2.1. Test Bench and Experimental Procedure
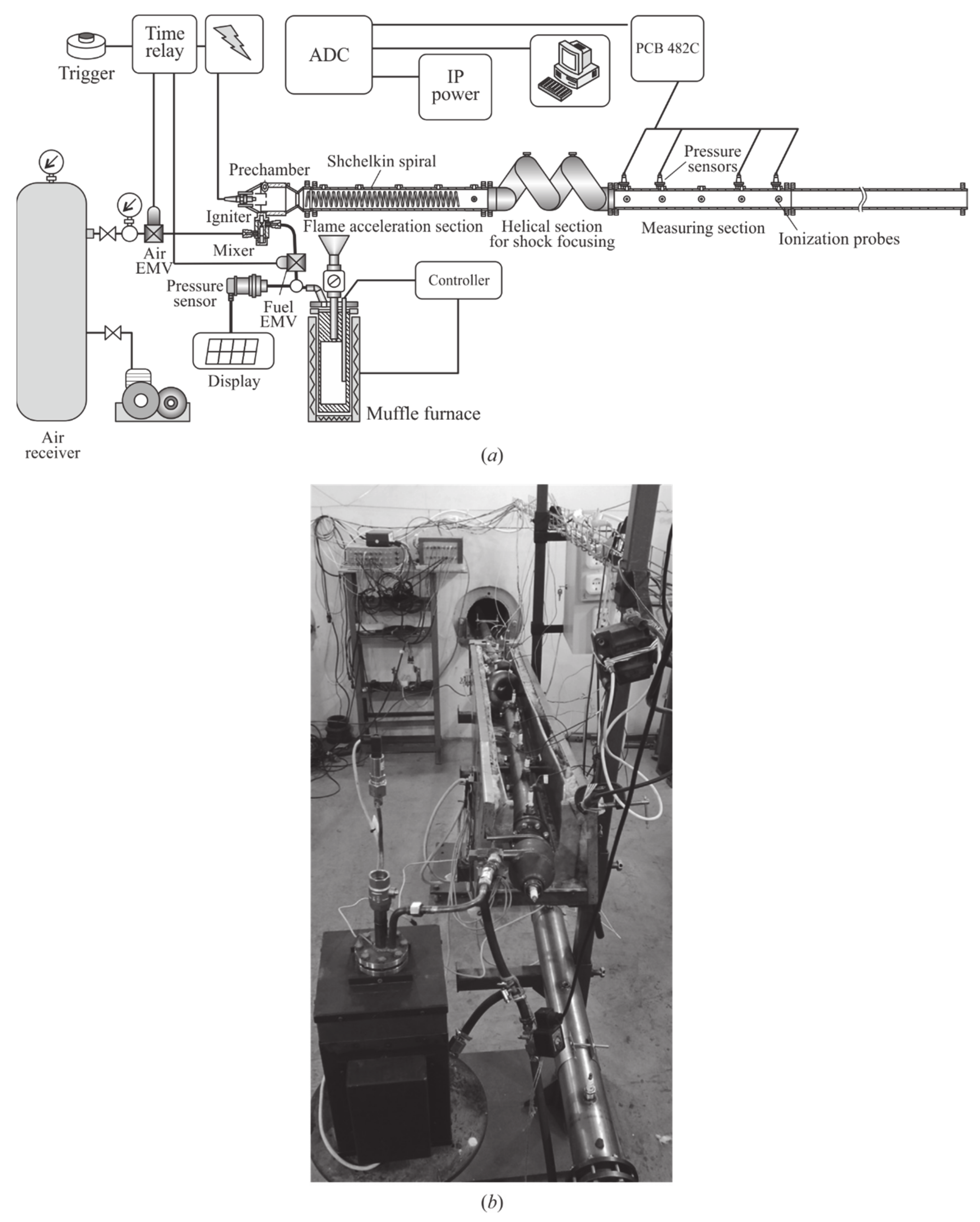
Experiments are performed on the test bench (
Figure 1) described in detail in [
23,
24,
25]. The test bench includes a standard pulsed detonation tube (PDT), thermostatic gas generator (GG), air supply system, mixer, control devices, ignition system, measuring probes and sensors, and data acquisition system. The main element of the test bench is the PDT (
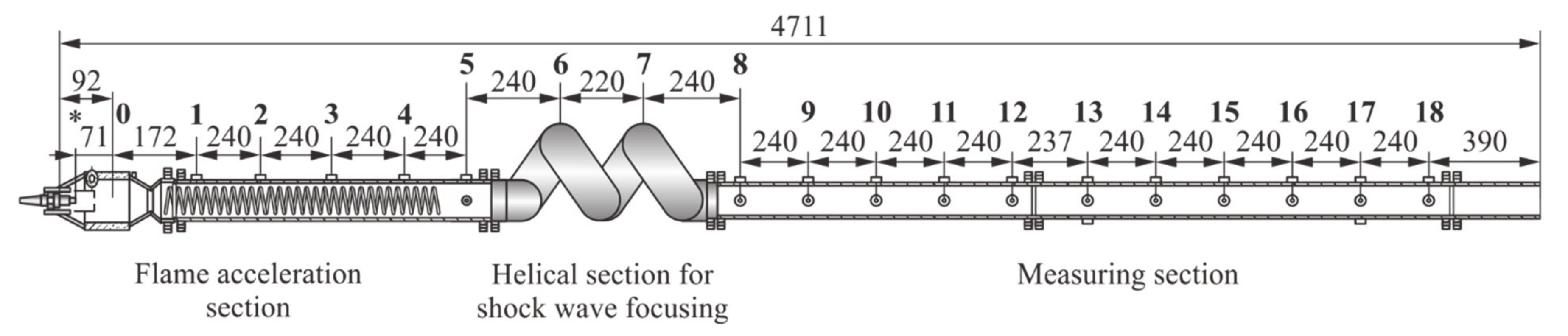
Figure 2), which consists of three sections: flame acceleration section with Shchelkin spiral; helical section for gasdynamic focusing of shock waves (SWs) running ahead of the flame; and measuring section. The internal diameter of the tube of all PDT sections is 50 mm. The total length of the PDT is about 5 m. The length of the measuring section is increased as compared to [
23,
24,
25]: instead of 12 (see
Figure 3 in [
22]), 18 measuring stations are used, in which high-frequency pressure sensors (PS, PCB 113b24) and/or ionization probes (IP) are installed. Compared with [
23,
24,
25], the design of the GG is also changed (
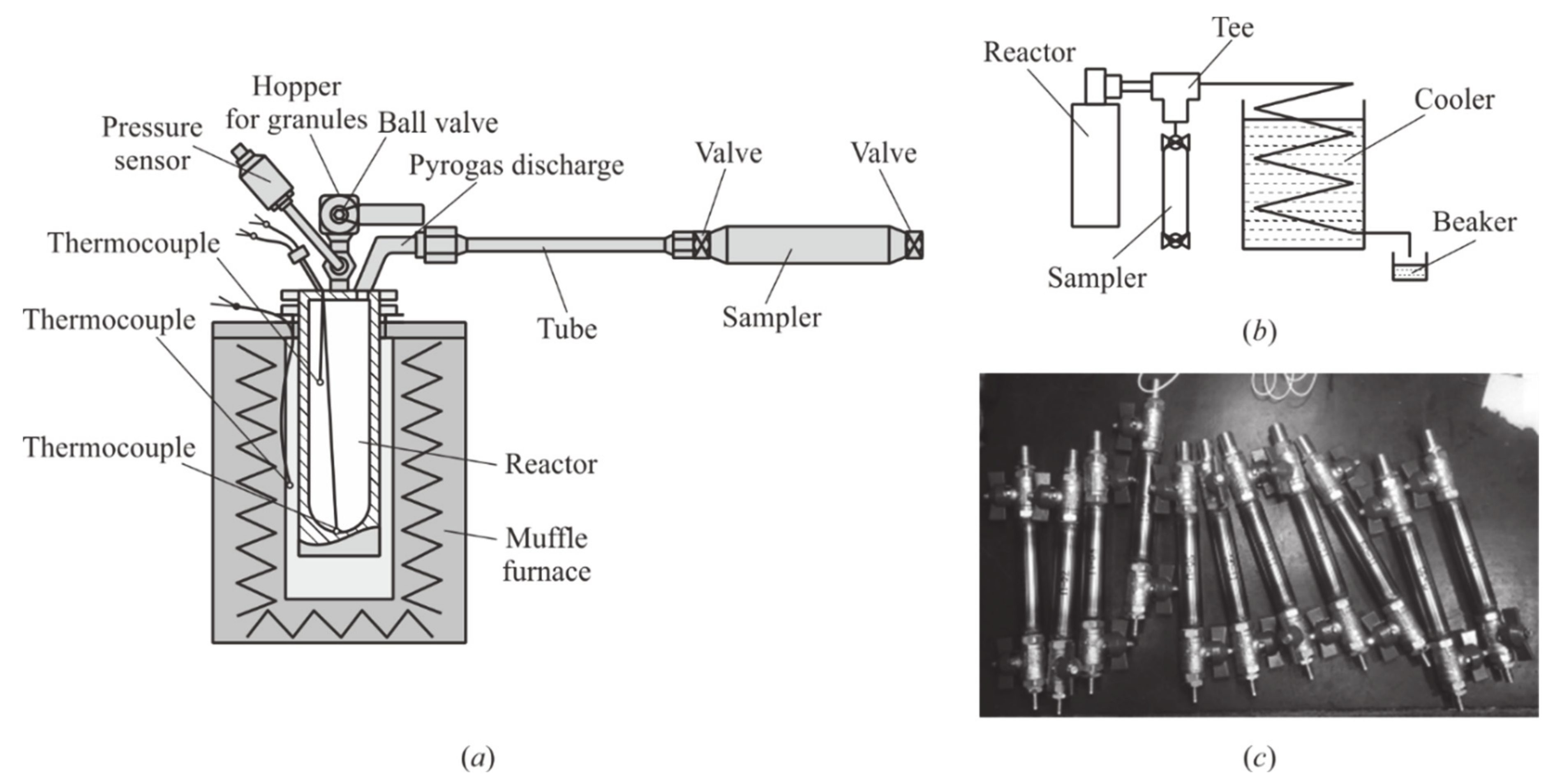
Figure 3): instead of a massive insert, it applies a massive bottom with a spherical recess, and an additional thermocouple is used for measuring the temperature of the pyrogas at the outlet from the GG. Besides, an additional electromagnetic valve (EMV) is installed in the fuel main to control the supply of pyrogas in the mixer. The rest of the test bench is the same as in [
23,
24,
25]. During the experiment, air and pyrogas are periodically supplied into the mixer through the EMVs in air and fuel mains, where they are effectively mixed. The mixture is then fed from the mixer to the PDT, where it is periodically ignited by a car spark plug. The resulting flame accelerates in the flame acceleration section and forms a leading SW, which enters the helical section. Multiple reflections of the SW in the helical section lead to DDT according to the mechanism of [
26] and the onset of a detonation wave (DW) which further propagates in the measuring section of the PDT and exits to the atmosphere. Note that the geometry of the PDT and ignition arrangement were thoroughly optimized in [
27] for obtaining the shortest possible DDT run-up distance.
