Natural Dyes from Mortiño (Vaccinium floribundum) as Sensitizers in Solar Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Dye Extraction and Characterization
2.3. DSSC Construction and Characterization
3. Results and Discussions
3.1. Monomeric Anthocyanin Content
3.2. UV-VIS Spectroscopy
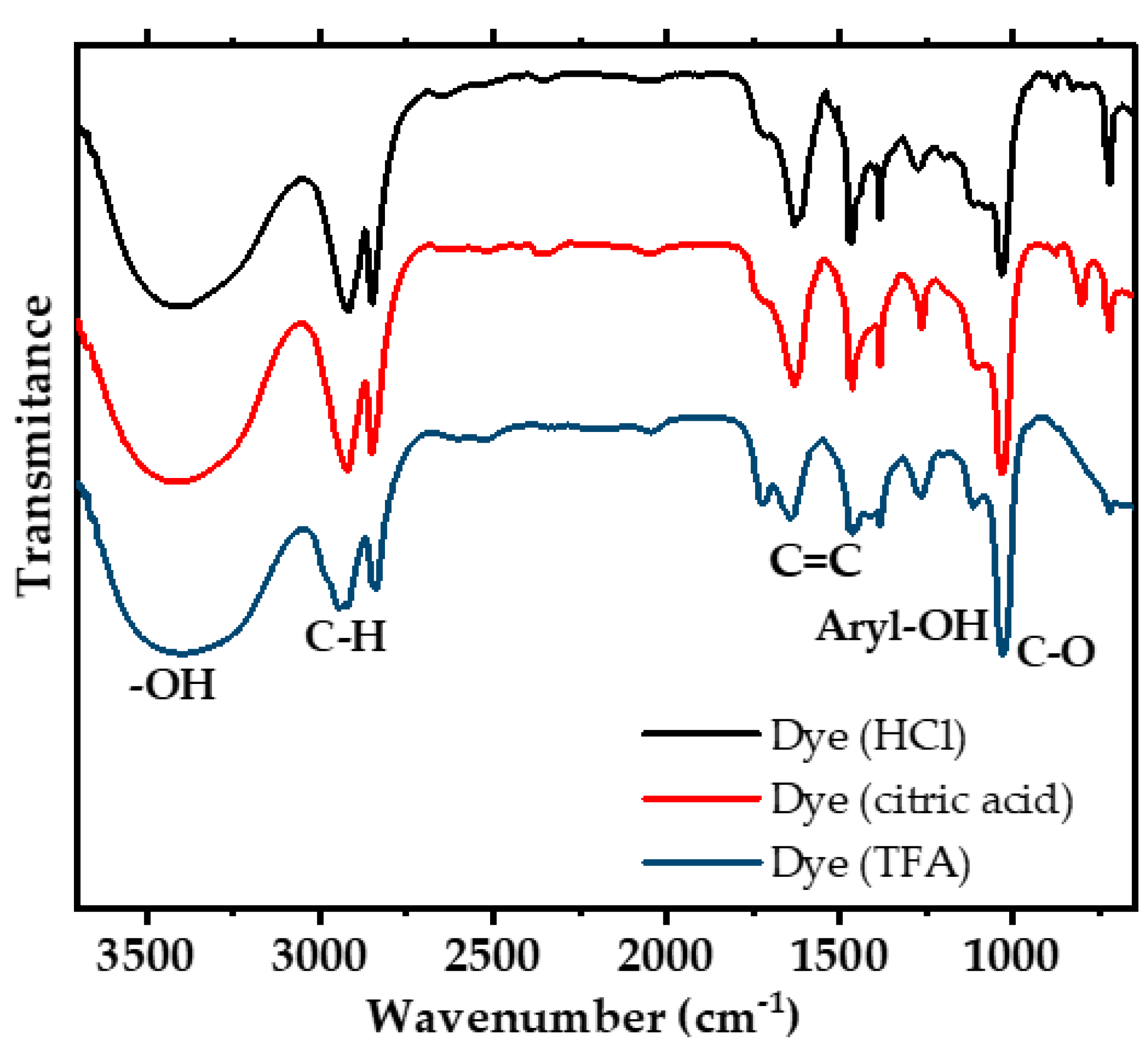
3.3. Infrared Spectroscopy
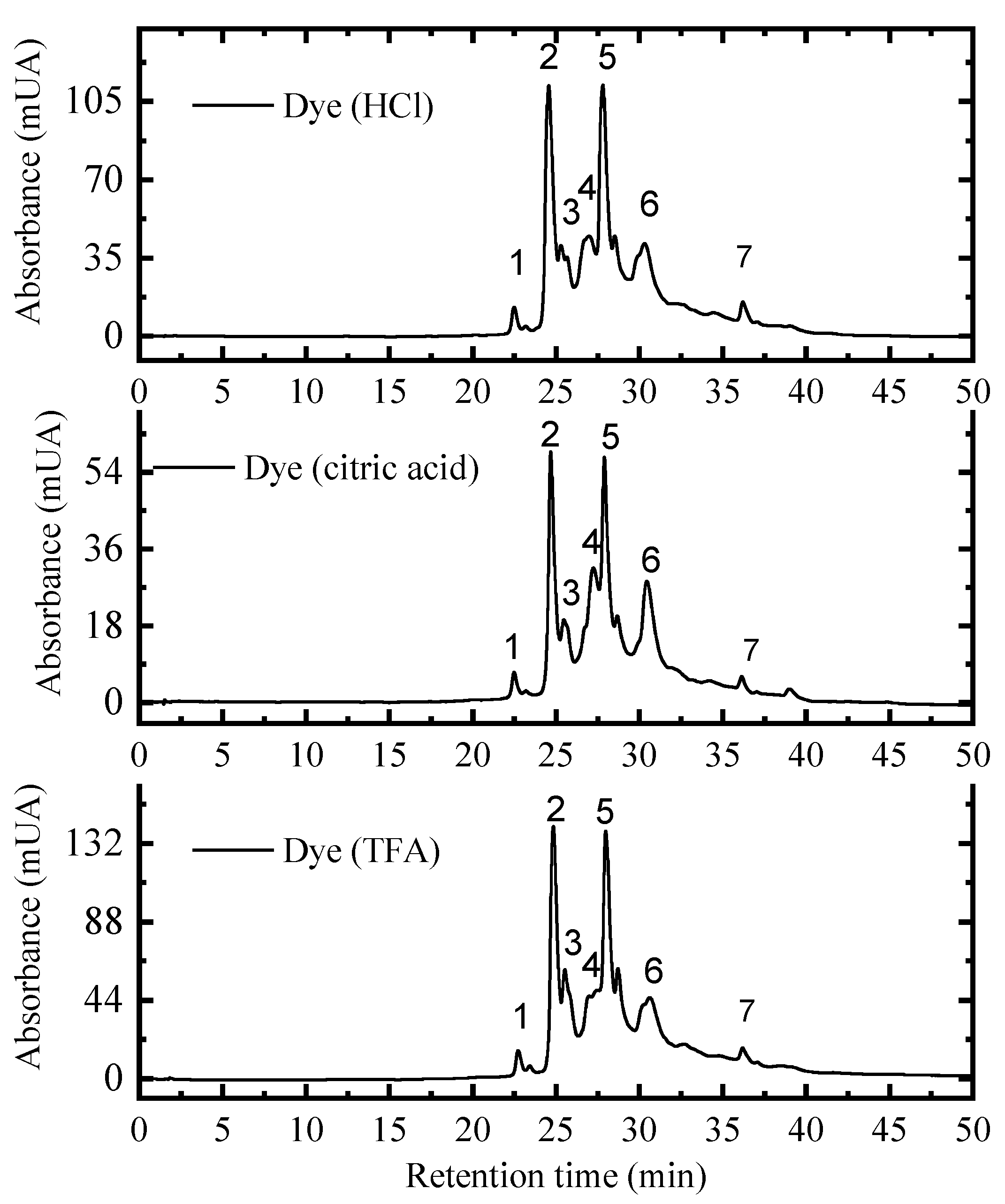
3.4. High Performance Liquid Chromatography (HPLC)
