Dynamics and Numerical Simulation of Contaminant Diffusion for a Non-Flushing Ecological Toilet
Abstract
1. Introduction
2. Methods
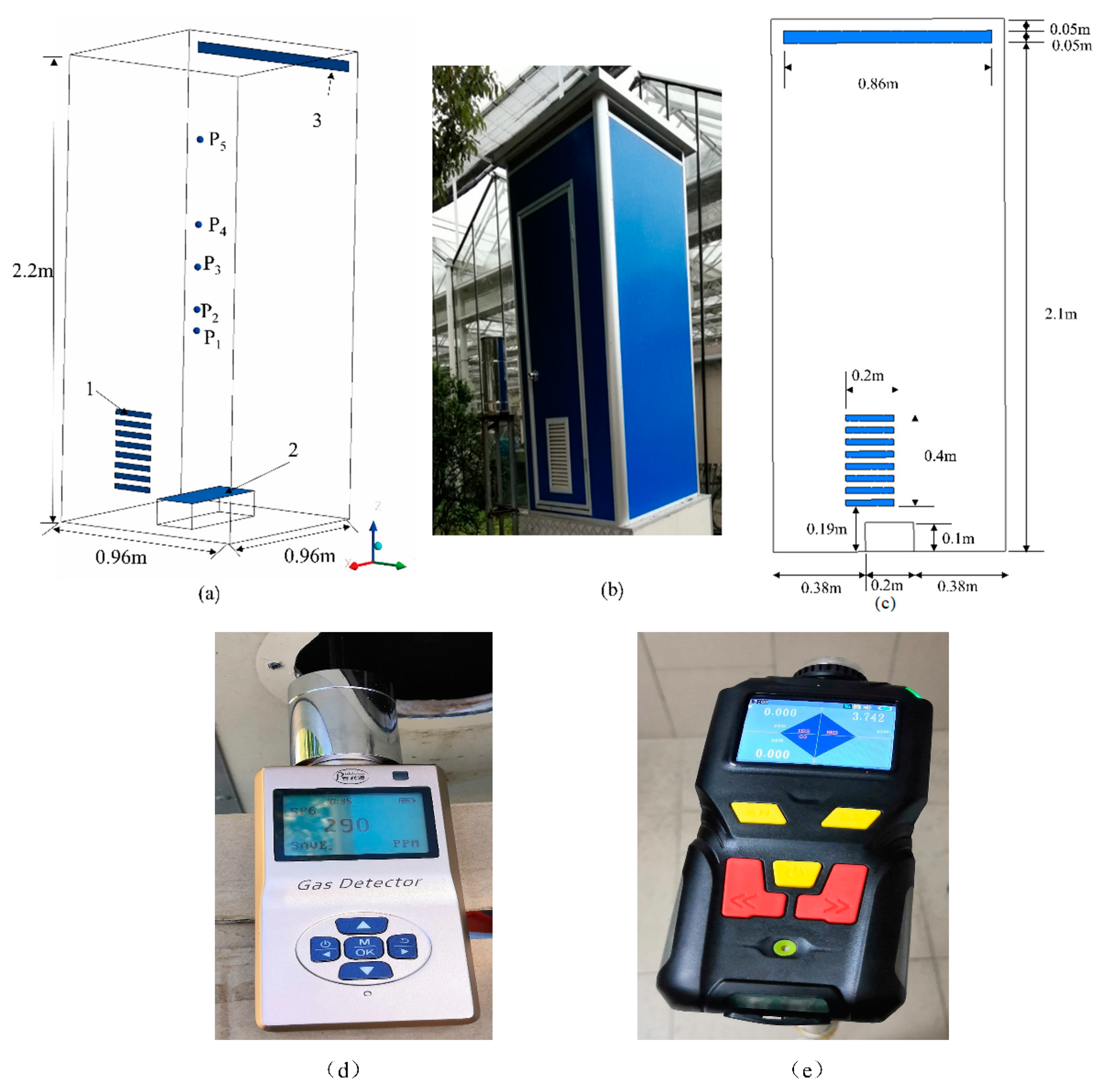
2.1. Physical Model
2.2. Tracer Gas Release and Measurement
2.3. Data Acquisition Equipment
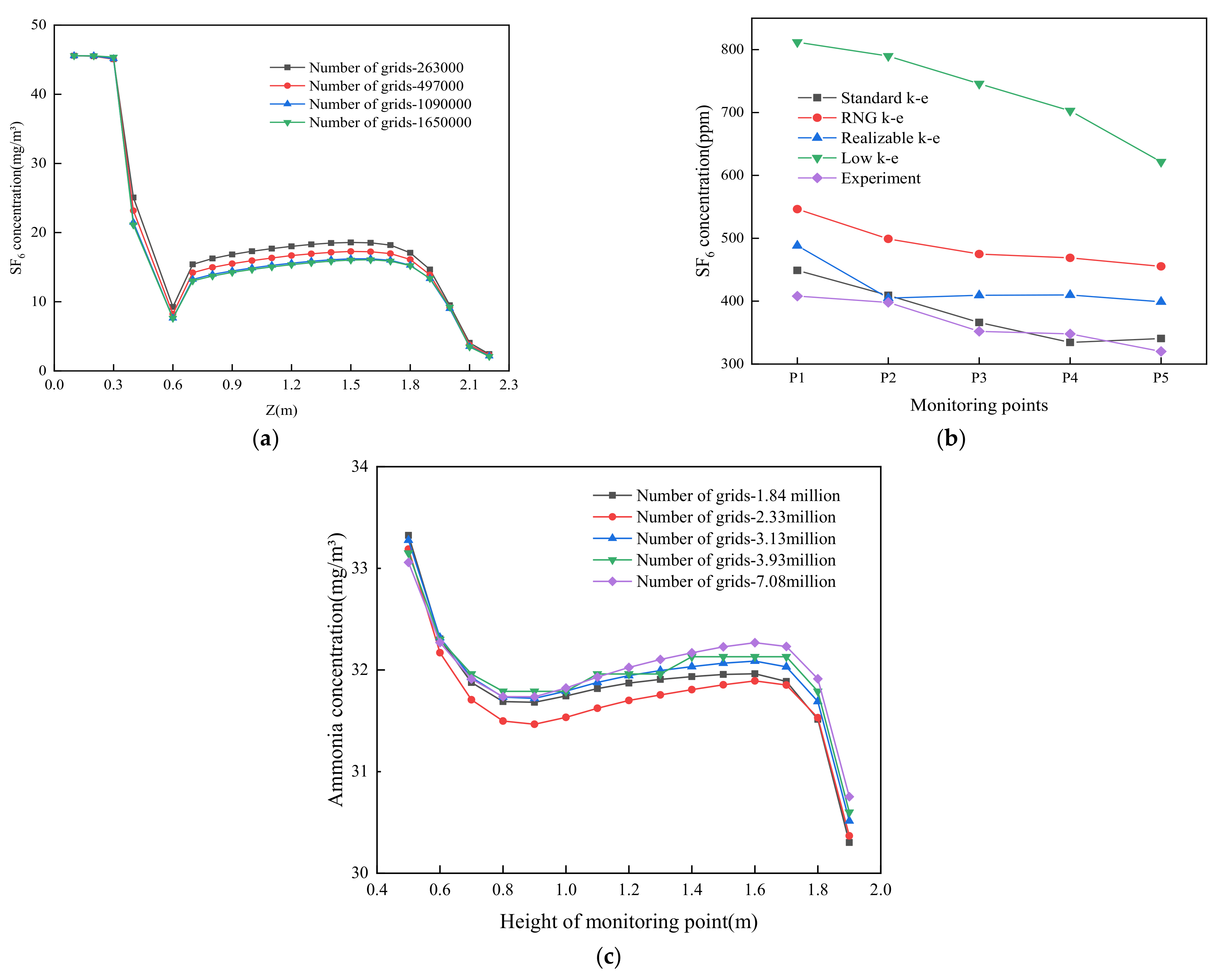