Before conducting experiments on DDT in the air mixtures of pyrogas, we have determined the characteristics of GG and the composition of pyrogas during pyrolysis of PE samples in a closed and open GG. The measured characteristics of the GG include: the mass of solid PE sample,
m0, decomposition temperature,
Tp, pyrolysis time,
tp, and the corresponding maximum pressure,
Pp, the minimum temperature during pyrolysis,
Tmin, and the time it is attained,
tmin, the mass of solid residue (pyrocarbon) in the GG,
mr, the mass of wax-like sediments in the mains at the outlet of the GG,
mw, and the volume fraction of the
i-th component in the pyrolysis gas,
Xi.
Table 1 shows the measurement errors of all listed characteristics. For the values measured directly we indicate the absolute instrumental errors. For pyrolysis time
tp, the error is evaluated based on the apparent indefinity of pyrolysis completion. For
Xi, the relative error is the error of gas chromatography. For
mr and
mw, the absolute errors take the methodical measurement error into account.
2.2. Polyethylene Pyrolysis in a Closed Gas Generator
The procedure for obtaining the characteristics of the GG and determining the pyrogas composition in a closed GG is as follows. The GG is heated up to a predetermined decomposition temperature
Tp = 650–850 °C and kept at a constant temperature at least for 10 min. Then, the data acquisition system based on the analog-to-digital converter (ADC in
Figure 1) is switched on (recording frequency 1 kHz), and a sample of granular PE grade HDPE 273-83 of a given mass (for example, 1 g) is poured into the GG.
White spherical PE granules have a diameter of 4 mm. Then the ball valve is closed and PE pyrolysis proceeds with an increase in pressure, which is controlled by a low-frequency PS (see
Figure 3a). After time interval
tp has elapsed, the pyrogas pressure in the GG reaches the
Pp value, and the pyrogas temperature approaches the preset value of
Tp, which is interpreted as the completion of pyrolysis: the exhaust valve opens, and the pyrogas expels into the atmosphere through the sampler (see
Figure 3b). At the end of experiment, the GG furnace is cooled with the outlet valve closed, the GG is disassembled, and all solid residue is removed from it. The solid residue obtained in experiment is weighed on a balance, and a pyrogas sample in a sampler (see
Figure 3c) is sent for chromatographic analysis.
