Experimental Characterization of an Alkaline Electrolyser and a Compression System for Hydrogen Production and Storage
Abstract
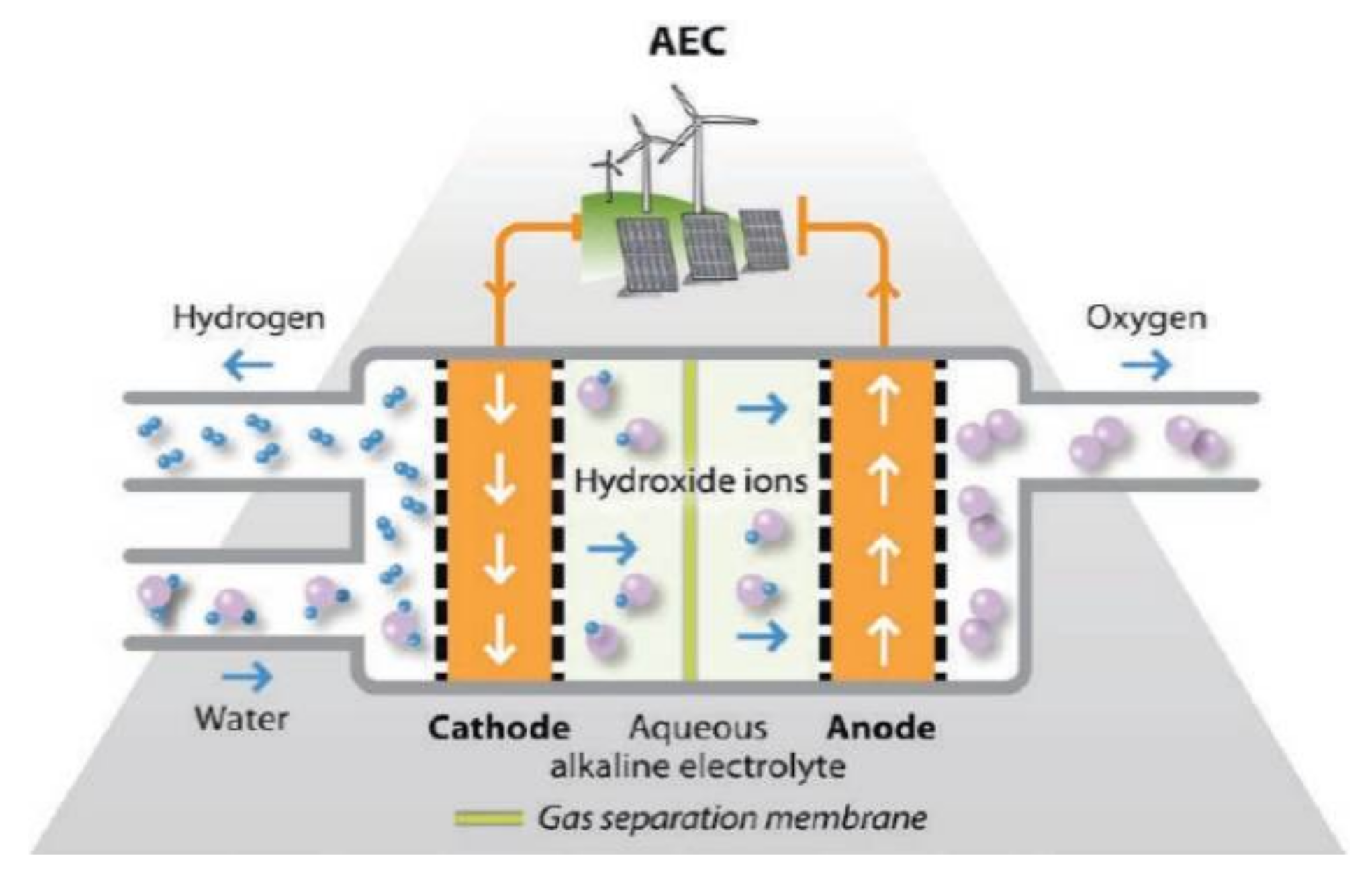
1. Introduction
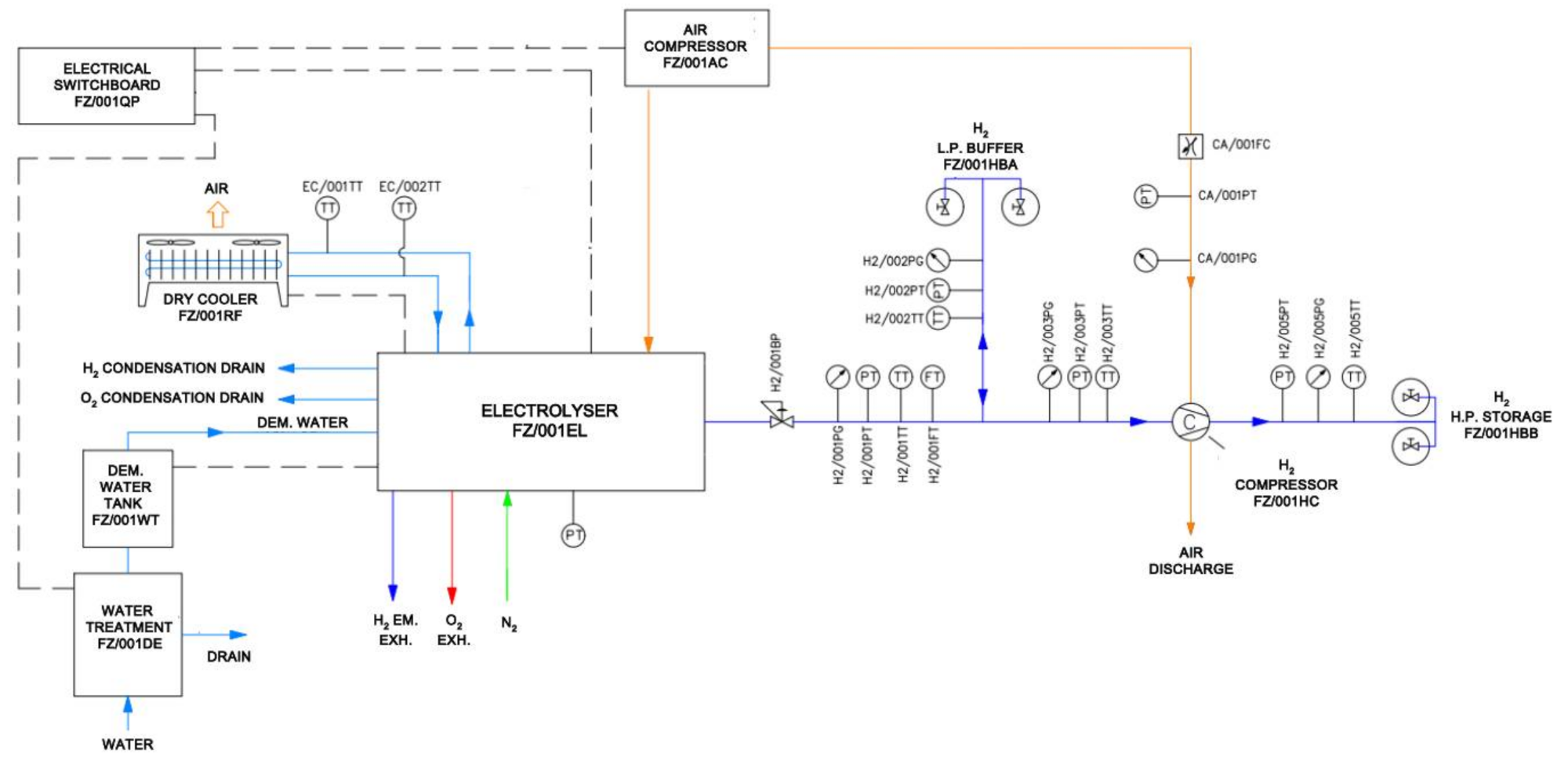
2. Test Rig Description
2.1. Process Flow
- Water is treated and appropriately demineralized using a water treatment system (FZ/001DE) in order to reduce its conductivity below the limit imposed by the electrolyser manufacturer;
- Demineralized water goes then to the electrolyser where is converted into hydrogen and oxygen. The electrolyser hydrogen production internal pressure is precisely controlled and limited by an external back-pressure regulator (H2/001BP);
- The produced hydrogen fills a set of buffer cylinders (FZ/001HBA), with a geometric volume of 50 l, interposed between the electrolyser and an air-driven hydrogen compressor (FZ/001HC). The buffer cylinders (FX/001HBA) are filled at a pressure of about 5 bar g. The purpose of these “low-pressure” buffer systems is to ensure smooth and continuous operation of the electrolyser and of the hydrogen compressor;
- The oxygen produced in the electrolysis reaction, which for the purpose of the project is considered a by-product, is suitably vented to the atmosphere in a safe location;
- Hydrogen pressure is then raised up to 200 bar g by an air-driven hydrogen reciprocating compressor (FZ/001HC);
- Flow of compressed hydrogen is then stored in high-pressure cylinders with a geometric volume of 50 l (FZ/001HBB).
2.2. Main Components Data
2.2.1. Alkaline Electrolyser (FZ/001EL)
2.2.2. Compressed Air Package (FZ/001AC)
2.2.3. Reciprocating Air-Driven Hydrogen Compressor (FZ/001HC)
2.3. Field Instrumentation
2.3.1. Hydrogen Line
- Between the electrolyser and the low-pressure buffer;
- Upstream the low-pressure buffer;
- Upstream the hydrogen compressor;
- Hydrogen compressor delivery side, upstream the high-pressure storage.
