Thermodynamic and Transport Properties of Biomass-Derived Furfural, Furfuryl Alcohol and Their Mixtures
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemicals
2.2. Apparatus and Procedure
3. Result and Discussion
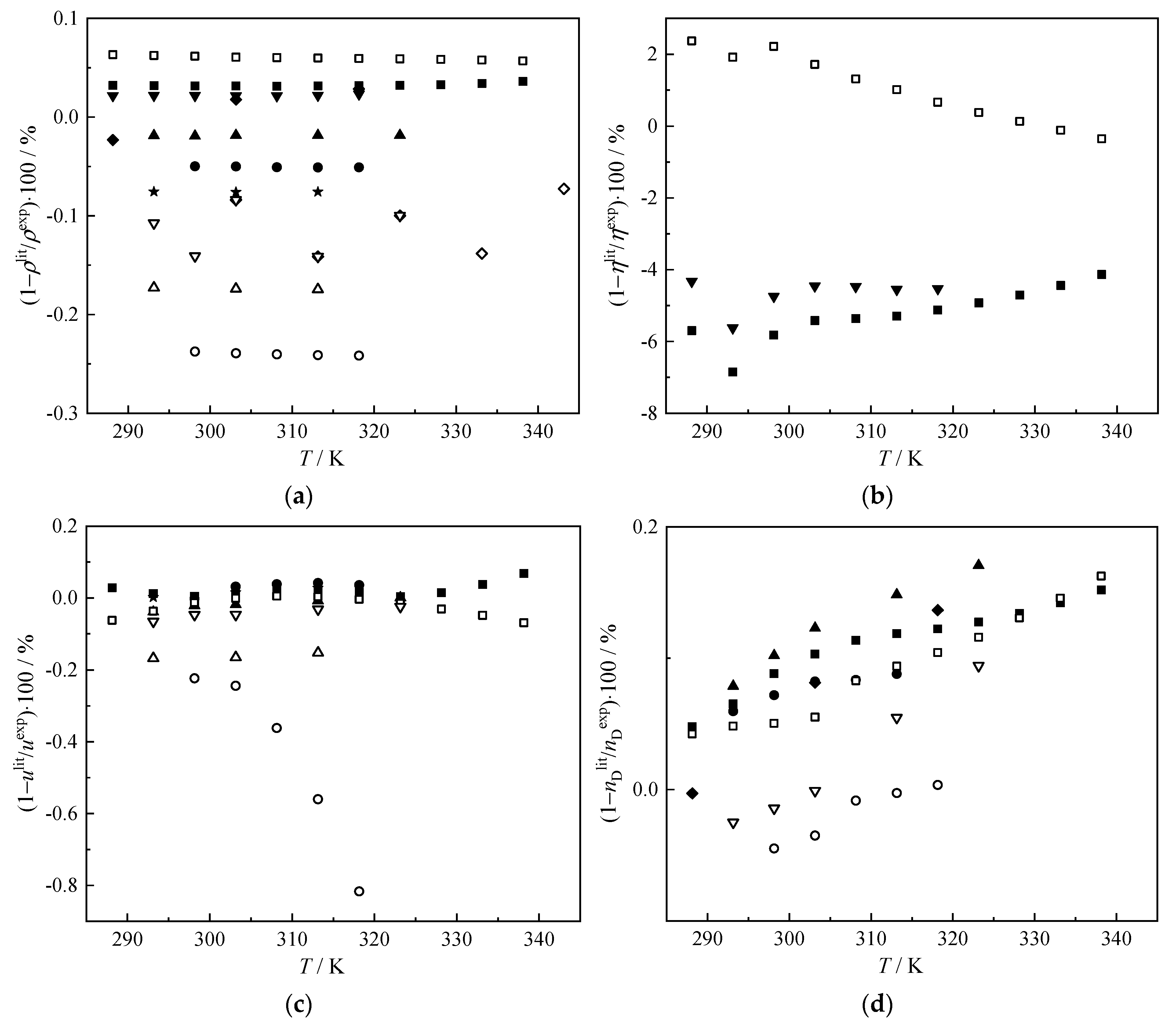
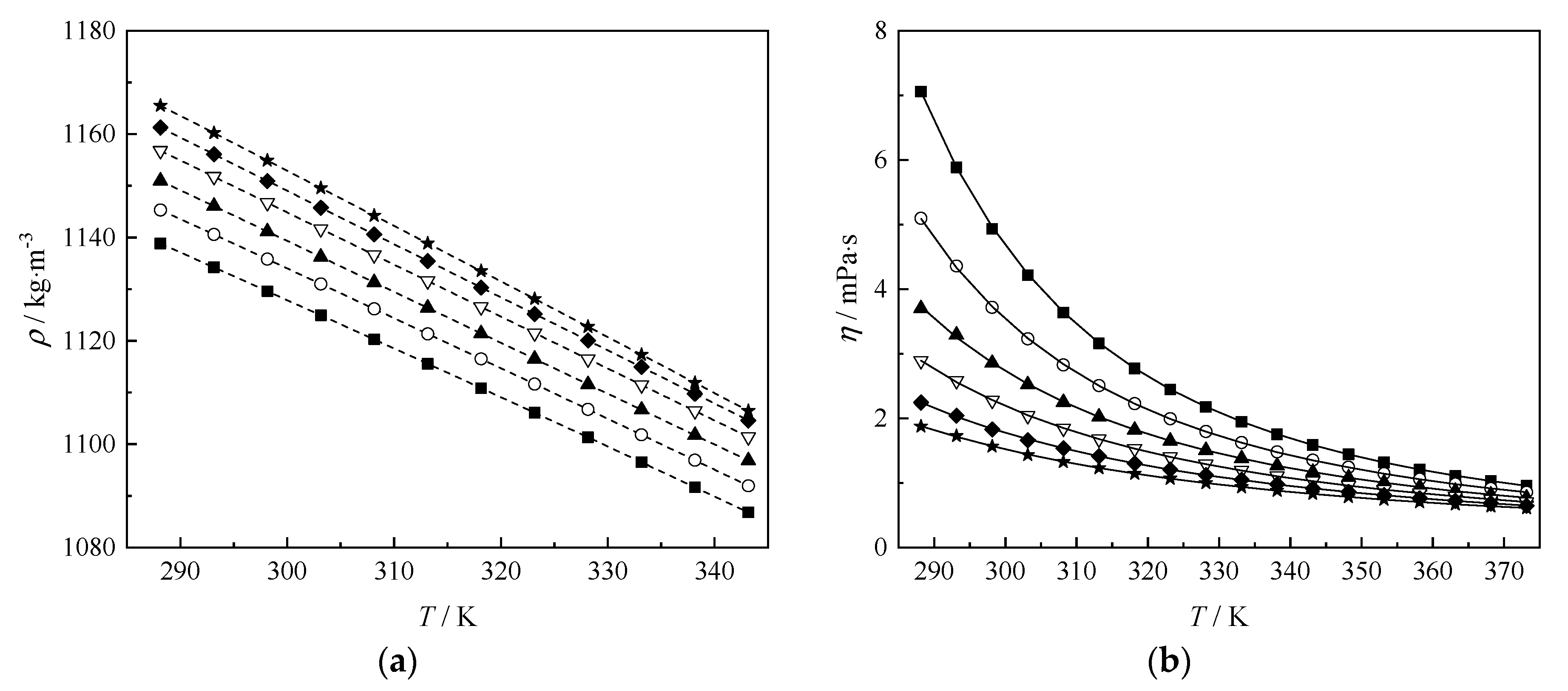
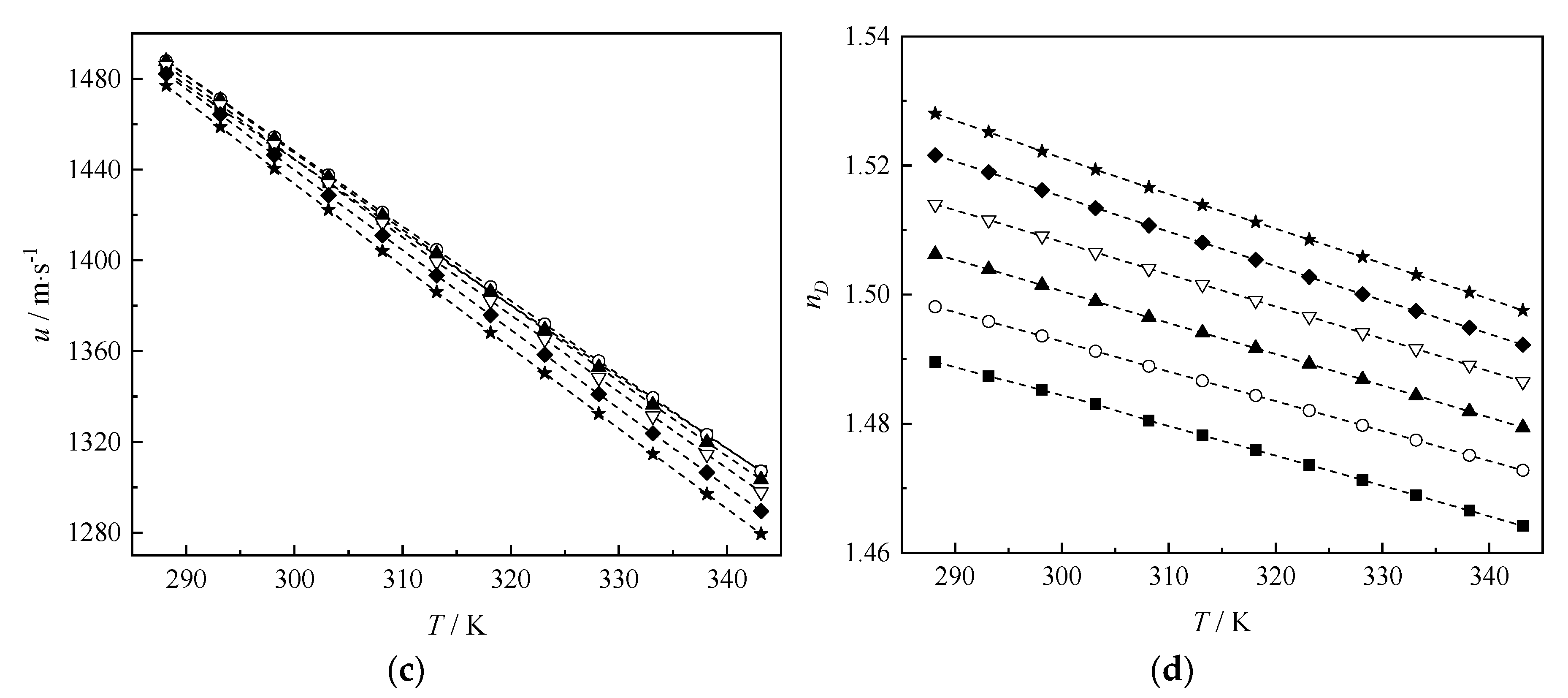
3.1. Experimental Results
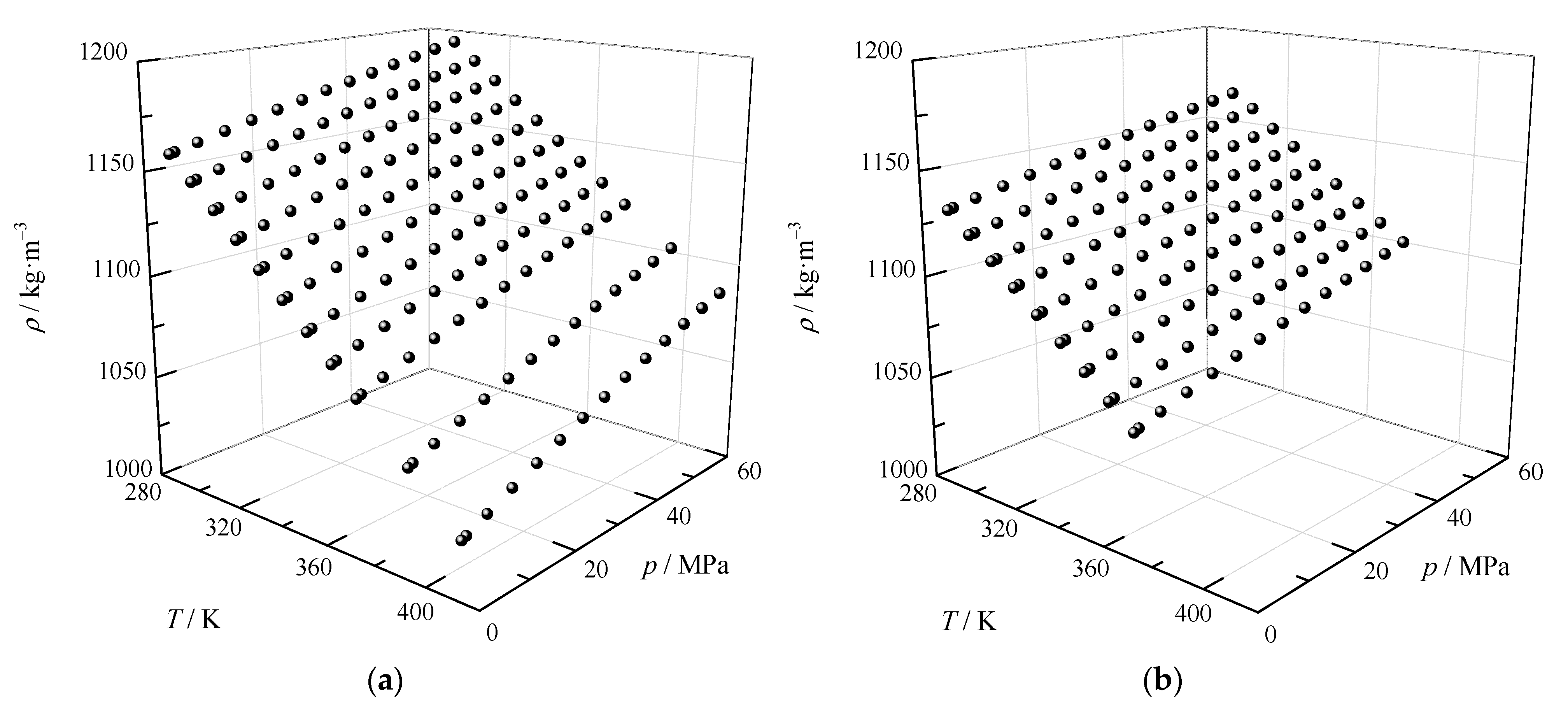
3.2. High Pressure Density Correlation
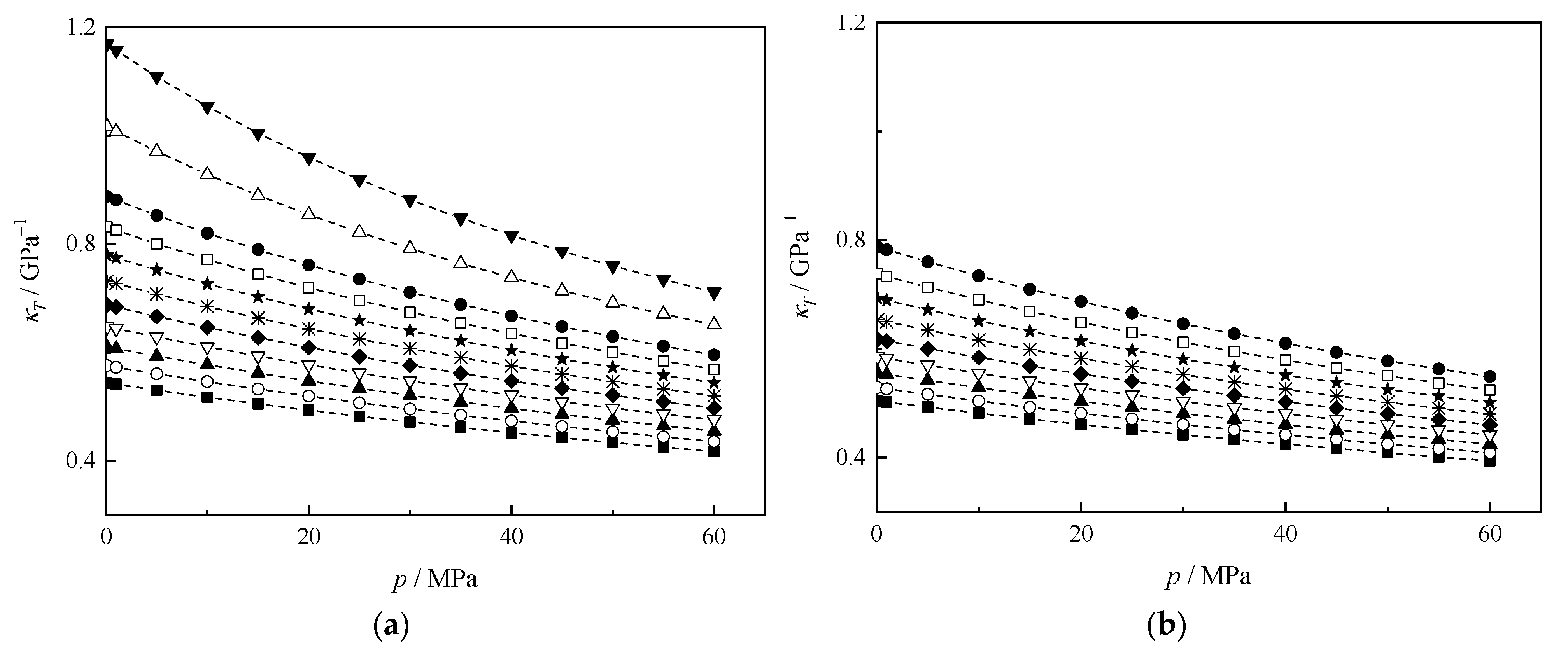
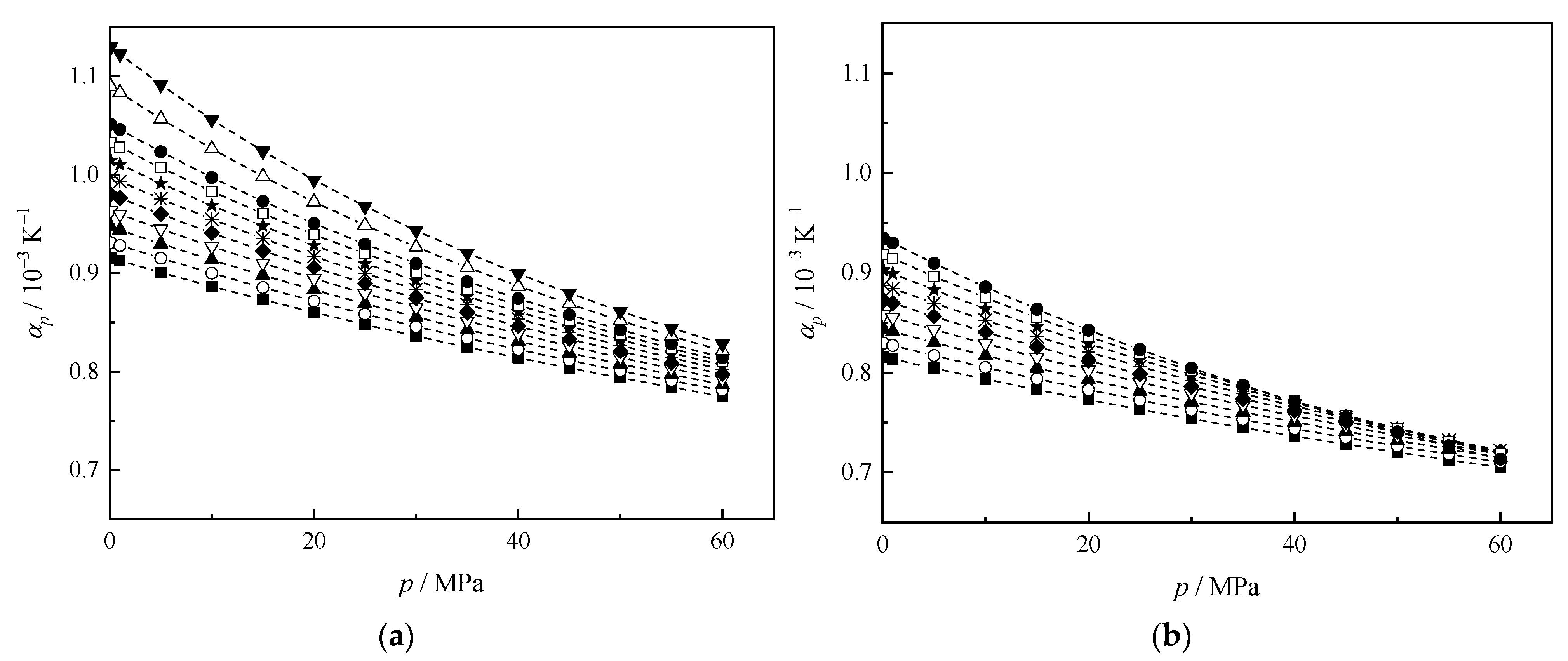
3.3. Derived Thermodynamic Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anastas, P.T.; Warner, J.C. Green Chemistry: Theory and Practice; Oxford University Press: New York, NY, USA, 2000. [Google Scholar]
- Isikgor, F.H.; Becer, C.R. Lignocellulosic biomass: A sustainable platform for the production of bio-based chemicals and polymers. Polym. Chem. 2015, 6, 4497–4559. [Google Scholar] [CrossRef] [Green Version]
- Somerville, C.; Youngs, H.; Taylor, C.; Davis, S.C.; Long, S.P. Feedstocks for lignocellulosic fuels. Science 2010, 329, 790–791. [Google Scholar] [CrossRef] [Green Version]
- Grilc, M.; Likozar, B.; Levec, J. Hydrodeoxygenation and hydrocracking of solvolysed lignocellulosic biomass by oxide, reduced and sulphide form of NiMo, Ni, Mo and Pd catalysts. Appl. Catal. B Environ. 2014, 150–151, 275–287. [Google Scholar] [CrossRef]
- Mariscal, R.; Maireles-Torres, P.; Ojeda, M.; Sádaba, I.; López Granados, M. Furfural: A renewable and versatile platform molecule for the synthesis of chemicals and fuels. Energy Environ. Sci. 2016, 9, 1144–1189. [Google Scholar] [CrossRef]
- Mamman, A.S.; Lee, J.M.; Kim, Y.C.; Hwang, I.T.; Park, N.J.; Hwang, Y.K.; Chang, J.S.; Hwang, J.S. Furfural: Hemicellulose/xylose-derived biochemical. Biofuels Bioprod. Bioref. 2008, 2, 438–454. [Google Scholar] [CrossRef]
- Zhao, Y.; Lu, K.; Xu, H.; Zhu, L.; Wang, S. A critical review of recent advances in the production of furfural and 5-hydroxymethylfurfural from lignocellulosic biomass through homogeneous catalytic hydrothermal conversion. Renew. Sust. Energ. Rev. 2021, 139, 110706. [Google Scholar] [CrossRef]
- Fele Žilnik, L.; Grilc, V.; Mirt, I.; Cerovečki, Ž. Study of the influence of key process parameters on furfural production. Acta Chim. Slov. 2016, 63, 298–308. [Google Scholar]
- Gómez Millán, G.; Bangalore Ashok, R.P.; Oinas, P.; Llorca, J.; Sixta, H. Furfural production from xylose and birch hydrolysate liquor in a biphasic system and techno-economic analysis. Biomass Convers. Biorefin. 2021, 11, 2095–2106. [Google Scholar] [CrossRef] [Green Version]
