PAH and POP Presence in Plastic Waste and Recyclates: State of the Art
Abstract
1. Introduction
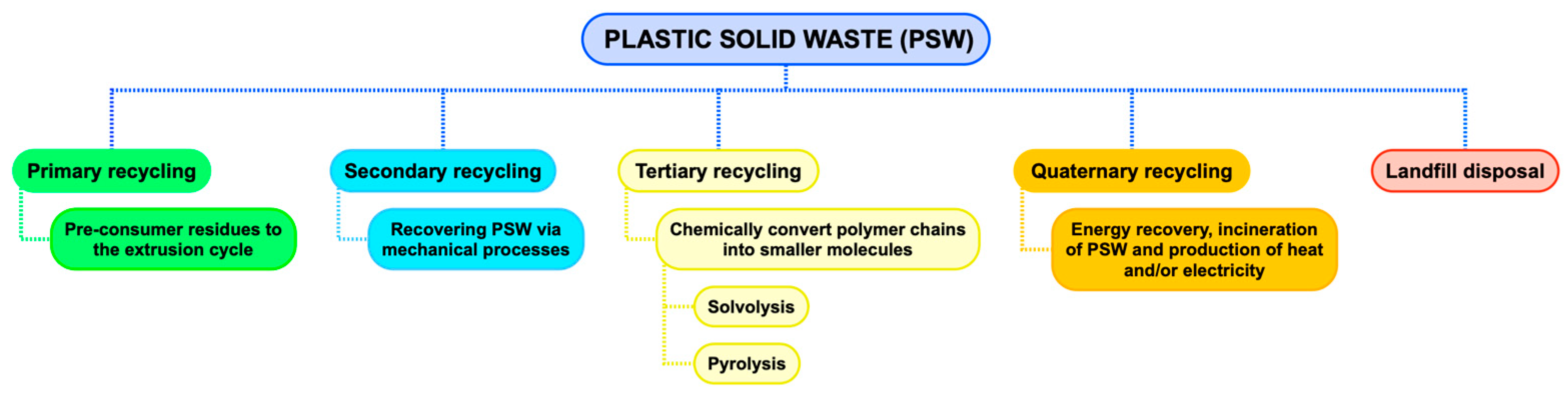
2. Plastic Waste Management
- Primary recycling, which consists of the reintroduction of pre-consumer waste (chopped plastics, edges and pieces of industrial plastic or a single polymer) in the extrusion cycle in order to produce products of the same material (closed-loop recycling or upcycling). Reusing plastics through primary recycling is the best way to work in a circular economy.
- Secondary recycling or mechanical recycling, which includes operations that seek to recover solid plastic waste through mechanical processes. In this way, the new recycled material can be converted into new plastic products, different from the original ones, substituting virgin polymers or a portion of virgin polymers (downcycling). Reusing plastics through primary or secondary recycling enhances resource efficiency and is part of a circular economy approach. The successful recycling of separated plastics counts on different available technologies, but the effective recycling of mixed plastics waste is the next major challenge for the plastics recycling sector [11].
- Tertiary recycling, raw material recycling, or chemical recycling are the terms used for processes that obtain smaller molecules from polymer chains. These small molecules can be used as feedstock for the production of fuels, new polymers, or other chemicals. The following two main groups of methods can be distinguished: solvolysis, i.e., the dissolution/reprecipitation of polymers [12], and pyrolysis, i.e., decomposition into low molecular weight compounds by thermal cracking.
- Quaternary recycling, or energy recovery, which consists of the production of electricity and/or heat from the sensible heat provided by the combustion gases obtained by incinerating plastic waste.
