Fatty Acid Methyl Esters of the Aerophytic Cave Alga Coccomyxa subglobosa as a Source for Biodiesel Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Algal Isolation
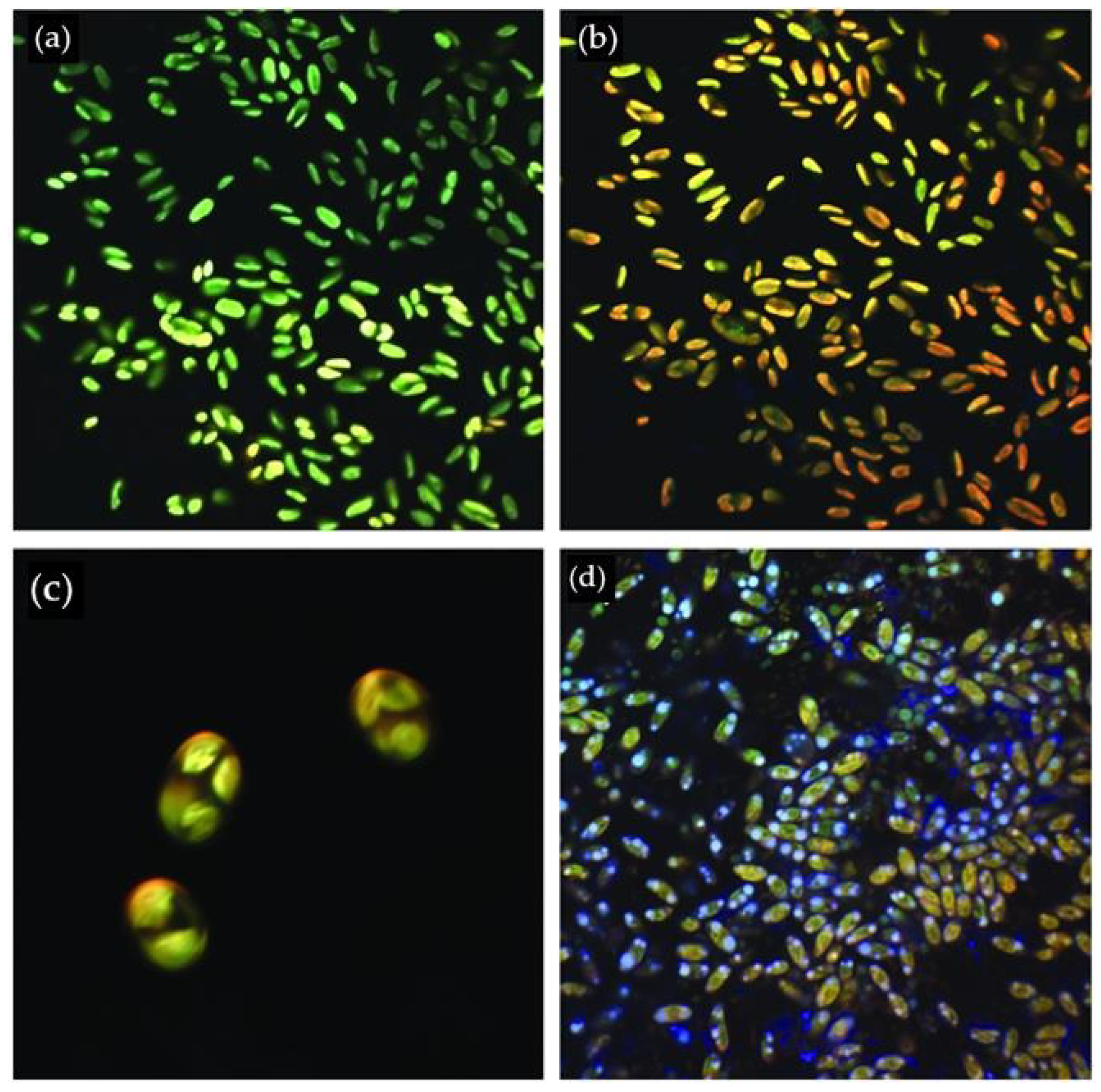
2.2. Microscopy
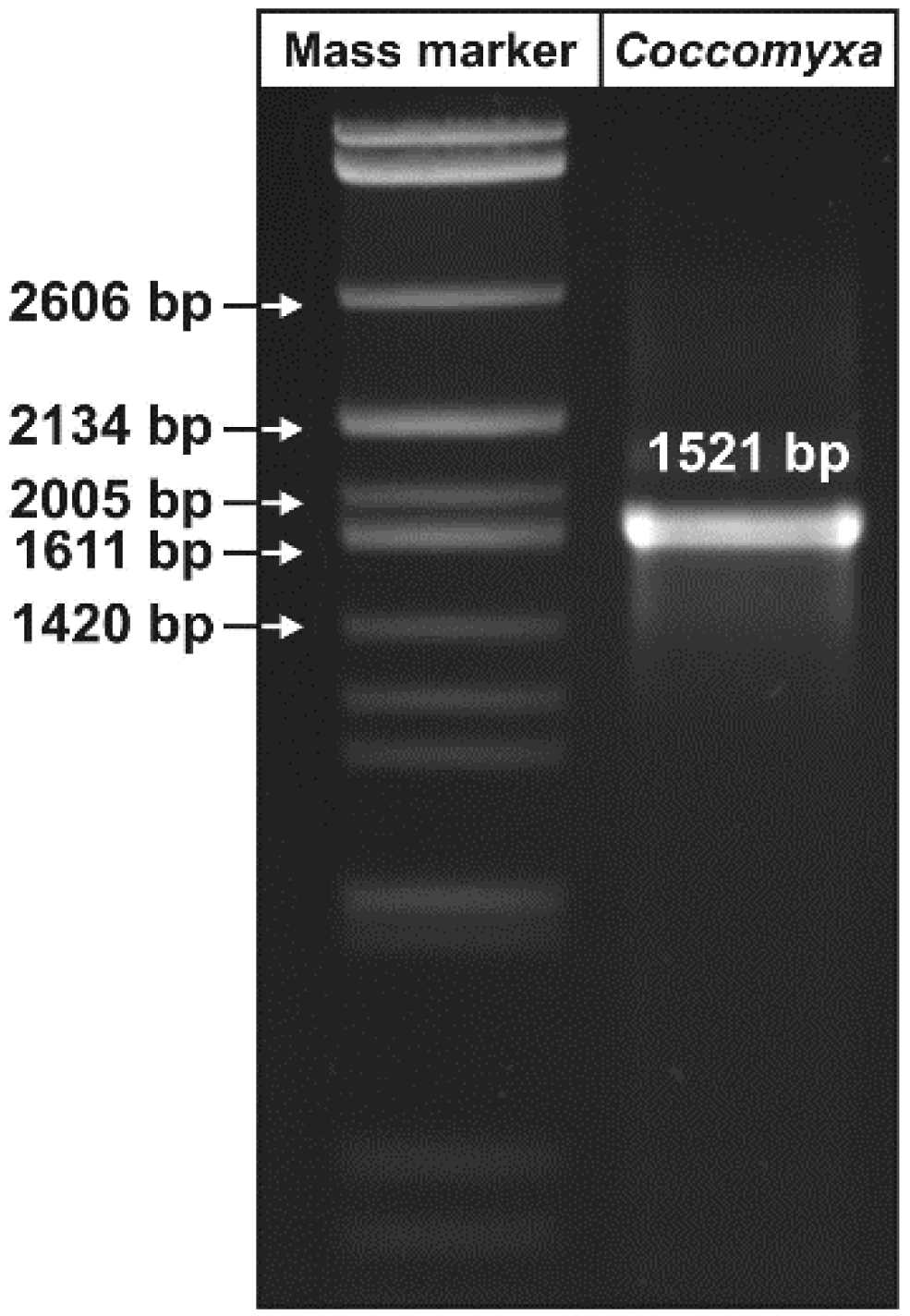
2.3. PCR
2.4. Lipid Extraction and Methyl Transesterification
2.5. GC–MS Analysis of FAMEs
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sobczuk, T.M.; Chisti, Y. Potential fuel oils from the microalga Choricystis minor. J. Chem. Technol. Biotechnol. 2010, 85, 100–108. [Google Scholar] [CrossRef]
- Fakhry, E.M.; Maghraby, D.M. El Fatty Acids Composition and Biodiesel Characterization of Dunaliella salina. J. Water Resour. Prot. 2013, 05, 894–899. [Google Scholar] [CrossRef]
- Van Gerpen, J. Biodiesel processing and production. Fuel Process. Technol. 2005, 86, 1097–1107. [Google Scholar] [CrossRef]
- Rizwanul Fattah, I.M.; Ong, H.C.; Mahlia, T.M.I.; Mofijur, M.; Silitonga, A.S.; Ashrafur Rahman, S.M.; Ahmad, A. State of the Art of Catalysts for Biodiesel Production. Front. Energy Res. 2020, 8, 1–17. [Google Scholar] [CrossRef]
- Gutnikov, G. Fatty acid profiles of lipid samples. J. Chromatogr. B Biomed. Sci. Appl. 1995, 671, 71–89. [Google Scholar] [CrossRef]
- Orsavova, J.; Misurcova, L.; Vavra Ambrozova, J.; Vicha, R.; Mlcek, J. Fatty acids composition of vegetable oils and its contribution to dietary energy intake and dependence of cardiovascular mortality on dietary intake of fatty acids. Int. J. Mol. Sci. 2015, 16, 12871–12890. [Google Scholar] [CrossRef] [PubMed]
- Lang, I.; Hodac, L.; Friedl, T.; Feussner, I. Fatty acid profiles and their distribution patterns in microalgae: A comprehensive analysis of more than 2000 strains from the SAG culture collection. BMC Plant Biol. 2011, 11, 124. [Google Scholar] [CrossRef]
- Knothe, G. Dependence of biodiesel fuel properties on the structure of fatty acid alkyl esters. Fuel Process. Technol. 2005, 86, 1059–1070. [Google Scholar] [CrossRef]
