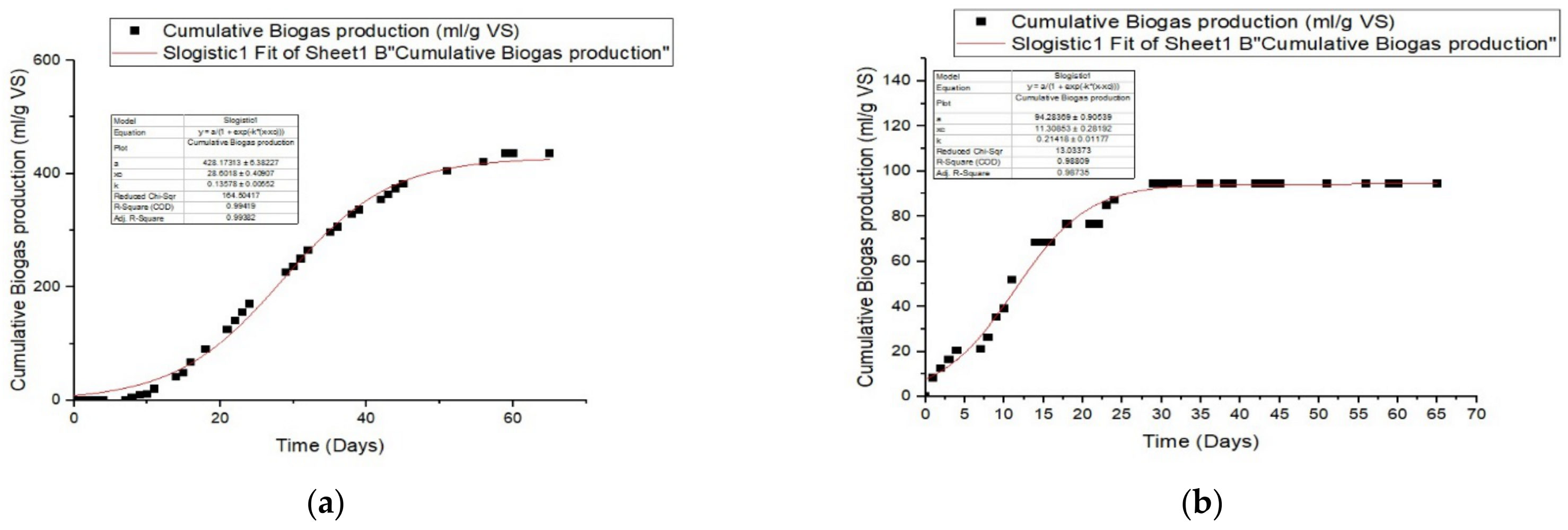
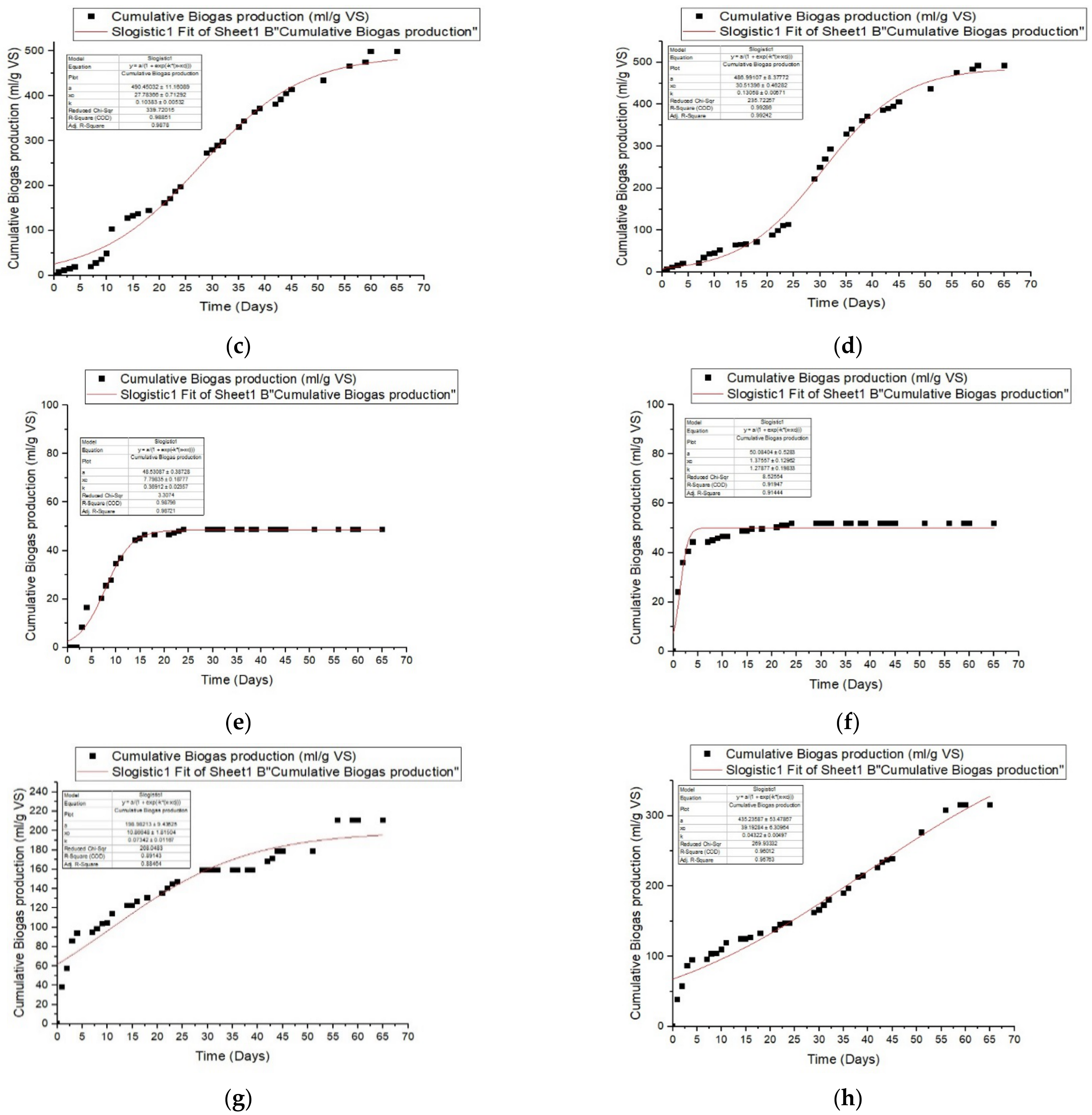
3.3. Impact of Pretreatment with Ozone on Anaerobic Digestion by Batch
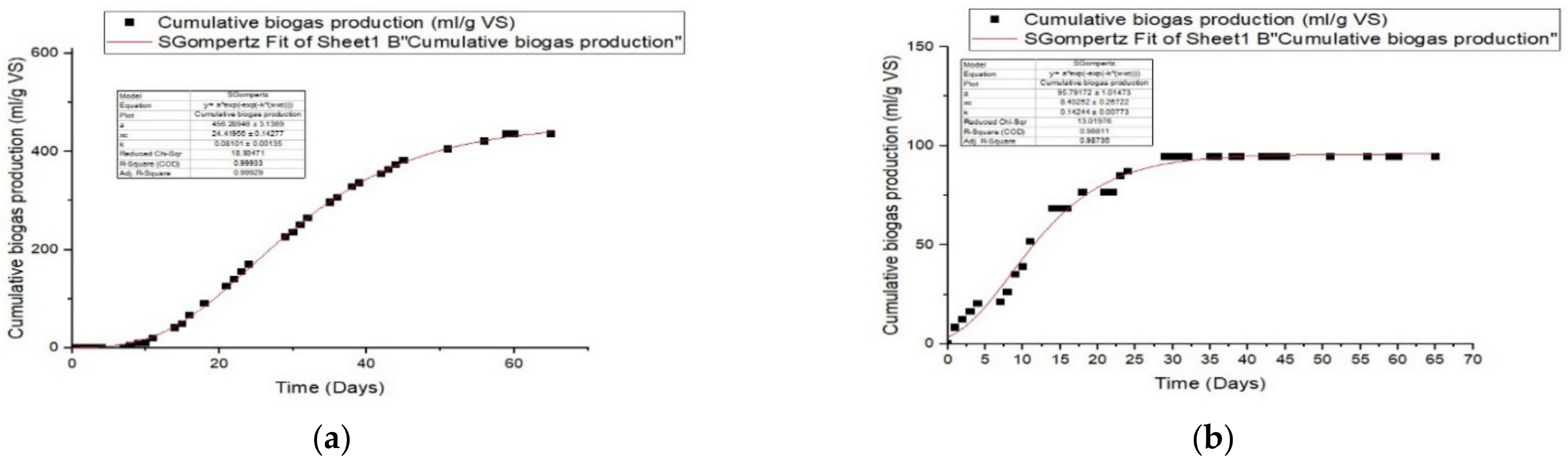
3.4. Kinetic Study
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| O3 | Ozone |
| AD | Anaerobic digestion |
| FTIR | Fourier transform infrared |
| SEM | Scanning electron microscope |
| TGA | Thermo gravimetric analysis |
| XRD | X-ray diffraction |
| TS | Total solids |
| VS | Volatile solids |
| Rm | The maximum biogas production rate (L/g VS added.d) |
| λ | The lag phase time (days) |
References
- Påledal, S.N.; Hellman, E.; Moestedt, J. The effect of temperature, storage time and collection method on biomethane potential of source separated household food waste. Waste Manag. 2018, 71, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Sarvari Horvath, I.; Tabatabaei, M.; Karimi, K.; Kumar, R. Recent Updates on Biogas Production Review. Biofuel Res. J. 2016, 3, 394–402. [Google Scholar] [CrossRef]
- Bruhn, A.; Dahl, J.; Nielsen, H.B.; Nikolaisen, L.; Rasmussen, M.B.; Markager, S.; Olesen, B.; Arias, C.; Jensen, P.D. Bioenergy potential of Ulva lactuca: Biomass yield, methane production and combustion. Bioresour. Technol. 2011, 102, 2595–2604. [Google Scholar] [CrossRef] [PubMed]
- Lora Grando, R.; de Souza Antune, A.M.; da Fonseca, F.V.; Sanchez, A.; Barrena, R.; Font, X. Technology Overview of Bio-gas Production in Anaerobic Digestion Plants: A European Evaluation of Research and Development. Renew. Sustain. Energy Rev. 2017, 80, 44–53. [Google Scholar] [CrossRef]
- Hao, H.; Liu, Z.; Zhao, F.; Ren, J.; Chang, S.; Rong, K.; Du, J. Biofuel for vehicle use in China: Current status, future potential and policy implications. Renew. Sustain. Energy Rev. 2018, 82, 645–653. [Google Scholar] [CrossRef]
- Cheng, J.J.; Timilsina, G.R. Status and barriers of advanced biofuel technologies: A review. Renew. Energy 2011, 36, 3541–3549. [Google Scholar] [CrossRef]
- Montingelli, M.; Tedesco, S.; Olabi, A. Biogas production from algal biomass: A review. Renew. Sustain. Energy Rev. 2015, 43, 961–972. [Google Scholar] [CrossRef]
- Zhu, L.; Li, Z.; Hiltunen, E. Theoretical Assessment of Biomethane Production from Algal Residues after Biodiesel Production: Biogas Production from Algal Residues. Wiley Interdiscip. Rev. Energy Environ. 2017, 7, e273. [Google Scholar] [CrossRef]
