Efficiency Improvement of MAPbI3 Perovskite Solar Cells Based on a CsPbBr3 Quantum Dot/Au Nanoparticle Composite Plasmonic Light-Harvesting Layer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Plasmonic AuNPs:QD-CsPbBr3 Perovskite Solution
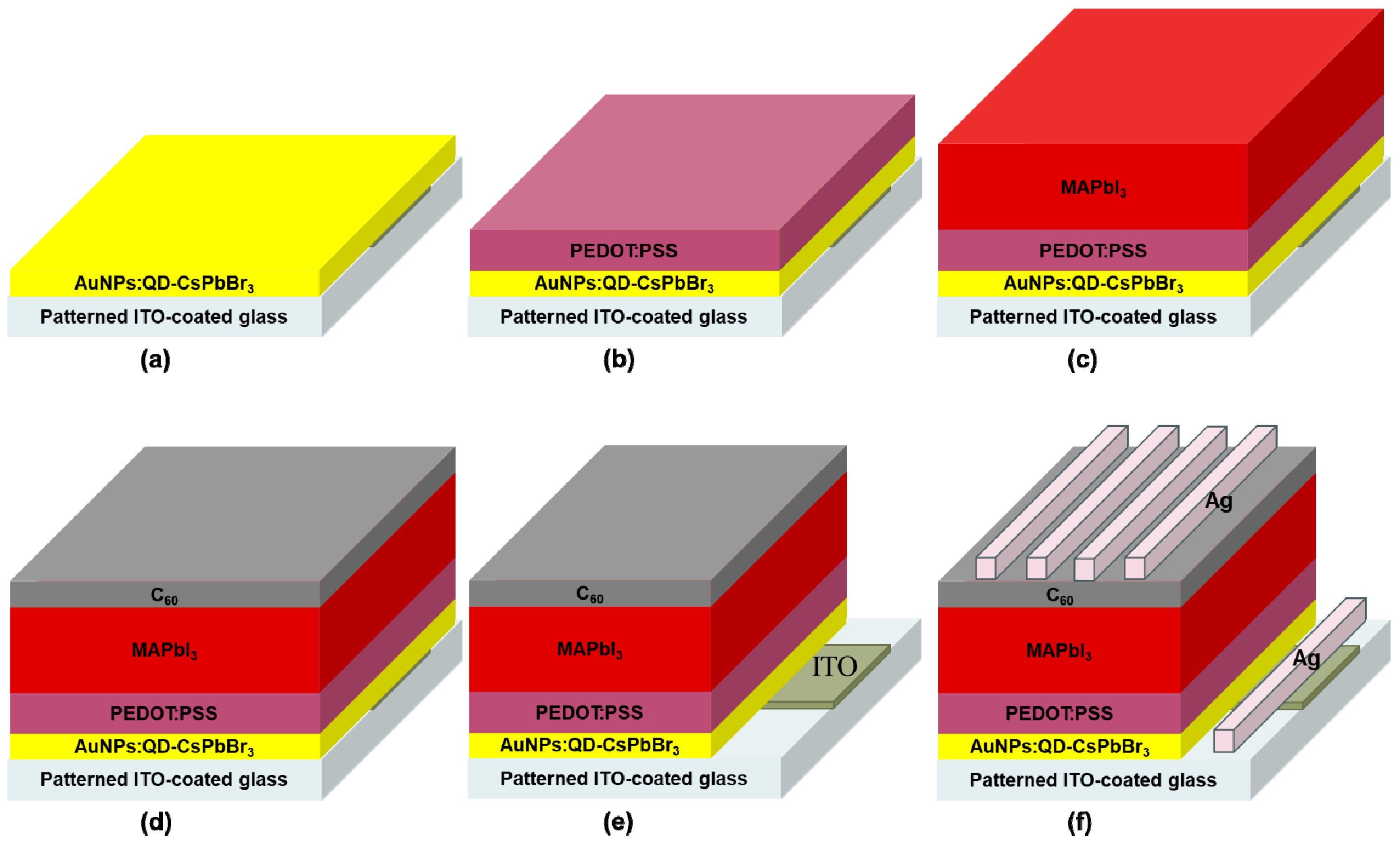
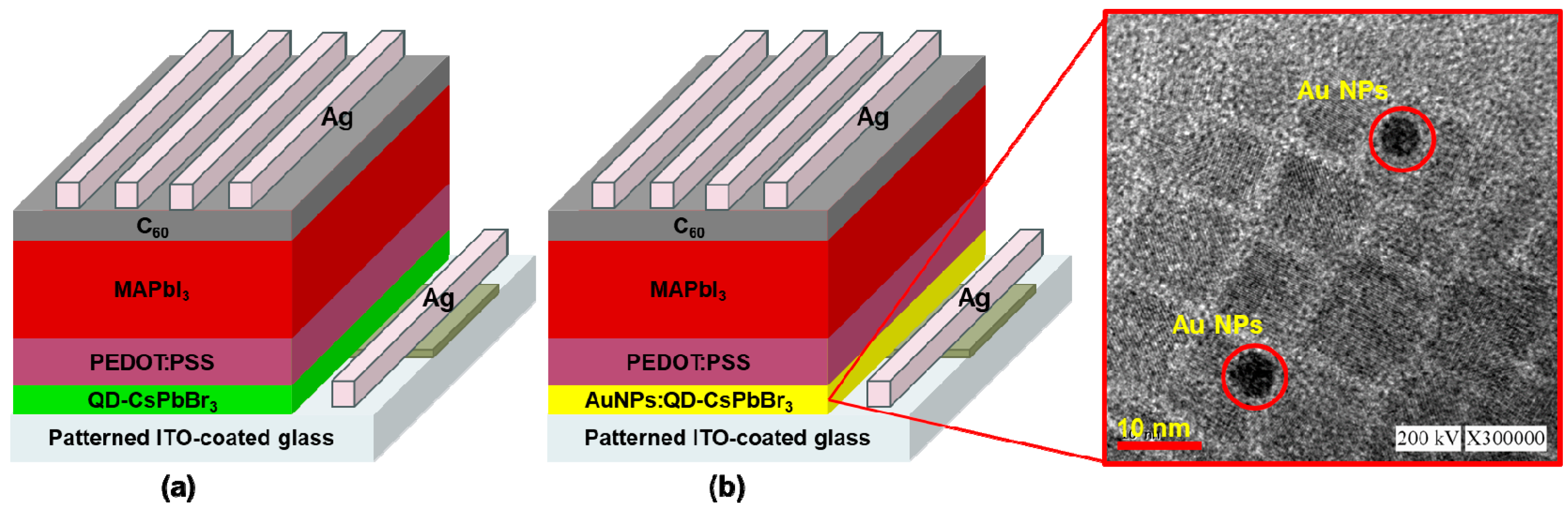
2.2. Fabrication of Plasmonic AuNPs:QD-CsPbBr3/MAPbI3 Perovskite Solar Cells
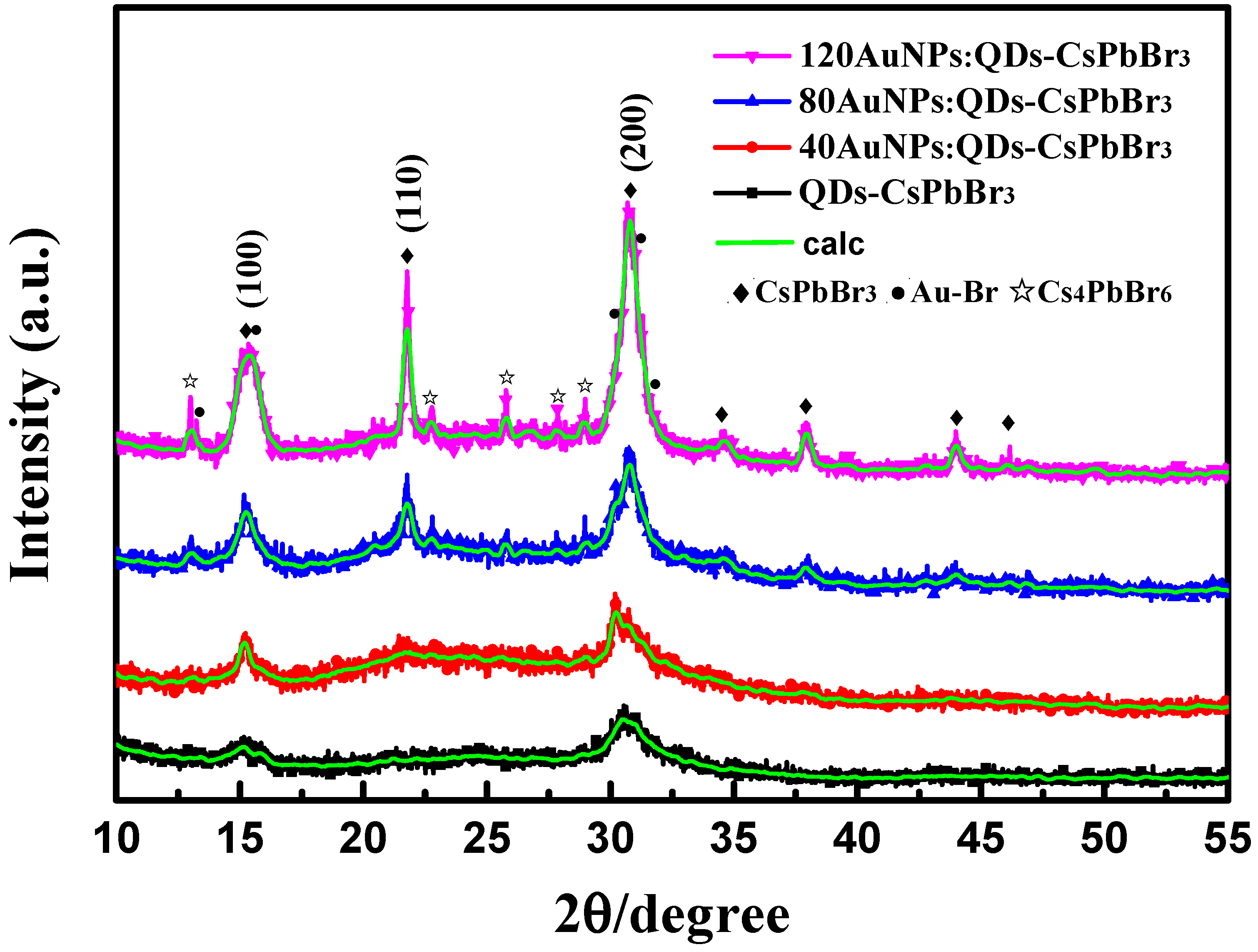
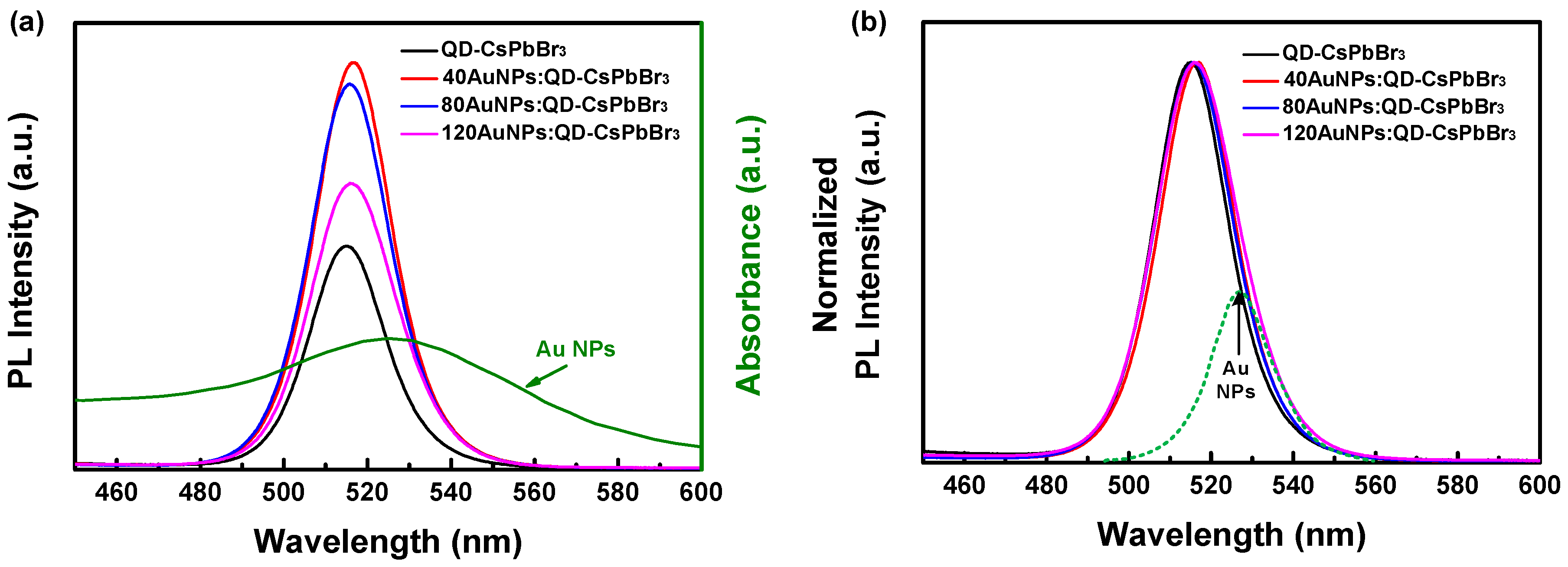
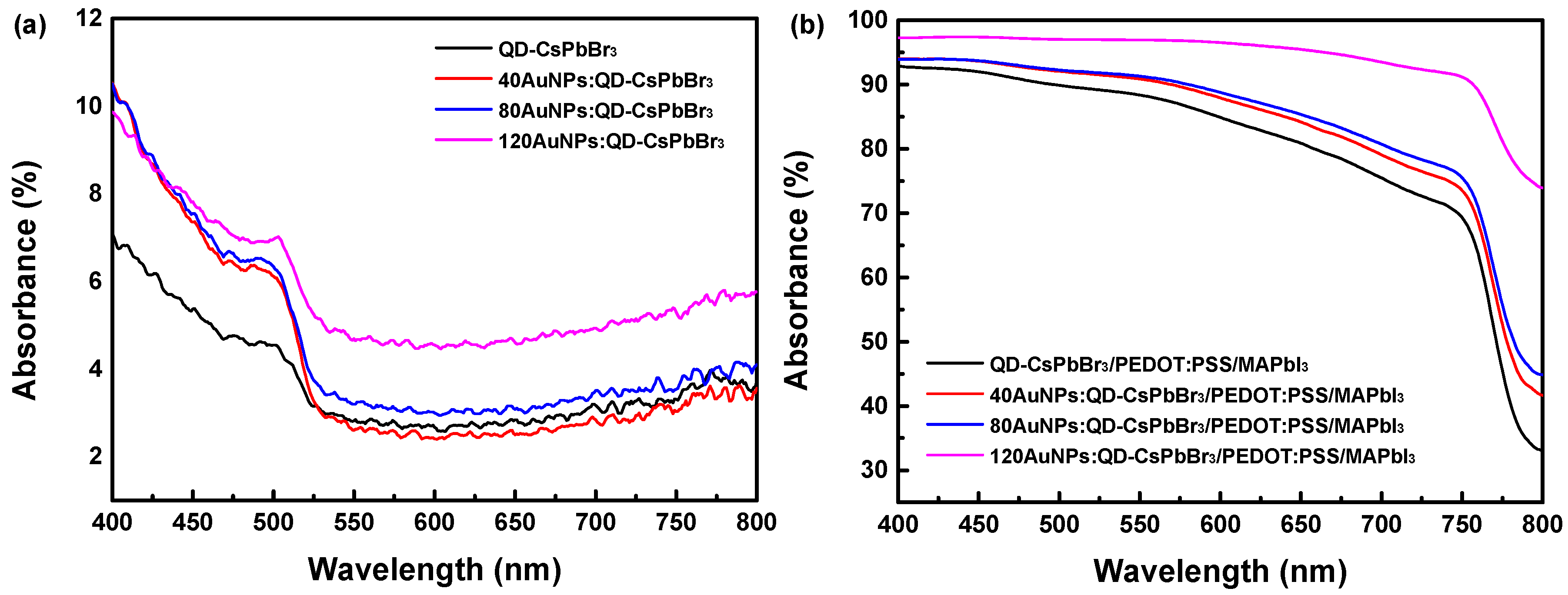
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lee, M.; Jo, Y.H.; Kim, D.S.; Jeong, H.Y.; Jun, Y.S. Efficient, durable and flexible perovskite photovoltaic devices with Ag-embedded ITO as the top electrode on a metal substrate. J. Mater. Chem. A 2015, 3, 14592–14597. [Google Scholar] [CrossRef]
- Prochowicz, D.; Tavakoli, M.M.; Solanki, A.; Goh, T.W.; Pandey, K.; Sum, T.C.; Salibag, M.; Yadav, P. Understanding the effect of chlorobenzene and isopropanol anti-solvent treatments on the recombination and interfacial charge accumulation in efficient planar perovskite solar cells. J. Mater. Chem. A 2018, 6, 14307–14314. [Google Scholar] [CrossRef]
- Chen, L.C.; Tien, C.H.; Tseng, Z.L.; Ruan, J.H. Enhance efficiency of MAPbI3 perovskite solar cells with FAPbX3 perovskite quantum dots. Nanomaterials 2019, 9, 121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, X.; Yang, D.; Yang, R.; Yang, B.; Yang, Z.; Ren, X.; Zhang, J.; Niu, J.; Feng, J.; Liu, S. Superior stability for perovskite solar cells with 20% efficiency using vacuum co-evaporation. Nanoscale 2017, 9, 12316–12323. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Liu, Z.; Lee, E.C. High-performance inverted planar perovskite solar cells using a pristine fullerene mixture as an electron-transport layer. J. Mater. Chem. C 2019, 7, 6956–6963. [Google Scholar] [CrossRef]