2.3.2. Compressed Air Lines
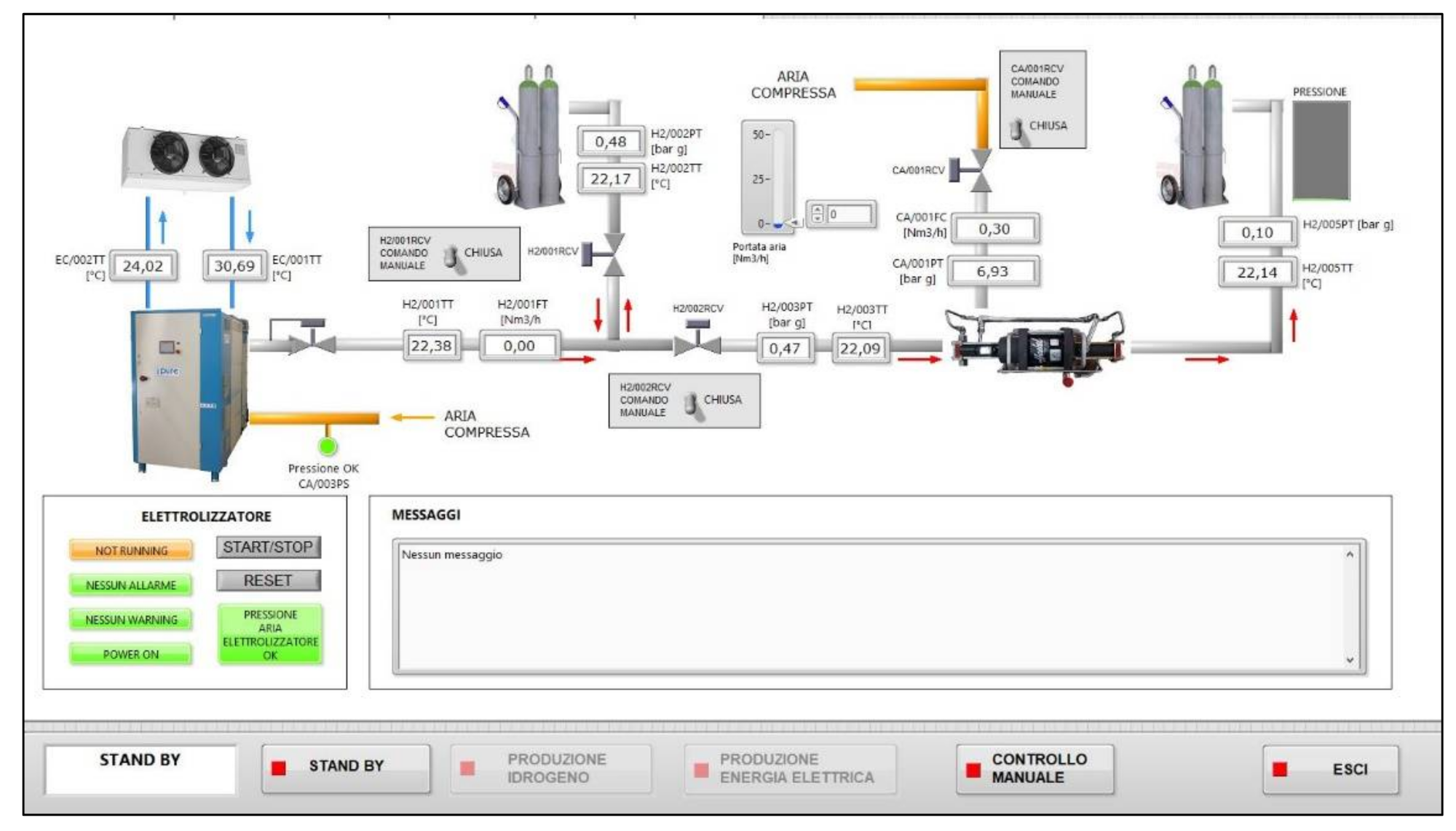
3. Methods Used for Carrying Out Tests and Results Obtained
3.1. Hydrogen Production Efficiency Characterization
3.1.1. Purpose of the Test
3.1.2. Test Procedure
3.1.3. Electrolyser Efficiency Calculation
3.1.4. Test Results: Hydrogen Production Efficiency
3.2. Hydrogen Production and Storage Plant Overall Characterisation
3.2.1. Purpose of the Test
3.2.2. Test Procedure
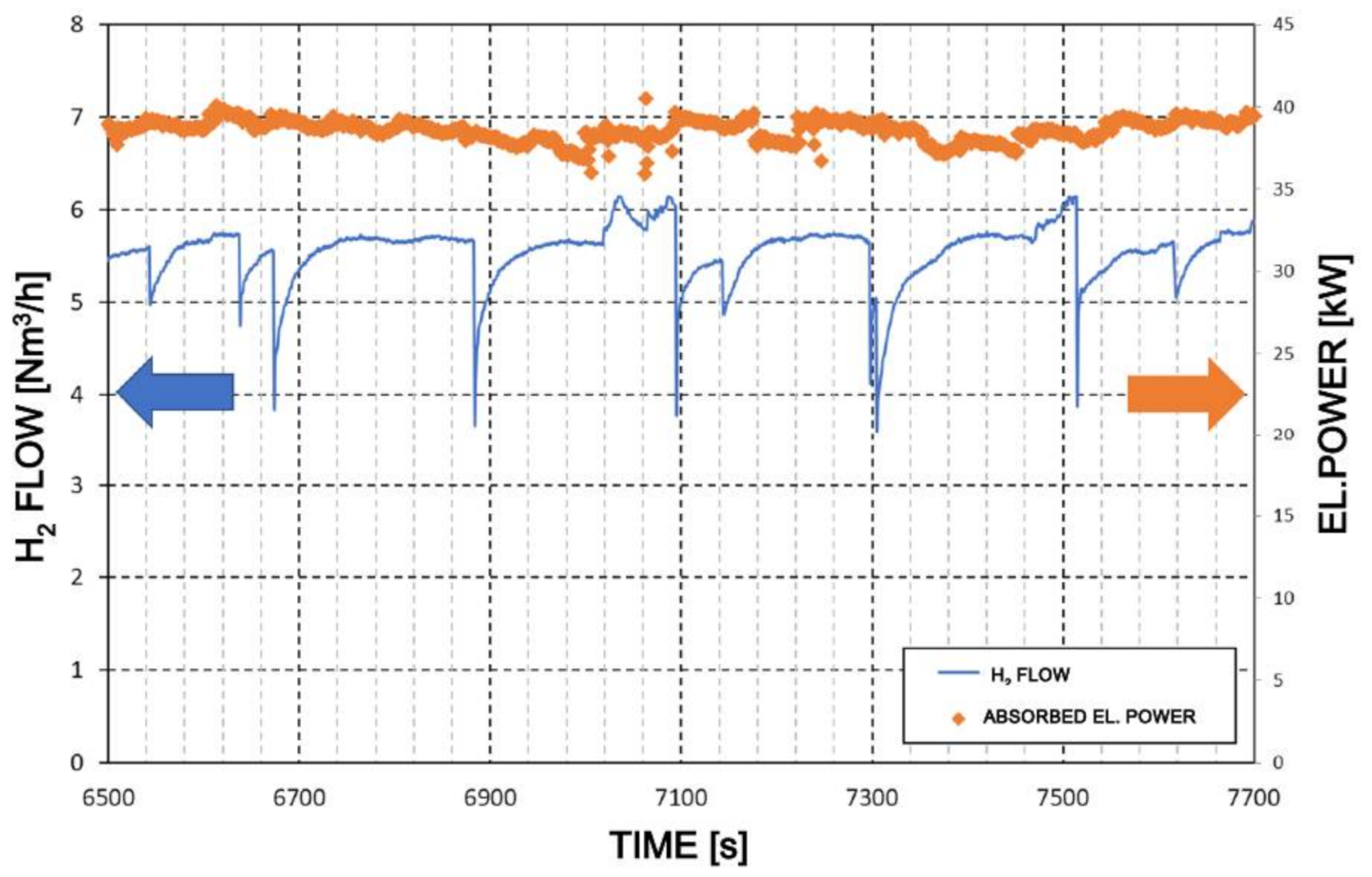
3.2.3. Test Results: Booster Hydrogen and Driving-Air Flow Rate Variation with Time and Booster Specific Energy Consumption
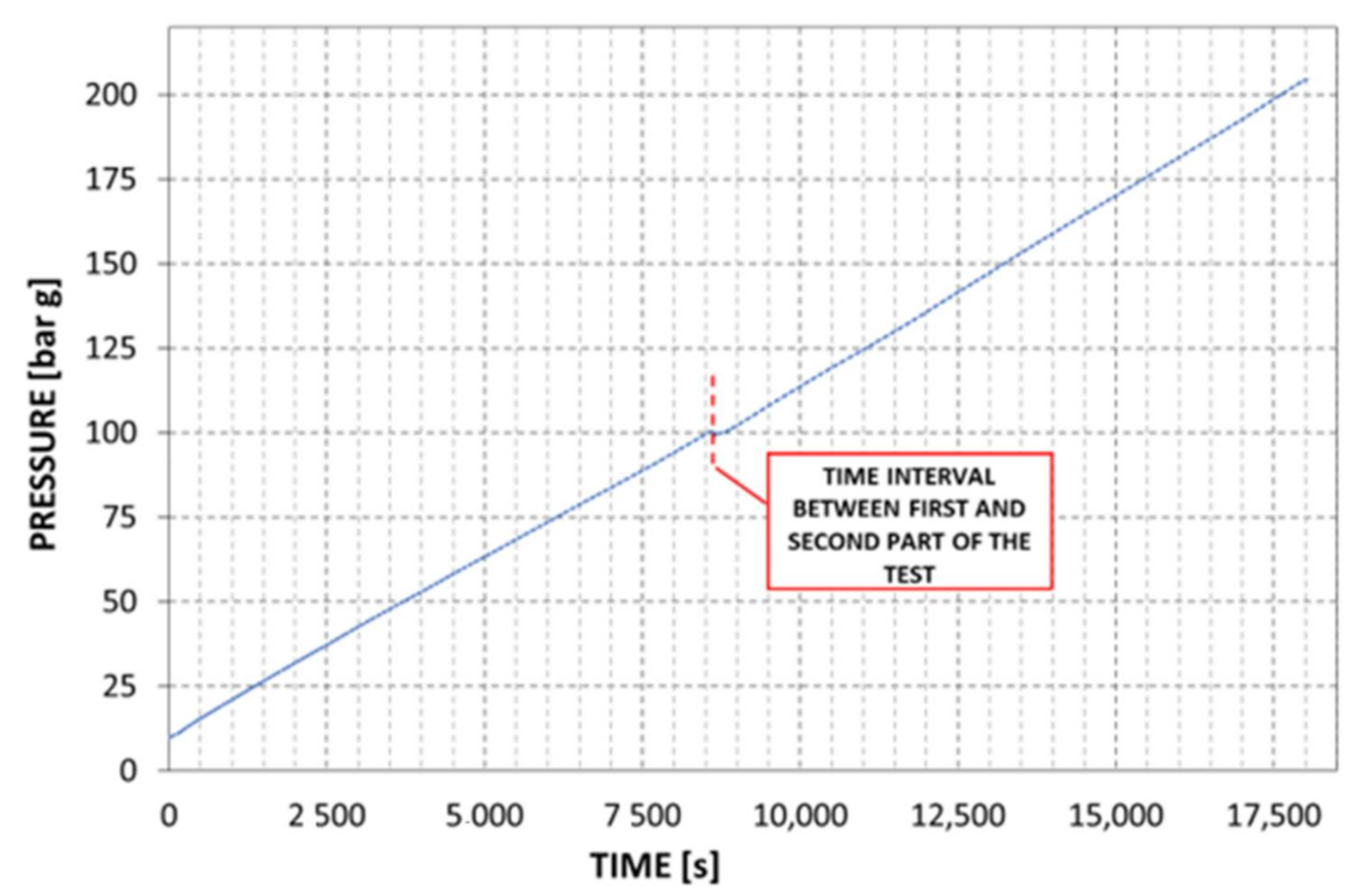
3.2.4. Test Results: Hydrogen Pressure Variations with Time
3.2.5. Test Results: Booster Running at 35 Cycles Per Minute
3.2.6. Test Results: Production and Storage Specific Energy Consumption Considerations
4. Conclusions
- Replacement of the hydrogen booster with a multistage unit capable of processing a higher flow rate;