2.1. Energy and Environmental Savings
2.2. Challenges in Plastic Recycling
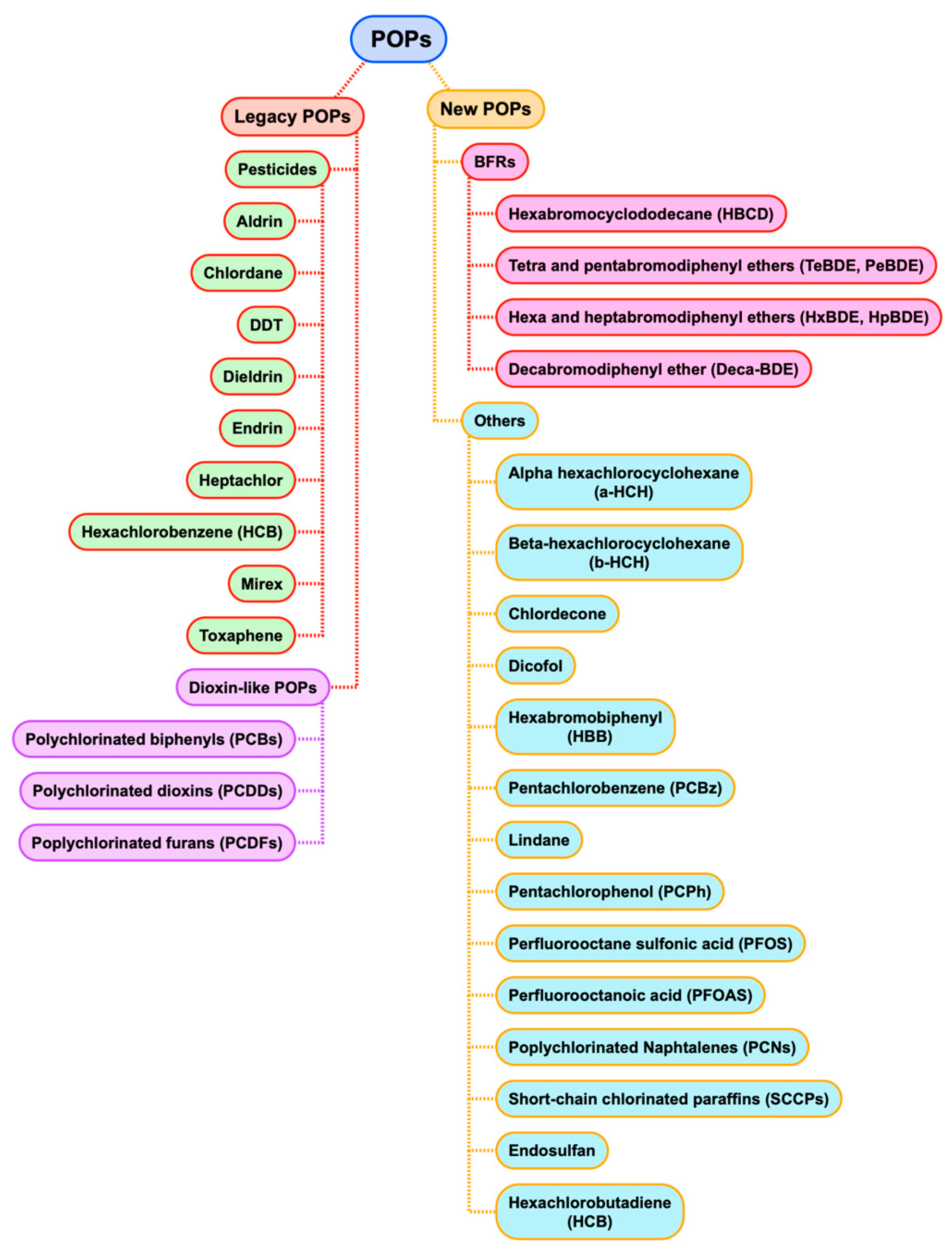
3. Substances of Concern in Recycled Plastics: A Focus on PAHs and POPs
4. Techniques for the Isolation of PAHs and POPs from Plastic Samples
5. Presence of Contaminants in Recycled Plastics
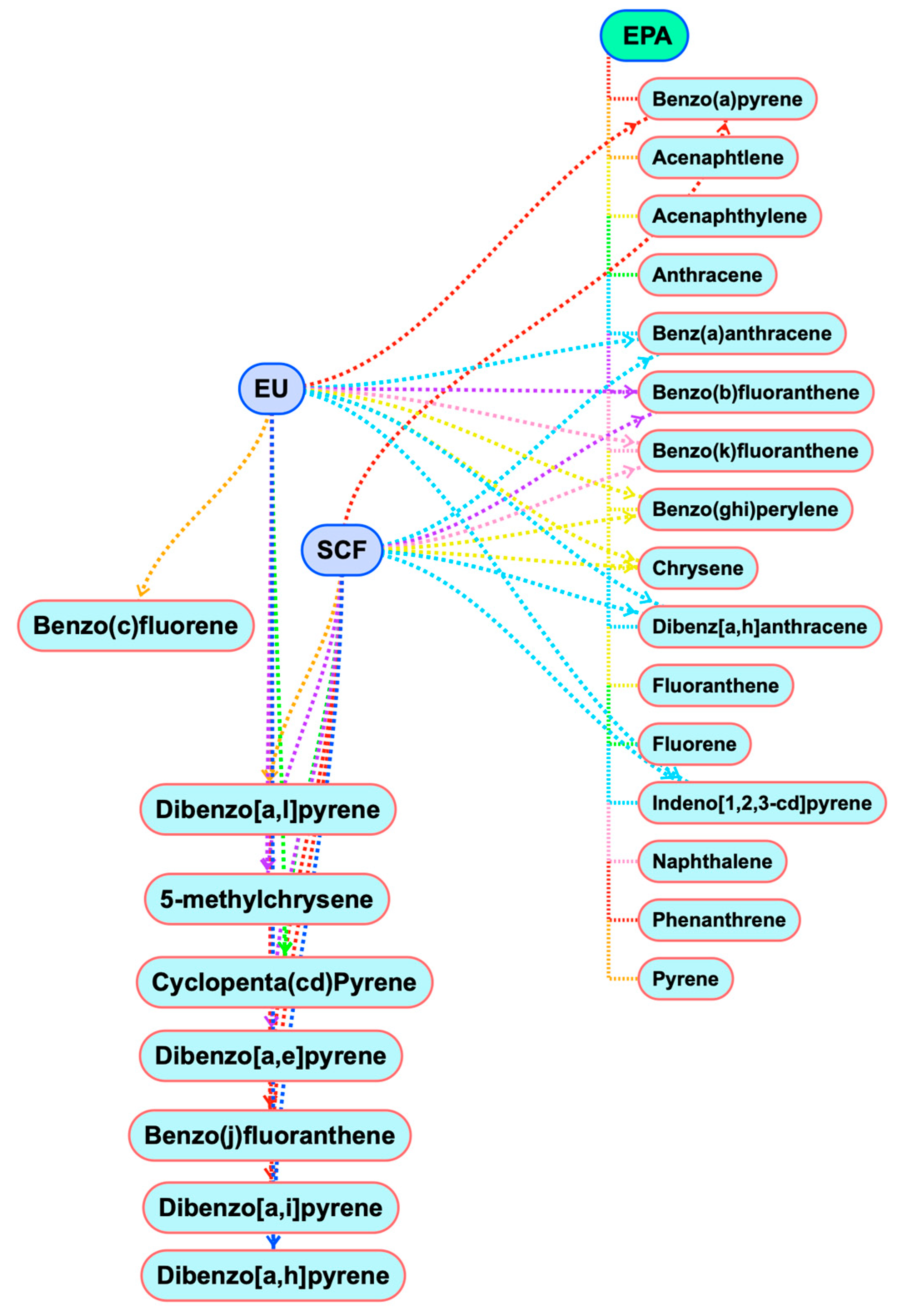
5.1. PAHs
5.2. Dioxin-Like POPs
6. Migration from PSW to Other Materials
7. Further Research Work
- (1)
- Identification of IASs/NIASs: It is necessary to deepen the study of microcontaminants (heavy metals, pesticides, polyaromatic hydrocarbons (PAHs), polychlorinated biphenyls (PCBs) and polychlorinated dioxins and furans (PCDD/Fs)), which have chemical structures very similar to those of IASs/NIASs, with an objective to identify the compounds present in recycled plastics that may negatively affect their application.
- (2)
- Removal of NIASs: In a way, recycled plastic can be considered a contaminated material, such as soil or water. Many of the techniques that are used for removing pollutants could also be used for IAS/NIAS elimination. The techniques used for odor elimination and contaminants’ reduction should be further explored, such as the extraction of organic substances by using environmentally friendly solvents, treatments with hot water, hot air, UV lamps and heavy solvents, among others.
- (3)
- Life Cycle Analysis (LCA): For the selection of sustainable solutions, it is necessary to make a life cycle analysis to prevent the proposed solution from having a higher environmental impact than the current situation.
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABS | acrylonitrile butadiene styrene |
| BDE | brominated diphenyl ether |
| BFRs | brominated flame retardants |
| BTBPE | 1,2-bis(2,4,6-tribromophenoxy)ethane |