2.4. Validation of the CFD Simulation Method
2.5. Boundary Conditions
3. Case Descriptions
3.1. Preliminary Study
3.2. Orthogonal Experimental Design
3.3. Evaluation Index
4. Results and Discussion
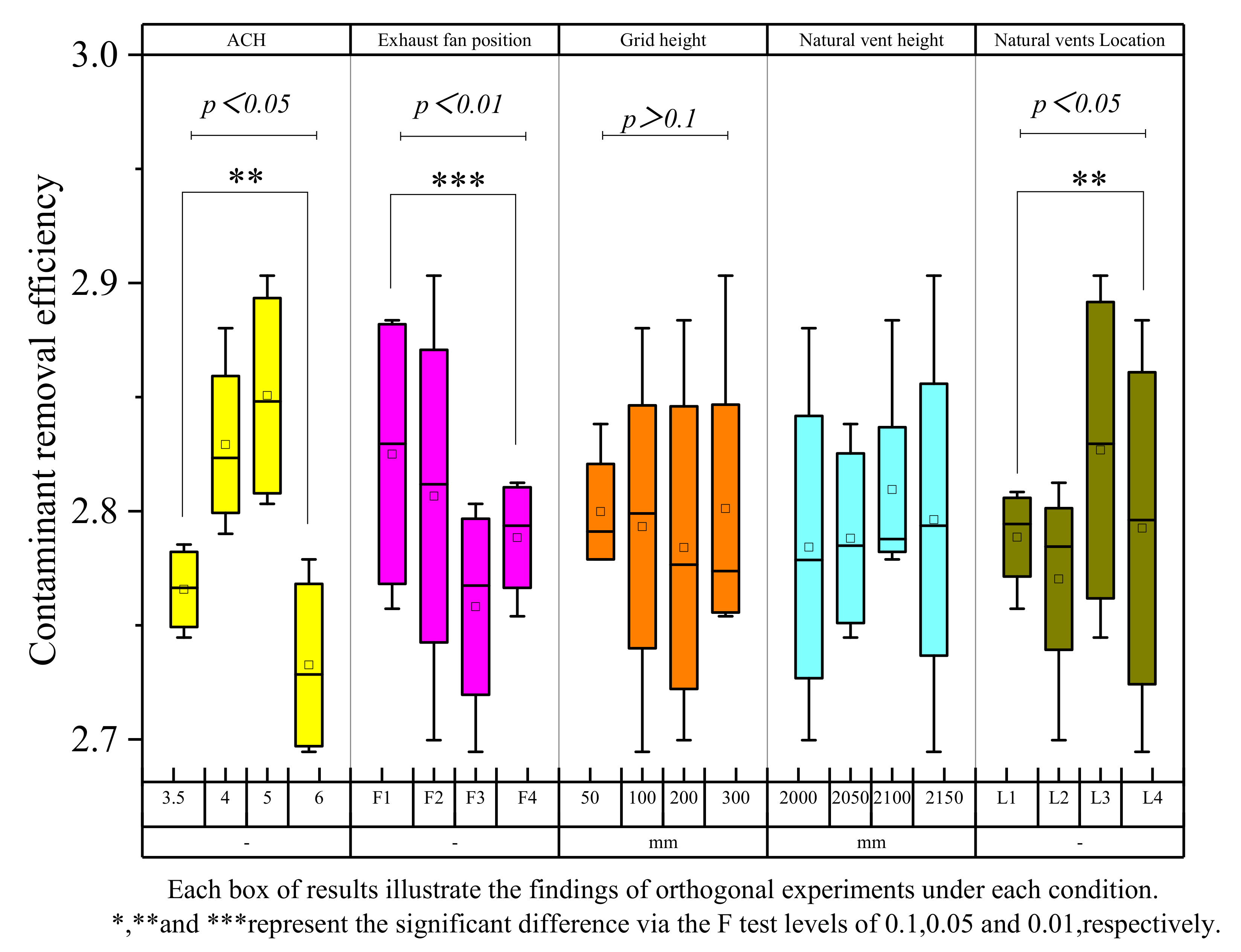
4.1. Orthogonal Significance Analysis of Relevant Factors
