Diesel Engine Performance, Emissions and Combustion Characteristics of Biodiesel and Its Blends Derived from Catalytic Pyrolysis of Waste Cooking Oil
Abstract
1. Introduction
Biodiesel Production from Pyrolysis of Waste Cooking Oil
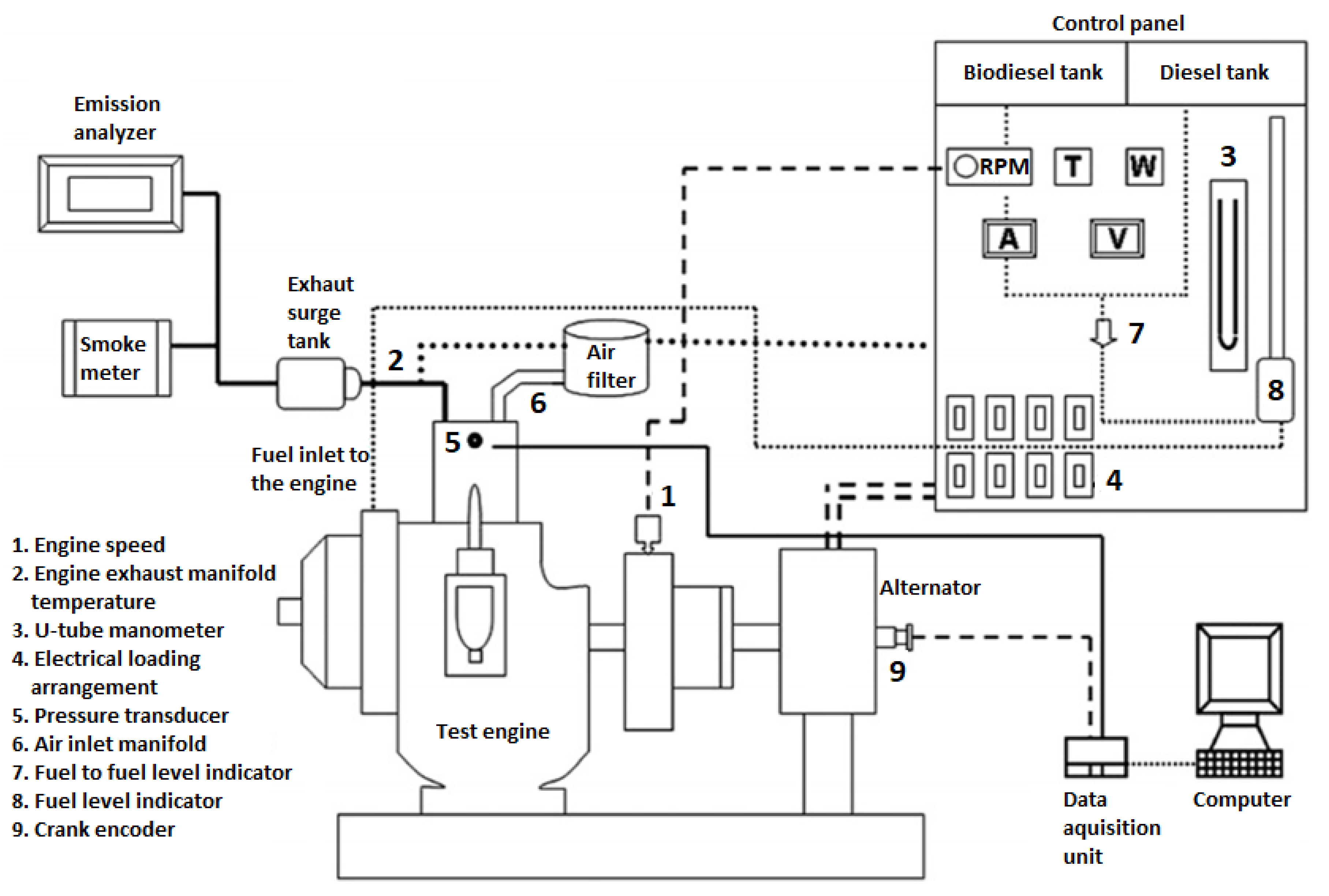
2. Experimental Setup for Engine Tests
- : Uncertainty of exhaust gas temperature.
- : Uncertainty of brake power.
- : Uncertainty of specific fuel consumption.
- : Uncertainty of engine speed.
- : Uncertainty of brake thermal efficiency.
- : Uncertainty of CO emission.
- : Uncertainty of HC emission.
- : Uncertainty of NOx emission.
- : Uncertainty of cylinder pressure transducer.
- : Uncertainty of TDC proximity switch.
3. Results and Discussion
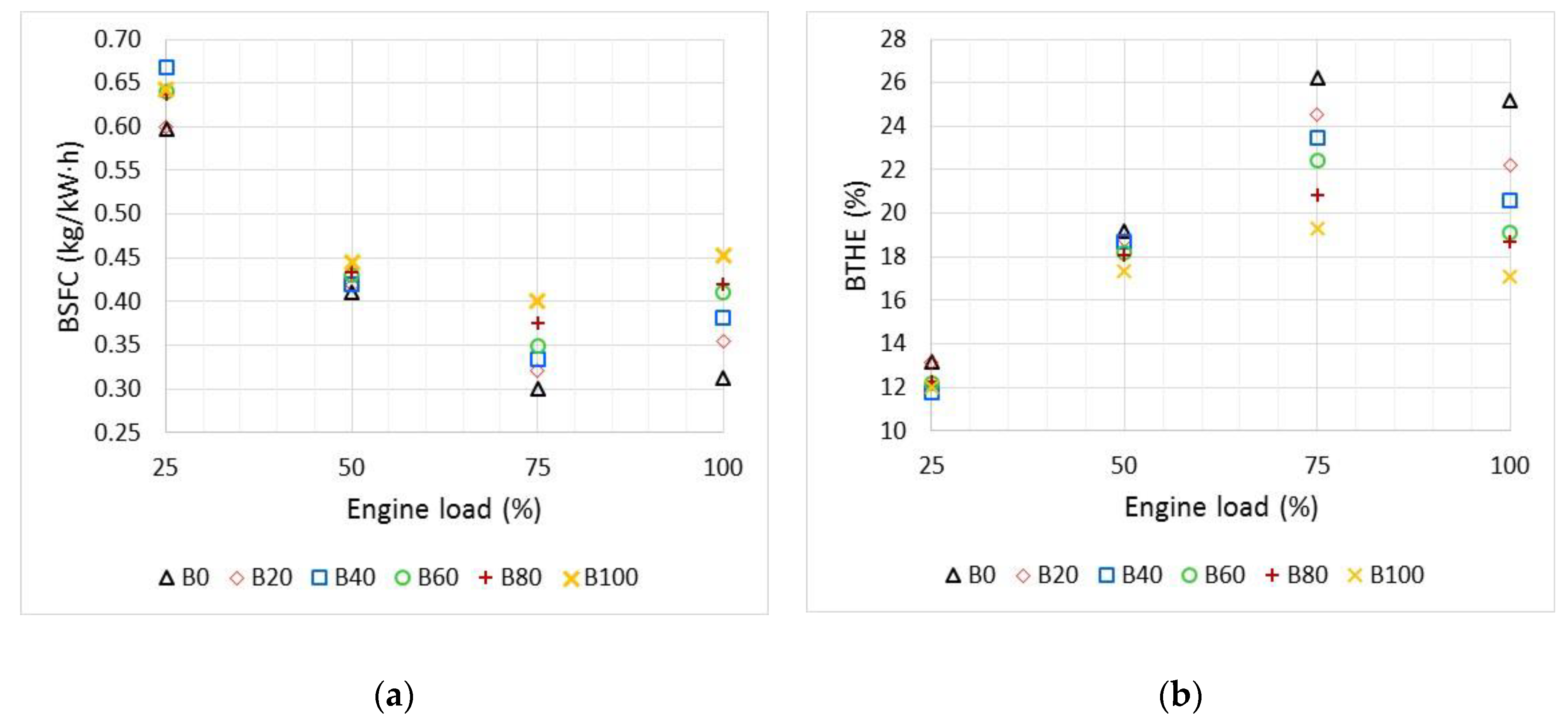
3.1. Engine Performance and Emissions Characteristics
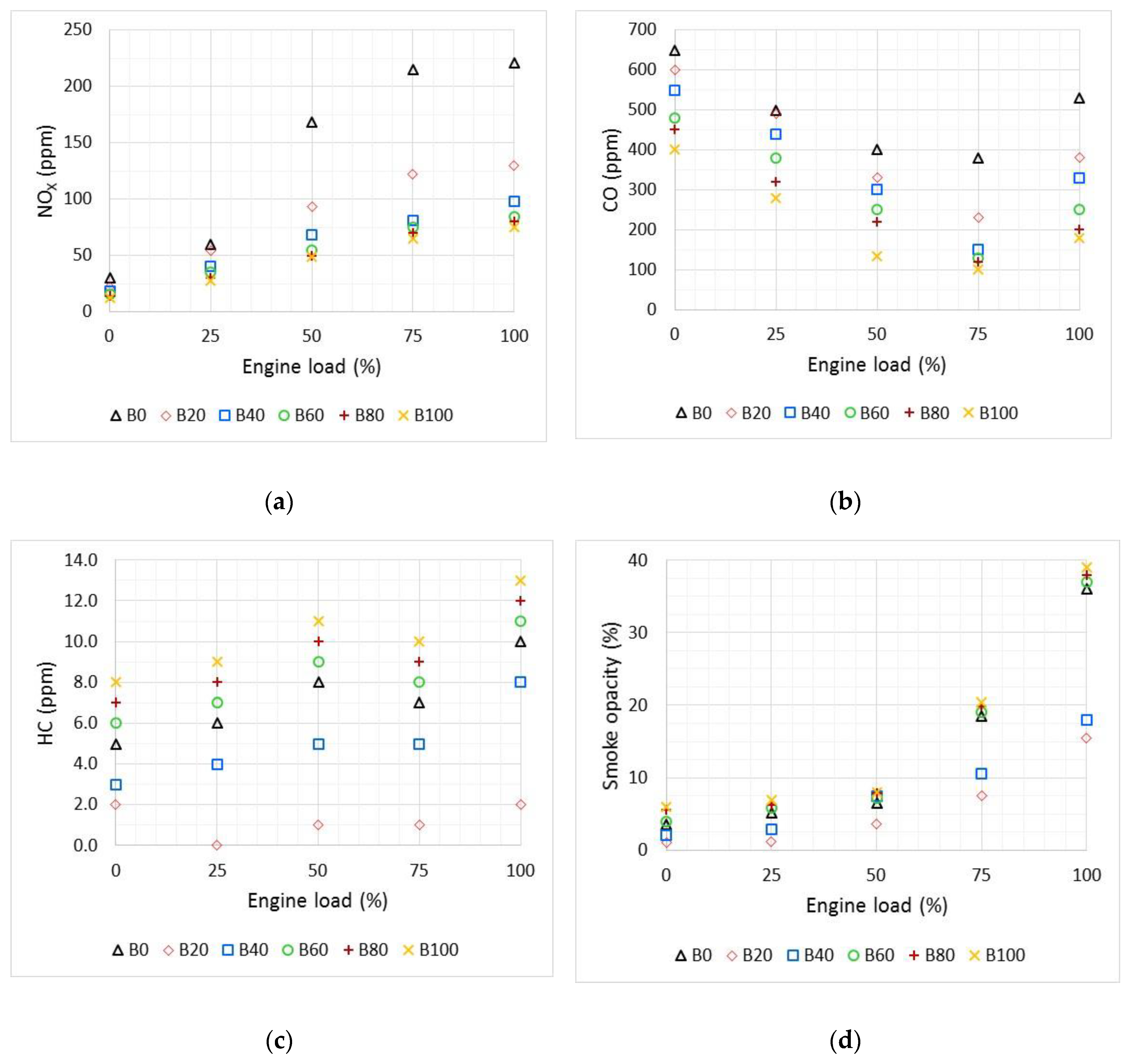
3.2. Engine Emissions Characteristics
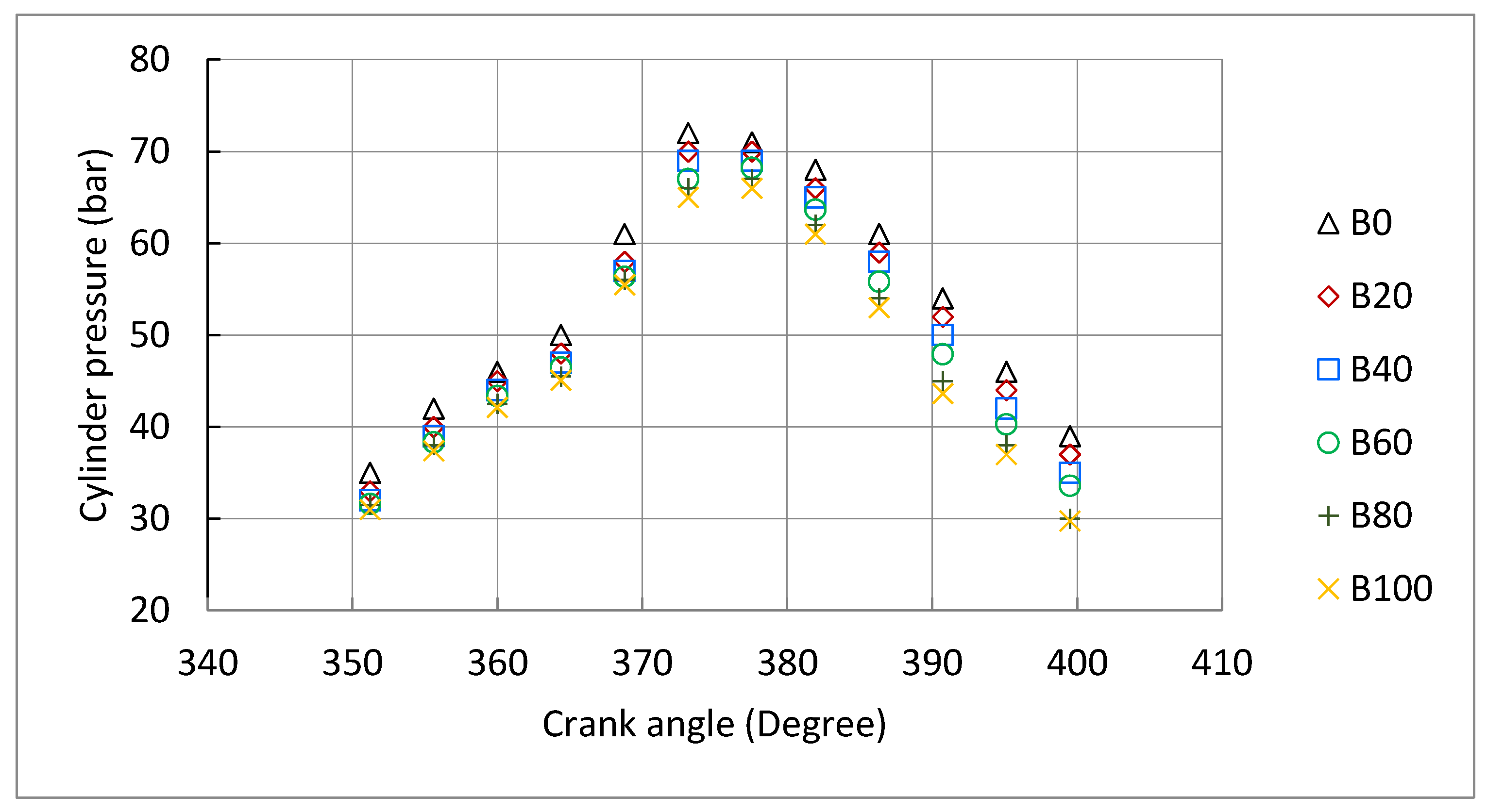
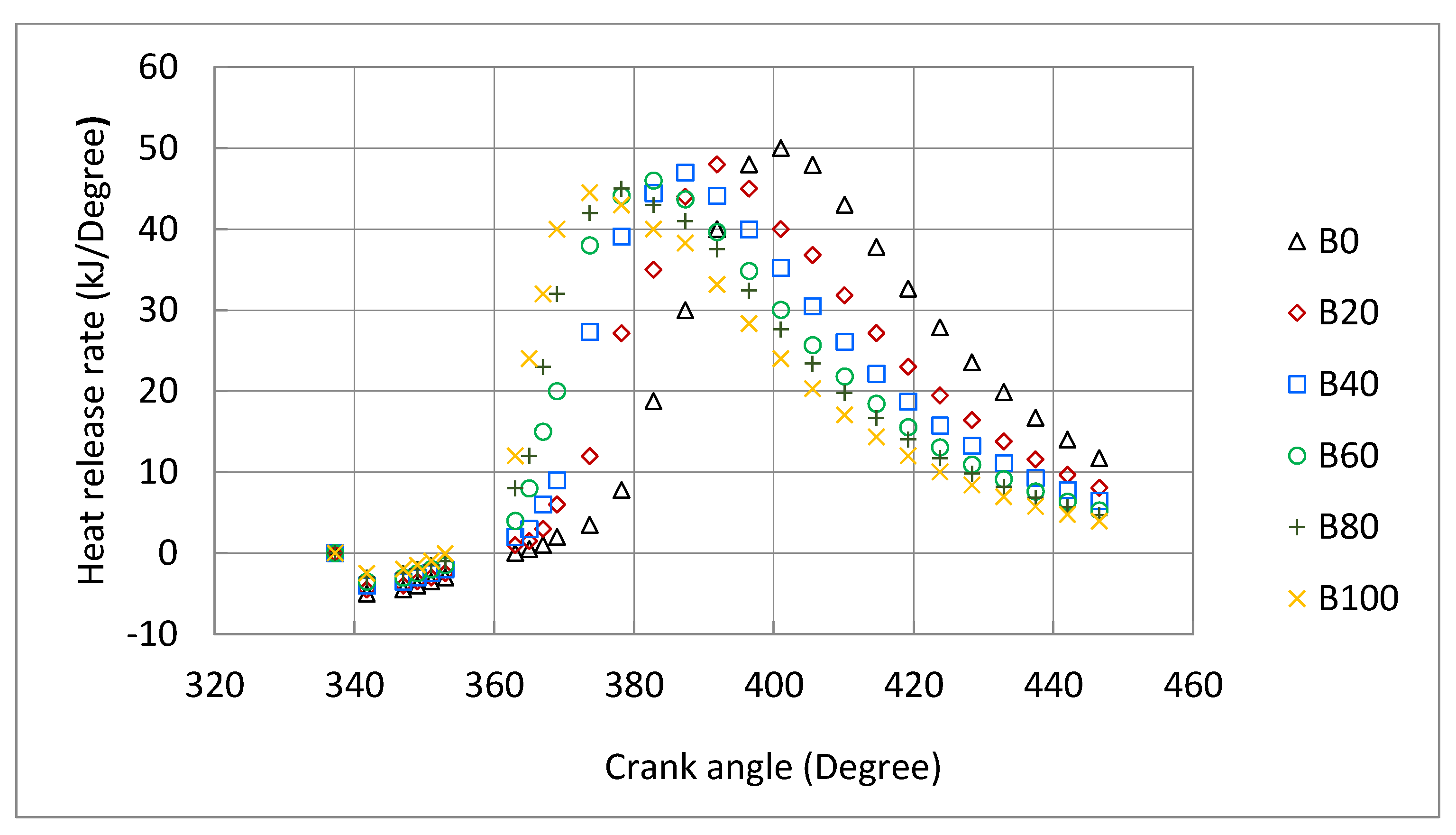
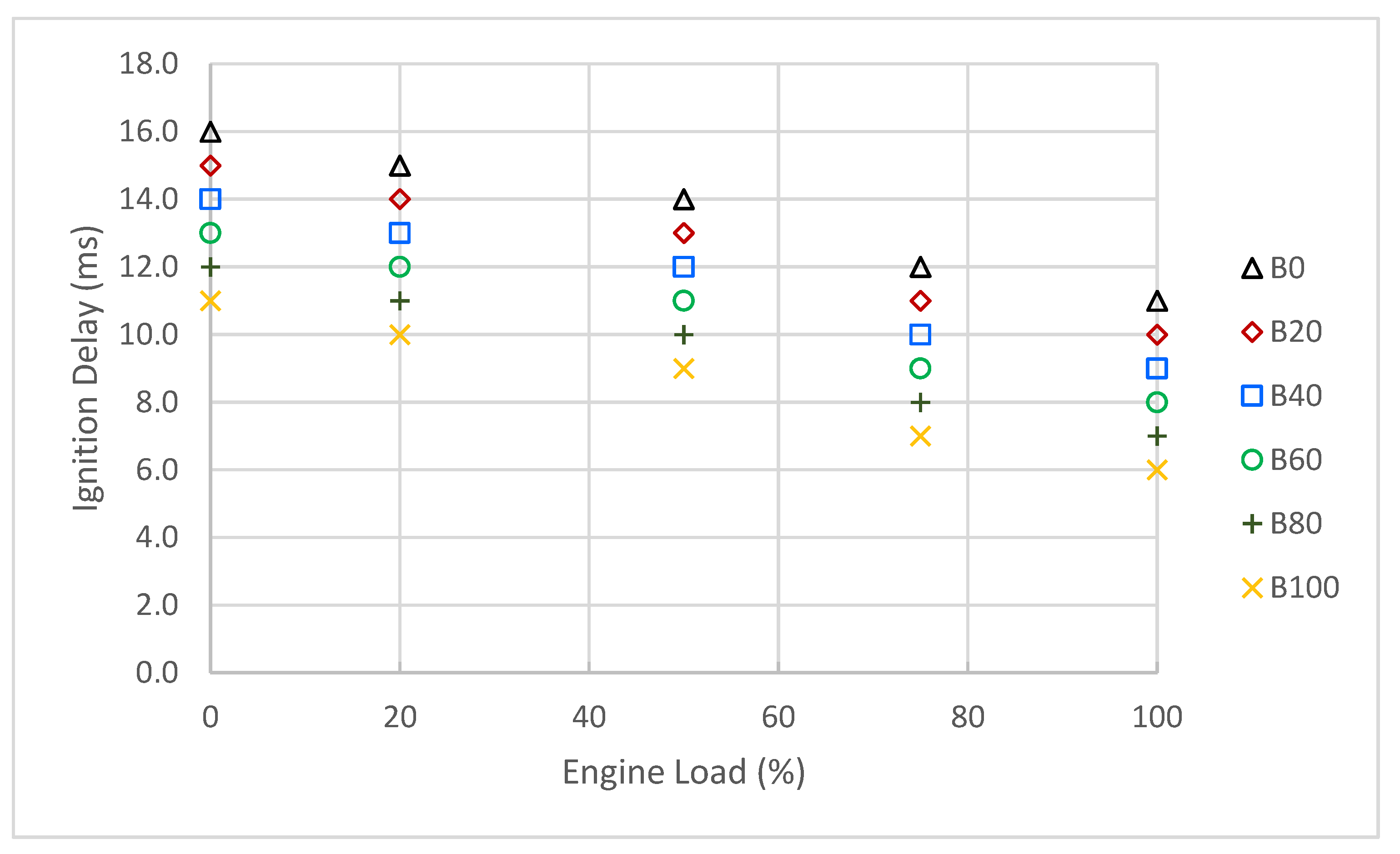
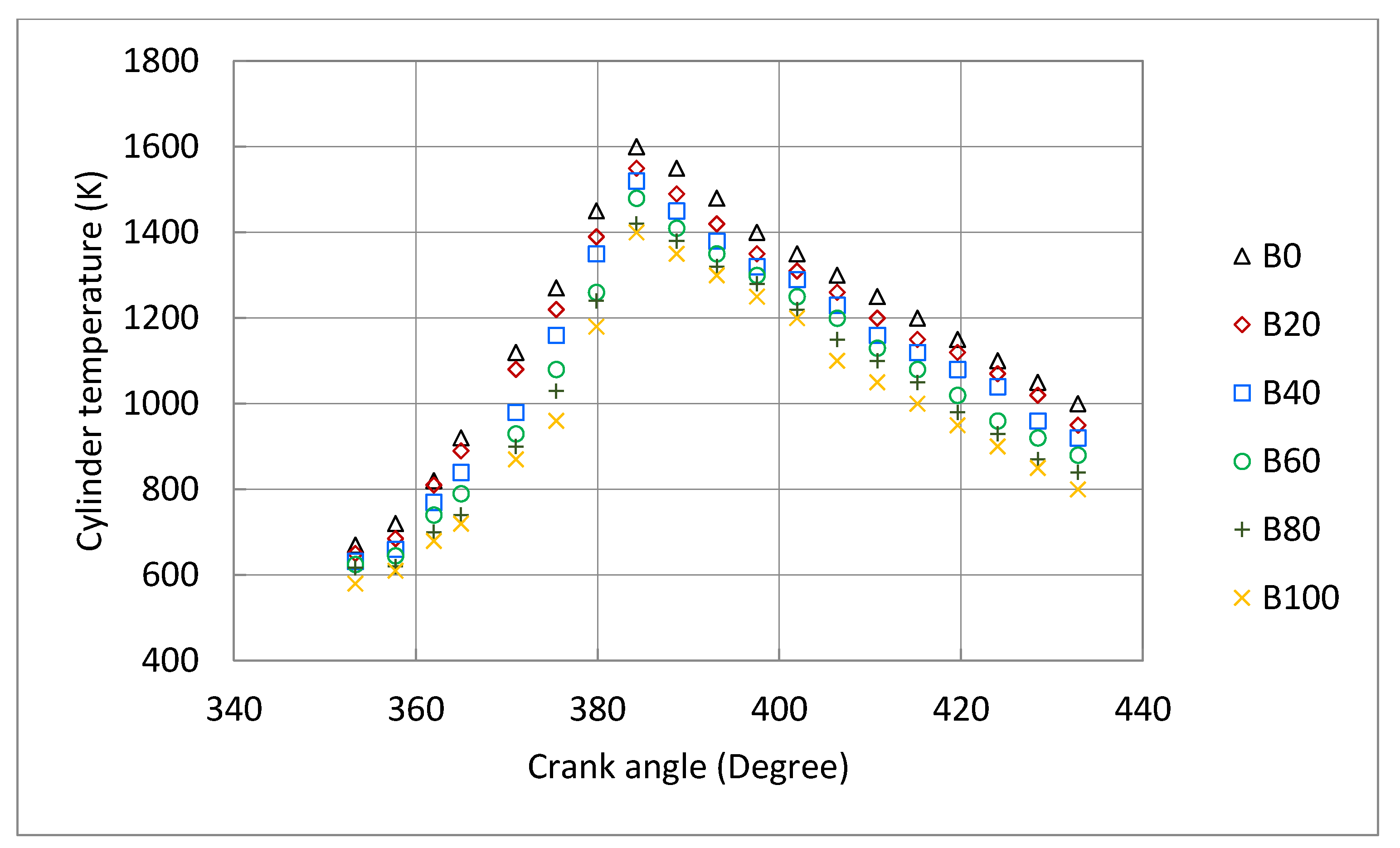
3.3. Engine Combustion Characteristics
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Avinash, A.; Subramaniam, D.; Murugesan, A. Bio-diesel—A global scenario. Renew. Sustain. Energy Rev. 2014, 29, 517–527. [Google Scholar] [CrossRef]
- Lin, L.; Zhou, C.S.; Vittayapadung , S.; Shen, X.; Dong, M. Opportunities and challenges for biodiesel fuel. Appl. Energy 2011, 88, 1020–1031. [Google Scholar] [CrossRef]
