1. Introduction
Global warming and air pollution are driving the development of new technological innovations that reduce carbon emissions by reducing the world’s dependence and use of fossil fuels. Two of the main ways of realizing this are by the electrification of almost all types of transportation systems and by the implementation of renewable energy generation. Large battery storage systems are key parts of the electrification of transport vehicles and stationary grid-tied and off-grid energy storage systems. The development of lithium-ion batteries, in particular, have paved the way for revolutionary changes in transport system developments, driven by the planned sales ban of new combustion engine vehicles by governments worldwide, in favour of battery-powered battery electric vehicles (BEVs), for example. Among all available electrochemical storage cells, the Li-ion battery (LIB) is favoured for high power-demanding applications [
1]. Compared to other types, lithium-ion batteries currently possess the best balance of high-power density, long battery life, fast charging rate, and fast discharging rate. This has made LIBs the technology of choice for large capacity battery systems for road and sea electric vehicles, solar power storage, and wind power storage [
1,
2]. However, safety, cost, and lifetime are some of the main challenges for many Li-ion battery material chemistries when used in energy storage systems [
2]. For solid-state LIB cells and advanced electrode materials, the safety issue is not a major concern as long as they are operated within specification. However, long-term cell performance is very sensitive to the cell operating temperature, and cell storage capacity can degrade quickly if the temperature is not maintained within a narrow range (25–50 °C) during charging and discharging. This degradation is caused primarily by a temperature rise due to electrochemical reactions in the electrolytes used and the degradation of anode materials, cathode materials, and internal short circuits caused by dendrites. The cells generate heat by current passing through their internal resistances. However, chemical reactions can generate additional, sometimes uncontrollable heat if the temperature within the cells reaches a reaction onset temperature. The onset temperatures for these reactions varies for different Li-ion chemistries but is usually in the region of 90–120 °C for common commercially available LIBs [
3] but can be as low as 50 °C for some chemistries. Therefore, numerical simulation and finite element analysis (FEA) have become essential tools for studying, developing, and optimizing the electro-thermal behaviour of LIBs [
2].
In the present work, the heat generation during the full discharge cycles of individual 18650 Li-polymer cells were investigated by FEA and experimentally for 1C, 2C, and 3C discharge rates. An understanding of the internal heat generation characteristics of LIBs is an important step towards designing and developing an optimal battery thermal management system (BTMS) for a lithium-ion battery [
3]. In addition, a hexagonal liquid cooled battery module with six parallel Li-polymer cells was prototyped with the aid of FEA, and it was experimentally evaluated to determine the cell temperature rises for the same 1C, 2C and 3C discharge rates. The hexagonal design enables an optimum packing density of these liquid-cooled modules for any cylindrical battery cells. The main aim was to develop a low cost, lightweight, and scalable liquid cooling system with high cooling performance for cylindrical LIBs. The optimization of the cooling system is required to avoid the LIBs experiencing capacity-degrading high and low temperatures and a destructive thermal runaway to ensure a high performance, a long life, and safe operation [
4,
5]. Other key design features are the ease of manufacture and assembly, recyclability, serviceability, and re-usability; these latter features reduce the cooling system whole life cost.
1.1. Heat Generation in Li-Ion Batteries (LIBs)
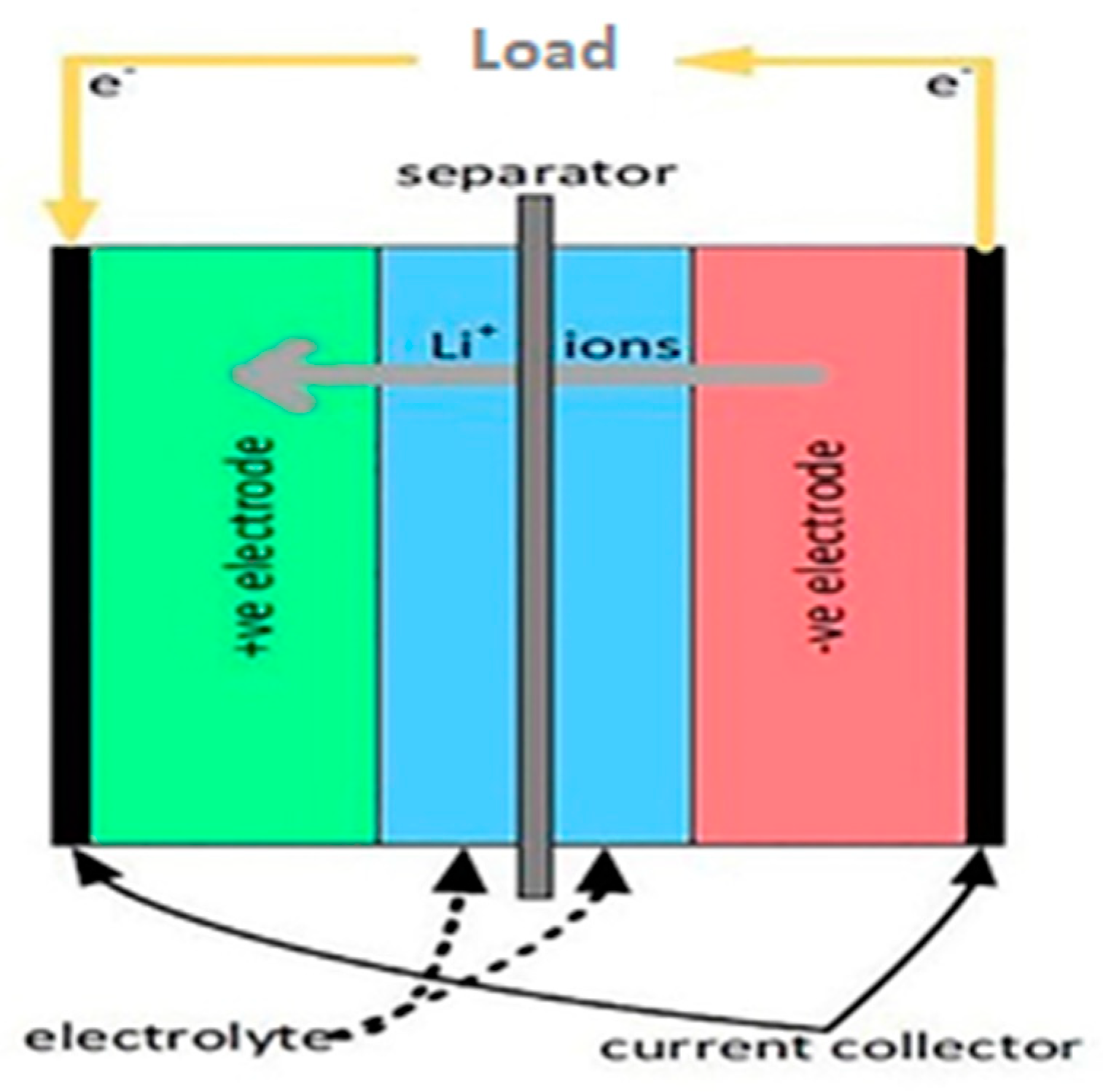
LIBs are energy storage systems that can convert stored chemical energy into electrical energy and vice versa as a result of insertion reactions in the cathode and anode materials, respectively. A lithium-ion battery consists of a positive electrode (cathode), a negative electrode (anode), an electrolyte, a separator, a current collector, and a stainless-steel case. The most commonly used anode materials are carbon or lithium titanate, Si, and Si-composites. The cathode materials are predominantly made of oxides of transition metals such as [
6,
7]:
Typical examples of electrochemical reactions (Equations (1) and (2)) are provided below, and the general construction of a conventional LIB is depicted in
Figure 1 [
7]. Electrodes are the principal components that determine the capacity and energy density of batteries. An aqueous/non-aqueous solution of lithium-containing salts in an organic liquid mixture is commonly used as the electrolyte [
8,
9]. A separator is used to separate cathode and anode to prevent internal short circuits [
10,
11].
Heat generation and temperature non-uniformity in the cells are the main concerns and drawbacks for large battery systems, as required for the propulsion system applications [
12]. The internal heat generation is derived from the thermodynamics Equations (3)–(6) [
12].
where Q is the internal heat generation per unit volume (W·m
−3), ∆G is Gibbs free energy (KJ), ∆S is the entropy change J·K
−1, F is Faraday’s constant C·mol
−1, n is the order of electrochemical reaction or the number of electrons involved in chemical reaction, E
eq is the equivalent electromotive force V, and W
el is the electric work J.
The total internal heat generation is given by Equation (7) [
13] as the sum of the reversible and irreversible heat:
where I is the current density (Am
−2), V
0 is the open circuit voltage, V is the voltage of the cell at each time interval of the discharge process, T is the temperature (K or °C), and I× (V
0 − V) and I × (T
) are the irreversible and reversible heat generation terms, respectively.
