Enhanced Hydrate-Based Geological CO2 Capture and Sequestration as a Mitigation Strategy to Address Climate Change
Abstract
:1. Introduction
2. Materials and Methods
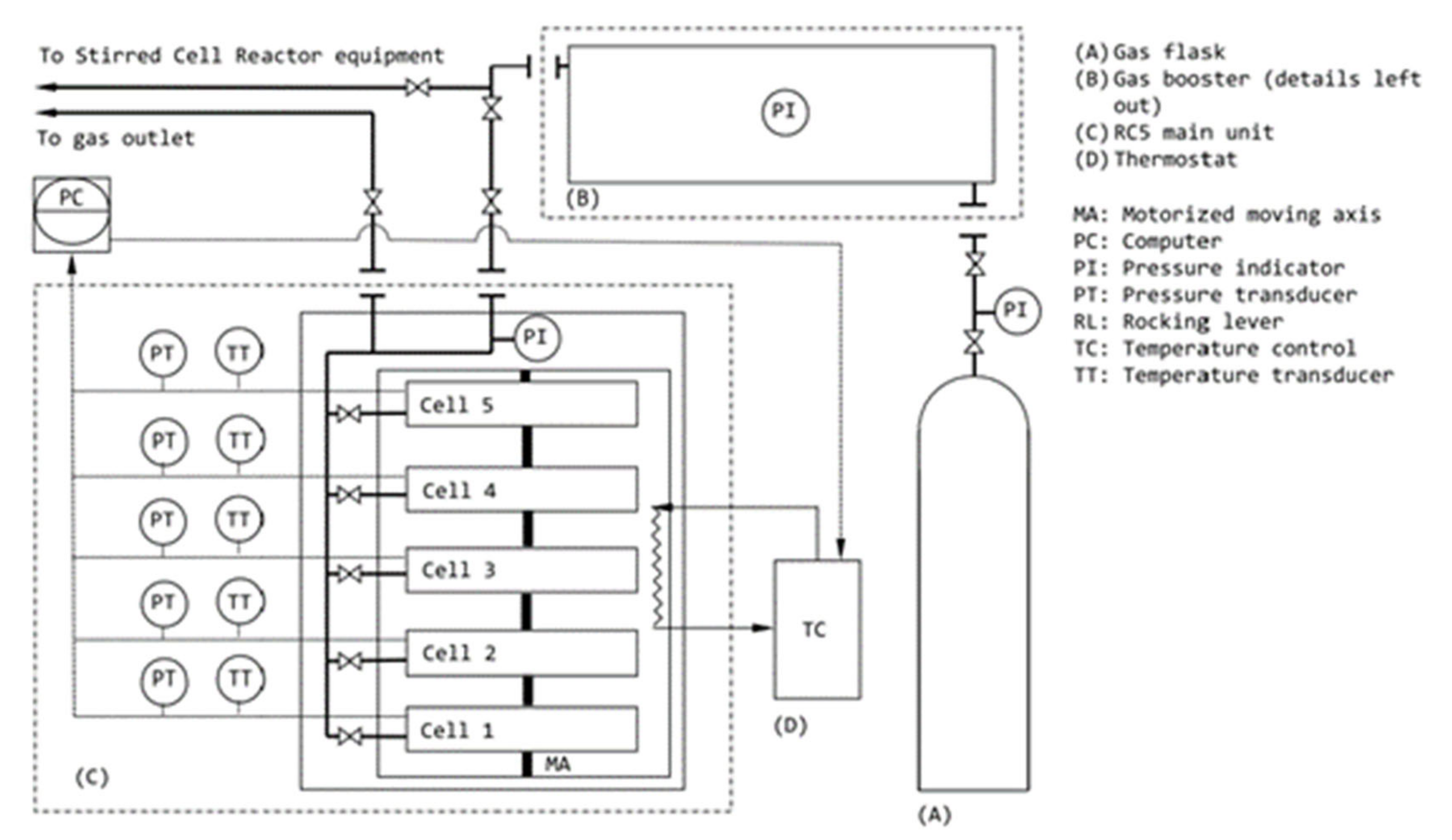
2.1. Setup and Materials
2.2. Procedure
2.3. Experimental Data Processing
3. Results and Discussion
3.1. Nucleation Temperature and Subcooling Measurement
3.2. Induction Time Measurement in Isothermal Experiments
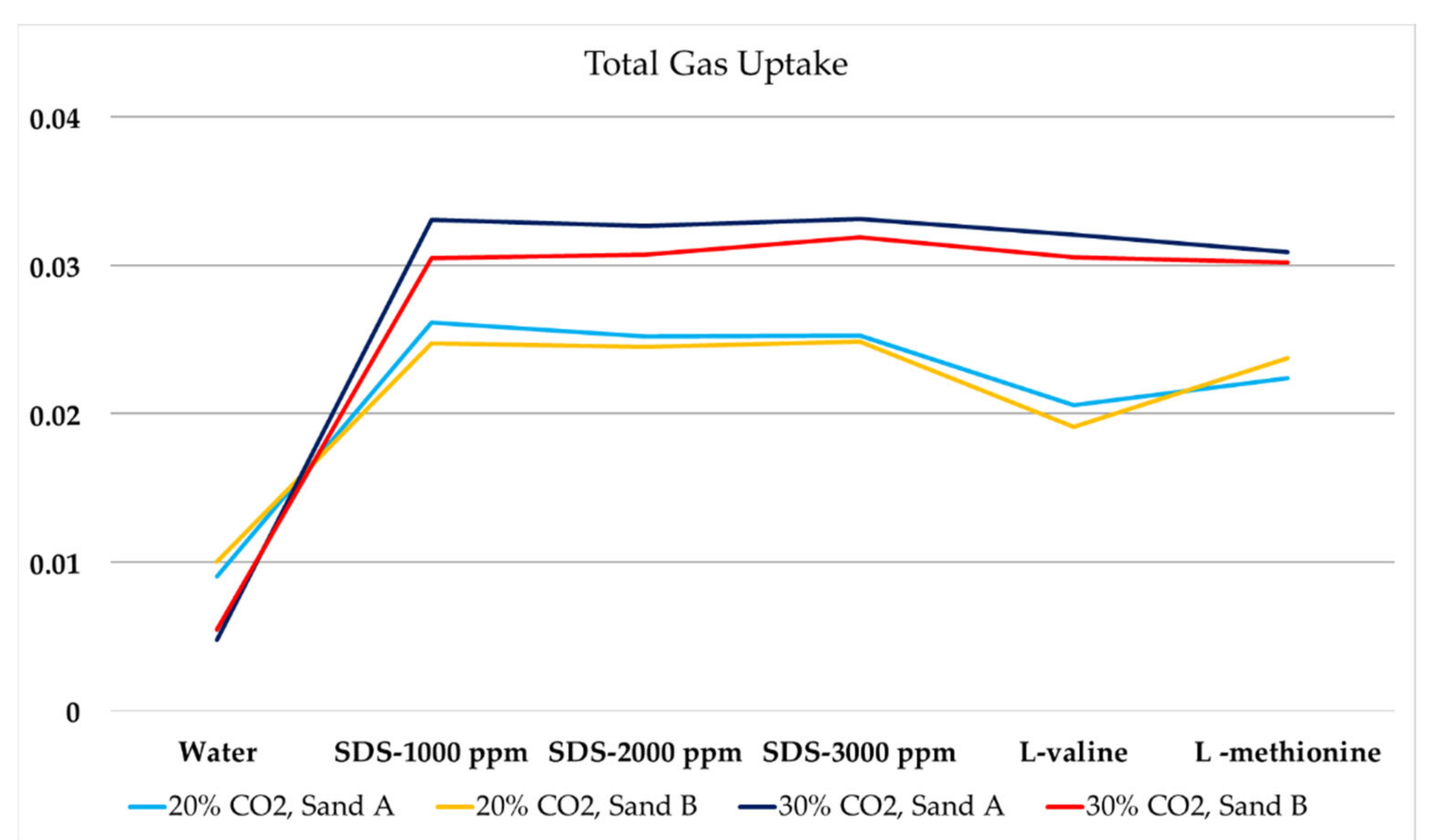
3.3. Gas Uptake Analysis
3.4. CO2 Split Fraction Analysis
3.5. Role of Amino Acids in Carbon Capture and Storage Applications
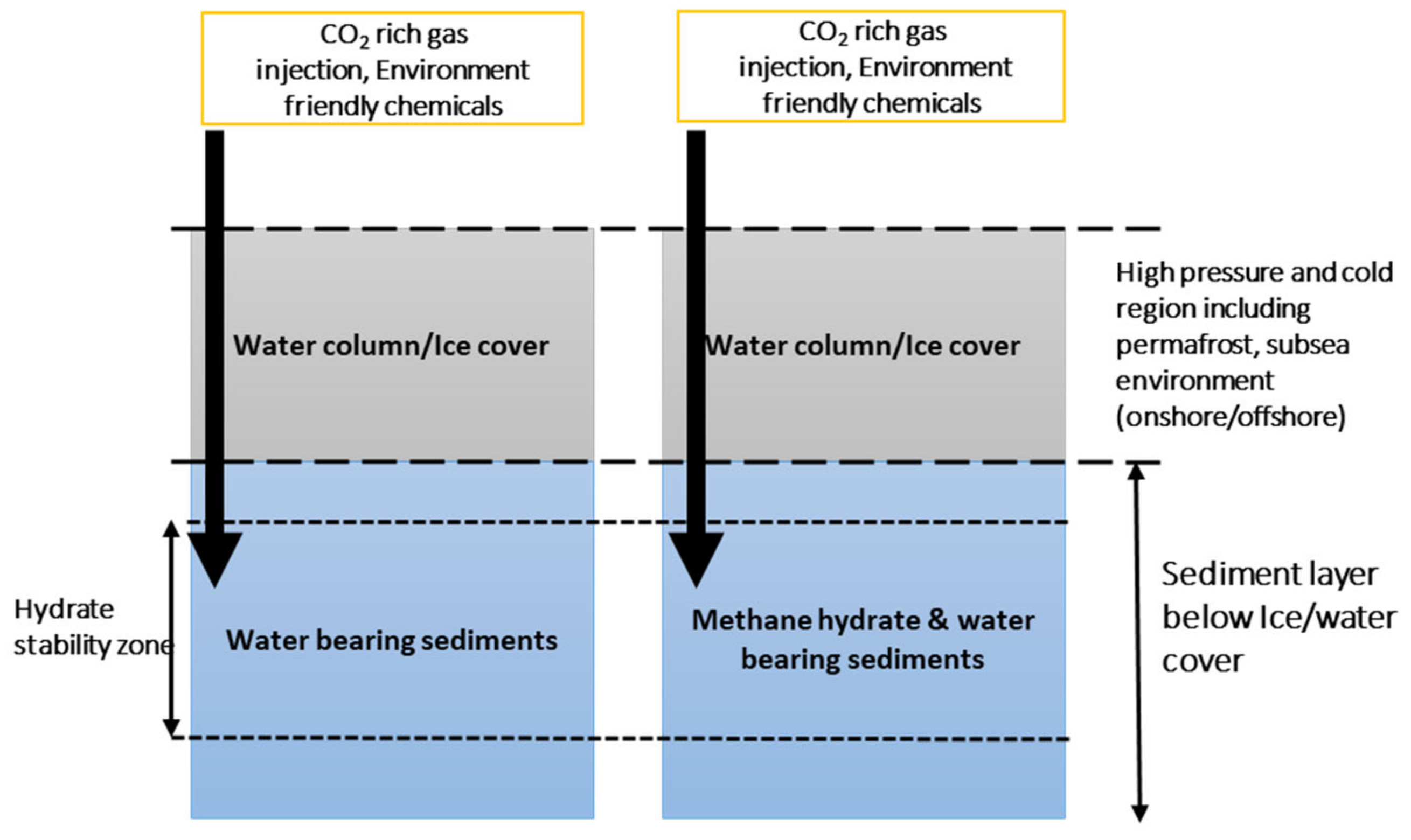
3.6. Challenge and Prospects in Geological Carbon Capture and Storage as Hydrates
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lelieveld, J.; Crutzen, P.J.; Dentener, F.J. Changing concentration, lifetime and climate forcing of atmospheric methane. Tellus Ser. B Chem. Phys. Meteorol. 1998, 50, 128–150. [Google Scholar] [CrossRef]
- Wuebbles, D.J.; Hayhoe, K. Atmospheric methane and global change. Earth-Sci. Rev. 2002, 57, 177–210. [Google Scholar] [CrossRef]
- Sloan, E., Jr.; Koh, C.A. Clathrate Hydrates of Natural Gases, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2007; ISBN 9780849390784. [Google Scholar]
- Koh, C.A.; Sloan, E.D.; Sum, A.K.; Wu, D.T. Fundamentals and applications of gas hydrates. Annu. Rev. Chem. Biomol. Eng. 2011, 2, 237–257. [Google Scholar] [CrossRef] [PubMed]
- Collett, T.; Bahk, J.J.; Baker, R.; Boswell, R.; Divins, D.; Frye, M.; Goldberg, D.; Husebø, J.; Koh, C.; Malone, M.; et al. Methane hydrates in nature-current knowledge and challenges. J. Chem. Eng. Data 2015, 60, 319–329. [Google Scholar] [CrossRef]
- Boswell, R.; Collett, T.S. Current perspectives on gas hydrate resources. Energy Environ. Sci. 2011, 4, 1206–1215. [Google Scholar] [CrossRef]
- Makogon, Y.F. Natural gas hydrates - A promising source of energy. J. Nat. Gas Sci. Eng. 2010, 2, 49–59. [Google Scholar] [CrossRef]
- Ketzer, M.; Praeg, D.; Rodrigues, L.F.; Augustin, A.; Pivel, M.A.G.; Rahmati-Abkenar, M.; Miller, D.J.; Viana, A.R.; Cupertino, J.A. Gas hydrate dissociation linked to contemporary ocean warming in the southern hemisphere. Nat. Commun. 2020, 11, 3788. [Google Scholar] [CrossRef] [PubMed]
- Chuvilin, E.; Bukhanov, B.; Ekimova, V.; Davletshina, D.; Sokolova, N. Evidence of gas emissions from permafrost in the russian arctic. Geosciences 2020, 10, 383. [Google Scholar] [CrossRef]
- Pandey, J.; Solms, N. Hydrate Stability and Methane Recovery from Gas Hydrate through CH4–CO2 Replacement in Different Mass Transfer Scenarios. Energies 2019, 12, 2309. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Wang, P.; Yang, M.; Zhao, Y.; Zhao, J.; Song, Y. CO2 sequestration in depleted methane hydrate sandy reservoirs. J. Nat. Gas Sci. Eng. 2018, 49, 428–434. [Google Scholar] [CrossRef]
- Englezos, P. Extraction of methane hydrate energy by carbon dioxide injection-key challenges and a paradigm shift. Chin. J. Chem. Eng. 2019, 27, 2044–2048. [Google Scholar] [CrossRef]
- Pandey, J.S.; Karantonidis, C.; Karcz, A.P.; von Solms, N. Enhanced CH4-CO2 Hydrate Swapping in the Presence of Low Dosage Methanol. Energies 2020, 13, 5238. [Google Scholar] [CrossRef]