- Gebremariam, S.N.; Marchetti, J.M. Economics of biodiesel production: Review. Energy Convers. Manag. 2018, 168, 74–84. [Google Scholar] [CrossRef]
- Theogene, N.; Ikwaba, P.D. The economic feasibility of Jatropha cultivation for biodiesel production in Rwanda: A case study of Kirehe district. Energy Sustain. Dev. 2019, 50, 27–37. [Google Scholar]
- Chen, Y.H.; Chiang, T.H.; Chen, J.H. Properties of soapnut (Sapindus mukorossi) oil biodiesel and its blends with diesel. Biomass Bioenergy 2013, 52, 15–21. [Google Scholar] [CrossRef]
- Abdelfattah, M.S.H.; Abu-Elyazeed, O.S.M.; Abdelazeem, M.A. On biodiesels from castor raw oil using catalytic pyrolysis. Energy 2018, 143, 950–960. [Google Scholar] [CrossRef]
- Alajmi, F.S.M.D.A.; Hairuddin, A.A.; Adam, N.M.; Abdullah, L.C. Recent trends in biodiesel production from commonly used animal fats. Int. J. Energy Res. 2018, 42, 885–902. [Google Scholar] [CrossRef]
- Fukuda, H.; Kondo, A.; Noda, H. Biodiesel fuel production by transesterification of oils. J. Biosci. Bioeng. 2001, 92, 405–416. [Google Scholar] [CrossRef]
- Takuya, I.; Yusuke, S.; Yusuke, K.; Motoyuki, S.; Katsumi, H. Biodiesel production from waste animal fats using pyrolysis method. Fuel Process. Technol. 2012, 94, 47–52. [Google Scholar]
- Buchori, L.; Istadi, I.; Purwanto, P. Advanced chemical reactor technologies for biodiesel production from vegetable oils—A review. Bull. Chem. React. Eng. Catal. 2016, 11, 406–430. [Google Scholar] [CrossRef]