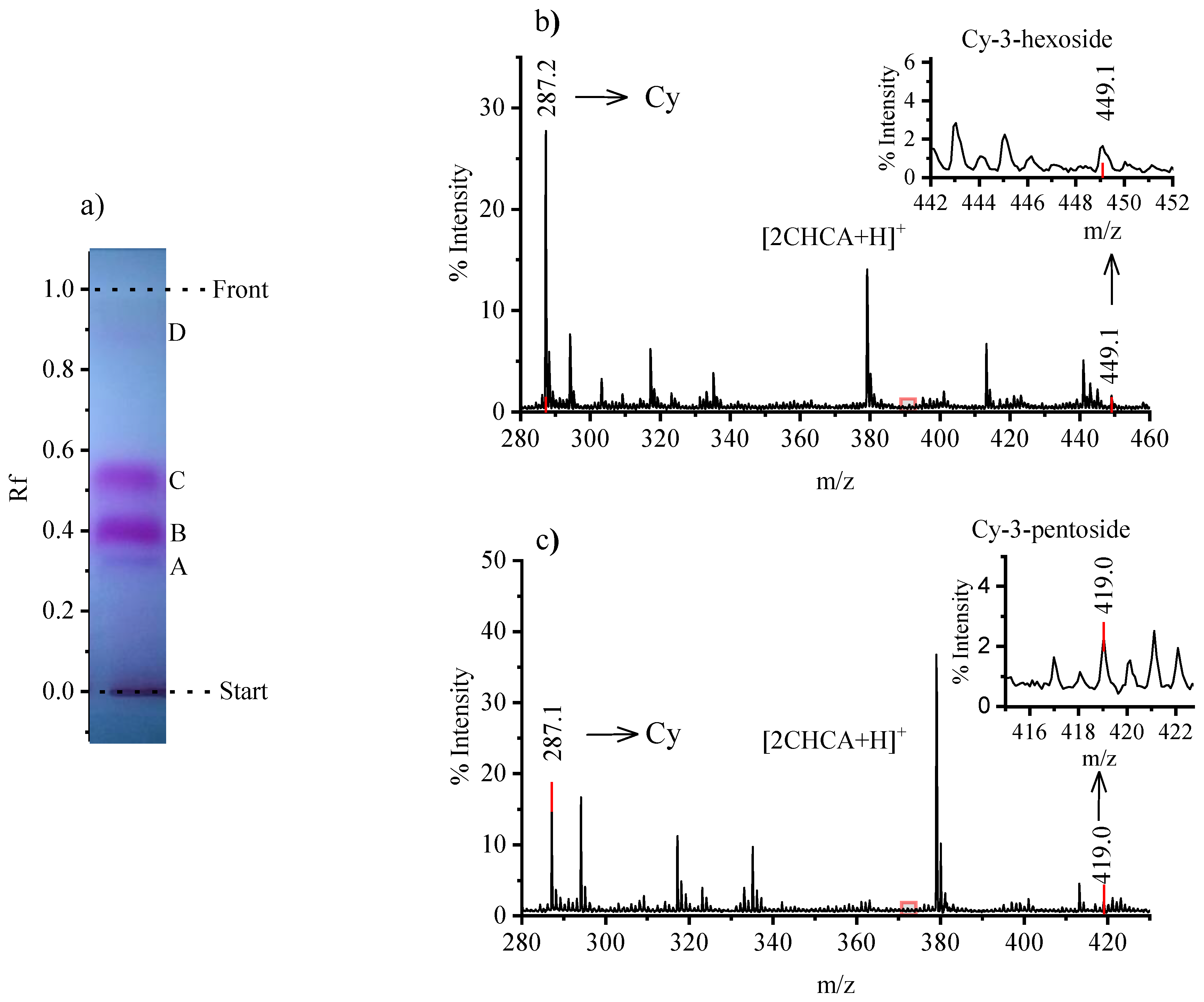
3.5. Thin Layer Chromatography (TLC) + MALDI
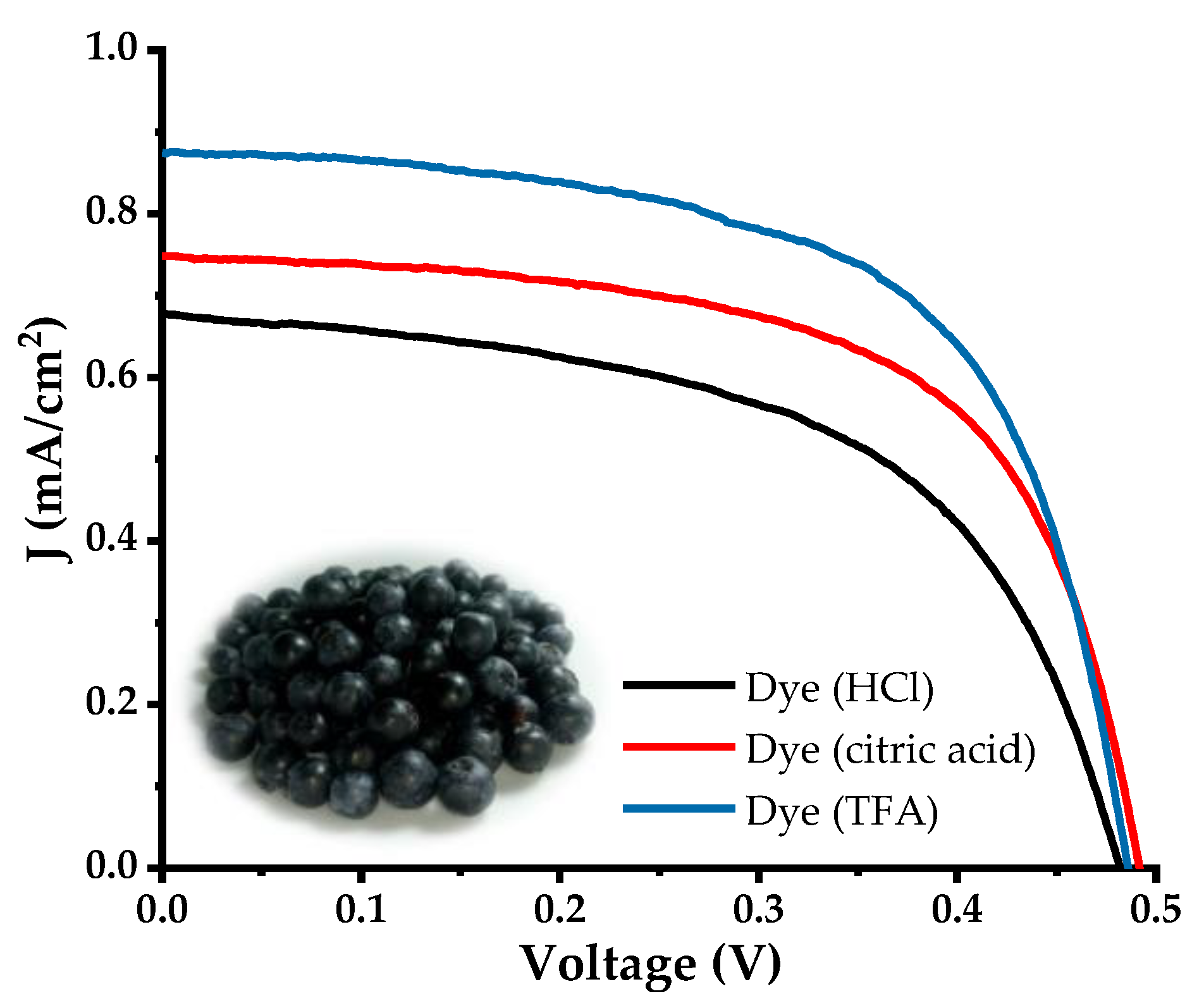
3.6. Characterization of DSSC
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- International Energy Agency. CO2 Emissions from Fuel Combustion Highlights. 2017. Available online: http://www.indiaenvironmentportal.org.in/files/file/CO2EmissionsfromFuelCombustionHighlights2017.pdf (accessed on 21 January 2020).