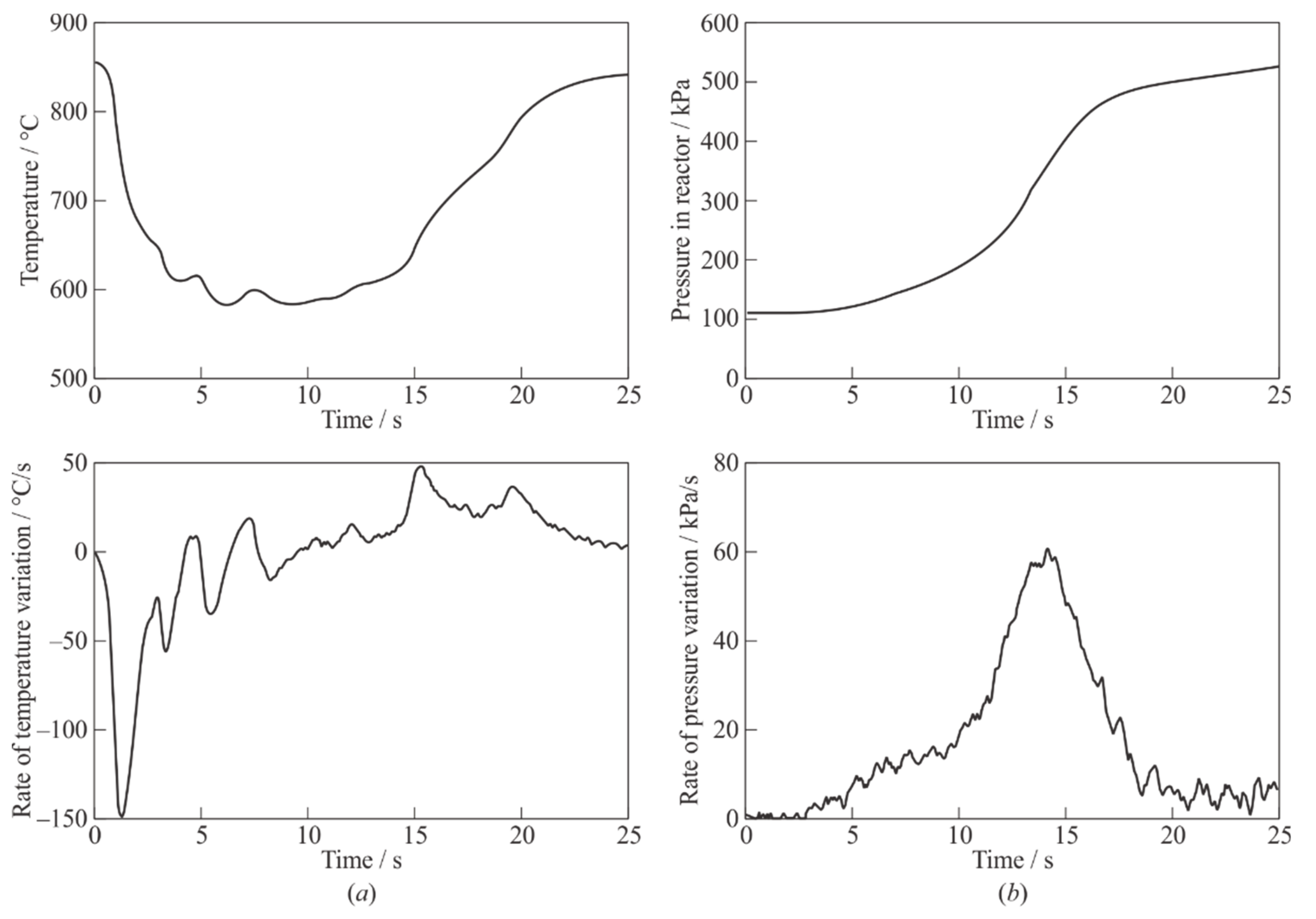
Figure 4 shows the examples of pyrogas temperature (
Figure 4a) and pressure (
Figure 4b) records in the GG, obtained using a thermocouple located on its bottom, and the PS, respectively, in an experiment with the decomposition temperature
Tp = 850 °C and with a sample of granular PE of
m0 = 1 g. For convenience,
Figure 4a,b also show the rates of temperature and pressure variation in the closed GG. The zero point in time corresponds to the moment of filling the PE granules into the GG. The temperature of the thermocouple junction at the bottom of the GG first decreases due to the prevailing heat removal into PE granules and the endothermic process of their pyrolysis, reaches a minimum value (
Tmin ≈ 580 °C) by the time
tmin ≈ 6 s, and then increases reaching the preset value
Tp ≈ 850 °C after complete transformation of PE granules and heating of pyrogas. The pyrogas pressure (see
Figure 4b) increases monotonically, reaching a value of
Pp ≈ 520 kPa at
tp ≈ 25 s.
From the time histories of pyrogas temperature and pressure,
T(
t) and
P(
t), it is possible to estimate the average molecular mass of pyrogas,
, at pyrolysis temperature
Tp using a simple formula based on the material balance equation for a closed GG:
where
R is the universal gas constant,
xr is the mass fraction of solid residue, and
V0 is the GG volume. The error in determining
by Equation (1) is estimated as 5%.
Table 2 shows the values of
Tmin,
tmin,
Pp,
tp,
xr and
for three values of
Tp: 650, 750, and 850 °C, when using a PE sample with
m0 = 1 g. With an increase in the decomposition temperature, the pyrolysis process in a closed GG proceeds more intensely and is completed in a shorter time. The mass fraction of the solid residue reaches 70% at
Tp = 850 °C, and the average molecular mass of pyrogas, on the contrary, decreases from ≈40 g/mol at
Tp = 650 °C to ≈10 g/mol at
Tp = 850 °C. Note that the values of
Tmin,
tmin,
Pp,
tp, and
xr also depend on the initial mass of a PE sample. Thus, with an increase in the sample mass to 1.5 g, the parameter
Pp takes on the values of 420, 775, and 695 kPa at
Tp = 650, 750, and 850 °C, respectively, i.e., the pressure of pyrogas in the GG increases significantly.
Table 3 shows the results of chromatographic analysis of pyrogas samples taken from a closed GG at decomposition temperatures
Tp = 650 and 850 °C. In metal samplers (see
Figure 3c), pyrogas samples were taken from the GG at the pyrolysis temperature after the end of the active phase of gas generation, when the pressure in the GG reached a plateau.
For chromatographic analysis, pyrogas samples were taken from the samplers at room temperature (19–20 °C). To reduce the contamination of chromatographs, samples were taken from samplers with a syringe through a filter made of chromatographic glass wool treated with dimethylchlorosilane (DMCS, (CH3)2SiHCl) and were introduced into the evaporators of the chromatographs heated to 150 °C. Analysis of C1–C9 hydrocarbons (detailed analysis of C1–C4) was carried out using a CP-Al2O3/KCl PLOT capillary column 50 m length × 0.53 mm inner diameter × 10 μm thickness of the adsorption layer and a flame ionization detector. Hydrogen was determined using a HayeSep Q 80/100 packed column 3 m long and 2 mm in inner diameter and a thermal conductivity detector.
It follows from
Table 3 that pyrogas mainly consists of hydrogen, methane, ethylene and ethane, and with the increase in the decomposition temperature from 650 to 850 °C, the content of hydrogen and methane increases significantly, and the discrepancy in the estimated average molecular mass of the pyrogas at the pyrolysis temperature (see
Table 2) and at room temperature (see
Table 3) decreases, which also indicates an increase in the content of substances in the pyrogas that remain gaseous at room temperature (19–20 °C).
2.3. Polyethylene Pyrolysis in an Open Gas Generator
The characteristics of the GG and pyrogas composition in an open GG are determined under conditions when the pressure in the GG is close to atmospheric. As in the procedure described earlier in this paper, the GG is heated to a decomposition temperature
Tp = 650–850 °C, and a weighed portion of granular PE of a given mass
m0 = 15 g is poured into it. The difference is that during the entire pyrolysis process, except for the moment of sampling, the inlet and outlet valves of a sampler remain open, and the generated pyrogas continuously expels to the atmosphere. To collect the liquid fraction of PE pyrolysis products, pyrogas is passed through the cooler (see
Figure 3b) in some experiments.
