Impact of Dropwise Condensation on the Biomass Production Rate in Covered Raceway Ponds
Abstract
1. Introduction
2. Background
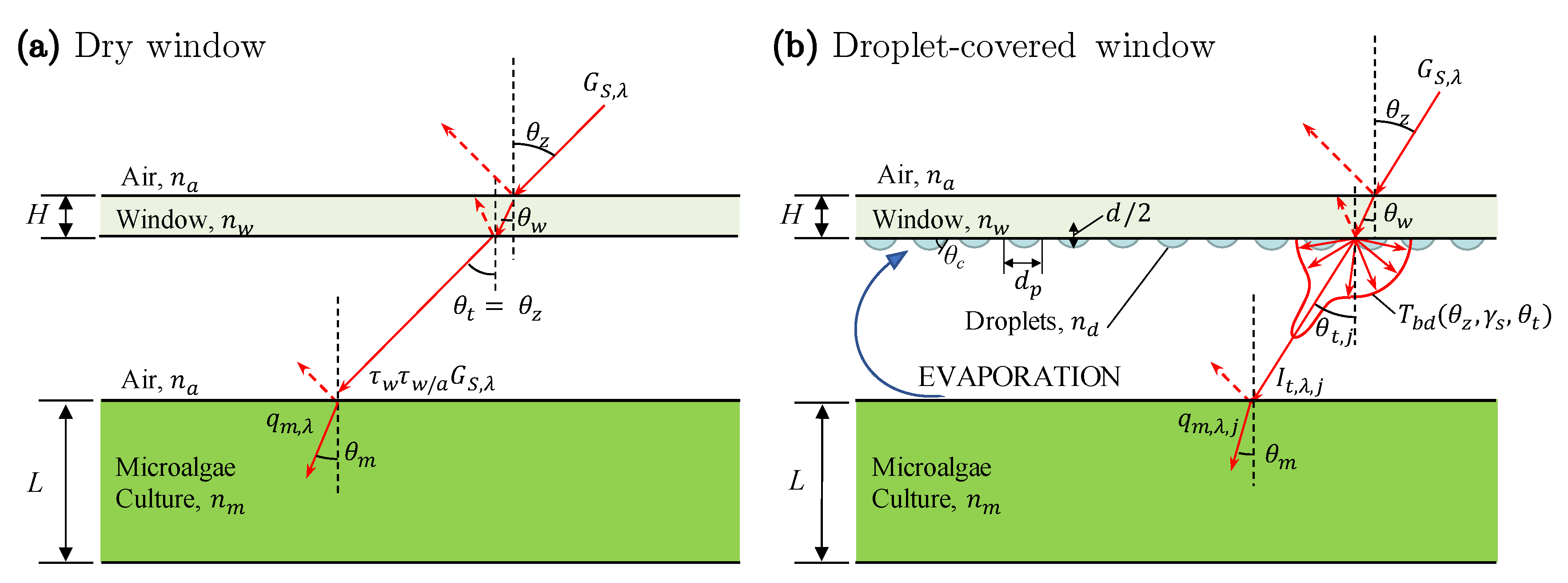
2.1. Light Transfer through Droplet-Covered PBR Windows
2.2. Light Transfer in Microalgae Culture
2.3. Microalgae Growth Kinetics
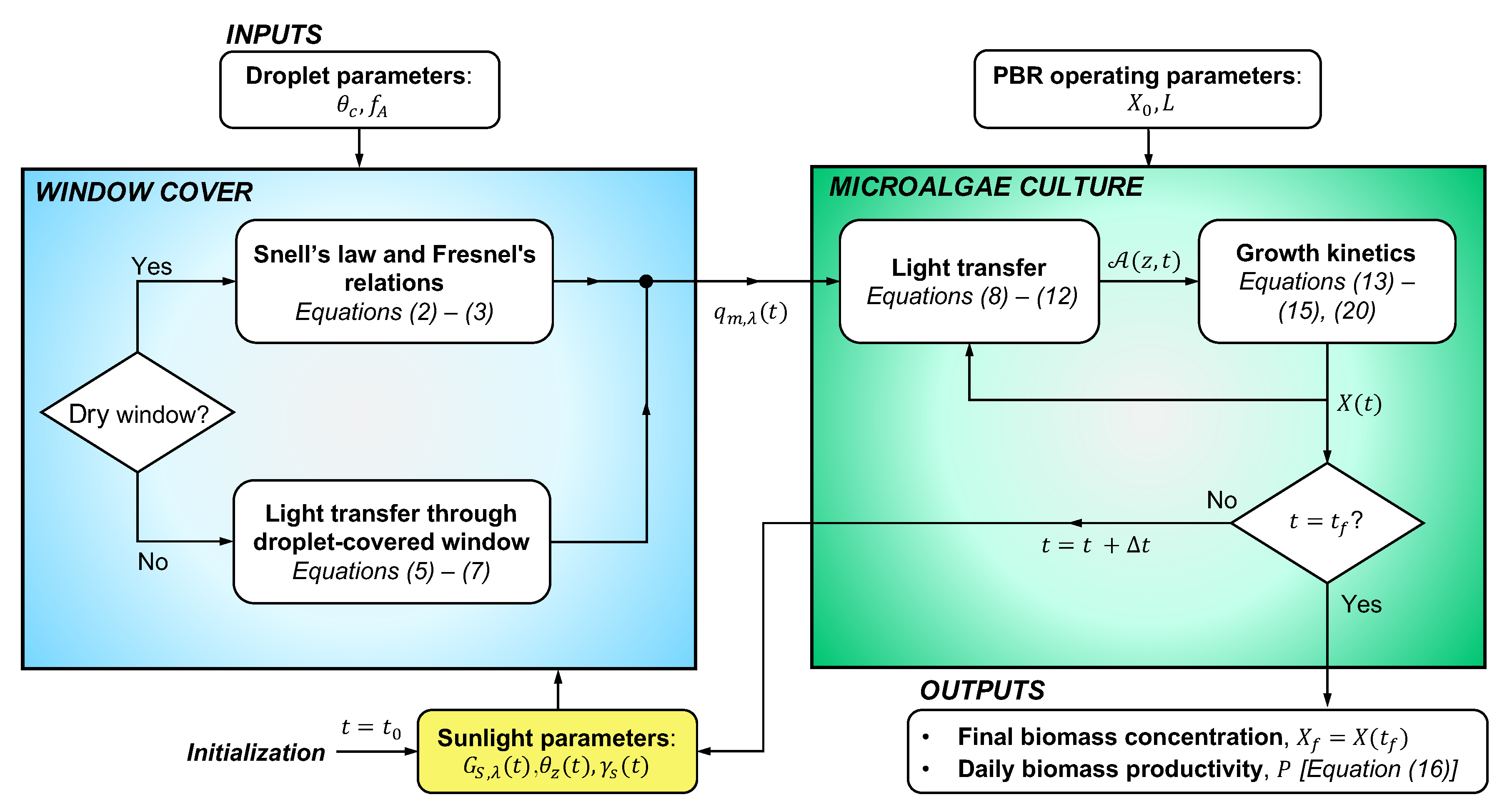
3. Methods
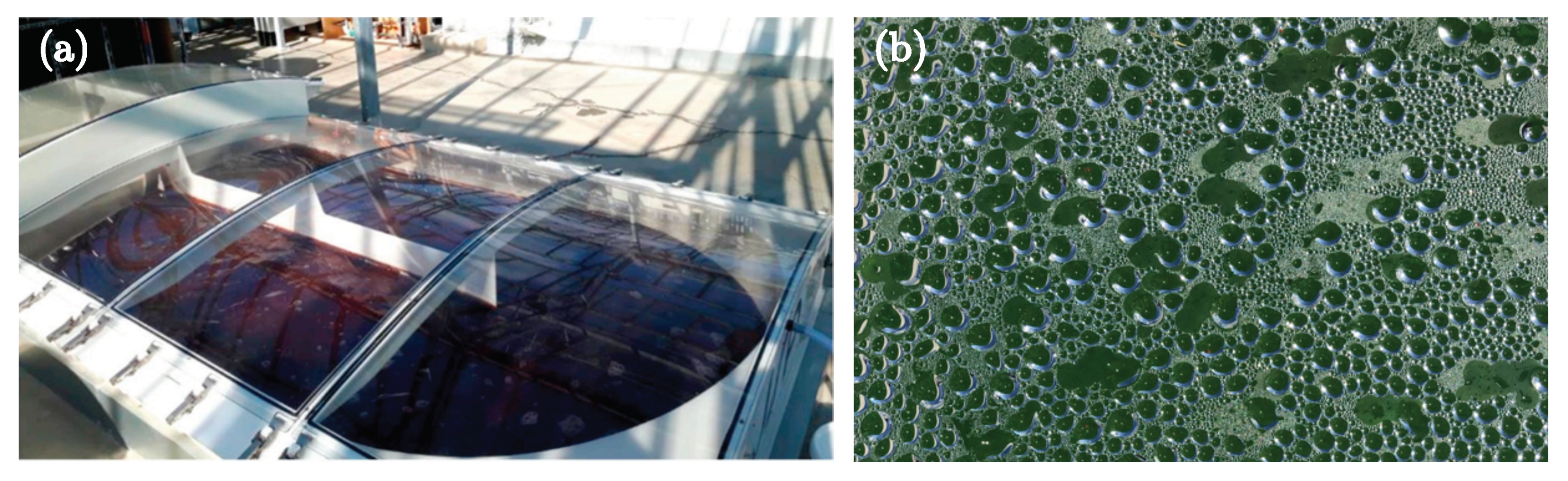
3.1. Problem Statement
3.2. Assumptions
3.3. Light Transfer through Droplet-Covered PBR Windows
3.4. Light Transfer in Microalgae Culture
3.5. Microalgae Growth Kinetics
3.6. Initial and Boundary Conditions
3.7. Method of Solution
4. Results and Discussion
4.1. Light Transfer through Droplet Covered PBR Windows
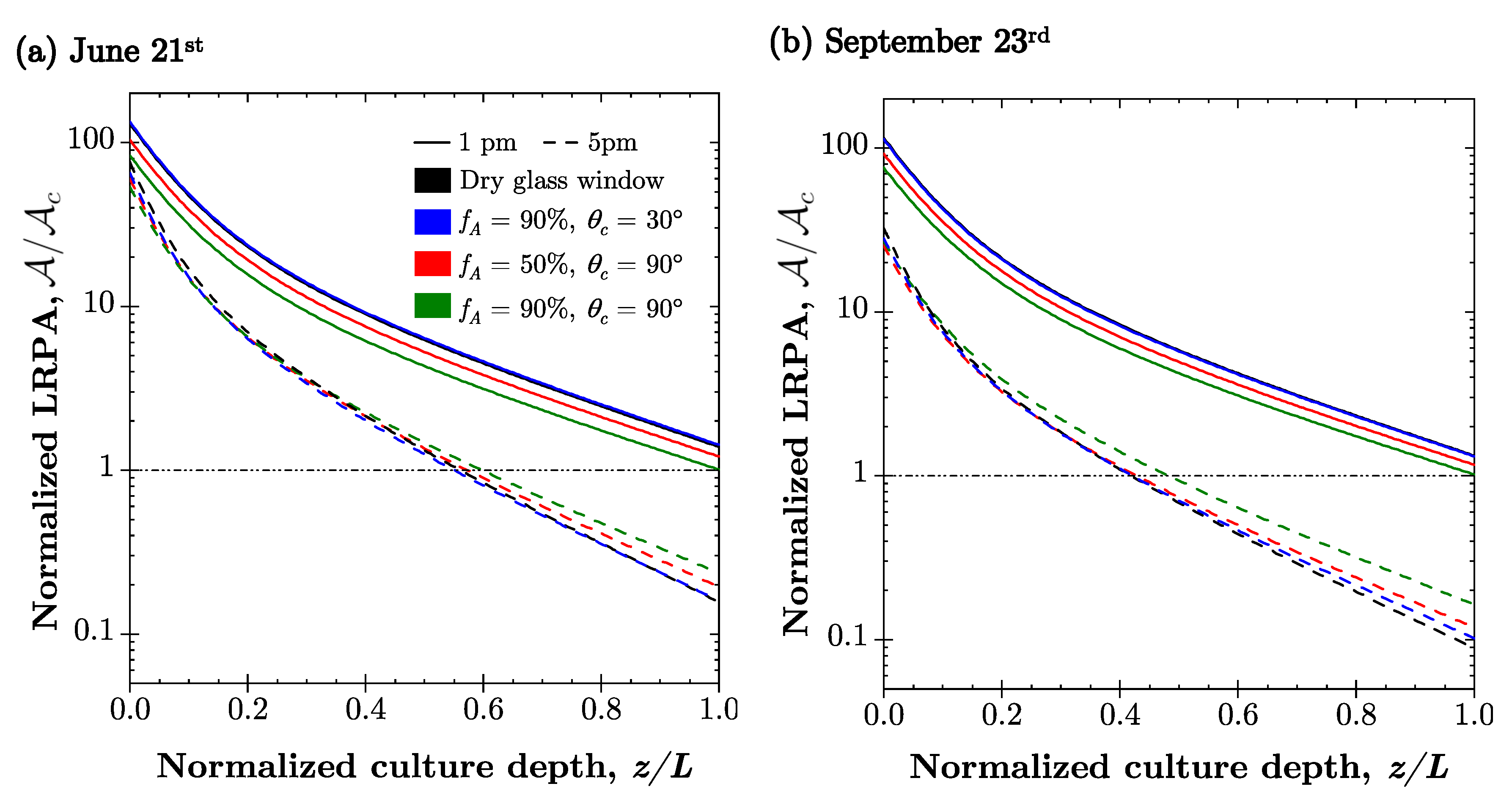
4.2. Light Transfer in Microalgae Culture
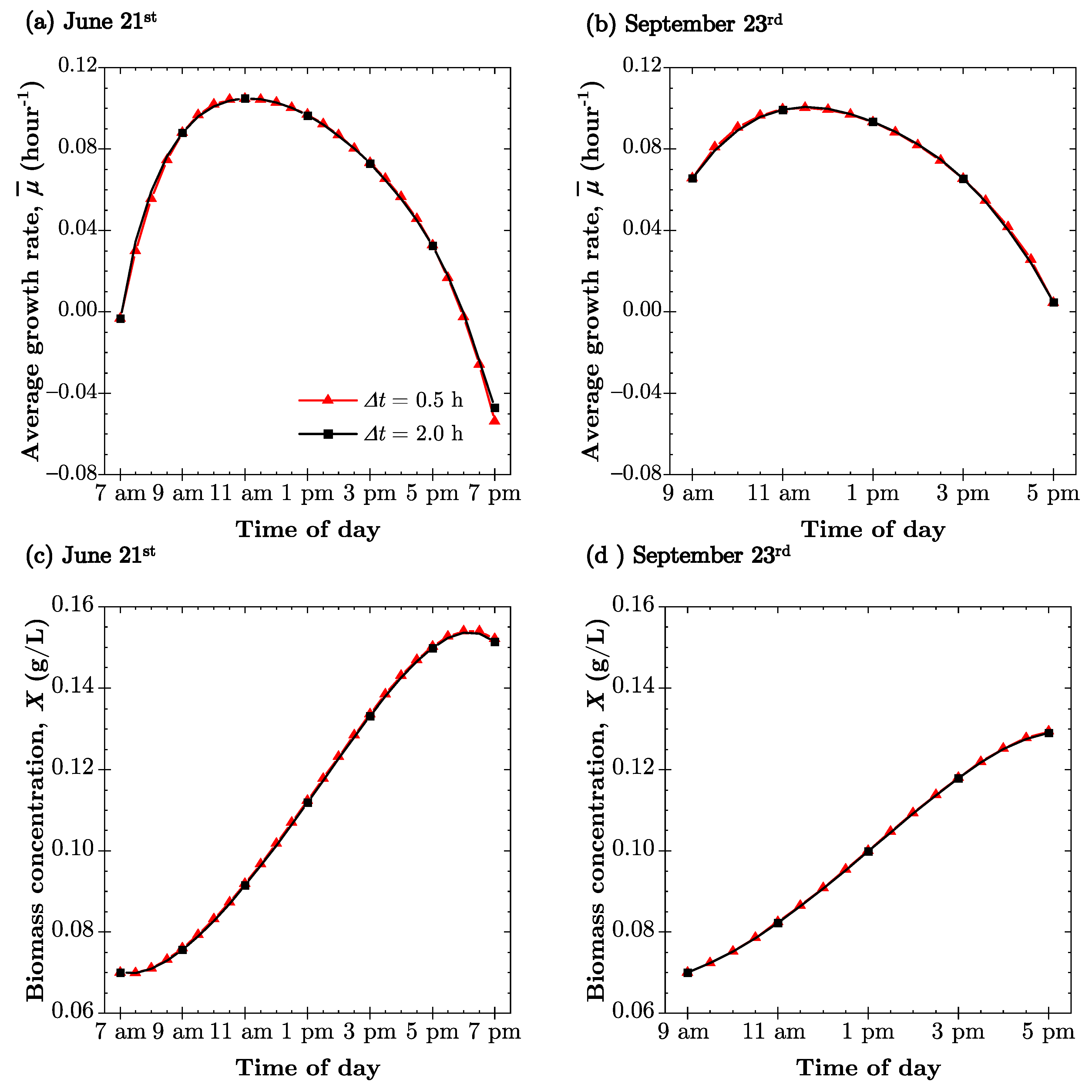
4.3. Microalgae Growth Kinetics
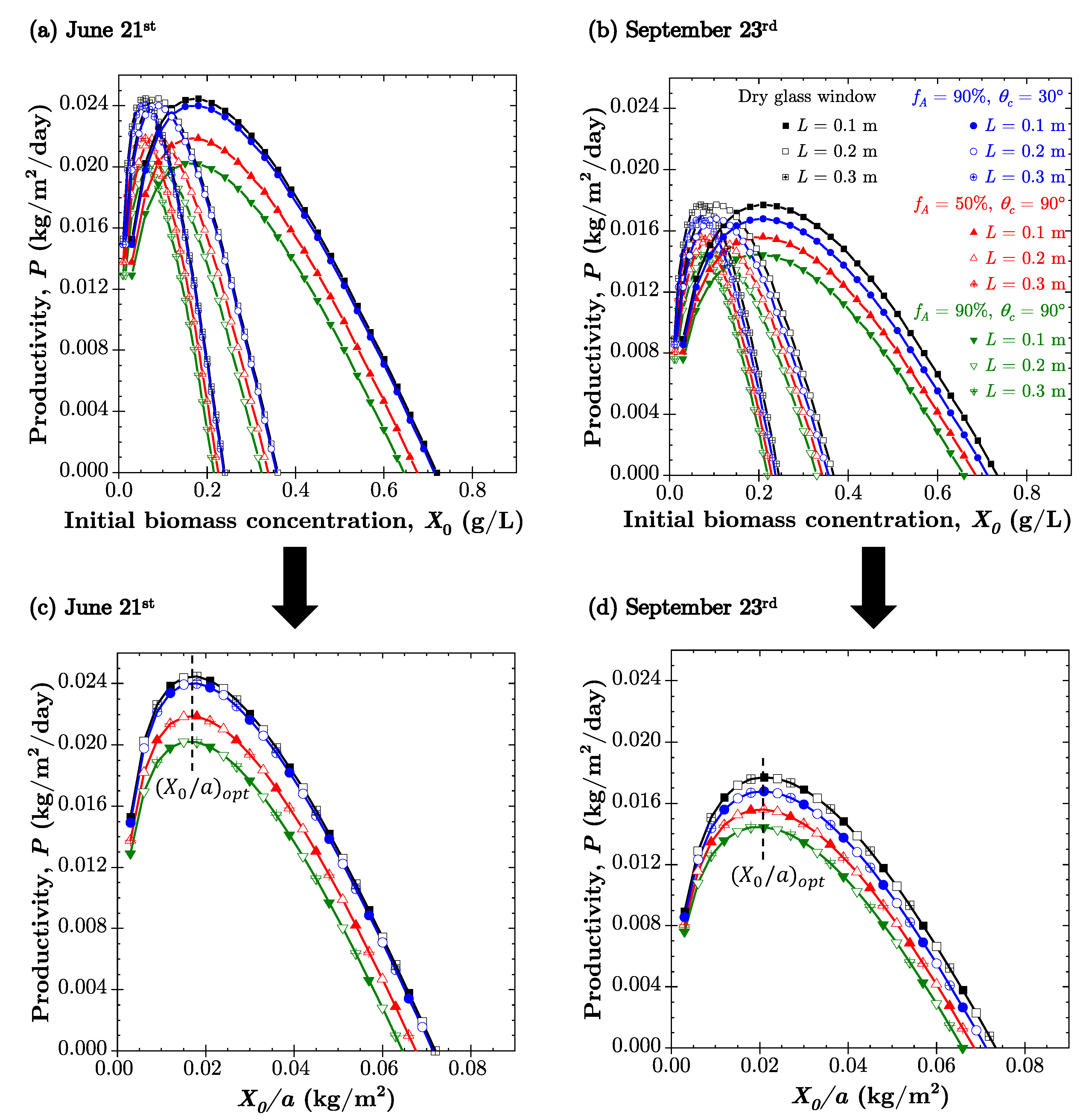
4.4. PBR Biomass Productivity
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| a | specific illuminated area, |
| average spectral mass absorption cross-section, | |
| local specific rate of photon absorption, | |
| specific rate of photon absorption at the compensation point, | |
| spectral back-scattering ratio | |
| d | droplet diameter, |
| droplet projected diameter, | |
| droplet surface area coverage, % | |
| G | fluence rate, |
| H | window thickness, m |
| I | radiative intensity, |
| specific rate of cofactor regeneration, | |
| local specific rate of oxygen production/consumption, in | |
| average specific rate of oxygen production/consumption, | |
| k | absorption index |
| K | half saturation constant for photosynthesis, |