- Babayigit, A.; Ethirajan, A.; Muller, M.; Conings, B. Toxicity of organometal halide perovskite solar cells. Nat. Mater. 2016, 15, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Hsu, D.D.; O’Donoughue, P.; Fthenakis, V.; Heath, G.A.; Kim, H.C.; Sawyer, P.; Choi, J.K.; Turney, D.E. Life Cycle Greenhouse Gas Emissions of Crystalline Silicon Photovoltaic Electricity Generation. J. Ind. Ecol. 2012, 16, S122–S135. [Google Scholar] [CrossRef]
- Tsoutsos, T.; Frantzeskaki, N.; Gekas, V. Environmental impacts from the solar energy technologies. Energy Policy 2005, 33, 289–296. [Google Scholar] [CrossRef]
- Grätzel, M. Dye-sensitized solar cells. J. Photochem. Photobiol. C Photochem. Rev. 2003, 4, 145–153. [Google Scholar] [CrossRef]
- Gong, J.; Sumathy, K.; Qiao, Q.; Zhou, Z. Review on dye-sensitized solar cells (DSSCs): Advanced techniques and research trends. Renew. Sustain. Energy Rev. 2017, 68, 234–246. [Google Scholar] [CrossRef]
- Bagher, A.M. Introduction to Natural Dye Sensitized Solar Cells. Eng. Phys. 2017, 1, 1–7. [Google Scholar]
- Clifford, J.N.; Martínez-Ferrero, E.; Viterisi, A.; Palomares, E. Sensitizer molecular structure-device efficiency relationship in dye sensitized solar cells. Chem. Soc. Rev. 2011, 40, 1635–1646. [Google Scholar] [CrossRef]
- Wang, P.; Klein, C.; Humphry-Baker, R.; Zakeeruddin, S.M.; Grätzel, M. A High Molar Extinction Coefficient Sensitizer for Stable Dye-Sensitized Solar Cells. J. Am. Chem. Soc. 2005, 127, 808–809. [Google Scholar] [CrossRef]
- Wongcharee, K.; Meeyoo, V.; Chavadej, S. Dye-sensitized solar cell using natural dyes extracted from rosella and blue pea flowers. Sol. Energy Mater. Sol. Cells 2005, 91, 566–571. [Google Scholar] [CrossRef]
- Ludin, N.A.; Mahmoud, A.M.A.A.; Mohamad, A.B.; Kadhum, A.A.H.; Sopian, K.; Karim, N.S.A. Review on the development of natural dye photosensitizer for dye-sensitized solar cells. Renew. Sustain. Energy Rev. 2014, 31, 386–396. [Google Scholar] [CrossRef]
- Calogero, G.; Yum, J.H.; Sinopoli, A.; Di Marco, G.; Grätzel, M.; Nazeeruddin, M.K. Anthocyanins and betalains as light-harvesting pigments for dye-sensitized solar cells. Sol. Energy 2012, 86, 1563–1575. [Google Scholar] [CrossRef]
- Calogero, G.; Di Marco, G. Red Sicilian orange and purple eggplant fruits as natural sensitizers for dye-sensitized solar cells. Sol. Energy Mater. Sol. Cells 2008, 92, 1341–1346. [Google Scholar] [CrossRef]
- San Esteban, A.C.M.; Enriquez, E.P. Graphene–anthocyanin mixture as photosensitizer for dye-sensitized solar cell. Sol. Energy 2013, 98, 392–399. [Google Scholar] [CrossRef]
- Nan, H.; Shen, H.P.; Wang, G.; Xie, S.D.; Yang, G.J.; Lin, H. Studies on the optical and photoelectric properties of anthocyanin and chlorophyll as natural co-sensitizers in dye sensitized solar cell. Opt. Mater. 2017, 73, 172–178. [Google Scholar] [CrossRef]
- Teoli, F.; Lucioli, S.; Nota, P.; Frattarelli, A.; Matteocci, F.; Di Carlo, A.; Caboni, E.; Forni, C. Role of pH and pigment concentration for natural dye-sensitized solar cells treated with anthocyanin extracts of common fruits. J. Photochem. Photobiol. A Chem. 2017, 316, 24–30. [Google Scholar] [CrossRef]