| DCM | dichloromethane |
| EPA | US Environmental Protection Agency |
| EU | European Union |
| HBCD | hexabromocyclododecane |
| HDPE | high density polyethylene |
| IAS | Intentionally Added Substances |
| LDPE | low density polyethylene |
| NIAS | non-intentionally added substances |
| PAHs | polycyclic aromatic hydrocarbons |
| PAN | polyacrylonitrile |
| PBDEs | polybrominated diphenyl ethers |
| PCBs | polychlorinated biphenyls |
| PBDD/Fs | polybrominated dioxins and furans |
| PCDD/Fs | polychlorinated dioxins and furans |
| PDMS | polydimethylsiloxane, silicone rubber |
| PE | polyethylene |
| PET | polyethylene terephthalate |
| PMMA | poly methyl methacrylate |
| POPs | persistent organic pollutants |
| PP | polypropylene |
| PS | polystyrene |
| PSW | plastic solid waste |
| PTFE | polytetrafluoroethylene, Teflon® |
| PVC | polyvinyl chloride |
| SCF | EU Scientific Committee for Food |
| SVHCs | substances of very high concern |
| TEQ | toxic equivalents |
| VOCs | volatile organic compounds |
| WEEE | waste from electrical and electronic equipment |
References
- Andrady, A.L.; Neal, M.A. Applications and societal benefits of plastics. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 1977–1984. [Google Scholar] [CrossRef] [PubMed]
- Association of Plastic Manufacturers. Plastics—the Facts 2020; PlasticEurope: London, UK, 2020; Volume 16. [Google Scholar]
- Miller, S.; Bolger, M.; Copello, L. Reusable Solutions: How Governments Can Help Stop Single-Use Plastic Pollution; Zero Waste Europe: Brussels, Belgium, 2009. [Google Scholar]
- European Commission. EuRIC—Plastic Recycling Factsheet/European Circular Economy Stakeholder Platform. Available online: https://circulareconomy.europa.eu/platform/en/knowledge/euric-plastic-recycling-factsheet (accessed on 26 May 2021).