| half saturation constant for respiration, | |
| L | microalgae culture depth, |
| M | interval number of transmission angles |
| carbon molar mass in the biomass, | |
| N | number of rays |
| n | refractive index |
| P | daily PBR biomass productivity, |
| q | radiative flux, |
| S | culture illuminated surface area, m2 |
| average spectral mass scattering cross-section, | |
| t | time, |
| bidirectional transmittance, | |
| one-dimensional bidirectional transmittance, | |
| directional-hemispherical transmittance | |
| normal-hemispherical transmittance | |
| V | culture volume, m3 |
| X | biomass concentration, |
| z | culture depth, |
Greak Symbols
| spectral linear scattering modulus for the two-flux approximation | |
| spectral extinction coefficient m−1 | |
| PBR illuminated fraction | |
| solar azimuth angle, | |
| spectral extinction coefficient for the two flux approximation, | |
| scattering angle, | |
| polar angle, | |
| droplet contact angle, | |
| critical angle for total internal reflection, | |
| average transmission angle, | |
| solar zenith angle, | |
| light wavelength, | |
| average specific growth rate, | |
| stoichiometric coefficient of cofactor regeneration | |
| stoichiometric coefficient of oxygen production | |
| maximum energy yield for photon conversion | |
| transmissivity | |
| scattering phase function | |
| mean mass quantum yield for the Z-scheme, | |
| azimuth angle, | |
| solid angle, |
Subscripts
| 0 | refers to initial conditions |
| a | refers to air |
| d | refers to droplet |
| f | refers to final conditions |
| i | refers to incidence |
