Abstract
Proton exchange membrane is an important factor affecting the power generation capacity and water purification effect of microbial fuel cells. The performance of microbial fuel cells can be improved by modifying the proton exchange membrane by some suitable method. Microbial fuel cells with membranes modified by SiO2/PVDF (polyvinylidene difluoride), sulfonated PVDF and polymerized MMA (methyl methacrylate) electrolyte were tested and their power generation capacity and water purification effect were compared. The experimental results show that the three membrane modification methods can improve the power generation capacity and water purification effect of microbial fuel cells to some extent. Among them, the microbial fuel cell with the polymerized MMA modified membrane showed the best performance, in which the output voltage was 39.52 mV, and the electricity production current density was 18.82 mA/m2, which was 2224% higher than that of microbial fuel cell with the conventional Nafion membrane; and the COD (chemical oxygen demand) removal rate was 54.8%, which was 72.9% higher than that of microbial fuel cell with the conventional Nafion membrane. Modifying the membrane with the polymerized MMA is a very effective way to improve the performance of microbial fuel cells.
1. Introduction
Energy crisis and environmental pollution are two important factors hindering the development of economy and society. On the one hand, due to over exploitation, the traditional fossil fuel has been nearly exhausted, and the competition around resources in the world is increasingly intensified; on the other hand, the use of fossil fuels will produce a large amount of gas and solid waste, which will further aggravate the environmental pollution. Therefore, the development of renewable energy which can replace fossil energy has become the global focus [1,2,3,4].
Microbial fuel cell (MFC) is a kind of bioreactor that directly converts chemical energy in organic matter into electrical energy by microbial metabolic activities. MFC, which uses organic waste as feed, can generate electric energy in the process of waste treatment, and will not release harmful substances to the environment in the process of power generation. It can solve the two major problems of energy and environment at the same time. Therefore, it is considered as a kind of sustainable energy with high efficiency, low consumption, clean and environmental protection, and has become a research hotspot in many fields such as environment and energy [5,6,7]. In recent years, great progress has been made in the research of MFCs, but its popularization and application are still restricted by the problems of low power generation capacity, unstable operation, high cost, etc.
The factors affecting the performance of MFCs include microbial population, exchange membrane, electrodes, internal and external resistance, substrate concentration, electrode spacing, etc. [8,9,10,11,12,13]. Among all these factors, proton exchange membrane, as an important part of separating anode chamber and cathode chamber and transferring protons from anode to cathode, has a direct impact on electron transfer, and its cost accounts for about 40% of the total cost of MFCs [14,15,16]. Therefore, proton exchange membrane is considered as the most important factor affecting the performance of MFCs, and it is also one of the important bottlenecks in the popularization and application of MFCs.
At present, almost all commercial proton exchange membranes are Nafion membranes. Nafion membrane has the advantages of high proton conductivity, good chemical and physical stability, good mechanical durability, etc., but the use of Nafion membrane also brings high oxygen permeability, poor thermal stability, biological deposition and some other problems, which further limits the performance of MFCs [17]. In order to promote the practical application of MFCs, it is necessary to improve the performance of the proton exchange membrane.
Membrane modification can effectively improve the proton conductivity and water retention of the membrane, reduce the internal resistance of MFCs, so as to improve the power generation performance and wastewater treatment effect of MFCs. Many research methods have been used to modify proton exchange membrane. The main modification methods include doping nanoparticles, nanofibers, organic and inorganic particles in the proton exchange membrane, or using some aromatic polymers, such as polyether ketone, polyether sulfone, polybenzimidazole, heteropoly acid, etc., to make blend membranes. Metal composite membrane prepared by depositing Pt or other metal oxides in Nafion membrane can reduce the influence of fuel penetration on the cathode and achieve the effect of humidification and water retention [18]. Heteropolyacids (HPAs) are a kind of inorganic proton conducting materials with high proton conductivity. Heteropoly acid/Nafion composite membrane is helpful to improve electrical conductivity, water retention and reduce fuel permeation [19]. Zirconium phosphate/Nafion composite membrane can significantly improve the conductivity of the membrane [20]. Most of these modification methods can effectively improve the electricity generation performance and wastewater treatment effect of MFCs to a certain extent. However, there are still some problems such as high manufacturing cost, poor durability.
