Impact of the Paleoclimate, Paleoenvironment, and Algae Bloom: Organic Matter Accumulation in the Lacustrine Lucaogou Formation of Jimsar Sag, Junggar Basin, NW China
Abstract
1. Introduction
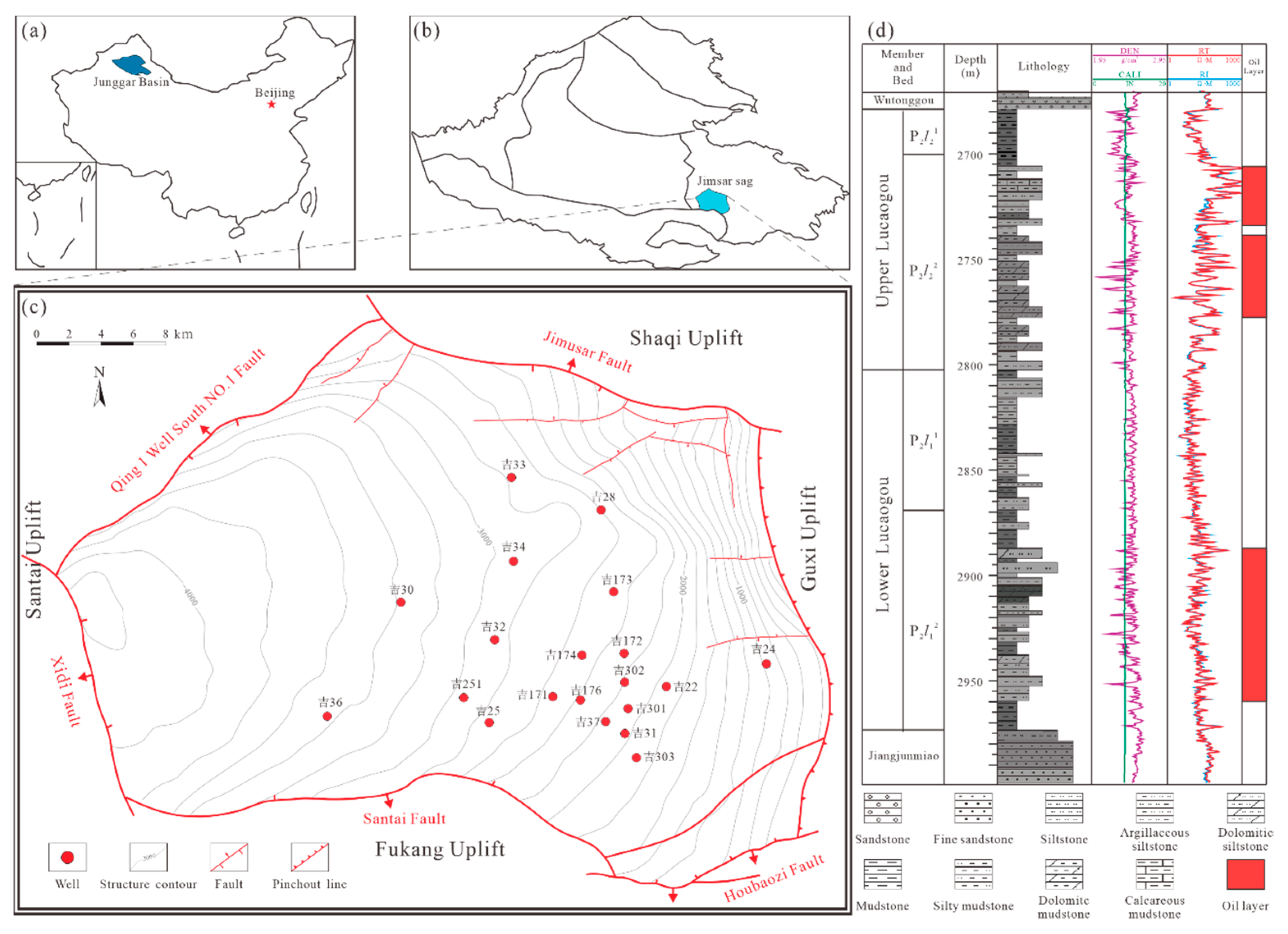
2. Geological Settings
3. Materials and Methods
4. Results
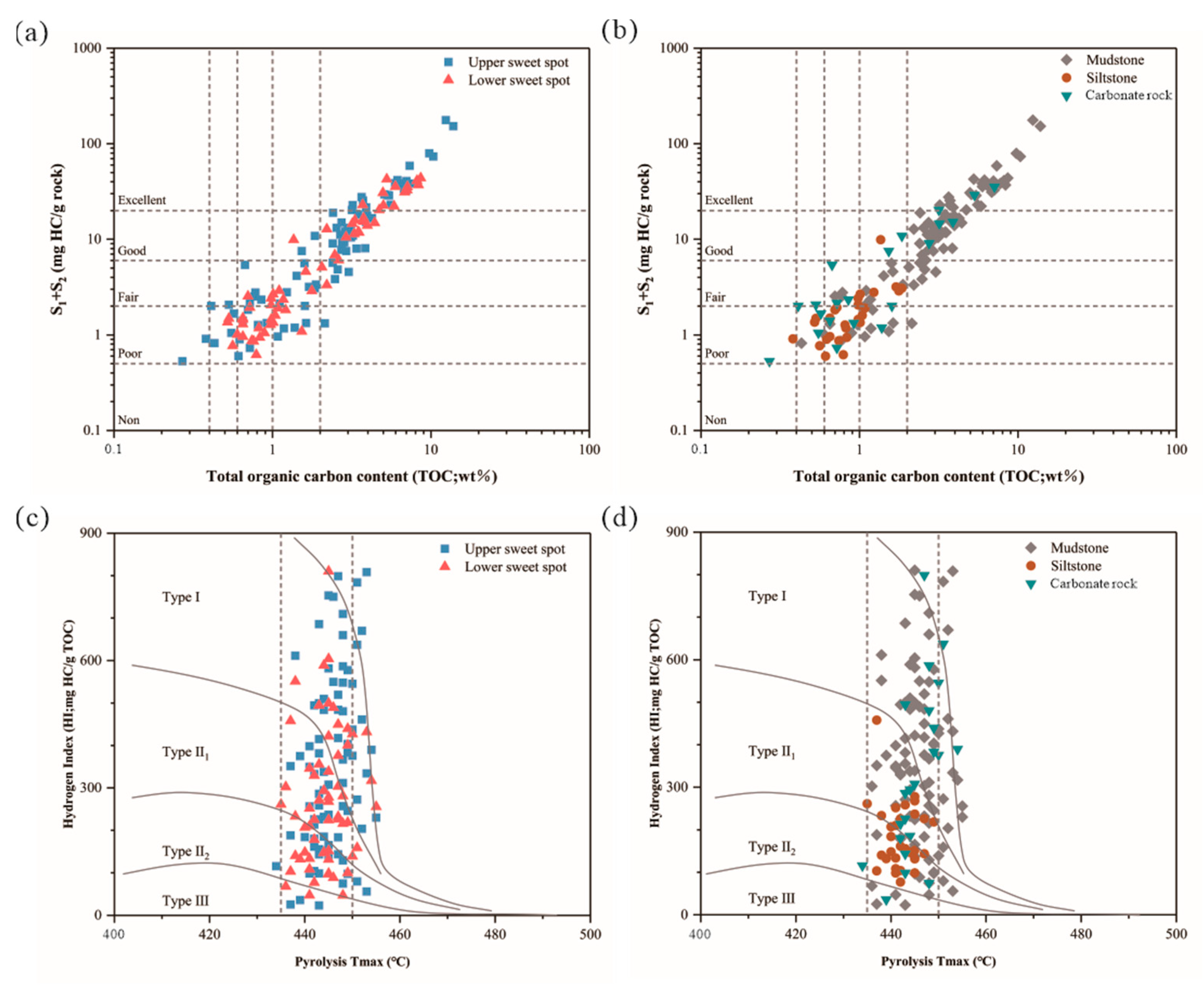
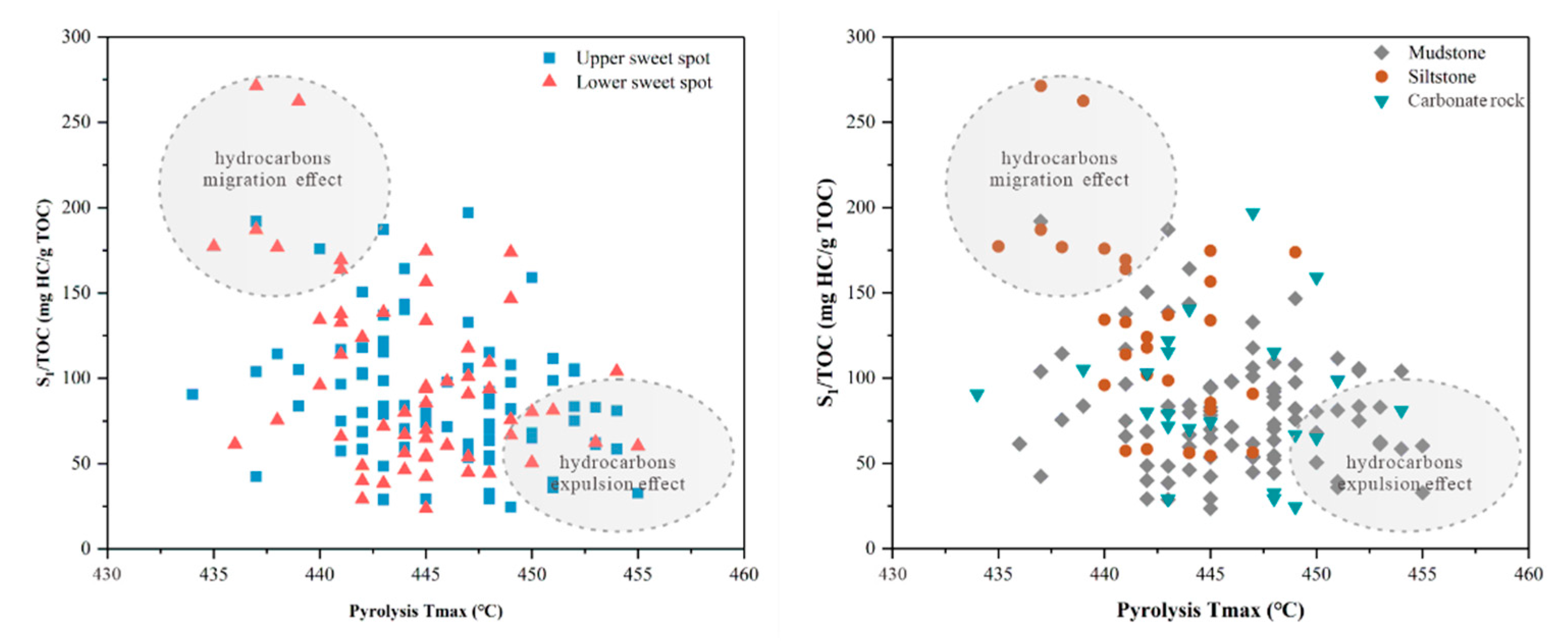
4.1. Total Organic Carbon and Rock-Eval Pyrolysis
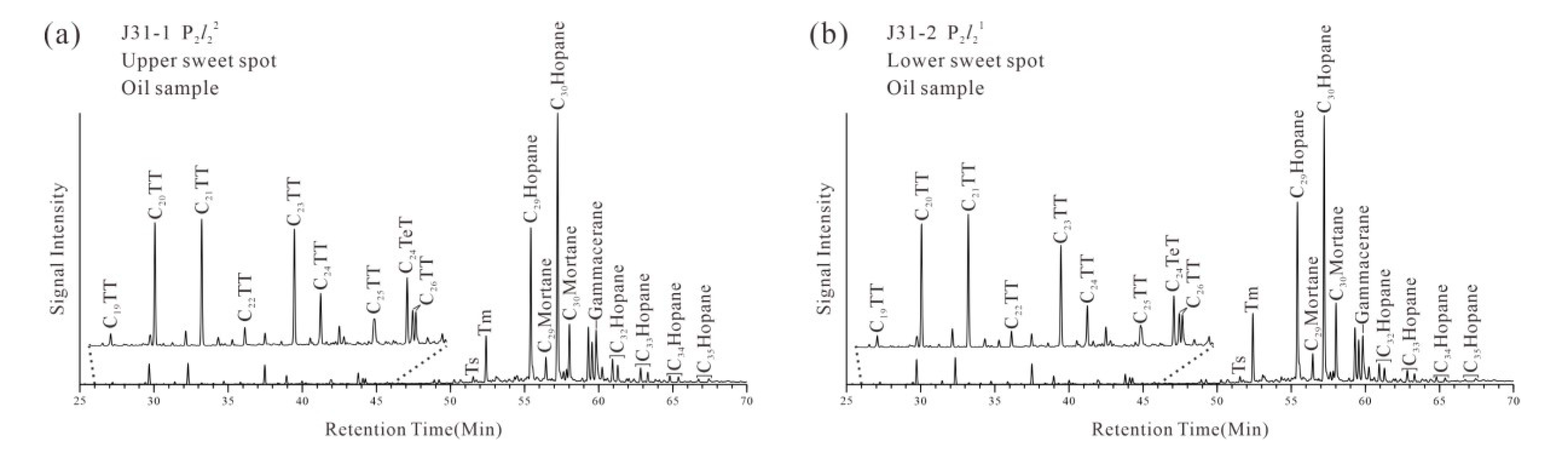
4.2. Molecular Marker Composition of Crude Oil Samples
4.2.1. N-Alkanes and Isoprenoids
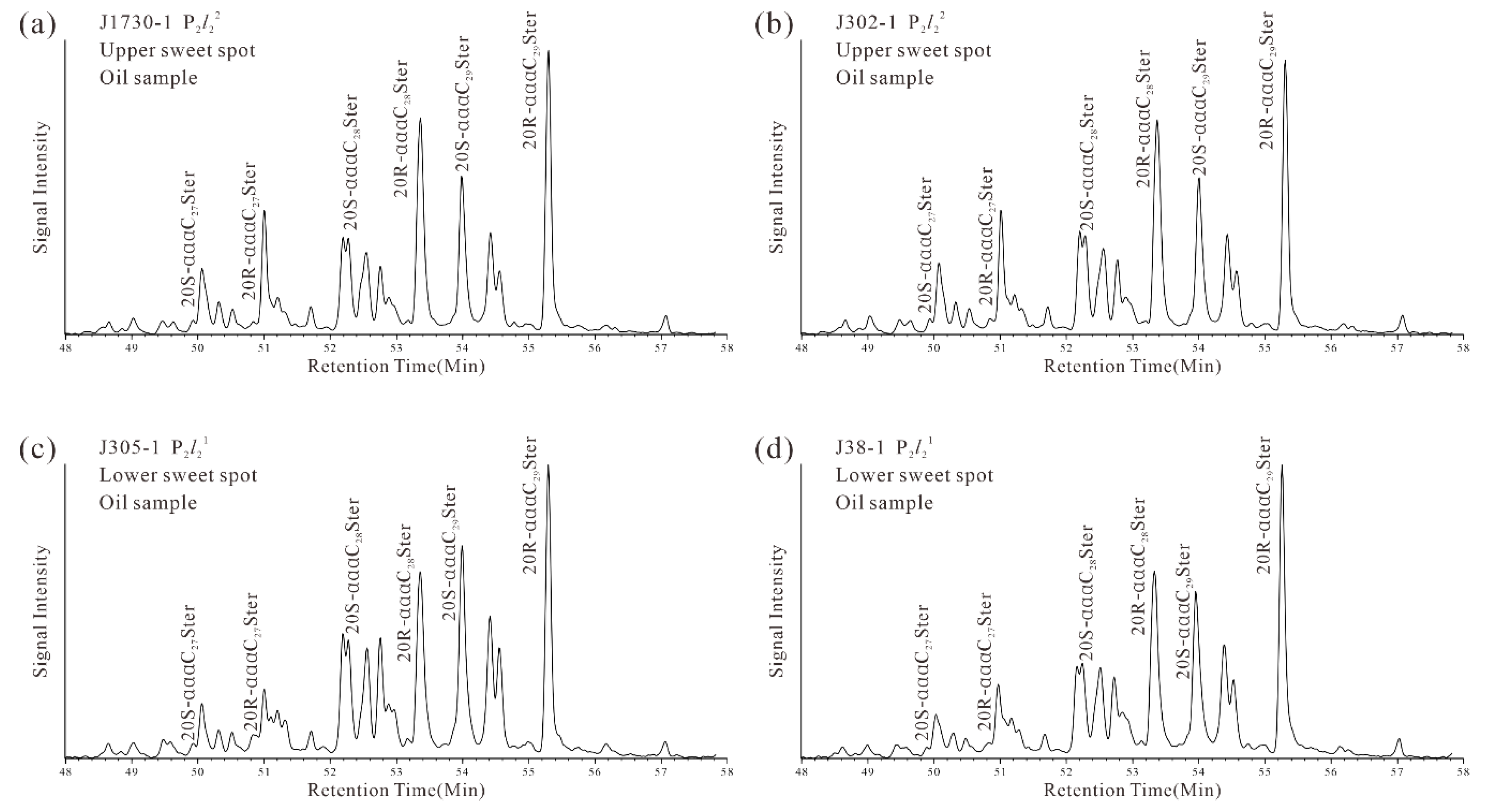
4.2.2. Terpanes and Steranes
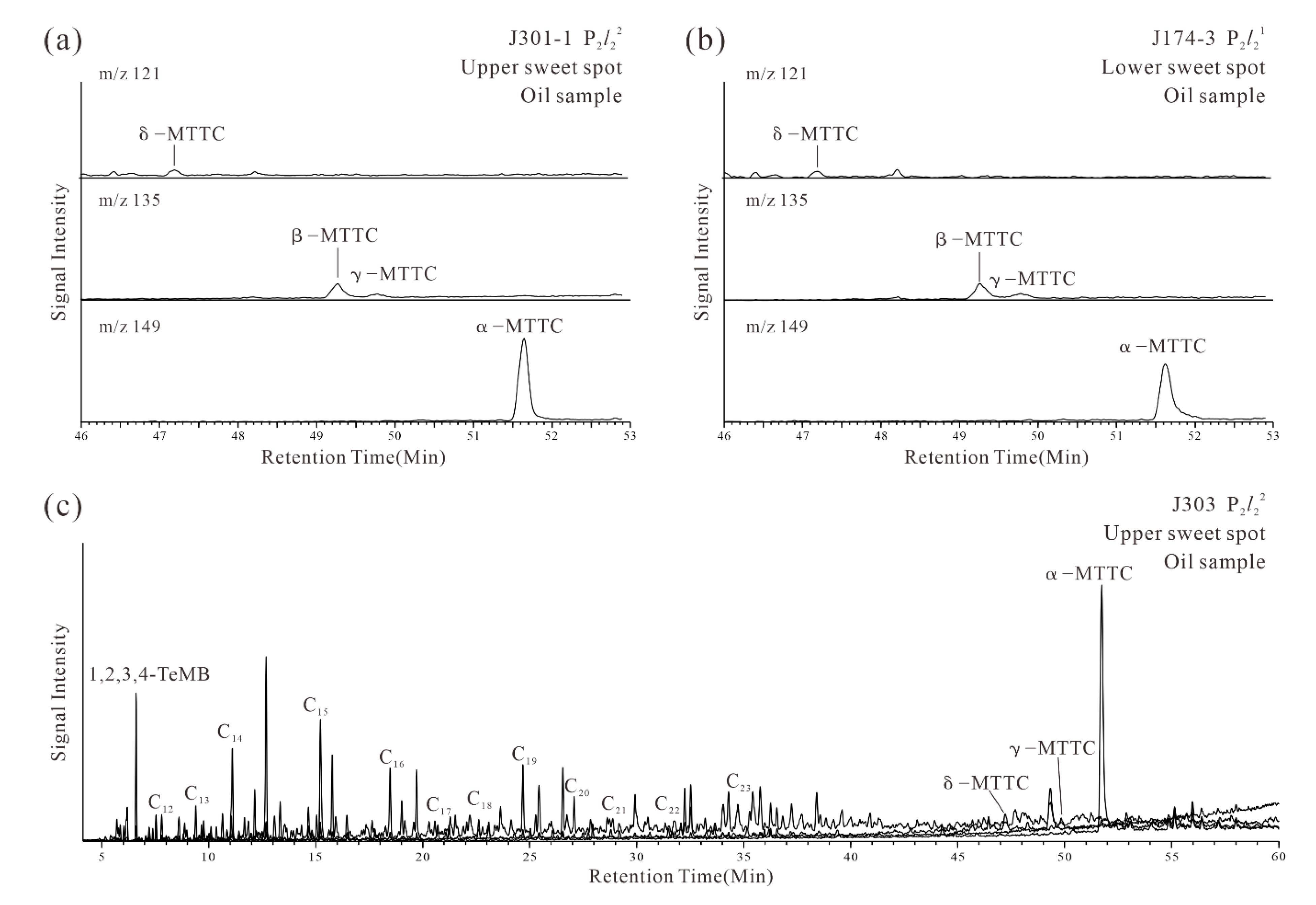
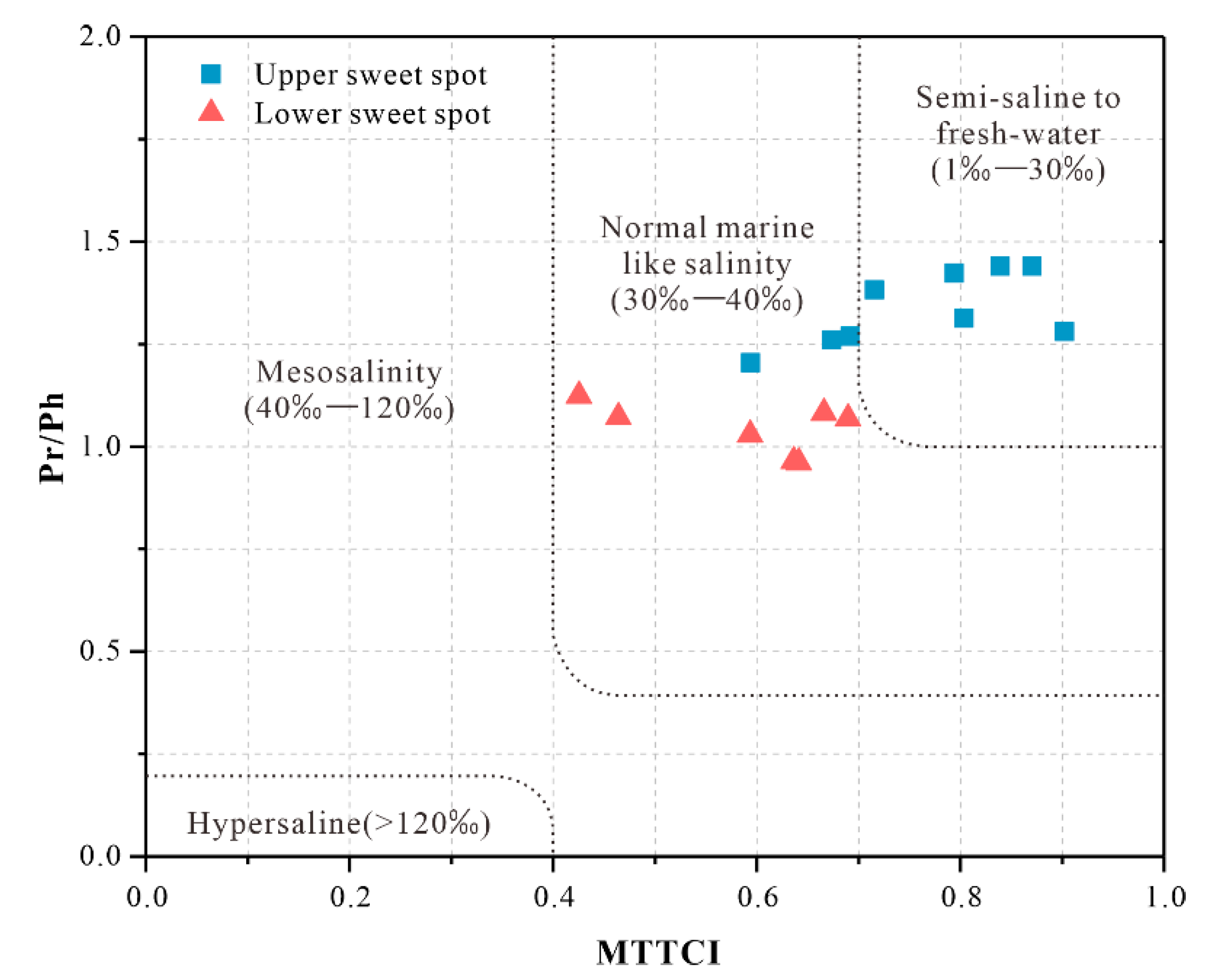
4.2.3. Chromans and Aryl Isoprenoids
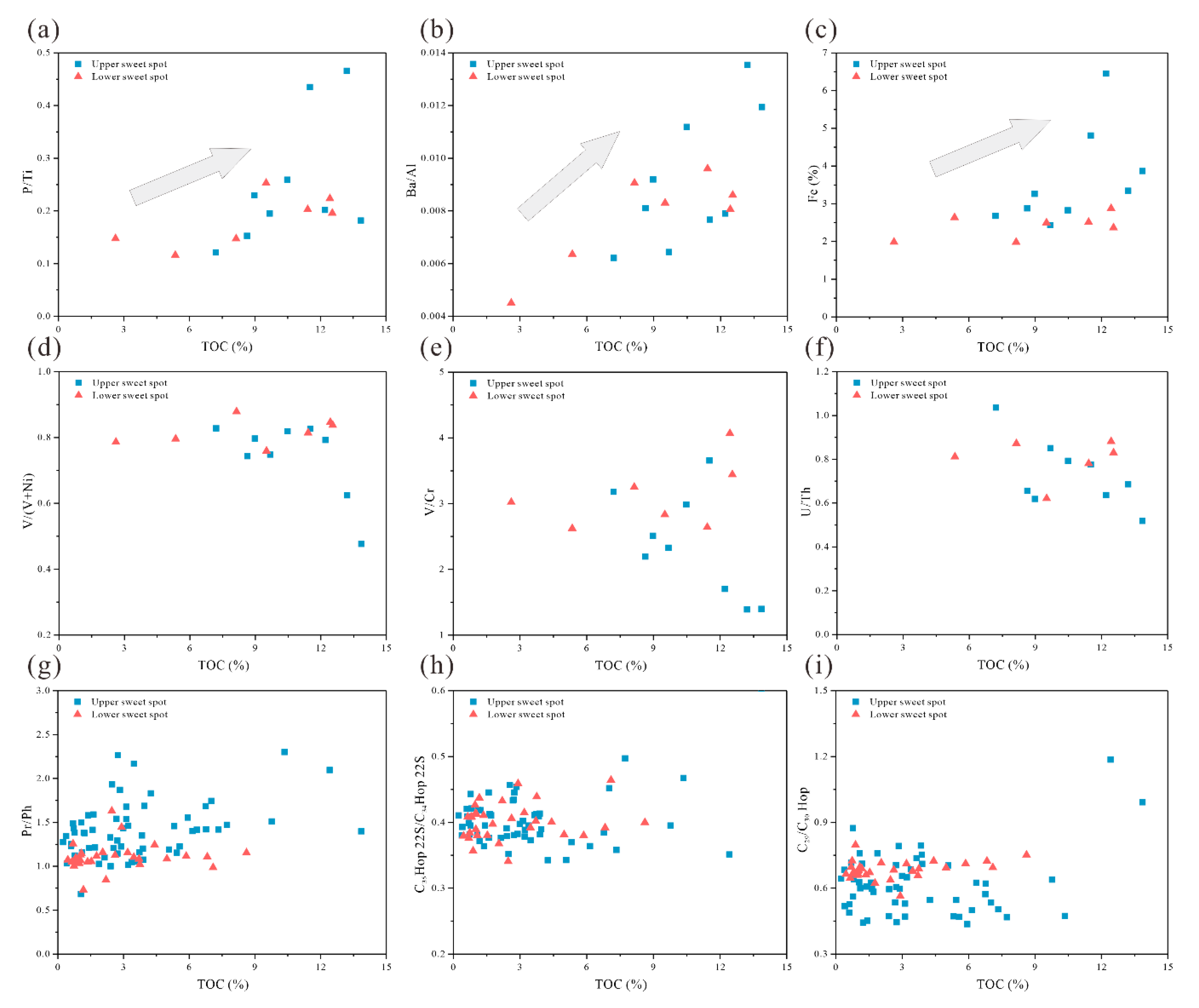
4.3. Elemental Geochemistry Characteristics of Core Samples
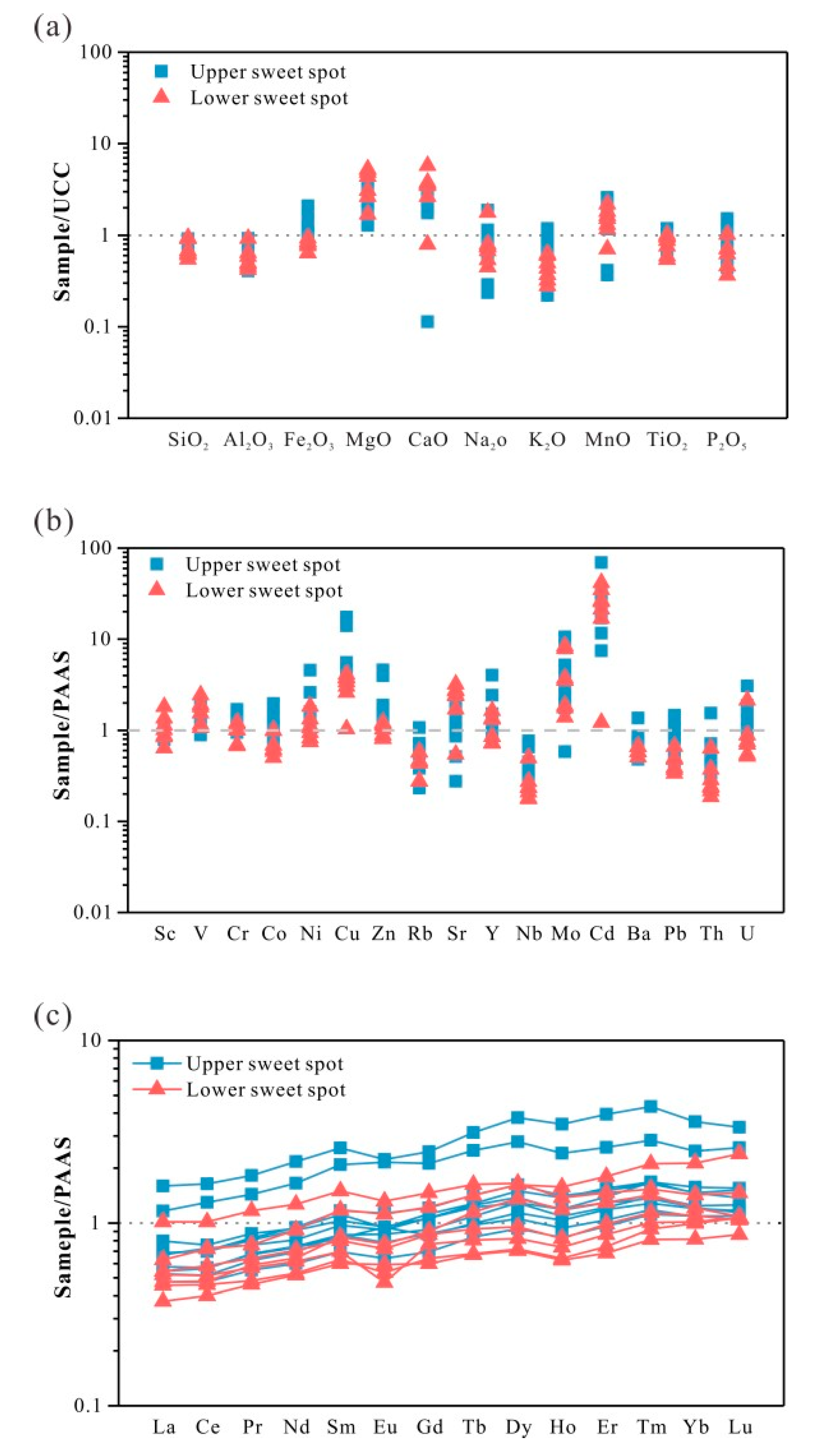
4.3.1. Distribution of Major and Trace Elements.
4.3.2. REE Distribution and PAAS Normalized Patterns.
5. Discussion
5.1. Paleoclimate and Paleoenvironment
5.2. The Provenance of Organic Matter
5.3. Factors Affecting the Enrichment of Organic Matter
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bradley, W. Oil shale formed in desert environment: Green River Formation, Wyoming. Geol. Soc. Am. Bull. 1973, 84, 1121–1124. [Google Scholar] [CrossRef]
- Carroll, A.R.; Brassell, S.C.; Graham, S.A. Upper Permian Lacustrine Oil Shales, Southern Junggar Basin, Northwest China (1). AAPG Bull. 1992, 76, 1874–1902. [Google Scholar]
- Bohacs, K.M.; Carroll, A.R.; Neal, J.E.; Mankiewicz, P.J. Lake-basin type, source potential, and hydrocarbon character: An integrated sequence-stratigraphic-geochemical framework. Lake Basins Through Space Time Aapg Stud. Geol. 2000, 46, 3–34. [Google Scholar]