- Kwietniewska, E.; Tys, J. Process characteristics, inhibition factors and methane yields of anaerobic digestion process, with particular focus on microalgal biomass fermentation. Renew. Sustain. Energy Rev. 2014, 34, 491–500. [Google Scholar] [CrossRef]
- Siddique, M.N.; Wahid, Z.A. Achievements and perspectives of anaerobic co-digestion: A review. J. Clean. Prod. 2018, 194, 359–371. [Google Scholar] [CrossRef]
- Kumar, G.; Sivagurunathan, P.; Zhen, G.; Kobayashi, T.; Kim, S.-H.; Xu, K. Combined Pretreatment of Electrolysis and Ul-traSonication towards Enhancing Solubilization and Methane Production from Mixed Microalgae Biomass. Bioresour. Technol. 2017, 245, 196–200. [Google Scholar] [CrossRef] [PubMed]
- Montingelli, M.; Benyounis, K.; Stokes, J.R.; Olabi, A. Pretreatment of macroalgal biomass for biogas production. Energy Convers. Manag. 2016, 108, 202–209. [Google Scholar] [CrossRef]
- Ayala-Parra, P.; Liu, Y.; Sierra-Alvarez, R.; Field, J.A. Pretreatments to Enhance the Anaerobic Biodegradability of Chlorella Protothecoides Algal Biomass. Environ. Prog. Sustain. Energy 2017, 37, 418–424. [Google Scholar] [CrossRef]
- Barbot, Y.; Thomsen, L.; Benz, R. Thermo-Acidic Pretreatment of Beach Macroalgae from Rügen to Optimize Biomethane Production-Double Benefit with Simultaneous Bioenergy Production and Improvement of Local Beach and Waste Management. Mar. Drugs 2015, 13, 5681–5705. [Google Scholar] [CrossRef]
- Jard, G.; Dumas, C.; Delgenes, J.P.; Marfaing, H.; Sialve, B.; Steyer, J.P.; Carrere, H. Effect of Thermochemical Pretreatment on the Solubilization and Anaerobic Biodegradability of the Red Macroalga Palmaria Palmata. Biochem. Eng. J. 2013, 79, 253–258. [Google Scholar] [CrossRef]
- Montingelli, M.; Benyounis, K.; Quilty, B.; Stokes, J.; Olabi, A. Influence of mechanical pretreatment and organic concentration of Irish brown seaweed for methane production. Energy 2017, 118, 1079–1089. [Google Scholar] [CrossRef]
- Tedesco, S.; Barroso, T.M.; Olabi, A. Optimization of mechanical pre-treatment of Laminariaceae spp. biomass-derived biogas. Renew. Energy 2014, 62, 527–534. [Google Scholar] [CrossRef]
- Cardeña, R.; Moreno, G.; Bakonyi, P.; Buitrón, G. Enhancement of methane production from various microalgae cultures via novel ozonation pretreatment. Chem. Eng. J. 2017, 307, 948–954. [Google Scholar] [CrossRef]