- Jiang, Q.; Chu, Z.; Wang, P.; Yang, X.; Liu, H.; Wang, Y.; Yin, Z.; Wu, J.; Zhang, X.; You, J. Planar-structure perovskite solar cells with efficiency beyond 21%. Adv. Mater. 2017, 29, 1703852. [Google Scholar] [CrossRef]
- Zhao, J.; Tavakoli, R.; Tavakoli, M.M. Synergistic interface and compositional engineering of inverted perovskite solar cells enables highly efficient and stable photovoltaic devices. Chem. Commun. 2019, 55, 9196–9199. [Google Scholar] [CrossRef]
- Zhou, H.; Chen, Q.; Li, G.; Luo, S.; Song, T.B.; Duan, H.S.; Hong, Z.; You, J.; Liu, Y.; Yang, Y. Interface engineering of highly efficient perovskite solar cells. Science 2014, 345, 542–546. [Google Scholar] [CrossRef]
- Tu, Y.; Wu, J.; He, X.; Guo, P.; Wu, T.; Luo, H.; Liu, Q.; Wang, K.; Lin, J.; Huang, M.; et al. Solvent engineering for forming stonehenge-like PbI2 nano-structures towards efficient perovskite solar cells. J. Mater. Chem. A 2017, 5, 4376–4383. [Google Scholar] [CrossRef]
- Patil, J.V.; Mali, S.S.; Shaikh, J.S.; Patil, A.P.; Patil, P.S.; Hong, C.K. Efficient mixed halide perovskite solar cells via solvent engineering process. Dyes Pigment. 2019, 168, 311–316. [Google Scholar] [CrossRef]
- Wang, D.L.; Cui, H.J.; Hou, G.J.; Zhu, Z.G.; Yan, Q.B.; Su, G. Highly efficient light management for perovskite solar cells. Sci Rep. 2016, 6, 18922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, K.; Zhang, S.; Ruan, Y.; Li, D.; Zhang, T.; Zhen, H. Optimization of light management layers for light harvest of perovskite solar cells. Opt. Express 2019, 27, A1004–A1013. [Google Scholar] [CrossRef] [PubMed]