- Summons, R.E.; Hope, J.M.; Swart, R.; Walter, M.R. Origin of Nama Basin bitumen seeps: Petroleum derived from a Permian lacustrine source rock traversing southwestern Gondwana. Org. Geochem. 2008, 39, 589–607. [Google Scholar] [CrossRef]
- Petersen, H.; Holme, A.; Thomsen, E.; Whitaker, M.; Brekke, T.; Bojesen-Koefoed, J.; Hansen, K.; Larsen, B. Hydrocarbon potential of Middle Jurassic coaly and lacustrine and Upper Jurassic–lowermost Cretaceous marine source rocks in the Søgne Basin, North Sea. J. Pet. Geol. 2011, 34, 277–304. [Google Scholar] [CrossRef]
- Ma, Y.; Fan, M.; Lu, Y.; Liu, H.; Hao, Y.; Xie, Z.; Liu, Z.; Peng, L.; Du, X.; Hu, H. Climate-driven paleolimnological change controls lacustrine mudstone depositional process and organic matter accumulation: Constraints from lithofacies and geochemical studies in the Zhanhua Depression, eastern China. Int. J. Coal Geol. 2016, 167, 103–118. [Google Scholar] [CrossRef]
- Demaison, G.J.; Moore, G.T. Anoxic environments and oil source bed genesis. Org. Geochem. 1980, 2, 9–31. [Google Scholar] [CrossRef]
- Calvert, S. Oceanographic controls on the accumulation of organic matter in marine sediments. Geol. Soc. Lond. Spec. Publ. 1987, 26, 137–151. [Google Scholar] [CrossRef]
- Pedersen, T.; Calvert, S. Anoxia vs. productivity: What controls the formation of organic-carbon-rich sediments and sedimentary Rocks? AAPG Bull. 1990, 74, 454–466. [Google Scholar]
- Meyers, P.A.; Ishiwatari, R. Lacustrine organic geochemistry—An overview of indicators of organic matter sources and diagenesis in lake sediments. Org. Geochem. 1993, 20, 867–900. [Google Scholar] [CrossRef]
- Murphy, A.E.; Sageman, B.B.; Hollander, D.J.; Lyons, T.W.; Brett, C.E. Black shale deposition and faunal overturn in the Devonian Appalachian Basin: Clastic starvation, seasonal water-column mixing, and efficient biolimiting nutrient recycling. Paleoceanography 2000, 15, 280–291. [Google Scholar] [CrossRef]