- Waite, W.F.; Santamarina, J.C.; Cortes, D.D.; Dugan, B.; Espinoza, D.N.; Germaine, J.; Jang, J.; Jung, J.W.; Kneafsey, T.J.; Shin, H.; et al. Physical properties of hydrate-bearing sediments. Rev. Geophys. 2009, 47, RG4003. [Google Scholar] [CrossRef]
- Uchida, T. Physical property measurements on CO2 clathrate hydratesReview of crystallography, hydration number, and mechanical properties. Waste Manag. 1998, 17, 343–352. [Google Scholar]
- Parrenin, F.; Masson-Delmotte, V.; Köhler, P.; Raynaud, D.; Paillard, D.; Schwander, J.; Barbante, C.; Landais, A.; Wegner, A.; Jouzel, J. Synchronous change of atmospheric CO2 and antarctic temperature during the last deglacial warming. Science 2013, 339, 1060–1063. [Google Scholar] [CrossRef] [PubMed]
- Boot-Handford, M.E.; Abanades, J.C.; Anthony, E.J.; Blunt, M.J.; Brandani, S.; Mac Dowell, N.; Fernández, J.R.; Ferrari, M.C.; Gross, R.; Hallett, J.P.; et al. Carbon capture and storage update. Energy Environ. Sci. 2014, 7, 130–189. [Google Scholar] [CrossRef]
- Dashti, H.; Zhehao Yew, L.; Lou, X. Recent advances in gas hydrate-based CO2 capture. J. Nat. Gas Sci. Eng. 2015, 23, 195–207. [Google Scholar] [CrossRef] [Green Version]
- Babu, P.; Linga, P.; Kumar, R.; Englezos, P. A review of the hydrate based gas separation (HBGS) process for carbon dioxide pre-combustion capture. Energy 2015, 85, 261–279. [Google Scholar] [CrossRef]
- Babu, P.; Kumar, R.; Linga, P. Pre-combustion capture of carbon dioxide in a fi xed bed reactor using the clathrate hydrate process. Energy 2013, 50, 364–373. [Google Scholar] [CrossRef]
- Wang, Y.; Lang, X.; Fan, S. Hydrate capture CO2 from shifted synthesis gas, flue gas and sour natural gas or biogas. J. Energy Chem. 2013, 22, 39–47. [Google Scholar] [CrossRef]
- Koide, H.; Takahashi, M.; Shindo, Y.; Tazaki, Y.; Iijima, M.; Ito, K.; Kimura, N.; Omata, K. Hydrate formation in sediments in the sub-seabed disposal of CO2. Energy 1997, 22, 279–283. [Google Scholar] [CrossRef]
- Koide, H.; Shindo, Y.; Tazaki, Y.; Iijima, M.; Ito, K.; Kimura, N.; Omata, K. Deep sub-seabed disposal of CO2: The most protective storage. Energy Convers. Manag. 1997, 38, 253–258. [Google Scholar] [CrossRef]
- Koide, H.; Takahashi, M.; Tsukamoto, H.; Shindo, Y. Self-trapping mechanisms of carbon dioxide in the aquifer disposal. Energy Convers. Manag. 1995, 36, 505–508. [Google Scholar] [CrossRef]
- Tohidi, B.; Yang, J.; Salehabadi, M.; Anderson, R.; Chapoy, A. CO2 hydrates could provide secondary safety factor in subsurface sequestration of CO2. Environ. Sci. Technol. 2010, 44, 1509–1514. [Google Scholar] [CrossRef] [PubMed]
- Kvamme, B.; Graue, A.; Buanes, T.; Kuznetsova, T.; Ersland, G. Storage of CO2 in natural gas hydrate reservoirs and the effect of hydrate as an extra sealing in cold aquifers. Int. J. Greenh. Gas Control 2007, 1, 236–246. [Google Scholar] [CrossRef]
- Zheng, J.; Chong, Z.R.; Qureshi, M.F.; Linga, P. Carbon Dioxide Sequestration via Gas Hydrates: A Potential Pathway toward Decarbonization. Energy Fuels 2020, 34, 10529–10546. [Google Scholar]