- Goel, R.; Tokutomi, T.; Yasui, H. Anaerobic digestion of excess activated sludge with ozone pretreatment. Water Sci. Technol. 2003, 47, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Yasui, H.; Komatsu, K.; Goel, R.; Matsuhashi, R.; Ohashi, A.; Harada, H. Minimization of greenhouse gas emission by application of anaerobic digestion process with biogas utilization. Water Sci. Technol. 2005, 52, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Upreti, F. Ein-Mozaffari, Ozone pretreatment of wheat straw for enhanced biohydrogen production. Int. J. Hydrogen Energy 2013, 38, 10270–10276. [Google Scholar] [CrossRef]
- Pilarski, G.; Kyncl, M.; Stegenta, S.; Piechota, G. Emission of Biogas from Sewage Sludge in Psychrophilic Conditions. Waste Biomass Valorization 2020, 11, 3579–3592. [Google Scholar] [CrossRef]
- Nguyen, T.L.; Lee, D.; Chang, J.; Liu, J. Effects of ozone and peroxone on algal separation via dispersed air flotation. Colloids Surfaces B Biointerfaces 2013, 105, 246–250. [Google Scholar] [CrossRef]
- Miao, H.; Tao, W. The mechanisms of ozonation on cyanobacteria and its toxins removal. Sep. Purif. Technol. 2009, 66, 187–193. [Google Scholar] [CrossRef]
- Huang, Y.; Hong, A.; Zhang, D.; Li, L. Comparison of cell rupturing by ozonation and ultrasonication for algal lipid extraction fromChlorella vulgaris. Environ. Technol. 2014, 35, 931–937. [Google Scholar] [CrossRef]
- Cea-Barcia, G.; Buitrón, G.; Moreno, G.; Kumar, G. A cost-effective strategy for the bio-prospecting of mixed microalgae with high carbohydrate content: Diversity fluctuations in different growth media. Bioresour. Technol. 2014, 163, 370–373. [Google Scholar] [CrossRef]
- Kalpana, V.N.; Rajeswari, V.D. A Review on Green Synthesis, Biomedical Applications, and Toxicity Studies of ZnO NPs. Bioinorg. Chem. Appl. 2018, 2018, 1–12. [Google Scholar] [CrossRef]
- Tian, Y.; Yang, K.; Zheng, L.; Han, X.; Xu, Y.; Li, Y.; Li, S.; Xu, X.; Zhang, H.; Zhao, L. Modelling Biogas Production Kinetics of Various Heavy Metals Exposed Anaerobic Fermentation Process Using Sigmoidal Growth Functions. Waste Biomass Valorization 2020, 11, 4837–4848. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC International, 17th ed; AOAC International: Gathersberg, MD, USA, 2000. [Google Scholar]
- Ortiz, J.; Romero, N.; Robert, P.; Araya, J.; Lopez-Hernández, J.; Bozzo, C.; Navarrete, E.; Osorio, A.; Rios, A. Dietary fiber, amino acid, fatty acid and tocopherol contents of the edible seaweeds Ulva lactuca and Durvillaea antarctica. Food Chem. 2006, 99, 98–104. [Google Scholar] [CrossRef]