- Gonçalves, F.T. Organic and isotope geochemistry of the Early Cretaceous rift sequence in the Camamu Basin, Brazil: Paleolimnological inferences and source rock models. Org. Geochem. 2002, 33, 67–80. [Google Scholar] [CrossRef]
- Bechtel, A.; Jia, J.; Strobl, S.A.; Sachsenhofer, R.F.; Liu, Z.; Gratzer, R.; Püttmann, W. Palaeoenvironmental conditions during deposition of the Upper Cretaceous oil shale sequences in the Songliao Basin (NE China): Implications from geochemical analysis. Org. Geochem. 2012, 46, 76–95. [Google Scholar] [CrossRef]
- Zeng, S.; Wang, J.; Fu, X.; Chen, W.; Feng, X.; Wang, D.; Song, C.; Wang, Z. Geochemical characteristics, redox conditions, and organic matter accumulation of marine oil shale from the Changliang Mountain area, northern Tibet, China. Mar. Pet. Geol. 2015, 64, 203–221. [Google Scholar] [CrossRef]
- Cecil, C.B. Paleoclimate controls on stratigraphic repetition of chemical and siliciclastic rocks. Geology 1990, 18, 533–536. [Google Scholar] [CrossRef]
- Meyers, P.A. Organic geochemical proxies of paleoceanographic, paleolimnologic, and paleoclimatic processes. Org. Geochem. 1997, 27, 213–250. [Google Scholar] [CrossRef]
- Xie, S.; Nott, C.J.; Avsejs, L.A.; Maddy, D.; Chambers, F.M.; Evershed, R.P. Molecular and isotopic stratigraphy in an ombrotrophic mire for paleoclimate reconstruction. Geochim. Cosmochim. Acta 2004, 68, 2849–2862. [Google Scholar] [CrossRef]
- Kohn, M.J. Carbon isotope compositions of terrestrial C3 plants as indicators of (paleo) ecology and (paleo) climate. Proc. Natl. Acad. Sci. USA 2010, 107, 19691–19695. [Google Scholar] [CrossRef]
- Wu, P.; Hou, D.; Gan, J.; Li, X.; Ding, W.; Liang, G.; Wu, B. Paleoenvironment and Controlling Factors of Oligocene Source Rock in the Eastern Deep-Water Area of the Qiongdongnan Basin: Evidences from Organic Geochemistry and Palynology. Energy Fuels 2018, 32, 7423–7437. [Google Scholar] [CrossRef]