Although silicon dioxide (SiO2) cannot be used as proton conductor like zirconium phosphate and heteropoly acid, nor can it play the role of hydrogen permeation and catalysis like Pt, the presence of SiO2 can improve the water retention of membrane and facilitates the transfer of H+ [21]. Methyl methacrylate (MMA) are also used for the modification of membranes, making them antifouling [22]. Polyvinylidene difluoride (PVDF) membranes have been extensively applied to scientific research and industrial process due to its outstanding properties such as high thermal stability, good chemical resistance and membrane forming properties [23]. The three materials have stable chemical properties and are widely circulated in the market, making them become good modification materials for proton exchange membrane. These three materials are much cheaper than Nafion membrane, therefore the overall cost performance of Nafion composite membrane modified by these three materials will be improved. It is a good choice to use these low-cost materials to optimize the performance of MFCs.
In this paper, SiO2, MMA and PVDF were used to modify the proton exchange membrane respectively, and the effects of these three different modification methods on the power generation performance and water purification capacity of MFCs were studied.
2. Materials and Methods
2.1. Structure of the Experimental System
A dual-chamber MFC was used as an experimental reaction system for this study, as shown in Figure 1. Both the volume of the cathode chamber and the anode chamber are 500 mL. The cathode and anode compartments are separated by a proton exchange membrane. The aerobic state of the cathode chamber and the anaerobic state of the anode should be maintained throughout the reaction process. The electrode materials of cathode and anode are carbon cloth (WOS1002, CeTech Co., Taiwan China) with thickness of 0.36 mm, and the surface area is 6 cm × 7 cm. The real-time output voltage generated by MFC was collected and transmitted online through a 16-channel multi-function USB data acquisition card (MPS-010602, Morpheus Electronics Technology Co., Beijing China) in parallel with the load, and displayed, stored and processed by the computer.
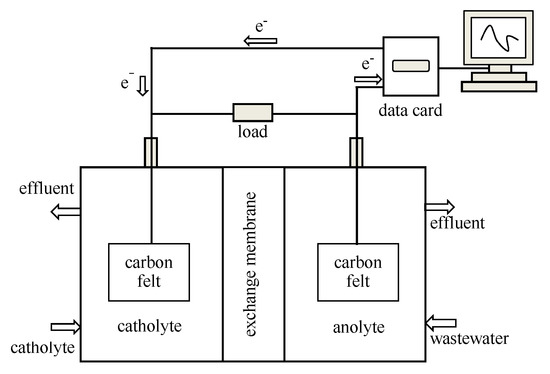
Figure 1.
Schematic diagram of the microbial fuel cell (MFC) experimental system.
2.2. Experimental Materials and Pretreatment
The reagents used in the experiment mainly include acetone (CH3COCH3) (Damao Co., Tianjin China), methanol (CH3OH) (Damao Co., Tianjin China), polyvinylidene fluoride (PVDF) (Arkema Co.,France), N,N-Dimethylformamide (DMF) (Damao Co., Tianjin China), sodium chloride (NaCl) (Damao Co., Tianjin China), potassium ferricyanide (K3[Fe(CN)6]) (Damao Co., Tianjin China), hydrogen peroxide (H2O2) (Damao Co., Tianjin China), sulfuric acid (H2SO4) (Damao Co., Tianjin China), silicon dioxide (SiO2) (Damao Co., Tianjin China), sodium bicarbonate (NaHCO3) (Damao Co., Tianjin China), silane coupling agent (KH550) (Axygen Co., USA), N-methyl pyrrolidone (C5H9NO) (Damao Co., Tianjin China), sodium sulfite (Na2SO3) (Damao Co., Tianjin China), ammonium chloride (NH4Cl) (Damao Co., Tianjin China), potassium chloride (KCl) (Damao Co., Tianjin China), Manganese (II) Sulfate Monohydrate (MnSO4·H2O) (Damao Co., Tianjin China), sodium dihydrogen phosphate (NaH2PO4·H2O) (Damao Co., Tianjin China), disodium phosphate dodecahydrate (Na2HPO4·12H2O) (Damao Co., Tianjin China), methyl methacrylate (MMA) (Damao Co., Tianjin China), etc., all of which are analytical pure reagents.
The cathode electrolyte used in the experiment was a mixed solution of K3 [Fe(CN)6] and NaCl. K3[Fe(CN)6] (632.9 g, 0.2 mol/L) was dissolved with PBS in a 500 mL volumetric flask, NaCl solution (11.7 g, 0.4 mol/L) was put into another 500 mL volumetric flask; then the above K3[Fe(CN)]6 solution and NaCl solution were mixed at a ratio of 1:1 to obtain the cathode electrolyte of the MFC.