| j | index for transmitted polar angle |
| k | index for transmitted azimuthal angle |
| m | refers to microalgae culture |
| s | refers to solar variable |
| t | refers to transmission |
| w | refers to window |
| X | refers to biomass |
| refers to a spectral variable |
Appendix A
| Parameter | Value | Units |
|---|---|---|
| 0.8 | - | |
| 2.8 | ||
| 1.13 | - | |
| 1.1 | ||
| 0.024 | ||
| 2 | - | |
| K | 40,000 | |
| 556.5 | ||
| 2800 |
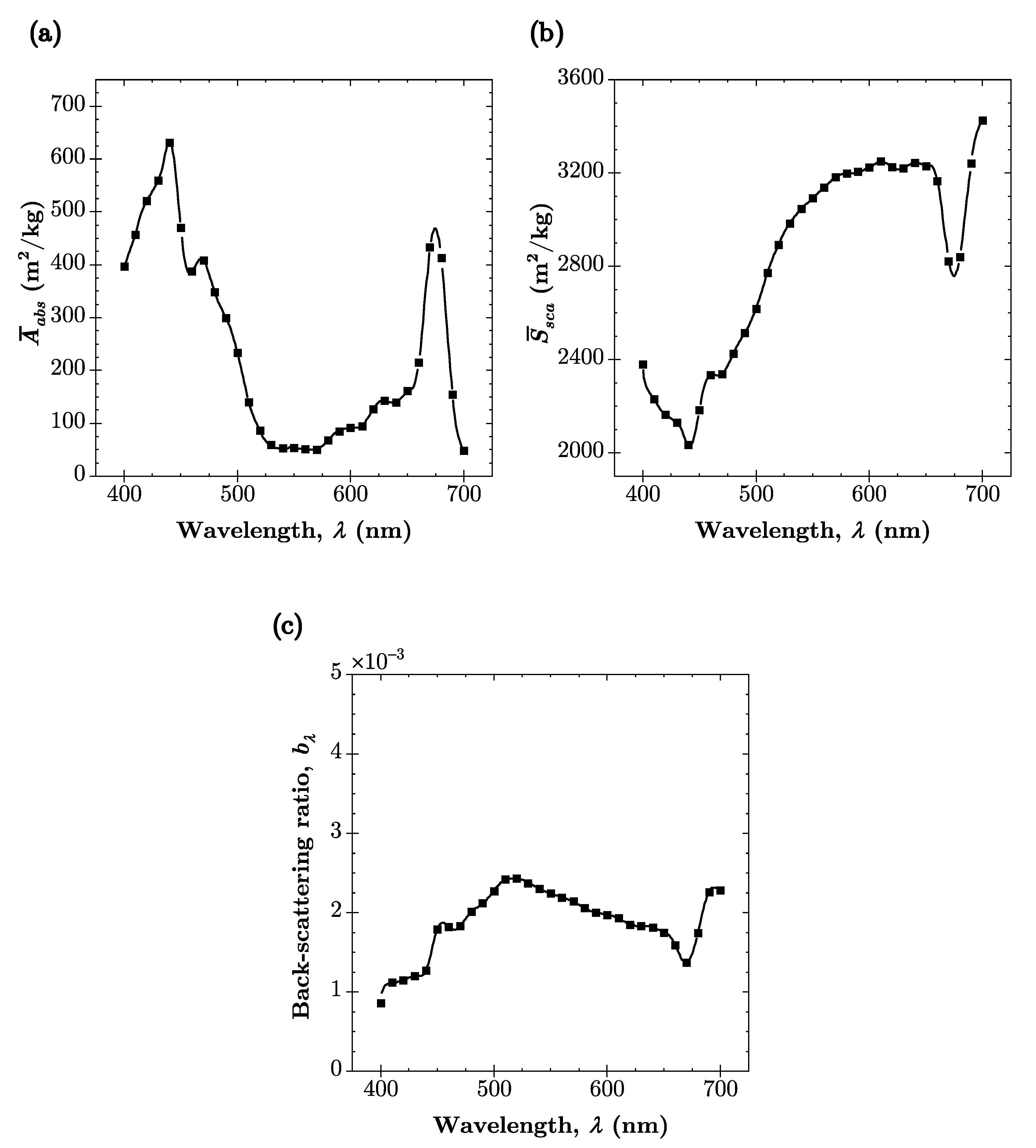
References
- Chisti, Y. Biodiesel from microalgae. Biotechnol. Adv. 2007, 25, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Chew, K.W.; Yap, J.Y.; Show, P.L.; Suan, N.H.; Juan, J.C.; Ling, T.C.; Lee, D.J.; Chang, J.S. Microalgae biorefinery: High value products perspectives. Bioresour. Technol. 2017, 229, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Tredici, M.R. Mass Production of Microalgae: Photobioreactors. In Handbook of Microalgal Culture: Biotechnology and Applied Phycology; Richmond, A., Ed.; Blackwell Publishing Ltd.: Cornwall, UK, 2004; Chapter 9; pp. 178–214. [Google Scholar]
- Pruvost, J.; Cornet, J.-F.; Pilon, L. Large-scale production of algal biomass: Photobioreactors. In Algae Biotechnology: Products and Processes; Bux, F., Chisti, Y., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 41–66. [Google Scholar]
- Pruvost, J.; Borgne, F.; Artu, A.; Legrand, J. Development of a thin-film solar photobioreactor with high biomass volumetric productivity (AlgoFilm©) based on process intensification principles. Algal Res. 2017, 21, 120–137. [Google Scholar] [CrossRef]
