1. Introduction
The unstable crude oil prices and global warming caused by greenhouse gases drive us to use alternative energy sources. Companies and governments have made substantial investments in new and renewable energy over the past few years [
1]. Among the new and renewable energy, hydrogen had begun to be translated into the alternative energy source area due to easy deployment into electricity infrastructure, diversified energy sources to produce hydrogen from fossil fuels to biomass, improvement of local air pollution, and recent matured technologies such as fuel cells and electrolyzers [
2,
3]. Using the electrochemistry-driven energy conversion technologies, hydrogen could be produced from water by using electricity and could be consumed to convert into the water along with generating electricity (H
2 + O
2 ↔ H
2O) [
4,
5,
6].
Fuel cells are the most promising technology utilizing hydrogen. Proton exchange membrane fuel cells (PEMFCs) using hydrogen as fuel show high efficiency during the direct conversion of chemical energy to electric energy. In addition, there is no greenhouse gas emission when hydrogen is produced by water electrolysis using electricity supplied from renewable energy sources such as wind, solar, biomass, etc. [
1,
7]. Nevertheless, the installation cost is still higher than conventional energy conversion technology such as internal combustion engine and the technical level of durability and reliability must be raised to a higher level to enter the full-fledged fuel cell market [
8,
9,
10]. Membrane-electrodeassembly (MEA) is a key component in PEMFCs, which consists of a piece of proton exchange membrane (PEM) sandwiched between two catalyst layers as an electrode [
11]. The recent approach for PEM development has shifted from more proton conductivity to more proton conductance (in other words, from less resistivity to less areal specific resistance) at low relative humidity and higher temperature. Thus, the development of thinner PEMs is crucial to minimize areal specific resistance by the trade-off between less thickness and mechanical/chemical stability. It directly results in a significant decrease in Ohmic losses which is an increase in stack power density and a decrease in material cost [
10]. Mechanical reinforcement of thin PEMs (ca. 10–20 μm) is one of the approaches to overcome less mechanical/chemical stability by thinning the thickness. The reinforcement could be achieved by the development of porous substrate-reinforced composite membranes. It could be done only by filling high-conductivity ionomers into porous substrates or by forming a three-layered structure (ionomer/ionomer filled substrate/ionomer) [
12,
13,
14,
15,
16,
17,
18,
19,
20]. Perfluorosuflonic acid (PFSA) ionomers are still the most frequently used material even though less expensive hydrocarbon membranes have been intensively developed [
7,
21,
22,
23] Among many reasons for the use of PFSA ionomers, the main one would be the good stability against mechanical and chemical stress occurring during fuel cell or water electrolysis operation. Since the less volume of PFSA is used compared to non-reinforced PFSA membranes, benefits can be obtained in terms of material cost [
12,
24,
25]. To prepare reinforced composite membranes, PFSA ionomer dispersion normally in a mixed solvent of water and alcohols and poly(tetrafluoroethylene) (PTFE) porous substrate is often used due to high proton conductivity of the ionomers and polymeric compatibility with PFSA, respectively [
26,
27,
28]. Nevertheless, the preparation process of porous PTFE-reinforced PFSA composite membranes is very difficult since not all the hydrophilic ionomer dispersions are compatible with hydrophobic PTFE porous substrates. The fabrication of the composite membranes with an incomplete filling of PFSA ionomers into the substrates causes them to lose mechanical strength and chemical stability as well as gas permeability [
29]. However, few studies to discuss the effect of materials for hydrophilic treatment of porous substrates for the preparation of porous PTFE-reinforced PFSA composite membranes have been reported.