- Homeier, J.; Werner, F.A.; Gradstein, S.R.; Breckle, S.W.; Richter, M. Flora and Fungi: Composition and Function BT—Gradients in a Tropical Mountain Ecosystem of Ecuador; Beck, E., Bendix, J., Kottke, I., Makeschin, F., Mosandl, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 87–100. [Google Scholar]
- Council, N.R. Lost Crops of the Incas: Little-Known Plants of the Andes with Promise for Worldwide Cultivation; National Academies Press: Washington, DC, USA, 1989. [Google Scholar]
- Solarte, C.U. Patrimonio Cultural Alimentario; Fondo Editorial Ministerio de Cultura: Quito, Ecuador, 2010. [Google Scholar]
- Vasco, C.; Riihinen, K.; Ruales, J.; Kamal-Eldin, A. Chemical Composition and Phenolic Compound Profile of Mortiño (Vaccinium floribundum Kunth). J. Agric. Food Chem. 2009, 57, 8274–8281. [Google Scholar] [CrossRef]
- Schreckinger, M.E.; Wang, J.; Yousef, G.; Lila, M.A.; De Mejia, E.G. Antioxidant Capacity and in Vitro Inhibition of Adipogenesis and Inflammation by Phenolic Extracts of Vaccinium floribundum and Aristotelia chilensis. J. Agric. Food Chem. 2010, 58, 8966–8976. [Google Scholar] [CrossRef]
- Vizuete, K.S.; Kumar, B.; Vaca, A.V.; Debut, A.; Cumbal, L. Mortiño (Vaccinium floribundum Kunth) berry assisted green synthesis and photocatalytic performance of Silver–Graphene nanocomposite. J. Photochem. Photobiol. A Chem. 2016, 329, 273–279. [Google Scholar] [CrossRef]
- Cherepy, N.J.; Smestad, G.P.; Grätzel, M.; Zhang, J.Z. Ultrafast Electron Injection: Implications for a Photoelectrochemical Cell Utilizing an Anthocyanin Dye-Sensitized TiO2 Nanocrystalline Electrode. J. Phys. Chem. B 1997, 101, 9342–9351. [Google Scholar] [CrossRef]
- Chien, C.Y.; Hsu, B.D. Optimization of the dye-sensitized solar cell with anthocyanin as photosensitizer. Sol. Energy 2013, 98, 203–211. [Google Scholar] [CrossRef]
- Ramamoorthy, R.; Radha, N.; Maheswari, G.; Anandan, S.; Manoharan, S.; Williams, R.V. Betalain and anthocyanin dye-sensitized solar cells. J. Appl. Electrochem. 2016, 46, 929–941. [Google Scholar] [CrossRef]
- Zolkepli, Z.; Lim, A.; Ekanayake, P.; Tennakoon, K. Efficiency Enhancement of Cocktail Dye of Ixora coccinea and Tradescantia spathacea in DSSC. J. Biophys. 2015, 2015, 582091. [Google Scholar] [CrossRef] [PubMed]
- Strauch, R.C.; Mengist, M.F.; Pan, K.; Yousef, G.G.; Iorizzo, M.; Brown, A.F.; Lila, M.A. Variation in anthocyanin profiles of 27 genotypes of red cabbage over two growing seasons. Food Chem. 2019, 301, 125289. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Durst, R.; Wrolstad, R. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: Collaborative Study. J. AOAC Int. 2005, 88, 1269–1278. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Saona, L.E.; Wrolstad, R.E. Extraction, Isolation, and Purification of Anthocyanins. Curr. Protoc. Food Anal. Chem. 2001. [Google Scholar] [CrossRef]
- Durst, R.W.; Wrolstad, R.E. Separation and Characterization of Anthocyanins by HPLC. Curr. Protoc. Food Anal. Chem. 2001. [Google Scholar] [CrossRef]
