Treatment of Light-Induced Degradation for Solar Cells in a p-PERC Solar Module via Induction Heating
Abstract
:1. Introduction
2. Materials and Methods
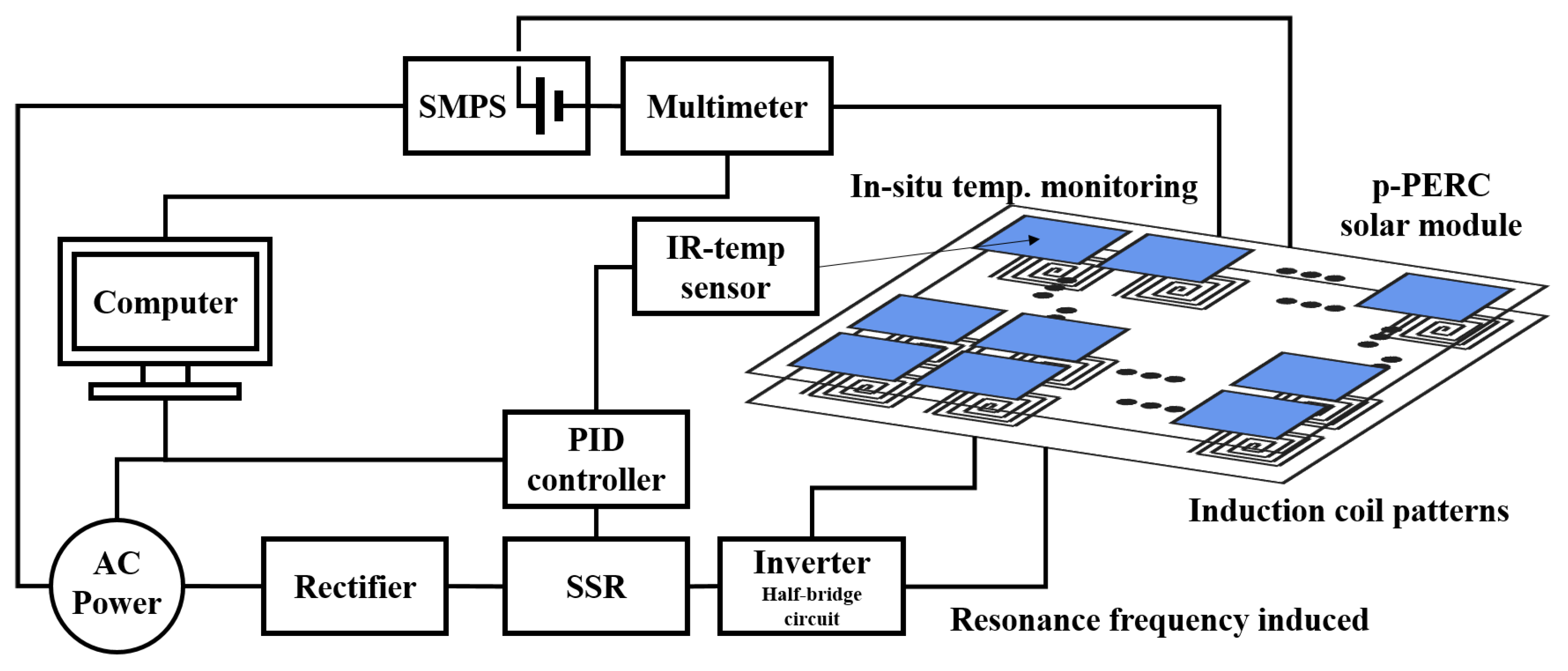
2.1. Treatment of LID Apparatus and Half-Bridge Resonance Circuit
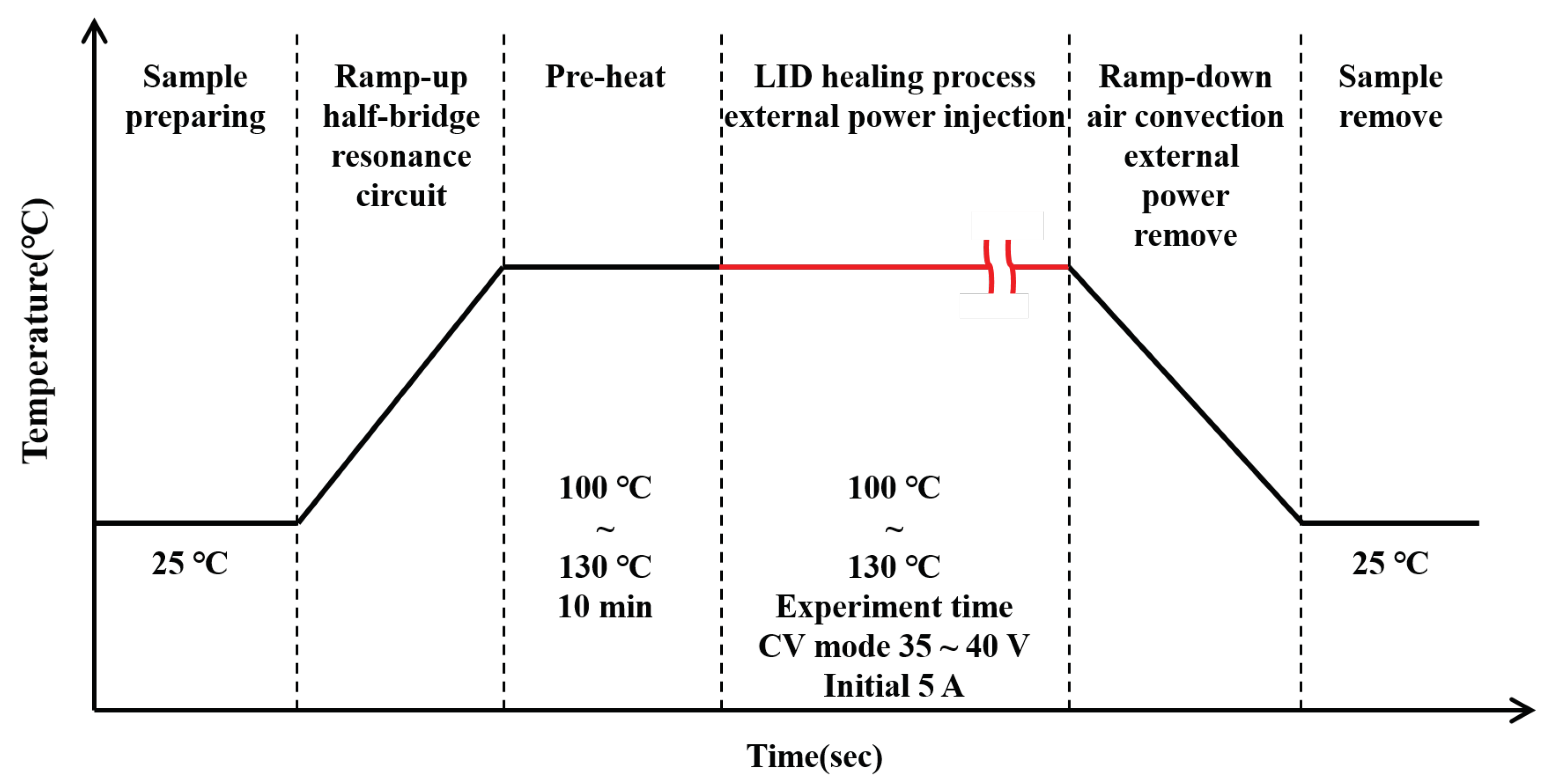
2.2. Solar Cell Module & LID Healing Process
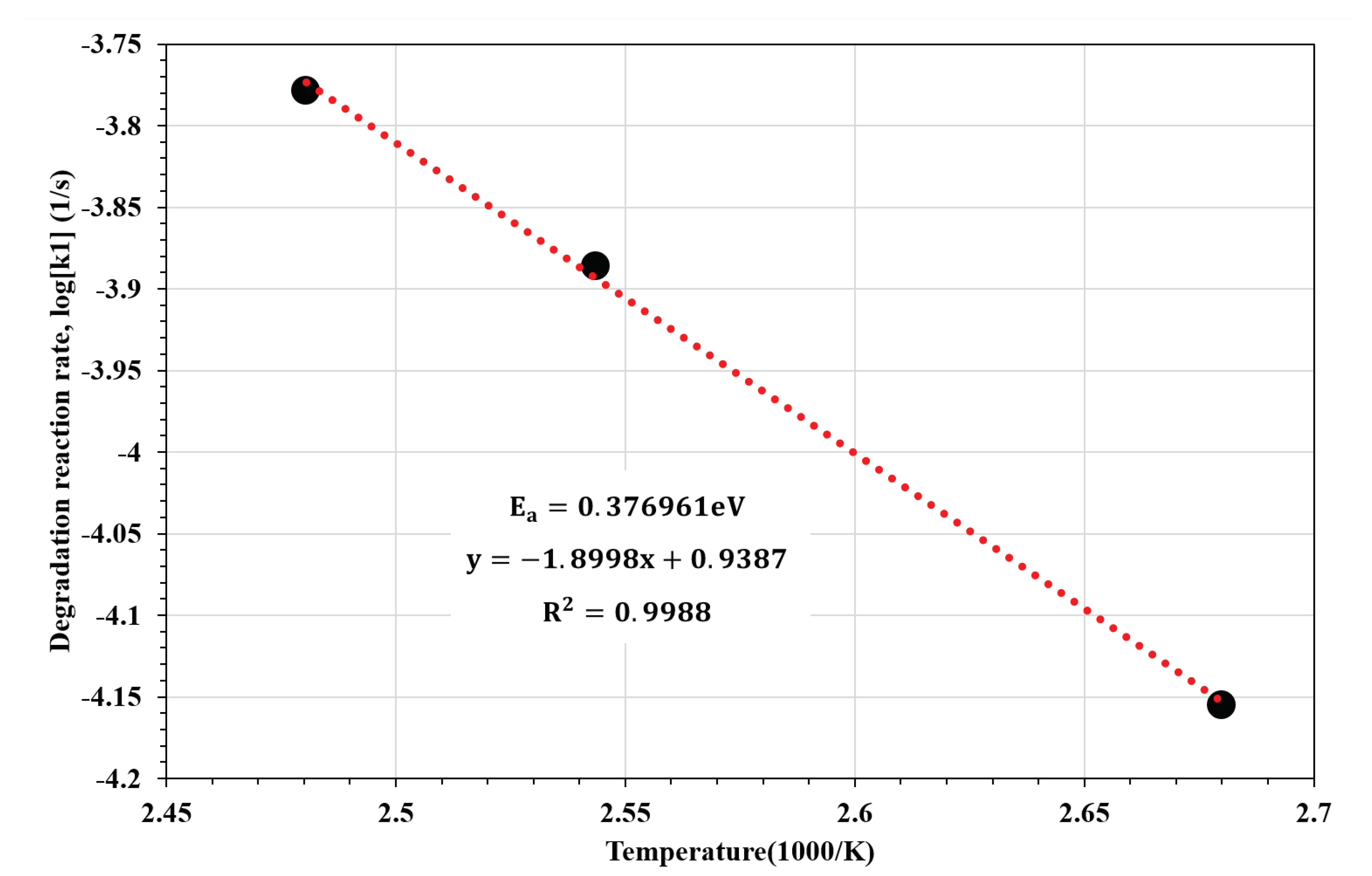
2.3. Kinetic Simulation Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| PERC | Passivated Emitter and Rear Contact |
| BSF | Back Surface Field |
| LID | Light-induced degradation |
| LCOE | Levelized Cost of Electricity |
| Cz | Czochralski |
| SSR | Solid-State Relay |
| PID | Proportional Integral Derivative |
| SMPS | Switching Mode Power Supply |
| EVA | Ethylene-Vinyl Acetate |
References
- Equipment, V.P. International Technology Roadmap for Photovoltaic (ITRPV). 2021. Available online: https://itrpv.vdma.org/en/ueber-uns (accessed on 21 July 2021).
- Kersten, F.; Doll, R.; Kux, A.; Huljic, D.M.; Gorig, M.A.; Breyer, C.; Müller, J.W.; Wawer, P. PV Learning Curves: Past and Future Drivers of Cost Reduction. 2011. Available online: https://www.eupvsec-proceedings.com/proceedings?paper=12834 (accessed on 21 July 2021).