- Lange, J.P. Lignocellulose conversion: An introduction to chemistry, process and economics. In Catalysis for Renewables: From Feedstock to Energy Production; Centi, G., van Santen, R.A., Eds.; Wiley-VCH: Weinheim, Germany, 2007; pp. 21–51. [Google Scholar]
- Zeitsch, K.J. The Chemistry and Technology of Furfural and Its Many By-Products; Elsevier: Amsterdam, The Netherlands, 2000. [Google Scholar]
- Lomba, L.; Giner, B.; Bandrès Lafuente, C.; Pino, R. Physicochemical properties of green solvents derived from biomass. Green Chem. 2011, 13, 2062–2070. [Google Scholar] [CrossRef]
- Bendiaf, L.; Bahadur, I.; Negadi, A.; Naidoo, P.; Ramjugernath, D.; Negadi, L. Effects of alkyl group and temperature on the interactions between furfural and alcohol: Insight from density and sound velocity studies. Thermochim. Acta 2015, 599, 13–22. [Google Scholar] [CrossRef]
- Zaoui-Djelloul-Daouadji, M.; Bendiaf, L.; Bahadur, I.; Negadi, A.; Ramjugernath, D. Volumetric and acoustic properties of binary systems (furfural or furfuryl alcohol + toluene) and (furfuryl alcohol + ethanol) at different temperatures. Thermochim. Acta 2015, 611, 47–55. [Google Scholar] [CrossRef]
- De Almeida, B.F.; Waldrigui, T.M.; Alves, T.C.; De Oliveira, L.H.; Aznar, M. Experimental and calculated liquid–liquid equilibrium data for water + furfural + solvents. Fluid Phase Equilib. 2012, 334, 97–105. [Google Scholar] [CrossRef]
- Hough, E.W.; Mason, D.M.; Sage, B.H. Heat capacities of several organic liquids. J. Am. Chem. Soc. 1950, 72, 5775–5777. [Google Scholar] [CrossRef]
- Qureshi, M.S.; Vrbka, P.; Dohnal, V. Thermodynamic properties of five biofuel-relevant compounds at infinite dilution in water. Fuel 2017, 191, 518–527. [Google Scholar] [CrossRef]
- Nduli, M.; Deenadayalu, N. Thermophysical properties of binary mixtures of (methanol or 1-ethyl-3-methylimidazolium acetate + furfural or furfuryl alcohol) at various temperatures. J. Mol. Liq. 2017, 241, 407–421. [Google Scholar] [CrossRef]
- Mahi, M.R.; Ouaar, F.; Negadi, A.; Bahadur, I.; Negadi, L. Excess/deviation properties of binary mixtures of 2,5-dimethylfuran with furfuryl alcohol, methyl isobutyl ketone, 1-butanol and 2-butanol at temperature range of (293.15–323.15) K. Oil Gas Sci. Technol. Rev. IFP Energ. Nouv. 2018, 73, 64–78. [Google Scholar] [CrossRef]