- de Carvalho, C.G.P.; Caldeira, A.; de Carvalho, L.M.; de Carvalho, H.W.L.; Ribeiro, J.L.; Mandarino, J.M.G.; de Resende, J.C.F.; dos Santos, A.R.; da Silva, M.R.; Arriel, N.H.C. Fatty Acid Profile of Sunflower Achene Oil From the Brazilian Semi-arid Region. J. Agric. Sci. 2018, 10, 144. [Google Scholar] [CrossRef]
- Chisti, Y. Biodiesel from microalgae. Biotechnol. Adv. 2007, 25, 294–306. [Google Scholar] [CrossRef]
- Resurreccion, E.P.; Colosi, L.M.; White, M.A.; Clarens, A.F. Comparison of algae cultivation methods for bioenergy production using a combined life cycle assessment and life cycle costing approach. Bioresour. Technol. 2012, 126, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, R.; Guieysse, B. Algal-bacterial processes for the treatment of hazardous contaminants: A review. Water Res. 2006, 40, 2799–2815. [Google Scholar] [CrossRef] [PubMed]
- Neustupa, J.; Štifterová, A. Distribution patterns of Subaerial corticolous microalgae in two European regions. Plant Ecol. Evol. 2013, 146, 279–289. [Google Scholar] [CrossRef]
- Falasco, E.; Ector, L.; Isaia, M.; Wetzel, C.E.; Hoffmann, L.; Bona, F. Diatom flora in subterranean ecosystems: A review. Int. J. Speleol. 2014, 43, 231–251. [Google Scholar] [CrossRef]
- Gorbushina, A.A.; Broughton, W.J. Microbiology of the atmosphere-rock interface: How biological interactions and physical stresses modulate a sophisticated microbial ecosystem. Annu. Rev. Microbiol. 2009, 63, 431–450. [Google Scholar] [CrossRef]
- Czerwik-Marcinkowska, J.; Pusz, W.; Zagożdżon, P. Cyanobacteria and algae in an old mine adit (Marcinków, sudety mountains, southwestern Poland). J. Cave Karst Stud. 2017, 79, 122–130. [Google Scholar] [CrossRef]
- Park, J.H.; Yoon, J.J.; Park, H.D.; Kim, Y.J.; Lim, D.J.; Kim, S.H. Feasibility of biohydrogen production from Gelidium amansii. Int. J. Hydrogen Energy 2011, 36, 13997–14003. [Google Scholar] [CrossRef]
