Experimental Study on Critical Membrane Water Content of Proton Exchange Membrane Fuel Cells for Cold Storage at −50 °C
Abstract
:1. Introduction
2. Experimental Section
2.1. MEA Manufacturing and Fuel Cell Setup
2.2. Equationuilibrium Purge
2.3. Dry purge and Freeze/Thaw Cycles
2.4. Electrochemical Measurements
2.5. Morphology and Pore Structure Analysis
3. Results and Discussion
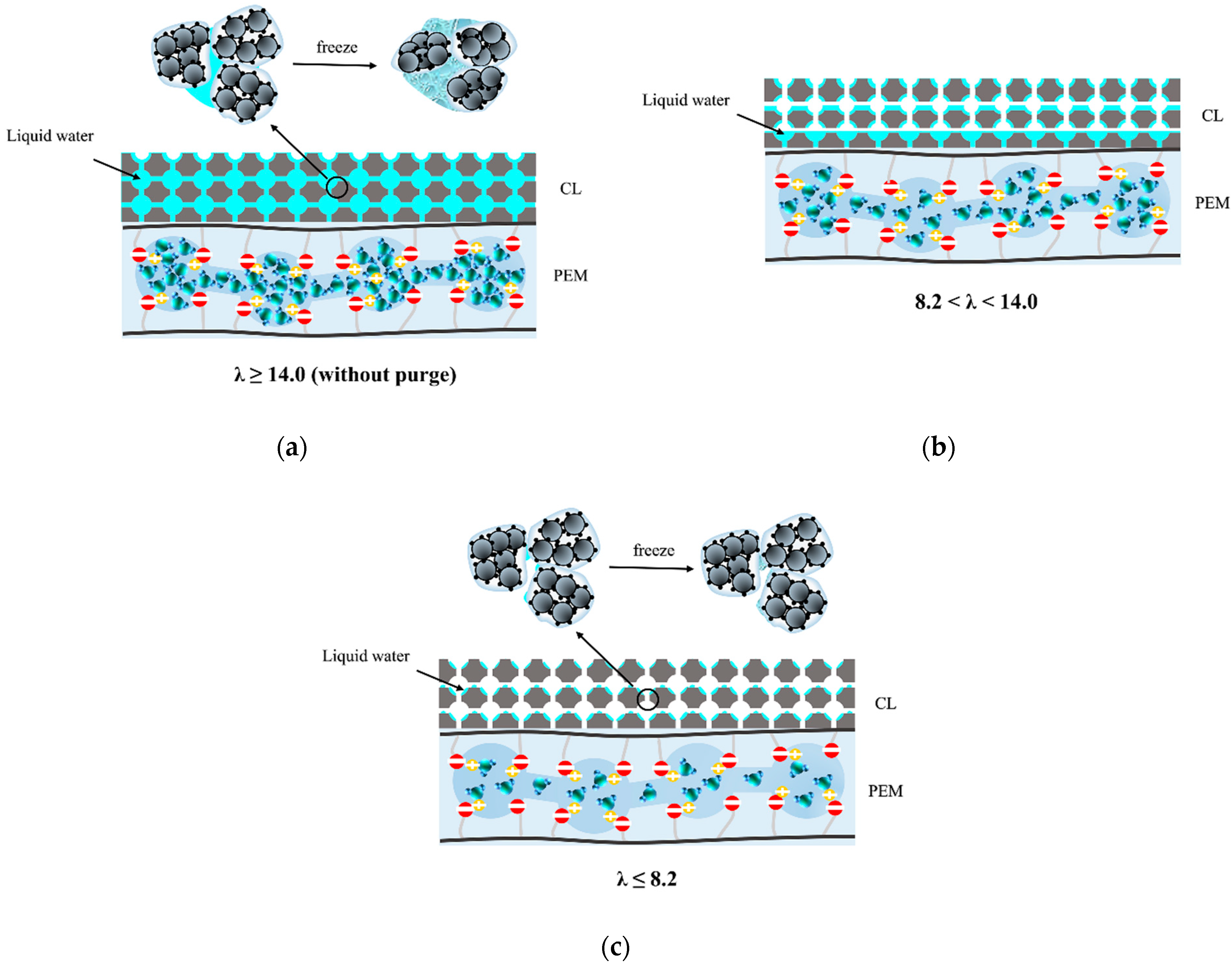
3.1. Calculation of Membrane Water Content
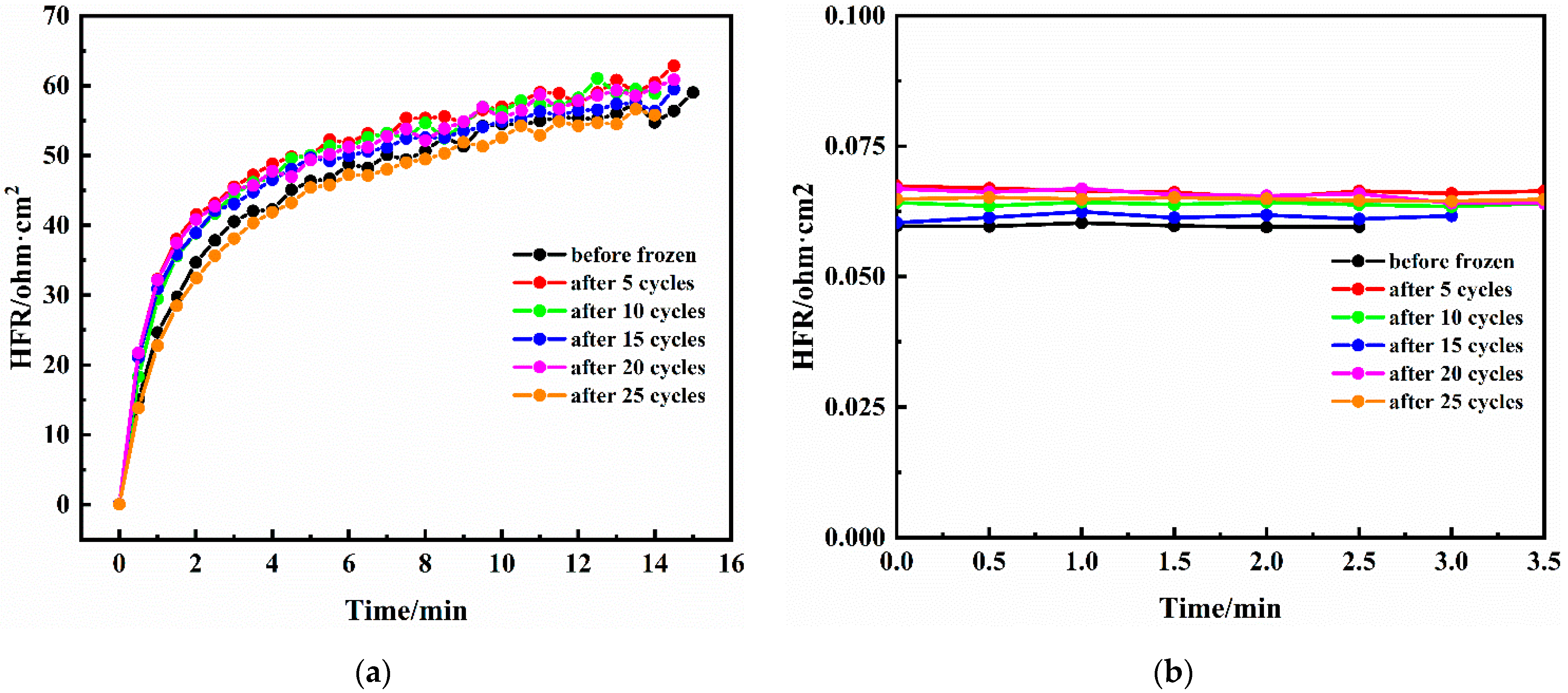
3.2. Dry Purge and HFR Relaxation
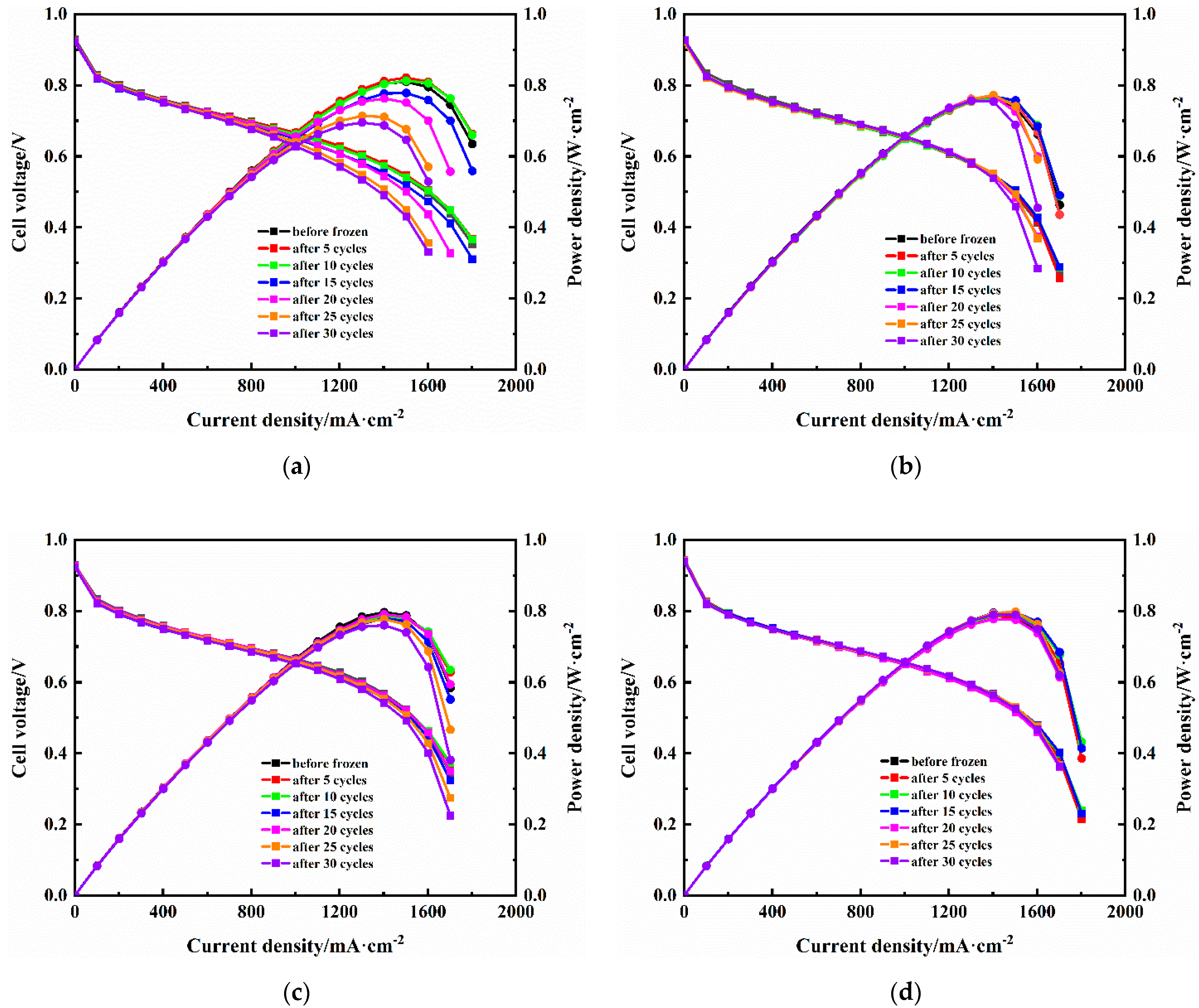
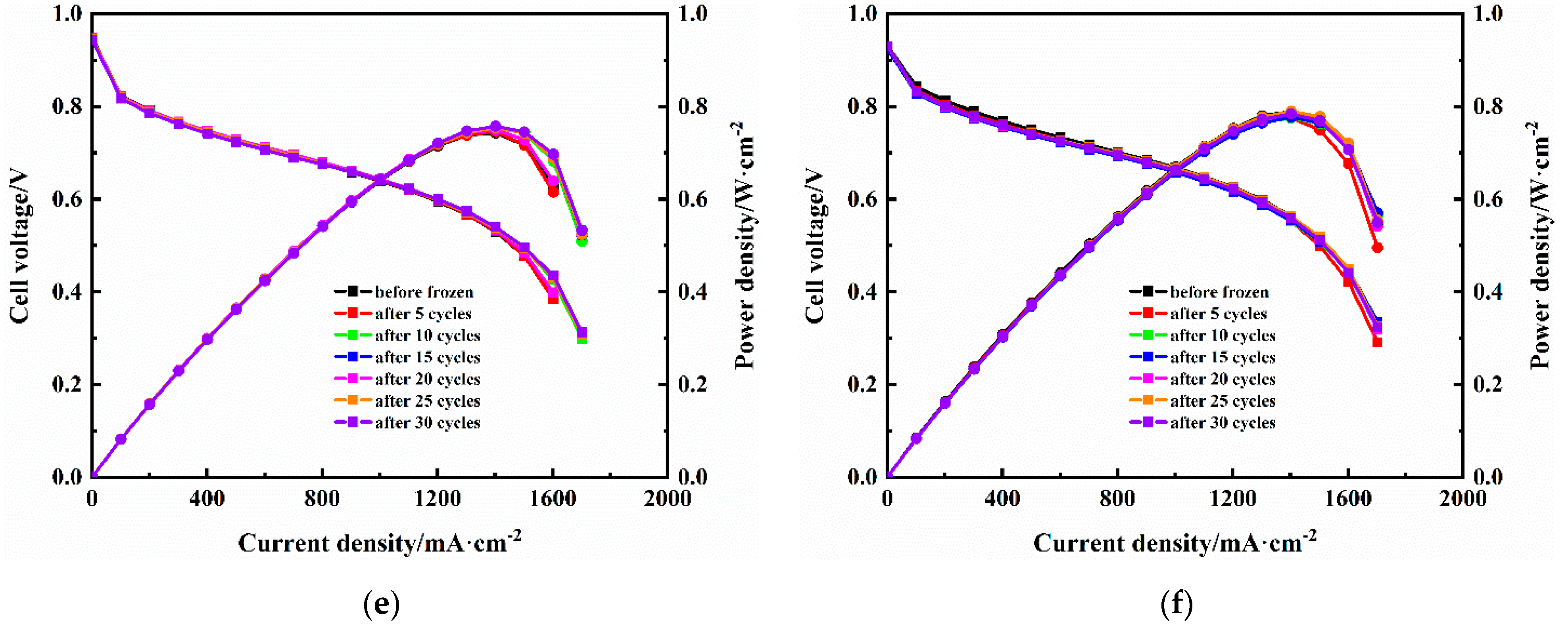
3.3. Fuel Cell Performance after Freeze/Thaw Cycles
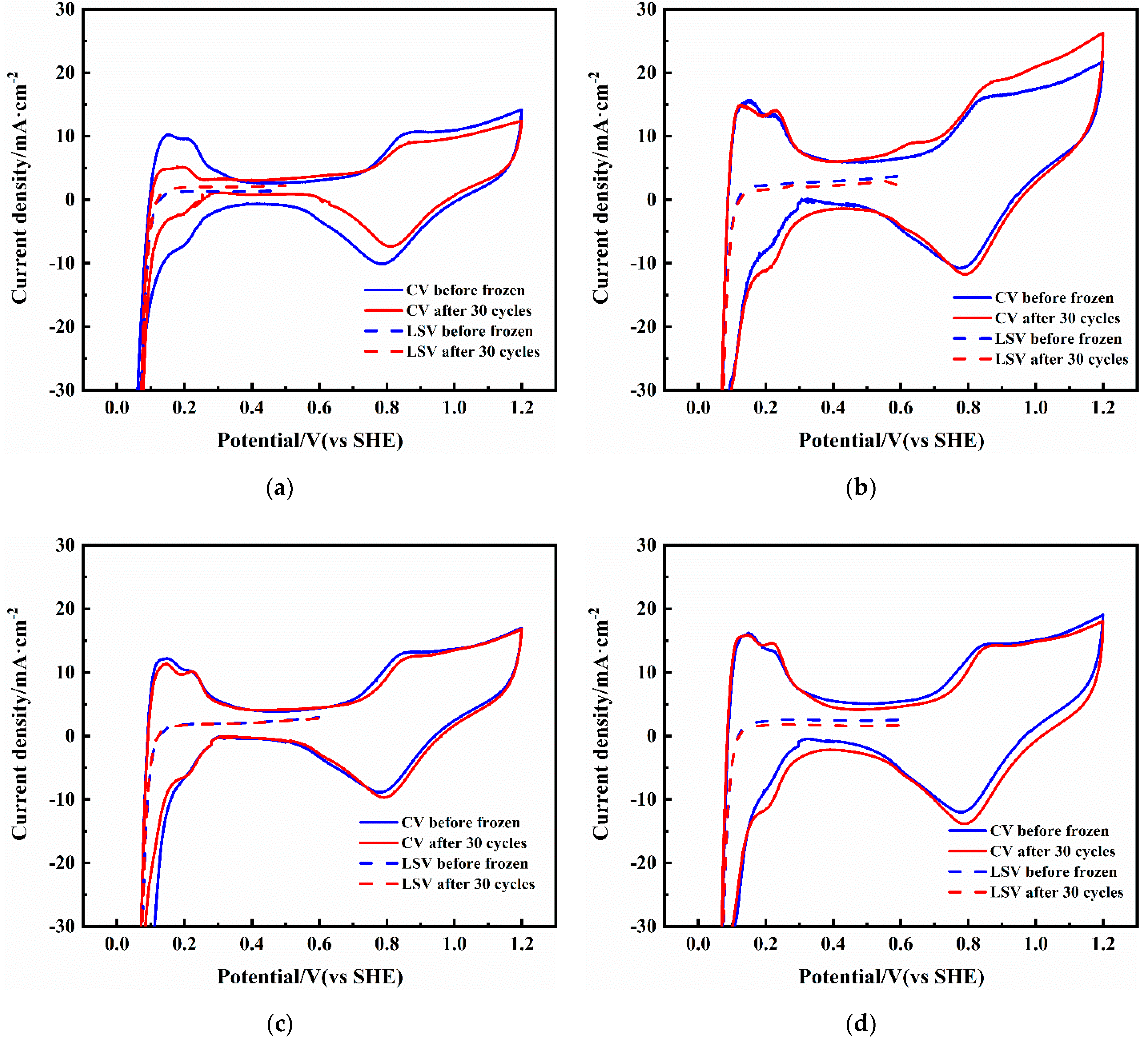
3.4. Electrochemical Active Surface Area and Hydrogen Crossover
3.5. Electrochemical Impedance Spectra
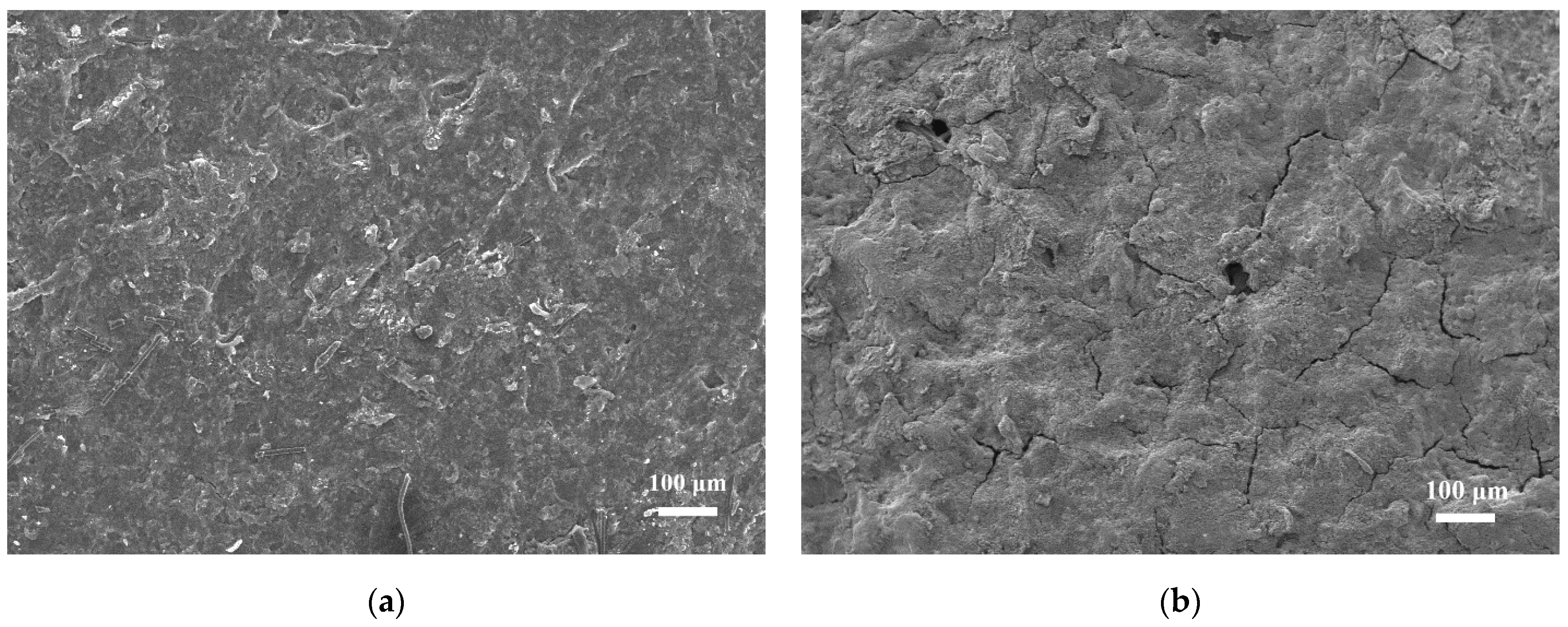
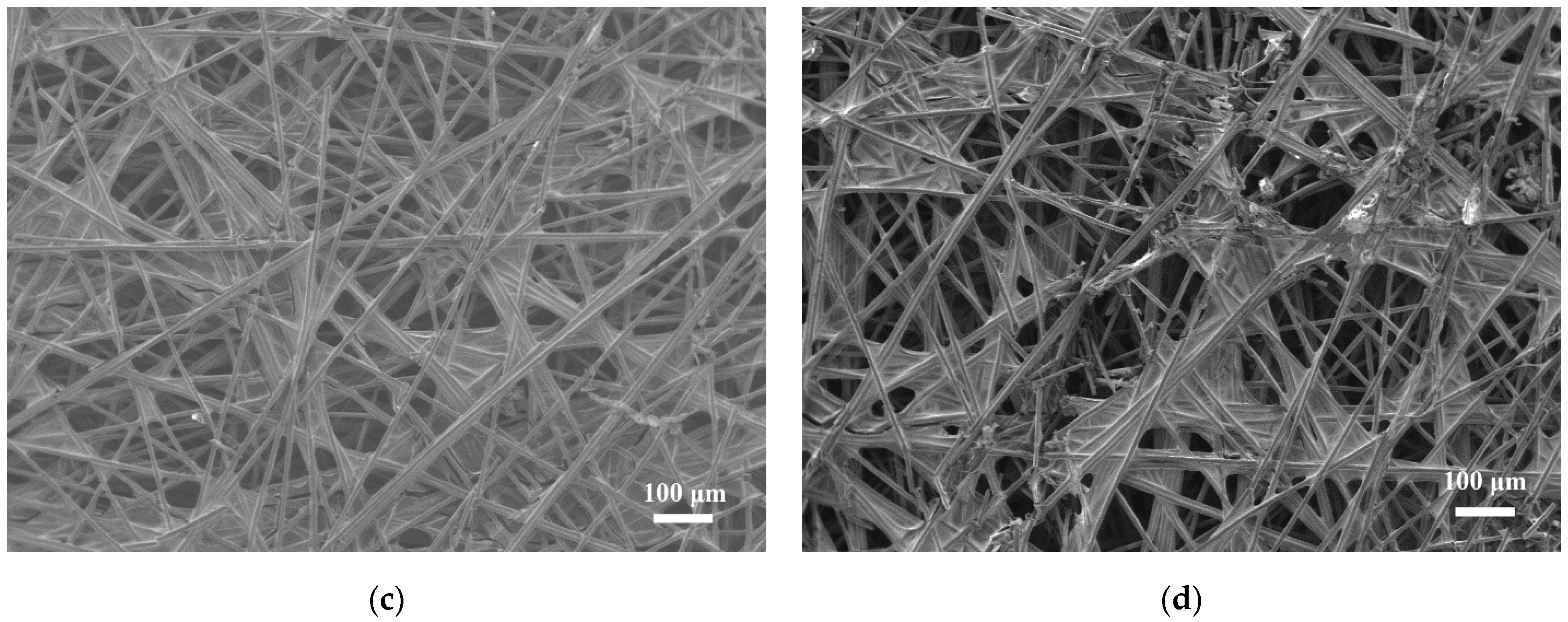
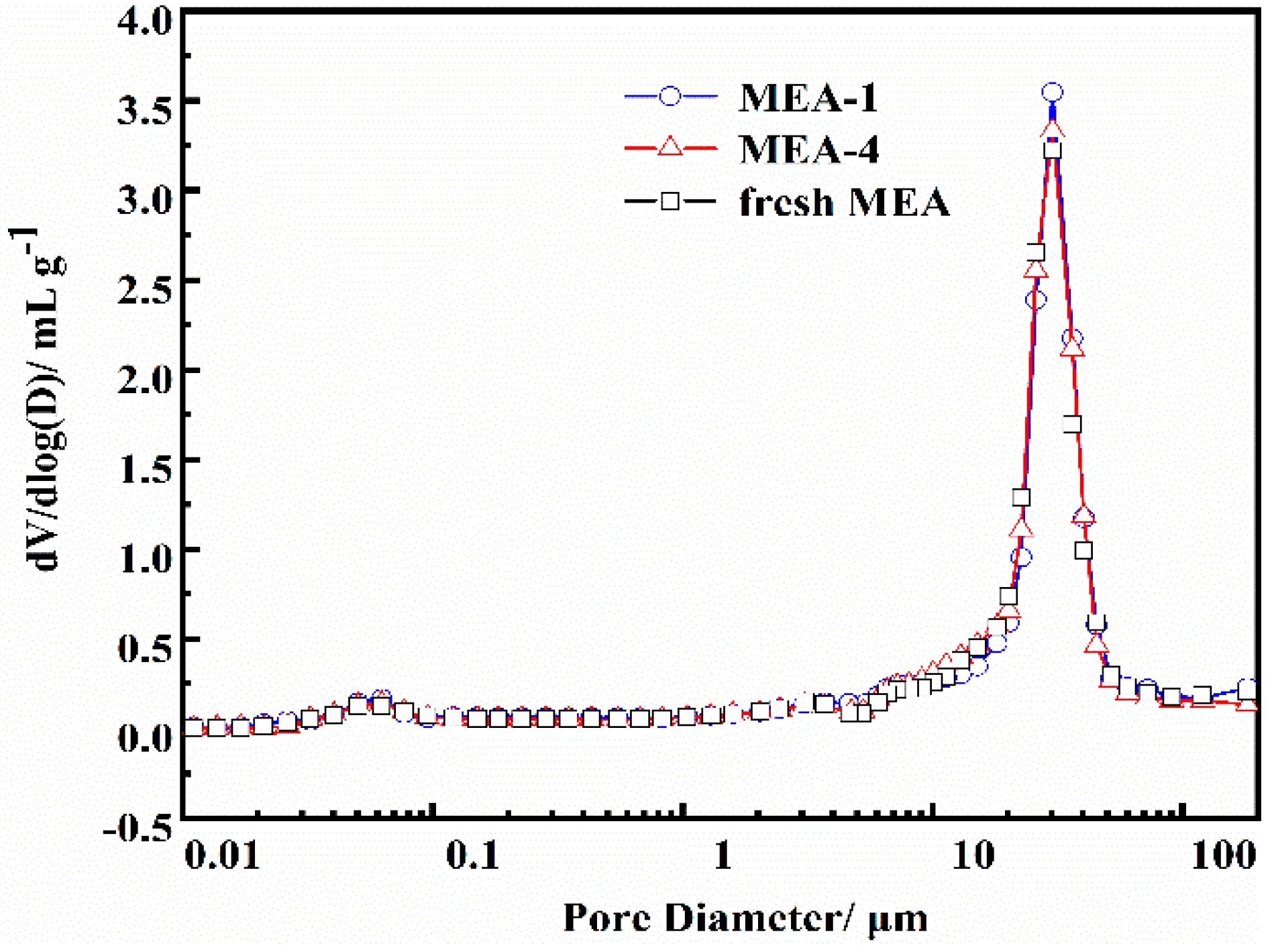
3.6. Morphology and Porosity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Debe, M.K. Electrocatalyst approaches and challenges for automotive fuel cells. Nature 2012, 486, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Diza, D.F.R.; Chen, K.S.; Wang, Z.; Adroher, X.C. Materials, technological status, and fundamentals of PEM fuel cells—A review. Mater. Today 2019, 32, 178–203. [Google Scholar] [CrossRef]