- Wilkins, D.; Rao, S.; Lu, X.; Lee, P.K. Effects of sludge inoculum and organic feedstock on active microbial commu-nities and methane yield during anaerobic digestion. Front. Microbiol. 2015, 6, 1114. [Google Scholar] [CrossRef]
- Hassaan, M.A.; Pantaleo, A.; Tedone, L.; Elkatory, M.R.; Ali, R.M.; Nemr, A.E.; Mastro, G.D. Enhancement of biogas produc-tion via green ZnO nanoparticles: Experimental results of selected herbaceous crops. Chem. Eng. Commun. 2019, 208, 1–14. [Google Scholar]
- Amirante, R.; Demastro, G.; Distaso, E.; Hassaan, M.A.; Mormando, A.; Pantaleo, A.M.; Tamburrano, P.; Tedone, L.; Clodo-veo, M.L. Effects of ultrasound and green synthesis ZnO nanoparticles on biogas production from Olive Pomace. Energy Proced. 2018, 148, 940–947. [Google Scholar]
- Hassaan, M.A.; Pantaleo, A.; Santoro, F.; Elkatory, M.R.; De Mastro, G.; El Sikaily, A.; Ragab, S.; El Nemr, A. Techno-Economic Analysis of ZnO Nanoparticles Pretreatments for Biogas Production from Barley Straw. Energies 2020, 13, 5001. [Google Scholar] [CrossRef]
- Remigi, E.U.; Buckley, C.A. Co-Digestion of High Strength/Toxic Organic Effluents in Anaerobic Digesters at Wastewater Treatment Works; Water Research Commission: Pretoria, South Africa, 2016. [Google Scholar]
- Hassaan, M.A.; Pantaleo, A.; Tedone, L.; Demastro, G. Biogas production from silage flour wheat influenced by chemical and green synthesized ZnO nanoparticles. In Proceedings of the XLVII Conference of Italian Society for Agronomy, Marsala, Italy, 12–14 September 2018. [Google Scholar]
- Nguyen, D.D.; Jeon, B.-H.; Jeung, J.H.; Rene, E.R.; Banu, J.R.; Ravindran, B.; Vu, C.M.; Ngo, H.H.; Guo, W.; Chang, S.W. Thermophilic anaerobic digestion of model organic wastes: Evaluation of biomethane production and multiple kinetic models analysis. Bioresour. Technol. 2019, 280, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Kafle, G.K.; Chen, L. Comparison on Batch Anaerobic Digestion of Five Different Livestock Manures and Prediction of Bio-chemical Methane Potential (BMP) Using Different Statistical Models. Waste Manag. 2016, 48, 492–502. [Google Scholar] [CrossRef] [PubMed]