- Belhadj, D.; Negadi, A.; Venkatesu, P.; Bahadur, I.; Negadi, L. Density, speed of sound, refractive index and related derived/excess properties of binary mixtures (furfural + dimethyl sulfoxide), (furfural + acetonitrile) and (furfural + sulfolane) at different temperatures. J. Mol. Liq. 2021, 330, 115436. [Google Scholar] [CrossRef]
- Yan, K.; Wu, G.; Lafleur, T.; Jarvis, C. Production, properties and catalytic hydrogenation of furfural to fuel additives and value-added chemicals. Renew. Sust. Energ. Rev. 2014, 38, 663–676. [Google Scholar] [CrossRef]
- Guerrero, H.; Lafuente, C.; Rayo, F.; Lomba, L.; Giner, B. PρT behavior of several chemicals from biomass. Energy Fuels 2011, 25, 3009–3013. [Google Scholar] [CrossRef]
- Baird, Z.S.; Uusi-Kyyny, P.; Pokki, J.P.; Pedegert, E.; Alopaeus, V. Vapor pressures, densities, and PC-SAFT parameters for 11 bio-compounds. Int. J. Thermophys. 2019, 40, 102–137. [Google Scholar] [CrossRef] [Green Version]
- Dymond, J.H.; Malhotra, R. The Tait equation: 100 years on. Int. J. Thermophys. 1988, 9, 941–951. [Google Scholar] [CrossRef]
- Kijevčanin, M.L.; Živković, E.M.; Djordjević, B.D.; Radović, I.R.; Jovanović, J.; Šerbanović, S.P. Experimental determination and modeling of excess molar volumes, viscosities and refractive indices of the binary systems (pyridine + 1-propanol, +1,2-propanediol, +1,3-propanediol, and +glycerol). New UNIFAC-VISCO parameters determination. J. Chem. Thermodyn. 2013, 56, 49–56. [Google Scholar] [CrossRef]
- Bajić, D.M.; Živković, E.M.; Jovanović, J.; Šerbanović, S.P.; Kijevčanin, M.L. Experimental measurements and modelling of volumetric properties, refractive index and viscosity of binary systems of ethyl lactate with methyl ethyl ketone, toluene and n-methyl-2-pirrolidone at 288.15–323.15 K and atmospheric pressure. New UNIFAC–VISCO and ASOG–VISCO interaction parameters. Fluid Phase Equilib. 2015, 399, 50–65. [Google Scholar]
- Chirico, R.D.; Frenkel, M.; Magee, J.W.; Diky, V.; Muzny, C.D.; Kazakov, A.F.; Kroenlein, K.; Abdulagatov, I.; Hardin, G.R.; Acree, W.E.J.; et al. Improvement of quality in publication of experimental thermophysical property data: Challenges, assessment tools, global implementation, and online support. J. Chem. Eng. Data 2013, 58, 2699–2716. [Google Scholar] [CrossRef] [Green Version]
- Comuñas, M.J.P.; Bazile, J.P.; Baylaucq, A.; Boned, C. Density of diethyl adipate using a new vibrating tube densimeter from (293.15 to 403.15) K and up to 140 MPa. Calibration and measurements. J. Chem. Eng. Data 2008, 53, 986–994. [Google Scholar] [CrossRef]