- Wang, Z.; Chang, H.; Shu, S.; Wang, Y.; Tang, H. A Review on Cold Start of Proton Exchange Membrane Fuel Cells. Energies 2014, 7, 3179–3203. [Google Scholar]
- Luo, Y.; Jiao, K. Cold start of proton exchange membrane fuel cell. Prog. Energy Combust. Sci. 2018, 64, 29–61. [Google Scholar] [CrossRef]
- Alaswad, A.; Omran, A.; Sodre, J.R.; Wilberforce, T.; Pignatelli, G.; Dassisti, M.; Baroutaji, A.; Olabi, A.G. Technical and Commercial Challenges of Proton-Exchange Membrane (PEM) Fuel Cells. Energies 2021, 14, 144. [Google Scholar] [CrossRef]
- Ozdan, A.; Shahgldi, S.; Li, X.G.; Hamdullahpur, F. The impact of ionomer type on the morphological and microstructure degradations of proton exchange membrane fuel cell electrodes under freeze-thaw cycles. Appl. Energy 2019, 238, 1048–1059. [Google Scholar] [CrossRef]
- Lee, S.Y.; Kim, H.J.; Cho, E.A.; Lee, K.S.; Lim, T.H.; Hwang, I.C.; Jiang, J.H. Performance degradation and microstructure changes in freeze–thaw cycling for PEMFC MEAs with various initial microstructures. Int. J. Hydrogen Energy 2010, 35, 12888–12896. [Google Scholar] [CrossRef]
- Sabawa, J.P.; Bandarenka, A.S. Investigation of degradation mechanisms in PEM fuel cells caused by low-temperature cycles. Int. J. Hydrogen Energy 2021, 46, 15951–15964. [Google Scholar] [CrossRef]
- Lim, S.J.; Park, G.G.; Park, J.S.; Sohn, Y.J.; Yim, S.D.; Yang, T.H.; Hong, B.K.; Kim, C.S. Investigation of freeze/thaw durability in polymer electrolyte fuel cells. Int. J. Hydrogen Energy 2010, 35, 13111–13117. [Google Scholar] [CrossRef]