- Donoso-Bravo, A.; Pérez-Elvira, S.; Fdz-Polanco, F. Application of simplified models for anaerobic biodegradability tests. Evaluation of pre-treatment processes. Chem. Eng. J. 2010, 160, 607–614. [Google Scholar] [CrossRef]
- Li, L.; He, Q.; Zhao, X.; Wu, D.; Wang, X.; Peng, X. Anaerobic digestion of food waste: Correlation of kinetic parameters with operational conditions and process performance. Biochem. Eng. J. 2018, 130, 1–9. [Google Scholar] [CrossRef]
- Soliman, E.A.; Elkatory, M.R.; Hashem, A.I.; Ibrahim, H.S. Synthesis and performance of maleic anhydride copolymers with alkyl linoleate or tetra-esters as pour point depressants for waxy crude oil. Fuel 2018, 211, 535–547. [Google Scholar] [CrossRef]
- Ali, R.M.; Elkatory, M.R.; Hamad, H.A. Highly active and stable magnetically recyclable CuFe2O4 as a heterogenous catalyst for efficient conversion of waste frying oil to biodiesel. Fuel 2020, 268, 117297. [Google Scholar] [CrossRef]
- Schneider, L.; Haverinen, J.; Jaakkola, M.; Lassi, U. Pretreatment and fractionation of lignocellulosic barley straw by mecha-nocatalysis. Chem. Eng. J. 2017, 327, 898–905. [Google Scholar] [CrossRef]
- Priya, D.B.; Asharani, I.V. Catalytic reduction in 4-nitrophenol using Actinodaphne madraspatana Bedd leaves-mediated palladium nanoparticles. IET Nanobiotechnol. 2018, 12, 116–126. [Google Scholar] [CrossRef]
- Sanchez-Silva, L.; López-González, D.; Villaseñor, J.; Sánchez, P.; Valverde, J.L. Thermogravimetric-mass spectrometric analysis of lignocellulosic and marine biomass pyrolysis. Bioresour. Technol. 2012, 109, 163–172. [Google Scholar] [CrossRef]
- Carrier, M.; Loppinet-Serani, A.; Denux, D.; Lasnier, J.-M.; Ham-Pichavant, F.; Cansell, F.; Aymonier, C. Thermogravimetric analysis as a new method to determine the lignocellulosic composition of biomass. Biomass Bioenergy 2011, 35, 298–307. [Google Scholar] [CrossRef]
- Chen, Z.; Hu, M.; Zhu, X.; Guo, D.; Liu, S.; Hu, Z.; Xiao, B.; Wang, J.; Laghari, M. Characteristics and kinetic study on pyrol-ysis of five lignocellulosic biomass via thermogravimetric analysis. Bioresour. Technol. 2015, 192, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Gai, C.; Zhang, Y.; Chen, W.T.; Zhang, P.; Dong, Y. Thermogravimetric and kinetic analysis of thermal decomposition char-acteristics of low-lipid microalgae. Bioresour. Technol. 2013, 150, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Ong, M.Y.; Latif, N.-I.S.A.; Leong, H.Y.; Salman, B.; Show, P.L.; Nomanbhay, S. Characterization and Analysis of Malaysian Macroalgae Biomass as Potential Feedstock for Bio-Oil Production. Energies 2019, 12, 3509. [Google Scholar] [CrossRef]