- Hermanrud, C.; Andresen, T.; Eiken, O.; Hansen, H.; Janbu, A.; Lippard, J.; Bolås, H.N.; Simmenes, T.H.; Teige, G.M.G.; Østmo, S. Storage of CO2 in saline aquifers–Lessons learned from 10 years of injection into the Utsira Formation in the Sleipner area. Energy Procedia 2009, 1, 1997–2004. [Google Scholar] [CrossRef] [Green Version]
- Eiken, O.; Ringrose, P.; Hermanrud, C.; Nazarian, B.; Torp, T.A.; Høier, L. Lessons Learned from 14 years of CCS Operations: Sleipner, In Salah and Snøhvit. Energy Procedia 2011, 4, 5541–5548. [Google Scholar] [CrossRef] [Green Version]
- Duong, C.; Bower, C.; Hume, K.; Rock, L.; Tessarolo, S. Quest carbon capture and storage offset project: Findings and learnings from 1st reporting period. Int. J. Greenh. Gas Control 2019, 89, 65–75. [Google Scholar] [CrossRef]
- Almenningen, S.; Betlem, P.; Hussain, A.; Roy, S.; Senger, K.; Ersland, G. Demonstrating the potential of CO2 hydrate self-sealing in Svalbard, Arctic Norway. Int. J. Greenh. Gas Control 2019, 89, 1–8. [Google Scholar] [CrossRef]
- Le Nindre, Y.M.; Allier, D.; Duchkov, A.; Altunina, L.K.; Shvartsev, S.; Zhelezniak, M.; Klerkx, J. Storing CO2 underneath the Siberian Permafrost: A win-win solution for long-term trapping of CO2 and heavy oil upgrading. Energy Procedia 2011, 4, 5414–5421. [Google Scholar] [CrossRef] [Green Version]
- Duchkov, A.D.; Permyakov, M.E.; Sokolova, L.S.; Ayunov, D.E. Assessment of possibility for carbon dioxide st orage in west siberian permafrost. Pet. Abstr. 2014, 55, 97. [Google Scholar]
- Qanbari, F.; Pooladi-Darvish, M.; Tabatabaie, S.H.; Gerami, S. CO2 disposal as hydrate in ocean sediments. J. Nat. Gas Sci. Eng. 2012, 8, 139–149. [Google Scholar] [CrossRef]
- Kvamme, B.; Aromada, S.A.; Saeidi, N. Heterogeneous and homogeneous hydrate nucleation in CO2/water systems. J. Cryst. Growth 2019, 522, 160–174. [Google Scholar] [CrossRef]
- Mu, L.; Von Solms, N. Methane Production and Carbon Capture by Hydrate Swapping. Energy Fuels 2017, 31, 3338–3347. [Google Scholar] [CrossRef]
- Kang, S.P.; Lee, H. Recovery of CO2 from flue gas using gas hydrate: Thermodynamic verification through phase equilibrium measurements. Environ. Sci. Technol. 2000, 34, 4397–4400. [Google Scholar] [CrossRef]
- Kang, S.P.; Lee, H.; Lee, C.S.; Sung, W.M. Hydrate phase equilibria of the guest mixtures containing CO2, N2 and tetrahydrofuran. Fluid Phase Equilib. 2001, 185, 101–109. [Google Scholar] [CrossRef]
- Ho, L.C.; Babu, P.; Kumar, R.; Linga, P. HBGS (hydrate based gas separation) process for carbon dioxide capture employing an unstirred reactor with cyclopentane. Energy 2013, 63, 252–259. [Google Scholar] [CrossRef]
- Tang, J.; Zeng, D.; Wang, C.; Chen, Y.; He, L.; Cai, N. Study on the influence of SDS and THF on hydrate-based gas separation performance. Chem. Eng. Res. Des. 2013, 91, 1777–1782. [Google Scholar] [CrossRef]
- Scott, M.J.; Jones, M.N. The biodegradation of surfactants in the environment. Biochim. Biophys. Acta Biomembr. 2000, 1508, 235–251. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharjee, G.; Sangwai, J.S.; Veluswamy, H.P.; Kumar, R.; Linga, P.; Pandey, G. Alleviation of Foam Formation in a Surfactant Driven Gas Hydrate System: Insights via a Detailed Morphological Study. ACS Appl. Energy Mater. 2018, 1, 6899–6911. [Google Scholar]