- Stüeken, E.E.; Kipp, M.A.; Koehler, M.C.; Buick, R. The evolution of Earth’s biogeochemical nitrogen cycle. Earth-Sci. Rev. 2016, 160, 220–239. [Google Scholar]
- Stüeken, E.E.; Martinez, A.; Love, G.; Olsen, P.E.; Bates, S.; Lyons, T.W. Effects of pH on redox proxies in a Jurassic rift lake: Implications for interpreting environmental records in deep time. Geochim. Cosmochim. Acta 2019, 252, 240–267. [Google Scholar] [CrossRef]
- Grant, W.; Gerday, C.; Glansdorff, N. Alkaline Environments and Biodiversity; Eolss Publishers: Oxford, UK, 2006. [Google Scholar]
- Katz, B. The Green River Shale: An Eocene carbonate lacustrine source rock. In Petroleum Source Rocks; Springer: Berlin/Heidelberg, Germany, 1995; pp. 309–324. [Google Scholar]
- Cumming, V.M.; Selby, D.; Lillis, P.G. Re–Os geochronology of the lacustrine Green River Formation: Insights into direct depositional dating of lacustrine successions, Re–Os systematics and paleocontinental weathering. Earth Planet. Sci. Lett. 2012, 359, 194–205. [Google Scholar] [CrossRef]
- Johnson, R.; Mercier, T.; Brownfield, M.; Self, J. Assessment of In-Place Oil Shale Resources of the Green River Formation, Uinta Basin, Utah and Colorado; US Geological Survey: Denver, CO, USA, 2010.
- Berg, M.D.V. Basin-wide Evaluation of the Uppermost Green River Formation’s Oil-shale Resource, Uinta Basin, Utah and Colorado; Utah Geological Survey: Salt Lake City, UT, USA, 2008; Volume 128. [Google Scholar]
- Collister, J.W.; Hayes, J. A Preliminary Study of the Carbon and Nitrogen Isotopic Biogeochemistry of Lacustrine Sedimentary Rocks from the Green River Formation, Wyoming, Utah, and Colorado; U.S. Geological Survey: Denver, CO, USA, 1991; pp. 265–276.