- Grobbelaar, J.U. Factors governing algal growth in photobioreactors: The “open” versus “closed” debate. J. Appl. Phycol. 2009, 21, 489–492. [Google Scholar] [CrossRef]
- Huang, Y.; Feng, C.; Hoeniges, J.; Zhu, K.; Pilon, L. Bidirectional transmittance of transparent windows with external or backside condensation of nonabsorbing cap-shaped droplets. J. Quant. Spectrosc. Radiat. Transf. 2020, 251, 107039. [Google Scholar] [CrossRef]
- Zhu, K.; Pilon, L. Transmittance of semitransparent windows with non-absorbing cap-shaped droplets condensed on their backside. J. Quant. Spectrosc. Radiat. Transf. 2017, 194, 98–107. [Google Scholar] [CrossRef]
- Souliès, A.; Legrand, J.; Marec, H.; Pruvost, J.; Castelain, C.; Burghelea, T.; Cornet, J.F. Investigation and modeling of the effects of light spectrum and incident angle on the growth of Chlorella Vulgaris in photobioreactors. Biotechnol. Prog. 2016, 32, 247–261. [Google Scholar] [CrossRef]
- Hsieh, C.K.; Rajvanshi, A.K. The effect of dropwise condensation on glass solar properties. Sol. Energy 1997, 19, 389–393. [Google Scholar] [CrossRef]
- Briscoe, B.J.; Galvin, K.P. The effect of surface fog on the transmittance of light. Sol. Energy 1991, 46, 191–197. [Google Scholar] [CrossRef]
- Zhu, K.; Li, S.; Pilon, L. Light transfer through windows with external condensation. J. Quant. Spectrosc. Radiat. Transf. 2018, 208, 164–171. [Google Scholar] [CrossRef]
- Pollet, I.V.; Pieters, J.G. Condensation and radiation transmittance of greenhouse cladding materials, Part 3: Results for glass plates and plastic films. J. Agric. Eng. Res. 2000, 77, 419–428. [Google Scholar] [CrossRef]
