A Comparative Study of Biofuels and Fischer–Tropsch Diesel Blends on the Engine Combustion Performance for Reducing Exhaust Gaseous and Particulate Emissions
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Engine and Fuel Properties
- To obtain a density based on the range for diesel fuel established in EN 590 by the European Committee for Standardization, with a value below biodiesel density and higher than F-T diesel;
- To obtain a lower heating value (LHV) resembling the value for diesel fuel, with a value between that of the biodiesel and the F-T diesel. This would possibly prevent substantial variation in injection duration in comparison with pump diesel and therefore maintain the injection pattern of the engine [11];
- To obtain a potential benefit related to the engine emissions by increasing oxygen content (both alternative fuels have the same oxygen content) and decreasing aromatic content;
- The biodiesel fraction that was introduced has been chosen to balance the lower lubricity of ethanol that could influence the final blend value.
| ULSD | F-T Diesel | Ethanol | Biodiesel | E15FTD50B35 | E15D50B35 | |
|---|---|---|---|---|---|---|
| Chemical formula | C14H26.09 | C16.89H35.77 | C2H5OH | C18.96H35.92O2.9 | C15.52H31.53O1.24 | C14.13H26.88O1.21 |
| Cetane number | 53.9 | 80 | 8 | 54.7 | 58.5 1 | 47.7 1 |
| Lower heating value [MJ/kg] | 42.7 | 43.9 | 26.8 | 37.4 | 38.9 1 | 38.5 1 |
| Latent heat of vaporization [kJ/kg] | 243 | 339 | 858 | 216 | − | − |
| Density at 20 °C [kg/m3] | 827.1 | 784.6 | 789.4 | 883.7 | 820.01 2 | 841.26 2 |