- European Commission. Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions Closing the Loop—An EU Action Plan for the Circular Economy; European Commission: Brussels, Belgium, 2015. [Google Scholar]
- European Commission. Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions—A European Strategy for Plastics in a Circular Economy; European Commission: Brussels, Belgium, 2018. [Google Scholar]
- European Comission. Changing the Way We Use Plastics; European Commission: Brussels, Belgium, 2018; Volume 4. [Google Scholar]
- European Parliament. Directive (EU) 2018/852 of the European Parliament and of the Council of 30 May 2018 amending Directive 94/62/EC on packaging and packaging waste. Off. J. Eur. Union 2018, 2018, 141–154. [Google Scholar]
- American Society for Testing Materials. ASTM D7209-06: Standard Guide for Waste Reduction, Resource Recovery, and Use of Recycled Polymeric Materials and Products; American Society for Testing and Materials: West Conshohocken, PA, USA, 2013; pp. 1–7. [Google Scholar]
- International Organization for Standarization—ISO. ISO 15270_2008 (en), Plastics—Guidelines for the Recovery and Recycling of Plastics Waste. Available online: https://www.iso.org/standard/45089.html (accessed on 3 May 2021).
- Hopewell, J.; Dvorak, R.; Kosior, E. Plastics recycling: Challenges and opportunities. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2115–2126. [Google Scholar] [CrossRef] [PubMed]
- Achilias, D.; Roupakias, C.; Megalokonomos, P.; Lappas, A.; Antonakou, Ε. Chemical recycling of plastic wastes made from polyethylene (LDPE and HDPE) and polypropylene (PP). J. Hazard. Mater. 2007, 149, 536–542. [Google Scholar] [CrossRef]
- Karmakar, G.P. Regeneration and Recovery of Plastics. Ref. Modul. Mater. Sci. Mater. Eng. 2020. [Google Scholar] [CrossRef]
- Vollmer, I.; Jenks, M.J.F.; Roelands, M.C.P.; White, R.J.; Van Harmelen, T.; De Wild, P.; Van Der Laan, G.P.; Meirer, F.; Keurentjes, J.T.F.; Weckhuysen, B.M. Beyond Mechanical Recycling: Giving New Life to Plastic Waste. Angew. Chem. Int. Ed. 2020, 59, 15402–15423. [Google Scholar] [CrossRef] [PubMed]
- Wagner, S.; Schlummer, M. Legacy additives in a circular economy of plastics: Current dilemma, policy analysis, and emerging countermeasures. Resour. Conserv. Recycl. 2020, 158, 104800. [Google Scholar] [CrossRef]
- Gullett, B.K.; Wyrzykowska, B.; Grandesso, E.; Touati, A.; Tabor, D.G.; Ochoa, G.S. PCDD/F, PBDD/F, and PBDE Emissions from Open Burning of a Residential Waste Dump. Environ. Sci. Technol. 2010, 44, 394–399. [Google Scholar] [CrossRef]
- Gullett, B.; Sarofim, A.; Smith, K.; Procaccini, C. The Role of Chlorine in Dioxin Formation. Process. Saf. Environ. Prot. 2000, 78, 47–52. [Google Scholar] [CrossRef]
- Conesa, J.A.; Fullana, A.; Font, R. De Novo Synthesis of PCDD/F by Thermogravimetry. Environ. Sci. Technol. 2002, 36, 263–269. [Google Scholar] [CrossRef]
- Ortuño, N.; Conesa, J.A.; Moltó, J.; Font, R. De Novo Synthesis of Brominated Dioxins and Furans. Environ. Sci. Technol. 2014, 48, 7959–7965. [Google Scholar] [CrossRef]
- Ortuño, N.; Moltó, J.; Conesa, J.A.; Font, R. Formation of brominated pollutants during the pyrolysis and combustion of tetrabromobisphenol A at different temperatures. Environ. Pollut. 2014, 191, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Ebert, J. Formation of PBDD/F from flame-retarded plastic materials under thermal stress. Environ. Int. 2003, 29, 711–716. [Google Scholar] [CrossRef]
- USEPA Waste Reduction Model (WARM). Available online: https://www.epa.gov/warm (accessed on 11 May 2021).
- Singh, N.; Hui, D.; Singh, R.; Ahuja, I.; Feo, L.; Fraternali, F. Recycling of plastic solid waste: A state of art review and future applications. Compos. Part B Eng. 2017, 115, 409–422. [Google Scholar] [CrossRef]
- Groh, K.J.; Backhaus, T.; Carney-Almroth, B.; Geueke, B.; Inostroza, P.; Lennquist, A.; Leslie, H.A.; Maffini, M.; Slunge, D.; Trasande, L.; et al. Overview of known plastic packaging-associated chemicals and their hazards. Sci. Total. Environ. 2019, 651, 3253–3268. [Google Scholar] [CrossRef] [PubMed]
- Horodytska, O.; Cabanes, A.; Fullana, A. Non-intentionally added substances (NIAS) in recycled plastics. Chemosphere 2020, 251, 126373. [Google Scholar] [CrossRef] [PubMed]
- European Commission. A Circular Economy for Plastics—Insights from Research and Innovation to Inform Policy and Funding Decisions; European Commission: Brussels, Belgium, 2019. [Google Scholar]
- Stenmarck, Å.; Belleza, E.L.; Fråne, A.; Busch, N.; Larsen, Å.; Wahlström, M. Hazardous Substances in Plastics-Ways to Increase Recycling—Ways to Increase Recycling; Nordic Council of Ministers: Copenhagen, Denmark, 2017; ISBN 9789188319517. [Google Scholar]
- Cabanes, A.; Valdés, F.; Fullana, A. A review on VOCs from recycled plastics. Sustain. Mater. Technol. 2020, 25, e00179. [Google Scholar] [CrossRef]
- Lerda, D. Polycyclic Aromatic Hydrocarbons (PAHs) Factsheet, 4th ed; JRC Technical Notes: Geel, Belgium, 2011. [Google Scholar]
- Stockholm Convention Home Page. Available online: http://chm.pops.int/Home/tabid/2121/Default.aspx (accessed on 10 May 2021).