- Best Research-Cell Efficiencies, National Renewable Energy Laboratory. Available online: https://www.nrel.gov/pv/assets/pdfs/best-research-cell-efficiencies.20200128.pdf (accessed on 27 January 2020).
- Yu, H.; Wang, F.; Xie, F.; Li, W.; Chen, J.; Zhao, N. The role of chlorine in the formation process of “CH3NH3PbI3-xClx” perovskite. Adv. Funct. Mater. 2014, 24, 7102–7108. [Google Scholar] [CrossRef]
- Nie, W.; Tsai, H.; Asadpour, R.; Blancon, J.C.; Neukirch, A.J.; Gupta, G.; Crochet, J.J.; Chhowalla, M.; Tretiak, S.; Alam, M.A.; et al. High-efficiency solution-processed perovskite solar cells with millimeter-scale grains. Science 2015, 347, 522–525. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Zhou, H.; Fang, Y.; Stieg, A.Z.; Song, T.B.; Wang, H.H.; Xu, X.; Liu, Y.; Lu, S.; You, J.; et al. The optoelectronic role of chlorine in CH3NH3PbI3(Cl)-based perovskite solar cells. Nat. Commun. 2015, 6, 7269. [Google Scholar] [CrossRef]
- Pascoe, A.R.; Yang, M.; Kopidakis, N.; Zhu, K.; Reese, M.O.; Rumbles, G.; Fekete, M.; Duffy, N.W.; Cheng, Y.B. Planar versus mesoscopic perovskite microstructures: The influence of CH3NH3PbI3 morphology on charge transport and recombination dynamics. Nano Energy 2016, 22, 439–452. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Zhu, K. Efficient planar perovskite solar cells based on 1.8 eV band gap CH3NH3PbI2Br nanosheets via thermal decomposition. J. Am. Chem. Soc. 2014, 136, 12241–12244. [Google Scholar] [CrossRef]