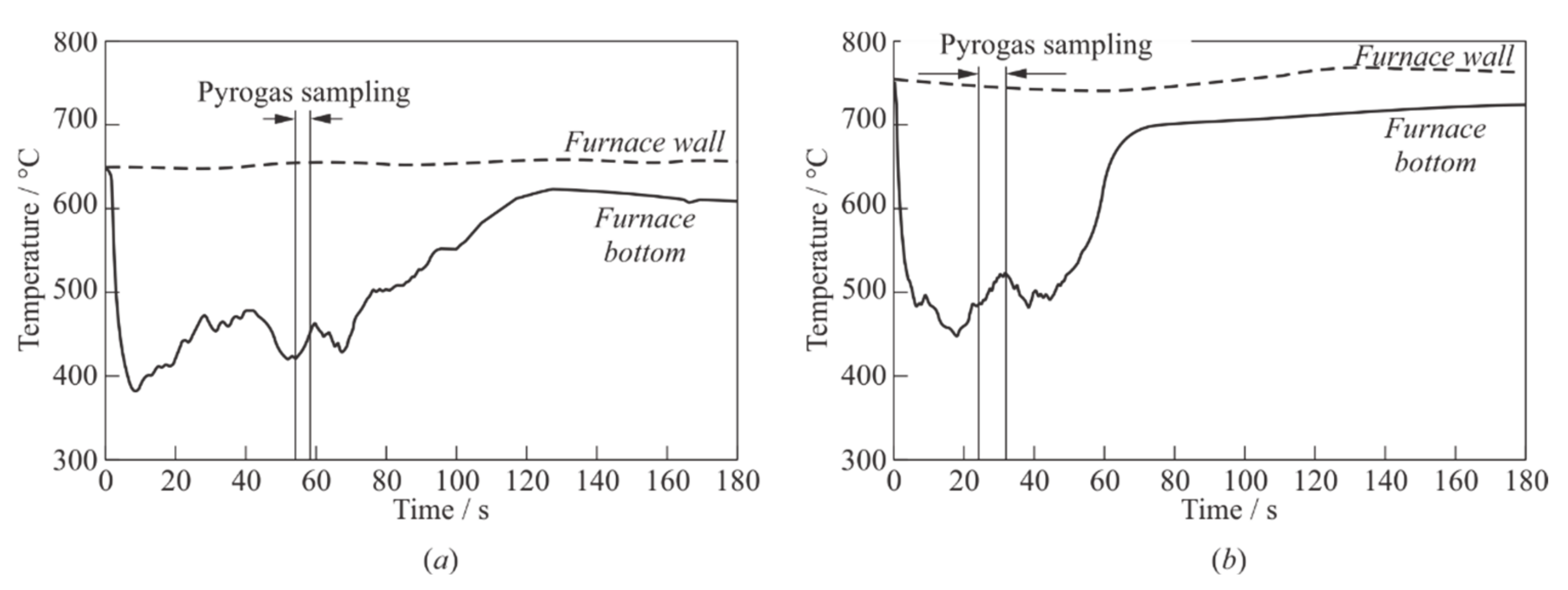
Figure 5 shows examples of the time histories of pyrogas temperature, obtained in an open GG using a thermocouple located at GG bottom at
Tp = 650 °C (
Figure 5a) and 750 °C (
Figure 5b) with PE samples of
m0 = 15 g. The zero point in time corresponds to the moment of filling the PE granules into the GG. The temperature of the thermocouple junction at the bottom of the GG first decreases due to heat removal into PE granules and the endothermic process of their pyrolysis, reaches a minimum value
Tmin by time
tmin, and then increases as the pyrolysis process is completed, but the preset value of
Tp during the observation time (180 s) is not reached.
Table 4 shows the values of
Tmin,
tmin, and
xr for three values of the decomposition temperature
Tp = 650, 750, and 850 °C, when using PE samples with a mass of
m0 = 15 g. Unlike pyrolysis in a closed GG, the mass fraction of the solid residue
xr during pyrolysis in an open GG is much less (2–7%).
Table 5 shows the results of chromatographic analysis of pyrogas in an open GG at
Tp = 650, 750, and 850 °C. As in the previous case, the sampling was made at the decomposition temperature, and the analysis of the composition was made at room temperature (19–20 °C). In contrast to pyrolysis in a closed GG (see
Table 3), the proportions of hydrogen and methane in the composition of pyrogas in an open GG significantly decreased and the proportions of ethylene, ethane, propylene, and propane increased. The average molecular mass of pyrogas is almost constant (
= 28–32 g/mol). Among the identified products of the C
4 fraction, the largest amounts are represented by butadiene-1,3 (3.9%) and butylene-1 (4.4%), the fraction C
5 mainly consists of unsaturated hydrocarbons–pentenes (C
5H
10), among which pentene-1 (1.9%) is present in the largest amount. The C
6 fraction mainly contains unsaturated hydrocarbons C
6H
12, cyclohexene and minor amounts of other hydrocarbons of the C
6H
10 group.
2.4. Experimental Technique and Processing of the Results
Experiments to determine the detonability of air mixtures of pyrogas are performed according to the technique described in detail in [
23,
24,
25]. We give here only a brief description of it. At the beginning of experiment, the muffle furnace is switched on, and the empty GG is heated to the preset decomposition temperature
Tp and kept at this temperature for at least 30 min. Next, a prepared sample of granulated PE is loaded into the GG through the upper loading hopper, the ball valve closes and the control program on the time relay is started: the unit starts operating in a cyclic mode.
Table 6 shows the settings of the time relay that controls the experiment. The operation cycle is set up implying that pyrogas and air are periodically supplied to the PDT in portions. A value of 0 in
Table 6 corresponds to the channel state “closed” or “disabled,” and a value of 1 corresponds to the channel state “open” or “on.” During periods T1 and T2, when the fuel and air EMVs are open, pyrogas from the GG and air from the air receiver flow through the mains and enter the mixer, where they are mixed, and the mixture formed is sent to the PDT. The duration of interval T1 is selected from the condition of filling the entire PDT volume with the mixture at a given air flow rate. The mixture in the PDT is ignited at time instant (T1 + T2), corresponding to the end of time interval T2. Mixture ignition (from now on referred to as “PDT shot”) generates either a deflagration wave or a DW. After ignition (for time interval T3), the fuel and air EMVs shut off the supply of pyrogas and air to the PDT: the GG is completely closed. In time interval T3 + T4 = 0.5 s, the rate of pyrogas formation and the composition of a new portion of fuel mixture for the next operation cycle are estimated based on the pressure rise in the closed GG. During time interval T4, a portion of purge air is supplied to the mixer. The process repeats cyclically until the time relay is stopped. The operation time of the test rig exceeds the pyrolysis time. At the end of experiment, a solid pyrocarbon residue is taken from the cooled GG, and unburned products in the form of wax-like deposits are taken from the mixer and PDT.
It follows from the described experimental procedure that the pyrolysis process proceeds under variable conditions of a closed and open GG.
The main parameters of the experiment are determined as follows. The average mass flow rate of air,
, is determined from the change in pressure in the air receiver Δ
P for time interval Δ
t according to the following formula:
where
and
PN are the air density and pressure under normal conditions;
Vb is the volume of the air receiver (50 L). The error in determining
is estimated at 10%. The constancy of the air flow rate is ensured by an air reducer that maintains a constant pressure in front of the air EMV.
The mass of solid residues in the GG,
mr, and wax-like deposits in the mixer and PDT,
mw, are determined by weighing on a precision balance at the end of experiment. The mass of pyrogas,
mp, supplied to the PDT is calculated using the material balance equation taking the mass fractions of solid residue,
xr, and wax-like deposits,
xw, into account:
The average mass flow rate of pyrogas,
, is calculated by the formula:
where
is the pyrolysis time determined from the pressure and temperature records in the GG. The error in determining
is estimated at 15%.
The average fuel-to-air equivalence ratio,
, is determined by the formula:
where
is the stoichiometric coefficient. For pyrogas,
L0 ≈ 14.8. The error of determining
is estimated at 20%. The temperature of pyrogas before entering the mixer,
Tin, is measured by a thermocouple installed in the main. The error in determining
Tin is estimated as 0.5%.