- Kim, S.; Mench, M.M. Physical Degradation of Membrane Electrode Assemblies undergoing Freeze/Thaw Cycling: Micro-Structure Effects. J. Power Sources 2007, 174, 206–220. [Google Scholar] [CrossRef]
- Kim, S.; Ahn, B.K.; Mench, M.M. Physical degradation of membrane electrode assemblies undergoing freeze/thaw cycling: Diffusion media effects. J. Power Sources 2008, 179, 140–146. [Google Scholar] [CrossRef]
- Ozdan, A.; Shahgldi, S.; Zhao, J.; Li, X.G.; Hamdullahpur, F. Degradations in porous components of a proton exchange membrane fuel cell under freeze-thaw cycles: Morphology and microstructure effects. Int. J. Hydrogen Energy 2020, 45, 3618–3631. [Google Scholar] [CrossRef]
- Ozdan, A.; Shahgldi, S.; Li, X.G.; Hamdullahpur, F. Degradations in the surface wettability and gas permeability characteristics of proton exchange membrane fuel cell electrodes under freeze-thaw cycles: Effects of ionomer type. Int. J. Hydrogen Energy 2020, 45, 29892–29903. [Google Scholar] [CrossRef]
- Song, K.Y.; Kim, H.T. Effect of air purging and dry operation on durability of PEMFC under freeze/thaw cycles. Int. J. Hydrogenen Energy 2011, 36, 12417–12426. [Google Scholar] [CrossRef]