| Lubricity at 60 °C [μm] | 312 | 560 | 656 | 233 | ND | ND |
| Stoichiometric fuel/air ratio | 1/14.55 | 1/14.91 | 1/9.00 | 1/11.77 | 1/13.20 | 1/12.92 |
| Aromatics (wt %) | 24.4 | 0.3 | 0 | ~0 | ND | ND |
| C (wt %) | 86.5 | 84.8 | 52.14 | 77.2 | 77.31 | 78.03 |
| H (wt %) | 13.5 | 15.2 | 13.13 | 12 | 13.68 | 12.96 |
| O (wt %) | 0 | 0 | 34.73 | 10.8 | 9.0 | 9.0 |
2.2. Experimental Setup and Materials
2.3. Error Analysis and Uncertainty Analysis
3. Results and Discussions
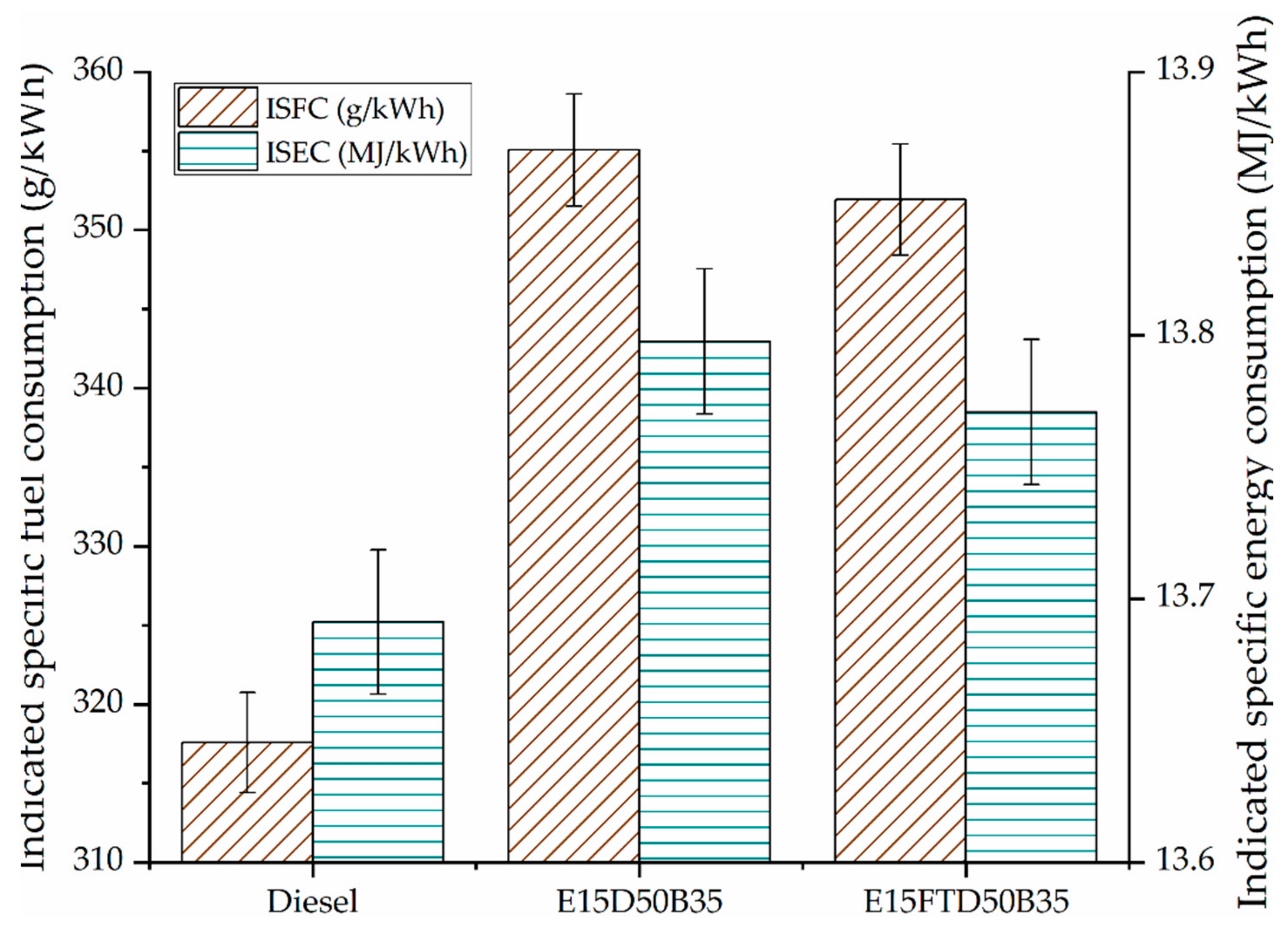
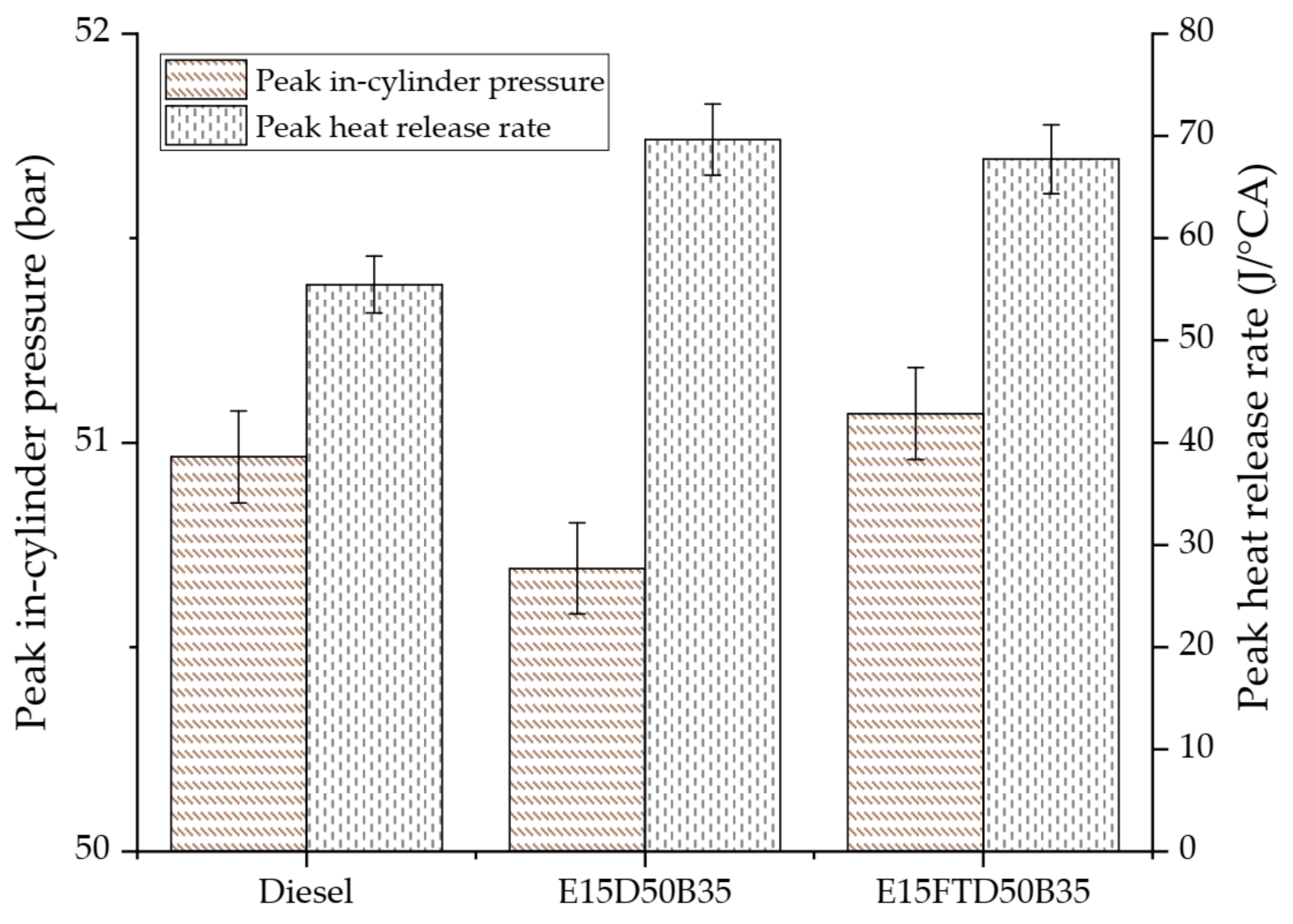
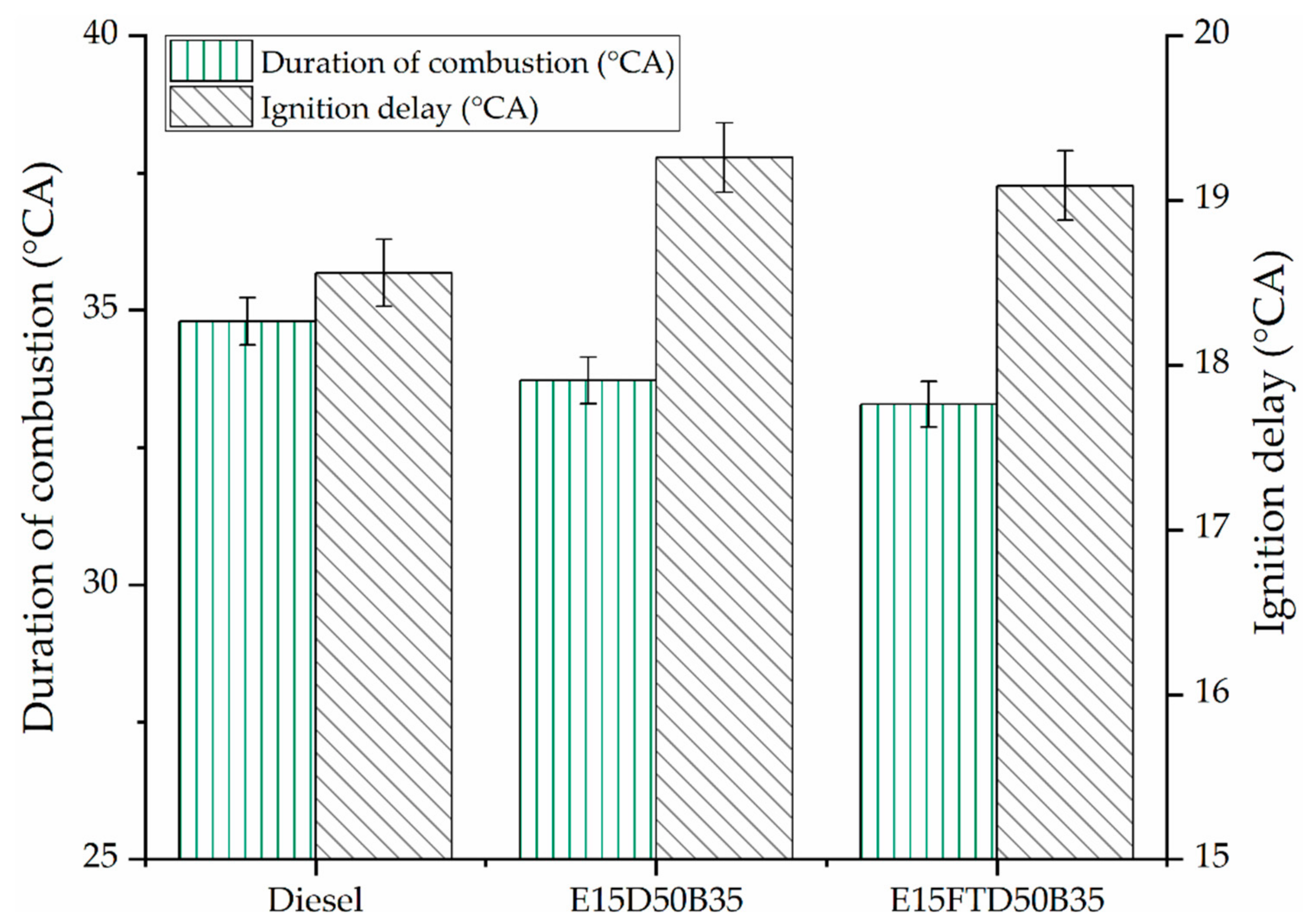
3.1. Performance and Combustion Characteristics
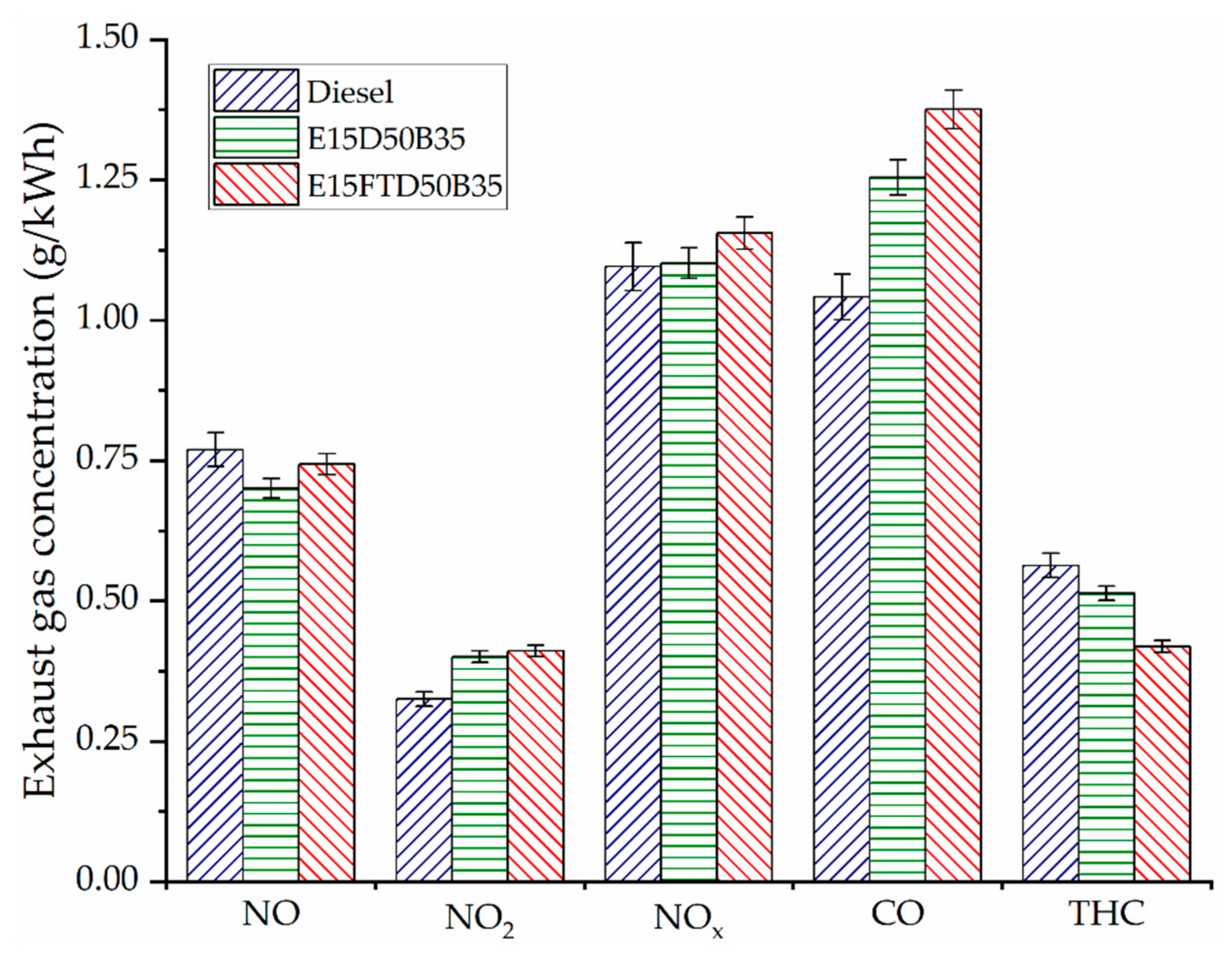
3.2. Engine Exhaust Emissions
3.3. Impact of Biofuels on a Diesel Oxidation Catalyst (DOC)