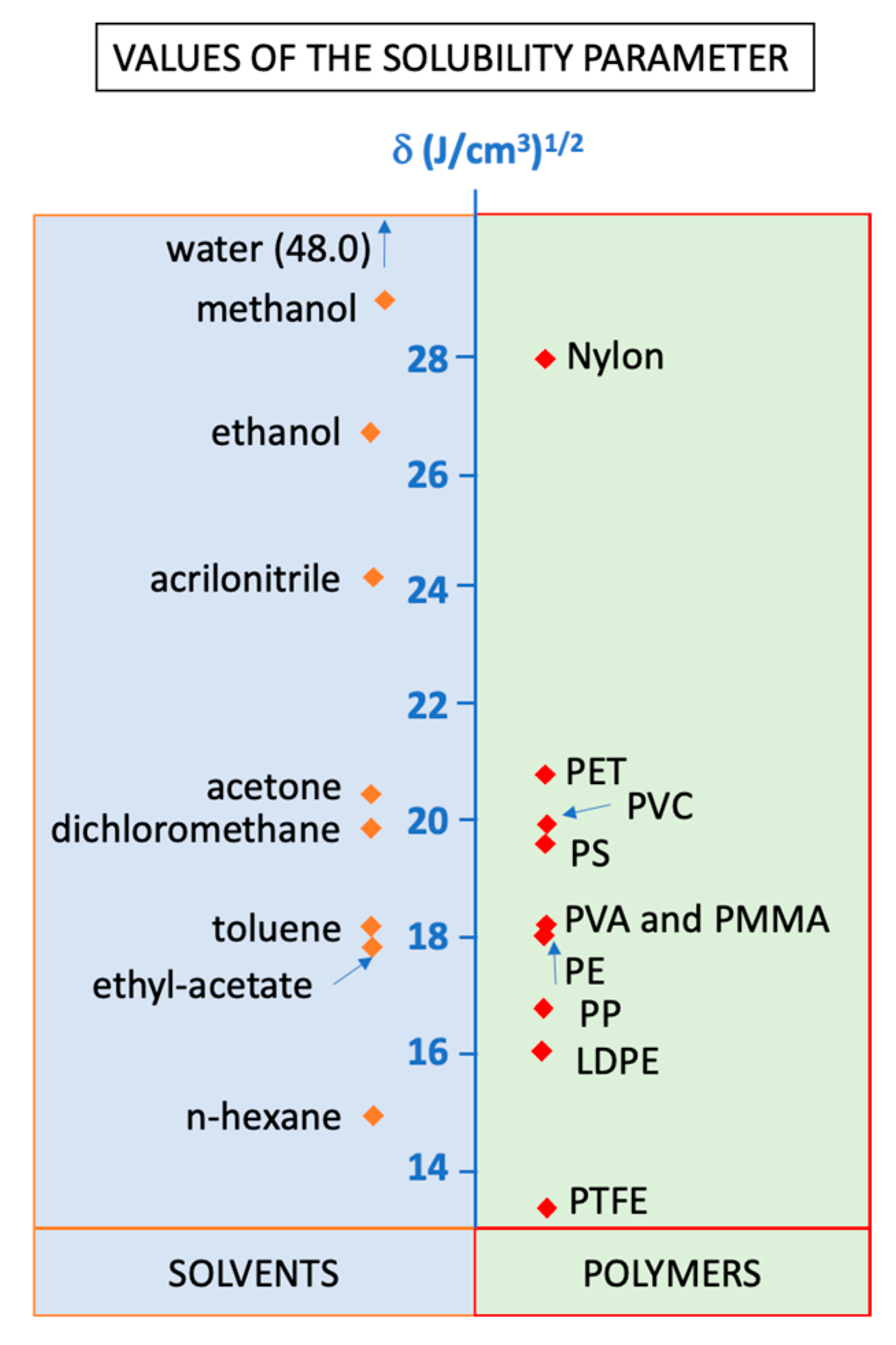
- Singla, M.; Díaz, J.; Broto-Puig, F.; Borrós, S. Sorption and release process of polybrominated diphenyl ethers (PBDEs) from different composition microplastics in aqueous medium: Solubility parameter approach. Environ. Pollut. 2020, 262, 114377. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Camargo, A.D.P.; Bueno, M.; Parada-Alfonso, F.; Cifuentes, A.; Ibáñez, E. Hansen solubility parameters for selection of green extraction solvents. TrAC Trends Anal. Chem. 2019, 118, 227–237. [Google Scholar] [CrossRef]
- Lu, Y.; Shi, J.-J.; Sun, L. Investigation of the selection of extraction solvent for extracting the n-alkane from diesel by means of solubility parameters theory. J. Fuel Chem. Technol. 2008, 36, 297–301. [Google Scholar] [CrossRef]
- Polymer Database Polymer Solubility. 2015. Available online: https://polymerdatabase.com/polymer%20physics/Polymer%20Solubility.html (accessed on 10 June 2021).