4.2. Single-Factor Analysis
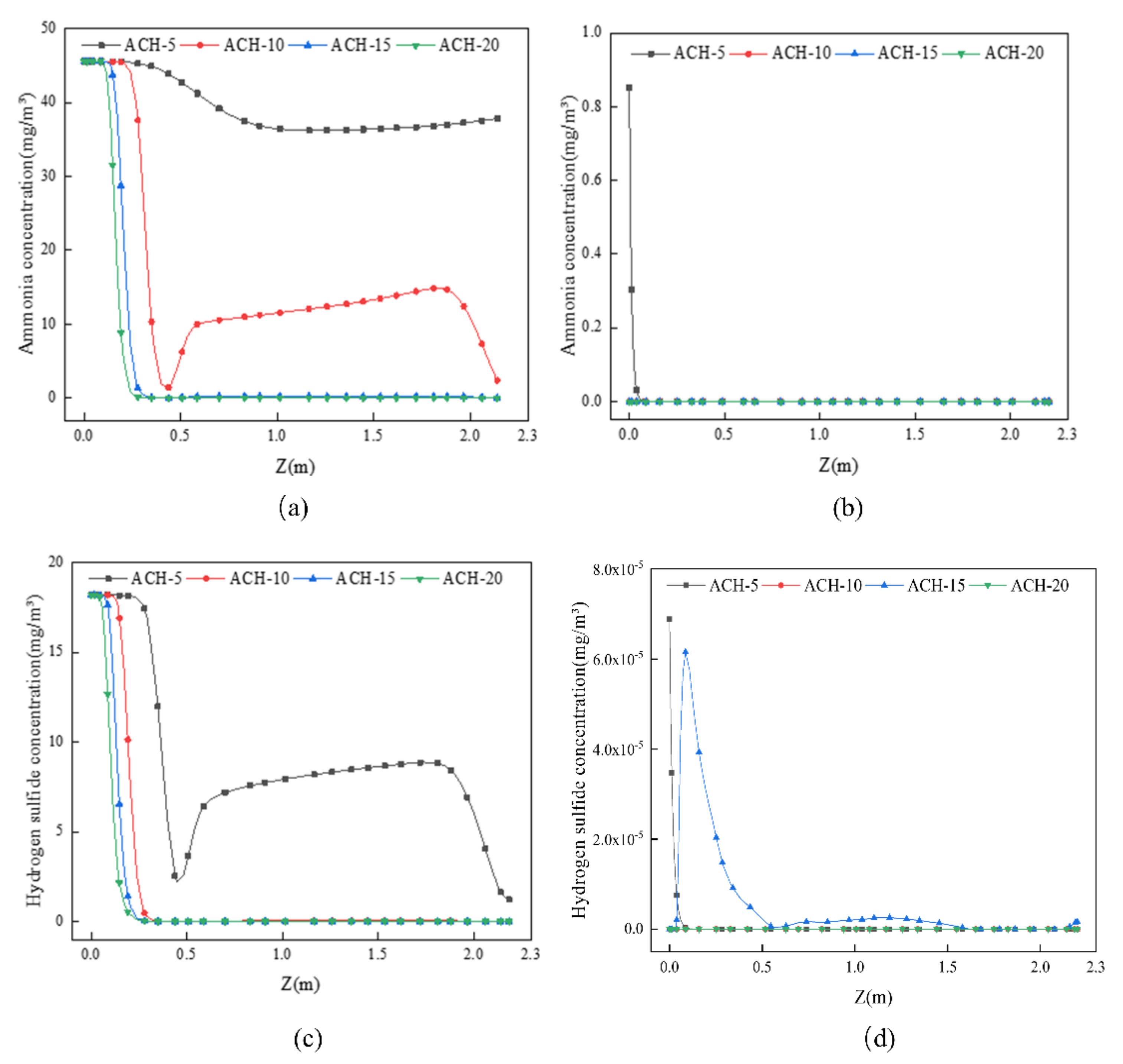
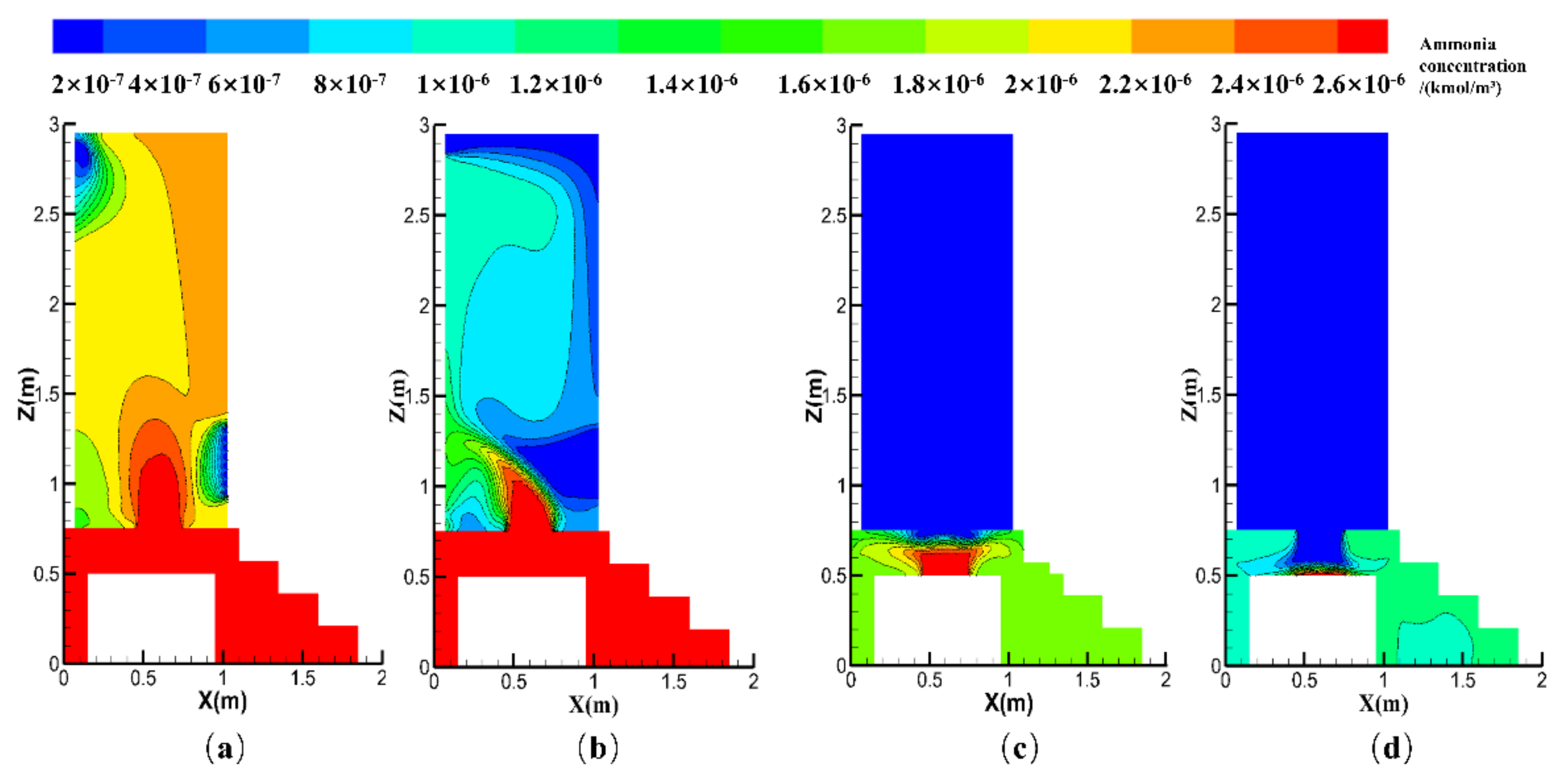
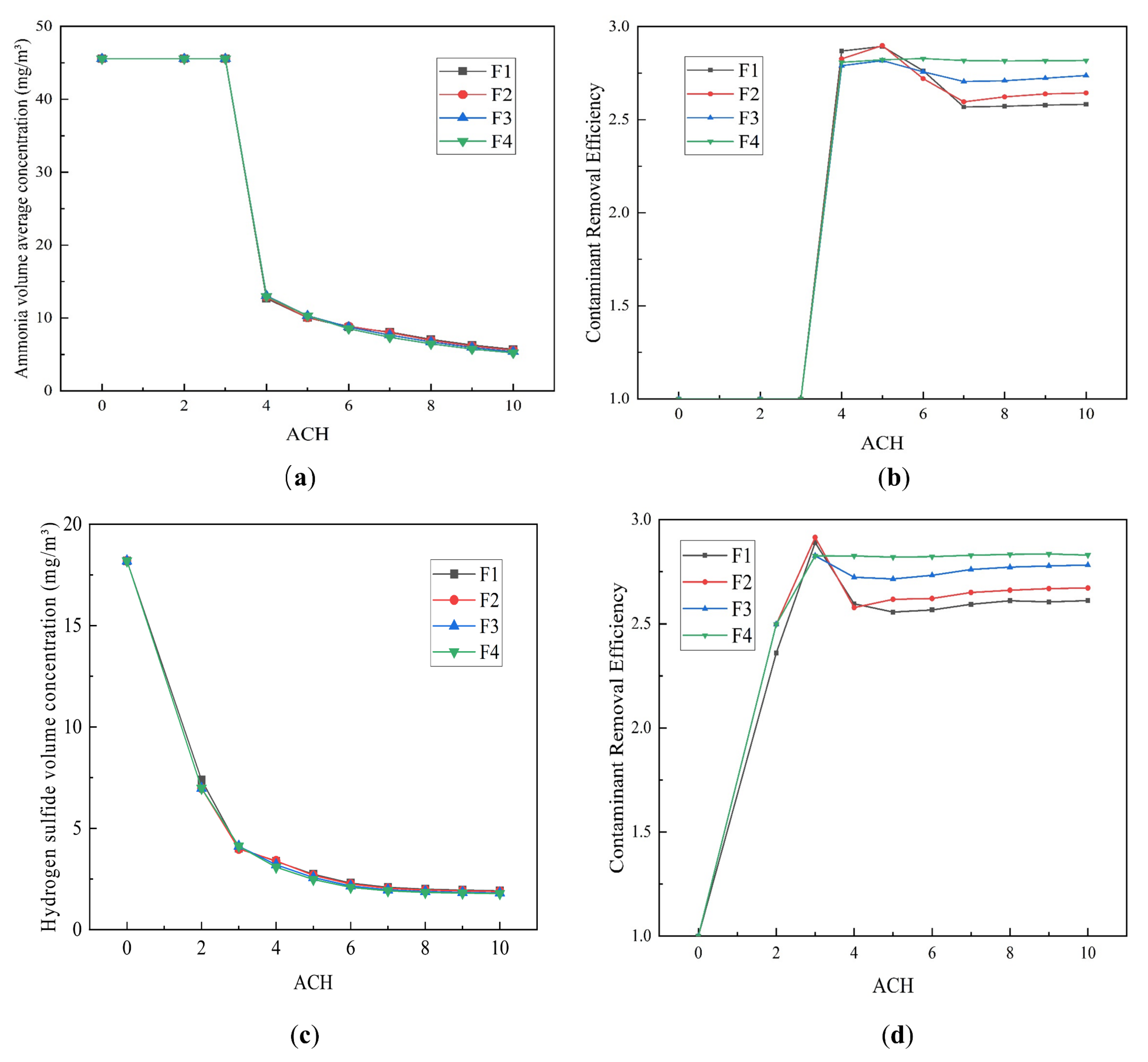
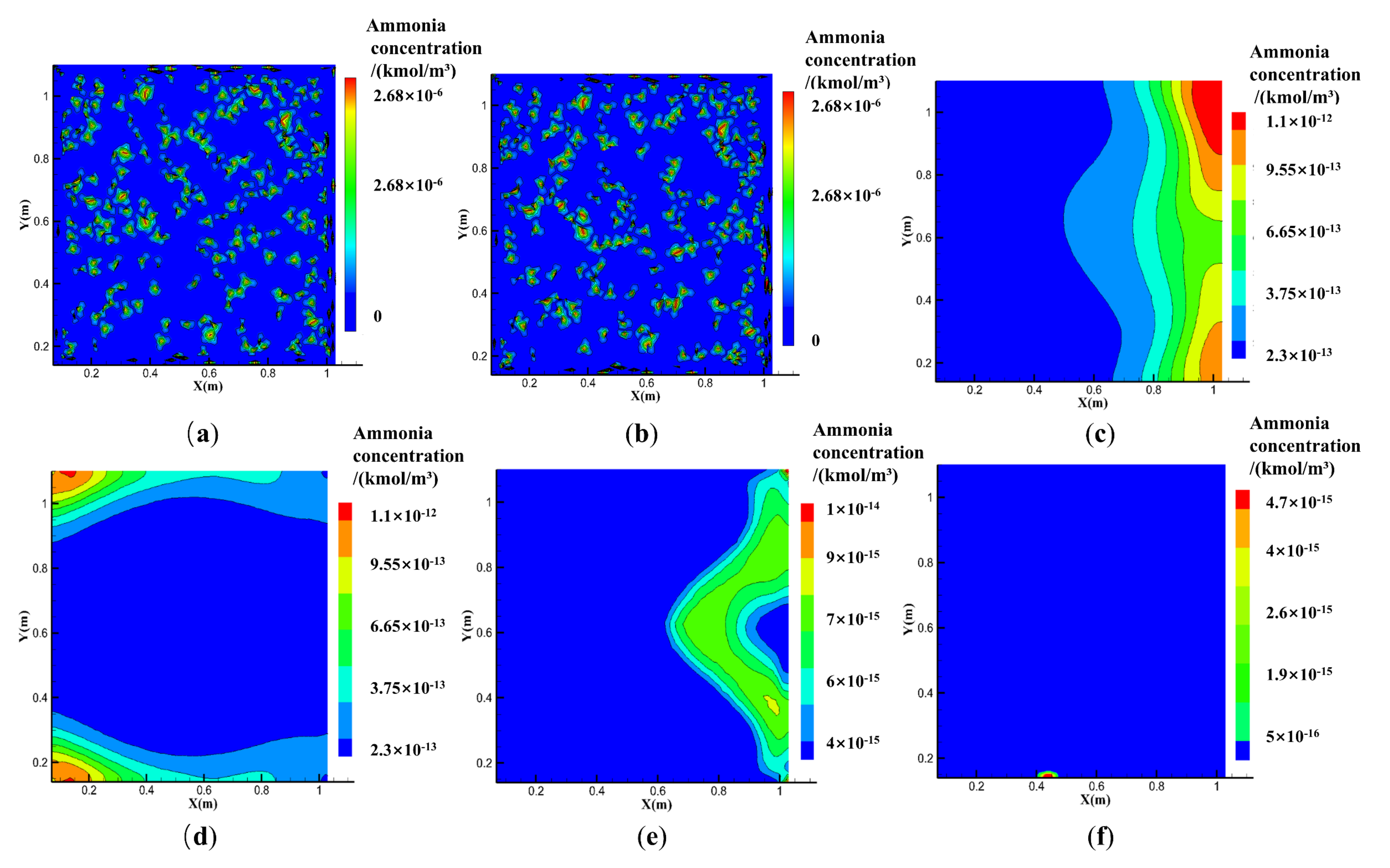
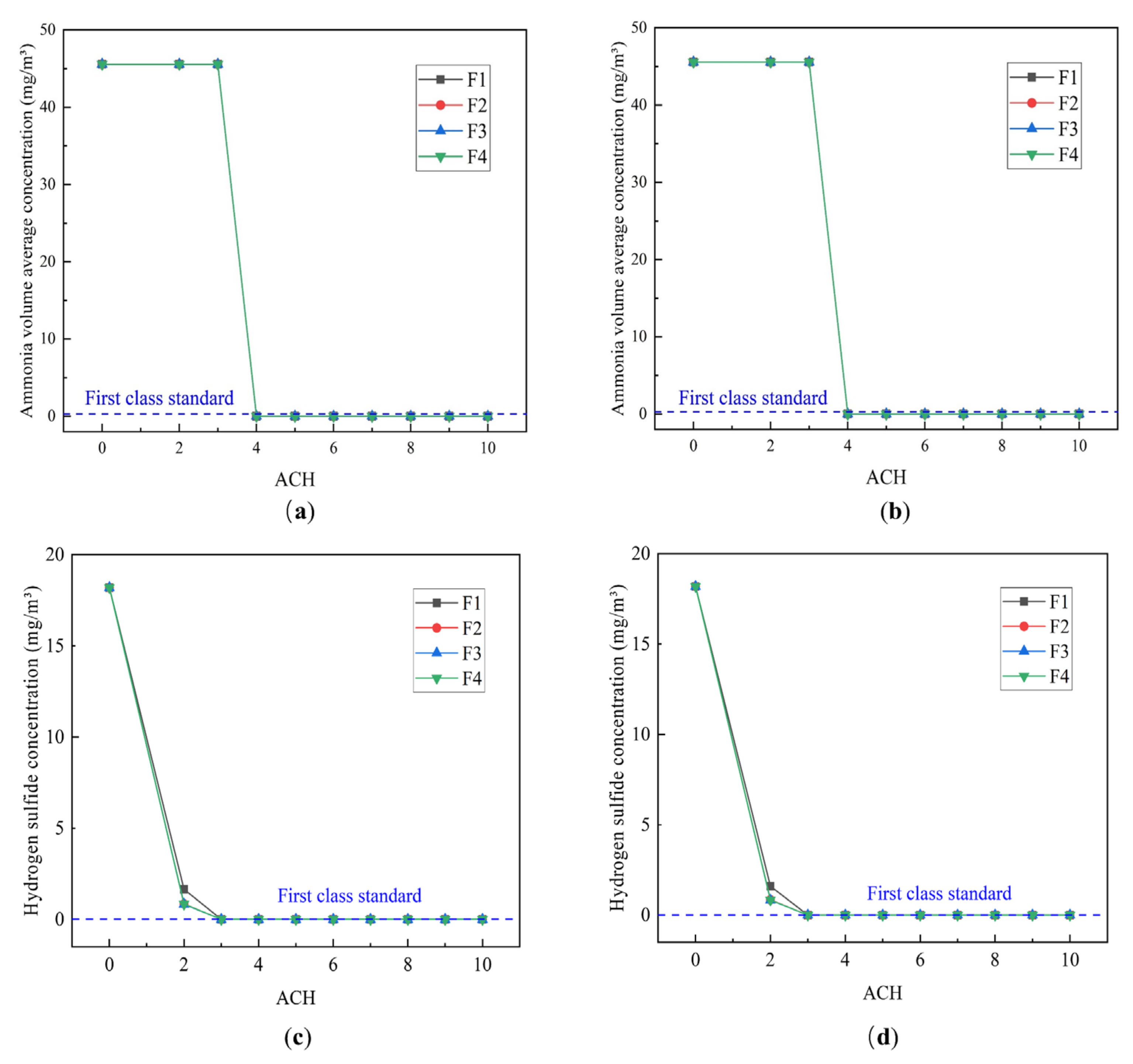
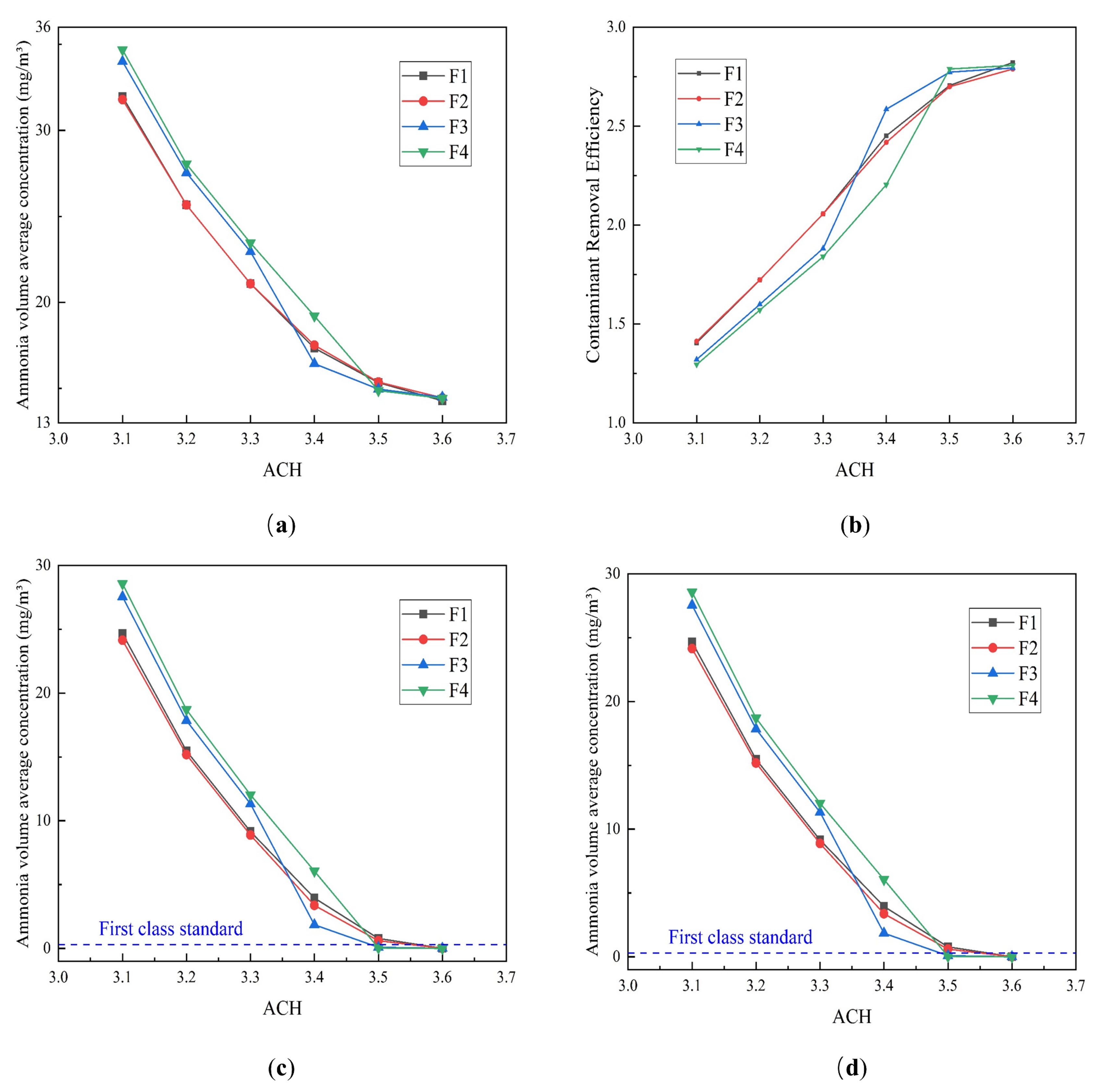
4.2.1. Effect of the ACH
