Application of Liquid Hydrogen Carriers in Hydrogen Steelmaking
Abstract
1. Introduction
2. Hydrogen Storage in Hydrogen Direct Reduction Context
- Low in investment cost (low CAPEX);
- Efficient, i.e., require little additional electricity and heat (low OPEX);
- Dynamic, i.e., it should be possible to change the operating mode of the storage (e.g., from filling to emptying) rapidly enough to respond to electricity price changes.
3. Liquid Hydrogen Carriers
3.1. Overview
- Import and export of the liquid H2 carrier to or from the H-DR site is readily achieved;
- Placement of the H2 production and storage units becomes less geographically constrained;
- Very large H2 storages are possible, covering, e.g., seasonal variations in electricity price.
- (1)
- Those based on a reaction between H2 and CO2 (e.g., methanol (CH3OH, MeOH) and formic acid (CH2O2, FA));
- (2)
- Those based on a reaction between H2 and nitrogen (N2) (e.g., ammonia (NH3));
- (3)
- Those based on a reaction between H2 and unsaturated liquid hydrocarbons (liquid organic hydrogen carriers (LOHCs)).
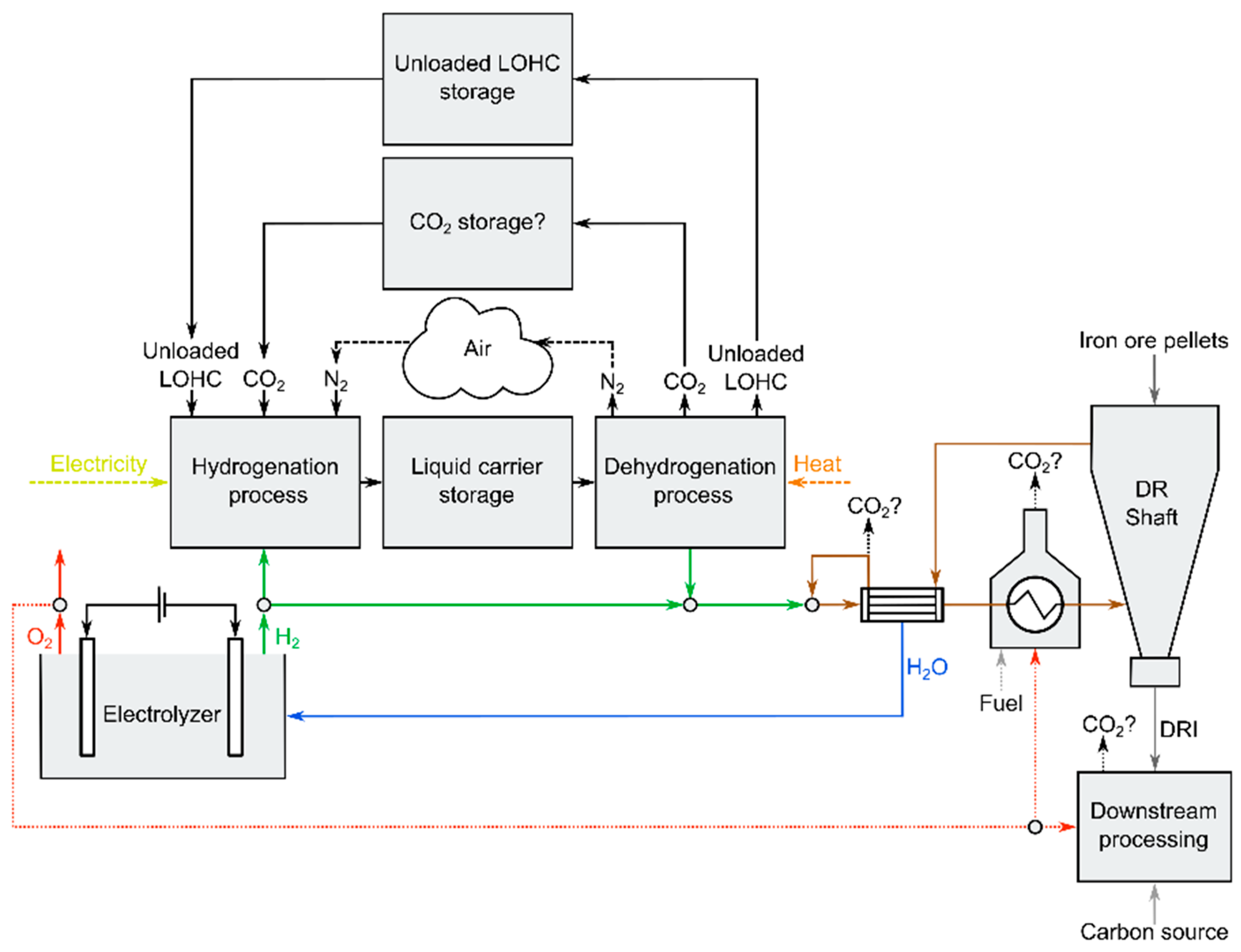
3.2. Integration of Liquid Hydrogen Carriers in Hydrogen Direct Reduction Processes
3.2.1. Management of Secondary Material
- (1)
- the separation of CO2 from the reducing gas in the case that in-shaft carburization would be applied;
- (2)
- the reducing gas pre-heating process;
- (3)
- the processing steps downstream of the DR shaft (e.g., the electric arc furnace (EAF)).
3.2.2. Management of Heat for Dehydrogenation
- Heat from electrolyzers;
- DRI product cooling in the case that cold DRI or hot-briquetted iron is produced [10];
- Excess heat from reduction gas pre-heating.
3.3. Carbon Dioxide-Based Carriers
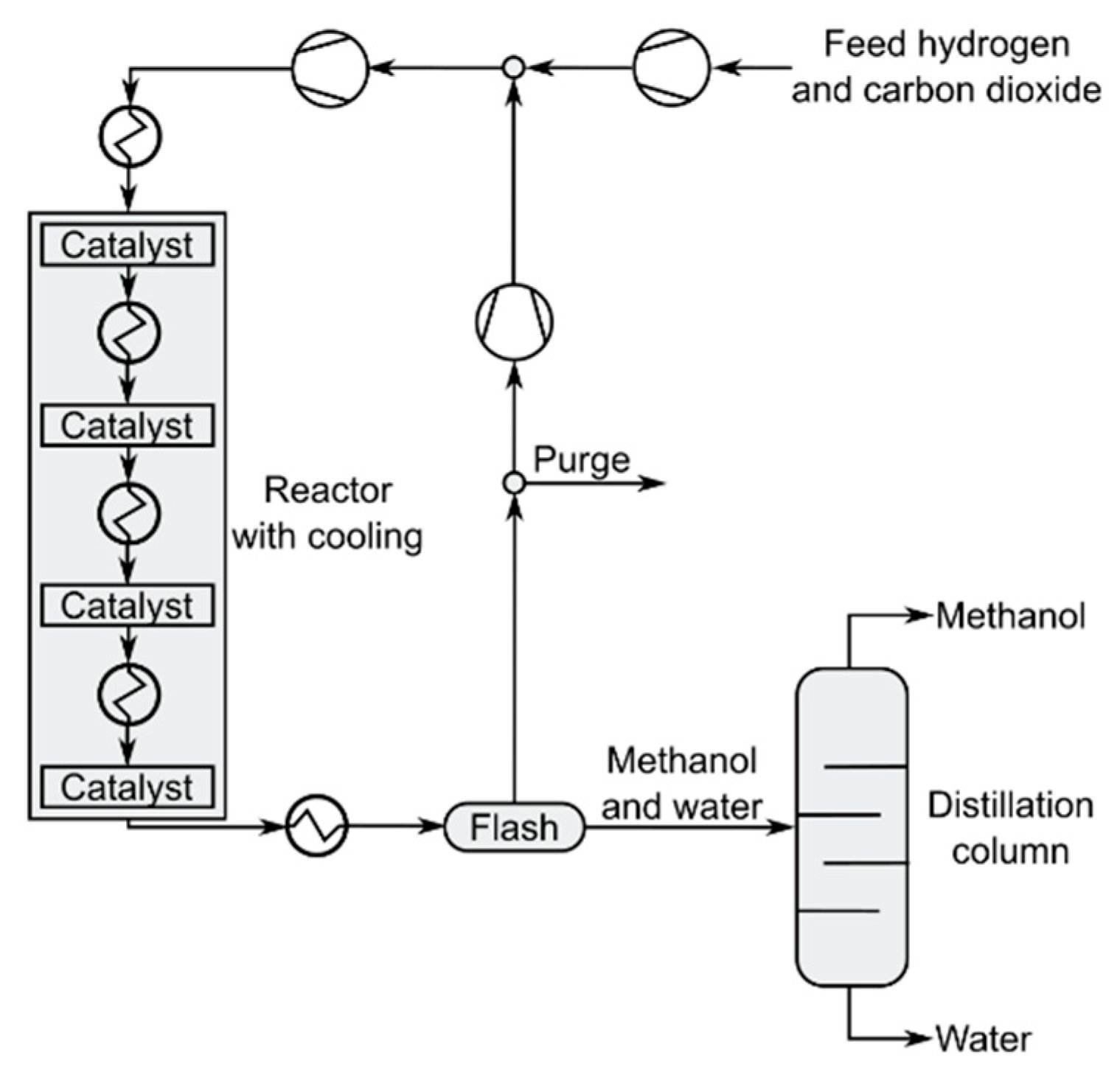
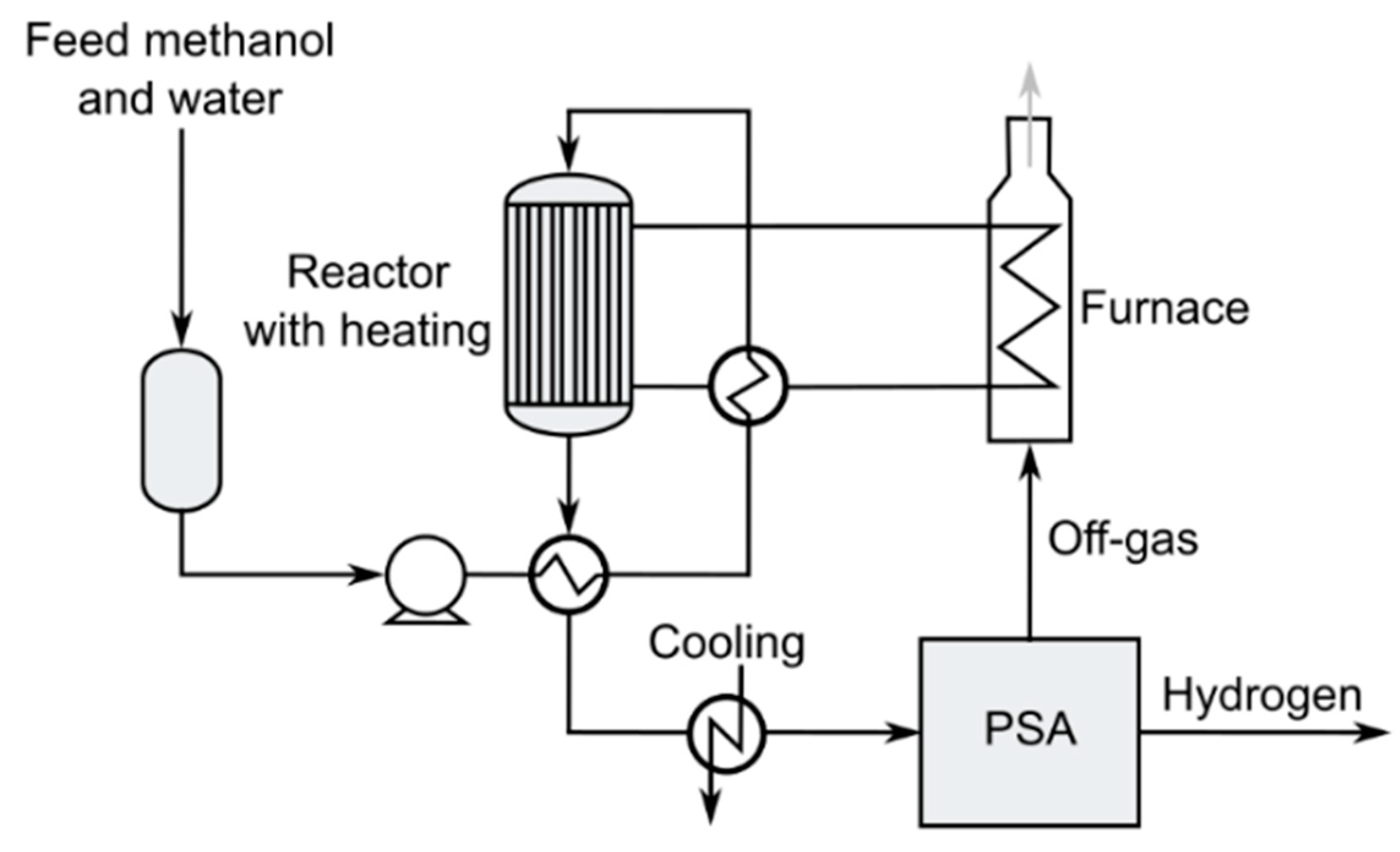
3.3.1. Methanol
3.3.2. Formic Acid
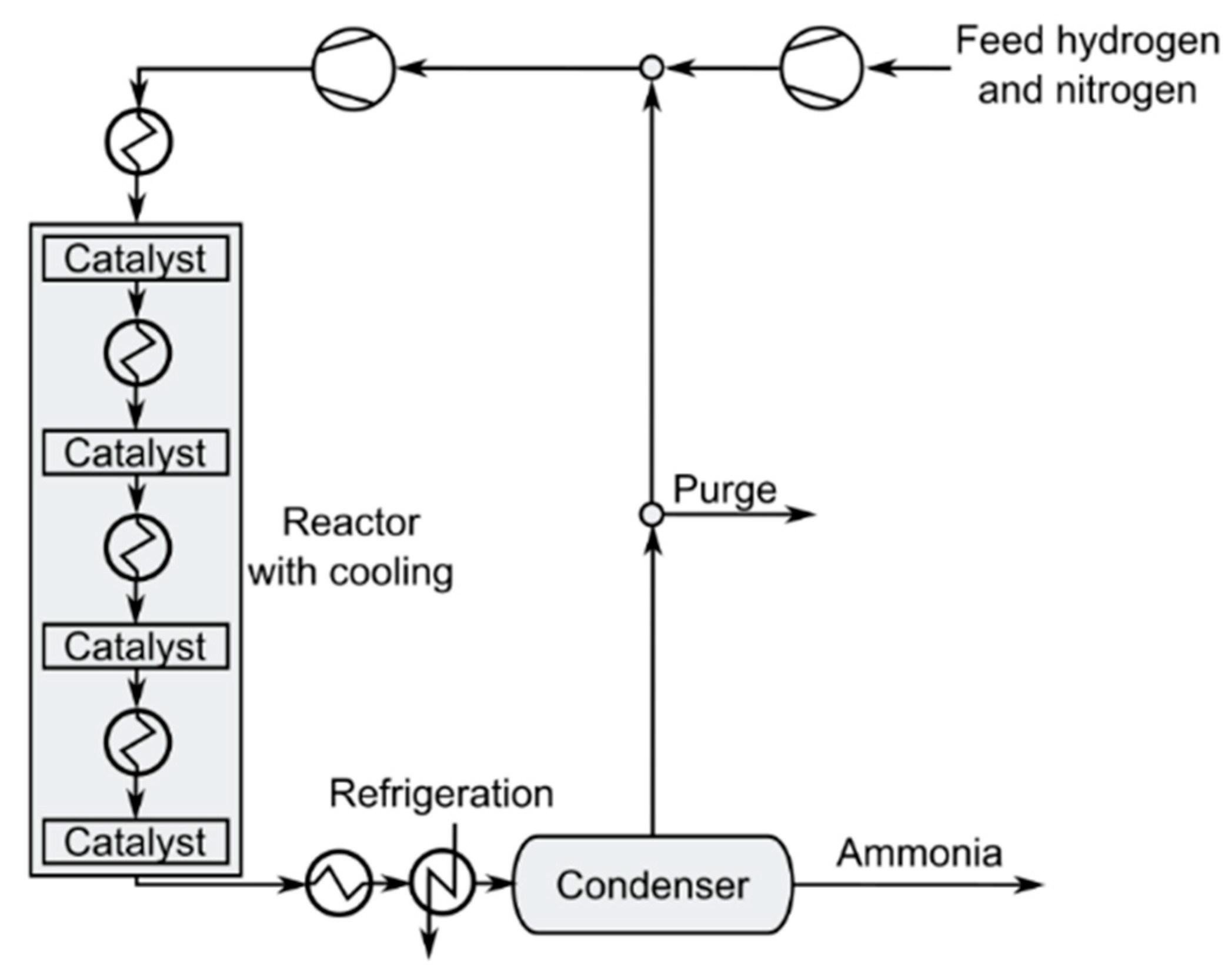
3.4. Ammonia
3.5. Liquid Organic Hydrogen Carriers
4. Comparison
4.1. Investment Costs
4.1.1. Hydrogenation Plants
4.1.2. Dehydrogenation Plants
4.1.3. Storage Units
4.1.4. Total Investment Costs
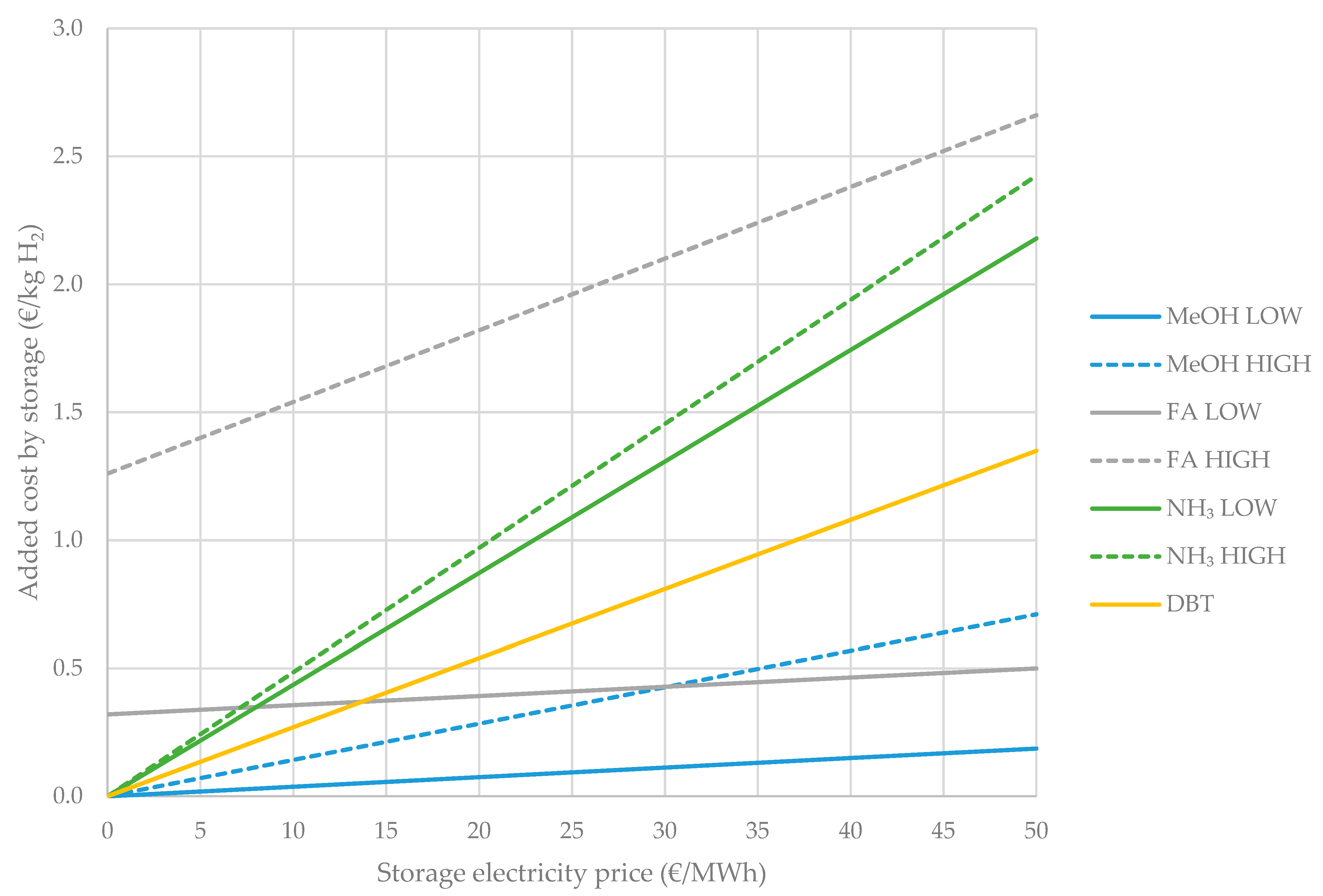
4.2. Operational Costs
4.3. Load Flexibility
5. Conclusions
Funding