- Kuang, L.; Yong, T.; Dewen, L.; Chang, Q.; Ouyang, M.; Lianhua, H.; Deguang, L. Formation conditions and exploration potential of tight oil in the Permian saline lacustrine dolomitic rock, Junggar Basin, NW China. Pet. Explor. Dev. 2012, 39, 700–711. [Google Scholar] [CrossRef]
- Zou, C.; Zhu, R.; Wu, S.; Yang, Z.; Tao, S.; Yuan, X.; Hou, L.; Yang, H.; Xu, C.; Li, D. Types, characteristics, genesis and prospects of conventional and unconventional hydrocarbon accumulations: Taking tight oil and tight gas in China as an instance. Acta Pet. Sin. 2012, 33, 173–187. [Google Scholar]
- Liang, H.; Xinning, L.; Qiang, M.; LIANG, H.; Quansheng, L.; Xuan, C.; Guojuan, B.; ZHANG, Q.; Yuanlin, M. Geological features and exploration potential of Permian Tiaohu Formation tight oil, Santanghu Basin, NW China. Pet. Explor. Dev. 2014, 41, 616–627. [Google Scholar] [CrossRef]
- Hu, T.; Pang, X.; Yu, S.; Wang, X.; Pang, H.; Guo, J.; Jiang, F.; Shen, W.; Wang, Q.; Xu, J. Hydrocarbon generation and expulsion characteristics of Lower Permian P1f source rocks in the Fengcheng area, northwest margin, Junggar Basin, NW China: Implications for tight oil accumulation potential assessment. Geol. J. 2016, 51, 880–900. [Google Scholar] [CrossRef]
- Frogner, P.; Gíslason, S.R.; Óskarsson, N. Fertilizing potential of volcanic ash in ocean surface water. Geology 2001, 29, 487–490. [Google Scholar] [CrossRef]
- Witham, C.S.; Oppenheimer, C.; Horwell, C.J. Volcanic ash-leachates: A review and recommendations for sampling methods. J. Volcanol. Geotherm. Res. 2005, 141, 299–326. [Google Scholar] [CrossRef]
- Kockum, P.C.F.; Herbert, R.B.; Gislason, S.R. A diverse ecosystem response to volcanic aerosols. Chem. Geol. 2006, 231, 57–66. [Google Scholar] [CrossRef]
- Hoffmann, L.J.; Breitbarth, E.; Ardelan, M.; Duggen, S.; Olgun, N.; Hassellöv, M.; Wängberg, S.-Å. Influence of trace metal release from volcanic ash on growth of Thalassiosira pseudonana and Emiliania huxleyi. Mar. Chem. 2012, 132, 28–33. [Google Scholar] [CrossRef]
- Brand, L.E.; Sunda, W.G.; Guillard, R.R. Reduction of marine phytoplankton reproduction rates by copper and cadmium. J. Exp. Mar. Biol. Ecol. 1986, 96, 225–250. [Google Scholar] [CrossRef]
- Paytan, A.; Mackey, K.R.; Chen, Y.; Lima, I.D.; Doney, S.C.; Mahowald, N.; Labiosa, R.; Post, A.F. Toxicity of atmospheric aerosols on marine phytoplankton. Proc. Natl. Acad. Sci. USA 2009, 106, 4601–4605. [Google Scholar] [CrossRef] [PubMed]
- Duggen, S.; Croot, P.; Schacht, U.; Hoffmann, L. Subduction zone volcanic ash can fertilize the surface ocean and stimulate phytoplankton growth: Evidence from biogeochemical experiments and satellite data. Geophys. Res. Lett. 2007, 34. [Google Scholar] [CrossRef]
- Langmann, B.; Zakšek, K.; Hort, M.; Duggen, S. Volcanic ash as fertiliser for the surface ocean. Atmos. Chem. Phys. 2010, 10, 3891–3899. [Google Scholar] [CrossRef]
- Hamme, R.C.; Webley, P.W.; Crawford, W.R.; Whitney, F.A.; DeGrandpre, M.D.; Emerson, S.R.; Eriksen, C.C.; Giesbrecht, K.E.; Gower, J.F.; Kavanaugh, M.T. Volcanic ash fuels anomalous plankton bloom in subarctic northeast Pacific. Geophys. Res. Lett. 2010, 37, L19604. [Google Scholar] [CrossRef]
- Clayton, J.L.; Yang, J.; King, J.D.; Lillis, P.G.; Warden, A. Geochemistry of oils from the Junggar basin, northwest China. AAPG Bull. -Am. Assoc. Pet. Geol. 1997, 81, 1926–1944. [Google Scholar]
- Wang, X.; Sun, L.; Zhu, R.; Jin, X.; Li, J.; Wu, S.; Bi, L.; Liu, X. Application of charging effects in evaluating storage space of tight reservoirs: A case study from Permian Lucaogou Formation in Jimusar sag, Junggar Basin, NW China. Pet. Explor. Dev. 2015, 42, 516–524. [Google Scholar] [CrossRef]
- Cao, Z.; Liu, G.; Zhan, H.; Gao, J.; Zhang, J.; Li, C.; Xiang, B. Geological roles of the siltstones in tight oil play. Mar. Pet. Geol. 2017, 83, 333–344. [Google Scholar] [CrossRef]
- Su, Y.; Zha, M.; Ding, X.; Qu, J.; Wang, X.; Yang, C.; Iglauer, S. Pore type and pore size distribution of tight reservoirs in the Permian Lucaogou Formation of the Jimsar Sag, Junggar Basin, NW China. Mar. Pet. Geol. 2018, 89, 761–774. [Google Scholar] [CrossRef]
- Zhang, S.; Cao, Y.; Liu, K.; Jahren, J.; Xi, K.; Zhu, R.; Yang, T.; Cao, X.; Wang, W. Characterization of lacustrine mixed fine-grained sedimentary rocks using coupled chemostratigraphic-petrographic analysis: A case study from a tight oil reservoir in the Jimusar Sag, Junggar Basin. Mar. Pet. Geol. 2019, 99, 453–472. [Google Scholar] [CrossRef]
- Fang, S.; Xu, H.; Song, Y.; Li, J. Characteristics and Evolution of the Composite Petroleum System in Jimsar Depression, Eastern Junggar Basin. Acta Geosci. Sin. 2005, 26, 259–264. [Google Scholar]
- Zhang, Y.; Zeng, L.; Luo, Q.; Zhang, C.; Wu, H.; Lv, W. Research on the types and genetic mechanisms of tight reservoir in the Lucaogou Formation in Jimusar Sag, Junggar Basin. Nat. Gas. Geosci. 2018, 29, 211–225. [Google Scholar]
- Kunag, L.; Wang, x.; Guo, X.; CHang, Q.; Jia, X. Geological characteristics and exploration practice of tight oil of Lucaogou Formation in Jimsar Sag. Xinjiang Pet. Geol. 2015, 36, 629–634. [Google Scholar]
- Xi, K.; Cao, Y.; Zhu, R.; Shao, Y.; Xue, X.; Wang, X. Rock types and characteristics of tight oil reservoir in Permian Lucaogou Formation, Jimsar sag. Acta Pet. Sin. 2015, 36, 1495–1507. [Google Scholar]
- Qiu, Z.; Shi, Z.; Dong, D.; Lu, B.; Zhang, C.; Zhou, J.; Wang, H.; Xiong, B.; Pang, Z.; Guo, H. Geological characteristics of source rock and reservoir of tight oil and its accumulation mechanism: A case study of Permian Lucaogou Formation in Jimusar sag, Junggar Basin. Pet. Explor. Dev. 2016, 43, 1013–1024. [Google Scholar] [CrossRef]
- Qiu, Z.; Tao, H.; Zou, C.; Wang, H.; Ji, H.; Zhou, S. Lithofacies and organic geochemistry of the Middle Permian Lucaogou Formation in the Jimusar Sag of the Junggar Basin, NW China. J. Pet. Sci. Eng. 2016, 140, 97–107. [Google Scholar] [CrossRef]
- Yang, Y.; Qiu, L.; Cao, Y.; Chen, C.; Lei, D.; Wan, M. Reservoir quality and diagenesis of the Permian Lucaogou Formation tight carbonates in Jimsar Sag, Junggar Basin, West China. J. Earth Sci. 2017, 28, 1032–1046. [Google Scholar] [CrossRef]
- Standardization Administration of the People’s Republic of China. GB/T 14506.28−2010, Method for Chemical Analysis of Silicate Rocks—Part 28: Determination of 16 Major and Minor Elements Content; Standardization Administration of the P.R.C.: Beijing, China, 2010.
- Standardization Administration of the People’s Republic of China. GB/T 14506.30−2010, Method for Chemical Analysis of Silicate Rocks—Part 30: Determination of 44 Elements; Standardization Administration of the P.R.C.: Beijing, China, 2010.
- Standardization Administration of the People’s Republic of China. GB/T 19145−2003, Determination of Total Organic Carbon in Sedimentary Rock; Standardization Administration of the P.R.C.: Beijing, China, 2003.
- Standardization Administration of the People’s Republic of China. GB/T 18602−2012, Rock Pyrolysis Analysis; Standardization Administration of the P.R.C.: Beijing, China, 2012.