- Radwan, M.S.; Ismail, M.A.; El-Feky, S.M.S.; Abu-Elyazeed, O.S.M. Jojoba Methyl Ester as a Diesel Fuel Substitute: Preparation and Characterization. Appl. Therm. Eng. 2017, 21, 314–322. [Google Scholar] [CrossRef]
- Lappi, H.; Alén, R. Pyrolysis of vegetable oil soaps—Palm, olive, rapeseed and castor oils. J. Anal. Appl. Pyrolysis 2011, 91, 154–158. [Google Scholar] [CrossRef]
- Aïda, B.H.T.; Kaouther, Z.; Withek, B.; Slim, N.; Aymen, O. Second generation biofuels production from waste cooking oil via pyrolysis process. Renew. Energy 2018, 126, 888–896. [Google Scholar]
- Phetyim, N.; Pivsa-Art, S. Prototype co-pyrolysis of used lubricant oil and mixed plasticwaste to produce a diesel-like fuel. Energies 2018, 11, 2973. [Google Scholar] [CrossRef]
- Wang, Y.P.; Dai, L.L.; Fan, L.L.; Cao, L.P.; Zhou, Y.; Zhao, Y.F.; Liu, Y.H.; Ruan, R. Catalytic co-pyrolysis of waste vegetable oil and high density polyethylene for hydrocarbon fuel production. Waste Manag. 2017, 61, 276–282. [Google Scholar] [CrossRef]
- Chen, G.Y.; Liu, C.; Ma, W.C.; Zhang, X.X.; Li, Y.B.; Yan, B.B.; Zhou, W.H. Co-pyrolysis of corn cob and waste cooking oil in a fixed bed. Bioresour. Technol. 2014, 166, 500–507. [Google Scholar] [CrossRef]
- Faisal Abnisa, F.; Arami-Niya, A.; Wan Daud, W.M.A.; Sahu, J.N. Characterization of bio-oil and bio-char from pyrolysis of palm oil wastes. Bioenergy Res. 2013, 6, 830–840. [Google Scholar] [CrossRef]