- Modification of the electrolyser unit in order to avoid using hydrogen to regenerate the filter cartridges;
- Increase of the electrolyser output pressure.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- REN21. Renewables 2020 Global Status Report; REN21: Paris, France, 2020. [Google Scholar]
- Parra, D.; Patel, M.K. Techno-economic implications of the electrolyser technology and size for power-to-gas systems. Int. J. Hydrog. Energy 2016, 41, 3748–3761. [Google Scholar] [CrossRef]
- Ferrero, D.; Gamba, M.; Lanzini, A.; Santarelli, M. Power-to-Gas Hydrogen: Techno-economic assessment of processes towards a multi-purpose energy carrier. Energy Procedia 2016, 101, 50–57. [Google Scholar] [CrossRef]
- Samsatli, S.; Samsatli, N.J. The role of renewable hydrogen and inter-seasonal storage in decarbonising heat—Comprehensive optimisation of future renewable energy value chains. Appl. Energy 2019, 233–234, 854–893. [Google Scholar] [CrossRef]
- Schmidt, O.; Gambhir, A.; Staffell, I.; Hawkes, A.; Nelson, J.; Few, S. Future cost and performance of water electrolysis: An expert elicitation study. Int. J. Hydrog. Energy 2017, 42, 30470–30492. [Google Scholar] [CrossRef]
- FCH JU. Development of Water Electrolysis in the European Union; Fuel Cells and Hydrogen Joint Undertaking (FCH JU): Brussels, Belgium, 2014. [Google Scholar]
- Kiaee, M.; Cruden, A.; Chladek, P.; Infield, D. Demonstration of the operation and performance of a pressurised alkaline electrolyser operating in the hydrogen fuelling station in Porsgrunn, Norway. Energy Convers. Manag. 2015, 94, 40–50. [Google Scholar] [CrossRef]
- Artuso, P.; Gammon, R.; Orecchini, F.; Watson, S.J. Alkaline Electrolisers: Model and real data analysis. Int. J. Hydrog. Energy 2011, 36, 7956–7962. [Google Scholar] [CrossRef]
- FuelCellToday. Water Electrolysis & Renewable Energy Systems; FuelCellToday: Royston, UK, 2013. [Google Scholar]
- FCH JU Addendum to the Multi-Annual Work Plan 2014–2020; Fuel Cells and Hydrogen Joint Undertaking (FCH JU): Brussels, Belgium, 2018.