- Prasad, P.S.R.; Kiran, B.S. Are the amino acids thermodynamic inhibitors or kinetic promoters for carbon dioxide hydrates? J. Nat. Gas Sci. Eng. 2018, 52, 461–466. [Google Scholar] [CrossRef]
- Lal, B.; Mukhtar, H.; Bavoh, C.B.; Osei, H.; Sabil, K.M. A Review on the Role of Amino Acids in Gas Hydrate Inhibition, CO2 Capture and Sequestration, and Natural Gas Storage. J. Nat. Gas Sci. Eng. 2019, 64, 52–71. [Google Scholar]
- Kyte, J.; Doolittle, R.F. A simple method for displaying the hydropathic character of a protein. J. Mol. Biol. 1982, 157, 105–132. [Google Scholar] [CrossRef] [Green Version]
- Linga, P.; Daraboina, N.; Ripmeester, J.A.; Englezos, P. Enhanced rate of gas hydrate formation in a fixed bed column filled with sand compared to a stirred vessel. Chem. Eng. Sci. 2012, 68, 617–623. [Google Scholar] [CrossRef]
- Linga, P.; Kumar, R.; Englezos, P. The clathrate hydrate process for post and pre-combustion capture of carbon dioxide. J. Hazard. Mater. 2007, 149, 625–629. [Google Scholar] [CrossRef]
- Lee, W.; Kim, Y.S.; Kang, S.P. Semiclathrate-based CO2 capture from fuel gas in the presence of tetra-n-butyl ammonium bromide and silica gel pore structure. Chem. Eng. J. 2018, 331, 1–7. [Google Scholar] [CrossRef]
- Kumar, R.; Linga, P.; Englezos, P. Pre and Post Combustion Capture of Carbon Dioxide via Hydrate Formation. In Proceedings of the 2006 IEEE EIC Climate Change Conference, Ottawa, ON, Canada, 10–12 May 2006; 2006; pp. 1–7. [Google Scholar]
- Linga, P.; Kumar, R.; Englezos, P. Gas hydrate formation from hydrogen/carbon dioxide and nitrogen/carbon dioxide gas mixtures. Chem. Eng. Sci. 2007, 62, 4268–4276. [Google Scholar] [CrossRef]
- Duc, N.H.; Chauvy, F.; Herri, J.M. CO2 capture by hydrate crystallization—A potential solution for gas emission of steelmaking industry. Energy Convers. Manag. 2007, 48, 1313–1322. [Google Scholar] [CrossRef]
- Daraboina, N.; Ripmeester, J.; Englezos, P. The impact of SO2 on post combustion carbon dioxide capture in bed of silica sand through hydrate formation. Int. J. Greenh. Gas Control 2013, 15, 97–103. [Google Scholar] [CrossRef]
- Kumar, A.; Sakpal, T.; Linga, P.; Kumar, R. Impact of fly ash impurity on the hydrate-based gas separation process for carbon dioxide capture from a flue gas mixture. Ind. Eng. Chem. Res. 2014, 53, 9849–9859. [Google Scholar] [CrossRef]
- Arora, A.; Kumar, A.; Bhattacharjee, G.; Balomajumder, C.; Kumar, P. Hydrate-Based Carbon Capture Process: Assessment of Various Packed Bed Systems for Boosted Kinetics of Hydrate Formation. J. Energy Resour. Technol. 2020, 143, 1–7. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, F.; Lipiński, W. Research progress and challenges in hydrate-based carbon dioxide capture applications. Appl. Energy 2020, 269, 114928. [Google Scholar]
- Pan, Z.; Liu, Z.; Zhang, Z.; Shang, L.; Ma, S. Effect of silica sand size and saturation on methane hydrate formation in the presence of SDS. J. Nat. Gas Sci. Eng. 2018, 56, 266–280. [Google Scholar]
- Daraboina, N.; Von Solms, N. The combined effect of thermodynamic promoters tetrahydrofuran and cyclopentane on the kinetics of flue gas hydrate formation. J. Chem. Eng. Data 2015, 60, 247–251. [Google Scholar] [CrossRef]