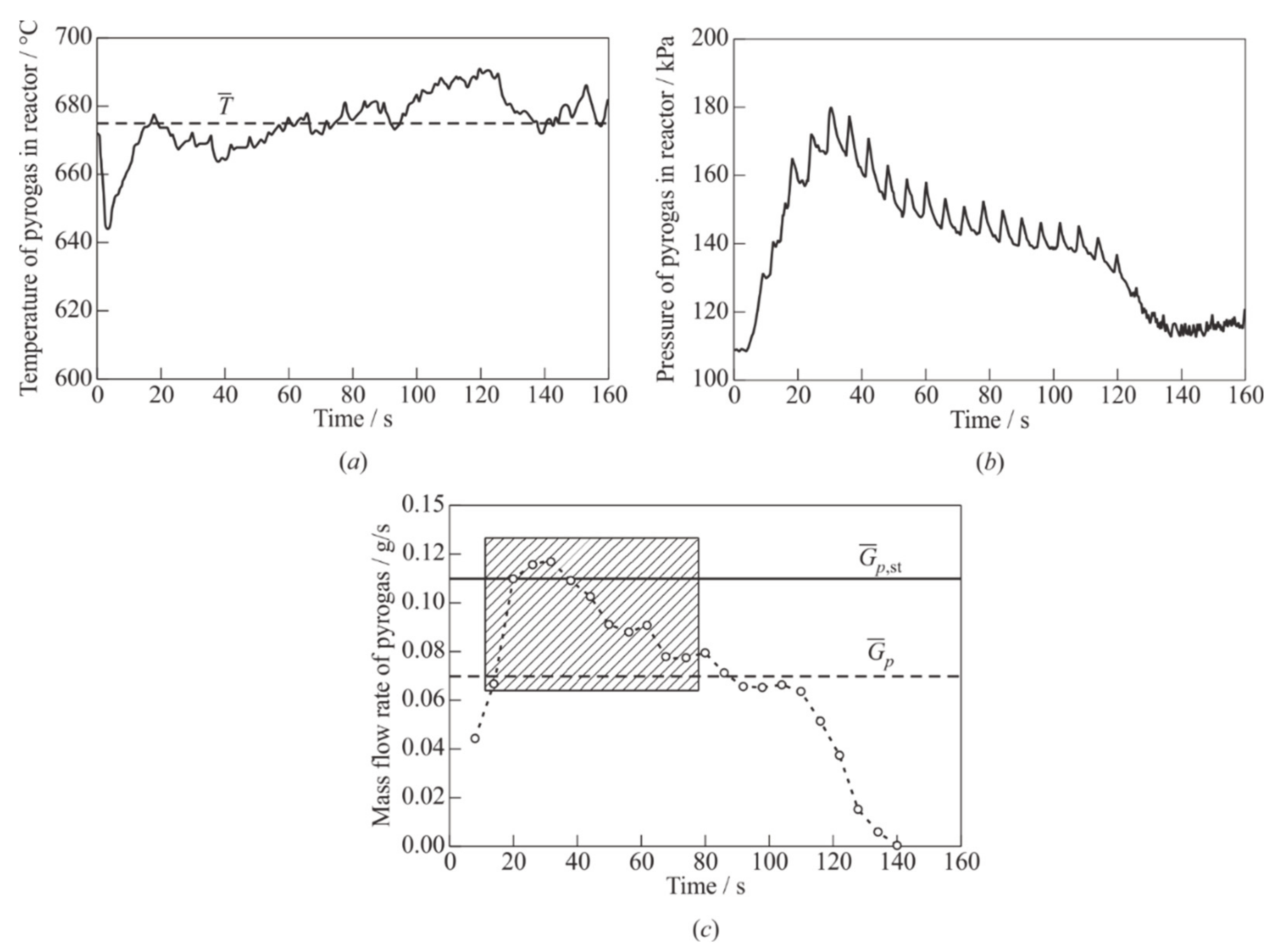
As an example,
Figure 6a shows temperature records
T(
t), and
Figure 6b shows pressure records
P(
t) in the GG in one of the experiments on the PDT with
Tp = 765 °C and
m0 = 10 g. It is seen from the temperature record that approximately 15s after loading the GG, pyrogas leaves it with an average temperature ≈ 675 °C, and temperature fluctuations are small (±10 °C). Note that in the main connecting the GG with mixer, the temperature of pyrogas significantly decreases and does not exceed 120 °C, and after mixing of pyrogas with air, it decreases to 30–40 °C. The pressure record (see
Figure 6b) shows regular pulsations with an amplitude of up to 20 kPa and a duration of 0.5 s, caused by the closing/opening of the fuel EMV at each PDT shot. Following the pressure curve, the pyrolysis time of the PE sample,
tp, under these conditions is about 130 s. In this experiment, the parameters
, (
xr +
xw),
, and
are approximately 1.66 g/s, 0.03, 0.07 g/s (see below), and 0.6, respectively. For this experiment
Figure 6c shows the calculated dependence of the average (per PDT shot) mass flow rate of pyrogas at the moments of time when the GG is open (92% of the cycle time), plotted based on the calibration dependence of the mass flow rate of pyrogas on the pressure in the GG. The solid horizontal line
= 0.11 g/s corresponds to a stoichiometric fuel mixture at
= 1.66 g/s. The dashed horizontal line corresponds to the integral averaged value of the pyrogas mass flow rate for a time interval of
tp = 130 s. In the considered experiment, the mixture is, on average, fuel lean (
≈ 0.6), while the instantaneous values of the fuel-to-air equivalence ratio in this experiment vary from the minimum value of
Φmin = 0.4 in the first PDT shot to the maximum value of
Φmax = 1.2 in the fifth PDT shot. In a series of cycles in the time interval from 15 to 80 s, the composition of the mixture is close to stoichiometric (0.7 ≤
≤ 1.1) (gray shaded area in
Figure 6c). It is in these cycles that DDT and the subsequent self-sustained steady-state propagation of the DW in the measuring section of the PDT are recorded in the experiment.