Conflicts of Interest
References
- Pérez-Fortes, M.; Moya, J.A.; Vatopoulos, K.; Tzimas, E. CO2 Capture and Utilization in Cement and Iron and Steel Industries. Energy Procedia 2014, 63, 6534–6543. [Google Scholar] [CrossRef]
- Allen, M.; Antwi-Agyei, P.; Aragon-Durand, F.; Babiker, M.; Bertoldi, P.; Bind, M.; Brown, S.; Buckeridge, M.; Camilloni, I.; Cartwright, A. Technical Summary: Global Warming of 1.5 °C. An IPCC Special Report on the Impacts of Global Warming of 1.5 °C above pre-Industrial Levels and Related Global Greenhouse Gas Emission Pathways, in the Context of Strengthening the Global Response to the Threat of Climate Change, Sustainable Development, and Efforts to Eradicate Poverty; Intergovernmental Panel on Climate Change: Geneve, Switzerland, 2019. [Google Scholar]
- IPCC Climate Change. Mitigation of climate change. In Contribution of Working Group III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: London, UK, 2014. [Google Scholar]
- Sasiain, A.; Rechberger, K.; Spanlang, A.; Kofler, I.; Wolfmeir, H.; Harris, C.; Bürgler, T. Green Hydrogen as Decarbonization Element for the Steel Industry. BHM Berg Hüttenmännische Mon. 2020, 165, 232–236. [Google Scholar] [CrossRef]
- Pei, M.; Petäjäniemi, M.; Regnell, A.; Wijk, O. Toward a Fossil Free Future with HYBRIT: Development of Iron and Steelmaking Technology in Sweden and Finland. Metals 2020, 10, 972. [Google Scholar] [CrossRef]
- Material Economics. Industrial Transformation 2050: Pathways to Net-Zero Emissions from EU Heavy Industry; Material Economics: Stockholm, Sweden, 2019. [Google Scholar]
- Ryan, N.A.; Miller, S.A.; Skerlos, S.J.; Cooper, D.R. Reducing CO2 Emissions from US Steel Consumption by 70% by 2050. Environ. Sci. Technol. 2020, 54, 14598–14608. [Google Scholar] [CrossRef]
- HYBRIT. Final Report HYBRIT—Hydrogen Breakthrough Ironmaking Technology; Energimyndigheten: Stockholm, Sweden, 2018. [Google Scholar]
- Béchara, R.; Hamadeh, H.; Mirgaux, O.; Patisson, F. Optimization of the iron ore direct reduction process through multiscale process modeling. Materials 2018, 11, 1094. [Google Scholar] [CrossRef]