3.3.1. CO Oxidation over a Diesel Oxidation Catalyst
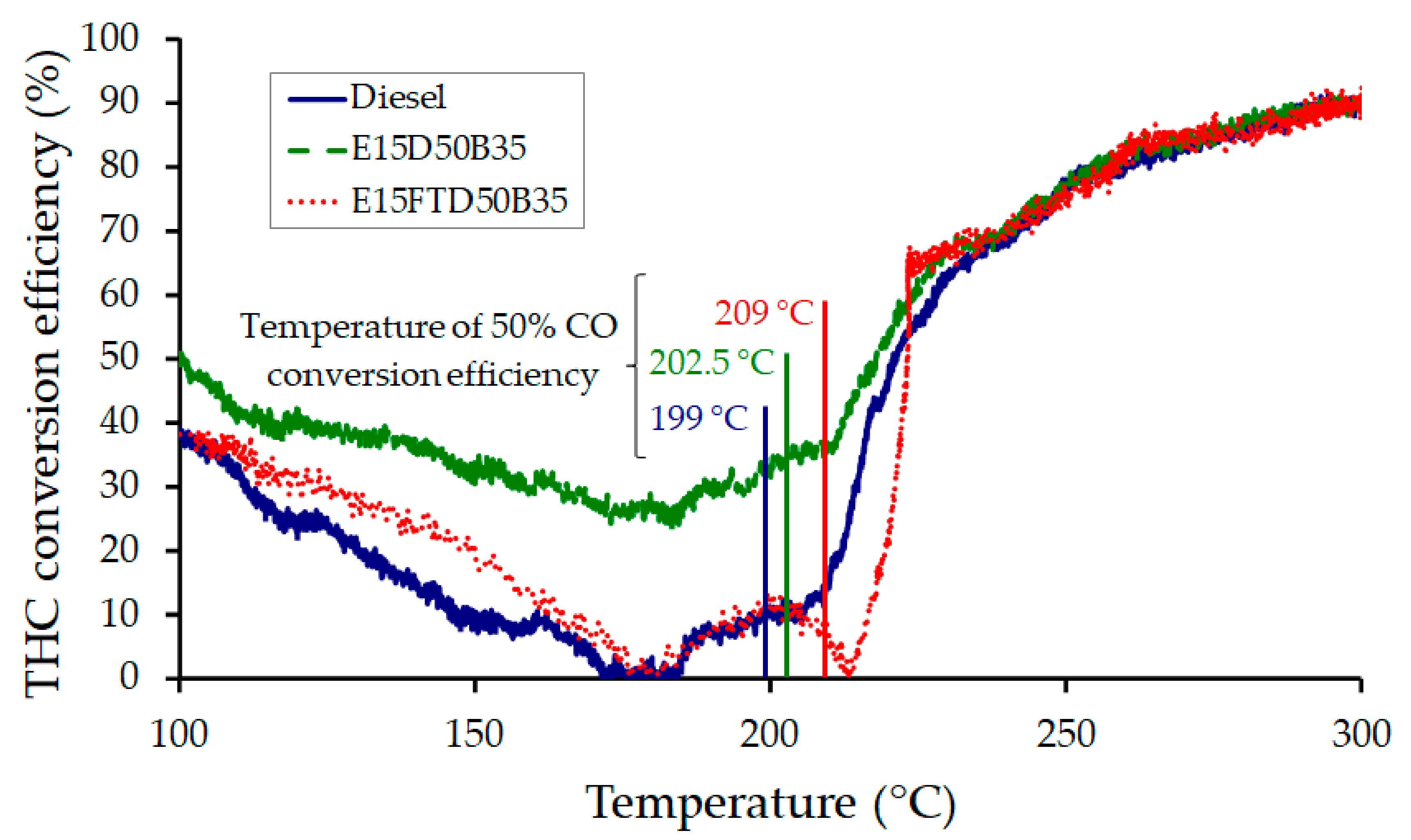
3.3.2. THC Oxidation over a Diesel Oxidation Catalyst
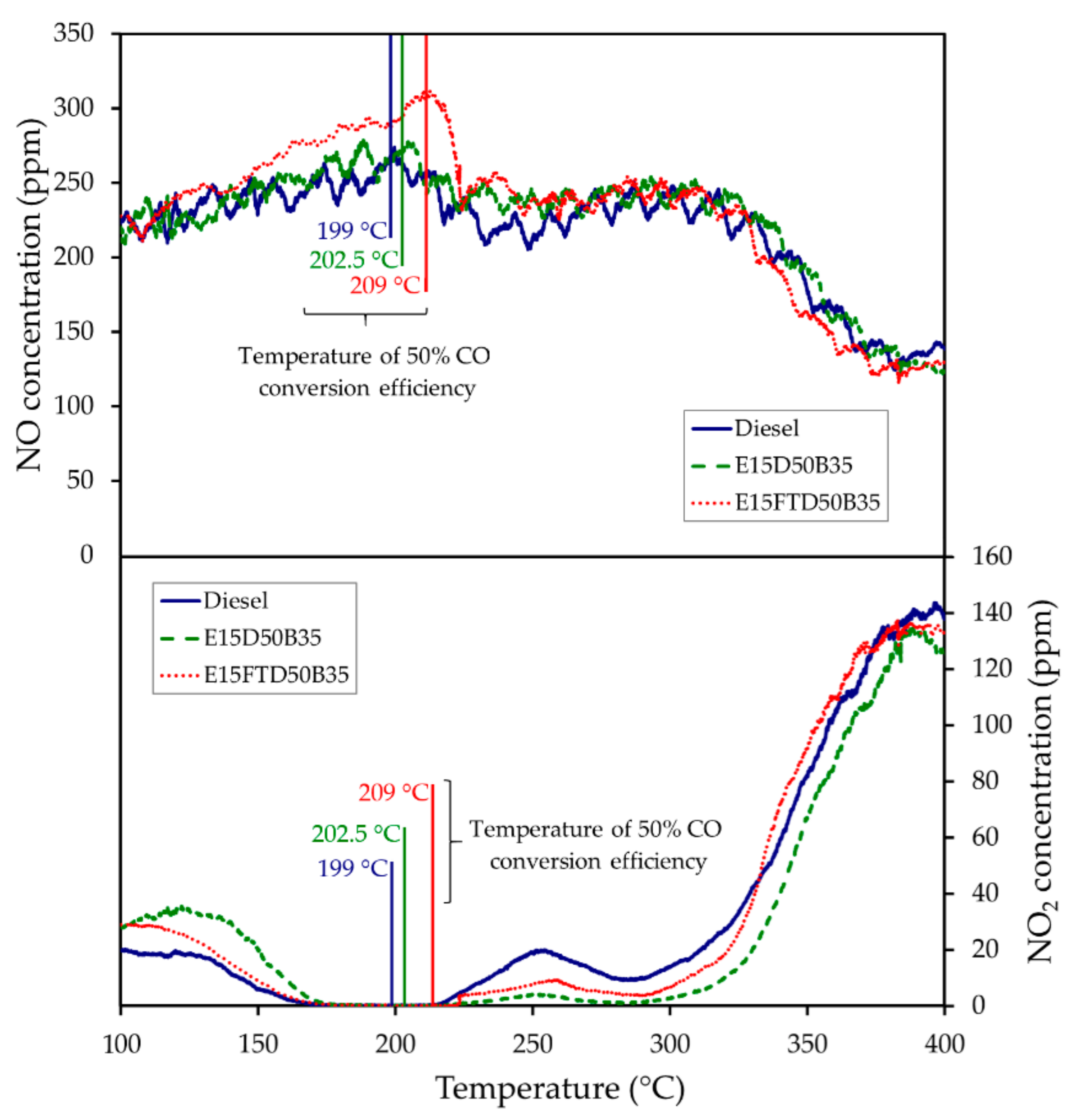
3.3.3. NO to NO2 Oxidation over DOC Catalyst
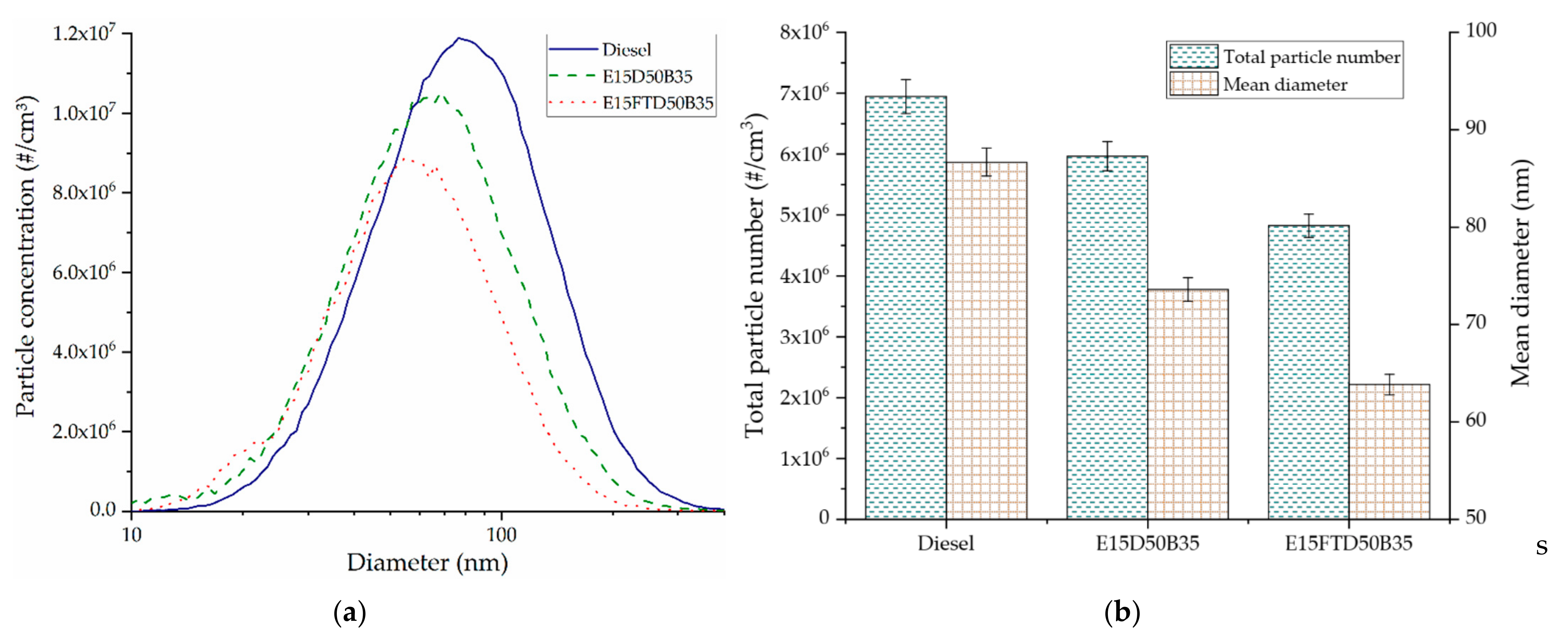
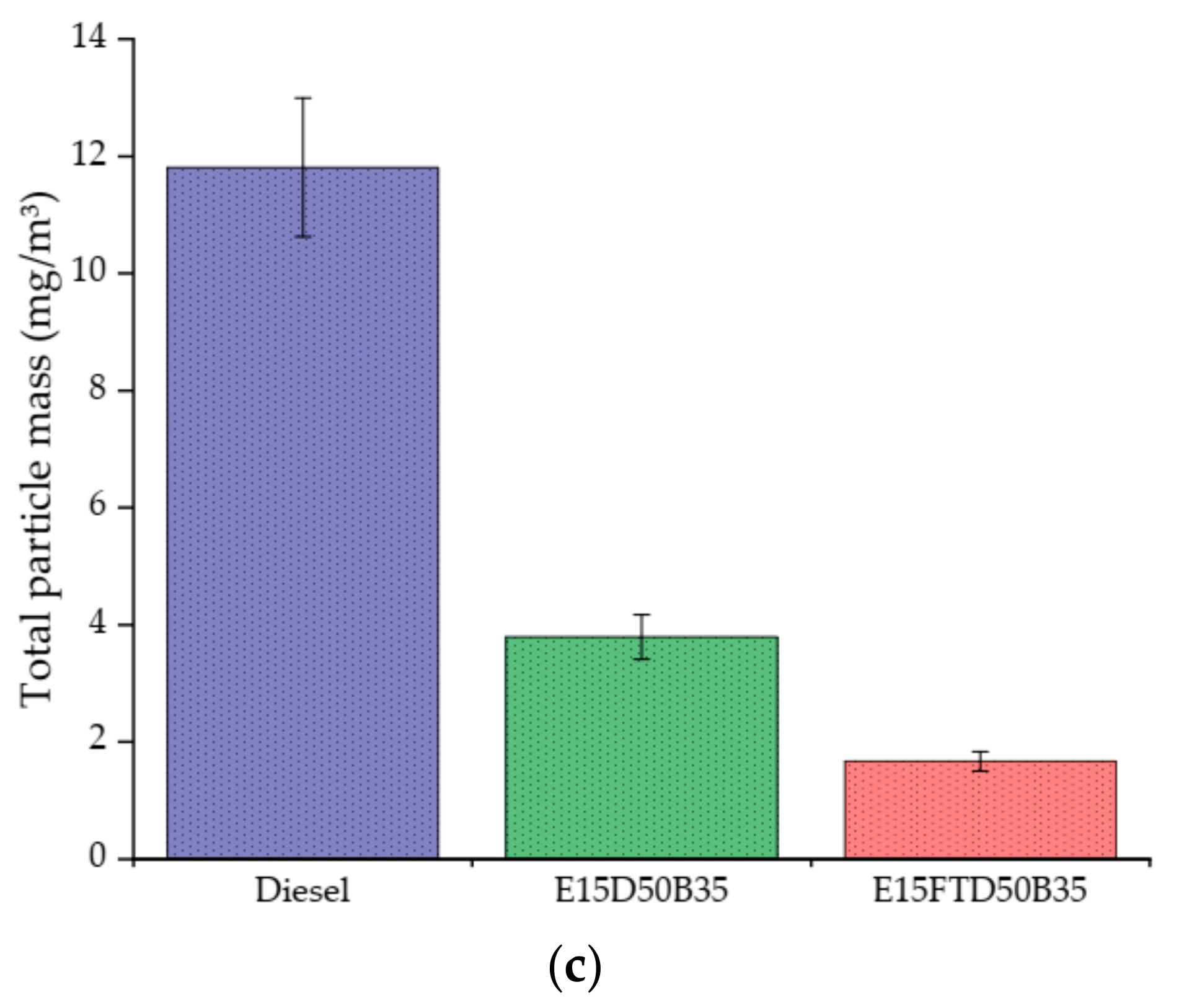
3.4. Particle Size Distribution
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BTDC | Before the top dead center |
| BTL | Biomass-to-liquid |
| CA | Crank angle |
| CAD | Crank angle degree |
| CN | Cetane number |
| CO | Carbon monoxide |
| CO2 | Carbon dioxide |
| CPC | Condensation particle counter |
| CTL | Coal-to-liquid |
| DMA | Differential mobility analyzer |
| DOC | Diesel oxidation catalyst |
| FTIR | Fourier Transform Infrared |
| GHSV | Gas hourly space velocity |
| GTL | Gas-to-liquid |
| HC | Hydrocarbon |
| HRR | Heat release rate |
| ICE | Internal combustion engines |
| ID | Ignition delay |
| IMEP | Indicated mean effective pressure |
| ISEC | Indicated specific energy consumption |
| ISFC | Indicated specific fuel consumption |
| LHV | Lower heating value |
| NO | Nitrogen oxide |
| NO2 | Nitrogen dioxide |
| NOx | Nitrogen oxides |
| PM | Particulate matter |
| PSD | Particle size distribution |
| SMPS | Scanning Mobility Particle Sizer |
| THC | Total hydrocarbons |
| ULSD | Ultra-low sulfur diesel |
References
- International Energy Agency (IEA). World Oil Final Consumption by Sector. Available online: https://www.iea.org/reports/key-world-energy-statistics-2020/final-consumption (accessed on 21 October 2020).