- Wang, W.C.; Thapaliya, N.; Campos, A.; Stikeleather, L.F.; Roberts, W.L. Hydrocarbon fuels from vegetable oils via hydrolysis and thermo-catalytic decarboxylation. Fuel 2012, 95, 622–629. [Google Scholar] [CrossRef]
- Wiggers, V.R.; Zonta, G.R.; França, A.P.; Scharf, D.R.; Simionatto, E.L.; Ender, L.; Meier, H.F. Challenges associated with choosing operational conditions for triglyceride thermal cracking aiming to improve biofuel quality. Fuel 2013, 107, 601–608. [Google Scholar] [CrossRef]
- Tamilselvan, P.; Nallusamy, N.; Rajkumar, S. A comprehensive review on performance, combustion and emission characteristics of biodiesel fuelled diesel engines. Renew. Sustain. Energy Rev. 2017, 79, 1134–1159. [Google Scholar] [CrossRef]
- Suresh, M.; Jawahar, C.P.; Richard, A. A review on biodiesel production, combustion, performance, and emission characteristics of non-edible oils in variable compression ratio diesel engine using biodiesel and its blends. Renew. Sustain. Energy Rev. 2018, 92, 38–49. [Google Scholar] [CrossRef]
- Abu-Elyazeed, O.S.M. On the ignition delay of two types of Castor oil bio-diesel using shock tube experiments. Fuel 2015, 144, 157–163. [Google Scholar] [CrossRef]
- Mohamed, M.A.; Hashim, A.M.; Abu-Elyazeed, O.S.; Elsayied, H.A. Biofuel production from used cooking oil using pyrolysis process. Int. J. Res. Appl. Sci. Eng. Technol. 2017, 5, 12. [Google Scholar] [CrossRef]