- Jarvie, D.M. Shale resource systems for oil and gas: Part 2—Shale-oil resource systems. AAPG Bull. 2012, 97, 89–119. [Google Scholar]
- Rudnick, R.L.; Gao, S. Composition of the continental crust. Treatise Geochem. 2003, 3, 659. [Google Scholar]
- Taylor, S.R.; McLennan, S.M. The Continental Crust: Its Composition and Evolution; Blackwell Scientific Publications: Hoboken, NJ, USA, 1985. [Google Scholar]
- Wright, J.; Schrader, H.; Holser, W.T. Paleoredox variations in ancient oceans recorded by rare earth elements in fossil apatite. Geochim. Cosmochim. Acta 1987, 51, 631–644. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhao, J.; Wang, H.; Liao, J.; Liu, C. Distribution characteristics and applications of trace elements in Junggar Basin. Nat. Gas Explor. Dev. 2007, 30, 30–33. [Google Scholar]
- Cao, J.; Wu, M.; Chen, Y.; Hu, K.; Bian, L.; Wang, L.; Zhang, Y. Trace and rare earth element geochemistry of Jurassic mudstones in the northern Qaidam Basin, northwest China. Chem. Der Erde-Geochem. 2012, 72, 245–252. [Google Scholar] [CrossRef]
- Moradi, A.V.; Sarı, A.; Akkaya, P. Geochemistry of the Miocene oil shale (Hançili Formation) in the Çankırı-Çorum Basin, Central Turkey: Implications for Paleoclimate conditions, source–area weathering, provenance and tectonic setting. Sediment. Geol. 2016, 341, 289–303. [Google Scholar] [CrossRef]
- Jiang, Z.; Fowler, M. Carotenoid-derived alkanes in oils from northwestern China. Org. Geochem. 1986, 10, 831–839. [Google Scholar] [CrossRef]
- Peters, K.E. The Biomarker Guide, 2, Biomarkers and Isotopes in Petroleum Systems and Earth History; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar]
- De Grande, S.; Neto, F.A.; Mello, M. Extended tricyclic terpanes in sediments and petroleums. Org. Geochem. 1993, 20, 1039–1047. [Google Scholar] [CrossRef]
- Moldowan, J.M.; Sundararaman, P.; Schoell, M. Sensitivity of biomarker properties to depositional environment and/or source input in the Lower Toarcian of SW-Germany. Org. Geochem. 1986, 10, 915–926. [Google Scholar] [CrossRef]
- Li, Z. Geochemical features and source analysis of crude oils from the western slope of Bayanhushu Sag, Hailaer Basin. Acta Pet. Sin. 2012, 33, 595–600. [Google Scholar]
- Zhang, Y.; Jiang, A.; Sun, Y.; Xie, L.; Chai, P. Stable carbon isotope compositions of isoprenoid chromans in Cenozoic saline lacustrine source rocks from the Western Qaidam Basin, NW China: Source implications. Chin. Sci. Bull. 2012, 57, 1013–1023. [Google Scholar] [CrossRef][Green Version]
- Schwark, L.; Vliex, M.; Schaeffer, P. Geochemical characterization of Malm Zeta laminated carbonates from the Franconian Alb, SW-Germany (II). Org. Geochem. 1998, 29, 1921–1952. [Google Scholar] [CrossRef]
- Yangming, Z.; Huanxin, W.; Aiguo, S.; Digang, L.; Dehua, P. Geochemical characteristics of Tertiary saline lacustrine oils in the Western Qaidam Basin, northwest China. Appl. Geochem. 2005, 20, 1875–1889. [Google Scholar] [CrossRef]
- Wang, L.; Song, Z.; Yin, Q.; George, S.C. Paleosalinity significance of occurrence and distribution of methyltrimethyltridecyl chromans in the Upper Cretaceous Nenjiang Formation, Songliao Basin, China. Org. Geochem. 2011, 42, 1411–1419. [Google Scholar] [CrossRef]
- Damsté, J.S.S.; Keely, B.J.; Betts, S.E.; Baas, M.; Maxwell, J.R.; de Leeuw, J.W. Variations in abundances and distributions of isoprenoid chromans and long-chain alkylbenzenes in sediments of the Mulhouse Basin: A molecular sedimentary record of palaeosalinity. Org. Geochem. 1993, 20, 1201–1215. [Google Scholar] [CrossRef]
- Li, M.; Larter, S.R.; Taylor, P.; Jones, D.M.; Bowler, B.; Bjorøy, M. Biomarkers or not biomarkers? A new hypothesis for the origin of pristane involving derivation from methyltrimethyltridecylchromans (MTTCs) formed during diagenesis from chlorophyll and alkylphenols. Org. Geochem. 1995, 23, 159–167. [Google Scholar] [CrossRef]
- Grice, K.; Schouten, S.; Peters, K.E.; Damsté, J.S.S. Molecular isotopic characterisation of hydrocarbon biomarkers in Palaeocene–Eocene evaporitic, lacustrine source rocks from the Jianghan Basin, China. Org. Geochem. 1998, 29, 1745–1764. [Google Scholar] [CrossRef]
- Kenig, F.; Damsté, J.S.S.; Frewin, N.L.; Hayes, J.; De Leeuw, J.W. Molecular indicators for palaeoenvironmental change in a Messinian evaporitic sequence (Vena del Gesso, Italy). II: High-resolution variations in abundances and 13C contents of free and sulphur-bound carbon skeletons in a single marl bed. Org. Geochem. 1995, 23, 485–526. [Google Scholar] [CrossRef]
- Van de Water, P.K.; Leavitt, S.W.; Betancourt, J. Trends in stomatal density and 13C/12C ratios of Pinus flexilis needles during last glacial-interglacial cycle. Science 1994, 264, 239–243. [Google Scholar] [CrossRef]