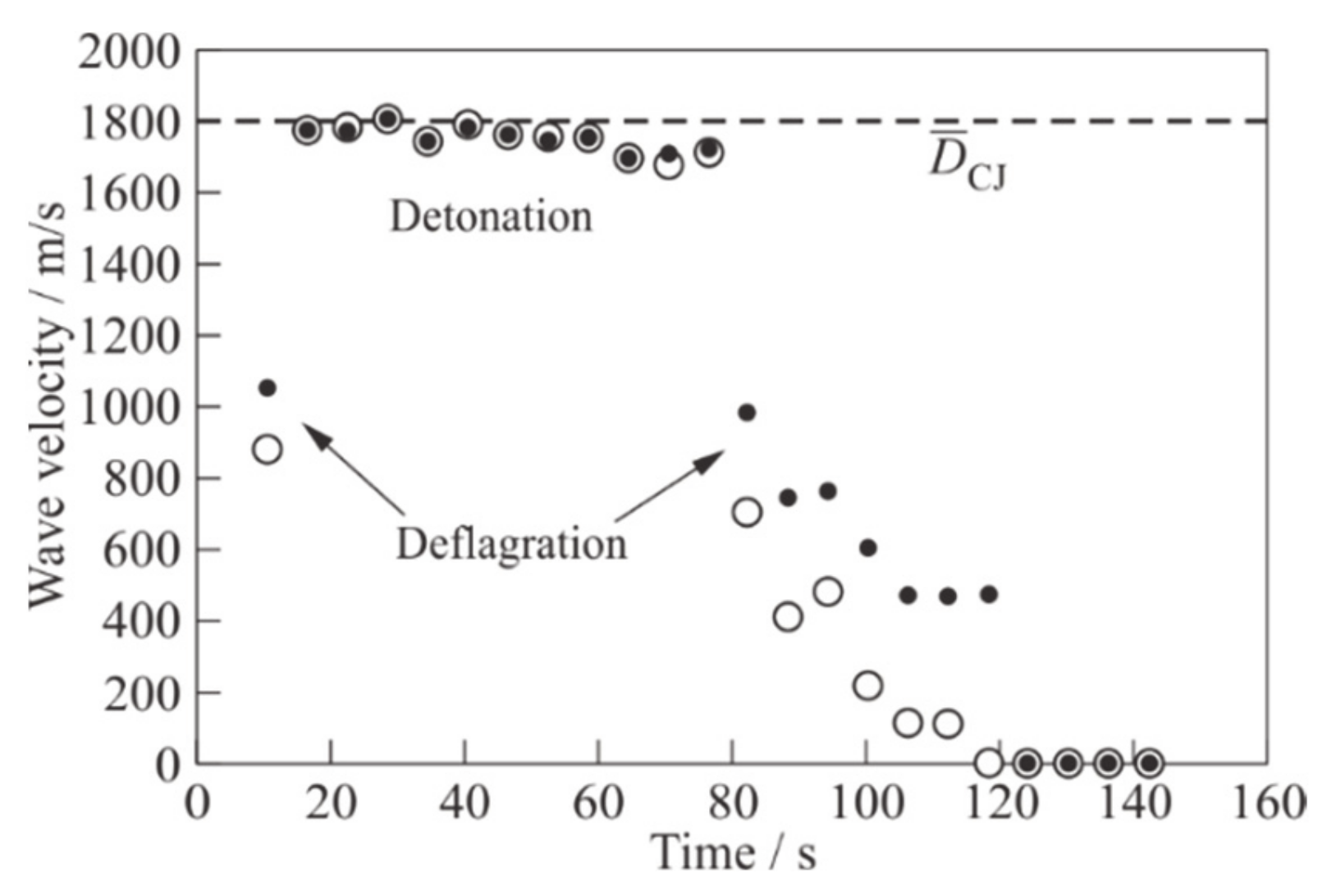
As a matter of fact,
Figure 7 shows the measured values of the mean velocities of the SW (dark circles) and the flame front (light circles) in each PDT shot of this experiment. The SW velocity at any measuring segment of the PDT is determined by the known distance between the measuring stations (see
Figure 2), in which the PSs are installed, and by the time interval between the moments of wave arrival at these PSs. The error of measuring the SW velocity is 3%. The flame front speed is determined in a similar way, but instead of PS records, IP records are used. The error of measuring the flame front velocity is 3%. The data in
Figure 7 are obtained by averaging the velocities over all measuring segments between the measuring stations 9 and 12. In (11) (detonation PDT shots) in the time interval 15 ≤
t ≤ 80 s, the SW and the flame front propagate at the same supersonic velocity, close to the typical value of the Chapman-Jouguet (CJ) detonation velocity
DCJ ≈ 1800 m/s in stoichiometric mixtures of hydrocarbon fuels (horizontal dashed line in
Figure 7). In one shot in the time interval
t < 15 s and in 7 shots at 80 ≤
t ≤ 120 s, the SW runs significantly ahead of the flame front, i.e., a deflagration wave propagates in the measuring section. In shots at
t > 120 s, a fuel mixture in the PDT is not ignited.
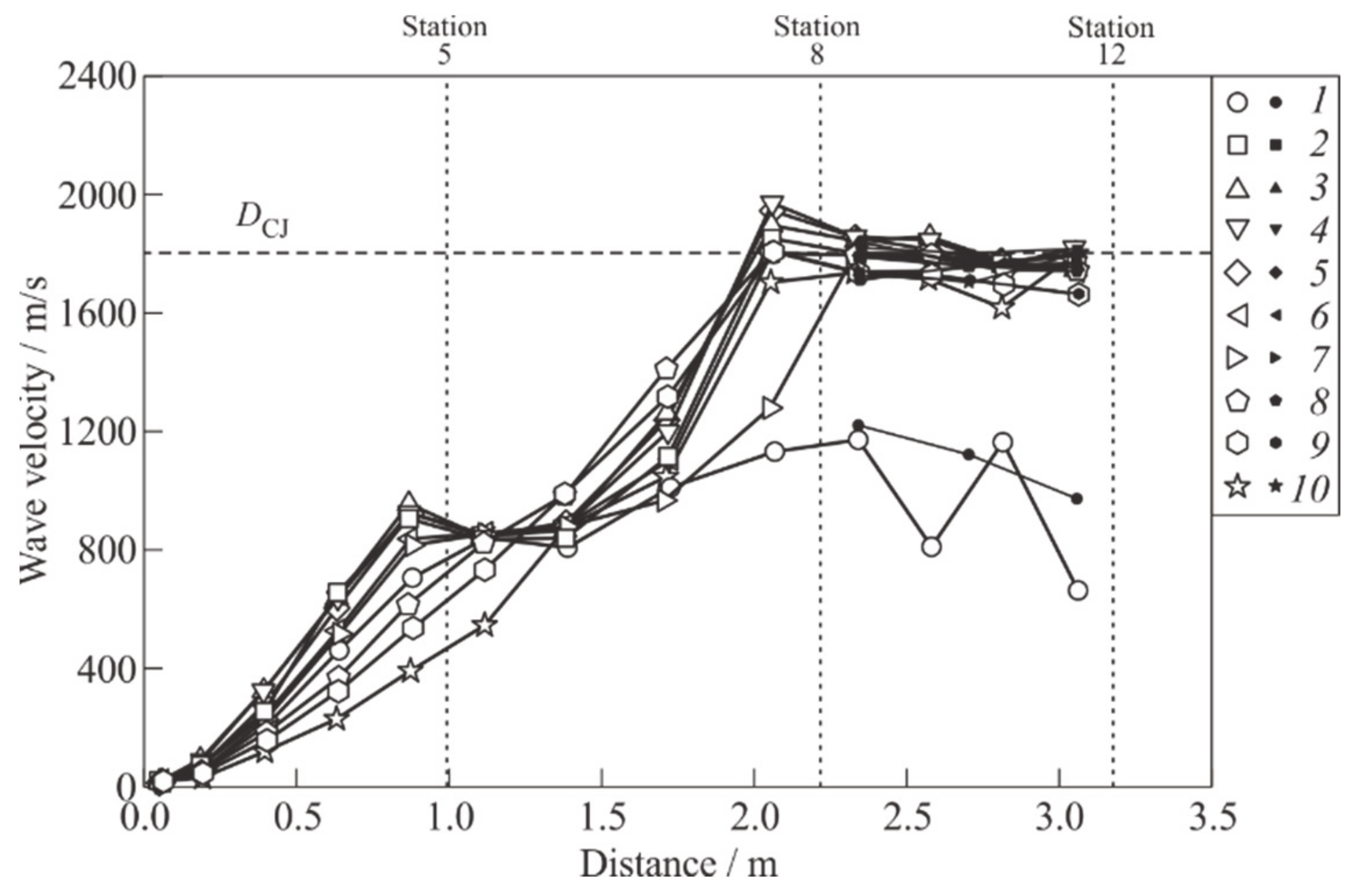
Figure 8 shows an example of the dependences of the SW and flame front velocities on the traveled distance in the considered experiment for a selection of 10 shots. The horizontal dashed line corresponds to
DCJ. In detonation shots, the velocity of the “SW–flame front” complex reaches the
DCJ value in the vicinity of measuring station 8 (at the exit from the PDT helical section) at about 2 m from the ignition source. As in [
23,
24,
25], the distance at which this occurs is called the DDT run-up distance,
LDDT. The time interval from the moment of mixture ignition to the moment of detonation onset is called the DDT run-up time,
tDDT. The DDT run-up time,
tDDT, is determined by joint consideration of wave velocity–distance and wave velocity–time dependences.