- He, N.; Smeds, A.; Friman, R.; Rosenholm, J.B. Solubility parameters of biopolymers. Phys. Chem. Liq. 2013, 51, 302–316. [Google Scholar] [CrossRef]
- Li, S.-Q.; Ni, H.-G.; Zeng, H. PAHs in polystyrene food contact materials: An unintended consequence. Sci. Total. Environ. 2017, 609, 1126–1131. [Google Scholar] [CrossRef] [PubMed]
- Nerín, C.; Tornés, A.R.; Domeño, C.; Cacho, J. Absorption of Pesticides on Plastic Films Used as Agricultural Soil Covers. J. Agric. Food Chem. 1996, 44, 4009–4014. [Google Scholar] [CrossRef]
- Pivnenko, K.; Granby, K.; Eriksson, E.; Astrup, T. Recycling of plastic waste: Screening for brominated flame retardants (BFRs). Waste Manag. 2017, 69, 101–109. [Google Scholar] [CrossRef]
- Rani, M.; Shim, W.J.; Han, G.M.; Jang, M.; Song, Y.K.; Hong, S.H. Hexabromocyclododecane in polystyrene based consumer products: An evidence of unregulated use. Chemosphere 2014, 110, 111–119. [Google Scholar] [CrossRef]
- León, V.M.; García-Agüera, I.; Moltó, V.; Fernández-González, V.; Llorca-Pérez, L.; Andrade, J.M.; Muniategui, S.; Campillo, J.A. PAHs, pesticides, personal care products and plastic additives in plastic debris from Spanish Mediterranean beaches. Sci. Total Environ. 2019, 670, 672–684. [Google Scholar] [CrossRef]
- Fisner, M.; Majer, A.; Taniguchi, S.; Bícego, M.; Turra, A.; Gorman, D. Colour spectrum and resin-type determine the concentration and composition of Polycyclic Aromatic Hydrocarbons (PAHs) in plastic pellets. Mar. Pollut. Bull. 2017, 122, 323–330. [Google Scholar] [CrossRef]
- Iñiguez, M.E.; Conesa, J.A.; Fullana, A. Pollutant content in marine debris and characterization by thermal decomposition. Mar. Pollut. Bull. 2017, 117, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Bouhroum, R.; Boulkamh, A.; Asia, L.; Lebarillier, S.; Ter Halle, A.; Syakti, A.; Doumenq, P.; Malleret, L.; Wong-Wah-Chung, P. Concentrations and fingerprints of PAHs and PCBs adsorbed onto marine plastic debris from the Indonesian Cilacap coast and theNorth Atlantic gyre. Reg. Stud. Mar. Sci. 2019, 29, 100611. [Google Scholar] [CrossRef]
- Budin, C.; Petrlik, J.; Strakova, J.; Hamm, S.; Beeler, B.; Behnisch, P.; Besselink, H.; van der Burg, B.; Brouwer, A. Detection of high PBDD/Fs levels and dioxin-like activity in toys using a combination of GC-HRMS, rat-based and human-based DR CALUX® reporter gene assays. Chemosphere 2020, 251, 126579. [Google Scholar] [CrossRef]
- DiGangi, J.; Strakova, J. A survey of PBDEs in recycled carpet padding. Organohalogen Compd. 2011, 73, 2067–2070. [Google Scholar]
- Vápenka, L.; Vavrouš, A.; Votavová, L.; Kejlová, K.; Dobiáš, J.; Sosnovcová, J. Contaminants in the paper-based food packaging materials used in the Czech Republic. J. Food Nutr. Res. 2016, 55, 361–373. [Google Scholar]
- Rochman, C.M.; Hoh, E.; Hentschel, B.T.; Kaye, S. Long-Term Field Measurement of Sorption of Organic Contaminants to Five Types of Plastic Pellets: Implications for Plastic Marine Debris. Environ. Sci. Technol. 2013, 47, 1646–1654. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, H.; Allgeier, A.; Zhou, Q.; Ouellet, J.D.; Crawford, S.; Luo, Y.; Yang, Y.; Shi, H.; Hollert, H. Marine microplastics bound dioxin-like chemicals: Model explanation and risk assessment. J. Hazard. Mater. 2019, 364, 82–90. [Google Scholar] [CrossRef]
- Fisner, M.; Taniguchi, S.; Majer, A.P.; Bícego, M.C.; Turra, A. Concentration and composition of polycyclic aromatic hydrocarbons (PAHs) in plastic pellets: Implications for small-scale diagnostic and environmental monitoring. Mar. Pollut. Bull. 2013, 76, 349–354. [Google Scholar] [CrossRef]
- Shaw, E.J.; Turner, A. Recycled electronic plastic and marine litter. Sci. Total Environ. 2019, 694, 133644. [Google Scholar] [CrossRef]
- Turner, A. Black plastics: Linear and circular economies, hazardous additives and marine pollution. Environ. Int. 2018, 117, 308–318. [Google Scholar] [CrossRef]
- Turner, A.; Filella, M. Bromine in plastic consumer products—Evidence for the widespread recycling of electronic waste. Sci. Total Environ. 2017, 601–602, 374–379. [Google Scholar] [CrossRef] [PubMed]
- Leslie, H.; Leonards, P.; Brandsma, S.; de Boer, J.; Jonkers, N. Propelling plastics into the circular economy—weeding out the toxics first. Environ. Int. 2016, 94, 230–234. [Google Scholar] [CrossRef] [PubMed]
- Teuten, E.L.; Saquing, J.M.; Knappe, D.R.U.; Barlaz, M.A.; Jonsson, S.; Björn, A.; Rowland, S.J.; Thompson, R.C.; Galloway, T.S.; Yamashita, R.; et al. Transport and release of chemicals from plastics to the environment and to wildlife. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2027–2045. [Google Scholar] [CrossRef] [PubMed]
- DiGangi, J.; Strakova, J.; Bell, L. POPs Recycling Contaminates Children’s Toys with Toxic Flame Retardants. 2017. Available online: https://ipen.org/sites/default/files/documents/toxic_toy_report_2017_update_v2_1-en.pdf (accessed on 10 June 2021).