- Pinton, E.; Fourneron, Y.; Rosini, S.; Antoni, L. Experimental and theoretical investigations on a proton exchange membrane fuel cell starting up at subzero temperatures. J. Power Sources 2009, 186, 80–88. [Google Scholar] [CrossRef]
- Sabawa, J.P.; Bandarenka, A.S. Degradation Mechanisms in Polymer Electrolyte Membrane Fuel Cells Caused by Freeze-Cycles: Investigation Using Electrochemical Impedance Spectroscopy. Electrochim. Acta 2019, 311, 21–29. [Google Scholar] [CrossRef]
- Hou, S.; Jiao, K.; Park, J.W. On the water transport behavior and phase transition mechanisms in cold start operation of PEM fuel cell. Appl. Energy 2019, 233–234, 776–788. [Google Scholar]
- Luo, Y.; Guo, Q.; Du, Q.; Yin, Y.; Jiao, K. Analysis of cold start processes in proton exchange membrane fuel cell stacks. J. Power Sources 2013, 224, 99–114. [Google Scholar] [CrossRef]
- Jiao, K.; Alaefour, I.E.; Karimi, G.; Li, X. Cold start characteristics of proton exchange membrane fuel cells. Int. J. Hydrogen Energy 2011, 36, 11832–11845. [Google Scholar] [CrossRef]
- Yao, L.; Ma, F.; Peng, J.; Zhang, J.; Zhang, Y.; Shi, J. Analysis of the Failure Modes in the Polymer Electrolyte Fuel Cell Cold-Start Process—Anode Dehydration or Cathode Pore Blockage. Energies 2020, 13, 256. [Google Scholar] [CrossRef]
- Jiao, K.; Li, X. Water transport in polymer electrolyte membrane fuel cells. Prog. Energy Combust. Sci. 2011, 37, 221–291. [Google Scholar] [CrossRef]