- Kalghatgi, G. Is it really the end of internal combustion engines and petroleum in transport? Appl. Energy 2018, 225, 965–974. [Google Scholar] [CrossRef]
- Unglert, M.; Bockey, D.; Bofinger, C.; Buchholz, B.; Fisch, G.; Luther, R.; Müller, M.; Schaper, K.; Schmitt, J.; Schröder, O.; et al. Action areas and the need for research in biofuels. Fuel 2020, 268, 117227. [Google Scholar] [CrossRef]
- Puškár, M.; Kopas, M. System based on thermal control of the HCCI technology developed for reduction of the vehicle NOX emissions in order to fulfil the future standard Euro 7. Sci. Total Environ. 2018, 643, 674–680. [Google Scholar] [CrossRef] [PubMed]
- Çelebi, Y.; Aydın, H. An overview on the light alcohol fuels in diesel engines. Fuel 2019, 236, 890–911. [Google Scholar] [CrossRef]
- De Oliveira, A.; de Morais, A.M.; Valente, O.S.; Sodré, J.R. Combustion characteristics, performance and emissions from a diesel power generator fuelled by B7-ethanol blends. Fuel Process. Technol. 2015, 139, 67–72. [Google Scholar] [CrossRef]
- Mahmoudi, H.; Jahangiri, H.; Doustdar, O.; Akbari, N.; Wood, J.; Tsolakis, A.; Wyszynski, M.L. Maximizing paraffin to olefin ratio employing simulated nitrogen-rich syngas via Fischer-Tropsch process over Co3O4/SiO2 catalysts. Fuel Process. Technol. 2020, 208, 106477. [Google Scholar] [CrossRef]
- Okoye-Chine, C.G.; Moyo, M.; Liu, X.; Hildebrandt, D. A critical review of the impact of water on cobalt-based catalysts in Fischer-Tropsch synthesis. Fuel Process. Technol. 2019, 192, 105–129. [Google Scholar] [CrossRef]
- Zhang, Y.; Mathieu, O.; Petersen, E.L.; Bourque, G.; Curran, H.J. Assessing the predictions of a NO x kinetic mechanism on recent hydrogen and syngas experimental data. Combust. Flame 2017, 182, 122–141. [Google Scholar] [CrossRef]
- Sajjad, H.; Masjuki, H.H.; Varman, M.; Khan, M.M.R.; Arbab, M.I.; Imtenan, S.; Sanjid, A. Comparative Study of Biodiesel, GTL Fuel and Their Blends in Context of Engine Performance and Exhaust Emission. Procedia Eng. 2014, 90, 466–471. [Google Scholar] [CrossRef]
- Lapuerta, M.; Armas, O.; Hernández, J.J.; Tsolakis, A. Potential for reducing emissions in a diesel engine by fuelling with conventional biodiesel and Fischer–Tropsch diesel. Fuel 2010, 89, 3106–3113. [Google Scholar] [CrossRef]
- Sajjad, H.; Masjuki, H.H.; Varman, M.; Kalam, M.A.; Arbab, M.I.; Imtenan, S.; Ashraful, A.M. Influence of gas-to-liquid (GTL) fuel in the blends of Calophyllum inophyllum biodiesel and diesel: An analysis of combustion–performance–emission characteristics. Energy Convers. Manag. 2015, 97, 42–52. [Google Scholar] [CrossRef]
- Soloiu, V.; Gaubert, R.; Moncada, J.; Wiley, J.; Williams, J.; Harp, S.; Ilie, M.; Molina, G.; Mothershed, D. Reactivity controlled compression ignition and low temperature combustion of Fischer-Tropsch Fuel Blended with n-butanol. Renew. Energy 2019, 134, 1173–1189. [Google Scholar] [CrossRef]
- Lapuerta, M.; Rodríguez-Fernandez, J.; García-Contreras, R.; Bogarra, M. Molecular interactions in blends of alcohols with diesel fuels: Effect on stability and distillation. Fuel 2015, 139, 171–179. [Google Scholar] [CrossRef]
- Sun, D.; Wang, T.; Cao, Y.; Yang, T.; Jiang, Q.; Fan, B. The Influence of F-T Diesel/Methanol Micro-Emulsified Fuels on Emission Characteristics of a Diesel Engine. Chin. Intern. Combust. Engine Eng. 2017, 38, 41–51. [Google Scholar]
- Yang, T.; Wang, T.; Qiao, J.; Gao, J.; Feng, Y.; Sun, D. Influence of the Methanol Proportion on the Combustion Characteristics of Methanol-Biodiesel -F-T Diesel Blended Fuel. In SAE Technical Papers; SAE International: Warrendale, PA, USA, 2017; Volume 1. [Google Scholar]
- Jiao, Y.; Liu, R.; Zhang, Z.; Yang, C.; Zhou, G.; Dong, S.; Liu, W. Comparison of combustion and emission characteristics of a diesel engine fueled with diesel and methanol-Fischer-Tropsch diesel-biodiesel-diesel blends at various altitudes. Fuel 2019, 243, 52–59. [Google Scholar] [CrossRef]
- Rodríguez-Fernández, J.; Tsolakis, A.; Theinnoi, K.; Snowball, J.; Sawtell, A.; York, A.P.E. Engine Performance and Emissions from Dual Fuelled Engine with In-Cylinder Injected Diesel Fuels and In-Port Injected Bioethanol. In SAE Technical Papers; SAE International: Warrendale, PA, USA, 2009. [Google Scholar] [CrossRef]
- May-Carle, J.-B.; Pidol, L.; Nicolle, A.; Anderlohr, J.M.; Togbé, C.; Dagaut, P. Experimental and Numerical Study of F-T/Biodiesel/Bioethanol Surrogate Fuel Oxidation in Jet-Stirred Reactor. Combust. Sci. Technol. 2012, 184, 901–915. [Google Scholar] [CrossRef]
- Pidol, L.; Lecointe, B.; Starck, L.; Jeuland, N. Ethanol–biodiesel–Diesel fuel blends: Performances and emissions in conventional Diesel and advanced Low Temperature Combustions. Fuel 2012, 93, 329–338. [Google Scholar] [CrossRef]
- Martins, J.; Brito, F.P. Alternative fuels for internal combustion engines. Energies 2020, 13, 4086. [Google Scholar] [CrossRef]
- Fayad, M.A.; Tsolakis, A.; Martos, F.J. Influence of alternative fuels on combustion and characteristics of particulate matter morphology in a compression ignition diesel engine. Renew. Energy 2020, 149, 962–969. [Google Scholar] [CrossRef]
- Fayad, M.A.; Herreros, J.M.; Martos, F.J.; Tsolakis, A. Role of Alternative Fuels on Particulate Matter (PM) Characteristics and Influence of the Diesel Oxidation Catalyst. Environ. Sci. Technol. 2015, 49, 11967–11973. [Google Scholar] [CrossRef]
- Herreros, J.M.; Schroer, K.; Sukjit, E.; Tsolakis, A. Extending the environmental benefits of ethanol–diesel blends through DGE incorporation. Appl. Energy 2015, 146, 335–343. [Google Scholar] [CrossRef]
- Fayad, M.A.; Fernàndez-Rodríguez, D.; Herreros, J.M.; Lapuerta, M.; Tsolakis, A. Interactions between aftertreatment systems architecture and combustion of oxygenated fuels for improved low temperature catalysts activity. Fuel 2018, 229, 189–197. [Google Scholar] [CrossRef]
- Hergueta, C.; Tsolakis, A.; Herreros, J.M.; Bogarra, M.; Price, E.; Simmance, K.; York, A.P.E.; Thompsett, D. Impact of bio-alcohol fuels combustion on particulate matter morphology from efficient gasoline direct injection engines. Appl. Energy 2018, 230, 794–802. [Google Scholar] [CrossRef]