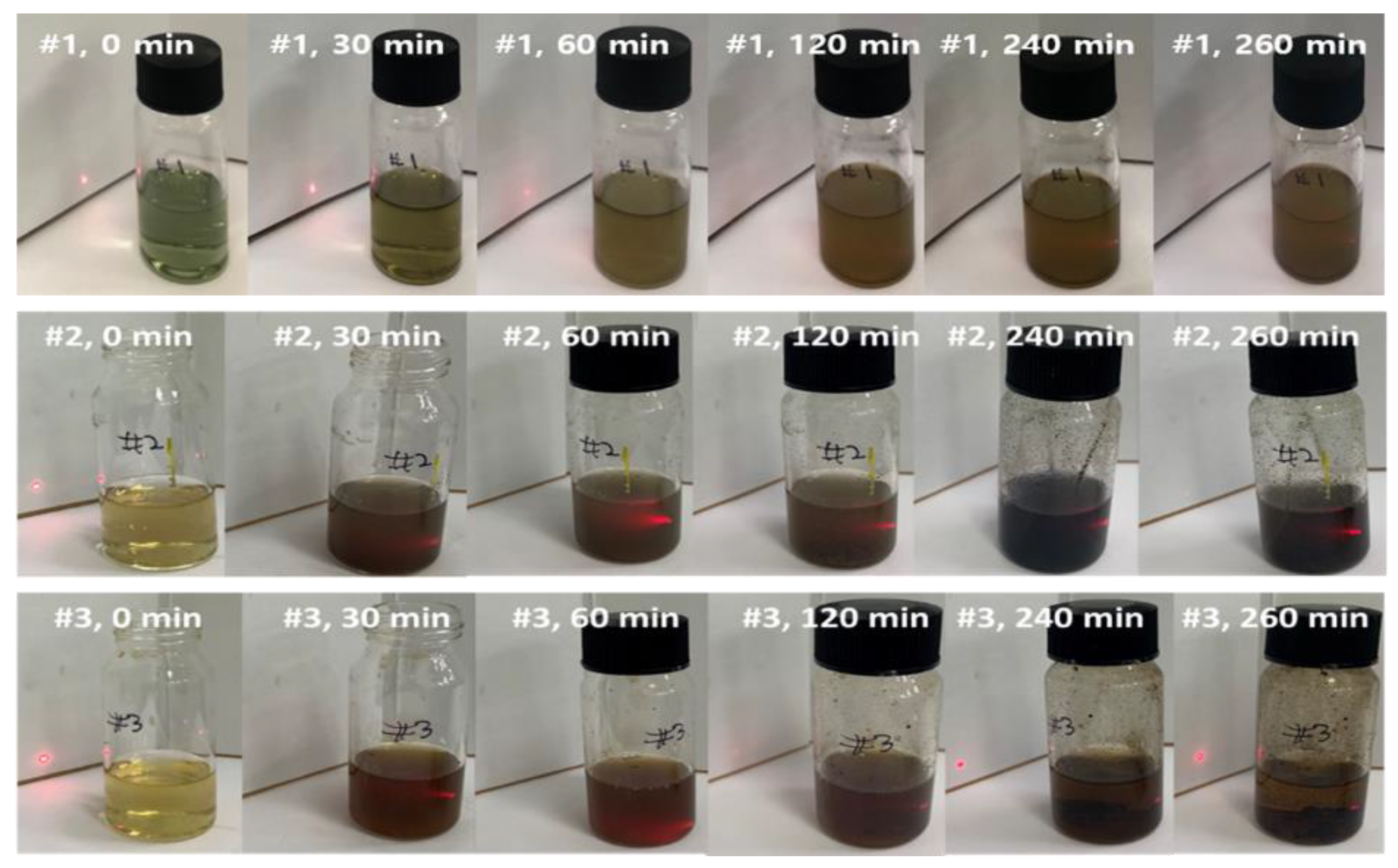
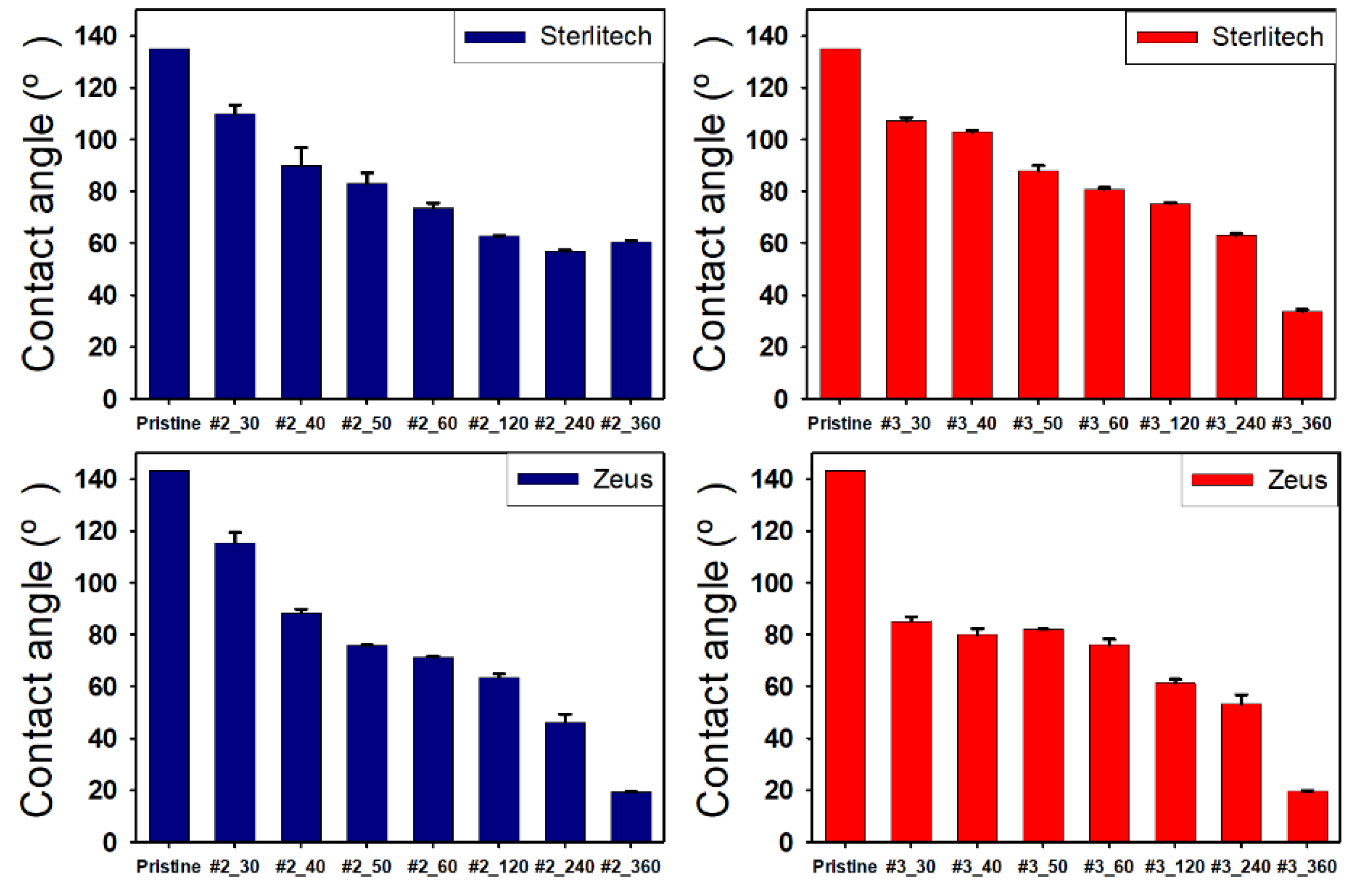
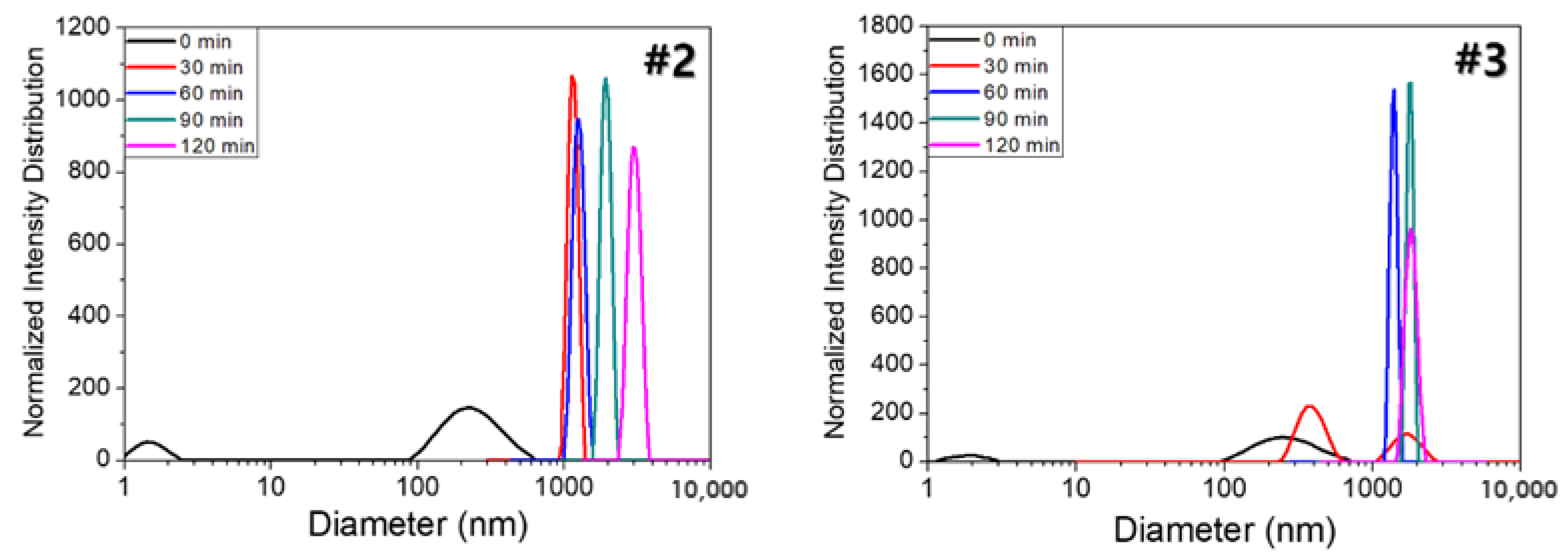
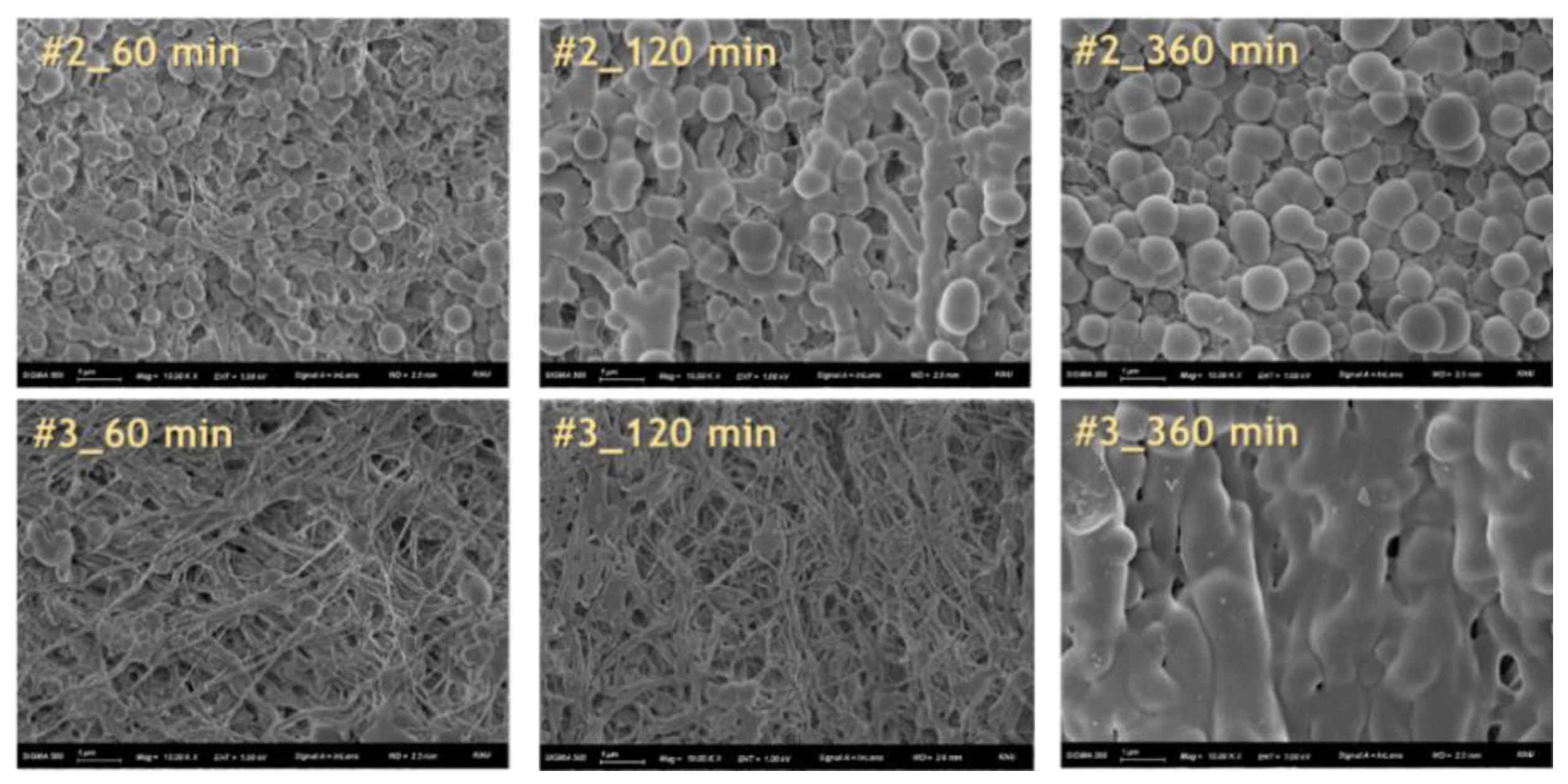
The hydrophilization of the hydrophobic material surface could be attained by oxygen plasma, UV radiation, grafting, surface oxidation by strong acids, hydrolysis, coating, or lamination. Hydrophilization treatment with plasma or UV radiation is effective because it directly exposes energy to the hydrophobic material surface. However, the hydrophilization using plasma has a limitation in that the process must be performed in a vacuum state, and the one using long-term or