- Ivaniš, G.R.; Tasić, A.Ž.; Radović, I.R.; Đorđević, B.D.; Šerbanović, S.P.; Kijevčanin, M.L. An apparatus proposed for density measurements in compressed liquid regions at the pressures 0.1-60 MPa and the temperatures 288.15-413.15 K. J. Serb. Chem. Soc. 2015, 80, 1073–1085. [Google Scholar] [CrossRef]
- Vogel, H. The temperature dependence law of the viscosity of fluids. Phys. Z. 1921, 22, 645. [Google Scholar]
- Fulcher, G.S. Analysis of recent measurements of the viscosity of glasses. J. Am. Ceram. Soc. 1925, 8, 339. [Google Scholar] [CrossRef]
- Tammann, G.; Hesse, W. Die Abhängigkeit der Viscosität von der Temperatur bie unterkühlten Flüssigkeiten. Z. Anorg. Allg. Chem. 1926, 156, 245. [Google Scholar] [CrossRef]
- Marquardt, D.W. An algorithm for least-squares estimation of nonlinear parameters. J. Appl. Math. 1963, 11, 431–441. [Google Scholar] [CrossRef]
- Ivaniš, G.R.; Tasić, A.Ž.; Radović, I.R.; Đorđević, B.D.; Šerbanović, S.P.; Kijevčanin, M.L. Modeling of density and calculations of derived volumetric properties for n-hexane, toluene and dichloromethane at pressures 0.1-60 MPa and temperatures 288.15-413.15 K. J. Serb. Chem. Soc. 2015, 80, 1423–1433. [Google Scholar] [CrossRef]
- Aissa, M.A.; Ivaniš, G.R.; Radović, I.R.; Kijevčanin, M.L. Experimental investigation and modeling of thermophysical properties of pure methyl and ethyl esters at high pressures. Energy Fuels 2017, 31, 7110–7122. [Google Scholar] [CrossRef]
- Aparicio, S.; Alcalde, R. The green solvent ethyl lactate: An experimental and theoretical characterization. Green Chem. 2009, 11, 65–78. [Google Scholar] [CrossRef]
| Chemical Name | CAS Reg. No. | Structure | Supplier | Purity, Mass Fraction |
|---|---|---|---|---|
| Furfural | 98-01-1 | 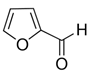 | Sigma-Aldrich | 0.99 |
| Furfuryl alcohol | 98-00-0 | 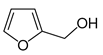 | Aldrich Chemicals | 0.98 |
| Furfural (1) + Furfuryl Alcohol (2) | ||||||
|---|---|---|---|---|---|---|
| ρb/kg·m−3 | ||||||
| Tc/K | ||||||
| x1d | 288.15 | 293.15 | 298.15 | 303.15 | 308.15 | 313.15 |
| 0.0000 | 1138.8 | 1134.2 | 1129.6 | 1124.9 | 1120.3 | 1115.6 |
| 0.2000 | 1145.3 | 1140.6 | 1135.8 | 1131.0 | 1126.2 | 1121.4 |
| 0.4000 | 1151.0 | 1146.1 | 1141.2 | 1136.2 | 1131.3 | 1126.4 |
| 0.6000 | 1156.8 | 1151.8 | 1146.7 | 1141.7 | 1136.6 | 1131.6 |
| 0.8001 | 1161.3 | 1156.1 | 1150.9 | 1145.8 | 1140.6 | 1135.4 |