- Moffat, R.J. Describing the uncertainties in experimental results. Exp. Therm. Fluid Sci. 1988, 1, 3–17. [Google Scholar] [CrossRef]
- Pradelle, F.; Leal Braga, S.; Fonseca de Aguiar Martins, A.R.; Turkovics, F.; Nohra Chaar Pradelle, R. Performance and combustion characteristics of a compression ignition engine running on diesel-biodiesel-ethanol (DBE) blends—Potential as diesel fuel substitute on an Euro III engine. Renew. Energy 2019, 136, 586–598. [Google Scholar] [CrossRef]
- Guarieiro, L.L.N.; de Almeida Guerreiro, E.T.; dos Santos Amparo, K.K.; Manera, V.B.; Regis, A.C.D.; Santos, A.G.; Ferreira, V.P.; Leão, D.J.; Torres, E.A.; de Andrade, J.B. Assessment of the use of oxygenated fuels on emissions and performance of a diesel engine. Microchem. J. 2014, 117, 94–99. [Google Scholar] [CrossRef]
- Chacko, N.; Jeyaseelan, T. Comparative evaluation of graphene oxide and graphene nanoplatelets as fuel additives on the combustion and emission characteristics of a diesel engine fuelled with diesel and biodiesel blend. Fuel Process. Technol. 2020, 204, 106406. [Google Scholar] [CrossRef]
- Jamuwa, D.K.; Sharma, D.; Soni, S.L. Experimental investigation of performance, exhaust emission and combustion parameters of stationary compression ignition engine using ethanol fumigation in dual fuel mode. Energy Convers. Manag. 2016, 115, 221–231. [Google Scholar] [CrossRef]
- Shahir, S.A.; Masjuki, H.H.; Kalam, M.A.; Imran, A.; Ashraful, A.M. Performance and emission assessment of diesel-biodiesel-ethanol/bioethanol blend as a fuel in diesel engines: A review. Renew. Sustain. Energy Rev. 2015, 48, 62–78. [Google Scholar] [CrossRef]
- Tse, H.; Leung, C.W.; Cheung, C.S. Investigation on the combustion characteristics and particulate emissions from a diesel engine fueled with diesel-biodiesel-ethanol blends. Energy 2015, 83, 343–350. [Google Scholar] [CrossRef]
- Gill, S.S.; Tsolakis, A.; Dearn, K.D.; Rodríguez-Fernández, J. Combustion characteristics and emissions of Fischer–Tropsch diesel fuels in IC engines. Prog. Energy Combust. Sci. 2011, 37, 503–523. [Google Scholar] [CrossRef]
- Aydın, F.; Öğüt, H. Effects of using ethanol-biodiesel-diesel fuel in single cylinder diesel engine to engine performance and emissions. Renew. Energy 2017, 103, 688–694. [Google Scholar] [CrossRef]
- Barabás, I.; Todoruţ, A.; Băldean, D. Performance and emission characteristics of an CI engine fueled with diesel–biodiesel–bioethanol blends. Fuel 2010, 89, 3827–3832. [Google Scholar] [CrossRef]
- Roy, M.M.; Calder, J.; Wang, W.; Mangad, A.; Diniz, F.C.M. Cold start idle emissions from a modern Tier-4 turbo-charged diesel engine fueled with diesel-biodiesel, diesel-biodiesel-ethanol, and diesel-biodiesel-diethyl ether blends. Appl. Energy 2016, 180, 52–65. [Google Scholar] [CrossRef]
- Khan, M.; Tafreshi, R.; Mokahal, A.J.; Mohamed, M.T.; Hanbal, M.Y.; Elturk, J. Single Cylinder GTL ENGINE: An Experimental Comparison between Traditional Diesel and GTL Diesel on Single Cylinder Engine. In SAE Technical Papers; SAE International: Warrendale, PA, USA, 2016. [Google Scholar] [CrossRef]
- Herreros, J.M.; Jones, A.; Sukjit, E.; Tsolakis, A. Blending lignin-derived oxygenate in enhanced multi-component diesel fuel for improved emissions. Appl. Energy 2014, 116, 58–65. [Google Scholar] [CrossRef]
- Zhu, L.; Cheung, C.S.; Zhang, W.G.; Huang, Z. Combustion, performance and emission characteristics of a DI diesel engine fueled with ethanol–biodiesel blends. Fuel 2011, 90, 1743–1750. [Google Scholar] [CrossRef]
- Ribeiro, N.; Pinto, A.C.; Quintella, C.M.; da Rocha, G.O.; Teixeira, L.S.G.; Guarieiro, L.L.N.; do Carmo Rangel, M.; Veloso, M.C.C.; Rezende, M.J.C.; da Cruz, R.S.; et al. The role of additives for diesel and diesel blended (ethanol or biodiesel) fuels: A review. Energy Fuels 2007, 21, 2433–2445. [Google Scholar] [CrossRef]
- Mofijur, M.; Rasul, M.G.; Hyde, J.; Azad, A.K.; Mamat, R.; Bhuiya, M.M.K. Role of biofuel and their binary (diesel-biodiesel) and ternary (ethanol-biodiesel-diesel) blends on internal combustion engines emission reduction. Renew. Sustain. Energy Rev. 2016, 53, 265–278. [Google Scholar] [CrossRef]
- Gülüm, M.; Bilgin, A. A comprehensive study on measurement and prediction of viscosity of biodiesel-diesel-alcohol ternary blends. Energy 2018, 148, 341–361. [Google Scholar] [CrossRef]
- Jamrozik, A.; Tutak, W.; Gnatowska, R.; Nowak, Ł. Comparative analysis of the combustion stability of diesel-methanol and diesel-ethanol in a dual fuel engine. Energies 2019, 12, 971. [Google Scholar] [CrossRef]
- Armas, O.; García-Contreras, R.; Ramos, Á. Pollutant emissions from New European Driving Cycle with ethanol and butanol diesel blends. Fuel Process. Technol. 2014, 122, 64–71. [Google Scholar] [CrossRef]
- Yilmaz, N.; Vigil, F.M.; Benalil, K.; Davis, S.M.; Calva, A. Effect of biodiesel–butanol fuel blends on emissions and performance characteristics of a diesel engine. Fuel 2014, 135, 46–50. [Google Scholar] [CrossRef]
- Carvalho, M.; Torres, F.; Ferreira, V.; Silva, J.; Martins, J.; Torres, E. Effects of Diethyl Ether Introduction in Emissions and Performance of a Diesel Engine Fueled with Biodiesel-Ethanol Blends. Energies 2020, 13, 3787. [Google Scholar] [CrossRef]
- Reijnders, J.; Boot, M.; de Goey, P. Impact of aromaticity and cetane number on the soot-NOx trade-off in conventional and low temperature combustion. Fuel 2016, 186, 24–34. [Google Scholar] [CrossRef]
- Glarborg, P.; Miller, J.A.; Ruscic, B.; Klippenstein, S.J. Modeling nitrogen chemistry in combustion. Prog. Energy Combust. Sci. 2018, 67, 31–68. [Google Scholar] [CrossRef]
- Ahmed, S.F.; Aghdam, A.C.; Dryer, F.L.; Farouk, T.I. Multidimensional simulations of Mckenna-driven flow tube configuration: Investigating non-ideality in NOx formation flow tube experiments. Combust. Flame 2021, 223, 511–524. [Google Scholar] [CrossRef]