- Gallandat, N.; Romanowicz, K.; Züttel, A. An Analytical Model for the Electrolyser Performance Derived from Materials Parameters. J. Power Energy Eng. 2017, 5, 34–49. [Google Scholar] [CrossRef]
- Buttler, A.; Spliethoff, H. Current status of water electrolysis for water storage, grid balancing and sector coupling via power-to-gas and power to liquids. A review. Renew. Sustain. Energy Rev. 2018, 82, 2440–2454. [Google Scholar] [CrossRef]
- Angstrom Advanced. Available online: https://angstromadvancedhydrogen.wordpress.com/2016/11/15/angstrom-advanced-hgh100001700085000170000-large-hydrogen-generator/ (accessed on 6 June 2021).
- Green Energy. Available online: https://greenhydrogen.dk/wp-content/uploads/2021/02/A-Series-brochure-120421.pdf (accessed on 6 June 2021).
- NEL Hydrogen. Available online: https://nelhydrogen.com/product/h-series/ (accessed on 6 June 2021).
- Sagim. Available online: https://sagim-gip.com/wp-content/uploads/M%205000_TECHNICAL%20SPECIFICATIONS.pdf (accessed on 6 June 2021).
- Zuttel, A. Hydrogen Storage methods. Naturwissenschaften 2004, 91, 157–172. [Google Scholar] [CrossRef] [PubMed]
- Nour, U.M.; Awad, S.; Yusup, S.; Sufian, S. Technical Evaluation of Current Hydrogen Storage Technologies for Vehicles. J. Appl. Sci. 2010, 10, 1200–1203. [Google Scholar] [CrossRef][Green Version]
- Sdanghi, G.; Maranzana, G.; Celzard, A.; Fierro, V. Review of the current technologies and performances of hydrogen compression for stationary and automotive applications. Renew. Sustain. Energy Rev. 2019, 102, 150–170. [Google Scholar] [CrossRef]
- Sanchez, C.; Gonzalez, D. Experimental characterization of a grid-connected hydrogen energy buffer: Hydrogen production. Int. J. Hydrog. Energy 2013, 38, 9741–9754. [Google Scholar] [CrossRef][Green Version]
- Riedl, S.M. Development of a Hydrogen Refueling Station Design Tool. Int. J. Hydrog. Energy 2020, 45, 1–9. [Google Scholar] [CrossRef]
- Saur, G.; Sprik, S.; Kurtz, J.; Onorato, S.; Gilleon, S.; Winkler, E. National Renewable Energy Laboratory. Hydrogen Station Data Collection and Analysis, DOE Hydrogen and Fuel Cells Program. In Proceedings of the 2019 Annual Merit Review and Peer Evaluation Meeting, Arlington, VA, USA, 29 April–1 May 2019. [Google Scholar]
- Ligen, Y.; Vrubel, H.; Arlettaz, J.; Girault, H. Experimental correlations and integration of gas boosters in a hydrogen refueling station. Int. J. Hydrog. Energy 2020, 45, e16663–e16671. [Google Scholar] [CrossRef]
- Sánchez-Diaz, C.; Monrabal, J.; González, D.; Alfonso, D.; Peñalvo-López, E. Experimental results of the hydrogen production control of a hydrogen energy buffer. Int. J. Hydrog. Energy 2015, 40, 5013–5024. [Google Scholar] [CrossRef]
- Cornish, A.J. Hydrogen Fueling Station Cost Reduction Study; Engineering, Procurement & Construction, LLC: Cheyenne, WY, USA, 2011. [Google Scholar]





| Electricity Consumption @ Nominal Capacity [kwh/kgh2] | ||||
|---|---|---|---|---|
| State-of-the-Art | FCH 2JU Target | |||
| 2012 | 2017 | 2020 | 2024 | 2030 |
| 57 | 51 | 50 | 49 | 48 |
| Company | Country | Type | Model | Capacity [Nm3/h] | H2 Output Pressure [bar g] | H2 Purity [%] | Electrical Consumption [kWh/kgH2] | HHV Efficiency [%] |
|---|---|---|---|---|---|---|---|---|
| Acta | Italy | AEM | EL1000 | 1 | 29 | 99.94 | 53.2 | 74% |
| Angstrom Advanced [13] | USA | PEM | HGH170000 | 10 | 4 | 99 to 99.9999 | 64.5 1 | 61.4% 2 |