- Carpio, R.B.; Zhang, Y.; Kuo, C.-T.; Chen, W.-T.; Schideman, L.C.; De Leon, R.L. Characterization and thermal decomposition of demineralized wastewater algae biomass. Algal Res. 2019, 38, 101399. [Google Scholar] [CrossRef]
- Friis, J.; Holm, C.; Halling-Sørensen, B. Evaluation of elemental composition of algal biomass as toxical endpoint. Chemosphere 1998, 37, 2665–2676. [Google Scholar] [CrossRef]
- Pang, Y.Z.; Liu, Y.P.; Li, X.J.; Wang, K.S.; Yuan, H.R. Improving Biodegradability and Biogas Production of Corn Stover through Sodium Hydroxide Solid State Pretreatment. Energy Fuels 2008, 22, 2761–2766. [Google Scholar] [CrossRef]
- Feng, L.; Perschke, Y.M.L.; Fontaine, D.; Ward, A.J.; Eriksen, J.; Sorensen, P.; Moller, H.B. Co-ensiling of cover crops and bar-ley straw for biogas production. Renew. Energy 2019, 142, 677–683. [Google Scholar] [CrossRef]
- Nyns, E.J. Biomethanation Processes; Wiley-VCH: Berlin, Germany, 1986; pp. 207–267. [Google Scholar]
- Kivaisi, A.K.; Mtila, M. Production of biogas from water hyacinth (Eichhorniacrassipes) in a two stage bioreactor. World J. Microbiol. Biotechnol. 1997, 14, 125–131. [Google Scholar] [CrossRef]
- Mshandete, A.; Kivaisi, A.; Rubindamayugi, M.; Mattiasson, B. Anaerobic batch co-digestion of sisal pulp and fish wastes. Bioresour. Technol. 2004, 95, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Ganzoury, M.A.; Allam, N.K. Impact of nanotechnology on biogas production: A mini-review. Renew. Sustain. Energy Rev. 2015, 50, 1392–1404. [Google Scholar] [CrossRef]
- Elijah, T.; Ibifuro, A.; Yahaya, S.M. The study of cow dung as co-substrate with rice husk in biogas production. Sci. Res. Essay 2009, 9, 861–866. [Google Scholar]
- Savithramma, N.; Linga Rao, M.; Venkateswarlu, P. Isolation and Identification of Phenolic Compounds from Boswellia ovalifoliolata Bal. & Henry and Their Free Radical Scavenger Activity. Int. J. Drug Deliv. Technol. 2014, 4, 14–21. [Google Scholar]
- Pérez, M.J.; Falqué, E.; Domínguez, H. Antimicrobial Action of Compounds from Marine Seaweed. Mar. Drugs 2016, 14, 52. [Google Scholar] [CrossRef]
- Tabassum, M.R.; Xia, A.; Murphy, J.D. Seasonal variation of chemical composition and biomethane production from the brown seaweed Ascophyllum nodosum. Bioresour. Technol. 2016, 216, 219–226. [Google Scholar] [CrossRef]