| 1.0000 | 1165.5 | 1160.2 | 1154.9 | 1149.6 | 1144.2 | 1138.9 |
| 318.15 | 323.15 | 328.15 | 333.15 | 338.15 | 343.15 | |
| 0.0000 | 1110.8 | 1106.1 | 1101.3 | 1096.5 | 1091.7 | 1086.8 |
| 0.2000 | 1116.5 | 1111.6 | 1106.7 | 1101.8 | 1096.9 | 1091.9 |
| 0.4000 | 1121.4 | 1116.5 | 1111.6 | 1106.6 | 1101.7 | 1096.8 |
| 0.6000 | 1126.6 | 1121.5 | 1116.5 | 1111.5 | 1106.4 | 1101.4 |
| 0.8001 | 1130.3 | 1125.2 | 1120.1 | 1114.9 | 1109.8 | 1104.6 |
| 1.0000 | 1133.5 | 1128.1 | 1122.7 | 1117.3 | 1111.9 | 1106.4 |
| ηe/mPa·s | ||||||
| T/K | ||||||
| x1 | 288.15 | 293.15 | 298.15 | 303.15 | 308.15 | 313.15 |
| 0.0000 | 7.056 | 5.886 | 4.934 | 4.216 | 3.635 | 3.160 |
| 0.2000 | 5.096 | 4.358 | 3.717 | 3.230 | 2.828 | 2.506 |
| 0.4000 | 3.702 | 3.292 | 2.856 | 2.524 | 2.251 | 2.022 |
| 0.6000 | 2.893 | 2.584 | 2.276 | 2.040 | 1.845 | 1.677 |
| 0.8001 | 2.244 | 2.042 | 1.827 | 1.665 | 1.538 | 1.414 |
| 1.0000 | 1.877 | 1.728 | 1.567 | 1.436 | 1.327 | 1.231 |
| 318.15 | 323.15 | 328.15 | 333.15 | 338.15 | 343.15 | |
| 0.0000 | 2.771 | 2.448 | 2.176 | 1.947 | 1.753 | 1.586 |
| 0.2000 | 2.227 | 1.994 | 1.795 | 1.626 | 1.480 | 1.353 |
| 0.4000 | 1.820 | 1.651 | 1.505 | 1.378 | 1.267 | 1.168 |
| 0.6000 | 1.529 | 1.402 | 1.291 | 1.193 | 1.107 | 1.030 |
| 0.8001 | 1.303 | 1.207 | 1.122 | 1.046 | 0.978 | 0.916 |
| 1.0000 | 1.145 | 1.068 | 1.000 | 0.938 | 0.883 | 0.832 |
| 348.15 | 353.15 | 358.15 | 363.15 | 368.15 | 373.15 | |
| 0.0000 | 1.442 | 1.317 | 1.208 | 1.112 | 1.027 | 0.955 |
| 0.2000 | 1.242 | 1.145 | 1.059 | 0.982 | 0.914 | 0.856 |
| 0.4000 | 1.082 | 1.005 | 0.936 | 0.874 | 0.818 | 0.770 |
| 0.6000 | 0.961 | 0.899 | 0.843 | 0.792 | 0.746 | 0.705 |
| 0.8001 | 0.861 | 0.810 | 0.764 | 0.722 | 0.683 | 0.649 |
| 1.0000 | 0.786 | 0.744 | 0.706 | 0.671 | 0.638 | 0.610 |
| uf/m·s−1 | ||||||
| T/K | ||||||
| x1 | 288.15 | 293.15 | 298.15 | 303.15 | 308.15 | 313.15 |
| 0.0000 | 1483.3 | 1466.9 | 1450.5 | 1434.3 | 1418.2 | 1402.1 |
| 0.2000 | 1487.9 | 1471.1 | 1454.3 | 1437.7 | 1421.1 | 1404.7 |
| 0.4000 | 1487.8 | 1470.7 | 1453.6 | 1436.7 | 1419.8 | 1403.0 |
| 0.6000 | 1485.9 | 1468.5 | 1451.1 | 1433.8 | 1416.5 | 1399.4 |
| 0.8001 | 1482.2 | 1464.3 | 1446.5 | 1428.7 | 1411.0 | 1393.4 |
| 1.0000 | 1477.1 | 1458.8 | 1440.5 | 1422.3 | 1404.2 | 1386.1 |
| 318.15 | 323.15 | 328.15 | 333.15 | 338.15 | 343.15 | |
| 0.0000 | 1386.2 | 1370.3 | 1354.5 | 1338.7 | 1322.9 | 1307.2 |
| 0.2000 | 1388.3 | 1372.0 | 1355.7 | 1339.4 | 1323.2 | 1307.1 |