Irreversible heat generation occurs at high discharge rates due to the cell internal resistance and joule heating. At lower discharge rates, reversible heat generation is caused by entropy changes at the cathode and anode. The methods of the precise determination of internal temperatures at different discharge rates and using the thermo–physical properties are essential for the optimized design of battery thermal management systems [
14,
15].
1.2. Thermal Runaway
When sufficient heat is generated due to chemical reactions within the batteries, an uncontrolled rise in temperature most commonly known as the thermal runaway can occur. The thermal runaway is one of the major failure mechanisms of Li-ion batteries, and it is a primary safety concern. The electrolytes are extremely flammable and can ignite if there is a sufficient rise in temperature, possibly resulting in an explosion or fire, capacity loss, property change with ageing, and short circuit. Repeated charge discharge cycling ages the lithium ion battery, which loses the capacity and the thermal runaway is one mode of failure in cells. It occurs when the exothermic reaction is uncontrollable, which increases the temperature, thus causing a further increase in temperature and then a further increase in reaction rate. The heat that is generated during an exothermic reaction within the batteries follows exponential functions, while heat removal is a linear function [
16]. The onset temperature for a chemical reaction is chemistry-dependent and is usually above 90 °C, which potentially leads to a thermal runaway [
3]. However, it can be as low as 50 °C for some chemistries such as Li–S. A thermal runaway occurs when the temperature rises above 130–150 °C, and the internal temperature increases as the exothermic chemical reactions set in between the electrolyte and electrode increases [
16].
J Seo et al. [
17] investigated the thermal characteristics of Li–sulphur and Li–sulphur composites to calculate the reversible entropy heat and irreversible resistive heat generated over the discharge process of the batteries. The onset temperature for the Li–sulphur and Li–sulphur composites during lithiation and de-lithiation are approximately in the range of 50–60 and 120–180 °C, respectively [
17]. Meanwhile, the onset temperature for the LIBs generally ranges between 90 and 120 °C, and it varies with the material chemistry of the cell [
18].
3. Design and Thermal Analysis of Prototype Battery Module
The present study mainly focused on the design of a novel reusable Li-ion battery module with integrated liquid cooling tubes.
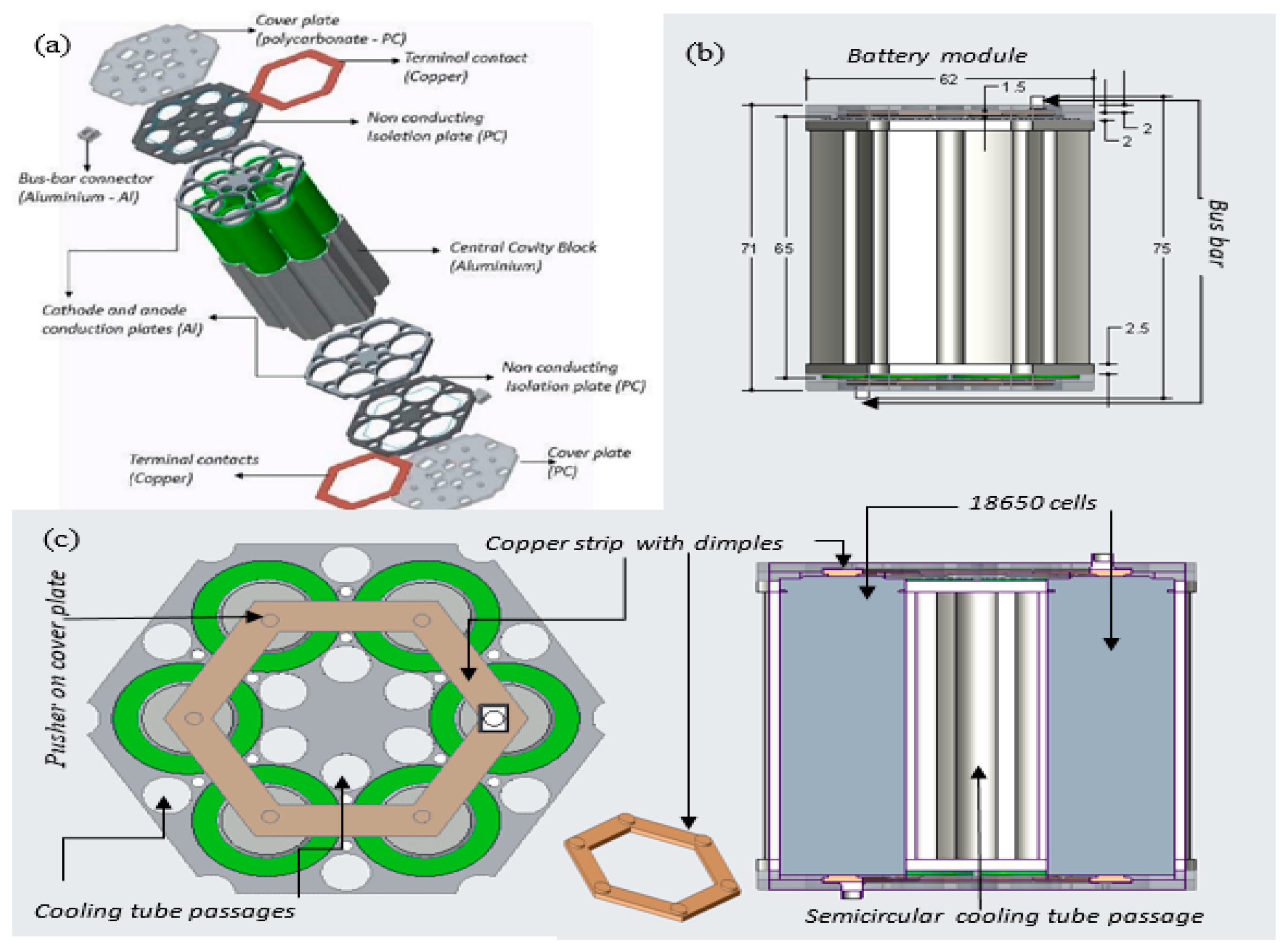
Figure 4 shows the virtual prototype of the module that incorporated six 18650 cells, supplying a nominal voltage of 3.6 V and an overall capacity of 15 Ah. This module was mainly designed for a Formula Student battery electric racing car. However, this design can also be used for stationary storage applications such as solar power and wind power.
This design is highly flexible, scalable, and easy to manufacture, and almost all of the components can be reused and are recyclable. This design is not completely optimized; a slight increase in size radially, for example, would enable one more LIBs to be accommodated at the centre of the module. The cooling tubes are accommodated in the space between adjacent cylindrical cells. The tube diameter can be increased if additional cooling performance is required, though this occurs by increasing the cell spacing and decreasing the power density of the module. The cooling tubes are in close contact with the central cavity block, which is in intimate contact with the surface of each LIB cell. The cavity block holds the cells firmly in position and conducts heat from their surface into the cooling tube and associated liquid coolant. Additionally, conductive plates extract heat from the terminal ends of the module while also holding the cooling tubes in place. Hexagonal copper terminal strips with dimpled surfaces make pressure contact with the positive and negative terminals and connect the cells parallel. There are many alternative connection methods for the electrical connections, including spring contacts and welded contacts. Plates made from insulating materials are used to cover the majority of the contact, but they enable a through-connection between a suitably sized bus-bar connection and the embedded copper terminals at both ends of the module.