- Pollet, I.V.; Pieters, J.G. Condensation and radiation transmittance of greenhouse cladding materials, Part 2: Results for a complete condensation cycle. J. Agric. Eng. Res. 2000, 75, 65–72. [Google Scholar] [CrossRef]
- Pollet, I.V.; Pieters, J.G. PAR transmittances of dry and condensate covered glass and plastic greenhouse cladding. Agric. For. Meteorol. 2002, 110, 285–298. [Google Scholar] [CrossRef]
- Bhardwaj, R.; Kortenaar, M.V.t.; Mudde, R.F. Influence of condensation surface on solar distillation. Desalination 2013, 326, 37–45. [Google Scholar] [CrossRef]
- Simsek, E.; Zhu, K.; Kashanchi, G.N.; Williams, M.J.; Galy, T.; Marszewski, M.; Tolbert, S.H.; Pilon, L. Light transfer through semi-transparent glass panes supporting pendant droplets. J. Quant. Spectrosc. Radiat. Transf. 2020. under review. [Google Scholar] [CrossRef]
- Zhu, K.; Pilon, L. Transmittance of semitransparent windows with absorbing cap-shaped droplets condensed on their backside. J. Quant. Spectrosc. Radiat. Transf. 2017, 201, 53–63. [Google Scholar] [CrossRef]
- Rubin, M. Optical properties of soda lime silica glasses. Sol. Energy Mater. 1985, 12, 275–288. [Google Scholar] [CrossRef]
- Hale, G.M.; Querry, M.R. Optical constants of water in the 200-nm to 200-μm wavelength region. Appl. Opt. 1973, 12, 555–563. [Google Scholar] [CrossRef]
- Pottier, L.; Pruvost, J.; Deremetz, J.; Cornet, J.-F.; Legrand, J.; Dussap, C.-G. A fully predictive model for one-dimensional light attenuation by Chlamydomonas Reinhardtii A Torus Photobioreactor. Biotechnol. Bioeng. 2005, 91, 569–582. [Google Scholar] [CrossRef]