| 0.4000 | 1386.2 | 1369.5 | 1352.9 | 1336.4 | 1319.8 | 1303.4 |
| 0.6000 | 1382.3 | 1365.3 | 1348.3 | 1331.4 | 1314.6 | 1297.8 |
| 0.8001 | 1375.9 | 1358.4 | 1341.1 | 1323.8 | 1306.6 | 1289.5 |
| 1.0000 | 1368.2 | 1350.3 | 1332.5 | 1314.7 | 1297.1 | 1279.5 |
| nDg | ||||||
| T/K | ||||||
| x1 | 288.15 | 293.15 | 298.15 | 303.15 | 308.15 | 313.15 |
| 0.0000 | 1.490 | 1.487 | 1.485 | 1.483 | 1.480 | 1.478 |
| 0.2000 | 1.498 | 1.496 | 1.494 | 1.491 | 1.489 | 1.487 |
| 0.4000 | 1.506 | 1.504 | 1.502 | 1.499 | 1.496 | 1.494 |
| 0.6000 | 1.514 | 1.512 | 1.509 | 1.506 | 1.504 | 1.502 |
| 0.8001 | 1.522 | 1.519 | 1.516 | 1.513 | 1.511 | 1.508 |
| 1.0000 | 1.528 | 1.525 | 1.522 | 1.519 | 1.517 | 1.514 |
| 318.15 | 323.15 | 328.15 | 333.15 | 338.15 | 343.15 | |
| 0.0000 | 1.476 | 1.474 | 1.471 | 1.469 | 1.466 | 1.464 |
| 0.2000 | 1.484 | 1.482 | 1.480 | 1.478 | 1.475 | 1.473 |
| 0.4000 | 1.492 | 1.489 | 1.487 | 1.484 | 1.482 | 1.479 |
| 0.6000 | 1.499 | 1.497 | 1.494 | 1.492 | 1.489 | 1.486 |
| 0.8001 | 1.505 | 1.503 | 1.500 | 1.498 | 1.495 | 1.492 |
| 1.0000 | 1.511 | 1.508 | 1.506 | 1.503 | 1.500 | 1.498 |
| Furfural (1) + Furfuryl Alcohol (2) | |||||||
|---|---|---|---|---|---|---|---|
| x1 | A | CIA a | B/K | CIB a/K | C/K | CIV a/K | AAD/% |
| 0 | −3.34 | ± 0.04 | 741.5 | ± 15 | 148.1 | ± 1.8 | 0.15 |
| 0.2000 | −3.192 | ± 0.06 | 697.6 | ± 22 | 143.4 | ± 2.7 | 0.16 |
| 0.4000 | −3.292 | ± 0.14 | 752.3 | ± 55 | 124.9 | ± 7.2 | 0.20 |
| 0.6000 | −3.113 | ± 0.12 | 696.6 | ± 47 | 121.3 | ± 6.8 | 0.22 |
| 0.8001 | −3.312 | ± 0.16 | 814.9 | ± 78 | 90.34 | ± 11.1 | 0.19 |
| 1 | −3.023 | ± 0.12 | 698 | ± 56 | 97.2 | ± 9.2 | 0.17 |
| ρa/kg·m−3 | ||||||
|---|---|---|---|---|---|---|
| Furfural | ||||||
| Tb/K | ||||||
| pc/MPa | 293.15 | 303.15 | 313.15 | 323.15 | 333.15 | 343.15 |
| 0.1 | 1160.5 | 1149.8 | 1139.1 | 1128.2 | 1117.3 | 1106.5 |
| 1 | 1161.0 | 1150.4 | 1139.7 | 1128.9 | 1118.0 | 1107.2 |
| 5 | 1163.5 | 1153.0 | 1142.4 | 1131.7 | 1121.0 | 1110.3 |
| 10 | 1166.6 | 1156.2 | 1145.7 | 1135.2 | 1124.7 | 1114.2 |
| 15 | 1169.5 | 1159.3 | 1149.0 | 1138.6 | 1128.2 | 1117.9 |
| 20 | 1172.5 | 1162.4 | 1152.2 | 1142.0 | 1131.8 | 1121.6 |
| 25 | 1175.3 | 1165.4 | 1155.3 | 1145.3 | 1135.2 | 1125.2 |
| 30 | 1178.2 | 1168.3 | 1158.4 | 1148.5 | 1138.5 | 1128.6 |
| 35 | 1180.9 | 1171.2 | 1161.4 | 1151.6 | 1141.8 | 1132.0 |
| 40 | 1183.6 | 1174.0 | 1164.3 | 1154.6 | 1145.0 | 1135.4 |
| 45 | 1186.3 | 1176.8 | 1167.1 | 1157.6 | 1148.1 | 1138.6 |