- Ficken, K.J.; Li, B.; Swain, D.; Eglinton, G. An n-alkane proxy for the sedimentary input of submerged/floating freshwater aquatic macrophytes. Org. Geochem. 2000, 31, 745–749. [Google Scholar] [CrossRef]
- Moldowan, J.M.; Dahl, J.; Huizinga, B.J.; Fago, F.J.; Hickey, L.J.; Peakman, T.M.; Taylor, D.W. The molecular fossil record of oleanane and its relation to angiosperms. Science 1994, 265, 768–771. [Google Scholar] [CrossRef]
- Cao, Z.; Liu, G.; Xiang, B.; Wang, P.; Niu, G.; Niu, Z.; Li, C.; Wang, C. Geochemical characteristics of crude oil from a tight oil reservoir in the Lucaogou Formation, Jimusar sag, Junggar Basin. AAPG Bull. 2017, 101, 39–72. [Google Scholar] [CrossRef]
- Ding, X.; Gao, C.; Zha, M.; Chen, H.; Su, Y. Depositional environment and factors controlling beta-carotane accumulation: A case study from the Jimsar Sag, Junggar Basin, northwestern China. Palaeogeogr. Palaeoclim. Palaeoecol. 2017, 485, 833–842. [Google Scholar] [CrossRef]
- Volkman, J.K. A Review of Sterol Markers for Marine and Terrigenous Organic-Matter. Org. Geochem. 1986, 9, 83–99. [Google Scholar] [CrossRef]
- Volkman, J.K.; Barrett, S.M.; Dunstan, G.A.; Jeffrey, S.W. Sterol Biomarkers for Microalgae from the Green Algal Class Prasinophyceae. Org. Geochem. 1994, 21, 1211–1218. [Google Scholar] [CrossRef]
- Moldowan, J.M.; Seifert, W.K.; Gallegos, E.J. Relationship between petroleum composition and depositional environment of petroleum source rocks. AAPG Bull. 1985, 69, 1255–1268. [Google Scholar]
- Moldowan, J.M.; Fago, F.J. Structure and Significance of a Novel Rearranged Monoaromatic Steroid Hydrocarbon in Petroleum. Geochim. Cosmochim. Acta 1986, 50, 343–351. [Google Scholar] [CrossRef]
- Föllmi, K. The phosphorus cycle, phosphogenesis and marine phosphate-rich deposits. Earth-Sci. Rev. 1996, 40, 55–124. [Google Scholar]
- Boyd, P.W.; Jickells, T.; Law, C.; Blain, S.; Boyle, E.; Buesseler, K.; Coale, K.; Cullen, J.; De Baar, H.J.; Follows, M. Mesoscale iron enrichment experiments 1993-2005: Synthesis and future directions. Science 2007, 315, 612–617. [Google Scholar] [CrossRef]
- Dehairs, F.; Chesselet, R.; Jedwab, J. Discrete suspended particles of barite and the barium cycle in the open ocean. Earth Planet. Sci. Lett. 1980, 49, 528–550. [Google Scholar] [CrossRef]
- Bishop, J.K. The barite-opal-organic carbon association in oceanic particulate matter. Nature 1988, 332, 341. [Google Scholar] [CrossRef]
- Hatch, J.; Leventhal, J. Relationship between inferred redox potential of the depositional environment and geochemistry of the Upper Pennsylvanian (Missourian) Stark Shale Member of the Dennis Limestone, Wabaunsee County, Kansas, USA. Chem. Geol. 1992, 99, 65–82. [Google Scholar] [CrossRef]
- Jones, B.; Manning, D.A. Comparison of geochemical indices used for the interpretation of palaeoredox conditions in ancient mudstones. Chem. Geol. 1994, 111, 111–129. [Google Scholar] [CrossRef]
- Adams, J.A.; Weaver, C.E. Thorium-to-uranium ratios as indicators of sedimentary processes: Example of concept of geochemical facies. AAPG Bull. 1958, 42, 387–430. [Google Scholar]
- Algeo, T.J.; Tribovillard, N. Environmental analysis of paleoceanographic systems based on molybdenum–uranium covariation. Chem. Geol. 2009, 268, 211–225. [Google Scholar] [CrossRef]
- Tribovillard, N.; Algeo, T.; Baudin, F.; Riboulleau, A. Analysis of marine environmental conditions based onmolybdenum–uranium covariation—Applications to Mesozoic paleoceanography. Chem. Geol. 2012, 324, 46–58. [Google Scholar] [CrossRef]
- Elderfield, H.; Greaves, M.J. The rare earth elements in seawater. Nature 1982, 296, 214–219. [Google Scholar] [CrossRef]
- De Baar, H.J.; Bacon, M.P.; Brewer, P.G.; Bruland, K.W. Rare earth elements in the Pacific and Atlantic Oceans. Geochim. Cosmochim. Acta 1985, 49, 1943–1959. [Google Scholar] [CrossRef]
- Li, H.; Liu, Y.; Zhang, L. Origin and Geological Significance of Sedimentary Exhalative Rocks with" Porphyritic" Structures in the Middle Permian Pingdiquan Formation, Eastern Junggar Basin. J. Paleogeography 2017, 19, 211–226. [Google Scholar]
- Ding, X.; Qu, J.; Imin, A.; Zha, M.; Su, Y.; Jiang, Z.; Jiang, H. Organic matter origin and accumulation in tuffaceous shale of the lower Permian Lucaogou Formation, Jimsar Sag. J. Pet. Sci. Eng. 2019, 179, 696–706. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, D.; Jiao, X.; Feng, Q.; Zhou, X. A preliminary study on the relationship between deep-sourced materials and hydrocarbon generation in lacustrine source rocks: An example from the Permian black rock series in Jimusar sag, Junggar Basin. J. Paleogeography 2019, 21, 9. [Google Scholar]