- Pandey, J.S.; Daas, Y.J.; Von Solms, N. Insights into Kinetics of Methane Hydrate Formation in the Presence of Surfactants. Processes 2019, 7, 598. [Google Scholar] [CrossRef] [Green Version]
- Pandey, J.S.; Daas, Y.J.; Von Solms, N. Screening of Amino Acids and Surfactant as Hydrate Promoter for CO2 Capture from Flue Gas. Processes 2020, 8, 124. [Google Scholar] [CrossRef] [Green Version]
- Berge, L.I.; Jacobsen, K.A.; Solstad, A. Measured acoustic wave velocities of R11 (CCl3F) hydrate samples with and without sand as a function of hydrate concentration. J. Geophys. Res. Solid Earth 1999, 104, 15415–15424. [Google Scholar] [CrossRef]
- Kumar, A.; Maini, B.; Bishnoi, P.R.; Clarke, M.; Zatsepina, O.; Srinivasan, S. Experimental determination of permeability in the presence of hydrates and its effect on the dissociation characteristics of gas hydrates in porous media. J. Pet. Sci. Eng. 2010, 70, 114–122. [Google Scholar]
- Gauteplass, J.; Almenningen, S.; Ersland, G.; Barth, T. Hydrate seal formation during laboratory CO2 injection in a cold aquifer. Int. J. Greenh. Gas Control 2018, 78, 21–26. [Google Scholar] [CrossRef]
- Ta, X.H.; Yun, T.S.; Muhunthan, B.; Kwon, T.-H. Observations of pore-scale growth patterns of carbon dioxide hydrate using X-ray computed microtomography. Geochem. Geophys. Geosyst. 2015, 16, 267–300. [Google Scholar]
- Lone, A.; Kelland, M.A. Exploring kinetic hydrate inhibitor test methods and conditions using a multicell steel rocker rig. Energy Fuels 2013, 27, 2536–2547. [Google Scholar] [CrossRef]
- Kelland, M.A.; Abrahamsen, E.; Ajiro, H.; Akashi, M. Kinetic Hydrate Inhibition with N-Alkyl-N-vinylformamide Polymers: Comparison of Polymers to n-Propyl and Isopropyl Groups. Energy Fuels 2015, 29, 4941–4946. [Google Scholar] [CrossRef]
- Molokitina, N.S.; Nesterov, A.N.; Podenko, L.S.; Reshetnikov, A.M. Carbon dioxide hydrate formation with SDS: Further insights into mechanism of gas hydrate growth in the presence of surfactant. Fuel 2019, 235, 1400–1411. [Google Scholar] [CrossRef]
- Gayet, P.; Dicharry, C.; Marion, G.; Graciaa, A.; Lachaise, J.; Nesterov, A. Experimental determination of methane hydrate dissociation curve up to 55 MPa by using a small amount of surfactant as hydrate promoter. Chem. Eng. Sci. 2005, 60, 5751–5758. [Google Scholar] [CrossRef]
- Hashimoto, H.; Yamaguchi, T.; Ozeki, H.; Muromachi, S. Structure-driven CO2 selectivity and gas capacity of ionic clathrate hydrates. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef]
- Rodriguez, C.T.; Le, Q.D.; Focsa, C.; Pirim, C.; Chazallon, B. Influence of crystallization parameters on guest selectivity and structures in a CO2-based separation process using TBAB semi-clathrate hydrates. Chem. Eng. J. 2020, 382, 122867. [Google Scholar] [CrossRef]
- Maeda, N. Nucleation Curve of Carbon Dioxide Hydrate from a Linear Cooling Ramp Method. J. Phys. Chem. A 2019, 123, 7911–7919. [Google Scholar] [CrossRef]
- Bagherzadeh, S.A.; Moudrakovski, I.L.; Ripmeester, J.A.; Englezos, P. Magnetic resonance imaging of gas hydrate formation in a bed of silica sand particles. Energy Fuels 2011, 25, 3083–3092. [Google Scholar] [CrossRef]