3.1. Simulation Analysis of the LIB Cell and the Module
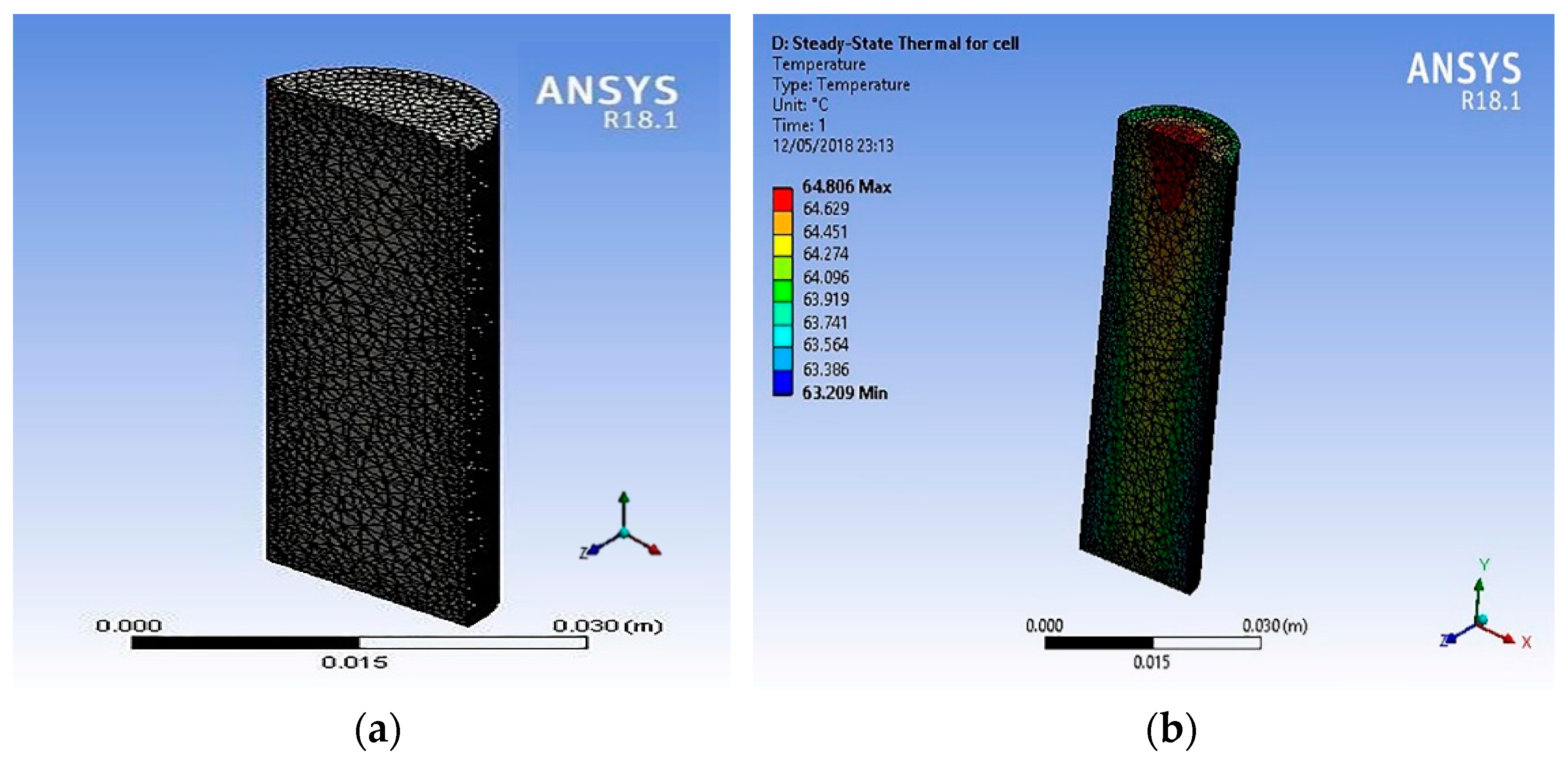
The 18650 LIB was modelled in Creo 3.0 and imported to the Ansys Workbench Fluent finite element simulation software for 2C dry discharge analysis (cooling pump off). The thermo-physical properties, and the values used are shown in
Table 1 [
25].
Figure 5 shows the simulation results for the 18650 LIBs as a cell when discharged at 2C rate. The cell model was imported to Ansys, and tetrahedral meshing was used. The internal heat generation values used in these simulation were calculated by using empirical relations Equations (8) and (9) provided for Sony 18650 LIBs [
10]. These equations were solved with MATLAB to estimate the heat generation values in W/m
3. These values were used for the steady state Ansys thermal analysis of the LIB single 18650 cell model. The simulation results showed that the maximum temperature was ~64 °C at the positive anode terminal end and gave an axial temperature difference of ~1 °C.
where
is the heat generation per unit volume W·m
−3, R
i is the internal equivalent resistance of the unit volume of the LIB Ω·m
−2, SOC is the state of charge, ∆S is the entropy change J·mol
−1·K
−1, I is the discharging electric current per unit volume A·m
−3, t is the time s, C
o is the battery capacity A.h, T is the temperature K, and F is Faraday’s constant 96,485 C·mol
−1.
The module 3D-model was also imported to Ansys Fluent for 2C wet discharge (i.e., cooling pump turned on) thermal analysis. The same calculated heat generation values were used for these wet discharge simulations because the 18650 cell model was the same for both the dry and wet discharges. A fluid mass flow rate of 0.001 Kg/s was modelled in the cooling tube for the steady state 2C wet discharge simulation. Instead of optimizing the fluid mass flow rate in the cooling tubes, the values were taken from previous publications [
27].
Figure 6 shows the simulation results for the battery module for 2C wet discharge.
3.2. Manufacturing of Prototype LIB Module
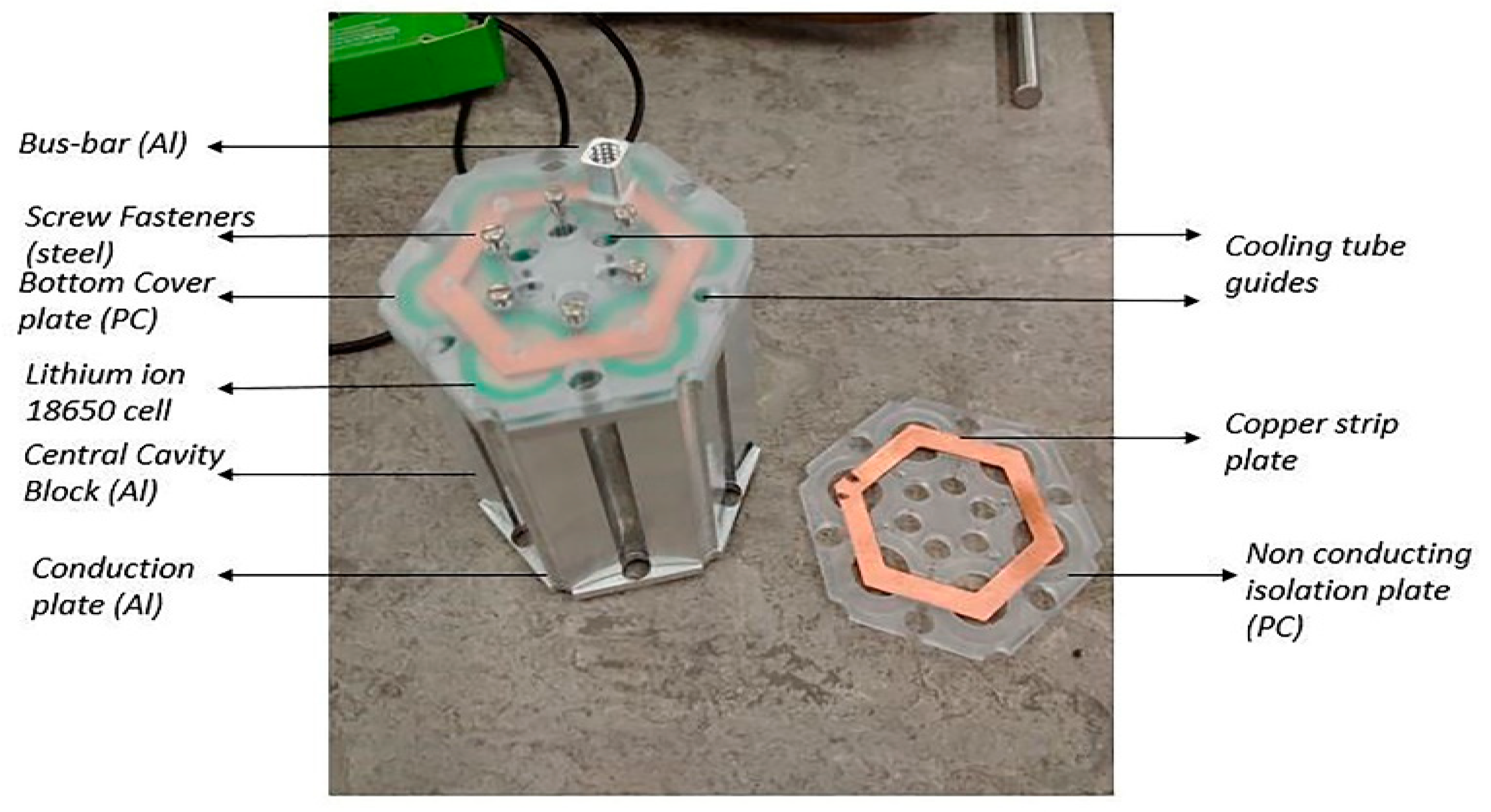
Figure 7 shows the manufactured prototype that was used for the experimental work in this research study. The majority of parts for the prototype module were manufactured with a HAAS Automation Inc. using computer numerically controlled (CNC) vertical milling machine.
The manufactured module, as shown in
Figure 7, was precisely the same as the modelled version. In addition, cooling tubes, coolant distribution, and collecting tanks were procured or manufactured to assemble a complete liquid cooled battery module for the experimental setup.
Figure 8a–c shows the association of the module with the cooling tubes, the cooling liquid distribution, and the collecting tank. The cooling tubes pass through the module that comes in contact with the cavity block and the conduction plates. The top half, bottom half, and the cooling tubes are made leak proof with the O-ring seals. The tanks helped in maintaining a constant pressure and a uniform velocity in each cooling tube.
4. Experimental Setup
Assumptions made in the experimental analysis:
Convection and radiation heat transfer were neglected.
Before each wet discharge, the cooling fluid temperature was cooled back to the ambient temperature.
Before each discharge, the module was charged to a 100% state of charge (SOC).
Electrical contact resistance and other resistances in the measurement circuit were neglected.
There were no air gaps between the cavity block and the cooling tube.