- Li, W.; Fan, J.; Li, J.; Mai, Y.; Wang, L. Controllable grain morphology of perovskite absorber film by molecular self-assembly toward efficient solar cell exceeding 17%. J. Am. Chem. Soc. 2015, 137, 10399–10405. [Google Scholar] [CrossRef]
- Yin, W.J.; Chen, H.; Shi, T.; Wei, S.H.; Yan, Y. Origin of high electronic quality in structurally disordered CH3NH3PbI3 and the passivation effect of Cl and O at grain boundaries. Adv. Electron. Mater. 2015, 1, 1500044. [Google Scholar] [CrossRef]
- Gaspera, E.D.; Peng, Y.; Hou, Q.; Spiccia, L.; Bach, U.; Jasieniak, J.J.; Cheng, Y.B. Ultra-thin high efficiency semitransparent perovskite solar cells. Nano Energy 2015, 13, 249–257. [Google Scholar] [CrossRef]
- Zhang, W.; Saliba, M.; Stranks, S.D.; Sun, Y.; Shi, X.; Wiesner, U.; Snaith, H.J. Enhancement of perovskite-based solar cells employing core–shell metal nanoparticles. Nano Lett. 2013, 13, 4505–4510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qi, J.; Dang, X.; Hammond, P.T.; Belcher, A.M. Highly efficient plasmon-enhanced dye-sensitized solar cells through metal@oxide core–shell nanostructure. ACS Nano 2011, 5, 7108–7116. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.L.; Juang, T.Y.; Chen, C.P.; Hsieh, C.M.; Yang, C.C.; Huang, C.L.; Jeng, R.J. Enhanced efficiency of organic and perovskite photovoltaics from shape-dependent broadband plasmonic effects of silver nanoplates. Sol. Energy Mater. Sol. Cells 2015, 140, 224–231. [Google Scholar] [CrossRef]
- Ye, L.; Fan, B.; Zhang, S.; Li, S.; Yang, B.; Qin, Y.; Zhang, H.; Hou, J. Perovskite-polymer hybrid solar cells with near-infrared external quantum efficiency over 40%. Sci. China Mater. 2015, 58, 953–960. [Google Scholar] [CrossRef]