- Pittman, J.K.; Dean, A.P.; Osundeko, O. The potential of sustainable algal biofuel production using wastewater resources. Bioresour. Technol. 2011, 102, 17–25. [Google Scholar] [CrossRef]
- Scott, S.A.; Davey, M.P.; Dennis, J.S.; Horst, I.; Howe, C.J.; Lea-Smith, D.J.; Smith, A.G. Biodiesel from algae: Challenges and prospects. Curr. Opin. Biotechnol. 2010, 21, 277–286. [Google Scholar] [CrossRef]
- Radakovits, R.; Jinkerson, R.E.; Darzins, A.; Posewitz, M.C. Genetic engineering of algae for enhanced biofuel production. Eukaryot. Cell 2010, 9, 486–501. [Google Scholar] [CrossRef]
- Sivakumar, G.; Xu, J.; Thompson, R.W.; Yang, Y.; Randol-Smith, P.; Weathers, P.J. Integrated green algal technology for bioremediation and biofuel. Bioresour. Technol. 2012, 107, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Vlysidis, A.; Binns, M.; Webb, C.; Theodoropoulos, C. A techno-economic analysis of biodiesel biorefineries: Assessment of integrated designs for the co-production of fuels and chemicals. Energy 2011, 36, 4671–4683. [Google Scholar] [CrossRef]
- Syasina, I.G.; Kukhlevsky, A.D.; Kovaleva, A.L.; Vaschenko, M.A. Phylogenetic and morphological characterization of the green alga infesting the horse mussel Modiolus modiolus from Vityaz Bay (Peter the Great Bay, Sea of Japan). J. Invertebr. Pathol. 2012, 111, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Bermúdez Menéndez, J.M.; Arenillas, A.; Menéndez Díaz, J.Á.; Boffa, L.; Mantegna, S.; Binello, A.; Cravotto, G. Optimization of microalgae oil extraction under ultrasound and microwave irradiation. J. Chem. Technol. Biotechnol. 2014, 89, 1779–1784. [Google Scholar] [CrossRef]
- Ríos, S.D.; Castañeda, J.; Torras, C.; Farriol, X.; Salvadó, J. Lipid extraction methods from microalgal biomass harvested by two different paths: Screening studies toward biodiesel production. Bioresour. Technol. 2013, 133, 378–388. [Google Scholar] [CrossRef]