- Hinatsu, J.T.; Mizuhata, M.; Takenaka, H. Water uptake of perfluorosulfonic acid membranes from liquid water and water vapor. J. Electrochem. Soc. 1994, 141, 1493–1498. [Google Scholar] [CrossRef]
- Springer, T.E.; Zawodzinski, T.A.; Gottesfeld, S. Polymer Electrolyte Fuel Cell Model. J. Electrochem. Soc. 1991, 138, 2334–2342. [Google Scholar] [CrossRef]
- Tajiri, K.; Tabuchi, Y.; Kagami, F.; Takahashi, S.; Yoshizawa, K.; Wang, C.-Y. Effects of operating and design parameters on PEFC cold start. J. Power Sources 2007, 165, 279–286. [Google Scholar] [CrossRef]
- Hou, J.; Yu, H.; Zhang, S.; Sun, S.; Wang, H.; Yi, B.; Ming, P. Analysis of PEMFC freeze degradation at −20 °C after gas purging. J. Power Sources 2006, 162, 513–520. [Google Scholar] [CrossRef]
- Luo, M.; Huang, C.; Liu, W.; Luo, Z.; Pan, M. Degradation behaviors of polymer electrolyte membrane fuel cell under freeze/thaw cycles. Int. J. Hydrogen Energy 2010, 35, 2986–2993. [Google Scholar] [CrossRef]
- Sinha, P.K.; Wang, C.-Y. Two-phase modeling of gas purge in a polymer electrolyte fuel cell. J. Power Sources 2008, 183, 609–618. [Google Scholar] [CrossRef]
- Ge, S.; Wang, C.-Y. Characteristics of subzero startup and water/ice formation on the catalyst layer in a polymer electrolyte fuel cell. Electrochim. Acta 2007, 52, 4825–4835. [Google Scholar] [CrossRef]