Firm contact between the copper strip plate and cell terminals was assumed to not be deformed or misaligned after each dry and wet discharges due to the effect of the temperature rise.
The equipment used for the experiment;
The battery module had a nominal capacity of 3.6 V (4.2 V max) and 15 Ah, comprising six parallel Samsung 24C 18650 Li–Po cells.
Cooling fluid was de-ionized water to avoid the chance of electrical conduction.
A pump was used to circulate the cooling fluid.
Flow sensor was used to measure the fluid flow rate.
Thermocouples and readers were used to measure the temperature rise continuously.
A heat exchanger (radiator) was used to transfer heat to ambient.
Cooling fans were used to cool the heat exchanger.
Collection and distribution tanks were used to maintain a uniform flow rate at a constant pressure.
Variable power resistors were used to set the discharge rates.
Meters were used to measure the voltages and currents.
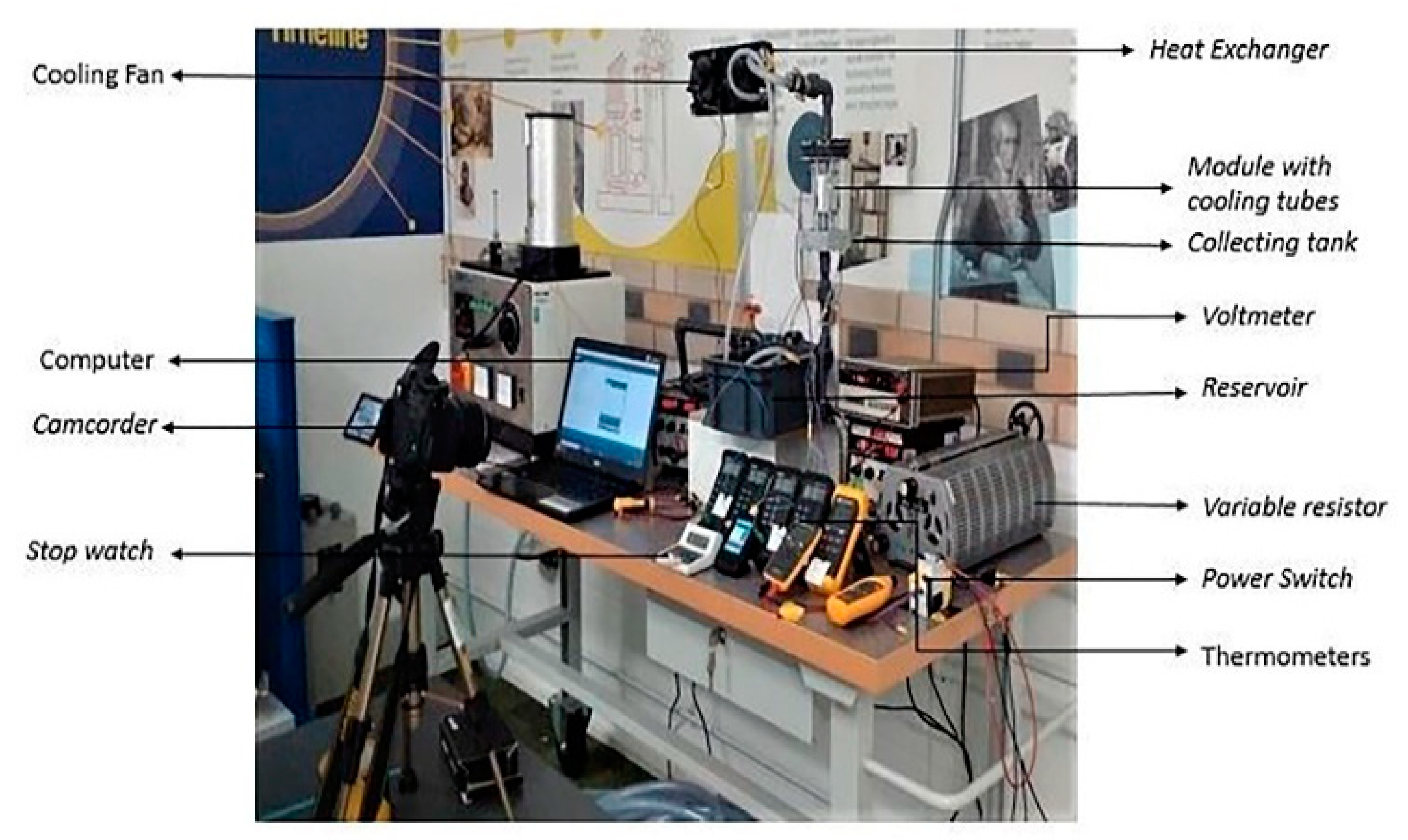
The experimental set up, shown in
Figure 9, consisted of electrical, electronic, and hydraulic systems. The hydraulic system consisted of a hydraulic pump, a reservoir, a flow sensor, fluid collecting and distribution tanks, cooling tubes, a heat exchanger, and pipe connections. The electrical system consisted of a power switch, a variable resistor, shunt resistors, and current and voltage measuring devices. The electronic system consisted of a laptop, an Arduino microcontroller, and thermostatically controlled cooling fans. During the dry discharge measurements, the hydraulic system and the electronic system were switched-off, whereas for the wet discharge all systems were on. K-type (nickel/chromium alloy type) thermocouples were attached to the surface of the module, on the conduction plates, and on the common terminal copper strip, as shown in
Figure 8c. Thermocouples were also used at the inlet and outlet of the heat exchanger to measure the coolant temperature.
Table 2 gives the specification of the test system components.
The module cells were charged to a 100% state of charge (SOC) before the start of the experiment, and, during the dry and wet discharge measurements, a constant current was set by adjusting the variable resistor/rheostat to give galvanostatic discharge rates of 1C, 2C or 3C. During wet discharge, a constant fluid mass flow rate of 0.001 Kg/s was maintained in the cooling tubes. De-ionized water was used as the cooling fluid because it is electrically neutral and safe to handle. A small reservoir was used to store the de-ionized water and for thermal expansion. A small hydraulic pump circulated the coolant from the reservoir to the heat exchanger by passing it through the module unit. Finally, the coolant was collected back to the reservoir from the heat exchanger. The coolant was pumped vertically upwards from the reservoir to eliminate air pockets in the cooling circuit.
The dry cell steady state thermal simulation results, as shown in
Figure 5, were used to position the thermocouples in the simulated high temperature zones in the cells. The cell level dry discharge was experimentally carried out for 1C, 2C and 3C. Before the experimental wet discharge measurements, the steady state wet discharge simulation results, shown in
Figure 6, were used to find suitable locations for the thermocouples. Then, the module was tested for 1C dry discharge prior to conducting the 2C and 3C discharges in order to check that the discharge time was correct—this gave confidence that the apparatus was set up correctly, including good electrical contact to each and every cell in the module.
An infrared (IR) camera was used to capture the measured temperatures over time, as indicated by multiple desktop and handheld meters, during the discharge experiments.