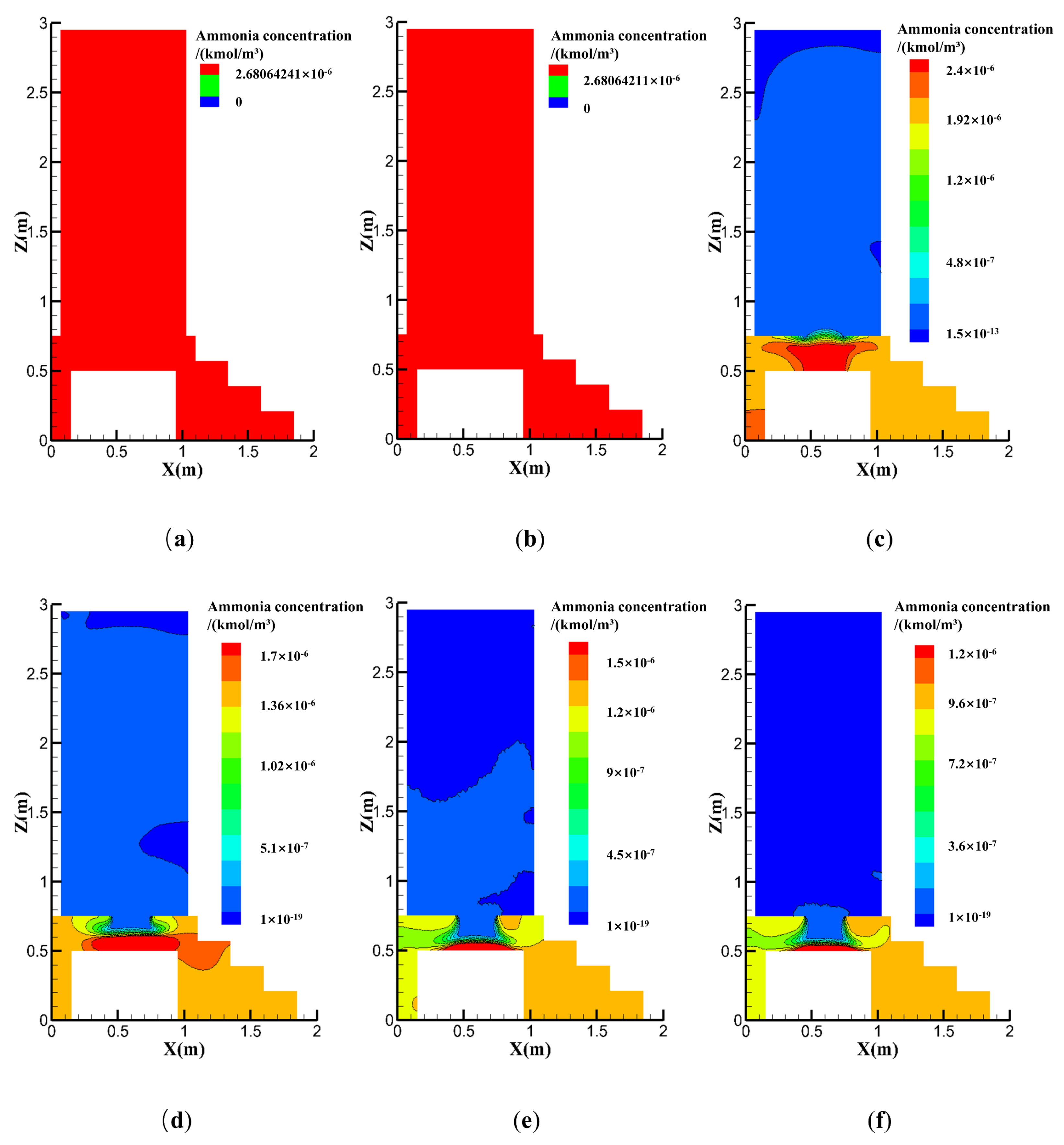
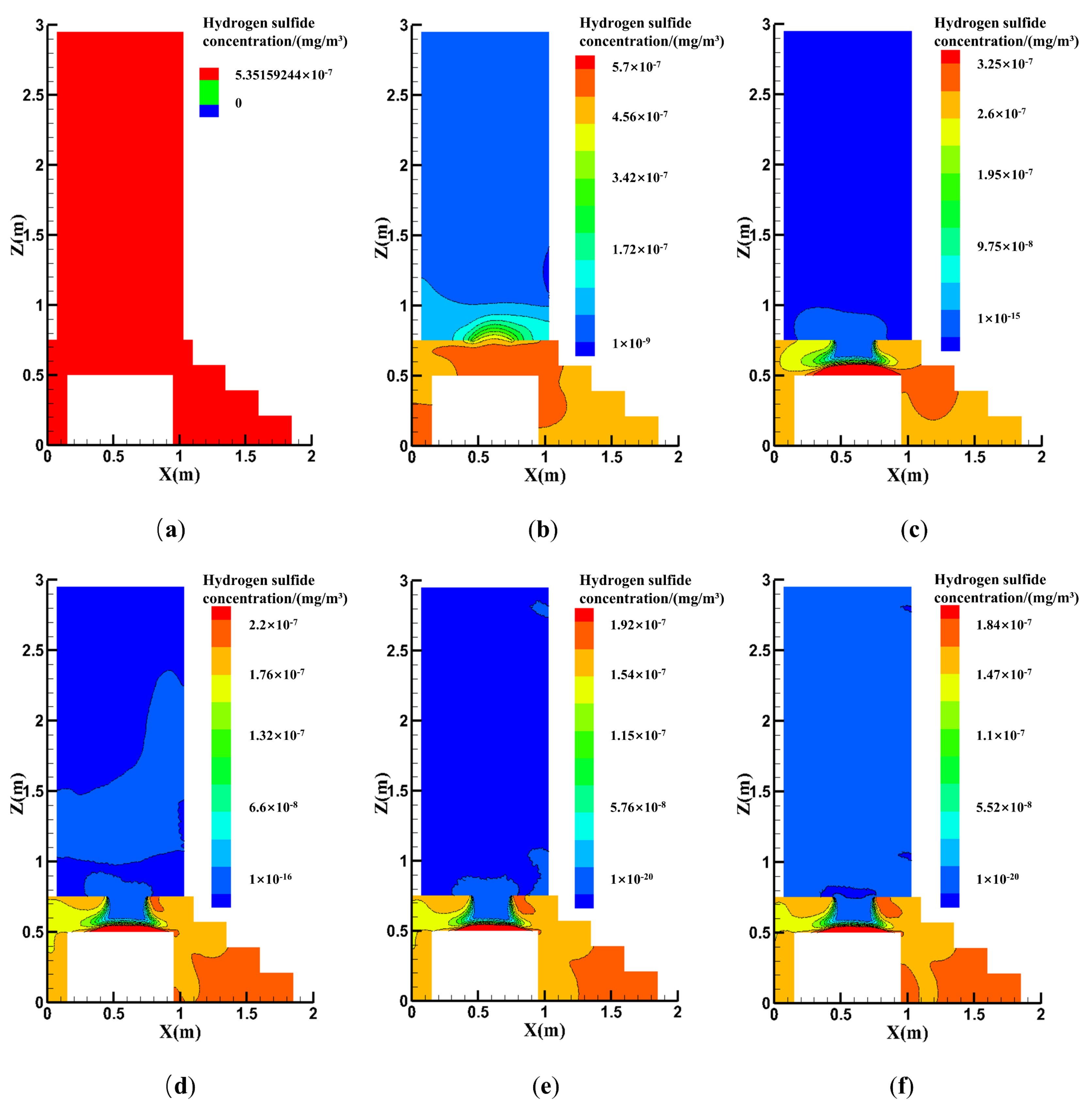
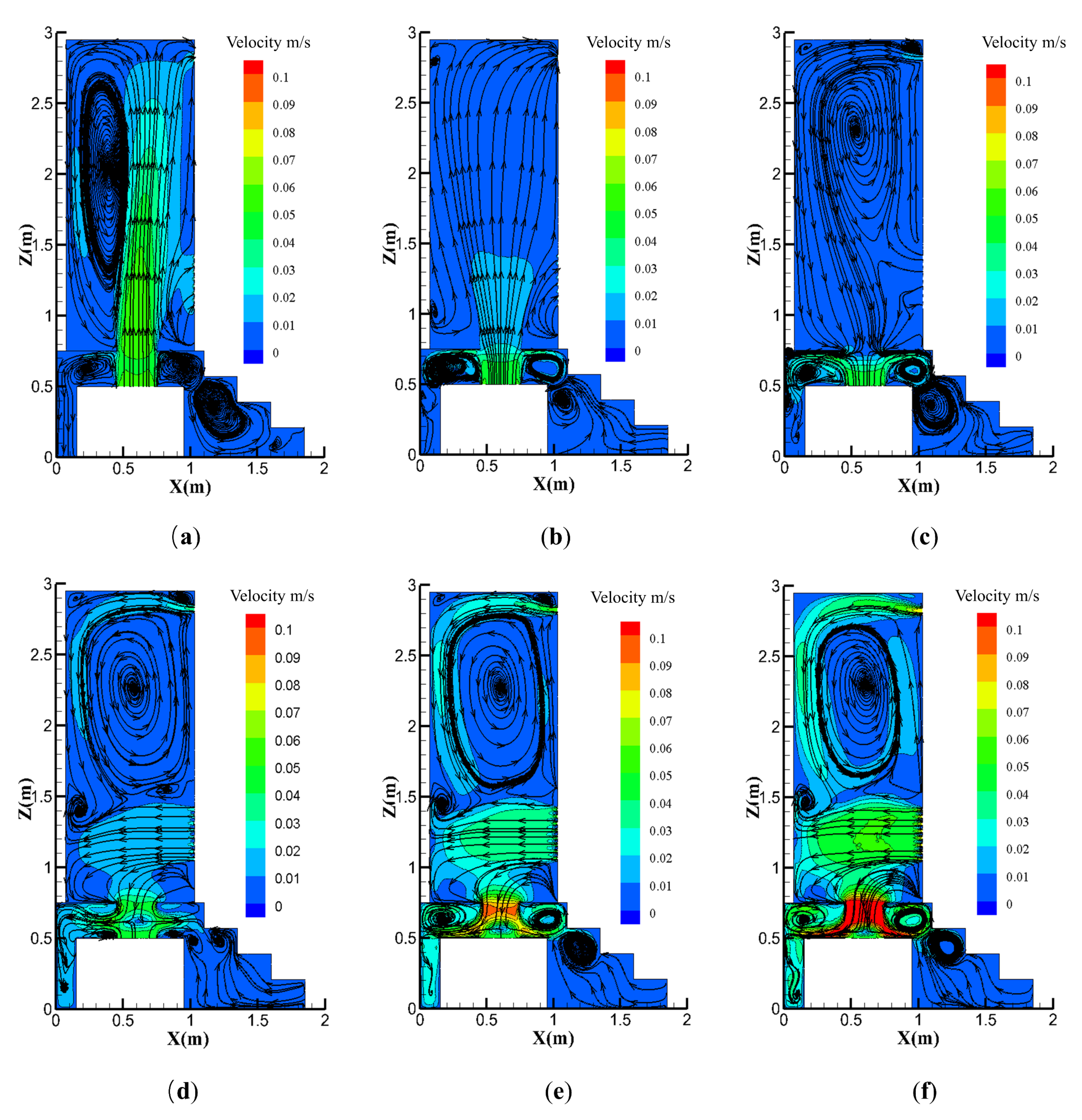
4.2.2. Effect of the Exhaust Fan Position
4.2.3. Effects of the Natural Vent Location
5. Conclusions
- Common toilet ventilation factors (e.g., the ACH) and factors specific to NFETs (e.g., the EFP, NVL, and G-h) were ranked based on their statistical significance as follows: EFP > ACH > NVL > G-h, with the EFP achieving statistical significance (p-value < 0.01) in the case of ammonia. The CRE is high when the exhaust fan is installed in the lower part of the toilet (near the fermentation tank).