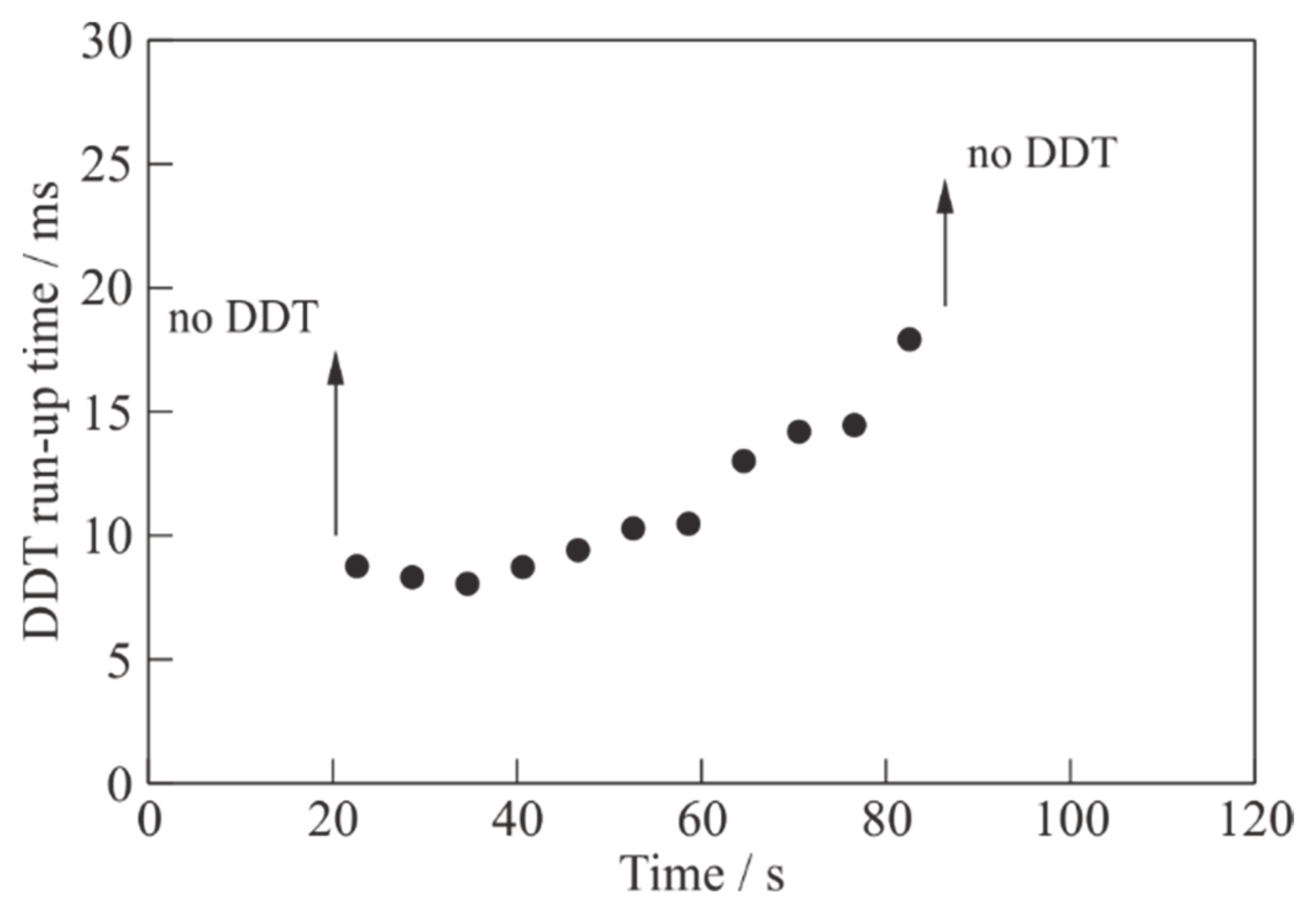
Figure 9 shows the measured values of the DDT run-up time in detonation shots in the considered experiment. The error in determining the DDT run-up time is estimated at 0.2 ms. The DDT run-up time varies from 8 to 18 ms. The minimum value of
tDDT ≈ 8 ms is achieved in a detonation shot at
t ≈ 35 s, when the instantaneous mass flow rate of pyrogas is close to the maximum value (see
Figure 7), and the mixture is slightly fuel rich (
= 1.05).
2.5. Results and Discussion
Using the procedures and techniques described above, we performed a large series of experiments to study the detonability of pyrogas.
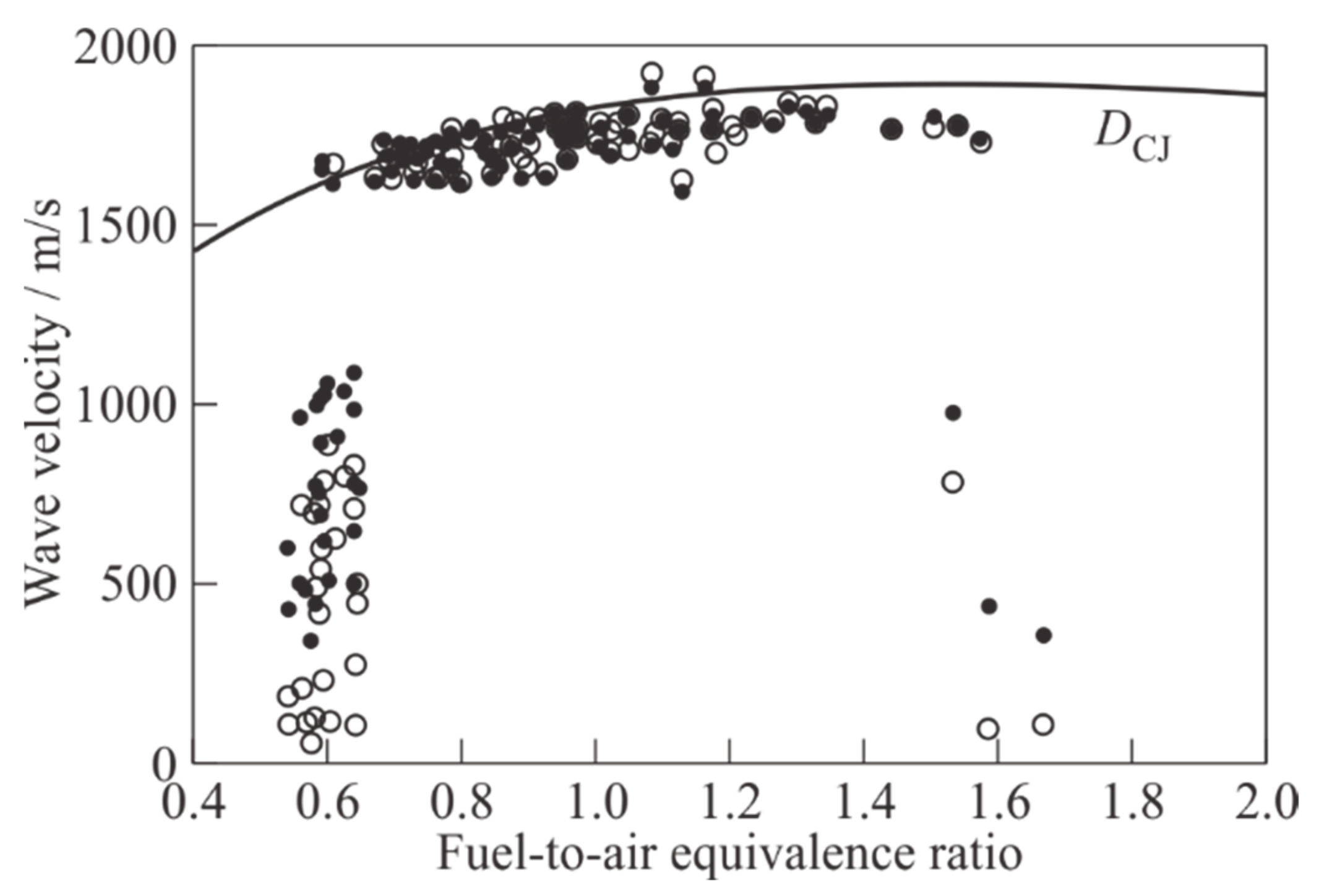
Figure 10 presents the cumulated experimental dependence of the average SW and flame front velocities in the measuring section of the PDT on the average fuel-to-air equivalence ratio in the mixture, determined for each individual shot. For comparison, the dependence of the theoretical detonation velocity
DCJ in a homogeneous ethylene–air mixture, obtained using the TDS code [
28], is also shown here.