Molasses wastewater was used as the anolyte of MFC. the molasses wastewater was prepared by the following chemicals: 3.13 g/L NaHCO3, 0.31 g/L NH4Cl, 6.338 g/L NaH2PO4·H2O, 6.8556 g/L Na2HPO4·12H2O, 0.13 g/L KCl, 0.2 g/L MgSO4·7H2O, 0.015 g/L CaCl2, 0.01 g/L MnSO4·H2O and 3 g/L brown sugar [24].
The microorganisms used in the anode chamber were prepared by sludge culture. The sludge was collected from the bottom of the lake in the campus (Shenyang, China). Under anaerobic conditions, the molasses wastewater and some nutrients (such as carbon, nitrogen, phosphorus, etc.) needed for microbial growth were put into the culture flask together with the collected sludge, and then domesticated in the biochemical incubator at a constant temperature of 20 °C. When the sludge in the culture bottle is suspended as flocculant, the cultivation of microorganisms can be considered successful.
Carbon cloth was used for both anode and cathode. Before use, the carbon cloth was pretreated by following methods: first, the carbon cloth was immersed in acetone solution for 2 h, then the immersed carbon cloth was washed repeatedly with deionized water until it was neutral, and then dried in an oven at 65–85 °C for reserve.
2.3. Preparation and Modification of Membranes
The basic material of proton exchange membrane used in the experiment was Nafion 117 which was produced by DuPont Company. Proton exchange membranes need to be pretreated before they are used. After boiling for 1 h in a 5% H2O2 solution, the organic impurities were removed by repeated washing with deionized water. Then the membrane was boiled in 1 mol/L H2SO4 for 1 h to convert the membrane into H+ type, then the membrane was washed repeatedly with deionized water to remove the impurities of metal ions. Finally, the membrane was boiled for 1 h with deionized water and then washed repeatedly with deionized water for 5 times to remove the residual H2SO4 in the membrane. The pretreated Nafion 117 membrane was put into deionized water for reserve.
The powdered PVDF was dried and added into DMF/acetone mixed solvent with the volume ratio of 4:6, then heated to 40–50 °C in a water bath and stirred until a uniform solution appeared. Then four parts of nano-SiO2 which the mass fractions of nano particles in PVDF were 2%, 5%, 8% and 10%, respectively, were added to the above solution, and a few drops of silane coupling agent (KH550) were added, then mechanical stirring was carried out with a stirrer (96-1A, Hongyi Instrument Equipment Co,. Shanghai China) for about 20 minutes (60 r/min), and then the SiO2/PVDF mixture was gained for use.
The PVDF was sulfonated with concentrated sulfuric acid for 12 h at 60 °C to obtain light brown particles, then rinsed and neutralized with deionized water. Finally, the PVDF was dissolved in N-methyl pyrrolidone at 80 °C to obtain the mixed solution of sulfonated PVDF for reserve.
Two Nafion membranes of 10 cm × 10 cm were immersed in methanol solution with volume ratio of 3:1 for 1 h, then were taken out and immersed in SiO2/PVDF mixture and sulfonated PVDF mixture respectively for 5 minutes at 60 °C; then were taken out and air-dried; then placed in a vacuum drying chamber (101-2BS, Lichen Co,. Shanghai China) to dry at 60 °C for 15 minutes. Four to five times of the above operations were repeated to obtain SiO2/PVDF modified and sulfonated PVDF modified proton exchange membranes [25].
Then, 0.2 mol/L MMA solution was put into a 500 mL beaker, another Nafion membrane was put into the MMA solution for 24 h, then the soaked Nafion membrane was taken out and immersed in the new MMA solution and a certain concentration of sodium sulfite was added as initiator. The polymerized MMA proton exchange membrane was obtained by sealed polymerization for 48 h. The prepared modified proton exchange membrane was dried and placed in deionized water for reserve.
2.4. Analysis Methods
In order to evaluate the performance of MFCs, the current density was measured and compared. The output current I of MFC can be calculated by Ohm’s law according to the measured voltage value U, that is:
where U is the output voltage of MFC, which is measured by data acquisition card; R is the load resistance. In the experiment, the external load resistance is always set at a fixed resistance value of 500 Ω. Since the load current is consistent with the internal current of the MFC, the current density IA of the MFC can be calculated according to its output current and the anode surface area A, that is:
I = U/R
The water absorption of the membrane can be determined by measuring the weight of the membrane before and after hydration. Firstly, the membrane was baked in an oven at 80 °C for 24 hours, then the membrane was taken out and cooled to room temperature and weighed. The weight of the dry membrane was recorded as W0. Then, the membrane was immersed in deionized water at room temperature for 24 hours, and the weight of the wet membrane was recorded as W1. The water absorption rate of the membrane was calculated by:
Scanning electron microscopy (SEM) (JSM-6360LV; JEOL Co., Japan) was used to characterize the membrane to further observe the surface morphology of the membrane.