- Lee, E.; Pruvost, J.; He, X.; Munipalli, R.; Pilon, L. Design tool and guidelines for outdoor photobioreactors. Chem. Eng. Sci. 2014, 106, 18–29. [Google Scholar] [CrossRef]
- Cornet, J.-F.; Dussap, C.G.; Dubertret, G. A structured model for simulation of cultures of the cyanobacterium Spirulina Platensis Photobioreactors: I. Coupling between Light Transf. Growth Kinet. Biotechnol. Bioeng. 1992, 40, 817–825. [Google Scholar] [CrossRef] [PubMed]
- Cornet, J.-F.; Dussap, C.G.; Gros, J.B.; Binois, C.; Lasseur, C. A simplified monodimensional approach for modeling coupling between radiant light transfer and growth kinetics in photobioreactors. Chem. Eng. Sci. 1995, 50, 1489–1500. [Google Scholar] [CrossRef]
- Pruvost, J.; Cornet, J.-F.; Goetz, V.; Legrand, J. Theoretical investigation of biomass productivities achievable in solar rectangular photobioreactors for the cyanobacterium Arthrospira platensis. Biotechnol. Prog. 2012, 28, 699–714. [Google Scholar] [CrossRef] [PubMed]
- Dunn, I.; Heinzle, E.; Ingham, J.; Prenosil, J. Biological Reaction Engineering: Dynamic Modelling Fundamentals with Simulation Examples, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2003. [Google Scholar]
- Takache, H.; Pruvost, J.; Cornet, J.-F. Kinetic modeling of the photosynthetic growth of Chlamydomonas Reinhardtii A Photobioreactor. Biotechnol. Prog. 2012, 28, 681–692. [Google Scholar] [CrossRef] [PubMed]
- Versyck, K.J.; Claes, J.E.; van Impe, J.-F. Practical identification of unstructured growth kinetics by application of optimal experimental design. Biotechnol. Prog. 1997, 13, 524–531. [Google Scholar] [CrossRef]