- Battle, T.; Srivastava, U.; Kopfle, J.; Hunter, R.; McClelland, J. The Direct Reduction of Iron. In Treatise on Process Metallurgy; Elsevier: Amsterdam, The Netherlands, 2014; pp. 89–176. [Google Scholar]
- Anameric, B.; Kawatra, S.K. Properties and features of direct reduced iron. Miner. Process. Extr. Metall. Rev. 2007, 28, 59–116. [Google Scholar] [CrossRef]
- Liu, B.N.; Li, Q.; Zou, Z.S.; Yu, A.B. Discussion on chemical energy utilisation of reducing gas in reduction shaft furnace. Ironmak. Steelmak. 2014, 41, 568–574. [Google Scholar] [CrossRef]
- Hübner, T.; Guminski, A.; Pichlmaier, S.; Höchtl, M.; Roon, S. European Steel with Hydrogen; Forschungsstelle für Energiewirtschaft: Munich, Germany, 2020. [Google Scholar]
- Lundgren, J.; Ekbom, T.; Hulteberg, C.; Larsson, M.; Grip, C.-E.; Nilsson, L.; Tunå, P. Methanol production from steel-work off-gases and biomass based synthesis gas. Appl. Energy 2013, 112, 431–439. [Google Scholar] [CrossRef]
- Nuber, D.; Eichberger, H.; Rollinger, B. Circored fine ore direct reduction. Millen. Steel 2006, 2006, 37–40. [Google Scholar]
- Holappa, L. A general vision for reduction of energy consumption and CO2 emissions from the steel industry. Metals 2020, 10, 1117. [Google Scholar] [CrossRef]
- Tang, J.; Chu, M.-S.; Li, F.; Feng, C.; Liu, Z.-G.; Zhou, Y.-S. Development and progress on hydrogen metallurgy. Int. J. Miner. Metall. Mater. 2020, 27, 713–723. [Google Scholar] [CrossRef]
- ArcelorMittal. Hydrogen-Based Steelmaking to Begin in Hamburg. 2021. Available online: https://corporate.arcelormittal.com/media/case-studies/hydrogen-based-steelmaking-to-begin-in-hamburg (accessed on 22 January 2021).
- Thyssenkrupp. Green Hydrogen for Steel Production: RWE and Thyssenkrupp Plan Partnership. 2020. Available online: https://www.thyssenkrupp.com/en/newsroom/press-releases/pressdetailpage/green-hydrogen-for-steel-production--rwe-and-thyssenkrupp-plan-partnership-82841 (accessed on 22 January 2021).
- Varriale, L. Germany’s Thyssenkrupp to Build DRI Plant Run on Hydrogen for Green Steel Production. 2020. Available online: https://www.spglobal.com/platts/en/market-insights/latest-news/metals/082820-germanys-thyssenkrupp-to-build-dri-plant-run-on-hydrogen-for-green-steel-production (accessed on 22 January 2021).