In order to further analyze the properties of the proton exchange membranes, cyclic voltammetry (CV) tests on the membrane were carried out with an electrochemical workstation (CHI660E, Shanghai China). The three-electrode system of electrochemical workstation was used in the test, and PBS (50 mmol/L, pH = 6.8) was used as the buffer solution; the potential scanning range was −1.10 V ~ 0.7 V, and the scanning speed was 10 mV/s. All the tests were carried out at room temperature.
The COD removal rate was tested to analyze the wastewater treatment effect of MFCs. The influent and effluent COD of the anode chamber were measured by a LH-NP2 type COD rapid detector (Lohand biological Co, Dalian China), and then the COD removal rates of MFC were calculated by:
where CODi denotes the influent COD and CODo denotes the effluent COD.
3. Results and Discussions
3.1. Effect of SiO2/PVDF Modified Membrane on Electricity Generation Performance of MFC
In the experiment, four groups of MFC experimental systems with the same basic configuration were operated simultaneously to ensure that all the experimental conditions were the same except for the comparison terms, thus ensuring a good contrast effect.
The output voltage curves of the four MFCs with the membranes modified by SiO2/PVDF in different mass fraction were shown in Figure 2. It can be seen that, the steady-state output voltage of MFC with ordinary Nafion membrane was about 1.70 mV, and when SiO2/PVDF with mass fractions of 2%, 5%, 8% and 10% were used to modify the Nafion membrane of MFCs, the steady-state output voltage of MFCs were about 14.26 mV, 18.17 mV, 22.40 mV and 15.28 mV, respectively, which were 738%, 969%, 1218% and 799% higher than that of MFC with ordinary Nafion membrane. So, membrane modification with SiO2/PVDF mixture can significantly improve the performance of MFC. When the mass fraction of the modified solution increases from 2% to 5% and 8%, the output voltage of MFC increased gradually; when the mass fraction of the modified solution was 8%, the output voltage reached the maximum and was relatively more stable; when the mass fraction of SiO2/PVDF continued to increase to 10%, the output voltage no longer increased but decreased, and it showed a significant downward trend after a sharp fluctuation in the period of 150 h to 170 h. Therefore, increasing the mass fraction of the modified solution does not mean that the improvement effect is better. Excessive mass fraction will inhibit the power generation capacity of MFCs. When the SiO2/PVDF mixture was used to modify the proton exchange membrane, the modification effect reached the best state when the mass fraction was 8%.
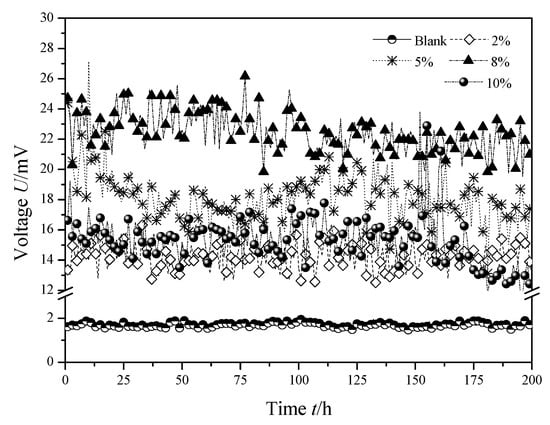
Figure 2.
Output voltage of MFC with the membranes modified by SiO2/Polyvinylidene difluoride (PVDF) in different mass fraction.
3.2. Effect of Sulfonated PVDF and Polymerized MMA Modified Membranes on Electricity Generation Performance of MFC
The output voltage curves of MFCs with sulfonated PVDF and polymerized MMA modified Nafion membrane were shown in Figure 3.
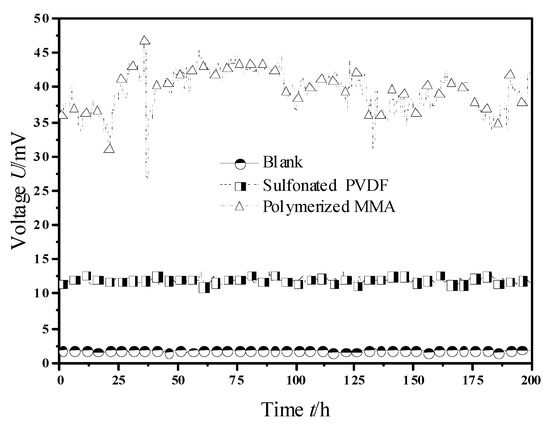
Figure 3.