strong UV radiation may damage the material surface to irreversibly change the properties of materials. In addition, there is a limit to completely hydrophilize the inner pores of porous substrates. Similarly, lamination is not good for materials with complicated structure. The coating is lower than the plasma and UV radiation in terms of durability, but it is much simpler than the aforementioned methods and inexpensive. Thus, it is frequently used in the industrial hydrophilization process [
30]. Initially, the increase in hydrophilicity of PTFE surfaces was obtained by surfactants, but there are too many parameters to be considered for good wettability [
31,
32]. Recently, biomimetic materials have been deposited on porous hydrophobic microfiltration/ultrafiltration membranes for better water flux from the oil-in-water emulsion and protein wastewater [
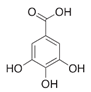
33]. It is found that the pyrogallol moiety in gallic acid (GA) with amino-terminated substances (ATS) such as siloxane generated a similar mussel-inspired adhesive coating via Michael addition/Schiff base reactions in alkaline conditions [
34,
35,
36]
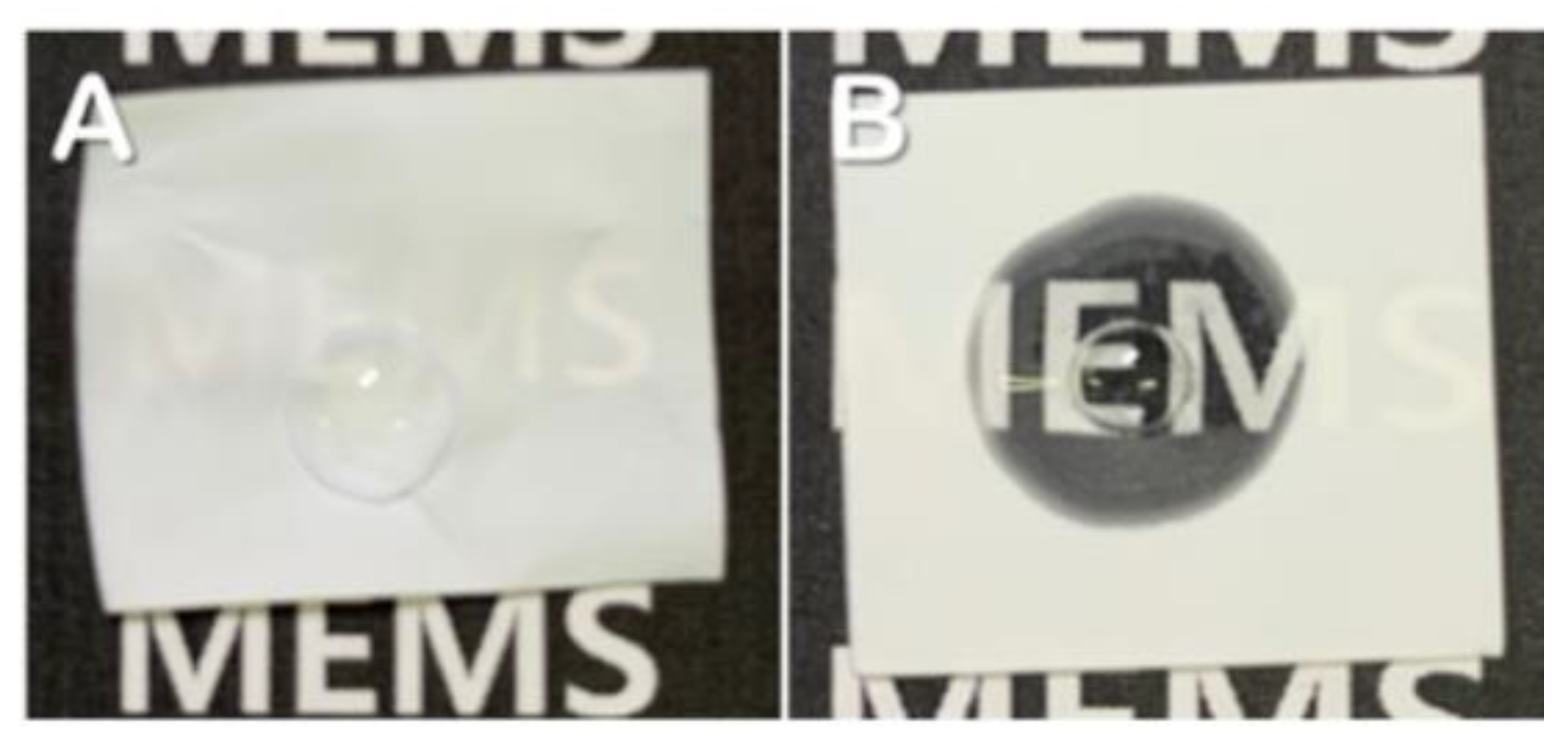
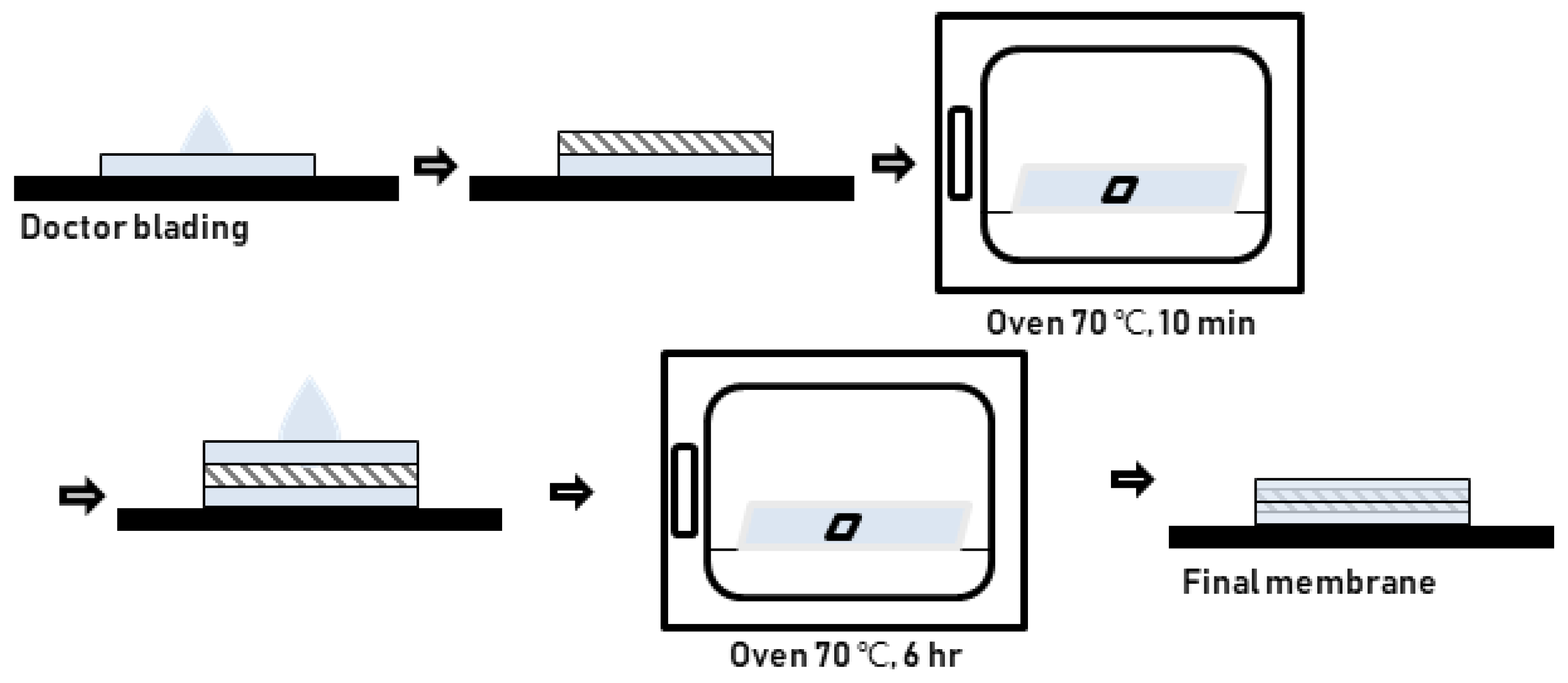
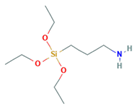
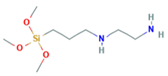
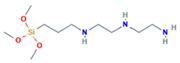
Herein, an approach to increase the wettability of hydrophobic PTFE substrates is investigated by using the nature-born materials, i.e., mussel-inspired silicified polysiloxane adhesive materials, to overcome the aforementioned incompatibility between hydrophobic PTFE substrates and hydrophilic ionomer dispersions. Hydrophilization on porous PTFE substrates (porosity 40‒90%) from the polymerization of GA with respect to the ATS, i.e., 3-aminopropyltriethoxysilane (APTES), N-[3-(trimethoxysilyl)propyl]ethylenediamine (TMPEDA), and (3-trimethoxysilylpropyl)diethylenetriamine (TMPDETA) is carried out, not on microfiltration (MF) or ultrafiltration (UF) which is less porous (porosity <40%) than PTFE. The properties of composite membranes using the porous substrates hydrophilically treated by GA and one of the amino-terminated substances are discussed in terms of water contact angle of hydrophilically treated porous substrates and proton conductivity of composite membranes. Afterward, fuel cell performance of composite membranes is measured and discussed to investigate the effect of the biomimetic coating materials on the properties of porous PTFE-reinforced composite membranes.