- Nikolaidis, P.; Poullikkas, A. A comparative overview of hydrogen production processes. Renew. Sustain. Energy Rev. 2017, 67, 597–611. [Google Scholar] [CrossRef]
- Buergler, T.; Prammer, J. Hydrogen steelmaking: Technology options and R&D projects. BHM Berg Hüttenmännische Mon. 2019, 164, 447–451. [Google Scholar]
- Matute, G.; Yusta, J.; Beyza, J.; Correas, L. Multi-state techno-economic model for optimal dispatch of grid connected hydrogen electrolysis systems operating under dynamic conditions. Int. J. Hydrogen Energy 2021, 46, 1449–1460. [Google Scholar] [CrossRef]
- Kruck, O.; Crotogino, F.; Prelicz, R.; Rudolph, T. Overview on all known underground storage technologies for hydrogen. In HyUnder (2013 August) Delivery; Springer: Berlin, Germany, 2013; Volume 3. [Google Scholar]
- Caglayan, D.G.; Weber, N.; Heinrichs, H.U.; Linßen, J.; Robinius, M.; Kukla, P.A.; Stolten, D. Technical potential of salt caverns for hydrogen storage in Europe. Int. J. Hydrogen Energy 2020, 45, 6793–6805. [Google Scholar] [CrossRef]
- Gabrielli, P.; Poluzzi, A.; Kramer, G.J.; Spiers, C.; Mazzotti, M.; Gazzani, M. Seasonal energy storage for zero-emissions multi-energy systems via underground hydrogen storage. Renew. Sustain. Energy Rev. 2020, 121, 109629. [Google Scholar] [CrossRef]
- Böttcher, N.; Görke, U.-J.; Kolditz, O.; Nagel, T. Thermo-mechanical investigation of salt caverns for short-term hydrogen storage. Environ. Earth Sci. 2017, 76, 98. [Google Scholar] [CrossRef]
- Johansson, F.; Spross, J.; Damasceno, D.; Johansson, J.; Stille, H. Investigation of Research Needs Regarding the Storage of Hydrogen Gas in Lined Rock Caverns: Prestudy for Work Package 2.3 in HYBRIT Research Program 1; KTH Royal Institute of Technology: Stockholm, Sweden, 2018. [Google Scholar]
- Sofregaz, U.; Gustafsväg, C. Commercial Potential of Natural Gas Storage in Lined Rock Caverns (LRC); US Department of Energy: Washington, DC, USA, 1999.
- Tengborg, P.; Johansson, J.; Durup, G. Storage of highly compressed gases in underground lined rock caverns–more than 10 years of experience. In Proceedings of the World Tunnel Congress 2014—Tunnels for a Better Life, Iguassu Falls, Brazil, 9–15 May 2014. [Google Scholar]
- Laban, M. Hydrogen Storage in Salt Caverns: Chemical Modelling and Analysis of Large-Scale Hydrogen Storage in Underground Salt Caverns; Delft University of Technology: Delft, The Netherlands, 2020. [Google Scholar]
- Davies, J.; Dolci, F.; Klassek-Bajorek, D.; Ortiz Cebolla, R.; Weidner, E. Current status of Chemical Energy Storage Technologies; Publications Office of the European Union: Luxembourg, 2020. [Google Scholar]
- Andersson, J.; Grönkvist, S. Large-scale storage of hydrogen. Int. J. Hydrogen Energy 2019, 44, 11901–11919. [Google Scholar] [CrossRef]
- Smith, A.; Klosek, J. A review of air separation technologies and their integration with energy conversion processes. Fuel Process. Technol. 2001, 70, 115–134. [Google Scholar] [CrossRef]
- Bos, M.J.; Kersten, S.R.A.; Brilman, D.W.F. Wind power to methanol: Renewable methanol production using electricity, electrolysis of water and CO2 air capture. Appl. Energy 2020, 264, 114672. [Google Scholar] [CrossRef]
- Keith, D.W.; Holmes, G.; St. Angelo, D.; Heidel, K. A Process for Capturing CO2 from the Atmosphere. Joule 2018, 2, 1573–1594. [Google Scholar] [CrossRef]
- Andersson, J.; Krüger, A.; Grönkvist, S. Methanol as a carrier of hydrogen and carbon in fossil-free production of direct reduced iron. Energy Convers. Manag. X 2020, 7, 100051. [Google Scholar] [CrossRef]
- Robinson, R.; Brabie, L.; Pettersson, M.; Amovic, M.; Ljunggren, R. An Empirical Comparative Study of Renewable Biochar and Fossil Carbon as Carburizer in Steelmaking. ISIJ Int. 2020. ISIJINT-2020-2135. [Google Scholar] [CrossRef]
- Echterhof, T. Review on the Use of Alternative Carbon Sources in EAF Steelmaking. Metals 2021, 11, 222. [Google Scholar] [CrossRef]
- Eggemann, L.; Escobar, N.; Peters, R.; Burauel, P.; Stolten, D. Life cycle assessment of a small-scale methanol production system: A Power-to-Fuel strategy for biogas plants. J. Clean. Prod. 2020, 271, 122476. [Google Scholar] [CrossRef]
- Buttler, A.; Spliethoff, H. Current status of water electrolysis for energy storage, grid balancing and sector coupling via power-to-gas and power-to-liquids: A review. Renew. Sustain. Energy Rev. 2018, 82, 2440–2454. [Google Scholar] [CrossRef]
- Bergins, C.; Buddenberg, T.; Koytsoumpa, E.-I.; Duarte, M.J.; Kakaras, E.; Schmidt, S.; Deierling, A. A Technology Review and Cost Analysis of the Production of Low Carbon Methanol and Following Methanol to Gasoline Process. In Zukünftige Kraftstoffe; Springer: Berlin/Heidelberg, Germany, 2019; pp. 433–463. [Google Scholar]
- Arpagaus, C.; Bless, F.; Uhlmann, M.; Schiffmann, J.; Bertsch, S.S. High temperature heat pumps: Market overview, state of the art, research status, refrigerants, and application potentials. Energy 2018, 152, 985–1010. [Google Scholar] [CrossRef]
- Wismann, S.T.; Engbæk, J.S.; Vendelbo, S.B.; Bendixen, F.B.; Eriksen, W.L.; Aasberg-Petersen, K.; Frandsen, C.; Chorkendorff, I.; Mortensen, P.M. Electrified methane reforming: A compact approach to greener industrial hydrogen production. Science 2019, 364, 756–759. [Google Scholar] [CrossRef]
- Rostrup-Nielsen, J.; Hansen, J.B. Steam reforming for fuel cells. In Fuel Cells: Technologies for Fuel Processing; Elsevier: Amsterdam, The Netherlands, 2011; pp. 49–71. [Google Scholar]
- Voldsund, M.; Jordal, K.; Anantharaman, R. Hydrogen production with CO2 capture. Int. J. Hydrogen Energy 2016, 41, 4969–4992. [Google Scholar] [CrossRef]
- Mortensen, P.M.; Østberg, M.; Nielsen, P.E.H. A Process for Producing Hydrogen or Syngas by Methanol Cracking. U.S. Patent Application 16/087,943, 18 April 2019. [Google Scholar]
- Friedmann, B.; FAN, Z.; Tang, K. Low-Carbon Heat Solutions for Heavy Industry: Sources, Options, and Costs Today; Columbia SIPA Center on Global Policy: New York, NY, USA, 2019. [Google Scholar]
- Energimyndigheten. Trädbränsle- och Torvpriser. 2019. Available online: https://www.energimyndigheten.se/statistik/den-officiella-statistiken/statistikprodukter/tradbransle--och-torvpriser/?currentTab=2#mainheading (accessed on 2 March 2021).