| Elogen (ex AREVA H2 Gen) | France | PEM | ELYTE 10 | 10 | 30 | 99.999 | 47.8–60.0 1 | 65.6–82.4% 4 |
| Enapter (ex Acta) | Italy | AEM | EL 2.1 | 0.5 | 35 | 99.9 | 53.3 1 | 73.9% 4 |
| Erredue | Italy | PEM | SIRIO 200 | 2 | 15 (Opt. 30) | 99.99 | 53.3 1 | 73.9% 4 |
| GreenHydrogen [14] | Denmark | PEM | HyProvide P1 | 1 | 50 | >99.995 | 61.2 1 | 64.9% 2 |
| H-TEC SYSTEMS | Germany | PEM | EL30/144 | 3.6 | 29 | N/A | 55.4 | 71.1% |
| Idroenergy | Italy | Alkaline | mod. 8.0 | 5.33 | 4 | >99.5 | 62.5 3 | 63.0% 4 |
| ITM Power | UK | PEM | HGAS3SP | 3.2 | N/A | 99.999 | N/A | N/A |
| Nel Hydrogen [15] | Norway | PEM | H6 | 6 | 15 (Opt. 30) | 99.9995 | 75.5 1 | 52.2% 4 |
| Pure Energy Centre | UK | Alkaline | 4Nm3 | 4 | Up to 12 | >99.3 | 62 | 63% 4 |
| Sagim [16] | France | Alkaline | M5000 | 5 | 7 | 99.9 | 55.6 1 | 70.8% 2 |
| Teledyne Energy Systems | USA | Alkaline | NH–450 | 450 | 10 | N/A | 65.6 1 | 60.2% 2 |
| Quantity | Unit | Value |
|---|---|---|
| Deionized water inlet | [l/h] | 4.7 max. |
| Hydrogen outlet | [Nm3/h] | 5.3 max. |
| [bar g] | 5 max. | |
| Oxygen outlet | [Nm3/h] | 2.5 max. |
| [bar g] | 5 max. | |
| Compressed air | [m3/h] | 1 |
| [bar g] | 5 to 8 |
| Quantity | Unit | Value |
|---|---|---|
| Output pressure | [bar g] | 10 max. |
| Air flow | [m3/min] | 1.59 |
| Efficiency at full load | [%] | 89 |
| Quantity | Unit | Value |
|---|---|---|
| Compression stages | - | 2 |
| Inlet pressure | [bar g] | 2 to 5 |
| Max. discharge pressure | [bar g] | 200 |
| Drive air pressure | [bar g] | 2.8 to 10.3 |
| Component | Feature | Value |
|---|---|---|
| Hydrogen flowmeter (H2/001FT), | Accuracy | ±0.5% Rd plus ± 0.1% FS |
| Repeatability | <0.2% Rd | |
| Pressure transmitter H2/001PT, H2/002PT, H2/003PT | Accuracy | ±0.50% FS |
| Repeatability | <0.1% FS | |
| Pressure transmitter (CA/001PT) | Linearity error | ≤±0.5% FS |
| Repeatability | ≤±0.1% FS | |
| Air flow meter/controller (CA/001FC) | Accuracy | ±(0.5% Rd plus 0.5% FS) |
| Portable network analyser | Voltage accuracy | 0.1% of Vnom |
| Ampere accuracy | ±(0.5% Rd plus 5% counts) |
| Quantity | Unit | Instrument | P&I Reference |
|---|---|---|---|
| Electrolyser H2 outlet flow rate | [Nm3/h] | Flow meter | H2/001FT |
| Electrolyser H2 outlet pressure | [bar g] | Electrolyser display | PT |
| Electrolyser power consumption | [kW] | Power analyser | - |
| Ambient temperature | [°C] | Weather station | - |
| Ambient pressure | [hPa] | Weather station | - |
| Quantity | Unit | Formula, Calculation Method or Value |
|---|---|---|
| [kW] | Equation (4) | |
| [MJ/kg] | 120 1 | |
| [Nm3/h] | Flow rate measured by the H2/001FT flowmeter referred to normal conditions: 101.325 Pa, 0 °C | |
| [kg/m3] | 0.0899 2 | |
| [kW] | The average electrical input power in the electrolyser is obtained as an average of the input power values provided from the power analyser |
| Quantity | Unit | Average @ 4.6 Bar g | Average @ 4.2 Bar g |
|---|---|---|---|
| Electrolyser H2 outlet flow rate | [Nm3/h] | 2.52 | 5.53 |
| Electrolyser H2 outlet pressure | [bar g] | 4.6 | 4.2 |
| Electrolyser power consumption | [kW] | 21.2 | 38.6 |
| Ambient temperature | [°C] | 25 | 25 |
| Ambient pressure | [hPa] | 1017 | 1017 |
| Electrolyser efficiency 1 | [%] | 36 | 43 |
| Specific Energy Consumption 1 | [kWh/kg] | 93 | 77 |