- Cai, B.; Peng, Y.; Cheng, Y.B.; Gu, M. 4-fold photocurrent enhancement in ultrathin nanoplasmonic perovskite solar cells. Opt. Express 2015, 23, A1700–A1706. [Google Scholar] [CrossRef]
- Cheng, K.; Wu, Y.; Meng, J.; Zhao, Y.; Wang, X.; Du, Z. Plasmon-enhanced photocurrent generation in quantum dots-sensitized solar cells by coupling of gold nanocrystals. Sci. Bull. 2015, 60, 541–548. [Google Scholar] [CrossRef] [Green Version]
- Atwater, H.A.; Polman, A. Plasmonics for improved photovoltaic devices. Nat. Mater. 2010, 9, 205–213. [Google Scholar] [CrossRef]
- Park, Y.; Lee, C.; Ryu, S.; Song, H. Ex situ and in situ surface plasmon monitoring of temperature-dependent structural evolution in galvanic replacement reactions at a single-particle level. J. Phys. Chem. C 2015, 119, 20125–20135. [Google Scholar] [CrossRef]
- Li, G.; Zhen, H.; Huang, Z.; Li, K.; Shen, W.; Liu, X. Silver clusters insert into polymer solar cell for enhancing light absorption. Chin. Opt. Lett. 2012, 10, 012401. [Google Scholar] [CrossRef]
- Lee, D.S.; Kim, W.; Cha, B.G.; Kwon, J.; Kim, S.J.; Kim, M.; Kim, J.; Wang, D.H.; Park, J.H. Self-position of Au NPs in perovskite solar cells: Optical and electrical contribution. ACS Appl. Mater. Interfaces 2016, 8, 449–454. [Google Scholar] [CrossRef]
- Wang, J.; Jia, X.; Zhou, J.; Pan, L.; Huang, S.; Chen, X. Improved performance of polymer solar cells by thermal evaporation of AgAl alloy nanostructures into the hole-transport layer. ACS Appl. Mater. Interfaces 2016, 8, 26098–26104. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.; Shen, P.; Liu, Y.; Chen, B.; Guo, W.; Ruan, S.; Shen, L. Performance improvement of polymer solar cells by surface-energy-induced dual plasmon resonance. ACS Appl. Mater. Interfaces 2016, 8, 6183–6189. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.D.; Suteewong, T.; Kumar, R.S.; D’Innocenzo, V.; Petrozza, A.; Lee, M.M.; Wiesner, U.; Snaith, H.J. Plasmonic dye-sensitized solar cells using core−shell metal−insulator nanoparticles. Nano Lett. 2011, 11, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Ding, I.K.; Zhu, J.; Cai, W.; Moon, S.J.; Cai, N.; Wang, P.; Zakeeruddin, S.M.; Grätzel, M.; Brongersma, M.L.; Cui, Y.; et al. Plasmonic dye-sensitized solar cells. Adv. Energy Mater. 2011, 1, 52–57. [Google Scholar] [CrossRef]
- Shalan, A.E.; Oshikiri, T.; Sawayanagi, H.; Nakamura, K.; Ueno, K.; Sun, Q.; Wu, H.P.; Diau, E.W.G.; Misawa, H. Versatile plasmonic-effects at the interface of inverted perovskite solar cells. Nanoscale 2017, 9, 1229–1236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, J.; Li, J.; Li, X.; Xu, L.; Dong, Y.; Zeng, H. Quantum dot light-emitting diodes based on inorganic perovskite cesium lead halides (CsPbX3). Adv. Mater. 2015, 27, 7162–7167. [Google Scholar] [CrossRef]
- Kulbak, M.; Cahen, D.; Hodes, G. How important is the organic part of lead halide perovskite photovoltaic cells? Efficient CsPbBr3 cells. J. Phys. Chem. Lett. 2015, 6, 2452–2456. [Google Scholar] [CrossRef]
- Stamplecoskie, K.G.; Kamat, P.V. Synergistic effects in the coupling of plasmon resonance of metal nanoparticles with excited gold clusters. J. Phys. Chem. Lett. 2015, 6, 1870–1875. [Google Scholar] [CrossRef] [Green Version]