6. Discussion
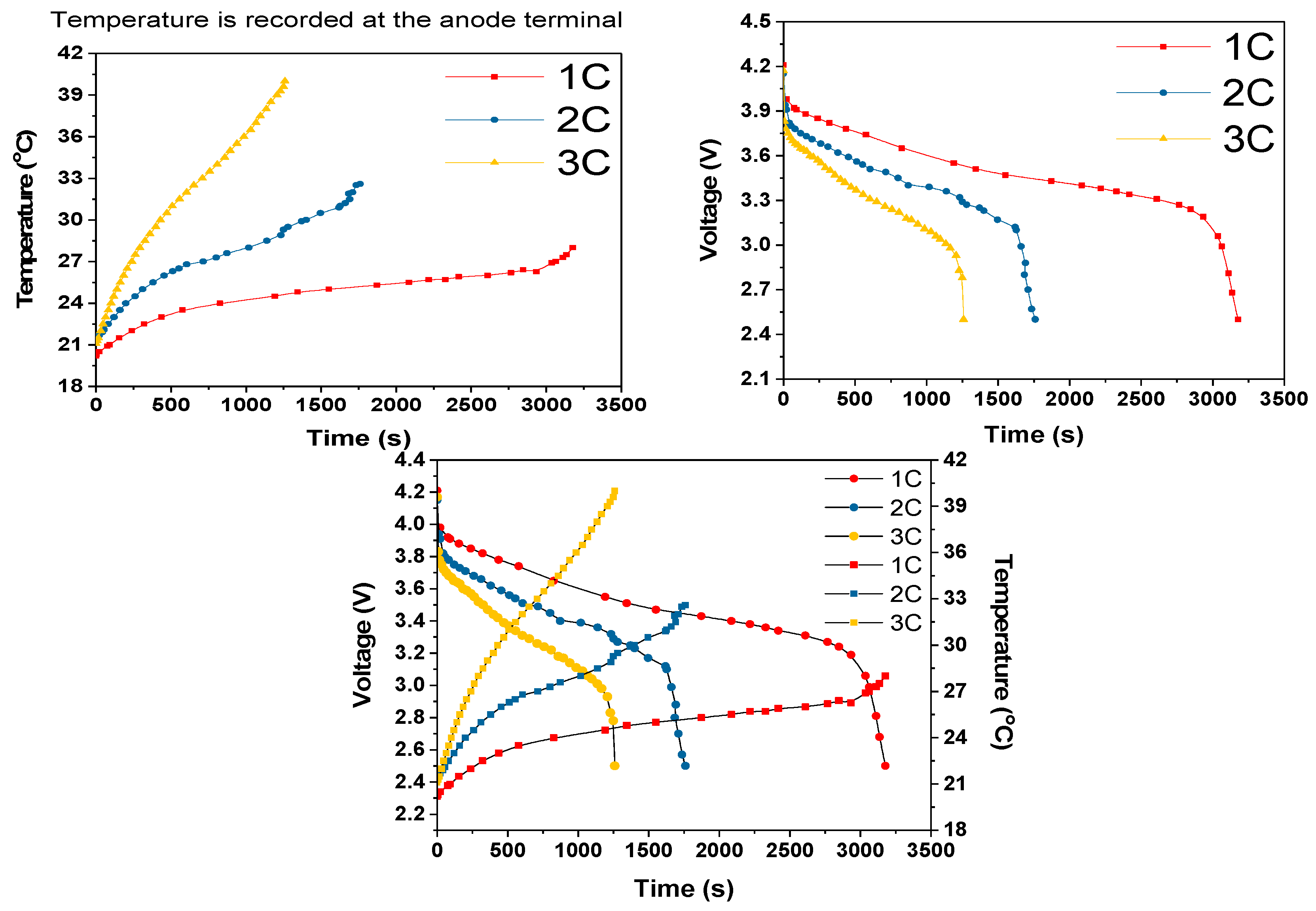
For the 2C and 3C dry discharges, the batteries discharge period agreed with their Ah rating. However, as shown in
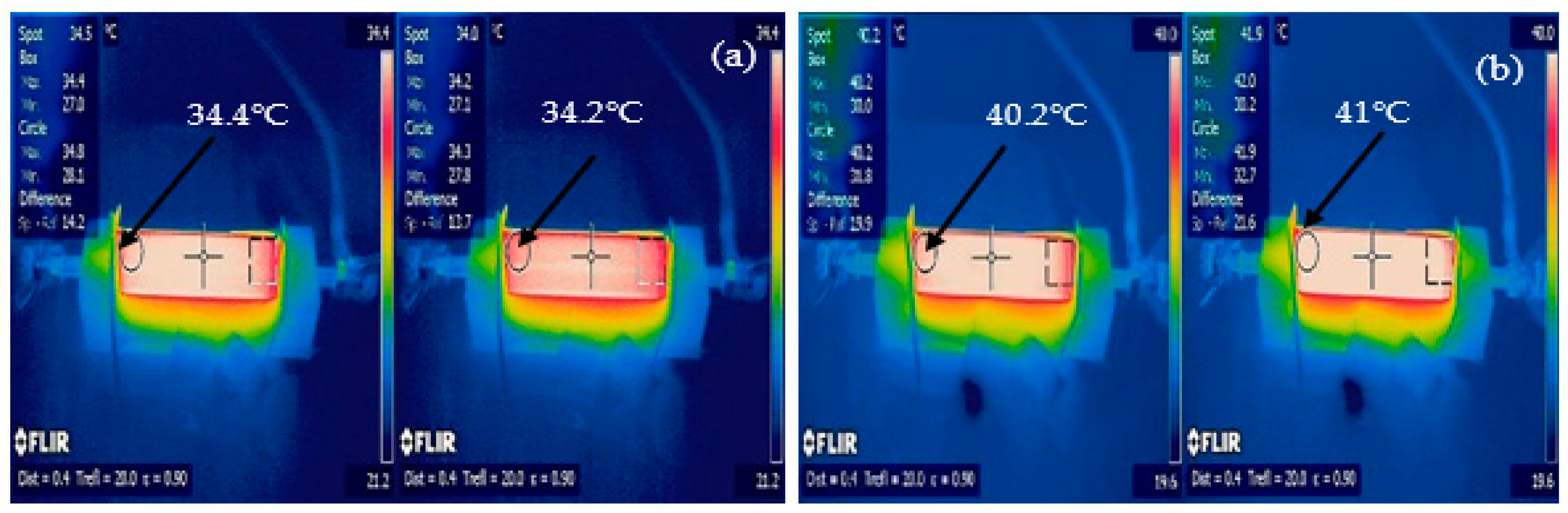
Figure 10, the battery discharged 300 s quicker at the 1C rate than the theoretical discharge period of 3600 s. We believe that this difference was due to one or more cells being at a low state of charge at the start of the 1C discharge cycle. The 2C and 3C experimental results correlated with the IR image results shown in
Figure 11.
As the discharge rate increased, a sharp increase in the voltage at turn on was observed, and this can mainly be attributed to internal resistance and to the electrical interconnections by lesser amount. This is evidenced by the fact that the voltage drop increased with discharge current increase, as governed by Ohms law, and temperature, as governed by the temperature dependence of the resistivity of all of the materials involved. Furthermore, the sharp voltage decreases approaching cut off is due to the internal resistance and high current draw, as well as to a change in entropy [
26]. Considering a higher cut-off voltage (3V), a sudden decrease in voltage and a sudden increase in temperature at the end of each discharge period could be eliminated.
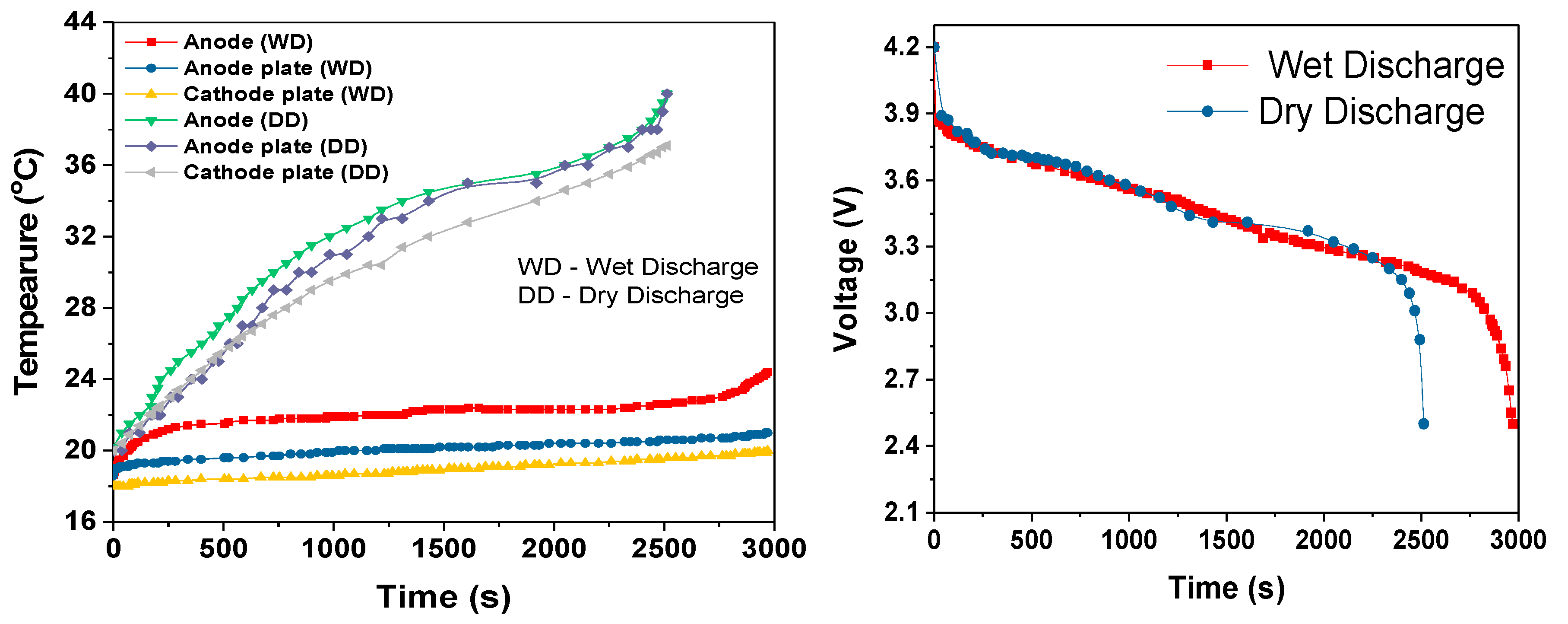
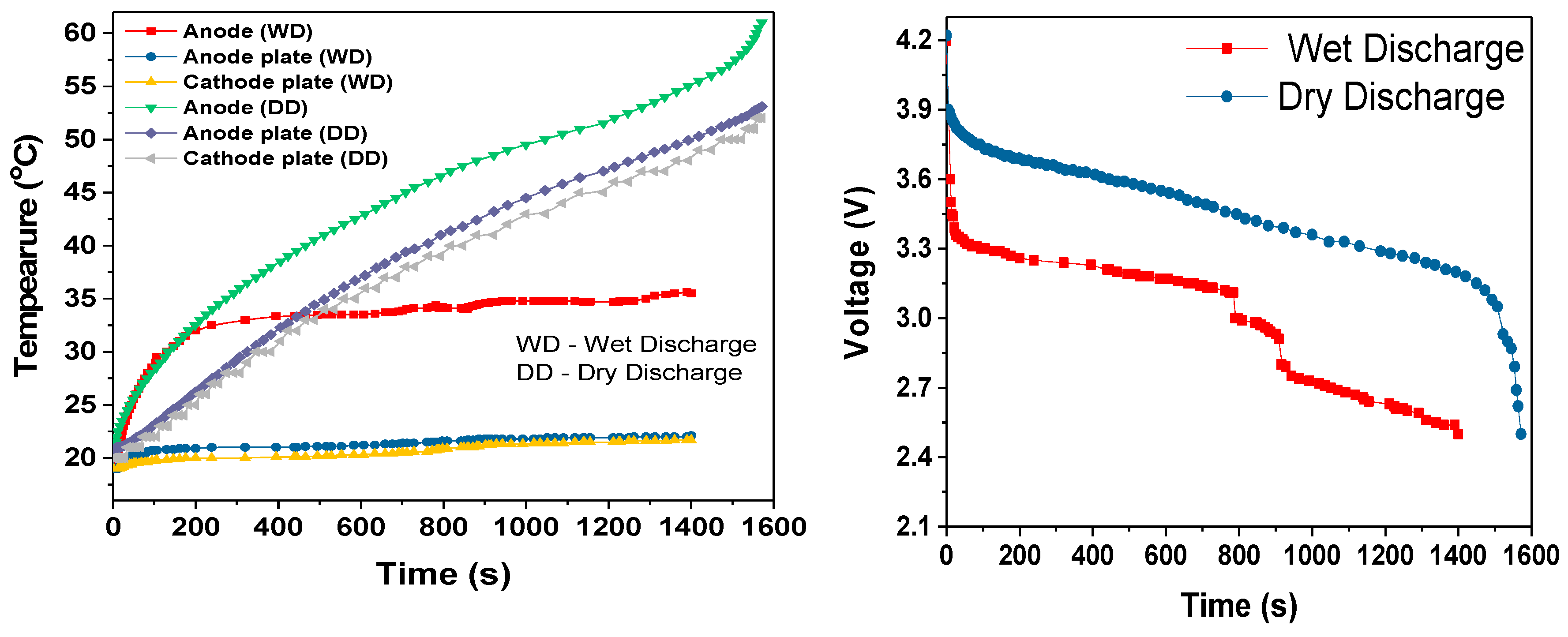
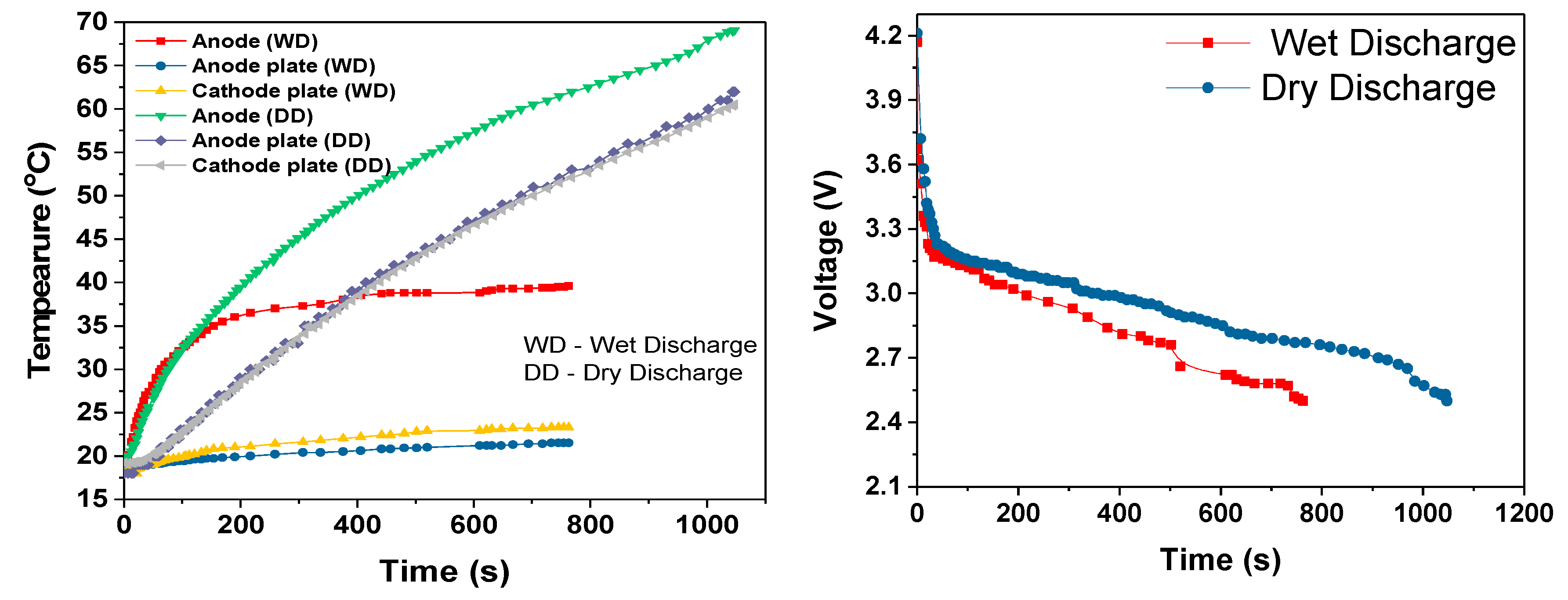
During the cell module discharges, the temperature rise increased with increased discharge rate, as shown in
Table 3. The slopes of the temperature rises were estimated after first 100-200s of discharge period. During this initial discharge period, there was a sudden increase in the temperature due to the internal resistance and discharge current rate. During the 3C dry discharge, the temperature