- Harwood, J.L.; Guschina, I.A. The versatility of algae and their lipid metabolism. Biochimie 2009, 91, 679–684. [Google Scholar] [CrossRef]
- Guzmán, H.M.; de la Valido, A.J.; Duarte, L.C.; Presmanes, K.F. Analysis of interspecific variation in relative fatty acid composition: Use of flow cytometry to estimate unsaturation index and relative polyunsaturated fatty acid content in microalgae. J. Appl. Phycol. 2011, 23, 7–15. [Google Scholar] [CrossRef]
- Silva, G.; Pereira, R.B.; Valentão, P.; Andrade, P.B.; Sousa, C. Distinct fatty acid profile of ten brown macroalgae. Braz. J. Pharmacogn. 2013, 23, 608–613. [Google Scholar] [CrossRef]
- Lapuerta, M.; Rodríguez-Fernández, J.; Armas, O. Correlation for the estimation of the density of fatty acid esters fuels and its implications. A proposed Biodiesel Cetane Index. Chem. Phys. Lipids 2010, 163, 720–727. [Google Scholar] [CrossRef]
- Allen, C.A.W.; Watts, K.C.; Ackman, R.G.; Pegg, M.J. Predicting the viscosity of biodiesel fuels from their fatty acid ester composition. Fuel 1999, 78, 1319–1326. [Google Scholar] [CrossRef]
- Ghosh, A.; Khanra, S.; Mondal, M.; Halder, G.; Tiwari, O.N.; Saini, S.; Bhowmick, T.K.; Gayen, K. Progress toward isolation of strains and genetically engineered strains of microalgae for production of biofuel and other value added chemicals: A review. Energy Convers. Manag. 2016, 113, 104–118. [Google Scholar] [CrossRef]
- Khotimchenko, S.V.; Vaskovsky, V.E.; Titlyanova, T.V. Fatty acids of marine algae from the pacific coast of North California. Bot. Mar. 2002, 45, 17–22. [Google Scholar] [CrossRef]
- Avula, S.G.C.; Belovich, J.M.; Xu, Y. Determination of fatty acid methyl esters derived from algae Scenedesmus dimorphus biomass by GC–MS with one-step esterification of free fatty acids and transesterification of glycerolipids. J. Sep. Sci. 2017, 40, 2214–2227. [Google Scholar] [CrossRef] [PubMed]