- Dong, Y.; Gu, Y.; Zou, Y.; Song, J.; Xu, L.; Li, J.; Xue, J.; Li, X.; Zeng, H. Improving all-inorganic perovskite photodetectors by preferred orientation and plasmonic effect. Small 2016, 12, 5622–5632. [Google Scholar] [CrossRef]
- Kirschner, M.S.; Diroll, B.T.; Guo, P.; Harvey, S.M.; Helweh, W.; Flanders, N.C.; Brumberg, A.; Watkins, N.E.; Leonard, A.A.; Evans, A.M.; et al. Photoinduced, reversible phase transitions in all-inorganic perovskite nanocrystals. Nat. Commun. 2019, 10, 504. [Google Scholar] [CrossRef]
- Wang, K.H.; Yang, J.N.; Ni, Q.K.; Yao, H.B.; Yu, S.H. Metal halide perovskite supercrystals: Gold–bromide complex triggered assembly of CsPbBr3 nanocubes. Langmuir 2018, 34, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Saidaminov, M.I.; Almutlaq, J.; Sarmah, S.; Dursun, I.; Zhumekenov, A.A.; Begum, R.; Pan, J.; Namchul Cho, N.; Mohammed, O.F.; Bakr, O.M. Pure Cs4PbBr6: Highly Luminescent Zero-Dimensional Perovskite Solids. ACS Energy Lett. 2016, 1, 840–845. [Google Scholar] [CrossRef] [Green Version]
- Mohammed, O.F. Outstanding Challenges of Zero-Dimensional Perovskite Materials. J. Phys. Chem. Lett. 2017, 8, 565–570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, G.; Ma, J.; Jiang, H.; Li, J.; Yang, D.; Gao, F.; Zeng, J.; Liu, Z.; Liu, S.F. Enhancing efficiency and stability of perovskite solar cells through Nb-doping of TiO2 at low temperature. ACS Appl. Mater. Interfaces 2017, 9, 10752–10758. [Google Scholar] [CrossRef] [PubMed]

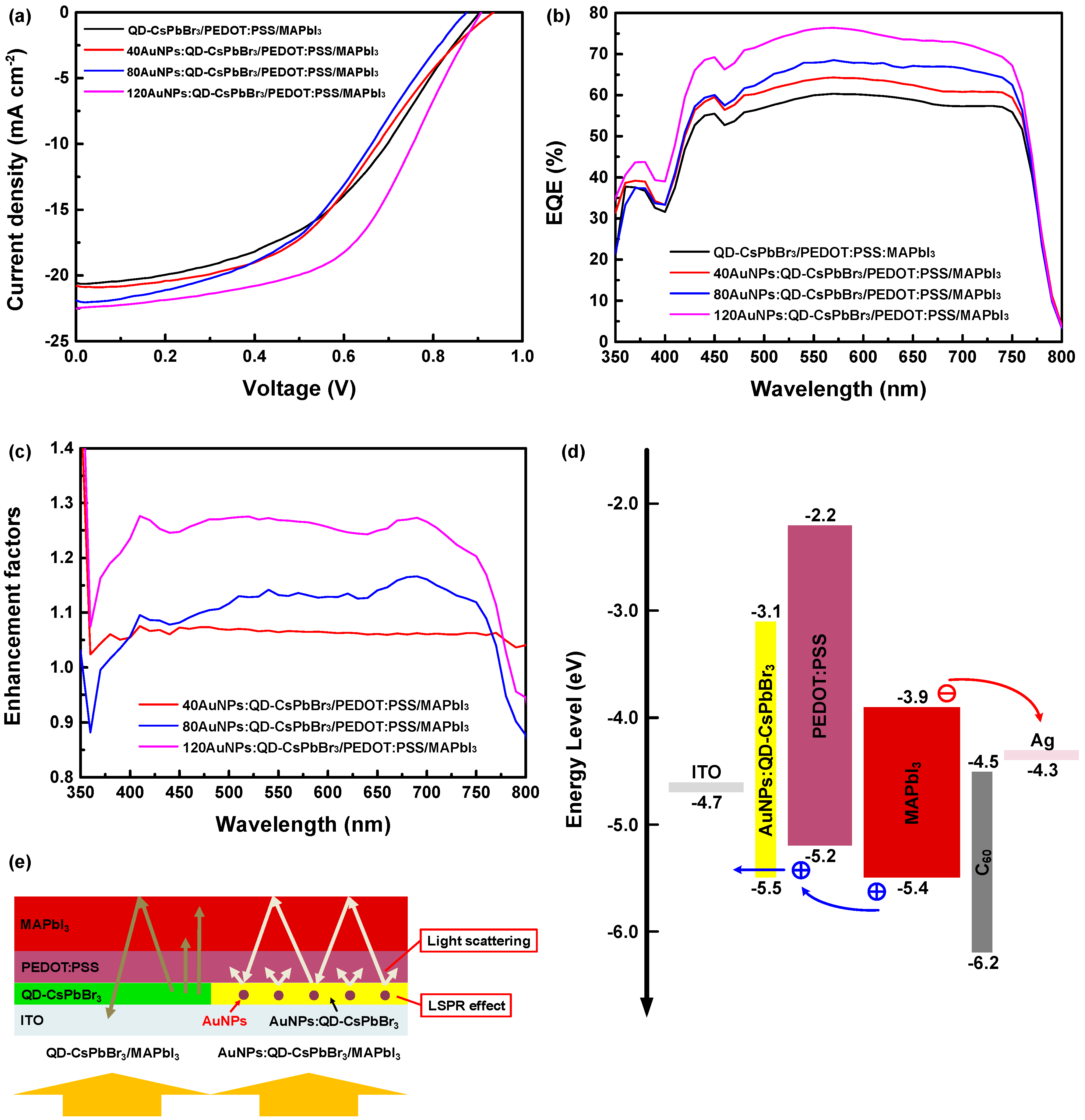
| Sample | Voc (V) | Jsc (mA cm−2) | FF (%) | PCE (%) | PCE Enhancement (%) |
|---|---|---|---|---|---|