- Tajiri, K.; Wang, C.-Y.; Tabuchi, Y. Water removal from a PEFC during gas purge. Electrochim. Acta 2008, 53, 6337–6343. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Kim, S.-U.; Kim, H.-J.; Jang, J.H.; Oh, I.-H.; Cho, E.A.; Hong, S.A.; Ko, J.; Lim, T.W.; Lee, K.Y.; et al. Water removal characteristics of proton exchange membrane fuel cells using a dry gas purging method. J. Power Sources 2008, 180, 784–790. [Google Scholar] [CrossRef]
- Tajiri, K.; Tabuchi, Y.; Wang, C.-Y. Isothermal cold start of polymer electrolyte fuel cells. J. Electrochem. Soc. 2007, 154, B147–B152. [Google Scholar] [CrossRef]
- Song, W.; Yu, H.M.; Hao, L.X.; Yi, B.L.; Shao, Z.G. Effect of catalytic ink on sub-freezing endurance of PEMFCs. Int. J. Hydrogen Energy 2010, 35, 11129–11137. [Google Scholar] [CrossRef]
- Ding, J.; Mu, Y.T.; Zhai, S.; Tao, W.Q. Numerical study of gas purge in polymer electrolyte membrane fuel cell. Int. J. Heat Mass Transf. 2016, 103, 744–752. [Google Scholar] [CrossRef]
- Jiao, K.; Li, X. Effects of various operating and initial conditions on cold start performance of polymer electrolyte membrane fuel cells. Int. J. Hydrogen Energy 2009, 34, 8171–8184. [Google Scholar] [CrossRef]
- Cho, K.T.; Mench, M.M. Fundamental characterization of evaporative water removal from fuel cell diffusion media. Int. J. Hydrogen Energy 2010, 195, 3858–3869. [Google Scholar] [CrossRef]
- Ciureanu, M.; Roberge, R. Electrochemical Impedance Study of PEM Fuel Cells. Experimental Diagnostics and Modeling of Air Cathodes. J. Phys. Chem. B 2001, 105, 3531–3539. [Google Scholar] [CrossRef]