- Green, M.; Dunlop, E.; Hohl-Ebinger, J.; Yoshita, M.; Kopidakis, N.; Hao, X. Solar cell efficiency tables (version 57). Prog. Photovolt. Res. Appl. 2021, 29, 3–15. [Google Scholar] [CrossRef]
- Du, M.H.; Branz, H.M.; Crandall, R.S.; Zhang, S.B. A New Mechanism for Non-Radiative Recombination at Light-Induced Boron-Oxygen Complexes in Silicon. In Proceedings of the 2005 DOE Solar Energy Technologies, Denver, CO, USA, 7–10 November 2005. [Google Scholar]
- Fischer, H. Investigation of photon and thermal changes in silicon solar cells. In Proceedings of the Conference Record of the 10th IEEE Photovoltaic Specialists Conference, Palo Alto, CA, USA, 13–15 November 1973. [Google Scholar]
- Knobloch, J.; Glunz, S.; Henninger, V.; Warta, W.; Wettling, W.; Schomann, F.; Schmidt, W.; Endrös, A.; Münzer, K. 21% efficient solar cells processed from Czochralski grown silicon. In Proceedings of the 13th European Photovoltaic Solar Energy Conference, WIP Renewable Energies, Nice, France, 23–27 October 1995; pp. 9–12. [Google Scholar]
- Sterk, S.; Münzer, K.; Glunz, S. Investigation of the degradation of crystalline silicon solar cells. In Proceedings of the 14th European Photovoltaic Solar Energy Conference, Barcelona, Spain, 30 June–4 July 1997; Volume 85. [Google Scholar]
- Zhao, J.; Wang, A.; Green, M.A.; Ferrazza, F. 19.8% efficient “honeycomb” textured multicrystalline and 24.4% monocrystalline silicon solar cells. Appl. Phys. Lett. 1998, 73, 1991–1993. [Google Scholar] [CrossRef]
- Herguth, A.; Schubert, G.; Kaes, M.; Hahn, G. A new approach to prevent the negative impact of the metastable defect in boron doped Cz silicon solar cells. In Proceedings of the 2006 IEEE 4th World Conference on Photovoltaic Energy Conference, Waikoloa, HI, USA, 7–12 May 2006; Volume 1, pp. 940–943. [Google Scholar]
- Herguth, A.; Schubert, G.; Kaes, M.; Hahn, G. Avoiding boron-oxygen related degradation in highly boron doped Cz silicon. In Proceedings of the 21st European Photovoltaic Solar Energy Conference: 21th EC PVSEC, Dresden, Germany, 4–8 September 2006; pp. 530–537. [Google Scholar]
- Fertig, F.; Greulich, J.; Broisch, J.; Biro, D.; Rein, S. Stability of the regeneration of the boron–oxygen complex in silicon solar cells during module integration. Sol. Energy Mater. Sol. Cells 2013, 115, 189–198. [Google Scholar] [CrossRef]
- Fertig, F.; Broisch, J.; Biro, D.; Rein, S. Stability of the regeneration of the boron–oxygen complex in silicon solar cells during module certification. Sol. Energy Mater. Sol. Cells 2014, 121, 157–162. [Google Scholar] [CrossRef]
- Ramspeck, K.; Zimmermann, S.; Nagel, H.; Metz, A.; Gassenbauer, Y.; Birkmann, B.; Seidl, A. Light Induced Degradation of Rear Passivated mc-Si Solar Cells. 2012. Available online: https://www.eupvsec-proceedings.com/proceedings?paper=18798 (accessed on 21 July 2021).