- Sa, J.H.; Kwak, G.H.; Han, K.; Ahn, D.; Lee, K.H. Gas hydrate inhibition by perturbation of liquid water structure. Sci. Rep. 2015, 5, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Sadeq, D.; Iglauer, S.; Lebedev, M.; Rahman, T.; Zhang, Y.; Barifcani, A. Experimental pore-scale analysis of carbon dioxide hydrate in sandstone via X-Ray micro-computed tomography. Int. J. Greenh. Gas Control 2018, 79, 73–82. [Google Scholar] [CrossRef]
- Almenningen, S.; Gauteplass, J.; Fotland, P.; Aastveit, G.L.; Barth, T.; Ersland, G. Visualization of hydrate formation during CO2 storage in water-saturated sandstone. Int. J. Greenh. Gas Control 2018, 79, 272–278. [Google Scholar] [CrossRef]
- Oya, S.; Aifaa, M.; Ohmura, R. Formation, growth and sintering of CO2 hydrate crystals in liquid water with continuous CO2 supply: Implication for subsurface CO2 sequestration. Int. J. Greenh. Gas Control 2017, 63, 386–391. [Google Scholar] [CrossRef]
- Kvamme, B. Feasibility of simultaneous CO2 storage and CH4 production from natural gas hydrate using mixtures of CO2 and N2. WSEAS Trans. Heat Mass Transf. 2015, 10, 21–30. [Google Scholar] [CrossRef]
- Singh, H.; Myshakin, E.M.; Seol, Y. A nonempirical relative permeability model for hydrate-bearing sediments. SPE J. 2019, 24, 547–562. [Google Scholar] [CrossRef]
- Yang, M.; Song, Y.; Jiang, L.; Zhu, N.; Liu, Y.; Zhao, Y.; Dou, B.; Li, Q. CO2 hydrate formation and dissociation in cooled porous media: A potential technology for CO2 capture and storage. Environ. Sci. Technol. 2013, 47, 9739–9746. [Google Scholar] [CrossRef]
- Massah, M.; Sun, D.; Sharifi, H.; Englezos, P. Demonstration of gas-hydrate assisted carbon dioxide storage through horizontal injection in lab-scale reservoir. J. Chem. Thermodyn. 2018, 117, 106–112. [Google Scholar] [CrossRef]
- Mekala, P.; Busch, M.; Mech, D.; Patel, R.S.; Sangwai, J.S. Effect of silica sand size on the formation kinetics of CO2hydrate in porous media in the presence of pure water and seawater relevant for CO2sequestration. J. Pet. Sci. Eng. 2014, 122, 1–9. [Google Scholar] [CrossRef]
- Malagar, B.R.C.; Lijith, K.P.P.; Singh, D.N.N. Formation & dissociation of methane gas hydrates in sediments: A critical review. J. Nat. Gas Sci. Eng. 2019, 65, 168–184. [Google Scholar]
- Liu, Z.; Sun, B.; Wang, Z.; Chen, L. New Mass-Transfer Model for Predicting Hydrate Film Thickness at the Gas-Liquid Interface under Different Thermodynamics-Hydrodynamics-Saturation Conditions. J. Phys. Chem. C 2019, 123, 20838–20852. [Google Scholar] [CrossRef]
- Jung, J.W.; Espinoza, D.N.; Santamarina, J.C. Properties and phenomena relevant to CH4-CO2 replacement in hydrate-bearing sediments. J. Geophys. Res. Solid Earth 2010, 115, 1–16. [Google Scholar] [CrossRef]
- Bhattacharjee, G.; Kumar, A.; Sakpal, T.; Kumar, R. Carbon dioxide sequestration: Influence of porous media on hydrate formation kinetics. ACS Sustain. Chem. Eng. 2015, 3, 1205–1214. [Google Scholar] [CrossRef]