- Hou, J.; Song, W.; Yu, H.; Fu, Y.; Shao, Z.; Yi, B. Electrochemical impedance investigation of proton exchange membrane fuel cells experienced subzero temperature. J. Power Sources 2007, 171, 610–616. [Google Scholar] [CrossRef]
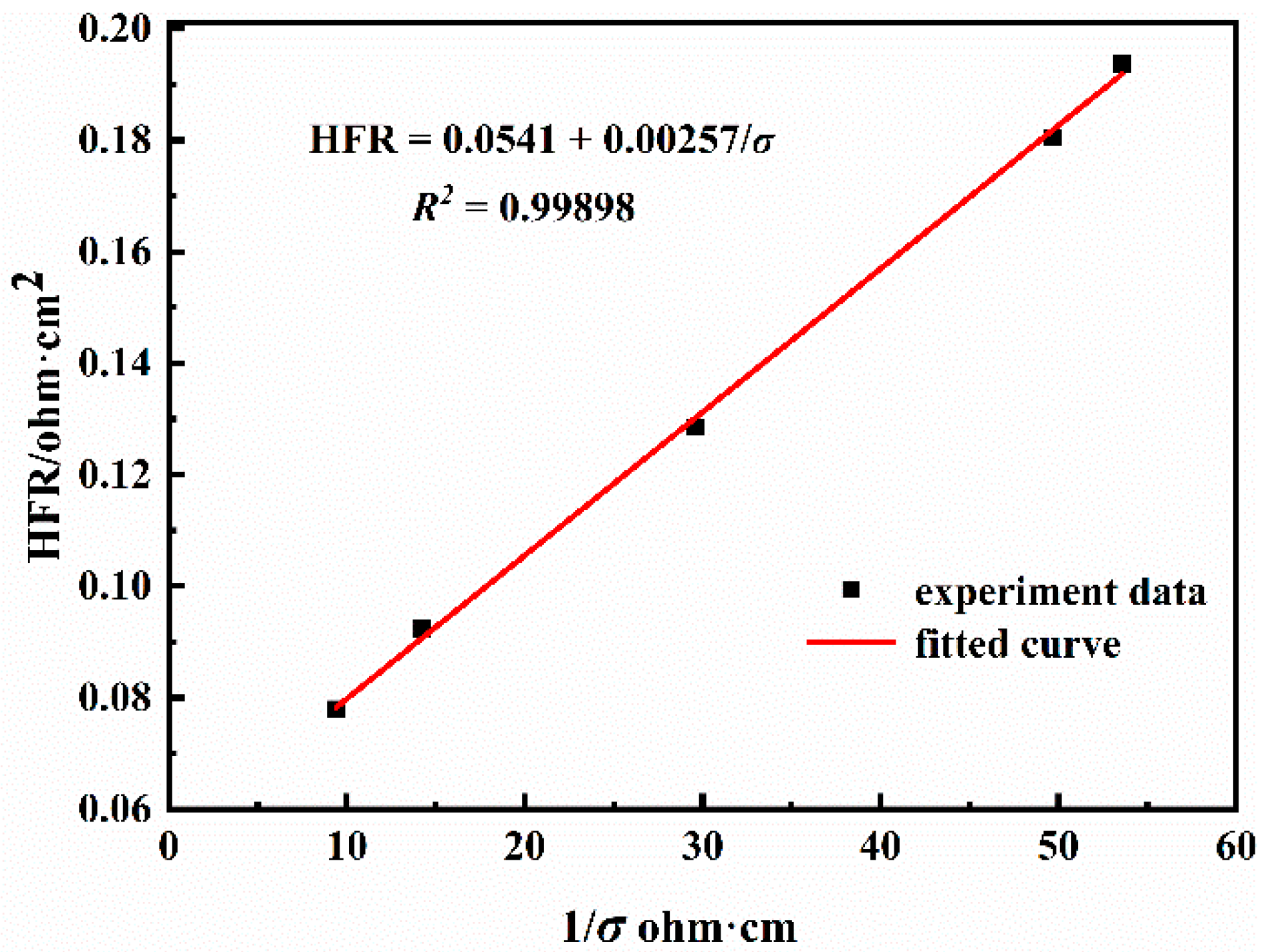



| Case No. | Gas Dew Point (°C) | Cell Temperature (°C) | Relative Humidity (%) | Membrane Water Content | Proton Conductivity ((ohm·cm)−1) | Final HFR (ohm·cm2) |
|---|---|---|---|---|---|---|
| 1 | 21 | 65 | 10 | 1.40 | 0.0061 | 2.4996 |
| 2 | 40 | 65 | 30 | 2.58 | 0.0155 | 0.3942 |
| 3 | 46 | 65 | 40 | 2.84 | 0.0175 | 0.2618 |
| 4 | 48 | 65 | 45 | 2.98 | 0.0187 | 0.1937 |
| 5 | 50 | 65 | 50 | 3.17 | 0.0201 | 0.1805 |
| 6 | 57 | 65 | 70 | 4.89 | 0.0338 | 0.1285 |
| 7 | 62.5 | 65 | 90 | 9.47 | 0.0702 | 0.0924 |
| 8 | 65 | 65 | 100 | 14.00 | 0.1061 | 0.0779 |
| Cell No. | Dry Purge | Relaxed HFR ( ) | ( ) | Membrane Water Content |
|---|---|---|---|---|
| 1 | No | - | - | 14.0 |
| 2 | Yes | 46.9 | 19.2 | 12.0 |
| 3 | Yes | 50.1 | 19.7 | 10.7 |
| 4 | Yes | 63.4 | 21.9 | 8.2 |
| 5 | Yes | 65.0 | 20.5 | 7.7 |
| Freeze/Thaw Cycles | Cell-1 | Cell-2 | Cell-3 | Cell-4 |
|---|---|---|---|---|
| ECSA(m2·g−1) | ECSA(m2·g−1) | ECSA(m2·g−1) | ECSA(m2·g−1) | |
| 0 | 26.09 | 33.81 | 28.39 | 38.76 |
| 30 | 5.10 | 32.28 | 24.57 | 40.49 |