- Ionas, A.C.; Ulevicus, J.; Ballesteros-Gómez, A.; Brandsma, S.H.; Leonards, P.E.; van de Bor, M.; Covaci, A. Children’s exposure to polybrominated diphenyl ethers (PBDEs) through mouthing toys. Environ. Int. 2016, 87, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-C.; Hsi, H.-C.; Wang, Y.-F.; Lin, S.-L.; Chang-Chien, G.-P. Distribution of polybrominated diphenyl ethers (PBDEs) and polybrominated dibenzo-p-dioxins and dibenzofurans (PBDD/Fs) in municipal solid waste incinerators. Environ. Pollut. 2010, 158, 1595–1602. [Google Scholar] [CrossRef] [PubMed]
- Ortuño, N.; Lundstedt, S.; Lundin, L. Emissions of PBDD/Fs, PCDD/Fs and PBDEs from flame-retarded high-impact polystyrene under thermal stress. Chemosphere 2015, 123, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Zhan, F.; Zhang, H.; Cao, R.; Fan, Y.; Xu, P.; Chen, J. Release and Transformation of BTBPE During the Thermal Treatment of Flame Retardant ABS Plastics. Environ. Sci. Technol. 2018, 53, 185–193. [Google Scholar] [CrossRef]
- Berg, M.V.D.; Denison, M.S.; Birnbaum, L.S.; DeVito, M.J.; Fiedler, H.; Falandysz, J.; Rose, M.; Schrenk, D.; Safe, S.; Tohyama, C.; et al. Polybrominated Dibenzo-p-Dioxins, Dibenzofurans, and Biphenyls: Inclusion in the Toxicity Equivalency Factor Concept for Dioxin-Like Compounds. Toxicol. Sci. 2013, 133, 197–208. [Google Scholar] [CrossRef]
- UNEP. Supporting Document for the Draft Technical Paper Developed in Accordance with the Work Programmes on New Persistent Organic Pollutants as Adopted by the Conference of the Parties; UNEP: Nairobi, Kenya, 2010. [Google Scholar]
- Weber, R.; Kuch, B. Relevance of BFRs and thermal conditions on the formation pathways of brominated and brominated-chlorinated dibenzodioxins and dibenzofurans. Environ. Int. 2003, 29, 699–710. [Google Scholar] [CrossRef]
- Roosen, M.; Mys, N.; Kusenberg, M.; Billen, P.; Dumoulin, A.; Dewulf, J.; Van Geem, K.M.; Ragaert, K.; De Meester, S. Detailed Analysis of the Composition of Selected Plastic Packaging Waste Products and Its Implications for Mechanical and Thermochemical Recycling. Environ. Sci. Technol. 2020, 54, 13282–13293. [Google Scholar] [CrossRef]
- Fujimori, T.; Takaoka, M.; Takeda, N. Influence of Cu, Fe, Pb, and Zn Chlorides and Oxides on Formation of Chlorinated Aromatic Compounds in MSWI Fly Ash. Environ. Sci. Technol. 2009, 43, 8053–8059. [Google Scholar] [CrossRef]
- Tongue, A.D.; Reynolds, S.J.; Fernie, K.J.; Harrad, S. Flame retardant concentrations and profiles in wild birds associated with landfill: A critical review. Environ. Pollut. 2019, 248, 646–658. [Google Scholar] [CrossRef]
- Gilbert, N.I.; Correia, R.A.; Silva, J.P.; Pacheco, C.; Catry, I.; Atkinson, P.W.; Gill, J.A.; Franco, A.M.A. Are white storks addicted to junk food? Impacts of landfill use on the movement and behaviour of resident white storks (Ciconia ciconia) from a partially migratory population. Mov. Ecol. 2016, 4, 1–13. [Google Scholar] [CrossRef]
- Hahladakis, J.N.; Velis, C.A.; Weber, R.; Iacovidou, E.; Purnell, P. An overview of chemical additives present in plastics: Migration, release, fate and environmental impact during their use, disposal and recycling. J. Hazard. Mater. 2018, 344, 179–199. [Google Scholar] [CrossRef]
- Guzzonato, A.; Puype, F.; Harrad, S. Improving the accuracy of hand-held X-ray fluorescence spectrometers as a tool for monitoring brominated flame retardants in waste polymers. Chemosphere 2016, 159, 89–95. [Google Scholar] [CrossRef]
- Cabanes, A.; Fullana, A. New methods to remove volatile organic compounds from post-consumer plastic waste. Sci. Total Environ. 2021, 758, 144066. [Google Scholar] [CrossRef]