- Schaub, T. CO2-based hydrogen storage: CO2 hydrogenation to formic acid, formaldehyde and methanol. Phys. Sci. Rev. 2018, 3. [Google Scholar] [CrossRef]
- Van Putten, R.; Wissink, T.; Swinkels, T.; Pidko, E.A. Fuelling the hydrogen economy: Scale-up of an integrated formic acid-to-power system. Int. J. Hydrogen Energy 2019, 44, 28533–28541. [Google Scholar] [CrossRef]
- Bulushev, D.A.; Ross, J.R. Heterogeneous catalysts for hydrogenation of CO2 and bicarbonates to formic acid and formates. Catal. Rev. 2018, 60, 566–593. [Google Scholar] [CrossRef]
- Goeppert, A.; Czaun, M.; Jones, J.-P.; Prakash, G.S.; Olah, G.A. Recycling of carbon dioxide to methanol and derived products–closing the loop. Chem. Soc. Rev. 2014, 43, 7995–8048. [Google Scholar] [CrossRef]
- Marlin, D.S.; Sarron, E.; Sigurbjörnsson, Ó. Process Advantages of Direct CO2 to Methanol Synthesis. Front. Chem. 2018, 6, 446. [Google Scholar] [CrossRef] [PubMed]
- Nyári, J.; Magdeldin, M.; Larmi, M.; Järvinen, M.; Santasalo-Aarnio, A. Techno-economic barriers of an industrial-scale methanol CCU-plant. J. CO2 Util. 2020, 39, 101166. [Google Scholar] [CrossRef]
- Dieterich, V.; Buttler, A.; Hanel, A.; Spliethoff, H.; Fendt, S. Power-to-liquid via synthesis of methanol, DME or Fischer–Tropsch-fuels: A review. Energy Environ. Sci. 2020, 13, 3207–3252. [Google Scholar] [CrossRef]
- Perez-Fortes, M.; Schoneberger, J.C.; Boulamanti, A.; Tzimas, E. Methanol synthesis using captured CO2 as raw material: Techno-economic and environmental assessment. Appl. Energy 2016, 161, 718–732. [Google Scholar] [CrossRef]
- Tenhumberg, N.; Büker, K. Ecological and Economic Evaluation of Hydrogen Production by Different Water Electrolysis Technologies. Chem. Ing. Tech. 2020, 92, 1586–1595. [Google Scholar] [CrossRef]
- Schittkowski, J.; Ruland, H.; Laudenschleger, D.; Girod, K.; Kähler, K.; Kaluza, S.; Muhler, M.; Schlögl, R. Methanol Synthesis from Steel Mill Exhaust Gases: Challenges for the Industrial Cu/ZnO/Al2O3 Catalyst. Chem. Ing. Tech. 2018, 90, 1419–1429. [Google Scholar] [CrossRef]
- Ruland, H.; Song, H.; Laudenschleger, D.; Stürmer, S.; Schmidt, S.; He, J.; Kähler, K.; Muhler, M.; Schlögl, R. CO2 hydrogenation with Cu/ZnO/Al2O3: A benchmark study. ChemCatChem 2020, 12, 3216–3222. [Google Scholar] [CrossRef]
- Zurbel, A.; Kraft, M.; Kavurucu-Schubert, S.; Bertau, M. Methanol synthesis by CO2 Hydrogenation over Cu/ZnO/Al2O3 catalysts under fluctuating conditions. Chem. Ing. Tech. 2018, 90, 721–724. [Google Scholar] [CrossRef]
- Stießel, S.; Berger, A.; Fernández Sanchis, E.M.; Ziegmann, M. Methodology for the Evaluation of CO2-Based Syntheses by Coupling Steel Industry with Chemical Industry. Chem. Ing. Tech. 2018, 90, 1392–1408. [Google Scholar] [CrossRef]
- Schweitzer, C. Small scale Methanol Plants: A chance for re-industrialisation. In Proceedings of the International Methanol Conference, Copenhagen, Demark, 8–10 May 2017. [Google Scholar]
- Klankermayer, J.; Wesselbaum, S.; Beydoun, K.; Leitner, W. Selective Catalytic Synthesis Using the Combination of Carbon Dioxide and Hydrogen: Catalytic Chess at the Interface of Energy and Chemistry. Angew. Chem. Int. Ed. 2016, 55, 7296–7343. [Google Scholar] [CrossRef]
- Szima, S.; Cormos, C.-C. Improving methanol synthesis from carbon-free H2 and captured CO2: A techno-economic and environmental evaluation. J. CO2 Util. 2018, 24, 555–563. [Google Scholar] [CrossRef]
- Frei, M.S.; Mondelli, C.; Short, M.I.; Pérez-Ramírez, J. Methanol as a Hydrogen Carrier: Kinetic and Thermodynamic Drivers for its CO2-Based Synthesis and Reforming over Heterogeneous Catalysts. ChemSusChem 2020, 13, 6330–6337. [Google Scholar] [PubMed]
- Palo, D.R.; Dagle, R.A.; Holladay, J.D. Methanol steam reforming for hydrogen production. Chem. Rev. 2007, 107, 3992–4021. [Google Scholar] [CrossRef]
- Behrens, M.; Armbrüster, M. Methanol Steam Reforming. In Catalysis for Alternative Energy Generation; Guczi, L., Erdôhelyi, A., Eds.; Springer: New York, NY, USA, 2012; pp. 175–235. [Google Scholar] [CrossRef]
- Agrell, J.; Lindstrom, B.; Pettersson, L.J.; Jaras, S.G. Catalytic hydrogen generation from methanol. Catalysis 2002, 16, 1–2. [Google Scholar]
- Agrell, J.; Birgersson, H.; Boutonnet, M. Steam reforming of methanol over a Cu/ZnO/Al2O3 catalyst: A kinetic analysis and strategies for suppression of CO formation. J. Power Sources 2002, 106, 249–257. [Google Scholar] [CrossRef]
- Biedermann, P.; Grube, T.; Höhlein, B. Methanol as an Energy Carrier; Forschungszentrum Jülich: Jülich, Germany, 2006. [Google Scholar]
- Stoll, R.; Von Linde, F. Hydrogen-what are the costs. Hydrocarb. Process 2000, 79, 42–46. [Google Scholar]
- Neumann, P.; von Linde, F. Options for economical supply of hydrogen. Metall. Plant Technol. Int. 2003, 2, 72–75. [Google Scholar]
- Haid, J.; Koss, U. Lurgi’s Mega-Methanol technology opens the door for a new era in down-stream applications. In Studies in Surface Science and Catalysis; Elsevier: Amsterdam, The Netherlands, 2001; Volume 136, pp. 399–404. [Google Scholar]
- Grubel, K.; Jeong, H.; Yoon, C.W.; Autrey, T. Challenges and opportunities for using formate to store, transport, and use hydrogen. J. Energy Chem. 2020, 41, 216–224. [Google Scholar] [CrossRef]
- Müller, K.; Brooks, K.; Autrey, T. Hydrogen Storage in Formic Acid: A Comparison of Process Options. Energy Fuels 2017, 31, 12603–12611. [Google Scholar] [CrossRef]
- Schaub, T.; Paciello, R.A. A process for the synthesis of formic acid by CO2 hydrogenation: Thermodynamic aspects and the role of CO. Angew. Chem. Int. Ed. 2011, 50, 7278–7282. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Han, J. Comprehensive analysis of two catalytic processes to produce formic acid from carbon dioxide. Appl. Energy 2020, 264, 114711. [Google Scholar] [CrossRef]
- Perez-Fortes, M.; Schoneberger, J.C.; Boulamanti, A.; Harrison, G.; Tzimas, E. Formic acid synthesis using CO2 as raw material: Techno-economic and environmental evaluation and market potential. Int. J. Hydrogen Energy 2016, 41, 16444–16462. [Google Scholar] [CrossRef]
- Van der Burg, L.; Reijerkerk, J. HyChain 1, 2 & 3: Energy Carriers and Hydrogen Supply Chain: A Management Summary; Institute for Sustainable Process Technology (ISPT): Amersfoort, The Netherlands, 2019. [Google Scholar]
- Hwang, Y.J.; Kwon, Y.; Kim, Y.; Sohn, H.; Nam, S.W.; Kim, J.; Autrey, T.S.; Yoon, C.W.; Jo, Y.S.; Jeong, H. Development of an autothermal formate-based hydrogen generator: From optimization of formate dehydrogenation conditions to thermal integration with Fuel Cells. ACS Sustain. Chem. Eng. 2020, 8, 9846–9856. [Google Scholar] [CrossRef]
- Müller, K.; Brooks, K.; Autrey, T. Releasing Hydrogen at High Pressures from Liquid Carriers: Aspects for the H2 Delivery to Fueling Stations. Energy Fuels 2018, 32, 10008–10015. [Google Scholar] [CrossRef]
- Stathi, P.; Solakidou, M.; Louloudi, M.; Deligiannakis, Y. From Homogeneous to Heterogenized Molecular Catalysts for H2 Production by Formic Acid Dehydrogenation: Mechanistic Aspects, Role of Additives, and Co-Catalysts. Energies 2020, 13, 733. [Google Scholar] [CrossRef]
- Theodorakopoulos, M.; Solakidou, M.; Deligiannakis, Y.; Louloudi, M. A Use-Store-Reuse (USR) Concept in Catalytic HCOOH Dehydrogenation: Case-Study of a Ru-Based Catalytic System for Long-Term USR under Ambient O2. Energies 2021, 14, 481. [Google Scholar] [CrossRef]
- Sun, R.; Liao, Y.; Bai, S.; Zheng, M.; Zhou, C.; Zhang, T.; Sels, B. Heterogeneous catalysts for CO2 hydrogenation to formic acid/formate: From nanoscale to single atom. Energy Environ. Sci. 2021. [Google Scholar] [CrossRef]
- Aziz, M.; Wijayanta, A.T.; Nandiyanto, A.B.D. Ammonia as Effective Hydrogen Storage: A Review on Production, Storage and Utilization. Energies 2020, 13, 3062. [Google Scholar] [CrossRef]
- Nørskov, J.; Chen, J.; Miranda, R.; Fitzsimmons, T.; Stack, R. Sustainable Ammonia Synthesis—Exploring the Scientific Challenges Associated with Discovering Alternative, Sustainable Processes for Ammonia Production; US DOE Office of Science: Washington, DC, USA, 2016. [Google Scholar]
- Smith, C.; Hill, A.K.; Torrente-Murciano, L. Current and future role of Haber-Bosch ammonia in a carbon-free energy landscape. Energy Environ. Sci. 2020, 13, 331–344. [Google Scholar] [CrossRef]
- Liu, X.; Elgowainy, A.; Wang, M. Life cycle energy use and greenhouse gas emissions of ammonia production from renewable resources and industrial by-products. Green Chem. 2020, 22, 5751–5761. [Google Scholar] [CrossRef]
- Lin, B.; Wiesner, T.; Malmali, M. Performance of a small-scale Haber process: A techno-economic analysis. ACS Sustain. Chem. Eng. 2020, 8, 15517–15531. [Google Scholar] [CrossRef]
- Hank, C.; Sternberg, A.; Köppel, N.; Holst, M.; Smolinka, T.; Schaadt, A.; Hebling, C.; Henning, H.-M. Energy efficiency and economic assessment of imported energy carriers based on renewable electricity. Sustain. Energy Fuels 2020, 4, 2256–2273. [Google Scholar] [CrossRef]
- Palys, M.J.; Daoutidis, P. Using hydrogen and ammonia for renewable energy storage: A geographically comprehensive techno-economic study. Comput. Chem. Eng. 2020, 136, 106785. [Google Scholar] [CrossRef]
- Wang, L.; Xia, M.; Wang, H.; Huang, K.; Qian, C.; Maravelias, C.T.; Ozin, G.A. Greening ammonia toward the solar ammonia refinery. Joule 2018, 2, 1055–1074. [Google Scholar] [CrossRef]
- Aneke, M.; Wang, M. Potential for improving the energy efficiency of cryogenic air separation unit (ASU) using binary heat recovery cycles. Appl. Therm. Eng. 2015, 81, 223–231. [Google Scholar] [CrossRef]
- Zhang, Z.; Liguori, S.; Fuerst, T.F.; Way, J.D.; Wolden, C.A. Efficient ammonia decomposition in a catalytic membrane reactor to enable hydrogen storage and utilization. ACS Sustain. Chem. Eng. 2019, 7, 5975–5985. [Google Scholar] [CrossRef]
- Jackson, C.; Fothergill, K.; Gray, P.; Haroon, F.; Makhloufi, C.; Kezibri, N.; Davey, A.; Lhote, O.; Zarea, M.; Davenne, T.; et al. Ammonia to Green Hydrogen Project: Feasibility Study; Business, Energy and Industrial Strategy: London, UK, 2020.