- Moen, E.; Horn, S.; Østgaard, K. Biological degradation of Ascophyllum nodosum. Environ. Biol. Fishes 1997, 9, 347–357. [Google Scholar] [CrossRef]
- Tsapekos, P.; Kougias, P.; Angelidaki, I. Biogas production from ensiled meadow grass; effect of mechanical pretreatments and rapid determination of substrate biodegradability via physicochemical methods. Bioresour. Technol. 2015, 182, 329–335. [Google Scholar] [CrossRef]
- Tsapekos, P.; Kougias, P.G.; Egelund, H.; Larsen, U.; Pedersen, J.; Trenel, P.; Angelidaki, I. Mechanical pretreatment at har-vesting increases the bioenergy output from marginal land grasses. Renew. Energy 2017, 111, 914–921. [Google Scholar] [CrossRef]
- Balsari, P.; Menardo, S.; Airoldi, G. Effect of Physical and Thermal Pre-Treatment on Biogas Yield of Some Agricultural By-Products. Progress in Biogas II; German Society for Sustainable Biogas and Bioenergy Utilisation: Stuttgart, Germany, 2011. [Google Scholar]
- Allen, E.; Browne, J.; Hynes, S.; Murphy, J. The potential of algae blooms to produce renewable gaseous fuel. Waste Manag. 2013, 33, 2425–2433. [Google Scholar] [CrossRef]
- Deepanraj, B.; Sivasubramanian, V.; Jayaraj, S. Experimental and Kinetic Study on Anaerobic Digestion of FoodWaste: The Effect of Total Solids and PH. J. Renew. Sustain. Energy 2015, 7, 063104. [Google Scholar] [CrossRef]
- Mao, C.; Wang, X.; Xi, J.; Feng, Y.; Ren, G. Linkage of Kinetic Parameters with Process Parameters and Operational Condi-tions during Anaerobic Digestion. Energy 2017, 135, 352–360. [Google Scholar] [CrossRef]

| Experiment | Pretreatment | Incubation Temp. (°C) | I/S Ratio |
|---|---|---|---|
| Batch 1 | Manure + algae untreated | 37 ± 1 | 20:1.5 |
| Batch 2 | Sludge + Untreated Algae | 37 ± 1 | 20:1.5 |
| Batch 3 | Manure + Algae O3 (10 min) | 37 ± 1 | 20:1.5 |
| Batch 4 | Manure + Algae O3 (15 min) | 37 ± 1 | 20:1.5 |
| Batch 5 | Manure + Algae O3 (30 min) | 37 ± 1 | 20:1.5 |
| Batch 6 | Sludge + Algae O3 (10 min) | 37 ± 1 | 20:1.5 |
| Batch 7 | Sludge + Algae O3 (15 min) | 37 ± 1 | 20:1.5 |
| Batch 8 | Sludge + Algae O3 (30 min) | 37 ± 1 | 20:1.5 |
| Proximate Tests | Ulva lactuca | Manure | Activated Sludge |
|---|---|---|---|
| DM% | 84.90 | 79.67 | 80.51 |
| Ash% | 29.21 | 17.78 | 16.85 |
| VS% | 70.79 | 82.22 | 83.15 |
| C% | 22.99 | 49.80 | - |