| 50 | 1188.9 | 1179.5 | 1170.0 | 1160.6 | 1151.1 | 1141.7 |
| 55 | 1191.4 | 1182.1 | 1172.8 | 1163.4 | 1154.0 | 1144.8 |
| 60 | 1193.9 | 1184.7 | 1175.4 | 1166.2 | 1156.9 | 1147.8 |
| Tb/K | ||||||
| pc/MPa | 353.15 | 363.15 | 373.15 | 393.15 | 413.15 | |
| 0.1 | 1095.3 | 1084.3 | 1072.6 | 1050.1 | 1027.2 | |
| 1 | 1096.1 | 1085.1 | 1073.7 | 1051.2 | 1028.1 | |
| 5 | 1099.4 | 1088.6 | 1077.4 | 1055.3 | 1032.7 | |
| 10 | 1103.4 | 1092.8 | 1081.8 | 1060.3 | 1038.3 | |
| 15 | 1107.4 | 1097.0 | 1086.2 | 1065.1 | 1043.7 | |
| 20 | 1111.2 | 1101.0 | 1090.4 | 1069.8 | 1048.8 | |
| 25 | 1115.0 | 1104.9 | 1094.6 | 1074.3 | 1053.9 | |
| 30 | 1118.6 | 1108.7 | 1098.5 | 1078.7 | 1058.7 | |
| 35 | 1122.2 | 1112.4 | 1102.4 | 1082.9 | 1063.3 | |
| 40 | 1125.6 | 1116.0 | 1106.2 | 1087.0 | 1067.8 | |
| 45 | 1129.0 | 1119.5 | 1109.8 | 1091.0 | 1072.1 | |
| 50 | 1132.2 | 1122.9 | 1113.3 | 1094.8 | 1076.2 | |
| 55 | 1135.4 | 1126.2 | 1116.7 | 1098.4 | 1080.1 | |
| 60 | 1138.5 | 1129.4 | 1120.0 | 1101.9 | 1083.9 | |
| Furfuryl alcohol | ||||||
| Tb/K | ||||||
| pc/MPa | 293.15 | 303.15 | 313.15 | 323.15 | 333.15 | 343.15 |
| 0.1 | 1134.6 | 1125.4 | 1115.9 | 1106.5 | 1097.0 | 1087.4 |
| 1 | 1135.1 | 1125.8 | 1116.5 | 1107.1 | 1097.6 | 1088.0 |
| 5 | 1137.4 | 1128.0 | 1118.9 | 1109.7 | 1100.2 | 1090.8 |
| 10 | 1140.2 | 1130.6 | 1121.9 | 1112.8 | 1103.5 | 1094.2 |
| 15 | 1142.9 | 1133.3 | 1124.8 | 1115.9 | 1106.7 | 1097.4 |
| 20 | 1145.5 | 1136.0 | 1127.7 | 1118.9 | 1109.8 | 1100.8 |
| 25 | 1148.2 | 1138.8 | 1130.5 | 1121.8 | 1112.9 | 1104.0 |
| 30 | 1150.7 | 1141.5 | 1133.3 | 1124.7 | 1115.9 | 1107.1 |
| 35 | 1153.2 | 1144.2 | 1136.0 | 1127.5 | 1118.8 | 1110.1 |
| 40 | 1155.7 | 1147.0 | 1138.6 | 1130.2 | 1121.6 | 1113.1 |
| 45 | 1158.1 | 1149.8 | 1141.2 | 1132.9 | 1124.4 | 1116.0 |
| 50 | 1160.5 | 1152.6 | 1143.8 | 1135.5 | 1127.1 | 1118.8 |
| 55 | 1162.8 | 1155.4 | 1146.3 | 1138.1 | 1129.8 | 1121.5 |
| 60 | 1165.1 | 1158.2 | 1148.7 | 1140.6 | 1132.4 | 1124.2 |
| Tb/K | ||||||
| pc/MPa | 353.15 | 363.15 | 373.15 | |||
| 0.1 | 1077.6 | 1068.0 | 1058.0 | |||
| 1 | 1078.3 | 1068.7 | 1059.0 | |||
| 5 | 1081.2 | 1071.8 | 1062.3 | |||
| 10 | 1084.8 | 1075.5 | 1066.2 | |||
| 15 | 1088.3 | 1079.2 | 1070.1 | |||
| 20 | 1091.7 | 1082.8 | 1073.8 | |||
| 25 | 1095.0 | 1086.2 | 1077.4 | |||
| 30 | 1098.3 | 1089.6 | 1081.0 | |||
| 35 | 1101.4 | 1092.9 | 1084.4 | |||
| 40 | 1104.5 | 1096.1 | 1087.8 | |||
| 45 | 1107.5 | 1099.2 | 1091.0 | |||
| 50 | 1110.4 | 1102.2 | 1094.1 | |||