- Contaminant concentration distributions were simulated for different exhaust positions and air changes, with the results showing that both the ACH and exhaust fan location must be considered in toilet exhaust design. Toilet ventilation efficiency may be optimized by installing the exhaust at the optimal location, thus maximizing the air quality improvement inside the toilet.
- The VAC decreases with increasing ACH, with the rate of decrease decelerating gradually. In contrast, the CRE first increases and then decreases before finally stabilizing in response to increasing ACH. The CRE varies between 1 and 3 but does not exceed 3, which may be related to the small size of the toilet investigated, and the relatively low pollutant concentrations contained within it.
- Single-factor analysis revealed three stages of exhaust behavior, namely, “ineffective”, “enhanced”, and “excessive”. Beyond guaranteeing sufficient air quality, an appropriate number of air changes should be selected to reduce the energy consumption and indoor air speed, thus reducing the blowing sensation and improving user comfort.
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| C | Average concentration of contaminants in the immediate environment (mg × m−3) |
| Ce | Concentration of contaminants at the exhaust outlet (mg × m−3) |
| Cin | Concentration of contaminants at the air supply outlet (mg × m−3) |
| IAQ | Indoor air quality |
| CRE | Contaminant removal efficiency |
| NFET | Non-flushing ecological toilet |
| CFD | Computational fluid dynamics |
| EFP | Exhaust fan position |
| ACH | Air change rate per hour (h−1) |
| NVL | Natural vent location |
| G-h | Grid height (m) |
References
- Cheng, S.K.; Li, Z.F.; Uddin, S.M.N.; Mang, H.P.; Zhou, X.Q.; Zhang, J.; Zheng, L.L.; Zhang, L.L. Toilet revolution in China. J. Environ. Manag. 2018, 216, 347–356. [Google Scholar] [CrossRef]
- Sato, H.; Hirose, T.; Kimura, T.; Moriyama, Y.; Nakashima, Y. Analysis of Malodorous Volatile Substances of Human Waste: Feces and Urine. J. Health Sci. 2001, 47, 483–490. [Google Scholar] [CrossRef]
- Zhang, F.D.; Zhang, S.Q.; Wang, U.J.; Dou, F.G.; Xiu-Mei, L.I.; Zou, S.W. Deodorization Techniques on Fleeting Continuous Zymosis of Organic Feces with High Temperature. J. Agro. Environ. Sci. 2004, 23, 796a800. [Google Scholar]
- Kikuchi, R. Pilot-scale test of a soil filter for treatment of malodorous gas. Soil Use Manag. 2006, 16, 211–214. [Google Scholar] [CrossRef]
- Lewkowska, P.; Cieślik, B.; Dymerski, T.; Konieczka, P.; Namieśnik, J. Characteristics of odors emitted from municipal wastewater treatment plant and methods for their identification and deodorization techniques. Environ. Res. 2016, 151, 573–586. [Google Scholar] [CrossRef]
- Kanjanarong, J.; Giri, B.S.; Jaisi, D.P.; de Oliveira, F.R.; Boonsawang, P.; Chaiprapat, S.; Singh, R.; Balakrishna, A.; Khanal, S.K. Removal of hydrogen sulfide generated during anaerobic treatment of sulfate-laden wastewater using biochar: Evaluation of efficiency and mechanisms. Bioresour. Technol. 2017, 234, 115–121. [Google Scholar] [CrossRef]
- Yamamoto, T.; Uchida, M.; Kurihara, Y. Deodorant compositions containing antibacterial zeolite and silicones. Zeolites 1997, 18, 235. [Google Scholar] [CrossRef]
- Wang, J.; Xiao, Y.; Tao, J.; Liang, H.; Wu, Z. An Introduction of Automatic Catalytic Oxidation Active Carbon Odor Control Technology. Guangdong Chem. Ind. 2008, 20, 141–144. [Google Scholar]
- Osako, M.; Nishida, K. Mechanisms of the sensory deodorization by aromatic deodorizers. Counteraction and masking effect of aromatic compounds. Jpn. J. Ergon. 1990, 26, 271–282. [Google Scholar] [CrossRef][Green Version]
- Fang, L.; Norris, C.; Johnson, K.; Cui, X.; Sun, J.; Teng, Y.; Tian, E.; Xu, W.; Li, Z.; Mo, J.; et al. Toxic volatile organic compounds in 20 homes in Shanghai: Concentrations, inhalation health risks, and the impacts of household air cleaning. Build. Environ. 2019, 157, 309–318. [Google Scholar] [CrossRef]
- Ao, Y.; Wang, L.; Jia, X.; Gu, C. Numerical Simulation of Pollutant Diffusion Law in Bathroom and Optimization of the Locations and Ways of Exhaust Outlet and Makeup Air, Shenyang Jianzhu Daxue Xuebao (Ziran Kexue Ban). J. Shenyang Jianzhu Univ. 2011, 27, 720–724. [Google Scholar]
- Han, Z.; Xu, Y.; Wang, H.; Tian, H.; Qiu, B.; Sun, D. Synthesis of ammonia molecularly imprinted adsorbents and ammonia adsorption separation during sludge aerobic composting. Bioresour. Technol. 2019, 300, 122670. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Liu, J.; Liu, L. On the capture of polar indoor air pollutants at sub-ppm level—A molecular simulation study. Build. Simul. 2020, 13, 989–997. [Google Scholar] [CrossRef]
- Vikrant, K.; Kailasa, S.K.; Tsang, D.; Lee, S.S.; Kumar, P.; Giri, B.S.; Singh, R.S.; Kim, K.-H. Biofiltration of hydrogen sulfide: Trends and challenges. J. Clean. Prod. 2018, 187, 131–147. [Google Scholar] [CrossRef]