- Sun, B.; Hu, Y.; Cheng, H.; Tao, S. Kinetics of Brominated Flame Retardant (BFR) Releases from Granules of Waste Plastics. Environ. Sci. Technol. 2016, 50, 13419–13427. [Google Scholar] [CrossRef]
- Sun, B.; Hu, Y.; Cheng, H.; Tao, S. Releases of brominated flame retardants (BFRs) from microplastics in aqueous medium: Kinetics and molecular-size dependence of diffusion. Water Res. 2019, 151, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Rusina, T.; Smedes, F.; Klanova, J. Diffusion coefficients of polychlorinated biphenyls and polycyclic aromatic hydrocarbons in polydimethylsiloxane and low-density polyethylene polymers. J. Appl. Polym. Sci. 2010, 116, 1803–1810. [Google Scholar] [CrossRef]
- Valderrama, J.F.N.; Baek, K.; Molina, F.J.; Allan, I.J. Implications of observed PBDE diffusion coefficients in low density polyethylene and silicone rubber. Environ. Sci. Process. Impacts 2015, 18, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Lin, S.; Chen, S.; Zhang, H. Determination of bisphenol A diglycidyl ether, novolac glycidyl ether and their derivatives migrated from can coatings into foodstuff by UPLC-MS/MS. Eur. Food Res. Technol. 2012, 235, 231–244. [Google Scholar] [CrossRef]
- Franz, R.; Welle, F. Contamination Levels in Recollected PET Bottles from Non-Food Applications and their Impact on the Safety of Recycled PET for Food Contact. Molecules 2020, 25, 4998. [Google Scholar] [CrossRef] [PubMed]
- Geueke, B. Dossier Non-intentionally added substances (NIAS). Food Packag. Forum 2018, 7. [Google Scholar] [CrossRef]
| Reference | Finding |
|---|---|
| Vapenka et al. [46] | PAHs in more than 50% of packaging materials. 1212 mg/kg of fluorene |
| Chen et al. [48] | Styrofoam presents a greater ability to adsorb POPs, compared to PE and polypropylene (PP). Higher PAH concentrations were found in plastic particles from coastal beaches (1722.9–31764.8 μg/kg) rather than in plastic particles from open ocean areas (nd-6298.8 μg/kg) |
| Fisner et al. [41] | Type (and color) of the polymers determine the concentration of PAHs. Increase in PAH concentrations along a spectrum of darkening pellets |
| Fisner et al. [49] | No correlation between the PAH content and the location of the plastic item |
| Bouhroum et al. [43] | Coastal debris contains high concentrations of PAHs and PCBs, while plastic debris found in remote sites showed much lower concentrations. |
| Budin et al. [44] | High levels of PBDD/Fs in plastic parts of some toys |
| Pivnenko et al. [38] | Presence of BFRs associated with ABS and PS |
| Rochman et al. [47] | HDPE, LDPE and PP sorb greater concentrations of PCBs and PAHs than PET and PVC |
| Rani et al. [39] | 0.475 mg/kg of HBCD in expanded PS items |
| Turner et al. [50,51,52] | Bromine in about 42% of WEEE samples, and 18% of non-WEEE |
| Leslie et al. [53] | BFRs in plastic materials destined for recycling markets |
| DiGangi et al. [45] | 1100 mg/kg PentaBDE and OctaBDE in recycled carpet padding |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Conesa, J.A.; Nuñez, S.S.; Ortuño, N.; Moltó, J. PAH and POP Presence in Plastic Waste and Recyclates: State of the Art. Energies 2021, 14, 3451. https://doi.org/10.3390/en14123451
Conesa JA, Nuñez SS, Ortuño N, Moltó J. PAH and POP Presence in Plastic Waste and Recyclates: State of the Art. Energies. 2021; 14(12):3451. https://doi.org/10.3390/en14123451
Chicago/Turabian StyleConesa, Juan A., Samuel S. Nuñez, Núria Ortuño, and Julia Moltó. 2021. "PAH and POP Presence in Plastic Waste and Recyclates: State of the Art" Energies 14, no. 12: 3451. https://doi.org/10.3390/en14123451
APA StyleConesa, J. A., Nuñez, S. S., Ortuño, N., & Moltó, J. (2021). PAH and POP Presence in Plastic Waste and Recyclates: State of the Art. Energies, 14(12), 3451. https://doi.org/10.3390/en14123451