rise was 135 °C/h, and during the 3C wet discharge, this rate was reduced to 13 °C/h and the temperature rise was reduced to 22 °C. This 3C wet discharge rise is the same as the 1C dry discharge rise, which demonstrates that our cooling solution enables a three-fold increase in current draw for the same temperature rise, when the coolant is flowing. It can be seen that during the dry discharges, the rate of surface temperature rise was higher than the rate of anode temperature rise, which is because the heat generated at the anode was conducted to the surface in addition to the heat at the surface. However, during the wet discharge, the rate of surface temperature rise was lower than the rate of the temperature rise at the anode; this can be completely attributed to the performance of the module cooling design.
The cooling efficiencies of the cooling system at the anode and the surface of the cell module during the discharges are shown in the
Table 4. The anode and surface efficiencies indicates the approximate fraction of heat generated that is absorbed by the coolant. The remaining fraction of the retained heat was the reason for the temperature rise at the anode and on the surface of the cell module during wet discharges. The cooling efficiency was higher on the surface and remained almost same at all three discharge rates, which indicates that, irrespective of the discharge rate and heat generated at the surface, the temperature rise was maintained well within the ideal temperature range. The anode cooling efficiency decreased with the increase in the discharge rate because the current and heat retained increased with the increase of the discharge current,
Table 5. The cooling efficiencies at the anode and the surface were used to calculate the heat transfer rate and the heat retaining rate.