- Preuster, P.; Papp, C.; Wasserscheid, P. Liquid Organic Hydrogen Carriers (LOHCs): Toward a Hydrogen-free Hydrogen Economy. Acc. Chem. Res. 2017, 50, 74–85. [Google Scholar] [CrossRef]
- Bulgarin, A.; Jorschick, H.; Preuster, P.; Bösmann, A.; Wasserscheid, P. Purity of hydrogen released from the Liquid Organic Hydrogen Carrier compound perhydro dibenzyltoluene by catalytic dehydrogenation. Int. J. Hydrogen Energy 2020, 45, 712–720. [Google Scholar] [CrossRef]
- Aakko-Saksa, P.; Repo, T.; Hurskainen, M.; Kaisalo, N.; Tallgren, J.; Keskiväli, L.; Auvinen, S.; Braunschweiler, A.; Simell, P.; Reinikainen, M. Liquid Organic Hydrogen Carriers; VTT Technical Research Centre of Finland: Espoo, Finland, 2020. [Google Scholar]
- Aakko-Saksa, P.T.; Cook, C.; Kiviaho, J.; Repo, T. Liquid organic hydrogen carriers for transportation and storing of renewable energy–Review and discussion. J. Power Sources 2018, 396, 803–823. [Google Scholar] [CrossRef]
- Modisha, P.M.; Ouma, C.N.; Garidzirai, R.; Wasserscheid, P.; Bessarabov, D. The prospect of hydrogen storage using liquid organic hydrogen carriers. Energy Fuels 2019, 33, 2778–2796. [Google Scholar] [CrossRef]
- Makepeace, J.W.; He, T.; Weidenthaler, C.; Jensen, T.R.; Chang, F.; Vegge, T.; Ngene, P.; Kojima, Y.; de Jongh, P.E.; Chen, P. Reversible ammonia-based and liquid organic hydrogen carriers for high-density hydrogen storage: Recent progress. Int. J. Hydrogen Energy 2019, 44, 7746–7767. [Google Scholar] [CrossRef]
- Rao, P.C.; Yoon, M. Potential Liquid-Organic Hydrogen Carrier (LOHC) Systems: A Review on Recent Progress. Energies 2020, 13, 6040. [Google Scholar] [CrossRef]
- Hurskainen, M. Liquid Organic Hydrogen Carriers (LOHC): Concept Evaluation and Techno-Economics; VTT Technical Research Centre of Finland: Espoo, Finland, 2019. [Google Scholar]
- Jorschick, H.; Preuster, P.; Durr, S.; Seidel, A.; Muller, K.; Bosmann, A.; Wasserscheid, P. Hydrogen storage using a hot pressure swing reactor. Energy Environ. Sci. 2017, 10, 1652–1659. [Google Scholar] [CrossRef]
- Fikrt, A.; Brehmer, R.; Milella, V.-O.; Müller, K.; Bösmann, A.; Preuster, P.; Alt, N.; Schlücker, E.; Wasserscheid, P.; Arlt, W. Dynamic power supply by hydrogen bound to a liquid organic hydrogen carrier. Appl. Energy 2017, 194, 1–8. [Google Scholar] [CrossRef]
- Jorschick, H.; Bulgarin, A.; Alletsee, L.; Preuster, P.; Bösmann, A.; Wasserscheid, P. Charging a Liquid Organic Hydrogen Carrier with Wet Hydrogen from Electrolysis. ACS Sustain. Chem. Eng. 2019, 7, 4186–4194. [Google Scholar] [CrossRef]
- Jorschick, H.; Vogl, M.; Preuster, P.; Bösmann, A.; Wasserscheid, P. Hydrogenation of liquid organic hydrogen carrier systems using multicomponent gas mixtures. Int. J. Hydrogen Energy 2019, 44, 31172–31182. [Google Scholar] [CrossRef]
- Jorschick, H.; Dürr, S.; Preuster, P.; Bösmann, A.; Wasserscheid, P. Operational Stability of a LOHC-Based Hot Pressure Swing Reactor for Hydrogen Storage. Energy Technol. 2019, 7, 146–152. [Google Scholar] [CrossRef]
- Hydrogenious.net. Hydrogenious LOHC Technologies GmbH. 2020. Available online: https://www.hydrogenious.net/index.php/en/hydrogen-2-2/ (accessed on 15 January 2020).
- Proost, J. Critical assessment of the production scale required for fossil parity of green electrolytic hydrogen. Int. J. Hydrogen Energy 2020, 45, 17067–17075. [Google Scholar] [CrossRef]
- Saba, S.M.; Müller, M.; Robinius, M.; Stolten, D. The investment costs of electrolysis–A comparison of cost studies from the past 30 years. Int. J. Hydrogen Energy 2018, 43, 1209–1223. [Google Scholar] [CrossRef]
- Mayyas, A.T.; Ruth, M.F.; Pivovar, B.S.; Bender, G.; Wipke, K.B. Manufacturing Cost Analysis for Proton Exchange Membrane Water Electrolyzers; National Renewable Energy Laboratory (NREL): Golden, CO, USA, 2019.
- Energy Sector Management Assistance Program. Green Hydrogen in Developing Countries; World Bank: Washington, DC, USA, 2020. [Google Scholar]
- Reuß, M.; Grube, T.; Robinius, M.; Preuster, P.; Wasserscheid, P.; Stolten, D. Seasonal storage and alternative carriers: A flexible hydrogen supply chain model. Appl. Energy 2017, 200, 290–302. [Google Scholar] [CrossRef]
- Bertau, M.; Wernicke, H.J.; Schmidt, F.; Standt, U.-D.; Seyfried, F.; Buchholz, S.; Busch, G.; Winterberg, M.; Reichelt, L.; Pätzold, C.; et al. Methanol Utilisation Technologies. In Methanol: The Basic Chemical and Energy Feedstock of the Future: Asinger’s Vision Today; Bertau, M., Offermanns, H., Plass, L., Schmidt, F., Wernicke, H.-J., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 327–601. [Google Scholar] [CrossRef]
- Towler, G.; Sinnott, R. Chemical Engineering Design: Principles, Practice and Economics of Plant and Process Design; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Drury, D.J. Formic acid. In Kirk-Othmer Encyclopedia of Chemical Technology; Wiley: Hoboken, NJ, USA, 2000. [Google Scholar]
- Eypasch, M.; Schimpe, M.; Kanwar, A.; Hartmann, T.; Herzog, S.; Frank, T.; Hamacher, T. Model-based techno-economic evaluation of an electricity storage system based on Liquid Organic Hydrogen Carriers. Appl. Energy 2017, 185, 320–330. [Google Scholar] [CrossRef]
- Tremel, A. Evaluation and Discussion. In Electricity-Based Fuels; Springer: Berlin/Heidelberg, Germany, 2018; pp. 75–83. [Google Scholar]
- Fischer, K.L.; Freund, H. On the Optimal Design of Load Flexible Fixed Bed Reactors: Integration of Dynamics into the Design Problem. Chem. Eng. J. 2020, 124722. [Google Scholar] [CrossRef]
- Nestler, F.; Krüger, M.; Full, J.; Hadrich, M.J.; White, R.J.; Schaadt, A. Methanol Synthesis–Industrial Challenges within a Changing Raw Material Landscape. Chem. Ing. Tech. 2019, 90, 1409–1418. [Google Scholar] [CrossRef]
- Supp, E. Improved Methanol Process. Hydrocarb. Process 1981, 71–75. [Google Scholar]
- Avgouropoulos, G.; Schlicker, S.; Schelhaas, K.P.; Papavasiliou, J.; Papadimitriou, K.D.; Theodorakopoulou, E.; Gourdoupi, N.; Machocki, A.; Ioannides, T.; Kallitsis, J.K.; et al. Performance evaluation of a proof-of-concept 70 W internal reforming methanol fuel cell system. J. Power Sources 2016, 307, 875–882. [Google Scholar] [CrossRef]
- Edwards, N.; Ellis, S.R.; Frost, J.C.; Golunski, S.E.; van Keulen, A.N.; Lindewald, N.G.; Reinkingh, J.G. On-board hydrogen generation for transport applications: The HotSpot™ methanol processor. J. Power Sources 1998, 71, 123–128. [Google Scholar] [CrossRef]
- Armijo, J.; Philibert, C. Flexible production of green hydrogen and ammonia from variable solar and wind energy: Case study of Chile and Argentina. Int. J. Hydrogen Energy 2020, 45, 1541–1558. [Google Scholar] [CrossRef]
- Ostuni, R.; Zardi, F. Method for Load Regulation of an Ammonia Plant. U.S. Patent Application 13/626,316, 25 September 2012. [Google Scholar]
- Cheema, I.I.; Krewer, U. Operating envelope of Haber-Bosch process design for power-to-ammonia. RSC Adv. 2018, 8, 34926–34936. [Google Scholar] [CrossRef]
- Schulte Beerbühl, S.; Fröhling, M.; Schultmann, F. Combined scheduling and capacity planning of electricity-based ammonia production to integrate renewable energies. Eur. J. Oper. Res. 2015, 241, 851–862. [Google Scholar] [CrossRef]
- Hawkins, S.; Joffe, D. Technological Characterisation of Hydrogen Storage and Distribution Technologies; Policy Studies Institute: London, UK, 2006. [Google Scholar]
- Hydrogenious LOHC Technologies GmbH. The StorageUNIT. 2020. Available online: https://www.hydrogenious.net/index.php/en/products/thestorageunit/#anchor_storageunit_sseries (accessed on 2 November 2020).