- Sibi, G. Inhibition of lipase and inflammatory mediators by Chlorella lipid extracts for antiacne treatment. J. Adv. Pharm. Technol. Res. 2015, 6, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Maltsev, Y.; Maltseva, I.; Maltseva, S.; Kociolek, J.P.; Kulikovskiy, M. Fatty Acid Content and Profile of the Novel Strain of Coccomyxa elongata (Trebouxiophyceae, Chlorophyta) Cultivated at Reduced Nitrogen and Phosphorus Concentrations. J. Phycol. 2019, 55, 1154–1165. [Google Scholar] [CrossRef]
- Lohtander, K.; Oksanen, I.; Rikkinen, J. Genetic diversity of green algal and cyanobacterial photobionts in Nephroma (Peltigerales). Lichenologist 2003, 35, 325–339. [Google Scholar] [CrossRef]
- Zoller, S.; Lutzoni, F. Slow algae, fast fungi: Exceptionally high nucleotide substitution rate differences between lichenized fungi Omphalina and their symbiotic green algae Coccomyxa. Mol. Phylogenet. Evol. 2003, 29, 629–640. [Google Scholar] [CrossRef]
- Darienko, T.; Gustavs, L.; Eggert, A.; Wolf, W.; Pröschold, T. Evaluating the species boundaries of green microalgae (Coccomyxa, Trebouxiophyceae, Chlorophyta) using integrative taxonomy and DNA barcoding with further implications for the species identification in environmental samples. PLoS ONE 2015, 10, e0127838. [Google Scholar] [CrossRef]
- Darienko, T.; Gustavs, L.; Mudimu, O.; Menendez, C.R.; Schumann, R.; Karsten, U.; Friedl, T.; PröSchold, T. Chloroidium, a common terrestrial coccoid green alga previously assigned to Chlorella (Trebouxiophyceae, Chlorophyta). Eur. J. Phycol. 2010, 45, 79–95. [Google Scholar] [CrossRef]
- Hwang, E.K.; Liu, F.; Lee, K.H.; Ha, D.S.; Park, C.S. Comparison of the cultivation performance between korean (Sugwawon no. 301) and Chinese strains (Huangguan no. 1) of kelp Saccharina japonica in an aquaculture farm in Korea. Algae 2018, 33, 101–108. [Google Scholar] [CrossRef]
- Juneja, A.; Ceballos, R.M.; Murthy, G.S. Effects of environmental factors and nutrient availability on the biochemical composition of algae for biofuels production: A review. Energies 2013, 6, 4607–4638. [Google Scholar] [CrossRef]