- Kersten, F.; Engelhart, P.; Ploigt, H.C.; Stekolnikov, A.; Lindner, T.; Stenzel, F.; Bartzsch, M.; Szpeth, A.; Petter, K.; Heitmann, J.; et al. A new mc-Si degradation effect called LeTID. In Proceedings of the 2015 IEEE 42nd Photovoltaic Specialist Conference (PVSC), New Orleans, LA, USA, 14–19 June 2015; pp. 1–5. [Google Scholar]
- Chen, D.; Hamer, P.G.; Kim, M.; Fung, T.H.; Bourret-Sicotte, G.; Liu, S.; Chan, C.E.; Ciesla, A.; Chen, R.; Abbott, M.D.; et al. Hydrogen induced degradation: A possible mechanism for light-and elevated temperature-induced degradation in n-type silicon. Sol. Energy Mater. Sol. Cells 2018, 185, 174–182. [Google Scholar] [CrossRef]
- Petter, K.; Hubener, K.; Kersten, F.; Bartzsch, M.; Fertig, F.; Kloter, B.; Muller, J. Dependence of LeTID on brick height for different wafer suppliers with several resistivities and dopants. Int. Work. Cryst. Silicon Sol. Cells 2016, 6, 1–17. [Google Scholar]
- Bredemeier, D.; Walter, D.C.; Heller, R.; Schmidt, J. Impact of hydrogen-rich silicon nitride material properties on light-induced lifetime degradation in multicrystalline silicon. Phys. Status Solidi (RRL)–Rapid Res. Lett. 2019, 13, 1900201. [Google Scholar] [CrossRef]
- Kersten, F.; Engelhart, P.; Ploigt, H.C.; Stekolnikov, A.; Lindner, T.; Stenzel, F.; Bartzsch, M.; Szpeth, A.; Petter, K.; Heitmann, J.; et al. Degradation of multicrystalline silicon solar cells and modules after illumination at elevated temperature. Sol. Energy Mater. Sol. Cells 2015, 142, 83–86. [Google Scholar] [CrossRef]
- Chan, C.E.; Payne, D.N.; Hallam, B.J.; Abbott, M.D.; Fung, T.H.; Wenham, A.M.; Tjahjono, B.S.; Wenham, S.R. Rapid stabilization of high-performance multicrystalline p-type silicon PERC cells. IEEE J. Photovolt. 2016, 6, 1473–1479. [Google Scholar] [CrossRef]
- Chen, D.; Vaqueiro Contreras, M.; Ciesla, A.; Hamer, P.; Hallam, B.; Abbott, M.; Chan, C. Progress in the understanding of light-and elevated temperature-induced degradation in silicon solar cells: A review. Prog. Photovolt. Res. Appl. 2020. [Google Scholar] [CrossRef]
- Ciesla, A.; Kim, M.; Wright, M.; Zafirovska, I.; Chen, D.; Hallam, B.; Chan, C. A case study on accelerated light-and elevated temperature-induced degradation testing of commercial multi-crystalline silicon passivated emitter and rear cell modules. Prog. Photovolt. Res. Appl. 2021. [Google Scholar] [CrossRef]
- Kersten, F.; Fertig, F.; Petter, K.; Klöter, B.; Herzog, E.; Strobel, M.B.; Heitmann, J.; Müller, J.W. System performance loss due to LeTID. Energy Procedia 2017, 124, 540–546. [Google Scholar] [CrossRef]
- Grant, N.E.; Scowcroft, J.R.; Pointon, A.I.; Al-Amin, M.; Altermatt, P.P.; Murphy, J.D. Lifetime instabilities in gallium doped monocrystalline PERC silicon solar cells. Sol. Energy Mater. Sol. Cells 2020, 206, 110299. [Google Scholar] [CrossRef]
- Grant, N.; Rougieux, F.; Macdonald, D.; Bullock, J.; Wan, Y. Grown-in defects limiting the bulk lifetime of p-type float-zone silicon wafers. J. Appl. Phys. 2015, 117, 055711. [Google Scholar] [CrossRef]
- Sperber, D.; Herguth, A.; Hahn, G. A 3-state defect model for light-induced degradation in boron-doped float-zone silicon. Phys. Status Solidi (RRL)–Rapid Res. Lett. 2017, 11, 1600408. [Google Scholar] [CrossRef] [Green Version]
- Niewelt, T.; Selinger, M.; Grant, N.; Kwapil, W.; Murphy, J.; Schubert, M. Light-induced activation and deactivation of bulk defects in boron-doped float-zone silicon. J. Appl. Phys. 2017, 121, 185702. [Google Scholar] [CrossRef]
- Payne, D.; Chan, C.; Hallam, B.; Hoex, B.; Abbott, M.; Wenham, S.; Bagnall, D. Acceleration and mitigation of carrier-induced degradation in p-type multi-crystalline silicon. Phys. Status Solidi (RRL)–Rapid Res. Lett. 2016, 10, 237–241. [Google Scholar] [CrossRef]