- Mendecka, B.; Lombardi, L.; Kozioł, J. Probabilistic multi-criteria analysis for evaluation of biodiesel production technologies from used cooking oil. Renew. Energy 2020, 147, 2542–2553. [Google Scholar] [CrossRef]
- Abed, K.A.; El-Morsi, A.K.; Sayed, M.M.; El-Shaib, A.A.; Gad, M.S. Effect of waste cooking-oil biodiesel on performance and exhaust emissions of a diesel engine. Egypt. J. Petroleum. 2018, 27, 985–989. [Google Scholar] [CrossRef]
- Hirkude, J.B.; Padalkar, A.S. Performance and emission analysis of a compression ignition Engine operated on waste fried oil methyl esters. Appl. Energy 2012, 90, 68–72. [Google Scholar] [CrossRef]
- Muralidharan, K.; Vasudevan, D.; Sheeba, K.N. Performance, emission and combustion characteristics of biodiesel fuelled variable compression ratio engine. Energy 2011, 36, 5385–5393. [Google Scholar] [CrossRef]
- Tesfa, B.; Mishra, R.; Zhang, C.; Gu, F.; Ball, A.D. Combustion and performance characteristics of CI (compression ignition) engine running with biodiesel. Energy 2013, 51, 101–115. [Google Scholar] [CrossRef]
- Das, M.; Sarkar, M.; Datta, A.; Santra, A.K. Study on viscosity and surface tension properties of biodiesel-diesel blends and their effects on spray parameters for CI engines. Fuel 2018, 220, 769–779. [Google Scholar] [CrossRef]
- Roskilly, A.P.; Nanda, S.K.; Wang, Y.D.; Chirkowski, J. The performance and the gaseous emissions of two small marine craft diesel engines fuelled with biodiesel. Appl. Therm. Eng. 2008, 28, 872–880. [Google Scholar] [CrossRef]