Output voltage of MFC with membranes modified by sulfonated PVDF and polymerized methyl methacrylate (MMA).
As can be seen from Figure 3, when sulfonated PVDF was used as a modified solution, the steady-state output voltage of MFC was about 11.97 mV, which was 604% higher than that of MFC with normal Nafion membrane. This shows that the sulfonated PVDF modified Nafion membrane has a good effect on improving the electric performance of MFC. When polymerized MMA was used as the modification solution of proton exchange membrane, the steady-state output voltage was about 39.52 mV, which was 2224% higher than that of MFC with ordinary Nafion membrane, and 230% higher than that of sulfonated PVDF, and the improvement effect was remarkable.
The current density curves of MFCs using the several different proton exchange membranes were shown in Figure 4, which makes it possible to compare the effects of different modification methods more clearly. The steady-state current densities of MFCs with common Nafion membrane, SiO2/PVDF (8% mass fraction) modified Nafion membrane, sulfonated PVDF modified Nafion membrane and polymerized MMA modified Nafion membrane were about 0.81 mA/m2, 5.66 mA/m2, 10.32 mA/m2 and 18.82 mA/m2, respectively. Therefore, modification of proton exchange membranes with SiO2/PVDF, sulfonated PVDF or polymerized MMA can improve the power generation capacity of MFCs in some certain extent. In general, polymerized MMA modified proton exchange membranes can make MFCs produce higher current density under the same conditions. Therefore, from the point of view of improving the electricity production performance of MFCs, polymerized MMA modified Nafion membranes can achieve better results.
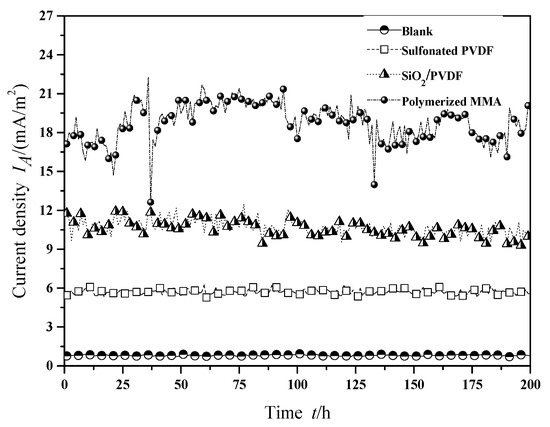
Figure 4.
Current density of MFC with different membranes.
3.3. Analysis of Water Absorption of Membranes
The hydrophilicity of membranes will directly affect the power generation performance of MFCs. When the water content of the membrane is high, the migration coefficient of the proton is higher than that of water, and its conductivity is higher. Therefore, the higher the water absorption of the membrane, the better its conductivity. By calculating the water absorption of the membranes, the water absorption properties of the several different proton exchange membranes used in the experiments can be compared.
The water absorption test data of the conventional Nafion membrane and the three different modified membranes were shown in Figure 5. The water absorption values of conventional Nafion membrane, sulfonated PVDF Nafion membrane, SiO2/PVDF (8% mass fraction) modified membrane and that of polymerized MMA modified membrane were 6.32%, 8.24%, 11.28% and 12.79%, respectively. The water absorption of Nafion membranes modified by three different methods were 30.4%, 78.5% and 102.4% higher than that of conventional Nafion membranes, which further verified the above experimental results that all the three modified methods can effectively improve the conductivity of membranes, and further proved that the modification of Nafion membrane by polymeric MMA was the best of the three methods.
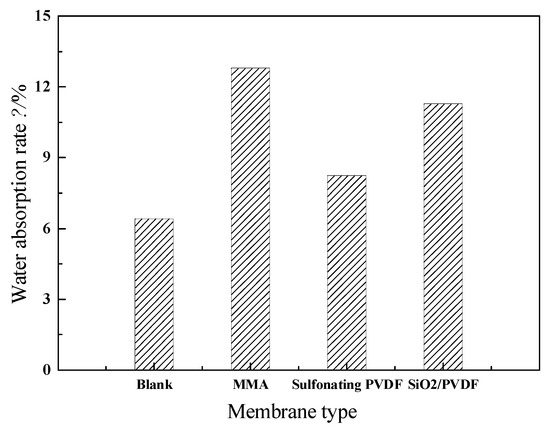
Figure 5.
Water absorption of different proton exchange membranes.