| Freeze/Thaw Cycles | Cell-1 | Cell-2 | Cell-3 | Cell-4 | ||||
|---|---|---|---|---|---|---|---|---|
| | | | | | | | | |
| 0 | 52.35 | 250.75 | 46.96 | 297.45 | 47.54 | 275.65 | 44.89 | 272.05 |
| 30 | 77.30 | 446.55 | 46.10 | 292.55 | 46.45 | 221.10 | 42.80 | 223.90 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Sun, J.; Jiang, G.; Sun, S.; Shao, Z.; Yu, H.; Duan, F.; Yang, Y. Experimental Study on Critical Membrane Water Content of Proton Exchange Membrane Fuel Cells for Cold Storage at −50 °C. Energies 2021, 14, 4520. https://doi.org/10.3390/en14154520
Yang X, Sun J, Jiang G, Sun S, Shao Z, Yu H, Duan F, Yang Y. Experimental Study on Critical Membrane Water Content of Proton Exchange Membrane Fuel Cells for Cold Storage at −50 °C. Energies. 2021; 14(15):4520. https://doi.org/10.3390/en14154520
Chicago/Turabian StyleYang, Xiaokang, Jiaqi Sun, Guang Jiang, Shucheng Sun, Zhigang Shao, Hongmei Yu, Fangwei Duan, and Yingxuan Yang. 2021. "Experimental Study on Critical Membrane Water Content of Proton Exchange Membrane Fuel Cells for Cold Storage at −50 °C" Energies 14, no. 15: 4520. https://doi.org/10.3390/en14154520
APA StyleYang, X., Sun, J., Jiang, G., Sun, S., Shao, Z., Yu, H., Duan, F., & Yang, Y. (2021). Experimental Study on Critical Membrane Water Content of Proton Exchange Membrane Fuel Cells for Cold Storage at −50 °C. Energies, 14(15), 4520. https://doi.org/10.3390/en14154520