- Wu, F.; Wang, J.; Chen, W.; Shuai, S. A study on emission performance of a diesel engine fuelled with five typical methyl ester biodiesels. Atmos. Environ. 2009, 43, 1481–1485. [Google Scholar] [CrossRef]
- Xue, J.L. Combustion characteristics, engine performances and emissions of waste edible oil biodiesel in diesel engine. Renew. Sustain. Energy Rev. 2013, 23, 350–365. [Google Scholar] [CrossRef]
- Rinaldini, C.A.; Mattarelli, E.; Savioli, T.; Cantore, G.; Garbero, M.; Bologna, A. Performance, emission and combustion characteristics of IDI engine running on waste plastic oil. Fuel 2016, 183, 292–303. [Google Scholar] [CrossRef]
- Baskovi, U.Z.; Vihar, R.; Seljak, T.; Katrasnik, T. Combustion and emission formation phenomena of tire pyrolysis oil in a common rail Diesel engine. Energy Convers. Manag. 2017, 149, 706–721. [Google Scholar]
- Rajak, U.; Nashine, P.; Chaurasiya, P.K.; Verma, T.N.; Patel, D.K.; Dwivedi, G. Experimental & predicative analysis of engine characteristics of various biodiesels. Fuel 2021, 285, 119097. [Google Scholar]
- Mirhashemi, F.S.; Sadrnia, H. NOx emissions of compression ignition engines fueled with various biodiesel blends: A review. J. Energy Inst. 2020, 93, 129–151. [Google Scholar] [CrossRef]
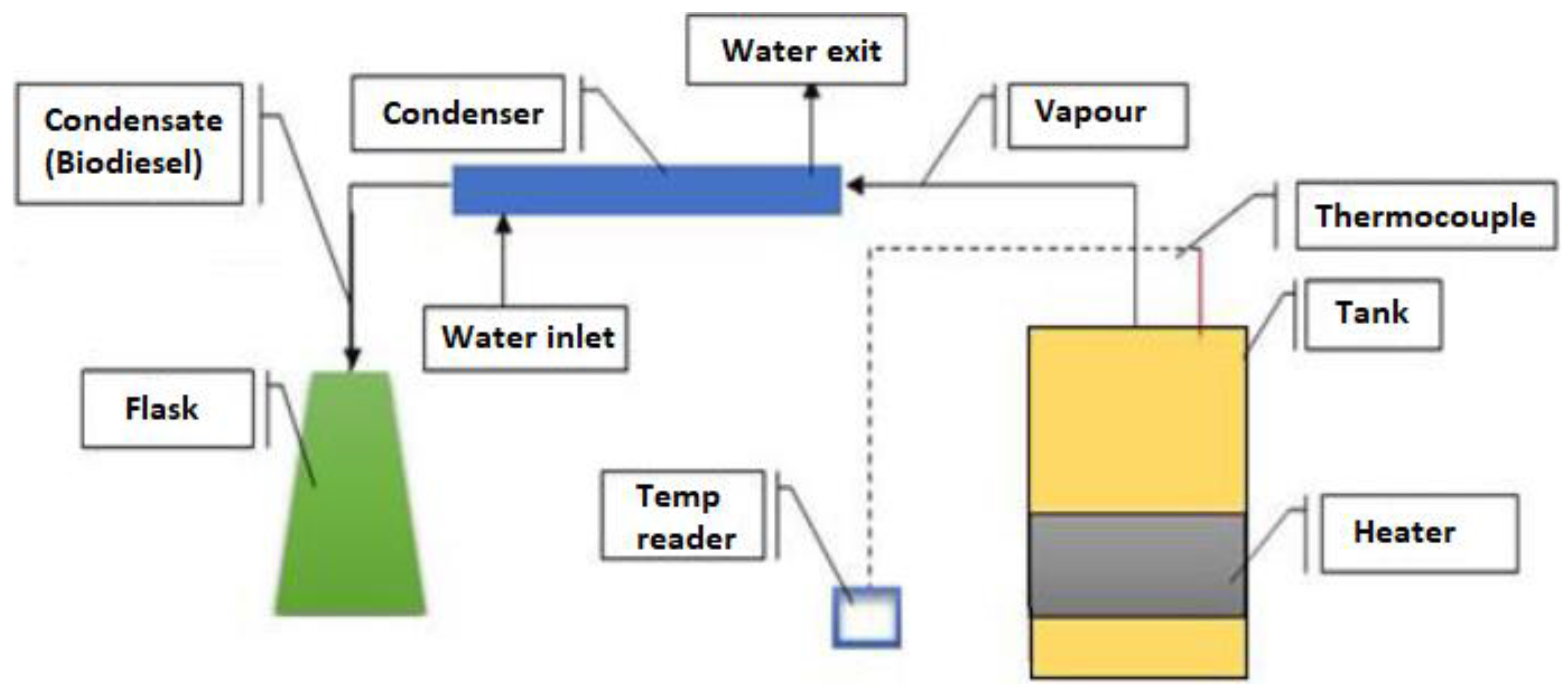
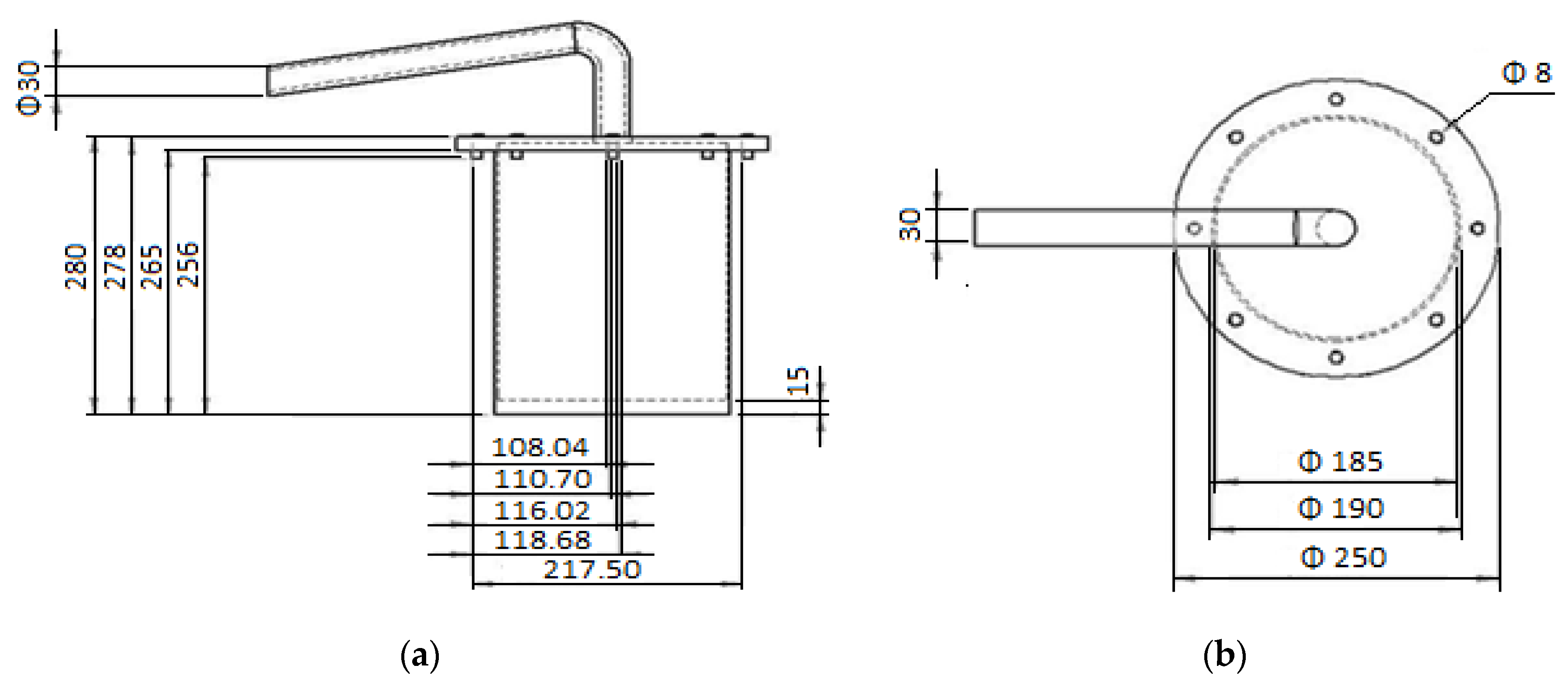
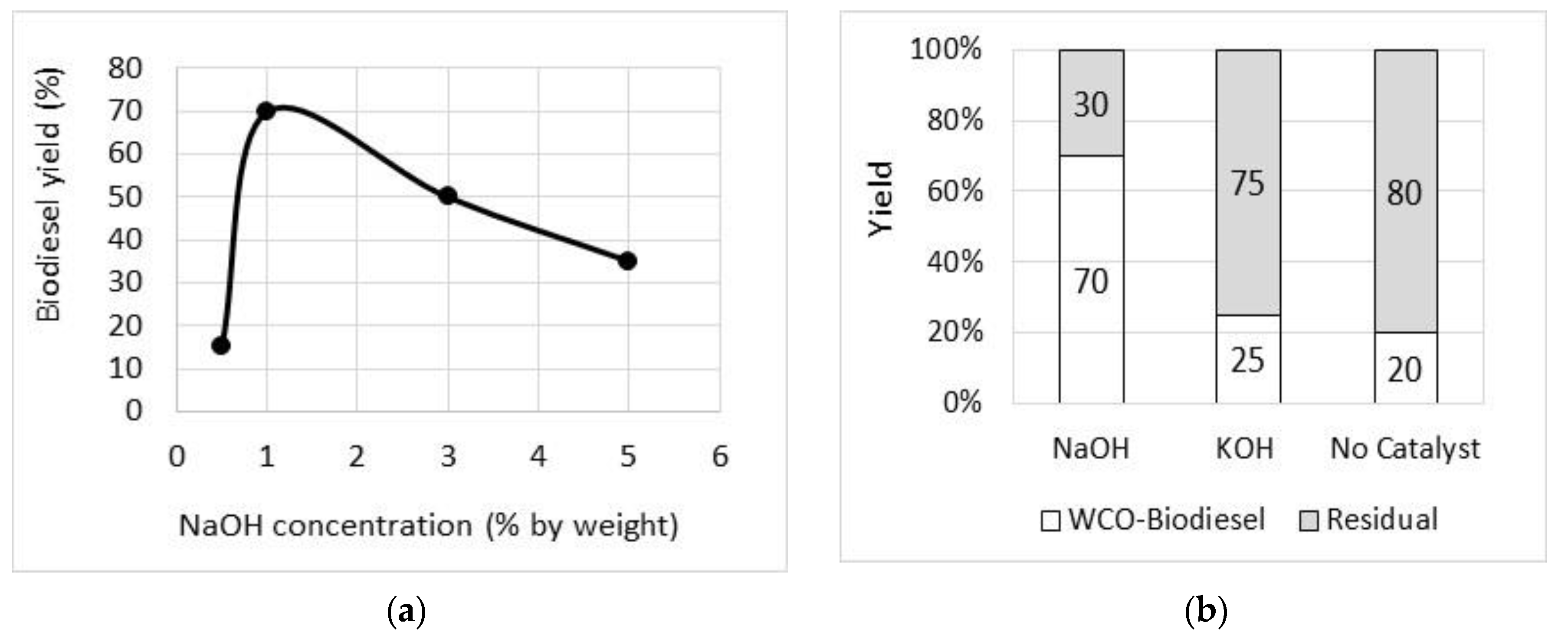