3.4. Water Quality Analysis
The anolyte of MFC was molasses wastewater. Table 1 shows the COD indices of influent and effluent of MFCs with different membranes. It can be seen from the table that the COD removal rate of MFC using conventional Nafion membrane was 31.7%, while the COD removal rates of MFCs with SiO2/PVDF (8% mass fraction) modified Nafion membrane, sulfonated PVDF modified Nafion membrane and polymerized MMA modified Nafion membrane were 41.6%, 42.2% and 54.8%, respectively. The COD removal rates of molasses wastewater in MFCs with the three modified membranes were 31.2%, 33.1% and 72.9% higher than that of MFC with conventional Nafion membrane, respectively. Therefore, when molasses wastewater is used as the anolyte of MFC, MFC can generate electrical energy through degradation of organic matter, and the molasses wastewater feed to the anode chamber of MFC can also be well purified. Moreover, the use of polymerized MMA modified membrane can significantly improve the water purification effect of MFC.

Table 1.
COD removal rate of MFCs with different membranes.
Molasses wastewater belongs to high concentration organic wastewater. The main treatment methods of molasses wastewater are some terminal degradation methods which usually provide low COD removal rate. For example, the COD removal rate of molasses wastewater by photodegradation method was only 2%, the COD removal rate of molasses wastewater in H2O2/TiO2/UV/zeolite system was about 15% [26], the COD removal rate of molasses wastewater in the anaerobic tank with better treatment effect was about 46.0% [27]. The COD removal rate of molasses wastewater in MFC with polymerized MMA modified membrane reached 54.8% in this experiment. Therefore, it is feasible to use MFC as an alternative to molasses wastewater treatment. On the one hand, molasses wastewater can be used as fuel to generate electricity by MFC; on the other hand, MFC can also be used as an effective alternative method to treat molasses wastewater.
3.5. SEM Analysis
Figure 6 shows the SEM photographs of conventional Nafion membrane, sulfonated PVDF (8% mass fraction) modified Nafion membrane and polymerized MMA modified Nafion membrane. It can be found from the photographs that both sulfonated PVDF and polymerized MMA can form adhesion layer on the surface of membrane. The sulfonated PVDF adhered sparsely to membrane, while the polymerized MMA adhered densely to the surface of membrane, which made the modified membrane have more complex morphology than the conventional Nafion membrane. The sulfonated PVDF or polymerized MMA with strong conductivity attached to the membrane, on the one hand, increased the specific surface area of the membrane, improved the membrane’s water retention and proton transfer rate; on the other hand, the good conductivity of sulfonated PVDF or polymerized MMA particles further improved the conductivity of proton exchange membranes. The polymerized MMA can be better attached to membrane, so it can improve the power generation capacity of MFC more effectively, which is consistent with the above experimental results.

Figure 6.
SEM images of conventional Nafion membrane (A), sulfonated PVDF modified Nafion membrane (B) and polymerized MMA modified Nafion membrane (C)
Further experimental results show that the conductivity of polymerized MMA modified Nafion membrane was 12.14 mS/cm at 20 °C, while that of sulfonated PVDF modified Nafion membrane was 4.76 mS/cm, which further proves that the polymerized MMA modified Nafion membrane has better proton conductivity than the sulfonated PVDF modified Nafion membrane.
3.6. Cyclic Voltammetric Characteristics
The cyclic voltammetry curves of the conventional Nafion membrane and the polymerized MMA modified Nafion membrane were shown in Figure 7.
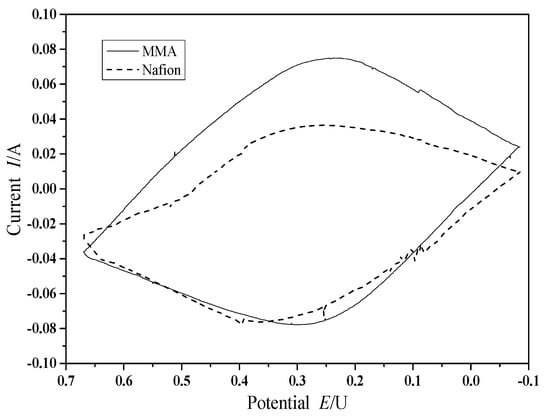
Figure 7.
Cyclic voltammetry (CV) curves of conventional membrane and polymerized MMA modified membrane.