The DDT in pyrogas–air mixtures is registered at 0.6 ≤ ≤ 1.6. For each given value of , the measured detonation velocities turn out to be quite close to the DCJ value for the ethylene–air mixture, and the relative detonation velocity deficit, defined as |D − DCJ|/DCJ, does not exceed 10–12%.
Near the limiting values
≈ 0.6 and 1.6, the mode of propagation of the reaction front is ambiguous: in some experiments, DDT is recorded with subsequent steady-state propagation of a DW in the measuring section of the PDT, in others, high-speed deflagration with a SW running noticeably ahead of the flame front is detected. Such effects were observed by other researchers during DDT in homogeneous fuel mixtures in the vicinity of concentration limits, for example, in hydrogen–air mixtures [
29]. They are usually explained by the stochastic nature of the DDT phenomenon, associated with the occurrence of self-ignition centers in the region between the leading SW and the highly perturbed surface of the turbulent flame, resulting in “the explosion in the explosion” (according to the terminology of [
30]). In addition to this factor, the reason for the ambiguity of the modes of propagation of the reaction front in
Figure 10 may be an error in determining a value of
associated with the time-varying composition of fuel mixture.
In view of it, some important issues, namely, the effect of PE properties (age, additives, etc.) prior to pyrolysis and the scale of the detonation tube on the DDT concentration limits, still must be addressed. These issues are important for hazard assessment of accompanying risks and are worth of special studies.
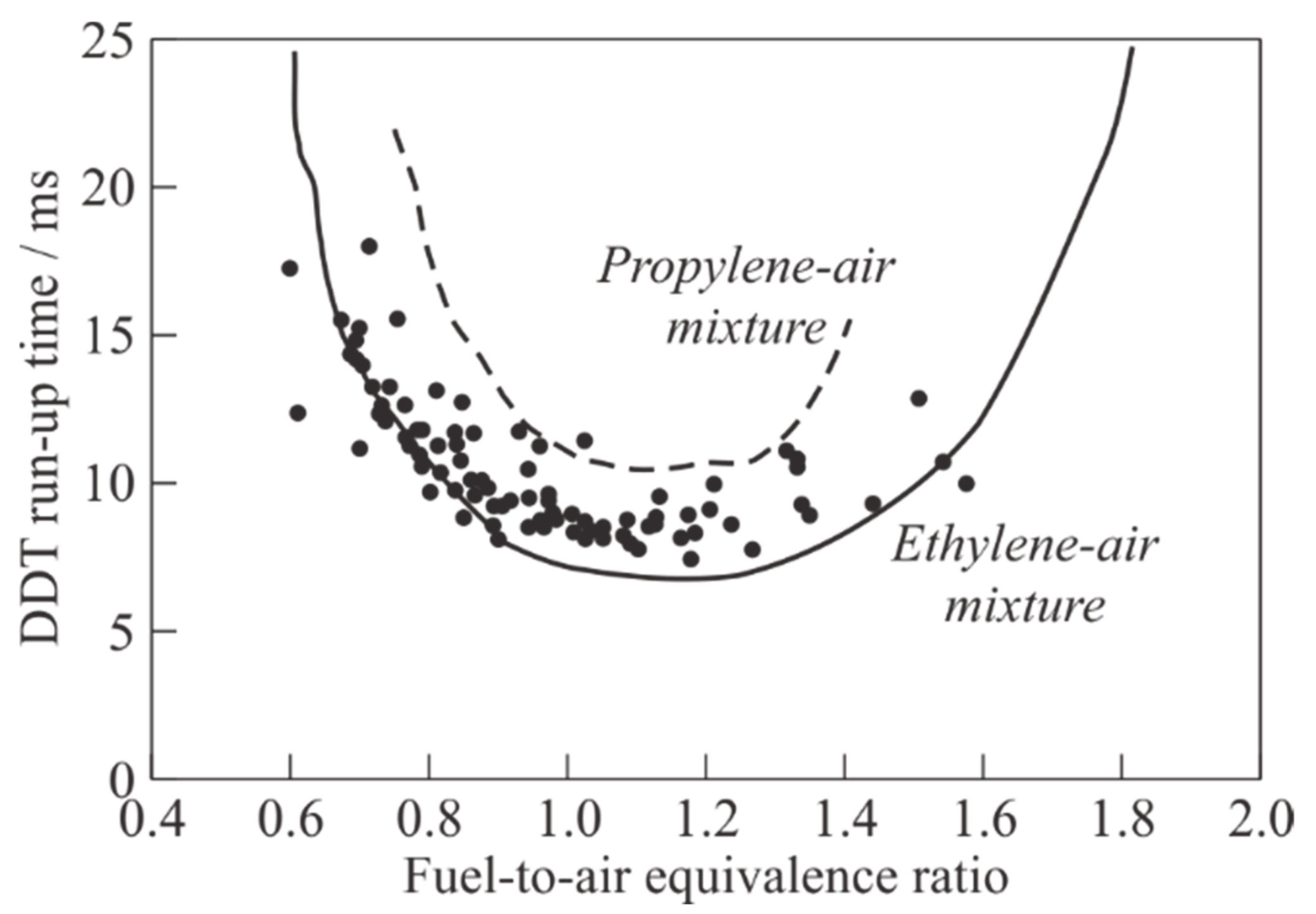
Figure 11 shows the cumulated dependence of the measured DDT run-up time in air mixtures of pyrogas (dark circles) on the average fuel-to-air equivalence ratio. Note that the DDT run-up distance,
LDDT, in all the experiments was nearly constant (≈2 m), i.e., DDT occurred at the exit from the PDT helical section. To estimate the relative detonability of pyrogas, we add to this plot the experimental dependences
tDDT(
Φ) obtained in [
25] for homogeneous ethylene-air (solid curve) and propylene-air (dashed curve) mixtures. It follows from considering
Figure 11 that the detonability of pyrogas is close to that for air mixtures of its monomer, ethylene, especially for fuel-lean compositions (
< 0.9). At
≥ 0.9, the fuel mixtures based on pyrogas exhibit the detonability that is intermediate between the detonability of air mixtures of ethylene and propylene. The DDT run-up time for fuel mixtures based on pyrogas varies from ≈ 7 to 18 ms. The mixtures of pyrogas with air with
= 1.1–1.2 have the maximum detonability (the minimum value
tDDT ≈ 7 ms). The concentration limits of detonation of pyrogas–air mixtures is seen to be somewhat narrower than those of homogeneous ethylene–air mixtures, but wider than those of homogeneous propylene–air mixtures.
The results obtained show that the pyrogas consisting mainly of a mixture of ethylene, propylene, methane, and hydrogen, has high detonability comparable to that of homogeneous ethylene–air and propylene–air mixtures. On the one hand, this indicates high explosibility of pyrogas, which can be formed, for example, during industrial and household fires. On the other hand, pyrogas, like PP pyrogas [
23], can be considered as a promising fuel for solid-fuel DRs. However, it should be kept in mind that the products of PE pyrolysis contain much hydrogen (see
Table 3) and the metal parts of such DRs may be prone to hydrogen embrittlement or high temperature hydrogen attack.