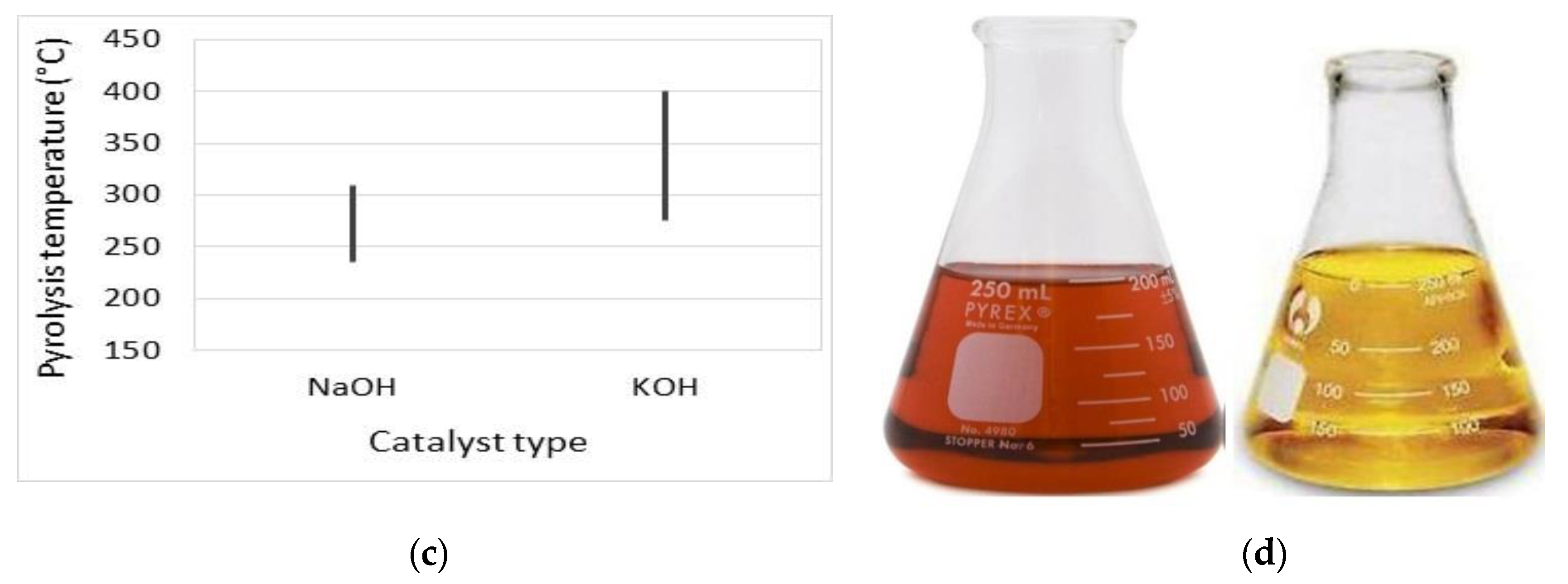
| Property | Unit | Diesel (as-Received) | Diesel Standards | B100 | Standard ASTM Method |
|---|---|---|---|---|---|
| Density at 15 °C | kg/m3 | 835.8 | 832.0 | 816.6 | D4052 |
| Colour | - | 2 | 0.85 | 6 | D6045 |
| Kinematic Viscosity at 40 °C | cSt | 4.95 | 1.0–6.7 | 2.288 | D7042 |
| Pensky Flashpoint Closed cup | °C | Minimum 55 | Minimum 55 | 28 | D93 |
| Pour point | °C | −9 | 3–15 | −27 | D6749 |
| Sulphur Content | wt.% | 1.5 | Maximum 1 | nil | D4294 |
| Ash | % vol. | nil | Maximum 0.01 | 0.00312 | D482 |
| Carbon Residue | wt.% | 0.1 | Maximum 0.1 | 1.031 | D4530 |
| Cetane Number | - | 51.86 | Minimum 46 | 54.6 | D4737 |
| Cu-strip corrosion at 50 °C for 3 h | - | Maximum 1 | Maximum 1 | 1 | D130 |
| Calorific value | MJ/kg | 45.62 | Minimum 44.3 | 46.62 | D4868 |
| Model | DEUTZ (FI L511) |
|---|---|
| Number of cylinders | 1 |
| Number of cycles | 4 |
| Bore, mm | 100 |
| Stroke, mm | 105 |
| Displacement, c.c | 825 |
| Connecting rod length, mm | 180 |
| Compression ratio | 17:1 |
| Idle speed, rpm | 900 |
| Rated speed, rpm | 1500 |
| Max. engine load, kW | 5.775 |
| Fuel injection timing | 24° Before TDC |
| Injection pressure, bar | 175 |
| Type of injection | Direct injection |
| Variable | Uncertainty |
|---|---|
| Engine speed | ±0.15% |
| Cylinder pressure | ±0.20% |
| Position of TDC | ±1.00% |
| Exhaust gas temperature | ±0.75% |