- Fouchard, S.; Pruvost, J.; Degrenne, B.; Titica, M.; Legrand, J. Kinetic modeling of light limitation and sulfur deprivation effects in the induction of hydrogen production with Chlamydomonas reinhardtii: Part I. Model Dev. Parameter Identification. Biotechnol. Bioeng. 2009, 102, 232–245. [Google Scholar] [CrossRef]
- Cornet, J.-F.; Dussap, C.-G. A simple and reliable formula for assessment of maximum volumetric productivities in photobioreactors. Biotechnol. Prog. 2009, 25, 424–435. [Google Scholar] [CrossRef]
- Pruvost, J.; Vooren, G.; Gouic, B.; Couzinet-Mossion, A.; Legrand, J. Systematic investigation of biomass and lipid productivity by microalgae in photobioreactors for biodiesel application. Bioresour. Technol. 2011, 2, 150–158. [Google Scholar] [CrossRef]
- Slegers, P.M.; Wijffels, R.H.; van Straten, G.; van Boxtel, A.J.B. Design scenarios for flat panel photobioreactors. Appl. Energy 2011, 88, 3342–3353. [Google Scholar] [CrossRef]
- Modest, M.F. Radiative Heat Transfer, 3rd ed.; Elsevier Inc.: Oxford, UK, 2013. [Google Scholar]
- Howell, J.R.; Mengüç, M.P.; Siegel, R. Thermal Radiation Heat Transfer, 6th ed.; CRC Press: Taylor and Francis: Boca Raton, FL, USA, 2016. [Google Scholar]
- Kandilian, R.; Pruvost, J.; Artu, A.; Lemasson, C.; Legrand, J.; Pilon, L. Comparison of experimentally and theoretically determined radiation characteristics of photosynthetic microorganisms. J. Quant. Spectrosc. Radiat. Transf. 2016, 175, 30–45. [Google Scholar] [CrossRef]
- Gueymard, C. Simple Model of the Atmospheric Radiative Transfer of Sunshine (SMARTS). Version 2.9.5. 2005. Available online: https://www.nrel.gov/grid/solar-resource/smarts.html (accessed on 30 December 2020).