- Jensen, M.D. Gender differences in regional fatty acid metabolism before and after meal ingestion. J. Clin. Investig. 1995, 96, 2297–2303. [Google Scholar] [CrossRef] [PubMed]
- Chao, J.; Wolfaardt, G.M.; Arts, M.T. Characterization of pseudomonas aeruginosa fatty acid profiles in biofilms and batch planktonic cultures. Can. J. Microbiol. 2010, 56, 1028–1039. [Google Scholar] [CrossRef] [PubMed]
- Darki, B.Z.; Seyfabadi, J.; Fayazi, S. Effect of nutrients on total lipid content and fatty acids profile of scenedesmus obliquus. Braz. Arch. Biol. Technol. 2017, 60, 1–12. [Google Scholar] [CrossRef]
- Rizwanul Fattah, I.M.; Noraini, M.Y.; Mofijur, M.; Silitonga, A.S.; Badruddin, I.A.; Yunus Khan, T.M.; Ong, H.C.; Mahlia, T.M.I. Lipid extraction maximization and enzymatic synthesis of biodiesel from microalgae. Appl. Sci. 2020, 10, 6103. [Google Scholar] [CrossRef]
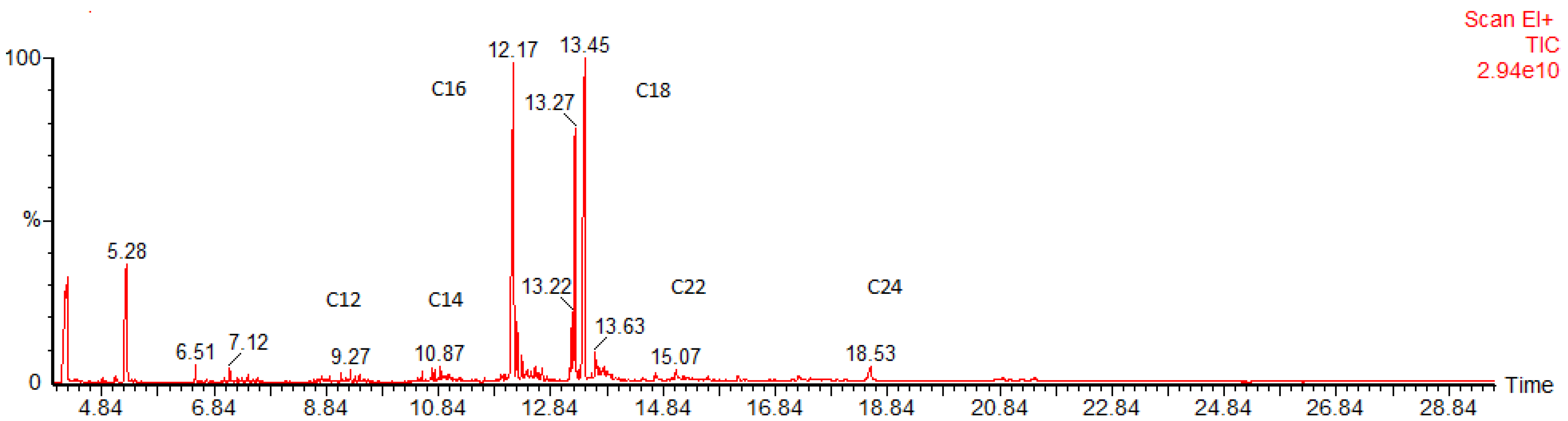

| Retention Time (min) | FAME | Name | Content (%) | Density * (kg/m3) | Viscosity ** (mPA s) |
|---|---|---|---|---|---|
| 5.12 | C6 | Caproate | 7.0 | 890.97 | - |
| 5.28 | C8 | Caprylate | 9.5 | 883.04 | 0.99 |
| 7.12 | C10 | Caprate | 2.5 | 876.31 | 1.40 |
| 9.27 | C12 | Laurate | 1.5 | 873.28 | 1.95 |
| 10.87 | C14 | Myristate | 1.5 | 868.18 | 2.69 |
| 12.17 | C16 | Palmitate | 28.5 | 864.19 | 3.60 |
| 13.45 | C18 | Stearate | 45.0 | 867.55 | 4.74 |
| 13.63 | C20 | Arachidate | 2.0 | 866.28 | - |
| 15.67 | C22 | Docosanoic | 1.0 | - | - |
| 18.53 | C24 | Tetracosanoic | 1.5 | - | - |
| Retention Time (min) | FAME | Name | Content (%) | Density * (kg/m3) | Viscosity ** (mPA s) |
|---|---|---|---|---|---|
| 12.17 | C16:0 | Palmitate | 27.3 | 864.19 | 3.60 |
| 12.24 | C16:1 | Palmitoleate | 3.1 | 882.39 | - |
| 12.33 | C16.2 | Hexadecadienoate | 0.6 | 903.85 | - |
| 13.22 | C18:2 | Linoleate | 2.8 | 893.18 | 3.05 |