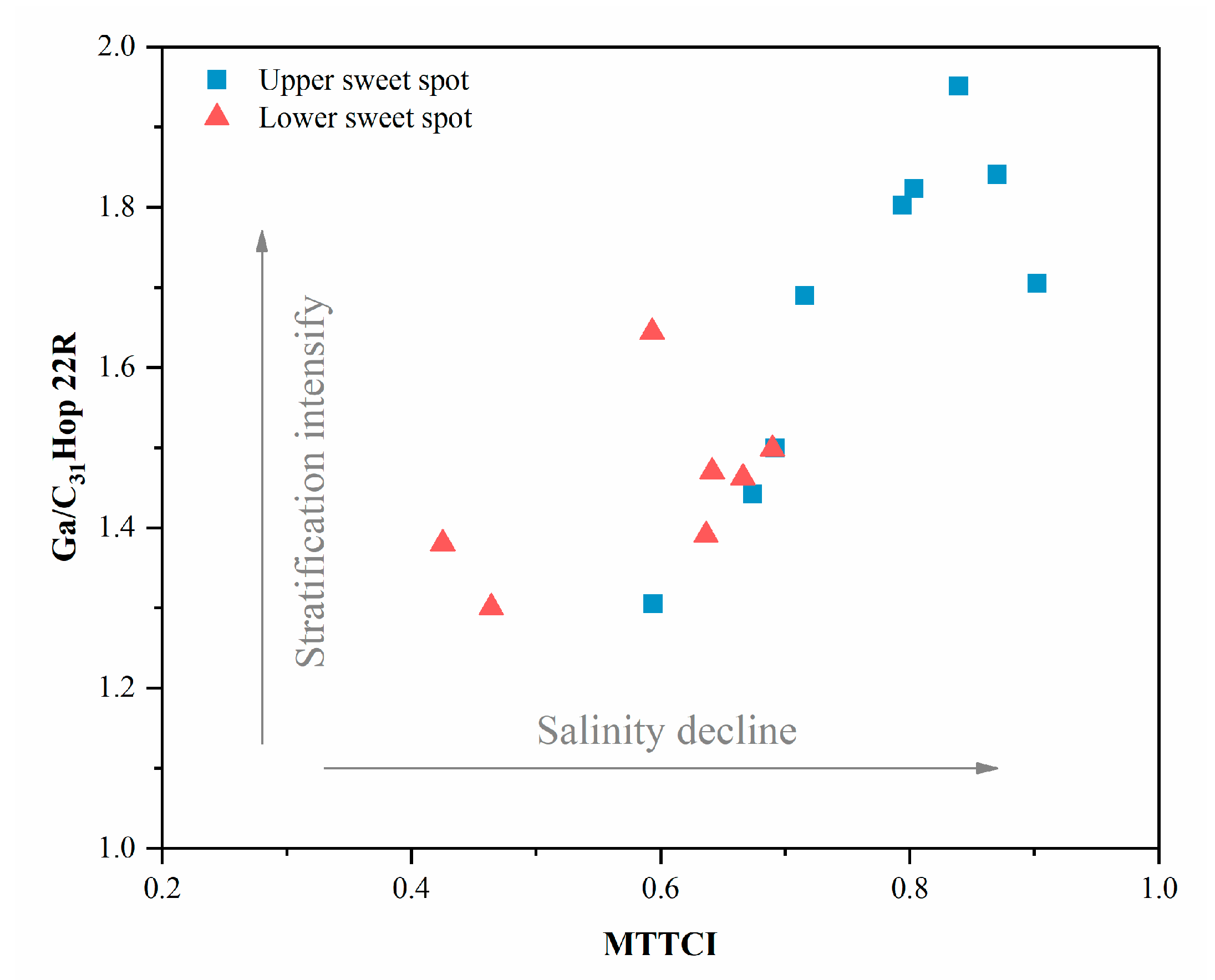
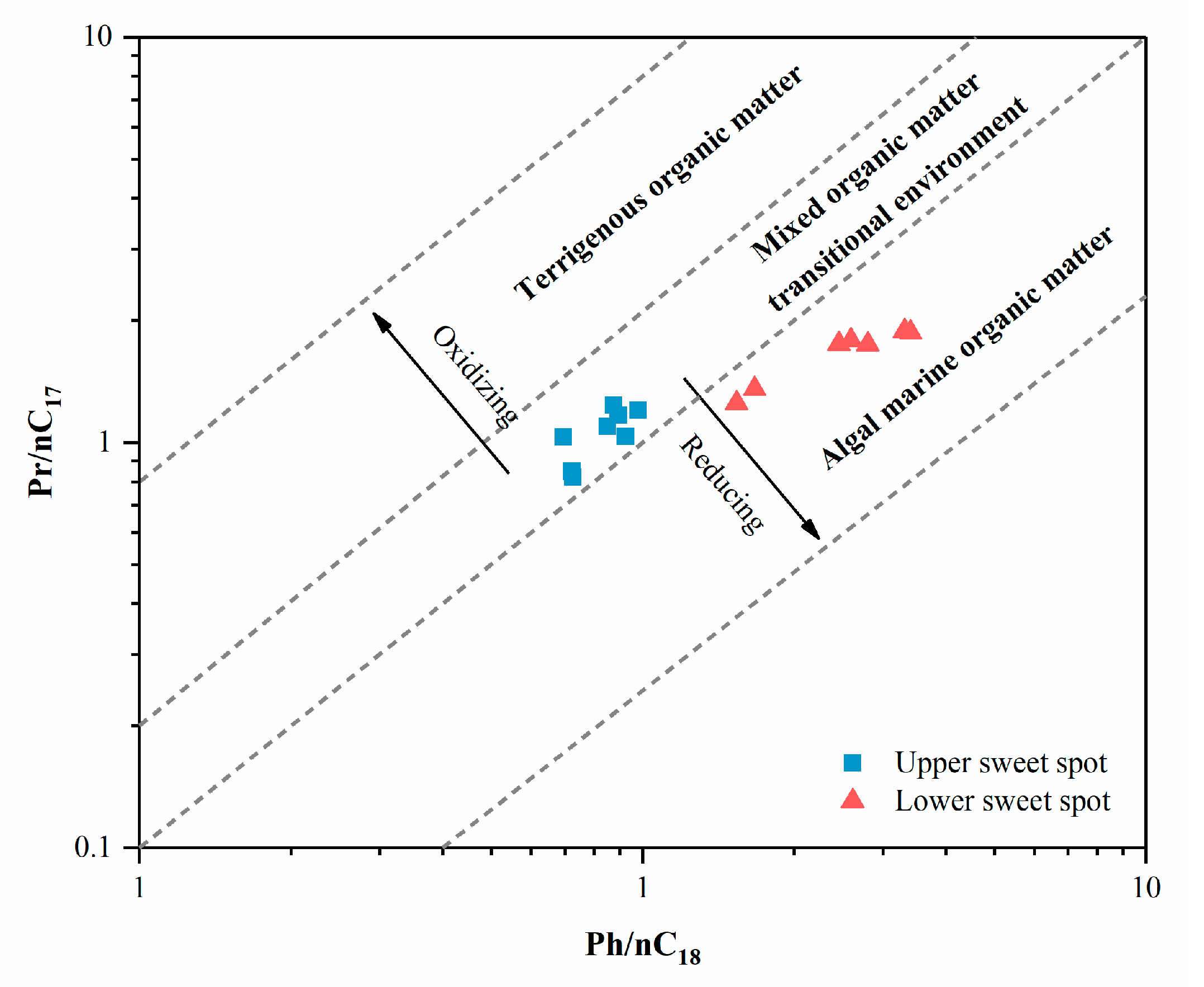
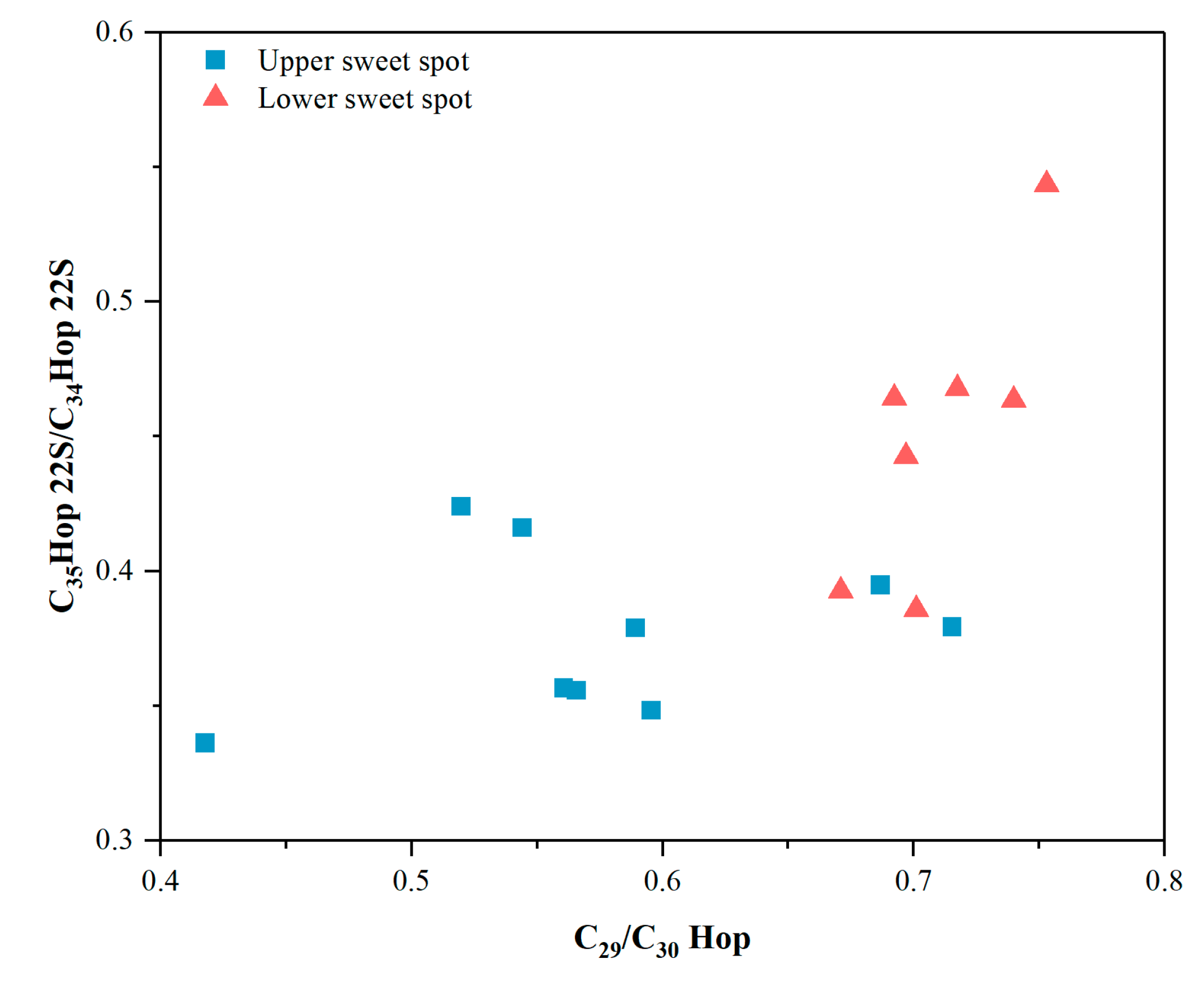
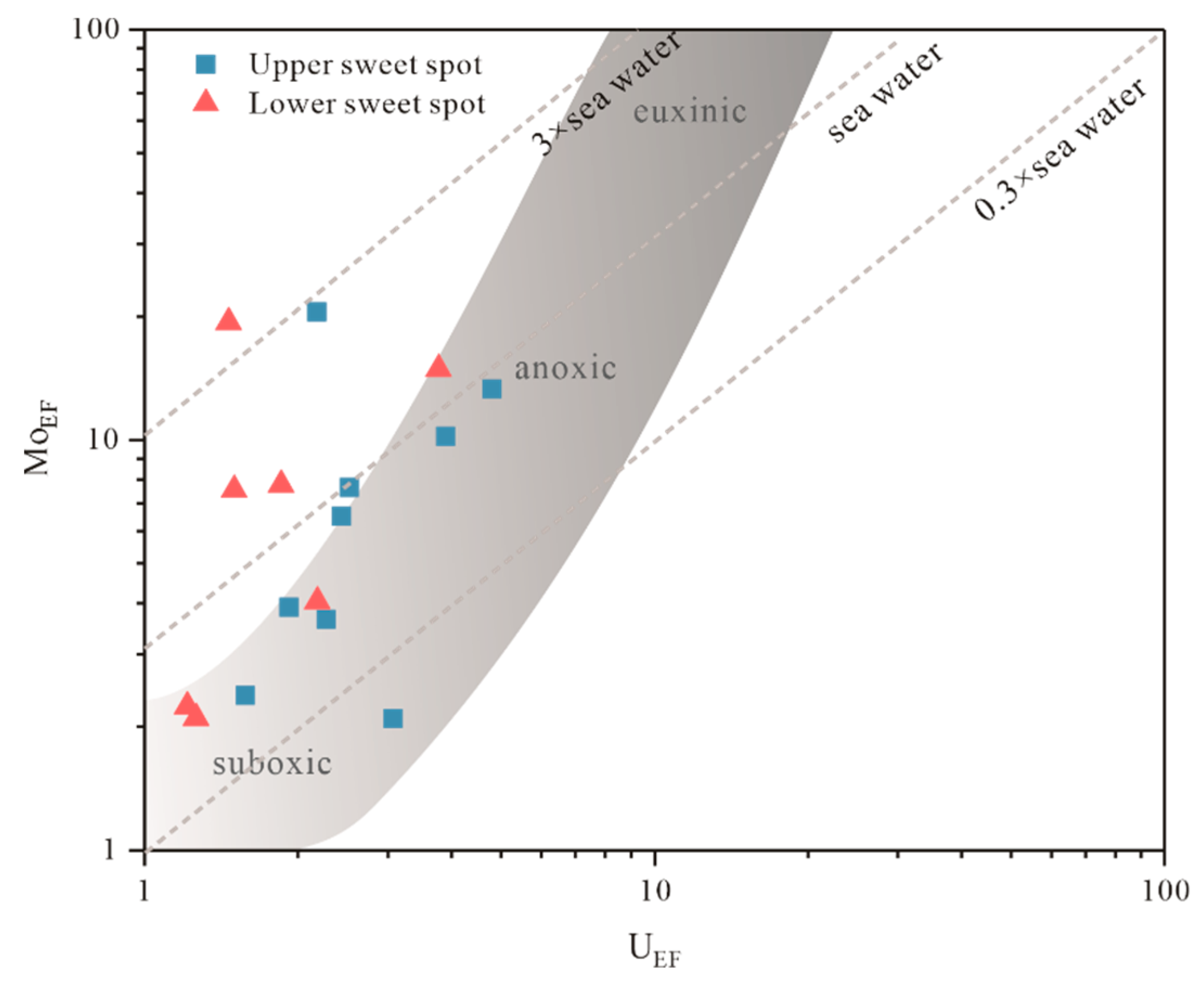
| Well | Sample Code | Sweet Spot | Pr/Ph | Pr/nC17 | Ph/nC18 | nC23-/nC24+ | Paq | CPI | β-Carotane/nCmax |
|---|---|---|---|---|---|---|---|---|---|
| J171 | 171-1 | upper | 1.27 | 1.03 | 0.93 | 2.28 | 0.77 | 1.34 | 0.47 |
| J173 | 173-1 | upper | 1.28 | 1.20 | 0.98 | 1.98 | 0.81 | 1.48 | 0.21 |
| J174 | 174-1 | upper | 1.26 | 0.85 | 0.72 | 2.50 | 0.84 | 1.37 | 0.16 |
| J25 | 25-1 | upper | 1.20 | 0.82 | 0.73 | 2.39 | 0.82 | 1.36 | 0.20 |
| J301 | 301-1 | upper | 1.31 | 1.10 | 0.85 | 1.90 | 0.76 | 1.37 | 0.35 |
| J302 | 302-1 | upper | 1.42 | 1.03 | 0.70 | 1.72 | 0.76 | 1.38 | 0.26 |
| J303 | 303-1 | upper | 1.44 | 1.23 | 0.87 | 1.71 | 0.75 | 1.39 | 0.46 |
| J31 | 31-1 | upper | 1.44 | 1.23 | 0.87 | 1.71 | 0.75 | 1.39 | 0.46 |
| J37 | 37-1 | upper | 1.38 | 1.17 | 0.89 | 1.78 | 0.75 | 1.36 | 0.37 |
| J174 | 174-2 | lower | 1.07 | 1.79 | 2.59 | 2.85 | 0.77 | 1.45 | 0.74 |
| J174 | 174-3 | lower | 1.08 | 1.75 | 2.45 | 1.82 | 0.69 | 1.26 | 1.27 |
| J305 | 305-1 | lower | 1.12 | 1.36 | 1.67 | 2.38 | 0.76 | 1.34 | 1.03 |
| J31 | 31-2 | lower | 0.96 | 1.88 | 3.31 | 2.13 | 0.75 | 1.52 | 1.99 |
| J31 | 31-3 | lower | 0.96 | 1.87 | 3.41 | 2.09 | 0.75 | 1.51 | 1.81 |
| J33 | 33-1 | lower | 1.07 | 1.25 | 1.54 | 2.57 | 0.77 | 1.32 | 0.96 |
| J38 | 38-1 | lower | 1.03 | 1.75 | 2.80 | 2.36 | 0.70 | 1.43 | 2.07 |
| Well | Depth (m) | Sweet Spot | A | B | C | D | E | F | G | H | I | J | K | L |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| J173 | 3083.83 | upper | 13.21 | 0.22 | 1.36 | 0.04 | 1.45 | 0.48 | 1.31×10−2 | 0.47 | 3.34 | 0.62 | 1.39 | 0.68 |
| J22 | 2541.76 | upper | 10.48 | 0.22 | 6.61 | 0.10 | 1.36 | 0.68 | 1.08×10−2 | 0.26 | 2.83 | 0.82 | 2.99 | 0.79 |
| J22 | 2542.81 | upper | 11.52 | 0.38 | 6.68 | 0.11 | 1.95 | 0.84 | 7.47×10−3 | 0.43 | 4.81 | 0.83 | 3.66 | 0.78 |
| J30 | 4047.21 | upper | 8.98 | 0.27 | 1.10 | 0.20 | 1.02 | 0.89 | 9.07×10−3 | 0.23 | 3.26 | 0.80 | 2.51 | 0.62 |
| J30 | 4050.83 | upper | 7.21 | 0.25 | 9.43 | 0.09 | 1.95 | 1.18 | 6.21×10−3 | 0.12 | 2.68 | 0.83 | 3.18 | 1.04 |
| J30 | 4056.00 | upper | 8.64 | 0.20 | 0.80 | 0.26 | 0.75 | 0.76 | 7.85×10−3 | 0.15 | 2.87 | 0.74 | 2.19 | 0.66 |
| J32 | 3563.05 | upper | 12.21 | 0.48 | 5.14 | 0.08 | 1.99 | 0.93 | 7.32×10−3 | 0.20 | 6.45 | 0.79 | 1.70 | 0.64 |
| J32 | 3579.60 | upper | 9.68 | 0.13 | 0.70 | 0.72 | 0.26 | 0.67 | 6.44×10−3 | 0.19 | 2.43 | 0.75 | 2.33 | 0.85 |
| J34 | 3685.63 | upper | 13.86 | 0.26 | 3.11 | 0.21 | 0.52 | 1.03 | 1.18×10−2 | 0.18 | 3.87 | 0.48 | 1.40 | 0.52 |
| J251 | 3751.34 | lower | 8.14 | 0.10 | 12.49 | 0.05 | 2.99 | 0.30 | 9.06×10−3 | 0.15 | 1.98 | 0.88 | 3.25 | 0.87 |
| J251 | 3764.05 | lower | 12.55 | 0.12 | 11.50 | 0.05 | 3.46 | 0.31 | 8.60×10−3 | 0.20 | 2.36 | 0.84 | 3.44 | 0.83 |
| J30 | 4144.56 | lower | 2.61 | 0.19 | 7.40 | 0.27 | 0.60 | 1.97 | 4.50×10−3 | 0.15 | 1.99 | 0.79 | 3.02 | 1.35 |
| J33 | 3663.38 | lower | 12.44 | 0.13 | 10.86 | 0.03 | 3.06 | 0.35 | 8.06×10−3 | 0.22 | 2.87 | 0.85 | 4.07 | 0.88 |
| J33 | 3665.70 | lower | 9.51 | 0.17 | 6.40 | 0.11 | 1.87 | 0.56 | 8.30×10−3 | 0.25 | 2.50 | 0.76 | 2.83 | 0.62 |
| J34 | 3781.75 | lower | 5.35 | 0.14 | 12.76 | 0.04 | 2.62 | 0.47 | 6.36×10−3 | 0.12 | 2.64 | 0.80 | 2.62 | 0.81 |
| J34 | 3814.26 | lower | 11.42 | 0.13 | 9.00 | 0.08 | 2.38 | 0.37 | 9.60×10−3 | 0.20 | 2.51 | 0.81 | 2.64 | 0.78 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Y.; Hou, D.; Li, H.; Zhang, Z.; Guo, R. Impact of the Paleoclimate, Paleoenvironment, and Algae Bloom: Organic Matter Accumulation in the Lacustrine Lucaogou Formation of Jimsar Sag, Junggar Basin, NW China. Energies 2020, 13, 1488. https://doi.org/10.3390/en13061488
Jiang Y, Hou D, Li H, Zhang Z, Guo R. Impact of the Paleoclimate, Paleoenvironment, and Algae Bloom: Organic Matter Accumulation in the Lacustrine Lucaogou Formation of Jimsar Sag, Junggar Basin, NW China. Energies. 2020; 13(6):1488. https://doi.org/10.3390/en13061488
Chicago/Turabian StyleJiang, Yuhan, Dujie Hou, Hang Li, Ziming Zhang, and Ruibo Guo. 2020. "Impact of the Paleoclimate, Paleoenvironment, and Algae Bloom: Organic Matter Accumulation in the Lacustrine Lucaogou Formation of Jimsar Sag, Junggar Basin, NW China" Energies 13, no. 6: 1488. https://doi.org/10.3390/en13061488
APA StyleJiang, Y., Hou, D., Li, H., Zhang, Z., & Guo, R. (2020). Impact of the Paleoclimate, Paleoenvironment, and Algae Bloom: Organic Matter Accumulation in the Lacustrine Lucaogou Formation of Jimsar Sag, Junggar Basin, NW China. Energies, 13(6), 1488. https://doi.org/10.3390/en13061488