It can be seen from the CV curves that the symmetry of the oxidation wave and the reduction wave of the polymerized MMA modified membrane has been improved, which indicates that the polymerized MMA improves the reversibility of electroactive substances. On the other hand, the polymerized MMA modified membrane has higher redox current than that of the conventional Nafion membrane. The results show that the proton exchange membrane modified by the polymerized MMA speeds up the rate of proton transfer, and such changes in electrochemical properties are beneficial to the performance improvement of MFC.
4. Conclusions
Modification of proton exchange membrane of MFC with some suitable electrolyte can increase the surface activity of the proton exchange membrane, improve the proton transfer ability and thus improve the power generation and water purification effect of MFCs. Modification of proton exchange membrane with SiO2/PVDF, sulfonated PVDF or polymerized MMA as electrolyte can improve the power generation ability and water purification effect of MFC in some certain extent. When the Nafion membrane was modified by the polymerized MMA, the performance of electricity generation and water purification of the MFC was the best. In this case, the steady-state output voltage was 39.52 mV, the current density was 18.82 mA/m2, which was 2224% higher than that of MFC with an ordinary Nafion membrane; the COD removal rate of molasses wastewater reached 54.8%, which was 72.9% higher than that of MFC with an ordinary Nafion membrane. Therefore, modifying the membrane with the polymerized MMA is a very effective way to improve the performance of MFCs.
Author Contributions
Conceptualization, L.F.; data curation, J.S. and T.G.; formal analysis, J.S. and T.G.; investigation, L.F. and J.S.; methodology, L.F.; project administration, L.F.; resources, L.F.; validation, J.S. and T.G.; visualization, L.F.; writing—original draft, T.G.; writing—review and editing, L.F. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the national natural science foundation of China, Grant 61143007; the Chinese-Macedonian scientific and technological cooperation project of ministry of science and technology of the China, Grant [2017] 25: 5-5, and the plan for distinguished professors in Liaoning Province, China, Grant [2014] 187.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ellabban, O.; Abu-rub, H.; Blaabjerg, F. Renewable energy resources: Current status, future prospects and their enabling technology. Renew. Sustain. Energy Rev. 2014, 39, 748–764. [Google Scholar] [CrossRef]
- Ivan, S.; Rene, P. Optimal sizing of renewable sources and energy storage in low-carbon microgrid nodes. Electr. Eng. 2018, 100, 1661–1674. [Google Scholar]
- Susana, S.; Isabel, S.; Carlos, P. Renewable energy subsidies versus carbon capture and sequestration support. Environ. Dev. Sustain. 2018, 20, 1213–1227. [Google Scholar]
- Nasirov, S.; Agostini, C.; Silva, C.; Caceres, G. Renewable energy transition: A market-driven solution for the energy and environmental concerns in Chile. Clean Technol. Environ. 2018, 20, 3–12. [Google Scholar] [CrossRef]
- Wang, H.; Park, J.D.; Ren, Z.J. Practical energy harvesting for microbial fuel cells: A review. Environ. Sci. Technol. 2015, 49, 3267–3277. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Khan, M.D.; Nizami, A.S.; Rehan, M.; Shaida, A.; Ahmad, A.; Khan, M.Z. Energy generation through bioelectrochemical degradation of pentachlorophenol in microbial fuel cell. RSC Adv. 2018, 8, 20726–20736. [Google Scholar] [CrossRef]
- Varanasi, L.J.; Sinha, P.; Das, D. Maximizing power generation from dark fermentation effluents in microbial fuel cell by selective enrichment of exoelectrogens and optimization of anodic operational parameters. Biotechnol. Lett. 2017, 39, 721–730. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.P.; Zheng, Y.J.; Miao, X.H. Effects of catholyte and dissolved oxygen on microbial fuel cell performance. J. Chem. Eng. Chin. Univ. 2016, 30, 491–496. [Google Scholar]