| QD-CsPbBr3/PEDOT:PSS/MAPbI3 | 0.9 | 20.6 | 46.0 | 8.53 | -- |
| Standard deviation | 0.009 | 0.309 | 0.380 | 0.104 | |
| 40AuNPs:QD-CsPbBr3/PEDOT:PSS/MAPbI3 | 0.93 | 20.8 | 44.9 | 8.68 | 1.2 |
| Standard Deviation | 0.015 | 0.248 | 0.269 | 0.052 | |
| 80AuNPs:QD-CsPbBr3/PEDOT:PSS/MAPbI3 | 0.91 | 21.9 | 47.1 | 9.4 | 10.2 |
| Standard Deviation | 0.014 | 0.245 | 0.286 | 0.048 | |
| 120AuNPs:QD-CsPbBr3/PEDOT:PSS/MAPbI3 | 0.9 | 22.5 | 54.0 | 10.9 | 27.8 |
| Standard Deviation | 0.011 | 0.280 | 0.230 | 0.065 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.-C.; Tien, C.-H.; Lee, K.-L.; Kao, Y.-T. Efficiency Improvement of MAPbI3 Perovskite Solar Cells Based on a CsPbBr3 Quantum Dot/Au Nanoparticle Composite Plasmonic Light-Harvesting Layer. Energies 2020, 13, 1471. https://doi.org/10.3390/en13061471
Chen L-C, Tien C-H, Lee K-L, Kao Y-T. Efficiency Improvement of MAPbI3 Perovskite Solar Cells Based on a CsPbBr3 Quantum Dot/Au Nanoparticle Composite Plasmonic Light-Harvesting Layer. Energies. 2020; 13(6):1471. https://doi.org/10.3390/en13061471
Chicago/Turabian StyleChen, Lung-Chien, Ching-Ho Tien, Kuan-Lin Lee, and Yu-Ting Kao. 2020. "Efficiency Improvement of MAPbI3 Perovskite Solar Cells Based on a CsPbBr3 Quantum Dot/Au Nanoparticle Composite Plasmonic Light-Harvesting Layer" Energies 13, no. 6: 1471. https://doi.org/10.3390/en13061471
APA StyleChen, L.-C., Tien, C.-H., Lee, K.-L., & Kao, Y.-T. (2020). Efficiency Improvement of MAPbI3 Perovskite Solar Cells Based on a CsPbBr3 Quantum Dot/Au Nanoparticle Composite Plasmonic Light-Harvesting Layer. Energies, 13(6), 1471. https://doi.org/10.3390/en13061471