- Santacruz, C.P.; Håkansson, P.; Barofsky, D.F.; Piyadasa, C.K.G. A Constant-Momentum/Energy-Selector Time-of-Flight Mass Spectrometer. J. Am. Soc. Mass Spectrom. 2007, 18, 92–101. [Google Scholar] [CrossRef][Green Version]
- Siegelman, H.W.; Hendricks, S.B. Photocontrol of Alcohol, Aldehyde, and Anthocyanin Production in Apple Skin. Plant Physiol. 1958, 33, 409–413. [Google Scholar] [CrossRef]
- Giusti, M.M.; Wrolstad, R.E. Characterization and Measurement of Anthocyanins by UV-Visible Spectroscopy. Curr. Protoc. Food Anal. Chem. 2001. [Google Scholar] [CrossRef]
- Harborne, J.B. Spectral methods of characterizing anthocyanins. Biochem. J. 1958, 70, 22–28. [Google Scholar] [CrossRef]
- Fernando, J.M.R.C.; Senadeera, G.K.R. Natural anthocyanins as photosensitizers for dye-sensitized solar devices. Curr. Sci. 2008, 95, 663–666. [Google Scholar]
- Hao, S.; Wu, J.; Huang, Y.; Lin, J. Natural dyes as photosensitizers for dye-sensitized solar cell. Sol. Energy 2006, 80, 209–214. [Google Scholar] [CrossRef]
- Jackman, R.L.; Yada, R.Y.; Tung, M.A. A review: Separation and chemival properties of anthocyanins used for their qualitative and quantitative analysys. J. Food Biochem. 1987, 11, 279–308. [Google Scholar] [CrossRef]
- Murayama, M.; Mori, T. Equivalent Circuit Analysis of Dye-Sensitized Solar Cell by Using One-Diode Model: Effect of Carboxylic Acid Treatment of TiO2 Electrode. Jpn. J. Appl. Phys. 2006, 45, 542–545. [Google Scholar] [CrossRef]
- Obasuyi, A.R.; Glossman-Mitnik, D.; Flores-Holguín, N. Electron injection in anthocyanidin and betalain dyes for dye-sensitized solar cells: A DFT approach. J. Comput. Electron. 2019, 18, 396–406. [Google Scholar] [CrossRef]
- Lakshmanakumar, M.; Sriram, S.; Balamurugan, D. Performance analysis of TiO2-flavylium compound-based dye-sensitized solar cell (DSSC): A DFT–TDDFT approach. J. Comput. Electron. 2018, 17, 1143–1152. [Google Scholar] [CrossRef]

| Extraction Acid | Efficiency (% η) | JSC (mA/cm2) | VOC (V) | FF |
|---|---|---|---|---|
| AC | 0.23 ± 0.05 a,b | 0.75 ± 0.15 a | 0.490 ± 0.007 a | 0.62 ± 0.03 a |
| HCl | 0.18 ± 0.03 a | 0.68 ± 0.20 a | 0.475 ± 0.012 b | 0.57 ± 0.06 a |
| TFA | 0.26 ± 0.05 b | 0.88 ± 0.17 a | 0.485 ± 0.007 a,b | 0.62 ± 0.04 a |
| ANOVA P | 0.027 | 0.181 | 0.035 | 0.156 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taco-Ugsha, M.A.; Santacruz, C.P.; Espinoza-Montero, P.J. Natural Dyes from Mortiño (Vaccinium floribundum) as Sensitizers in Solar Cells. Energies 2020, 13, 785. https://doi.org/10.3390/en13040785
Taco-Ugsha MA, Santacruz CP, Espinoza-Montero PJ. Natural Dyes from Mortiño (Vaccinium floribundum) as Sensitizers in Solar Cells. Energies. 2020; 13(4):785. https://doi.org/10.3390/en13040785
Chicago/Turabian StyleTaco-Ugsha, Miguel A., Cristian P. Santacruz, and Patricio J. Espinoza-Montero. 2020. "Natural Dyes from Mortiño (Vaccinium floribundum) as Sensitizers in Solar Cells" Energies 13, no. 4: 785. https://doi.org/10.3390/en13040785
APA StyleTaco-Ugsha, M. A., Santacruz, C. P., & Espinoza-Montero, P. J. (2020). Natural Dyes from Mortiño (Vaccinium floribundum) as Sensitizers in Solar Cells. Energies, 13(4), 785. https://doi.org/10.3390/en13040785