- Figgis, B.; Nouviaire, A.; Wubulikasimu, Y.; Javed, W.; Guo, B.; Ait-Mokhtar, A.; Belarbi, R.; Ahzi, S.; Rémond, Y.; Ennaoui, A. Investigation of factors affecting condensation on soiled PV modules. Sol. Energy 2018, 159, 488–500. [Google Scholar] [CrossRef]

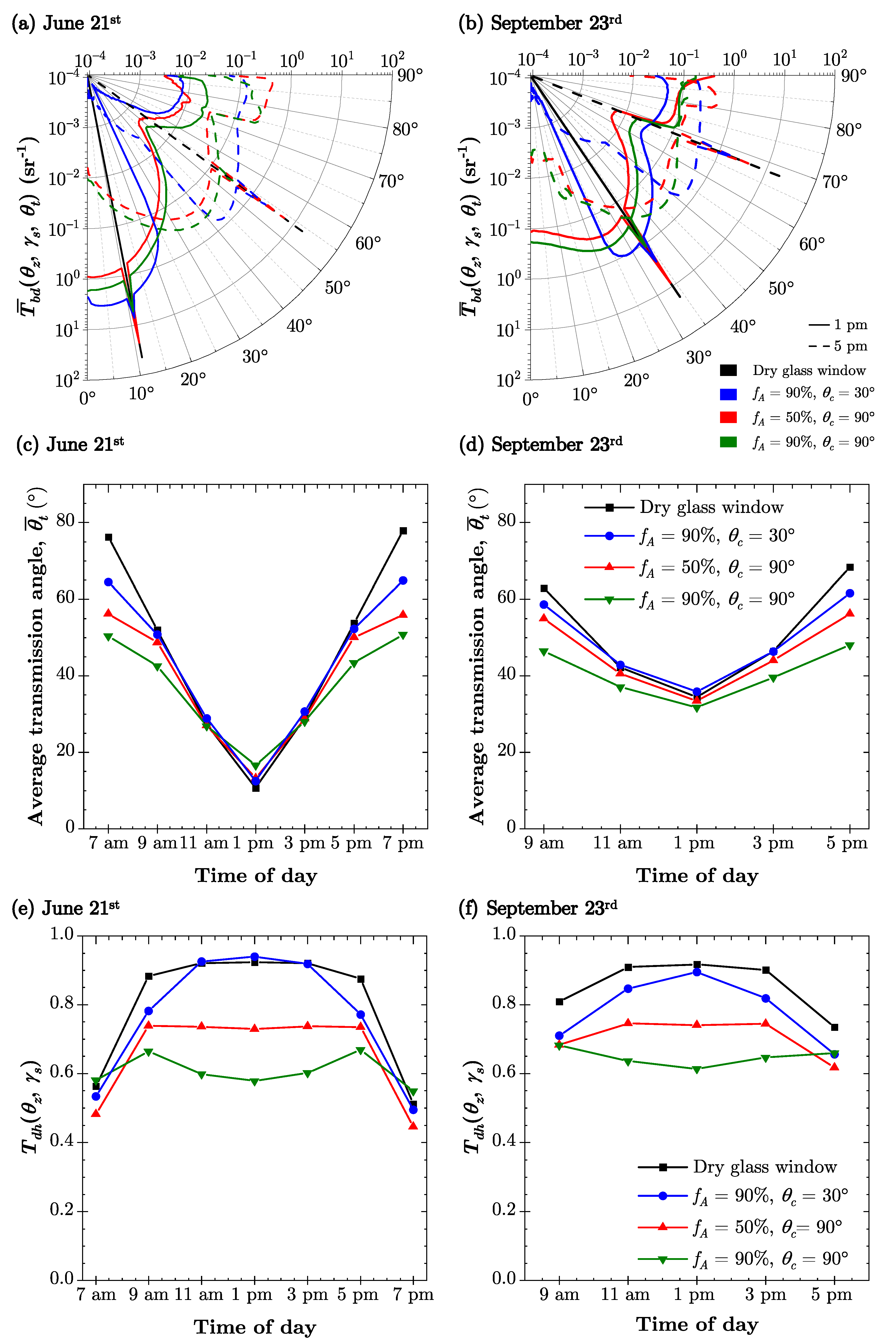

| Time | 21 June | 23 September | ||
|---|---|---|---|---|
| 7 a.m. | 76.2 | −109.2 | - | - |
| 9 a.m. | 52.0 | −94.7 | 62.9 | −69.6 |
| 11 a.m. | 27.3 | −74.8 | 42.3 | −41.6 |
| 1 p.m. | 10.7 | 5.5 | 34.4 | 6.2 |
| 3 p.m. | 29.1 | 76.9 | 46.5 | 49.5 |
| 5 p.m. | 53.8 | 95.8 | 68.4 | 74.2 |
| 7 p.m. | 77.9 | 110.3 | - | - |
| 21 June | ||||
|---|---|---|---|---|
| Change | ||||
| 0% | N/A | 0.018 | 0.0245 | 0% |
| 90% | 30 | 0.018 | 0.0240 | −2.0% |
| 50% | 90 | 0.018 | 0.0219 | −10.6% |
| 90% | 90 | 0.018 | 0.0202 | −17.5% |
| 23 September | ||||
| Change | ||||
| 0% | N/A | 0.021 | 0.0177 | 0% |
| 90% | 30 | 0.021 | 0.0168 | −5.1% |
| 50% | 90 | 0.021 | 0.0156 | −11.9% |
| 90% | 90 | 0.021 | 0.0145 | −18.1% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoeniges, J.; Zhu, K.; Pruvost, J.; Legrand, J.; Si-Ahmed, E.-k.; Pilon, L. Impact of Dropwise Condensation on the Biomass Production Rate in Covered Raceway Ponds. Energies 2021, 14, 268. https://doi.org/10.3390/en14020268
Hoeniges J, Zhu K, Pruvost J, Legrand J, Si-Ahmed E-k, Pilon L. Impact of Dropwise Condensation on the Biomass Production Rate in Covered Raceway Ponds. Energies. 2021; 14(2):268. https://doi.org/10.3390/en14020268
Chicago/Turabian StyleHoeniges, Jack, Keyong Zhu, Jeremy Pruvost, Jack Legrand, El-khider Si-Ahmed, and Laurent Pilon. 2021. "Impact of Dropwise Condensation on the Biomass Production Rate in Covered Raceway Ponds" Energies 14, no. 2: 268. https://doi.org/10.3390/en14020268
APA StyleHoeniges, J., Zhu, K., Pruvost, J., Legrand, J., Si-Ahmed, E.-k., & Pilon, L. (2021). Impact of Dropwise Condensation on the Biomass Production Rate in Covered Raceway Ponds. Energies, 14(2), 268. https://doi.org/10.3390/en14020268