4. Conclusions
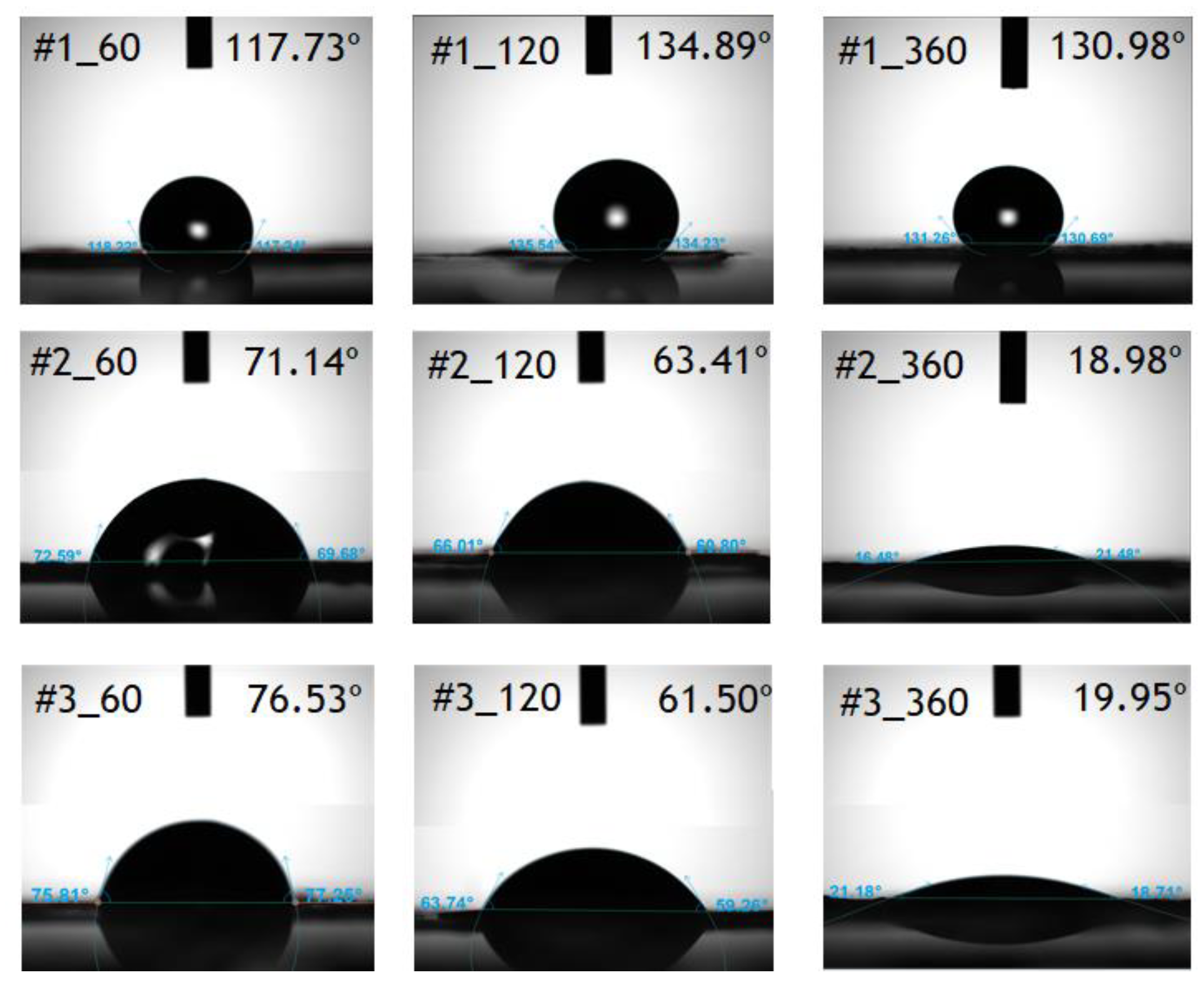
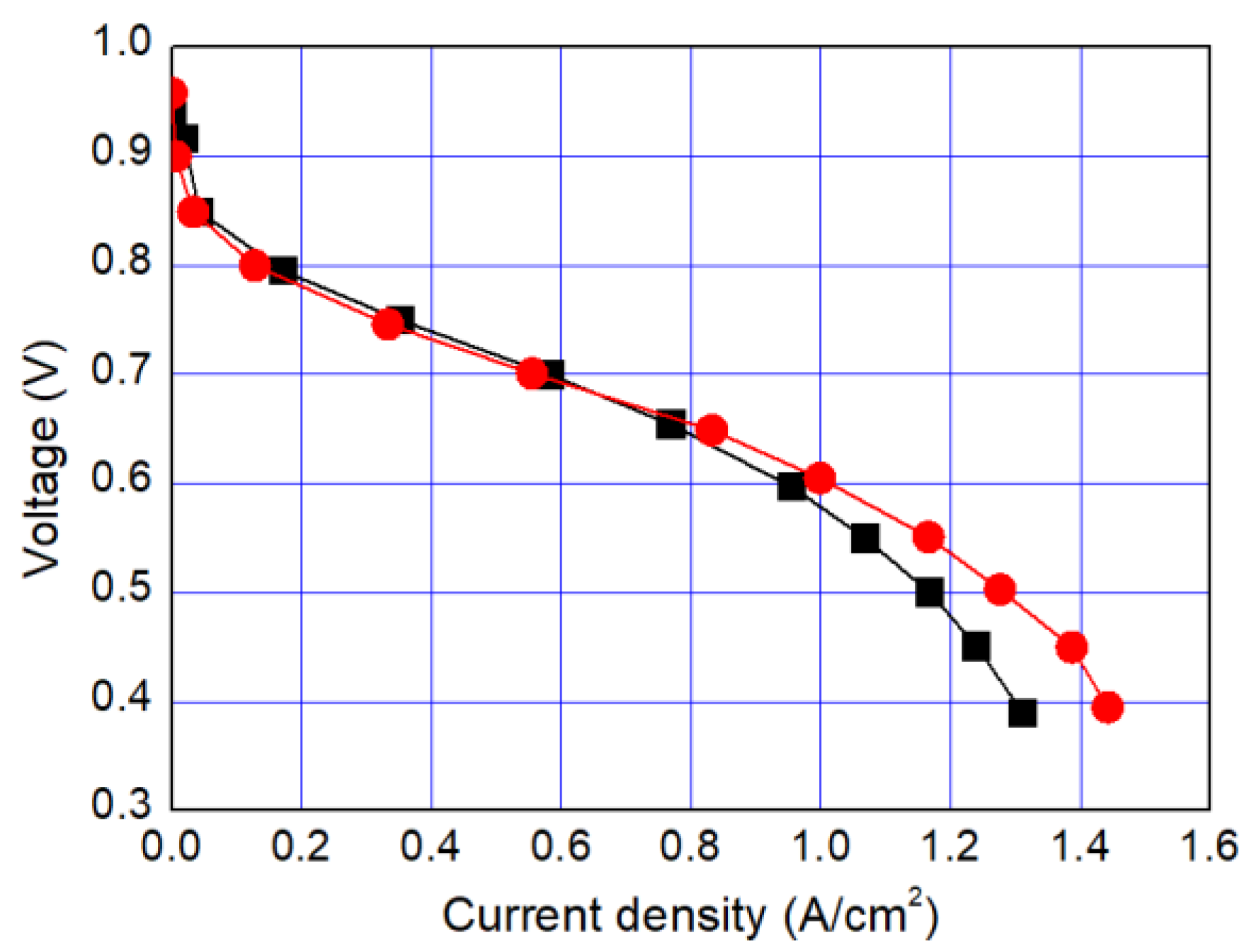
In this study, we have developed a new hydrophilic treatment method for PTFE porous substrates to impregnate PFSA ionomers into strongly hydrophobic porous substrates. Two different PFTE substrates were used: the one has the thickness of ~5 μm and the porosity of ~80% and another has the thickness of ~25 μm and the porosity of ~60%. For hydrophilic treatment, we have used in-situ biomimetic silicification which the pyrogallol moiety in gallic acid (GA) with amino-terminated substances (ATS) such as siloxane generated a similar mussel-inspired adhesive coating via Michael addition/Schiff base reactions in alkaline conditions. We investigated three different ATS materials, i.e., 3-aminopropyltriethoxysilane (APTES), N-[3-(trimethoxysilyl)propyl]ethylenediamine (TMPEDA), and (3-trimethoxysilylpropyl)diethylenetriamine (TMPDETA) with GA. It was found that GA/APTES showed no hydrophilic treatment on both substrates with higher porosity than microfiltration or ultrafiltration membranes. On the other hand, GA/TMPEDA and GA/TMPDETA using ATS with the longer amino moieties showed effective hydrophilic treatment on both substrates. However, GA/TMPDETA has obtained the best contact angle result at less incubation time for the thinner substrates. The thinner substrate having ~5 μm treated by the GA/TMPDETA solution with the incubation time of 30 min shows the best hydrophilic treatment result. In addition, the composite membranes using the porous substrates show the highest proton conductivity and the lowest water uptake and swelling ratio. MEAs using the composite membranes (thinner and lower proton conductivity) and Nafion 212 (thicker and higher proton conductivity), which have similar areal resistance, are compared in I–V polarization curves. The I–V polarization curves of two MEAs in activation and Ohmic region are very identical. However, higher mass transport limitation is observed for Nafion 212 since the composite membrane with less thickness than Nafion 212 would result in higher back diffusion of water and mitigate cathode flooding. It could be concluded that the composite membrane would be advantageous in proton exchange membrane fuel cell application since it has similar areal resistance to Nafion 212 to obtain similar Ohmic loss and less thickness than Nafion 212 to allow higher water flux from cathode to anode to obtain lower mass transport loss in I–V polarization curves.