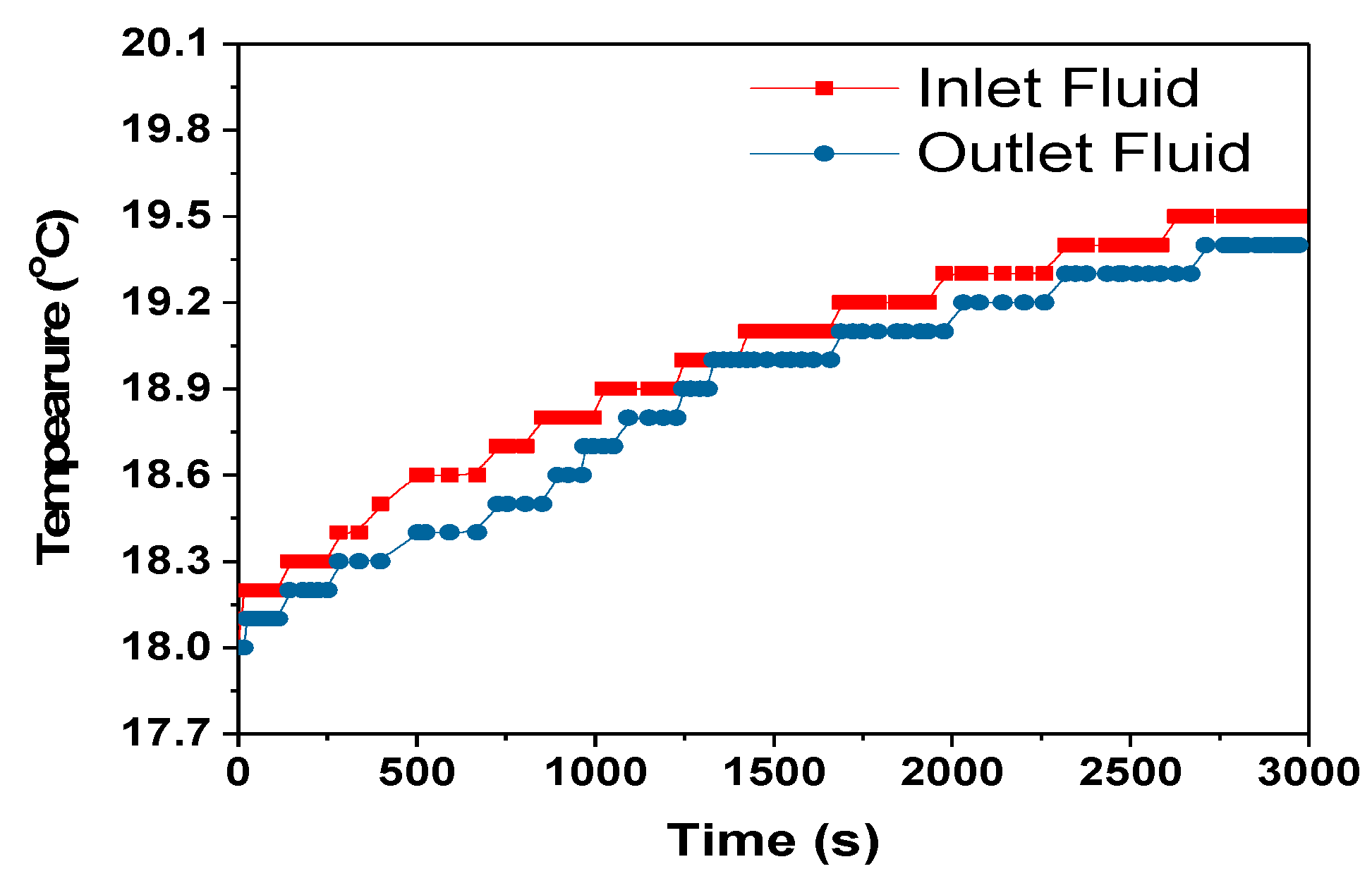
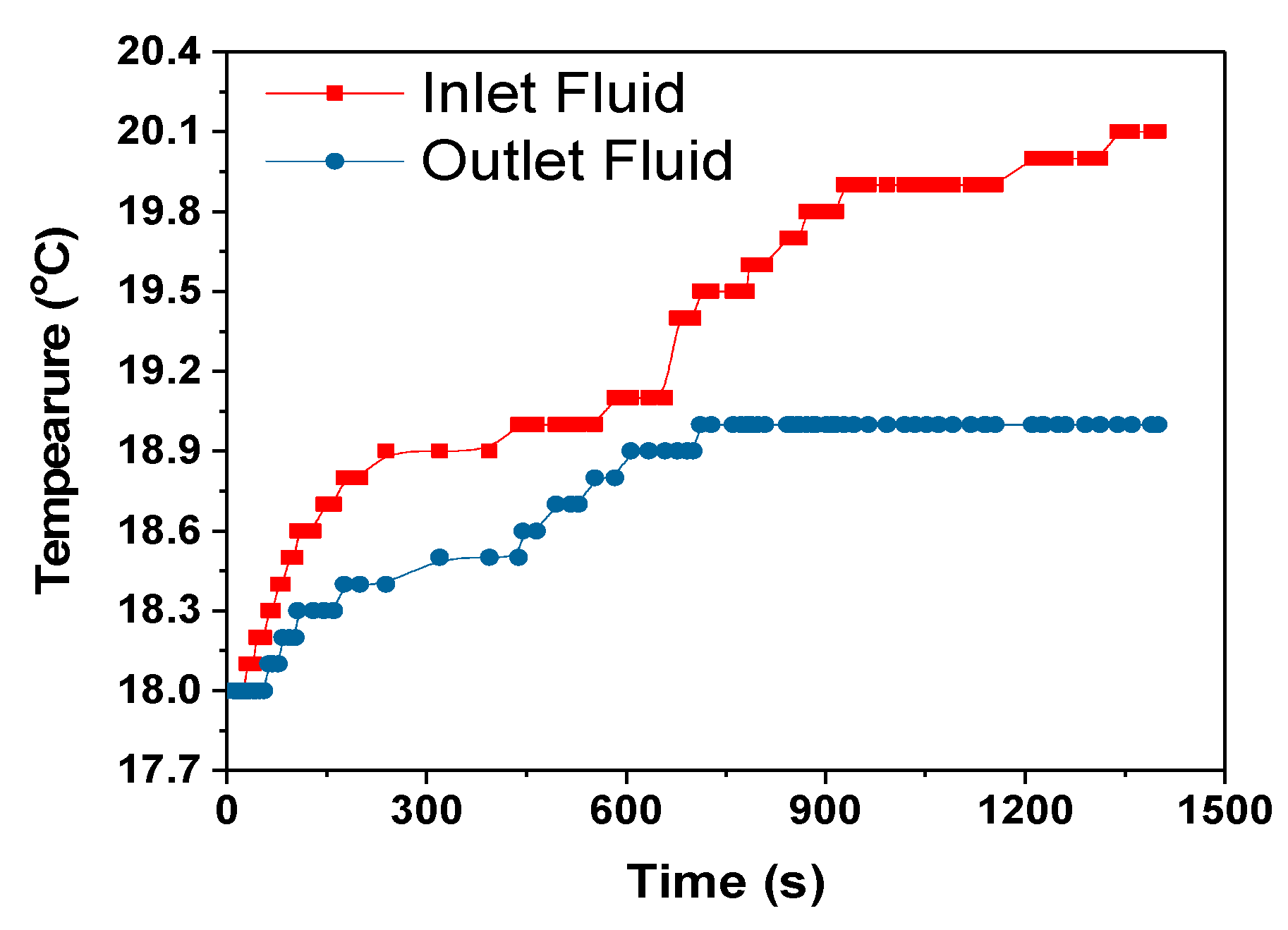
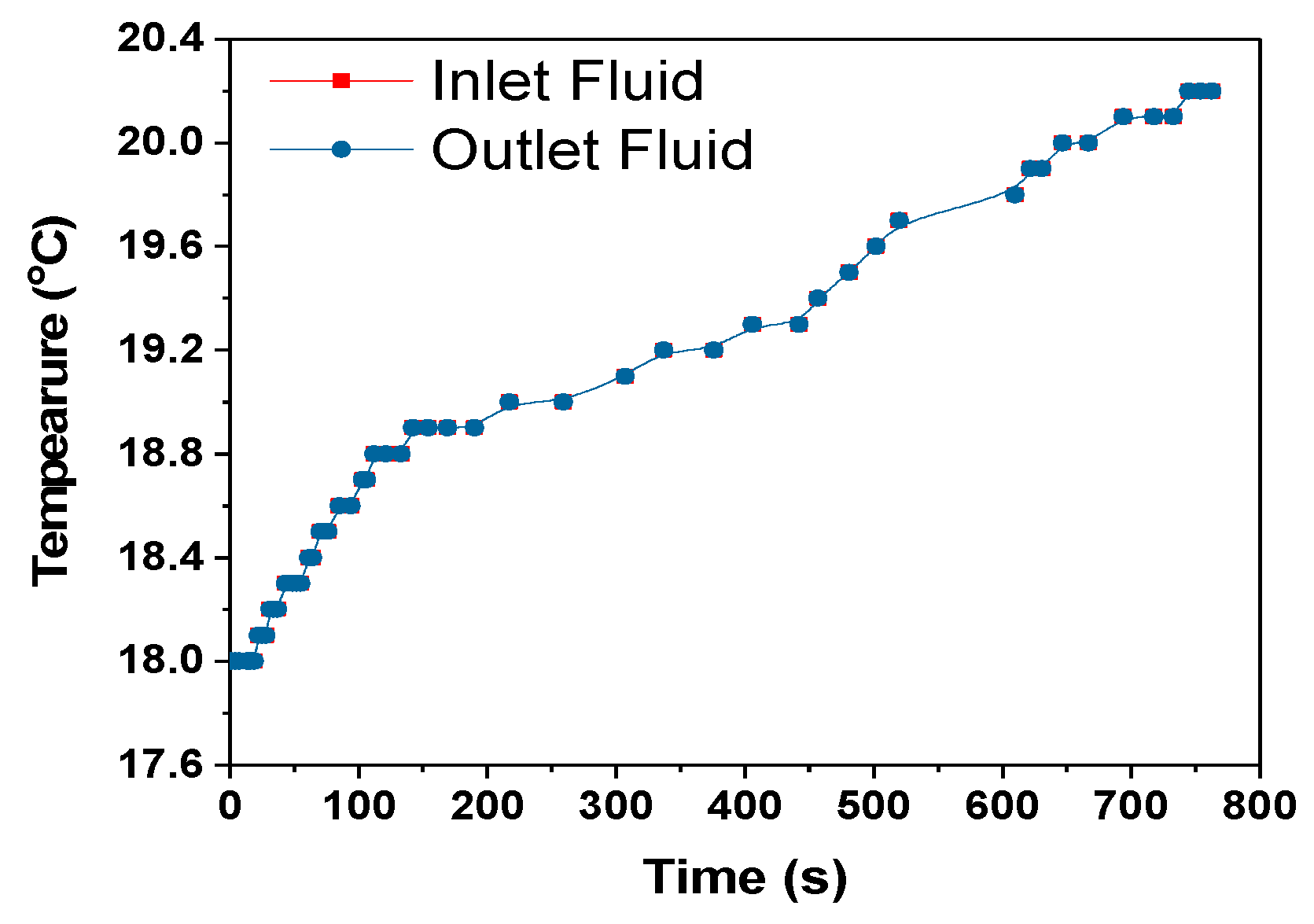
The heat transfer rate between the heat exchanger and surrounding is calculated using the Equation (12) assuming no radiation heat transfer between the heat exchanger and surrounding.