- Tian, K.; Wang, X.X.; Yu, Z.Y.; Li, H.; Xin, G. Hierarchical and hollow Fe2O3 nano-boxes derived from metal-organic frameworks with excellent sensitivity to H2S. Acs Appl. Mater. Interfaces 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Wu, L. Trend in H2S Biology and Medicine Research—A Bibliometric Analysis. Molecules 2017, 22, 2087. [Google Scholar] [CrossRef]
- Wei, Y.; Xu, Z.; Gao, J.; Cao, G.; Xiang, Z.; Xing, S. Indoor air pollutants, ventilation rate determinants and potential control strategies in Chinese dwellings: A literature review. Sci. Total Environ. 2017, 586, 696–729. [Google Scholar]
- Kato, S.; Yang, J.-H. Study on inhaled air quality in a personal air-conditioning environment using new scales of ventilation efficiency. Build. Environ. 2008, 43, 494–507. [Google Scholar] [CrossRef]
- Novoselac, A.; Srebric, J. Comparison of Air Exchange Efficiency and Contaminant Removal Effectiveness as IAQ Indices. ASHRAE Trans. 2003, 109, 339–349. [Google Scholar]
- Laverge, J.; Spilak, M.; Novoselac, A. Experimental assessment of the inhalation zone of standing, sitting and sleeping persons. Build. Environ. 2014, 82, 258–266. [Google Scholar] [CrossRef]
- Sandberg, M.; Blomqvist, C. Displacement ventilation systems in office rooms. ASHRAE Trans. 1989, 95, 1041–1049. [Google Scholar]
- American Society of Heating. Refrigerating and air-conditioning engineers. Int. J. Refrig. 1979, 2, 56–57. [Google Scholar] [CrossRef]
- Lin, Y.-P. Natural Ventilation of Toilet Units in K–12 School Restrooms Using CFD. Energies 2021, 14, 4792. [Google Scholar] [CrossRef]
- Ponechal, R.; Krušinský, P.; Kysela, P.; Pisca, P. Simulations of Airflow in the Roof Space of a Gothic Sanctuary Using CFD Models. Energies 2021, 14, 3694. [Google Scholar] [CrossRef]
- Khosravi, S.N.; Mahdavi, A. A CFD-Based Parametric Thermal Performance Analysis of Supply Air Ventilated Windows. Energies 2021, 14, 2420. [Google Scholar] [CrossRef]
- Seduikyte, L.; Stasiulienė, L.; Prasauskas, T.; Martuzevičius, D.; Černeckienė, J.; Ždankus, T.; Dobravalskis, M.; Fokaides, P. Field Measurements and Numerical Simulation for the Definition of the Thermal Stratification and Ventilation Performance in a Mechanically Ventilated Sports Hall. Energies 2019, 12, 2243. [Google Scholar] [CrossRef]
- Yang, G.; Li, X.; Ding, L.; Zhu, F.; Wang, Z.; Wang, S.; Xu, Z.; Xu, J.; Qiu, P.; Guo, Z. CFD Simulation of Pollutant Emission in a Natural Draft Dry Cooling Tower with Flue Gas Injection: Comparison between LES and RANS. Energies 2019, 12, 3630. [Google Scholar] [CrossRef]
- Chung, S.-C.; Lin, Y.-P.; Yang, C.; Lai, C.-M. Natural Ventilation Effectiveness of Awning Windows in Restrooms in K-12 Public Schools. Energies 2019, 12, 2414. [Google Scholar] [CrossRef]
- Conceio, E.; Awbi, H. Evaluation of Integral Effect of Thermal Comfort, Air Quality and Draught Risk for Desks Equipped with Personalized Ventilation Systems. Energies 2021, 14, 3235. [Google Scholar] [CrossRef]
- Cho, J. Investigation on the contaminant distribution with improved ventilation system in hospital isolation rooms: Effect of supply and exhaust air diffuser configurations. Appl. Therm. Eng. 2018, 148, 208–218. [Google Scholar] [CrossRef]
- Tung, Y.-C.; Hu, S.-C.; Tsai, T.-Y. Influence of bathroom ventilation rates and toilet location on odor removal. Build. Environ. 2009, 44, 1810–1817. [Google Scholar] [CrossRef]
- Tung, Y.-C.; Hu, S.-C.; Tsai, T.-I.; Chang, I.-L. An experimental study on ventilation efficiency of isolation room. Build. Environ. 2009, 44, 271–279. [Google Scholar] [CrossRef]
- Tung, Y.-C.; Shih, Y.-C.; Hu, S.-C.; Chang, Y.-L. Experimental performance investigation of ventilation schemes in a private bathroom. Build. Environ. 2010, 45, 243–251. [Google Scholar] [CrossRef]
- Quitzau, M.B. Water-flushing toilets: Systemic development and path-dependent characteristics and their bearing on techno-logical alternatives. Technol. Soc. 2007, 29, 351–360. [Google Scholar] [CrossRef]
- Sutherland, C.; Reynaert, E.; Dhlamini, S.; Magwaza, F.; Lienert, J.; Riechmann, M.E.; Buthelezi, S.; Khumalo, D.; Morgenroth, E.; Udert, K.M.; et al. Socio-technical analysis of a sanitation innovation in a peri-urban household in Durban, South Africa. Sci. Total. Environ. 2020, 755, 143284. [Google Scholar] [CrossRef]
- Tada, S.; Itoh, Y.; Kiyoshi, K.; Yoshida, N. Isolation of ammonia gas-tolerant extremophilic bacteria and their application to the elimination of malodorous gas emitted from outdoor heat-treated toilets. J. Biosci. Bioeng. 2021, 131, 509–517. [Google Scholar] [CrossRef]
- Bhagwan, J.N.; Pillay, S.; Koné, D. Sanitation game changing: Paradigm shift from end-of-pipe to off-grid solutions. Water Pract. Technol. 2019, 14, 497–506. [Google Scholar] [CrossRef]
- Anand, C.K.; Apul, D.S. Composting toilets as a sustainable alternative to urban sanitation—A review. Waste Manag. 2014, 34, 329–343. [Google Scholar] [CrossRef]