| 13.27 | C18:1 | Oleate | 18.9 | 877.46 | 3.73 |
| 13.45 | C18.0 | Stearate | 47.3 | 867.55 | 4.74 |
| Fraction of Algal Lipid Extract (Free FAs) | FA Chain | FA Content in Algal Dry Mass (%) | References |
|---|---|---|---|
| Scenedesmus dimorphus | C16:0 | 0.685 | [33] |
| C16:1 | 0.327 | ||
| C18:0 | 0.174 | ||
| C18:1 | 0.804 | ||
| C18:2 | 1.19 | ||
| C18:3 | 1.26 | ||
| Chlorella vulgaris | C8:0 | 0.25 | [34] |
| C14:0 | 0.95 | ||
| C15:0 | 0.29 | ||
| C16:0 | 15.58 | ||
| C17:1 | 0.86 | ||
| C18:2 | 0.15 | ||
| C18:3 | 0.23 | ||
| C20:4 | 0.17 | ||
| C20:5 | 0.17 | ||
| Coccomyxa subglobosa | C12:0 | 0.721 | [35] |
| C14:0 | 0.734 | ||
| C16:1 | 1.05 | ||
| C18:1 | 1.32 | ||
| C20:0 | 0.54 | ||
| C22:0 | 0.89 | ||
| C24:0 | 0.654 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czerwik-Marcinkowska, J.; Gałczyńska, K.; Oszczudłowski, J.; Massalski, A.; Semaniak, J.; Arabski, M. Fatty Acid Methyl Esters of the Aerophytic Cave Alga Coccomyxa subglobosa as a Source for Biodiesel Production. Energies 2020, 13, 6494. https://doi.org/10.3390/en13246494
Czerwik-Marcinkowska J, Gałczyńska K, Oszczudłowski J, Massalski A, Semaniak J, Arabski M. Fatty Acid Methyl Esters of the Aerophytic Cave Alga Coccomyxa subglobosa as a Source for Biodiesel Production. Energies. 2020; 13(24):6494. https://doi.org/10.3390/en13246494
Chicago/Turabian StyleCzerwik-Marcinkowska, Joanna, Katarzyna Gałczyńska, Jerzy Oszczudłowski, Andrzej Massalski, Jacek Semaniak, and Michał Arabski. 2020. "Fatty Acid Methyl Esters of the Aerophytic Cave Alga Coccomyxa subglobosa as a Source for Biodiesel Production" Energies 13, no. 24: 6494. https://doi.org/10.3390/en13246494
APA StyleCzerwik-Marcinkowska, J., Gałczyńska, K., Oszczudłowski, J., Massalski, A., Semaniak, J., & Arabski, M. (2020). Fatty Acid Methyl Esters of the Aerophytic Cave Alga Coccomyxa subglobosa as a Source for Biodiesel Production. Energies, 13(24), 6494. https://doi.org/10.3390/en13246494