where C
p = 4186 J/kg- specific heat capacity of coolant, mass flow rate of coolant-m = 0.001 kg/s, dT = temperature difference between the heat exchanger and surrounding, 0.1 °C, 1 °C and 0 °C for 1C, 2C and 3C respectively from
Figure 13,
Figure 15 and
Figure 17.
The heat transfer rate from the heat exchanger to the surroundings for the 1C, 2C and 3C discharges were approximately 1.6, 4.2 and 0 W, respectively. For the 3C discharge, the heat transfer between the heat exchanger and the surroundings is zero even if 0.446 W of heat was absorbed by the cooling fluid,
Table 5. This is a subject of future interest to investigate this behaviour of HXs. The amount of heat taken by the cooling fluid increased with the increase in discharge rate, but the cooling efficiency at the HX was decreased as can be seen in
Table 5. The cooling efficiency can be increased with the increase in the discharge rate by optimizing the module and cooling system design.
The internal heat generation and Ohmic heat (Joules heat) generation was calculated with the experimental results, theoretical concepts of heat capacity of materials and Joules law by assuming that the cell module was uniformly heated up and the internal heat generated was stored as the internal energy during all the dry discharges. The total energy, which includes the internal energy and the Joule energy, is responsible for the temperature rise on the surface, and the Joule heating is responsible for the temperature rise at the anode. The internal energy and Joule heating were calculated with Equations (13) and (14). From
Table 5, it is clear that theoretically calculated increase in measured temperature is approximately same as the experimental results; see
Figure 12,
Figure 14 and
Figure 16 for the 1C, 2C and 3C discharges. When comparing the theoretical and experimental temperature rise at the anode and surface of the cell module, the difference was only approximately 1 to 2 °C. This difference was due to convection and radiation heat transfer; temperature difference in
Table 6.
where Q is the total internal energy J, Cp is the specific heat capacity J/kg·K (for specific heat values, see
Table 1), m is the mass of the material Kg, I is the discharge current A, and R is the resistance of the copper hexagonal plate −3.86 × 10
−5 Ω.
Adding this theoretical temperature difference to the ambient temperature gave the measured rise in temperature, which was approximately the same as the experimental temperature rise at the anode and on the surface. This similarity implies that the calculated internal heat generation values and joule heating values were validated with the experimental values. These heat generation values and efficiencies could be used for the further optimization and the accurate numerical analysis of the cell module.
The difference in temperature rise between the theoretical and experimental results were due to convection and radiation heat transfer between the cell module and the atmosphere. The anode was not exposed to the atmosphere. Therefore, the convection and radiation heat transfer at the anode decreased to zero for the 3C discharge because the 3C discharge period was much shorter than for the other discharge rates and the cooling system was not optimized. However, the convection and radiation heat transfer at the surface increased with the increase in the discharge rate, because more heat was generated at higher discharge rates and the higher the temperature difference between the cell module and the surroundings, the larger the convection and radiation heat transfer rate will be.
For all three discharge rates, there was sudden increase in temperature when the discharge duration was close to the cut-off voltage; this rapid increase in temperature can be attributed to the rapid decrease in the terminal voltage, which was most likely due to the entropy change that further depended on the SOC of the cell [
26]. This could also be attributed to the decrease in discharge duration when comparing the single 18650 cell dry discharges (
Figure 10) and the module dry discharges seen in
Figure 12,
Figure 14 and
Figure 16.
The thermal resistance between the heat source and sink determines the heat transfer rate of a system. The thermal resistance of the battery module for the wet and dry 1C, 2C and 3C discharge rates was calculated with Equations 15 and 16 and captured in the
Table 7.
The thermal resistance of the cooling system is estimated to be 0.2 K/W, based on the 3C discharge results, which came closest to reaching the steady state temperature.
7. Conclusions
A novel liquid-cooled cylindrical cell battery module design was prototyped and evaluated to measure its ability to control the battery cell temperature rise over complete discharge cycles at rates of 1C, 2C and 3C. Finite element simulations results for 3D modelled 18650 type battery cells and the prototype module were used to find the optimum measurement points for the experimental work and to assist with the results analysis. The module experimental performance was compared to single cells without cooling and the six-cell module with and without the coolant pump turned on. It was shown that temperature rise over the complete discharge period for the cooled six 18650 cell module was only approximately 6, 17 and 22 °C for the 1C, 2C and 3C discharge rates. These systems had not reached the final steady-state temperature by the time the cells reached the cut-off voltage, because the overall cooling system never had time to reach its steady-state temperature. However, under the same circumstances, in comparison, the rise was 22, 44 and 52 °C for the same discharge rates with the cooling pump turned off. These rises were dominated by large temperature rises at the beginning of the discharge cycles because the temperature increase was exponential. With the coolant pump turned off, the slope of temperature increase was still quite steep because the heat generation rate greatly exceeded the heat removal rate. However, with the pump turned on, the effectiveness of our cooling solution could be clearly seen because the temperatures quickly began to approach steady-state, and then the temperature and temperature rises were very low for the entire discharge periods and for all three discharge rates.
Since the wet discharge temperatures were pseudo steady-state, it was possible to estimate the thermal resistance of the entire cooling system based on the average battery voltage, the constant discharge currents, and the final temperatures reached. The estimated thermal resistances were 0.12, 0.17, and 0.146 K/W for the 1C, 2C and 3C discharge rates and a coolant mass flow rate of 0.001 Kg/s. Clearly, these values should all be equal, because the thermal resistance of the cooling system was a constant. The differences in calculated values can mainly be attributed to the fact that the measured temperature values were not true steady-state measurements (the system can never reach steady-state for these high discharge rates). Further work is required to determine the thermal resistance of the module on its own.
Since the long-term cell performance of LIBs is very sensitive to the cell operating temperature and is understood to degrade quickly if the temperature is not maintained within an optimum chemistry-specific narrow range, a high-performance cooling system is essential. The prototype design proposed in this work was shown to maintain the cells close to the optimum temperature range for discharge rates up to 3C. In addition, we discussed the chemistry-specific thermal runaway onset temperature ranges and causes. The cooling performance of the proposed design also provides a greater safety margin and balance between the highest possible discharge rate and the onset temperature for the thermal runaway. This will be especially important for batteries, such as Li–S, whose construction and chemistry feature low onset temperatures; this design helps to address this serious safety issue. The design may be suitable for some mobile and fixed battery energy storage applications that may see either intermittent or continuous high discharging or charging rates, such as electric vehicles or storage for renewable energy sources. It might also be suitable for applications where the batteries need to be pre-heated or maintained at a temperature above or below the ambient temperature. The module design is also simple and lightweight, and its hexagonal design enables it to scale up to produce very large battery systems with exceptional power densities. The design also lends itself to mass production, using casting or extrusion and stamping processes, is made from recyclable materials, and is modular and serviceable.
There still appears to be some margin to increase the discharge rate even further for this design while still maintaining modest temperature increases. In future work, this design will be optimized and evaluated at higher and possibly lower discharge rates, and the whole cooling system will be modelled to support the future experimental work and results analysis. Finally, the module cooling and system cooling will be investigated under real application conditions by using continuously varying load mission profiles such as world-wide harmonized light vehicle test (WLTP) to investigate the temperature profiles and cooling system requirements for different standardized charging and discharge current profiles for cars, trucks, etc. This will provide a clearer understanding about the relationships between the cell’s temperatures and application load profiles. Because an under-sized battery system would see higher than optimum temperatures and therefore have a shorter lifetime, this future work will enable the optimization of the compromise between the battery system capacity and the battery cell lifetime for those load profiles.