- Nielsen, P.V. Fifty years of CFD for room air distribution. Build. Environ. 2015, 91, 78–90. [Google Scholar] [CrossRef]
- Rong, L.; Nielsen, P.V.; Bjerg, B.; Zhang, G. Summary of best guidelines and validation of CFD modeling in livestock buildings to ensure prediction quality. Comput. Electron. Agric. 2016, 121, 180–190. [Google Scholar] [CrossRef]
- Shen, C.; Gao, N.; Wang, T. CFD study on the transmission of indoor pollutants under personalized ventilation. Build. Environ. 2013, 63, 69–78. [Google Scholar] [CrossRef]
- Lv, L.; Gao, J.; Zeng, L.; Cao, C.; Zhang, J.; He, L. Performance assessment of air curtain range hood using contaminant removal efficiency: An experimental and numerical study. Build. Environ. 2020, 188, 107456. [Google Scholar] [CrossRef]
- Lv, L.; Zeng, L.; Wu, Y.; Gao, J.; Xie, W.; Cao, C.; Chen, Y.; Zhang, J. Effects of human walking on the capture efficiency of range hood in residential kitchen. Build. Environ. 2021, 196, 107821. [Google Scholar] [CrossRef]
- Zeng, L.; Liu, G.; Gao, J.; Du, B.; Lv, L.; Cao, C.; Ye, W.; Tong, L.; Wang, Y. A circulating ventilation system to concentrate pollutants and reduce exhaust volumes: Case studies with experiments and numerical simulation for the rubber refining process. J. Build. Eng. 2020, 35, 101984. [Google Scholar] [CrossRef]
- Yong, Z.A.; Xia, Y.A.; Hy, A.; Kz, A.; Xw, B.; Zl, C.; Jian, H.; Sz, A. Numerical investigations of reactive pollutant dispersion and personal exposure in 3D urban-like models. Build. Environ. 2019, 169, 106569. [Google Scholar]
- Yang, X.; Yang, H.; Ou, C.; Luo, Z.; Hang, J. Airborne transmission of pathogen-laden expiratory droplets in open outdoor space. Sci. Total. Environ. 2021, 773, 145537. [Google Scholar] [CrossRef]
- Tong, L.; Gao, J.; Luo, Z.; Wu, L.; Zeng, L.; Liu, G.; Wang, Y. A novel flow-guide device for uniform exhaust in a central air exhaust ventilation system. Build. Environ. 2019, 149, 134–145. [Google Scholar] [CrossRef]
- Shaojie, J.; Jinfeng, M. Pollutant Concentration Research of Toilet in Natural Ventilation Conditions. Contam. Control. Air-Cond. Technol. 2012, 4. [Google Scholar]
- The Standardization Administration of the People’s Republic of China, Hygienic Standards for Communal Toilet in City, Domestic-National Standards-State Administration for Market Regulation CN-GB. 1998. Available online: https://max.book118.com/html/2019/0429/8125027036002021.shtm (accessed on 15 August 2021).
- Wu, W.Z. Yuanfang, Urban public toilet design standards, Domestic-Industry Standard-Industry Standard-Urban Construction CN-CJ, China Construction Industry Press. 2016. Available online: http://www.mohurd.gov.cn/wjfb/201703/t20170306_230861.html (accessed on 15 August 2021).
- Lu, Y. HVAC Design Guidelines; China Construction Industry Press: Beijing, China, 1996. [Google Scholar]
- Liu, W.; Liu, D.; Gao, N. CFD study on gaseous pollutant transmission characteristics under different ventilation strategies in a typical chemical laboratory. Build. Environ. 2017, 126, 238–251. [Google Scholar] [CrossRef]
- Duci, A.; Papakonstantinou, K.; Chaloulakou, A.; Markatos, N. Numerical approach of carbon monoxide concentration dis-persion in an enclosed garage. Build. Environ. 2004, 39, 1043–1048. [Google Scholar] [CrossRef]
- Chung, K.-C.; Hsu, S.-P. Effect of ventilation pattern on room air and contaminant distribution. Build. Environ. 2001, 36, 989–998. [Google Scholar] [CrossRef]

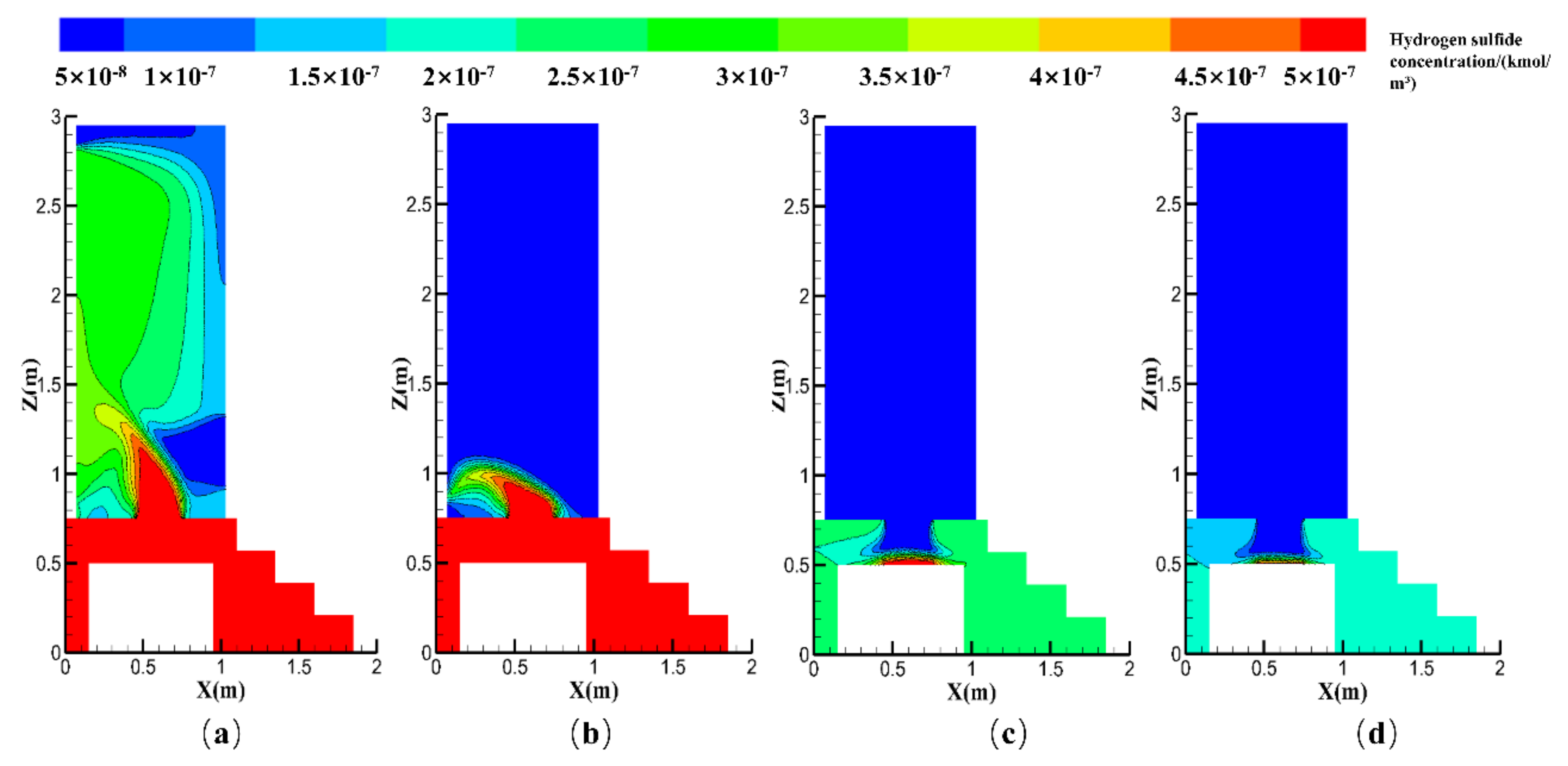
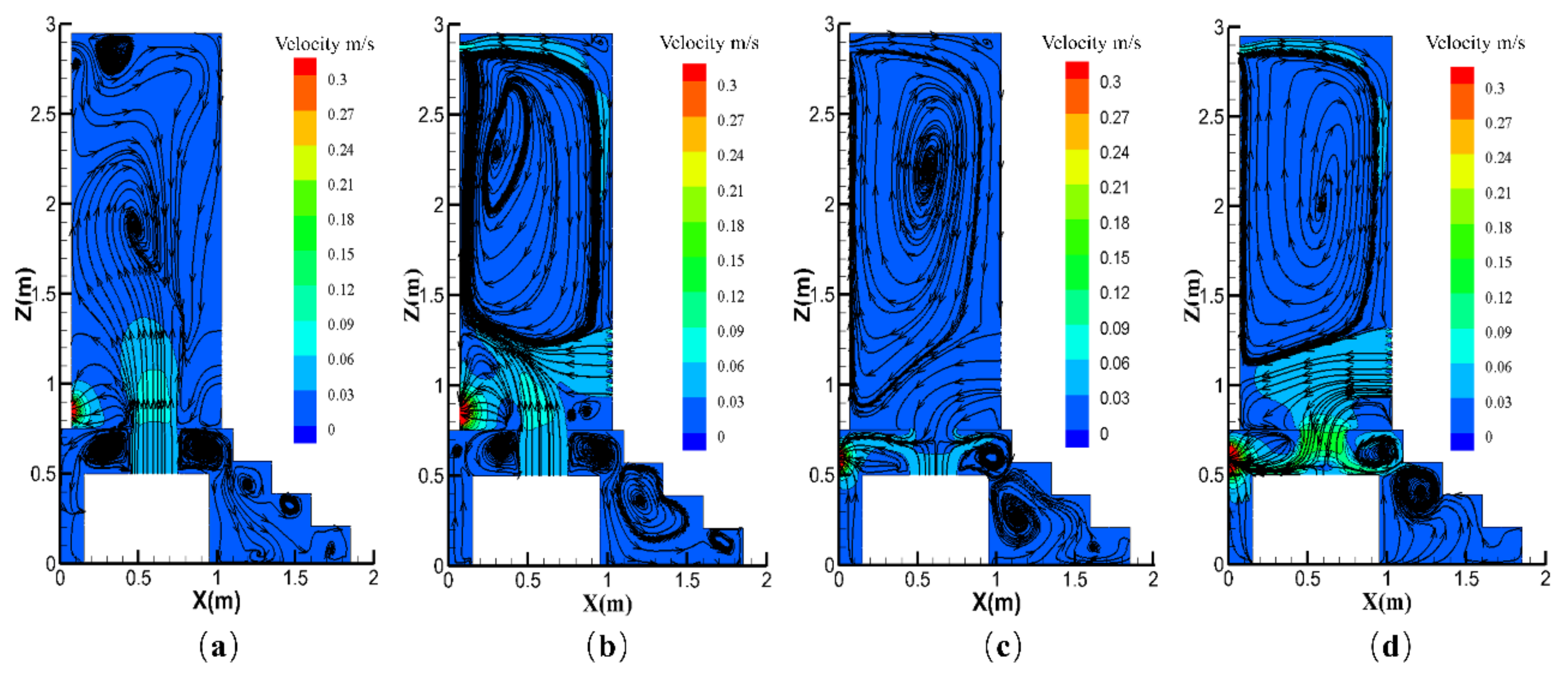
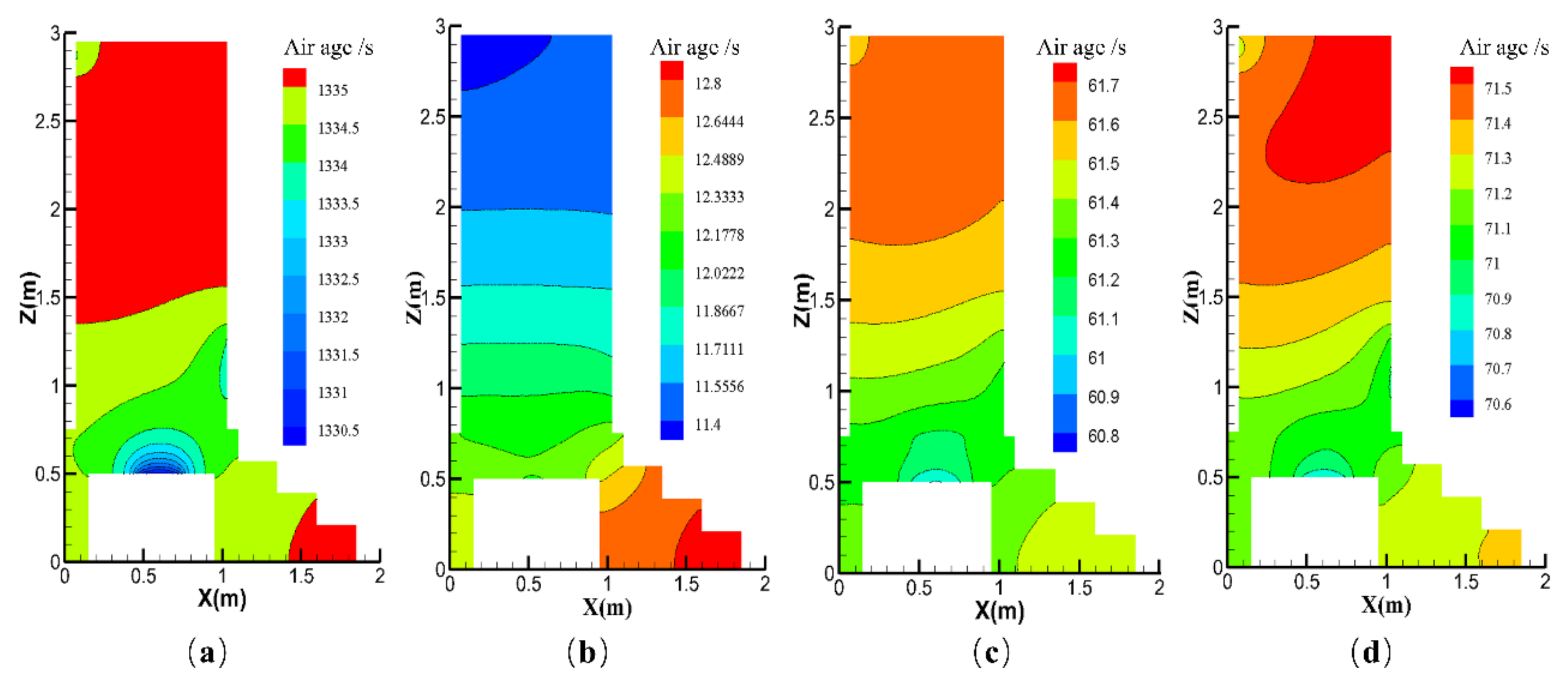

| Contaminants | First Class Standard | Second Class Standard | Third Class Standard |
|---|---|---|---|
| Ammonia (mg/m³) | 0.3 | 1 | 3 |
| Hydrogen sulfide (mg/m³) | 0.01 | 0.01 | 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Zeng, L.; Shi, H.; Yang, G.; Yu, Z.; Yin, W.; Gao, J.; Wang, L.; Zhang, Y.; Zhou, X. Dynamics and Numerical Simulation of Contaminant Diffusion for a Non-Flushing Ecological Toilet. Energies 2021, 14, 7570. https://doi.org/10.3390/en14227570
Zhang Z, Zeng L, Shi H, Yang G, Yu Z, Yin W, Gao J, Wang L, Zhang Y, Zhou X. Dynamics and Numerical Simulation of Contaminant Diffusion for a Non-Flushing Ecological Toilet. Energies. 2021; 14(22):7570. https://doi.org/10.3390/en14227570
Chicago/Turabian StyleZhang, Zhonghua, Lingjie Zeng, Huixian Shi, Gukun Yang, Zhenjiang Yu, Wenjun Yin, Jun Gao, Lina Wang, Yalei Zhang, and Xuefei Zhou. 2021. "Dynamics and Numerical Simulation of Contaminant Diffusion for a Non-Flushing Ecological Toilet" Energies 14, no. 22: 7570. https://doi.org/10.3390/en14227570
APA StyleZhang, Z., Zeng, L., Shi, H., Yang, G., Yu, Z., Yin, W., Gao, J., Wang, L., Zhang, Y., & Zhou, X. (2021). Dynamics and Numerical Simulation of Contaminant Diffusion for a Non-Flushing Ecological Toilet. Energies, 14(22), 7570. https://doi.org/10.3390/en14227570