- Watling, T.C.; Ahmadinejad, M.; Ţuţuianu, M.; Johansson, Å.; Paterson, M.A.J. Development and Validation of a Pt-Pd Diesel Oxidation Catalyst Model. SAE Int. J. Engines 2012, 5, 1420–1442. [Google Scholar] [CrossRef]
- Kim, I.H.; Seo, H.O.; Park, E.J.; Han, S.W.; Kim, Y.D. Low temperature CO oxidation over iron oxide nanoparticles decorating internal structures of a mesoporous alumina. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef]
- Ye, S.; Yap, Y.H.; Kolaczkowski, S.T.; Robinson, K.; Lukyanov, D. Catalyst ‘light-off’ experiments on a diesel oxidation catalyst connected to a diesel engine—Methodology and techniques. Chem. Eng. Res. Des. 2012, 90, 834–845. [Google Scholar] [CrossRef]
- Al-Harbi, M.; Hayes, R.; Votsmeier, M.; Epling, W.S. Competitive no, co and hydrocarbon oxidation reactions over a diesel oxidation catalyst. Can. J. Chem. Eng. 2012, 90, 1527–1538. [Google Scholar] [CrossRef]
- Oh, H.; Luo, J.; Epling, W.S. NO oxidation inhibition by hydrocarbons over a diesel oxidation catalyst: Reaction between surface nitrates and hydrocarbons. Catal. Lett. 2011, 141, 1746–1751. [Google Scholar] [CrossRef]
- Lefort, I.; Herreros, J.M.; Tsolakis, A. Reduction of low temperature engine pollutants by understanding the exhaust species interactions in a diesel oxidation catalyst. Environ. Sci. Technol. 2014, 48, 2361–2367. [Google Scholar] [CrossRef]
- Aydin, H.; Ilkiliç, C. Effect of ethanol blending with biodiesel on engine performance and exhaust emissions in a CI engine. Appl. Therm. Eng. 2010, 30, 1199–1204. [Google Scholar] [CrossRef]
- Patterson, M.J.; Angove, D.E.; Cant, N.W. The effect of carbon monoxide on the oxidation of four C6 to C8 hydrocarbons over platinum, palladium and rhodium. Appl. Catal. B Environ. 2000, 26, 47–57. [Google Scholar] [CrossRef]
- Demidyuk, V.; Hardacre, C.; Burch, R.; Mhadeshwar, A.; Norton, D.; Hancu, D. Aromatic hydrocarbons and sulfur based catalyst deactivation for selective catalytic reduction of NOx. Catal. Today 2011, 164, 515–519. [Google Scholar] [CrossRef]
- Irani, K.; Epling, W.S.; Blint, R. Effect of hydrocarbon species on no oxidation over diesel oxidation catalysts. Appl. Catal. B Environ. 2009, 92, 422–428. [Google Scholar] [CrossRef]
- Johnson, W.L.; Fisher, G.B.; Toops, T.J. Mechanistic investigation of ethanol SCR of NOx over Ag/Al2O3. Catal. Today 2012, 184, 166–177. [Google Scholar] [CrossRef]
- Ghadikolaei, M.A.; Wei, L.; Cheung, C.S.; Yung, K.F.; Ning, Z. Particulate emission and physical properties of particulate matter emitted from a diesel engine fueled with ternary fuel (diesel-biodiesel-ethanol) in blended and fumigation modes. Fuel 2020, 263, 116665. [Google Scholar] [CrossRef]
- Sukjit, E.; Herreros, J.M.; Dearn, K.; Tsolakis, A. Improving Ethanol-Diesel Blend Through the Use of Hydroxylated Biodiesel. In SAE Technical Papers; SAE International: Warrendale, PA, USA, 2014. [Google Scholar] [CrossRef]
- Di, Y.; Cheung, C.S.; Huang, Z. Comparison of the Effect of Biodiesel-Diesel and Ethanol-Diesel on the Particulate Emissions of a Direct Injection Diesel Engine. Aerosol Sci. Technol. 2009, 43, 455–465. [Google Scholar] [CrossRef]
- Lapuerta, M.; Armas, O.; Gómez, A. Diesel particle size distribution estimation from digital image analysis. Aerosol Sci. Technol. 2003, 37, 369–381. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Balasubramanian, R. Effects of oxygenated fuel blends on the composition of size-segregated engine-out diesel particulate emissions and on the toxicity of quasi-ultrafine particles. Fuel 2018, 215, 161–170. [Google Scholar] [CrossRef]
- Tse, H.; Leung, C.W.; Cheung, C.S. Performances, Emissions and Soot Properties from a Diesel-Biodiesel-Ethanol Blend Fuelled Engine. Adv. Automob. Eng. 2016, 1, 1–7. [Google Scholar] [CrossRef]




| Engine Parameters | Specifications |
|---|---|
| Engine type | Diesel single-cylinder |
| Stroke type | Four-stroke |
| Number of cylinders | 1 |
| Cylinder bore × stroke (mm) | 84 × 90 |
| Connecting rod length (mm) | 160 |
| Compression ratio | 16:1 |
| Displacement (cm3) | 499 |
| Engine speed range (rpm) | 900–2000 |
| IMEP range (bar) | <7 |
| Fuel pressure range (bar) | 500–1500 |
| Injection system | Common rail |
| Fuel | Injection Pressure (bar) | Pilot Injection Timing (CAD BTDC) | Injection Duration (ms) | Main Injection Timing (CAD BTDC) | Injection Duration (ms) | Engine-Out Exhaust Temperature (°C) |
| Diesel | 550 | 15 | 0.150 | 5 | 0.499 | 236 ± 2 |
| E15D50B35 | 550 | 15 | 0.150 | 5 | 0.529 | 232 ± 2 |
| E15FTD50B35 | 550 | 15 | 0.150 | 5 | 0.546 | 235 ± 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andrade Torres, F.; Doustdar, O.; Herreros, J.M.; Li, R.; Poku, R.; Tsolakis, A.; Martins, J.; Vieira de Melo, S.A.B. A Comparative Study of Biofuels and Fischer–Tropsch Diesel Blends on the Engine Combustion Performance for Reducing Exhaust Gaseous and Particulate Emissions. Energies 2021, 14, 1538. https://doi.org/10.3390/en14061538
Andrade Torres F, Doustdar O, Herreros JM, Li R, Poku R, Tsolakis A, Martins J, Vieira de Melo SAB. A Comparative Study of Biofuels and Fischer–Tropsch Diesel Blends on the Engine Combustion Performance for Reducing Exhaust Gaseous and Particulate Emissions. Energies. 2021; 14(6):1538. https://doi.org/10.3390/en14061538
Chicago/Turabian StyleAndrade Torres, Felipe, Omid Doustdar, Jose Martin Herreros, Runzhao Li, Robert Poku, Athanasios Tsolakis, Jorge Martins, and Silvio A. B. Vieira de Melo. 2021. "A Comparative Study of Biofuels and Fischer–Tropsch Diesel Blends on the Engine Combustion Performance for Reducing Exhaust Gaseous and Particulate Emissions" Energies 14, no. 6: 1538. https://doi.org/10.3390/en14061538
APA StyleAndrade Torres, F., Doustdar, O., Herreros, J. M., Li, R., Poku, R., Tsolakis, A., Martins, J., & Vieira de Melo, S. A. B. (2021). A Comparative Study of Biofuels and Fischer–Tropsch Diesel Blends on the Engine Combustion Performance for Reducing Exhaust Gaseous and Particulate Emissions. Energies, 14(6), 1538. https://doi.org/10.3390/en14061538