- Logan, B.E.; Zikmund, E.; Yang, W.; Rossi, R.; Kim, K.Y.; Saikaly, P.E.; Zhang, F. Impact of ohmic resistance on measured electrode potentials and maximum power production in microbial fuel cells. Environ. Sci. Technol. 2018, 52, 8977–8985. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhao, J.; Liu, S.; Zhao, R.; Hu, B. Effect of Temperature on Nitrogen Removal and Electricity Generation of a Dual-Chamber Microbial Fuel Cell. Water Air Soil Pollut. 2018, 229, 244. [Google Scholar] [CrossRef]
- Fan, L.P.; Miao, X.H. Study on the performance of microbial fuel cell for restaurant wastewater treatment and simultaneous electricity generation. J. Fuel Chem. Technol. 2014, 42, 1506–1512. [Google Scholar]
- Liu, J.D.; Tian, C.; Xiong, J.X.; Wang, L.J. Polypyrrole blending modification for PVDF conductive membrane preparing and fouling mitigation. J. Colloid Interface Sci. 2017, 494, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Kloch, M.; Toczylowska-Maminska, R. Toward optimization ofwood industry wastewater treatment in microbial fuel cells—mixed wastewaters approach. Energies 2020, 13, 263. [Google Scholar] [CrossRef]
- Kong, X.Y.; Li, L.H.; Ma, L.L.; Sun, Y.M.; Li, Y.; Yuan, Z.H. Electricity generation comparison of two-chamber microbial fuel cells with different membrane. Acta Energ. Sol. Sin. 2012, 33, 882–887. [Google Scholar]
- Srinophakun, P.; Thanapimmetha, A.; Plangsri, S.; Vetchayakunchai, S.; Saisriyoot, M. Application of modified chitosan membrane for microbial fuel cell: Roles of proton carrier site and positive charge. J. Clean. Prod. 2017, 142, 1274–1282. [Google Scholar] [CrossRef]
- Hernández-Flores, G.; Poggi-Varaldo, H.M.; Solorza-Feria, O. Comparison of alternative membranes to replace high cost Nafion ones in microbial fuel cells. Int. J. Hydrog. Energy 2016, 41, 23354–23362. [Google Scholar] [CrossRef]
- Hasani-Sadrabadi, M.M.; Dashtimoghadam, E.; Eslami, S.N.S.; Bahlakeh, G.; Shokrgozar, M.A.; Jacob, K.I. Air-breathing microbial fuel cell with enhanced performance using nanocomposite proton exchange membranes. Polymer 2014, 55, 6102–6109. [Google Scholar] [CrossRef]
- Uchida, H.; Mizuno, Y.; Watanabe, M. Suppression of methanol crossover and distribution of ohmic resistance in Pt-dispersed PEMs under DMFC operation experimental analyses. J. Electrochem. Soc. 2002, 149, 682–687. [Google Scholar] [CrossRef]
- Fan, L.P.; Zhang, L.L. Effect of heteropolyacid and heteropolyacid salt on the performance of nanometer proton membrane microbial fuel cell. Int. J. Electrochem. Sci. 2017, 12, 699. [Google Scholar] [CrossRef]
- Yang, C.; Srinivasan, S.; Bocarsly, A.B.; Tulyani, S.; Benziger, J.B. A comparison of physical properties and fuel cell performance of Nafion and zirconium phosphate/Nafion composite membranes. J. Membr. Sci. 2004, 237, 145–161. [Google Scholar] [CrossRef]
- Mauritz, K.A.; Payne, J.T. [Perfluorosulfonate ionomer]/silicate hybrid membranes via base-catalyzed in situ sol-gel processes for tetraethylorthosilicate. J. Membr. Sci. 2000, 168, 39–51. [Google Scholar] [CrossRef]
- Shen, X.; Liu, P.; Xia, S.; Liu, J.; Wang, R.; Zhao, H.; Liu, Q.; Xu, J.; Wang, F. Anti-fouling and anti-bacterial modification of poly(vinylidene fluoride) membrane by blending with the capsaicin-based copolymer. Polymers 2019, 11, 323. [Google Scholar] [CrossRef] [PubMed]
- Kang, G.D.; Cao, Y.M. Application and modification of poly(vinylidene fluoride) (PVDF) membranes—A review. J. Membr. Sci. 2014, 463, 145–165. [Google Scholar] [CrossRef]
- Fan, L.P.; Xu, D.D.; Li, C.; Xue, S. Molasses wastewater treatment by microbial fuel cell with MnO2-Modified cathode. Pol. J. Environ. Stud. 2016, 25, 2349–3256. [Google Scholar] [CrossRef]
- Ma, Z.; Lu, X.; Wu, C.; Gao, Q.; Zhao, L.; Zhang, H.; Liu, Z. Functional surface modification of PVDF membrane for chemical pulse cleaning. J. Membr. Sci. 2017, 524, 389–399. [Google Scholar] [CrossRef]
- Apollo, S.; Onyongo, M.S.; Ochieng, A. UV/H2O2/TiO2/Zeolite hybrid system for treatment of molasses wastewater. Iran. J. Chem. Chem. Eng. 2014, 33, 107–117. [Google Scholar]
- Sirianuntapiboon, S.; Phothilangka, P.; Ohmomo, S. Decolorization of molasses wastewater by a strain No. BP103 of acetogenic bacteria. Bioresour. Technol. 2004, 92, 31–39. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).