| Exhaust gas analyser | NO: ±1.00% |
| HC: ±1.00% | |
| CO: ±0.01% | |
| Brake power | ±0.85% |
| Brake thermal efficiency | ±1.50% |
| Specific fuel consumption | ±2.20% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohamed, M.; Tan, C.-K.; Fouda, A.; Gad, M.S.; Abu-Elyazeed, O.; Hashem, A.-F. Diesel Engine Performance, Emissions and Combustion Characteristics of Biodiesel and Its Blends Derived from Catalytic Pyrolysis of Waste Cooking Oil. Energies 2020, 13, 5708. https://doi.org/10.3390/en13215708
Mohamed M, Tan C-K, Fouda A, Gad MS, Abu-Elyazeed O, Hashem A-F. Diesel Engine Performance, Emissions and Combustion Characteristics of Biodiesel and Its Blends Derived from Catalytic Pyrolysis of Waste Cooking Oil. Energies. 2020; 13(21):5708. https://doi.org/10.3390/en13215708
Chicago/Turabian StyleMohamed, Mohamed, Chee-Keong Tan, Ali Fouda, Mohammed Saber Gad, Osayed Abu-Elyazeed, and Abdel-Fatah Hashem. 2020. "Diesel Engine Performance, Emissions and Combustion Characteristics of Biodiesel and Its Blends Derived from Catalytic Pyrolysis of Waste Cooking Oil" Energies 13, no. 21: 5708. https://doi.org/10.3390/en13215708
APA StyleMohamed, M., Tan, C.-K., Fouda, A., Gad, M. S., Abu-Elyazeed, O., & Hashem, A.-F. (2020). Diesel Engine Performance, Emissions and Combustion Characteristics of Biodiesel and Its Blends Derived from Catalytic Pyrolysis of Waste Cooking Oil. Energies, 13(21), 5708. https://doi.org/10.3390/en13215708