- Pandey, J.S.; Daas, Y.J.; Von Solms, N. Methane Hydrate Formation, Storage and Dissociation Behavior inUnconsolidated Sediments in the Presence of Environment-FriendlyPromoters. In Proceedings of the SPE Europec featured at 82nd EAGE Conference and Exhibition, Amsterdam, The Netherlands, 8–11 December 2020. [Google Scholar]
- Ohno, H.; Susilo, R.; Gordienko, R.; Ripmeester, J.; Walker, V.K. Interaction of antifreeze proteins with hydrocarbon hydrates. Chem. A Eur. J. 2010, 16, 10409–10417. [Google Scholar] [CrossRef] [PubMed]
- Pivezhani, F.; Roosta, H.; Dashti, A.; Mazloumi, S.H. Investigation of CO2 hydrate formation conditions for determining the optimum CO2 storage rate and energy: Modeling and experimental study. Energy 2016, 113, 215–226. [Google Scholar] [CrossRef]
- Adeyemo, A.; Kumar, R.; Linga, P.; Ripmeester, J.; Englezos, P. Capture of carbon dioxide from flue or fuel gas mixtures by clathrate crystallization in a silica gel column. Int. J. Greenh. Gas Control 2010, 4, 478–485. [Google Scholar] [CrossRef] [Green Version]
- Linga, P.; Adeyemo, A.; Englezos, P. Medium-pressure clathrate hydrate/membrane hybrid process for postcombustion capture of carbon dioxide. Environ. Sci. Technol. 2008, 42, 315–320. [Google Scholar] [CrossRef]
- Mu, D.; Liu, Z.S.; Huang, C.; Djilali, N. Determination of the effective diffusion coefficient in porous media including Knudsen effects. Microfluid. Nanofluidics 2008, 4, 257–260. [Google Scholar] [CrossRef]
- Sun, S.C.; Liu, C.L.; Ye, Y.G.; Liu, Y.F. Phase behavior of methane hydrate in silica sand. J. Chem. Thermodyn. 2014, 69, 118–124. [Google Scholar] [CrossRef]
- Every, D. Pore structure of silica membranes. Membr. Technol. 1999, 1999, 16. [Google Scholar]
- Yang, M.; Song, Y.; Jiang, L.; Zhao, Y.; Ruan, X.; Zhang, Y.; Wang, S. Hydrate-based technology for CO2 capture from fossil fuel power plants. Appl. Energy 2014, 116, 26–40. [Google Scholar] [CrossRef]
- Lirio da Silva, C.F.; Pessoa, F.L.P.; Uller, A.M.C. torage capacity of carbon dioxide hydrates in the presence of sodium dodecyl sulfate (SDS) and tetrahydrofuran (THF). Chem. Eng. Sci. 2013, 96, 118–123. [Google Scholar]
- Mech, D.; Gupta, P.; Sangwai, J.S. Kinetics of methane hydrate formation in an aqueous solution of thermodynamic promoters (THF and TBAB) with and without kinetic promoter (SDS). J. Nat. Gas Sci. Eng. 2016, 35, 1519–1534. [Google Scholar] [CrossRef]
- Seo, Y.T.; Moudrakovski, I.L.; Ripmeester, J.A.; Lee, J.W.; Lee, H. Efficient recovery of CO2 from flue gas by clathrate hydrate formation in porous silica gels. Environ. Sci. Technol. 2005, 39, 2315–2319. [Google Scholar] [CrossRef] [Green Version]
- Park, J.; Seo, Y.T.; Lee, J.W.; Lee, H.; Lee, H. Spectroscopic analysis of carbon dioxide and nitrogen mixed gas hydrates in silica gel for CO2 separation. Catal. Today 2006, 115, 279–282. [Google Scholar] [CrossRef]
- Hassanpouryouzband, A.; Yang, J.; Tohidi, B.; Chuvilin, E.; Istomin, V.; Bukhanov, B. Geological CO2 Capture and Storage with Flue Gas Hydrate Formation in Frozen and Unfrozen Sediments: Method Development, Real Time-Scale Kinetic Characteristics, Efficiency, and Clathrate Structural Transition. ACS Sustain. Chem. Eng. 2019, 7, 5338–5345. [Google Scholar] [CrossRef]