| 55 | 1113.2 | 1105.2 | 1097.1 | |||
| 60 | 1116.0 | 1108.0 | 1100.0 | |||
| Furfural | Furfuryl Alcohol | |
|---|---|---|
| a0/kg·m−3 | 1436.76 | 1371.86 |
| a1/kg m−3·K−1 | −0.82255 | −0.69307 |
| a2/kg·m−3·K−2 | −4.0892·10−4 | −3.9628·10−4 |
| b0/MPa | 610.517 | 489.736 |
| b1/MPa·K−1 | −1.98354 | −1.15038 |
| b2/MPa·K−2 | 1.7018·10−3 | 4.4122·10−4 |
| C | 0.095393 | 0.096069 |
| AADa/% | 0.005 | 0.009 |
| MDb/% | 0.028 | 0.10 |
| Biasc/% | 0.005 | 0.006 |
| σd/kg·m−3 | 0.078 | 0.199 |
| T/K | Furfural | Furfuryl Alcohol | ||||
|---|---|---|---|---|---|---|
| κS/GPa−1 | cp/kJ·kg−1·K−1 | cV/kJ·kg−1·K−1 | κS/GPa−1 | cp/kJ·kg−1·K−1 | cV/kJ·kg−1·K−1 | |
| 288.15 | 0.3933 | 1.502 | 1.117 | 0.3991 | 1.769 | 1.433 |
| 293.15 | 0.4050 | 1.525 | 1.135 | 0.4098 | 1.819 | 1.479 |
| 298.15 | 0.4173 | 1.548 | 1.154 | 0.4208 | 1.869 | 1.523 |
| 303.15 | 0.4300 | 1.569 | 1.172 | 0.4321 | 1.916 | 1.565 |
| 308.15 | 0.4433 | 1.590 | 1.190 | 0.4438 | 1.960 | 1.605 |
| 313.15 | 0.4570 | 1.610 | 1.206 | 0.4560 | 2.001 | 1.642 |
| 318.15 | 0.4713 | 1.630 | 1.223 | 0.4685 | 2.038 | 1.675 |
| 323.15 | 0.4862 | 1.649 | 1.238 | 0.4815 | 2.072 | 1.705 |
| 328.15 | 0.5017 | 1.666 | 1.253 | 0.4949 | 2.102 | 1.731 |
| 333.15 | 0.5178 | 1.684 | 1.267 | 0.5089 | 2.128 | 1.753 |
| 338.15 | 0.5346 | 1.700 | 1.281 | 0.5234 | 2.149 | 1.771 |
| 343.15 | 0.5521 | 1.716 | 1.294 | 0.5385 | 2.165 | 1.785 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simić, Z.V.; Kijevčanin, M.L.; Radović, I.R.; Grilc, M.; Ivaniš, G.R. Thermodynamic and Transport Properties of Biomass-Derived Furfural, Furfuryl Alcohol and Their Mixtures. Energies 2021, 14, 7769. https://doi.org/10.3390/en14227769
Simić ZV, Kijevčanin ML, Radović IR, Grilc M, Ivaniš GR. Thermodynamic and Transport Properties of Biomass-Derived Furfural, Furfuryl Alcohol and Their Mixtures. Energies. 2021; 14(22):7769. https://doi.org/10.3390/en14227769
Chicago/Turabian StyleSimić, Zoran V., Mirjana Lj. Kijevčanin, Ivona R. Radović, Miha Grilc, and Gorica R. Ivaniš. 2021. "Thermodynamic and Transport Properties of Biomass-Derived Furfural, Furfuryl Alcohol and Their Mixtures" Energies 14, no. 22: 7769. https://doi.org/10.3390/en14227769
APA StyleSimić, Z. V., Kijevčanin, M. L., Radović, I. R., Grilc, M., & Ivaniš, G. R. (2021). Thermodynamic and Transport Properties of Biomass-Derived Furfural, Furfuryl Alcohol and Their Mixtures. Energies, 14(22), 7769. https://doi.org/10.3390/en14227769