| N% | 2.44 | 4.25 | - |
| H% | 4.55 | 5.43 | - |
| C/N | 9.42 | 13.70 | - |
| Modified Gompertz Model | ||||||
| Manure | ||||||
| R2 | Predicted P (ml/g VS) | Differences (%) | Rmax mL/gVS.day | λ(day) | RMSE | |
| untreated | 0.999 | 456.29 | 0.87 | 24.42 | 0.081 | 4.16 |
| 10 O3 | 0.987 | 95.79 | 1.33 | 8.40 | 0.142 | 3.45 |
| 15 O3 | 0.993 | 539.00 | 0.43 | 23.06 | 0.059 | 12.98 |
| 30 O3 | 0.988 | 532.44 | 1.98 | 26.71 | 0.074 | 18.58 |
| Sludge | ||||||
| untreated | 0.989 | 48.88 | 0.26 | 5.88 | 0.240 | 1.59 |
| 10 O3 | 0.932 | 50.17 | 3.06 | 0.90 | 0.923 | 2.49 |
| 15 O3 | 0.901 | 202.96 | 6.80 | 4.55 | 0.057 | 14.51 |
| 30 O3 | 0.960 | 602.90 | 4.53 | 40.13 | 0.020 | 15.24 |
| Logistic function model | ||||||
| Manure | ||||||
| R2 | Predicted P (ml/g VS) | Differences (%) | Rmax mL/gVS.day | λ(day) | RMSE | |
| untreated | 0.994 | 428.17 | 2.44 | 28.60 | 0.136 | 12.26 |
| 10 O3 | 0.987 | 94.28 | 0.23 | 11.31 | 0.214 | 3.45 |
| 15 O3 | 0.988 | 490.45 | 3.61 | 27.78 | 0.104 | 17.62 |
| 30 O3 | 0.992 | 486.99 | 2.10 | 30.51 | 0.131 | 14.68 |
| Sludge | ||||||
| untreated | 0.987 | 48.53 | 0.45 | 7.80 | 0.369 | 1.74 |
| 10 O3 | 0.914 | 50.08 | 3.22 | 1.38 | 1.28 | 2.79 |
| 15 O3 | 0.885 | 198.98 | 7.32 | 10.87 | 0.073 | 15.56 |
| 30 O3 | 0.958 | 435.24 | 4.06 | 39.19 | 0.043 | 15.71 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hassaan, M.A.; El Nemr, A.; Elkatory, M.R.; Eleryan, A.; Ragab, S.; El Sikaily, A.; Pantaleo, A. Enhancement of Biogas Production from Macroalgae Ulva latuca via Ozonation Pretreatment. Energies 2021, 14, 1703. https://doi.org/10.3390/en14061703
Hassaan MA, El Nemr A, Elkatory MR, Eleryan A, Ragab S, El Sikaily A, Pantaleo A. Enhancement of Biogas Production from Macroalgae Ulva latuca via Ozonation Pretreatment. Energies. 2021; 14(6):1703. https://doi.org/10.3390/en14061703
Chicago/Turabian StyleHassaan, Mohamed A., Ahmed El Nemr, Marwa R. Elkatory, Ahmed Eleryan, Safaa Ragab, Amany El Sikaily, and Antonio Pantaleo. 2021. "Enhancement of Biogas Production from Macroalgae Ulva latuca via Ozonation Pretreatment" Energies 14, no. 6: 1703. https://doi.org/10.3390/en14061703
APA StyleHassaan, M. A., El Nemr, A., Elkatory, M. R., Eleryan, A., Ragab, S., El Sikaily, A., & Pantaleo, A. (2021). Enhancement of Biogas Production from Macroalgae Ulva latuca via Ozonation Pretreatment. Energies, 14(6), 1703. https://doi.org/10.3390/en14061703