- Chen, D.; Kim, M.; Stefani, B.V.; Hallam, B.J.; Abbott, M.D.; Chan, C.E.; Chen, R.; Payne, D.N.; Nampalli, N.; Ciesla, A.; et al. Evidence of an identical firing-activated carrier-induced defect in monocrystalline and multicrystalline silicon. Sol. Energy Mater. Sol. Cells 2017, 172, 293–300. [Google Scholar] [CrossRef]
- Chan, C.; Fung, T.H.; Abbott, M.; Payne, D.; Wenham, A.; Hallam, B.; Chen, R.; Wenham, S. Modulation of carrier-induced defect kinetics in multi-crystalline silicon PERC cells through dark annealing. Sol. Rrl 2017, 1, 1600028. [Google Scholar] [CrossRef]
- Kim, S.M.; Jung, S.; Kim, Y.; Kim, J. Anti-LID Process with a Remote Direct Heating Method Using a Half-Bridge Resonance Circuit for a PERC Solar Cell Module. Energies 2020, 13, 110. [Google Scholar] [CrossRef] [Green Version]
- Edwards, T.C.; Steer, M.B. Foundations for Microstrip Circuit Design; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Connors, K.A. Chemical Kinetics; VCH Publishers, Inc.: New York, NJ, USA, 2014. [Google Scholar]
- Espenson, J.H. Chemical Kinetics and Reaction Mechanisms, 2nd ed.; McGraw-Hill, Inc.: New York, NY, USA, 2014. [Google Scholar]
- Kim, S.M.; Chun, S.; Bae, S.; Park, S.; Kang, M.G.; Song, H.E.; Kang, Y.; Lee, H.S.; Kim, D. Light-induced degradation and metastable-state recovery with reaction kinetics modeling in boron-doped Czochralski silicon solar cells. Appl. Phys. Lett. 2014, 105, 083509. [Google Scholar] [CrossRef]
- Bothe, K.; Hezel, R.; Schmidt, J. Understanding and reducing the boron-oxygen-related performance degradation in Czochralski silicon solar cells. In Solid State Phenomena; Trans Tech Publications: Stafa-Zurich, Switzerland, 2004; Volume 95, pp. 223–228. [Google Scholar]
- Dubois, S.; Enjalbert, N.; Garandet, J. Slow down of the light-induced-degradation in compensated solar-grade multicrystalline silicon. Appl. Phys. Lett. 2008, 93, 103510. [Google Scholar] [CrossRef]
- Schmidt, J.; Bothe, K.; Hezel, R. Formation and annihilation of the metastable defect in boron-doped Czochralski silicon. In Proceedings of the Conference Record of the Twenty-Ninth IEEE Photovoltaic Specialists Conference, New Orleans, LA, USA, 19–24 May 2002; pp. 178–181. [Google Scholar]
- Glunz, S.; Schaffer, E.; Rein, S.; Bothe, K.; Schmidt, J. Analysis of the defect activation in Cz-silicon by temperature-dependent bias-induced degradation of solar cells. In Proceedings of the 3rd World Conference onPhotovoltaic Energy Conversion, Osaka, Japan, 11–18 May 2003; Volume 1, pp. 919–922. [Google Scholar]
- Palmer, D.W.; Bothe, K.; Schmidt, J. Kinetics of the electronically stimulated formation of a boron-oxygen complex in crystalline silicon. Phys. Rev. B 2007, 76, 035210. [Google Scholar] [CrossRef] [Green Version]
- Bothe, K.; Schmidt, J. Electronically activated boron-oxygen-related recombination centers in crystalline silicon. J. Appl. Phys. 2006, 99, 013701. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seok, M.-g.; Kim, J.; Lee, Y.; Kim, Y.; Kim, Y.; Kim, S.M. Treatment of Light-Induced Degradation for Solar Cells in a p-PERC Solar Module via Induction Heating. Energies 2021, 14, 6352. https://doi.org/10.3390/en14196352
Seok M-g, Kim J, Lee Y, Kim Y, Kim Y, Kim SM. Treatment of Light-Induced Degradation for Solar Cells in a p-PERC Solar Module via Induction Heating. Energies. 2021; 14(19):6352. https://doi.org/10.3390/en14196352
Chicago/Turabian StyleSeok, Min-gwang, Junhee Kim, Yonghwan Lee, Yoonkap Kim, Yangdo Kim, and Soo Min Kim. 2021. "Treatment of Light-Induced Degradation for Solar Cells in a p-PERC Solar Module via Induction Heating" Energies 14, no. 19: 6352. https://doi.org/10.3390/en14196352
APA StyleSeok, M.-g., Kim, J., Lee, Y., Kim, Y., Kim, Y., & Kim, S. M. (2021). Treatment of Light-Induced Degradation for Solar Cells in a p-PERC Solar Module via Induction Heating. Energies, 14(19), 6352. https://doi.org/10.3390/en14196352