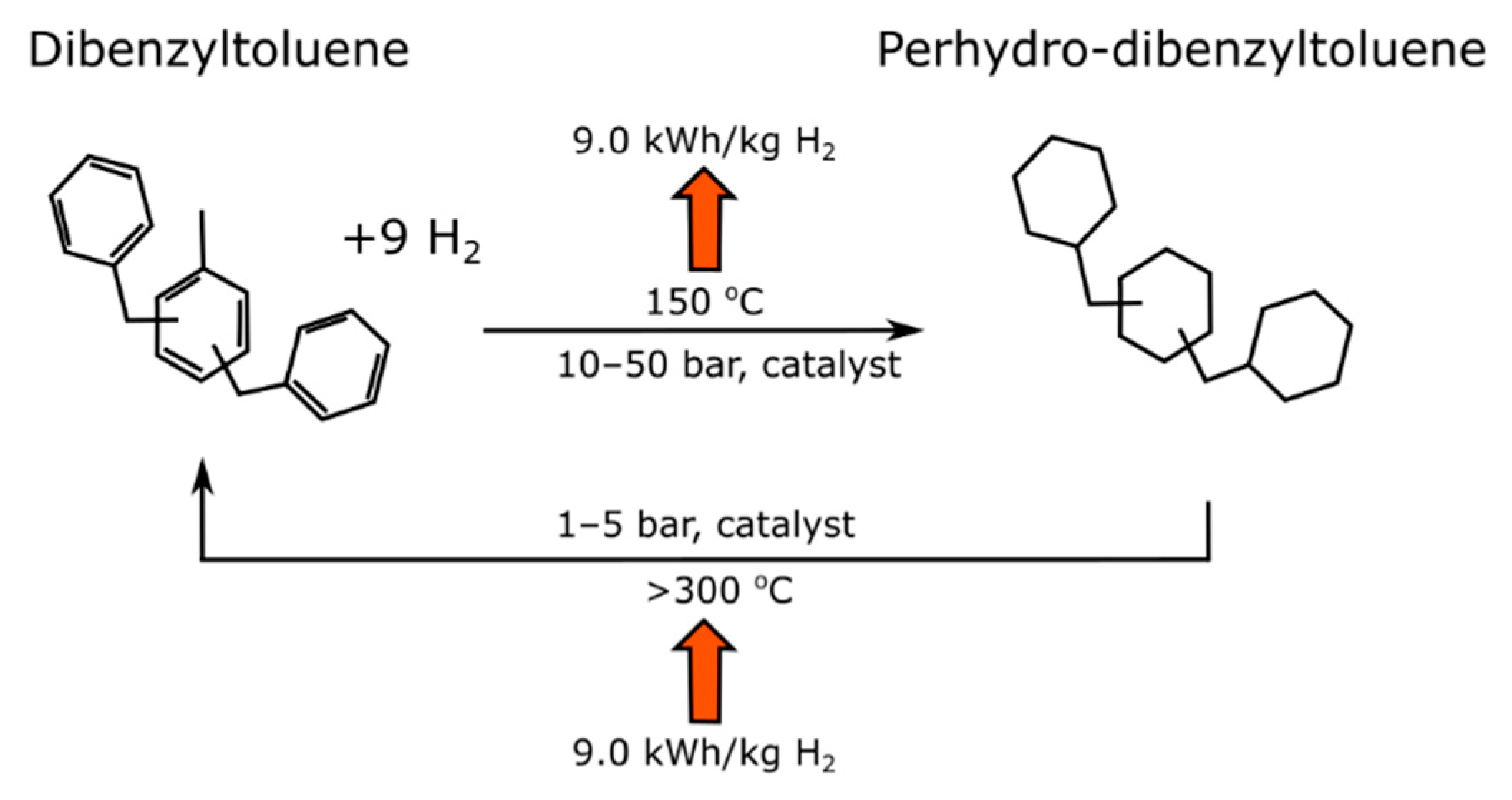
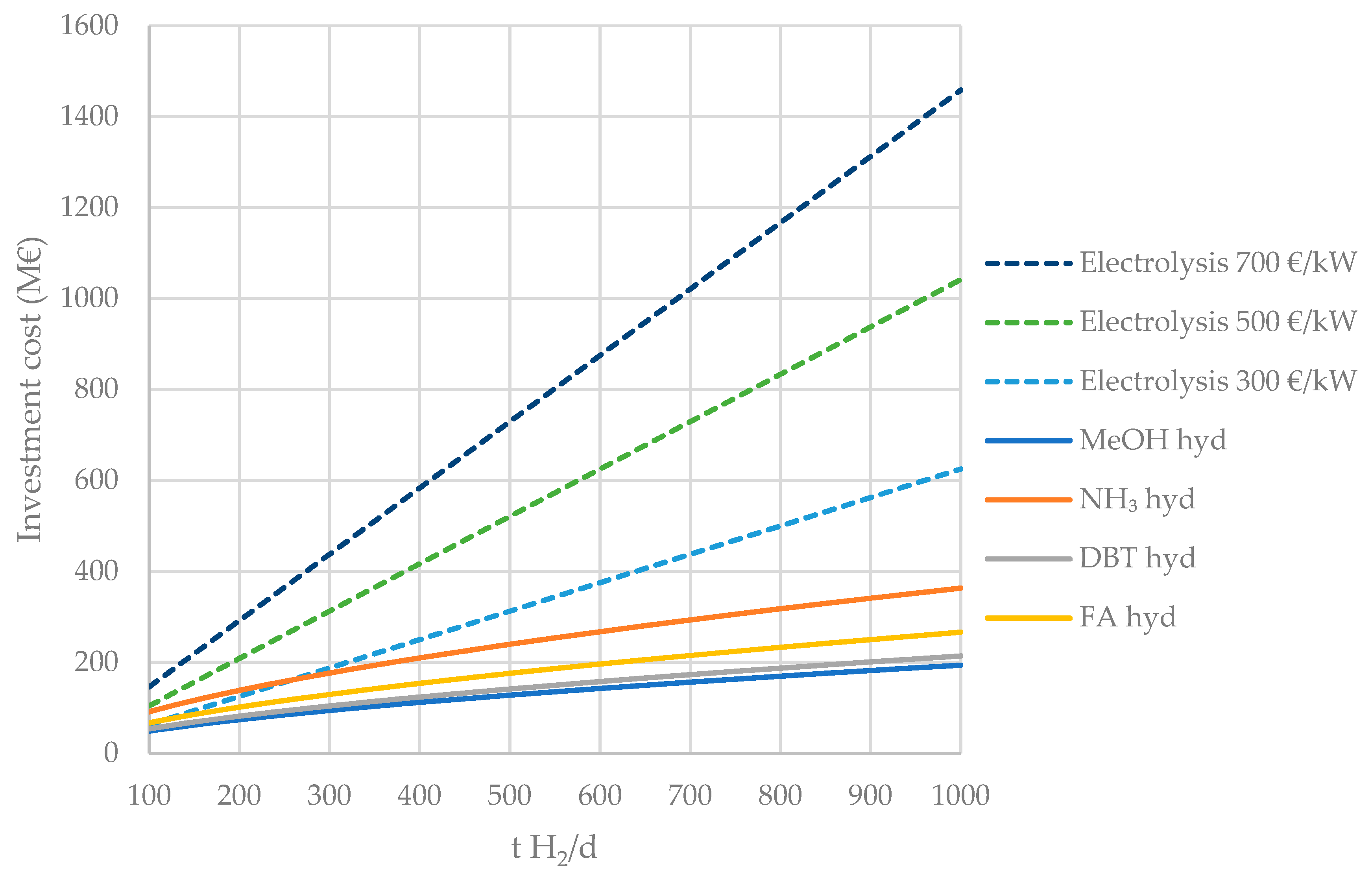
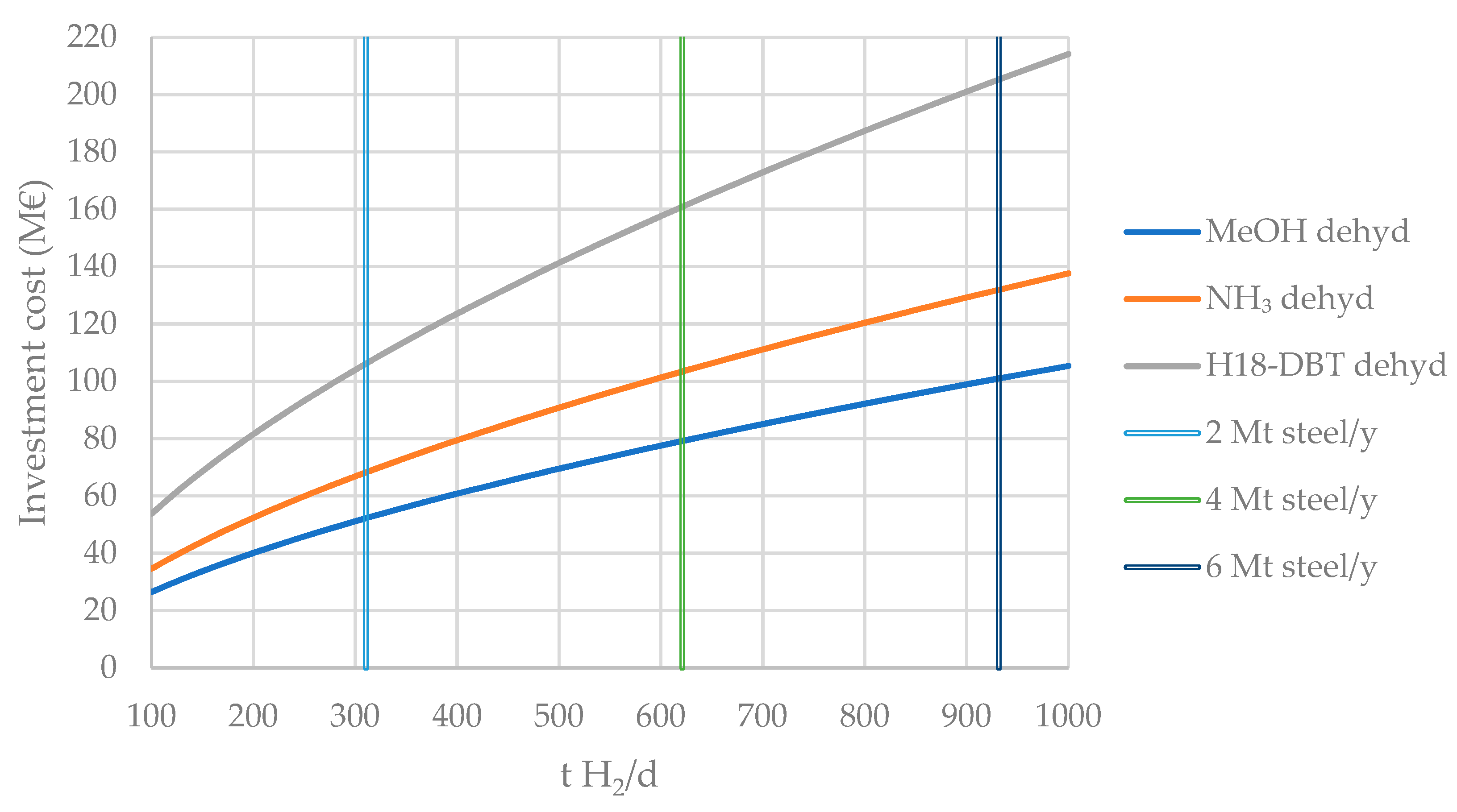
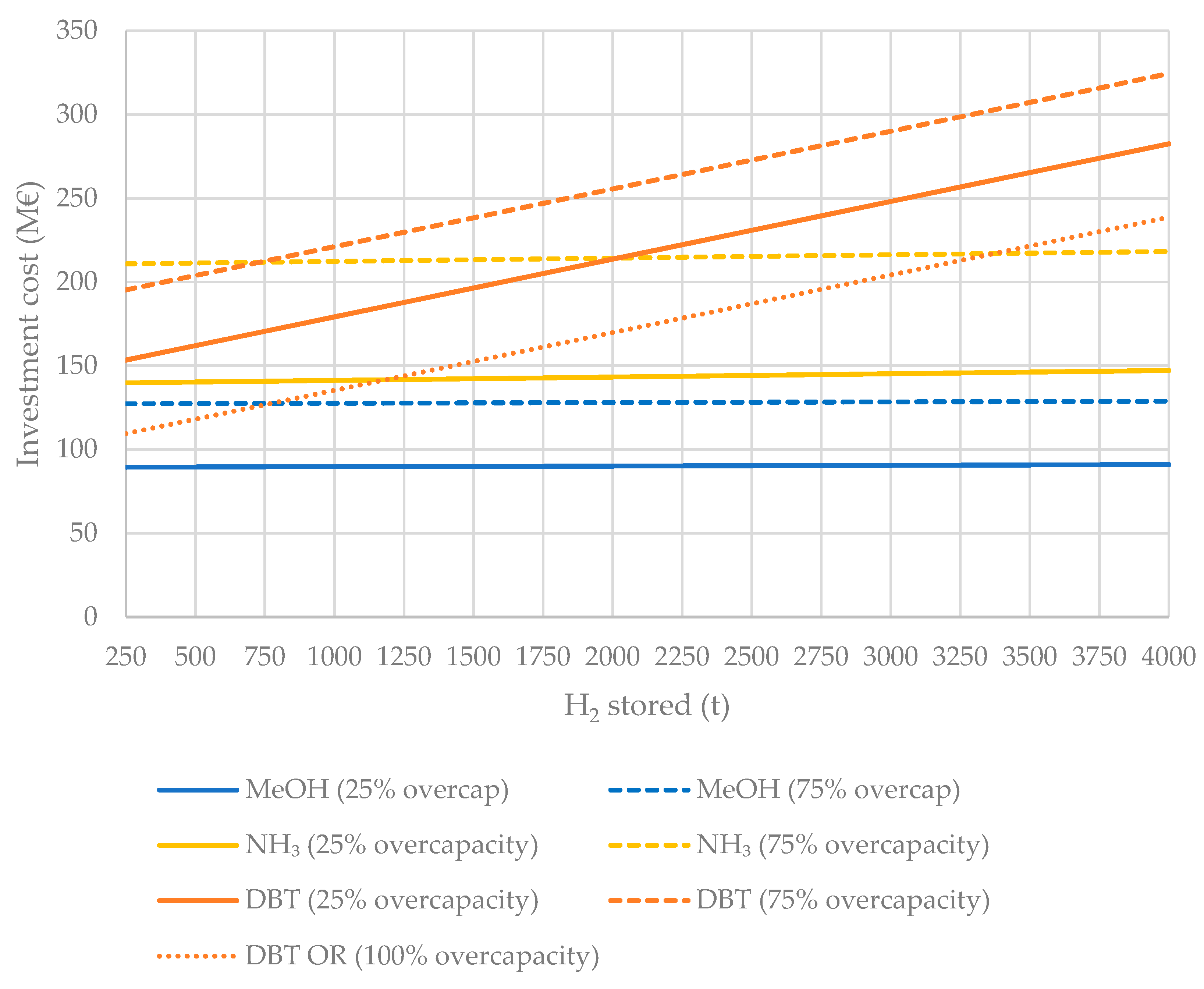
| Liquid H2 Carrier | €/t Carrier | €/t H2 |
|---|---|---|
| MeOH | 75 €/t MeOH | 397 |
| FA | 63 €/t FA | 1439 * |
| NH3 | 350 €/t NH3 | 1977 |
| (H18-)DBT | 68 €/t (H18-)DBT for storage tanks, 2 €/kg DBT material | 34,452 # |
| Liquid H2 Carrier | Electricity Demand Hydrogenation (kWh/kg H2) | Heat Demand Dehydrogenation (kWh/kg H2) | Temperature Dehydrogenation |
|---|---|---|---|
| MeOH | <0.0–0.9 | 2.5–6.9 # | 64% < 100 °C, rest > 200 °C |
| FA | 3.6–6.7 (+16–63 heat) | 2.8–9.1 | <100 °C |
| NH3 | 3.4–6.2 | 14.3 (−1.6 electricity generated) | >500 °C |
| H18-DBT/DBT | 0.4 | 11.5¤ | >300 °C |
| Liquid H2 Carrier | Advantages | Disadvantages | Questions to Resolve before Large-Scale Deployment |
|---|---|---|---|
| MeOH |
|
|
|
| FA |
|
|
|
| NH3 |
|
|
|
| H18-DBT/DBT |
|
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andersson, J. Application of Liquid Hydrogen Carriers in Hydrogen Steelmaking. Energies 2021, 14, 1392. https://doi.org/10.3390/en14051392
Andersson J. Application of Liquid Hydrogen Carriers in Hydrogen Steelmaking. Energies. 2021; 14(5):1392. https://doi.org/10.3390/en14051392
Chicago/Turabian StyleAndersson, Joakim. 2021. "Application of Liquid Hydrogen Carriers in Hydrogen Steelmaking" Energies 14, no. 5: 1392. https://doi.org/10.3390/en14051392
APA StyleAndersson, J. (2021). Application of Liquid Hydrogen Carriers in Hydrogen Steelmaking. Energies, 14(5), 1392. https://doi.org/10.3390/en14051392