| Quantity | Unit | Instrument | P&I Reference |
|---|---|---|---|
| Electrolyser H2 outlet flow rate | [Nm3/h] | Flow meter | H2/001FT |
| Electrolyser H2 outlet pressure | [bar g] | Electrolyser display | PT |
| Electrolyser power consumption | [kW] | Network analyser | - |
| Air compr. power consumption | [kW] | Network analyser | - |
| H2 compr. driving air flow rate | [Nm3/h] | Flow controller | CA/001FC |
| H2 compr. speed | [Cycles/min] | Stopwatch | - |
| H2 buffer pressure | [bar g] | Pressure transducer | H2/002PT |
| H2 storage pressure | [bar g] | Pressure transducer | H2/005PT |
| Ambient temperature | [°C] | Weather station | - |
| Ambient pressure | [hPa] | Weather station | - |
| Quantity | Unit | From 10 to 100 Bar g | From 100 to 200 Bar g | Overall Process: from 10 to 200 Bar g |
|---|---|---|---|---|
| Electrolyser H2 outlet flow rate (average) | [Nm3/h] | 1.54 | 1.43 | 1.51 |
| Electrolyser H2 outlet pressure (average) | [bar g] | 4.6 | 4.6 | 4.6 |
| Electrolyser power consumption (average) | [kW] | 21.2 | 21.2 | 21.2 |
| Air compressor power consumption (average) | [kW] | 8 | 9.7 | 8.85 |
| Peak air compressor power consumption | [kW] | 12.4 | 12.7 | - |
| Peak H2 compressor driving air flow rate | [Nm3/h] | 35 | 56.5 | - |
| Storage initial pressure | [bar g] | 10 | 99.3 | - |
| Storage final pressure | [bar g] | 100 | 204.5 | - |
| Buffer pressure (average) | [bar g] | 4.53 | 4.51 | 4.52 |
| H2 compressor booster speed | [cycles/min] | 44 | 44 | 44 |
| Quantity | Unit | From 0 to 172 Bar g | From 172 to 200 Bar g | From 0 to 200 Bar g |
|---|---|---|---|---|
| Electrolyser H2 outlet flow rate (average) | [Nm3/h] | 1.26 | 1.12 | 1.19 |
| Electrolyser H2 outlet pressure (average) | [bar g] | 4.6 | 4.6 | 4.6 |
| Electrolyser power consumption (average) | [kW] | 21.2 | 21.2 | 21.2 |
| Air compressor power consumption (average) | [kW] | 8 | 9.4 | 8.7 |
| Peak air compressor power consumption | [kW] | 12.7 | 12.7 | - |
| Peak H2 compressor driving air flow rate | [Nm3/h] | 35.7 | 43.7 | - |
| Storage initial pressure | [bar g] | 0 | 167 | - |
| Storage final pressure | [bar g] | 172 | 202 | - |
| Buffer pressure (average) | [bar g] | 4.58 | 4.46 | 4.52 |
| H2 compressor booster speed | [cycles/min] | 35 | 35 | 35 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pietra, A.; Gianni, M.; Zuliani, N.; Malabotti, S.; Taccani, R. Experimental Characterization of an Alkaline Electrolyser and a Compression System for Hydrogen Production and Storage. Energies 2021, 14, 5347. https://doi.org/10.3390/en14175347
Pietra A, Gianni M, Zuliani N, Malabotti S, Taccani R. Experimental Characterization of an Alkaline Electrolyser and a Compression System for Hydrogen Production and Storage. Energies. 2021; 14(17):5347. https://doi.org/10.3390/en14175347
Chicago/Turabian StylePietra, Andrea, Marco Gianni, Nicola Zuliani, Stefano Malabotti, and Rodolfo Taccani. 2021. "Experimental Characterization of an Alkaline Electrolyser and a Compression System for Hydrogen Production and Storage" Energies 14, no. 17: 5347. https://doi.org/10.3390/en14175347
APA StylePietra, A., Gianni, M., Zuliani, N., Malabotti, S., & Taccani, R. (2021). Experimental Characterization of an Alkaline Electrolyser and a Compression System for Hydrogen Production and Storage. Energies, 14(17), 5347. https://doi.org/10.3390/en14175347