- Cai, Y.; Chen, Y.; Li, Q.; Li, L.; Huang, H.; Wang, S.; Wang, W. CO2 Hydrate Formation Promoted by a Natural Amino Acid l-Methionine for Possible Application to CO2 Capture and Storage. Energy Technol. 2017, 5, 1195–1199. [Google Scholar] [CrossRef]
- Sa, J.H.; Kwak, G.H.; Lee, B.R.; Park, D.H.; Han, K.; Lee, K.H. Hydrophobic amino acids as a new class of kinetic inhibitors for gas hydrate formation. Sci. Rep. 2013, 3, 1–7. [Google Scholar] [CrossRef]
- Alireza Bagherzadeh, S.; Alavi, S.; Ripmeester, J.A.; Englezos, P. Why ice-binding type i antifreeze protein acts as a gas hydrate crystal inhibitor. Phys. Chem. Chem. Phys. 2015, 17, 9984–9990. [Google Scholar] [CrossRef]
- Nasir, Q.; Suleman, H.; Elsheikh, Y.A. A review on the role and impact of various additives as promoters/ inhibitors for gas hydrate formation. J. Nat. Gas Sci. Eng. 2020, 76, 103211. [Google Scholar] [CrossRef]
- Ide, M.; Maeda, Y.; Kitano, H. Effect of hydrophobicity of amino acids on the structure of water. J. Phys. Chem. B 1997, 101, 7022–7026. [Google Scholar] [CrossRef]
- Bavoh, C.B.; Nashed, O.; Khan, M.S.; Partoon, B.; Lal, B.; Sharif, A.M. The impact of amino acids on methane hydrate phase boundary and formation kinetics. J. Chem. Thermodyn. 2018, 117, 48–53. [Google Scholar] [CrossRef]
- Perfeldt, C.M.; Chua, P.C.; Daraboina, N.; Friis, D.; Kristiansen, E.; Ramløv, H.; Woodley, J.M.; Kelland, M.A.; Von Solms, N. Inhibition of gas hydrate nucleation and growth: Efficacy of an antifreeze protein from the longhorn beetle rhagium mordax. Energy Fuels 2014, 28, 3666–3672. [Google Scholar] [CrossRef]
- Roosta, H.; Dashti, A.; Mazloumi, S.H.; Varaminian, F. Inhibition properties of new amino acids for prevention of hydrate formation in carbon dioxide-water system: Experimental and modeling investigations. J. Mol. Liq. 2016, 215, 656–663. [Google Scholar] [CrossRef]
- Veluswamy, H.P.; Lee, P.Y.; Premasinghe, K.; Linga, P. Effect of Biofriendly Amino Acids on the Kinetics of Methane Hydrate Formation and Dissociation. Ind. Eng. Chem. Res. 2017, 56, 6145–6154. [Google Scholar] [CrossRef]
- Bavoh, C.B.; Partoon, B.; Lal, B.; Gonfa, G.; Foo Khor, S.; Sharif, A.M. Inhibition effect of amino acids on carbon dioxide hydrate. Chem. Eng. Sci. 2017, 171, 331–339. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, B.; Chen, Y.; Zhang, S.; Guo, W.; Cai, Y.; Tan, B.; Wang, W. Methane Storage in a Hydrated Form as Promoted by Leucines for Possible Application to Natural Gas Transportation and Storage. Energy Technol. 2015, 3, 815–819. [Google Scholar] [CrossRef]
- Bavoh, C.B.; Khan, M.S.; Lal, B.; Bt Abdul Ghaniri, N.I.; Sabil, K.M. New methane hydrate phase boundary data in the presence of aqueous amino acids. Fluid Phase Equilib. 2018, 478, 129–133. [Google Scholar] [CrossRef]
- Li, A.; Wang, J.; Bao, B. High-efficiency CO2 capture and separation based on hydrate technology: A review. Greenh. Gases Sci. Technol. 2019, 9, 175–193. [Google Scholar] [CrossRef]
- Clennell, M.B.; Henry, P.; Hovland, M.; Booth, J.S.; Winters, W.J.; Thomas, M. Formation of Natural Gas Hydrates in Marine Sediments: Gas Hydrate Growth and Stability Conditioned by Host Sediment Properties. Ann. N. Y. Acad. Sci. 2006, 912, 887–896. [Google Scholar] [CrossRef]


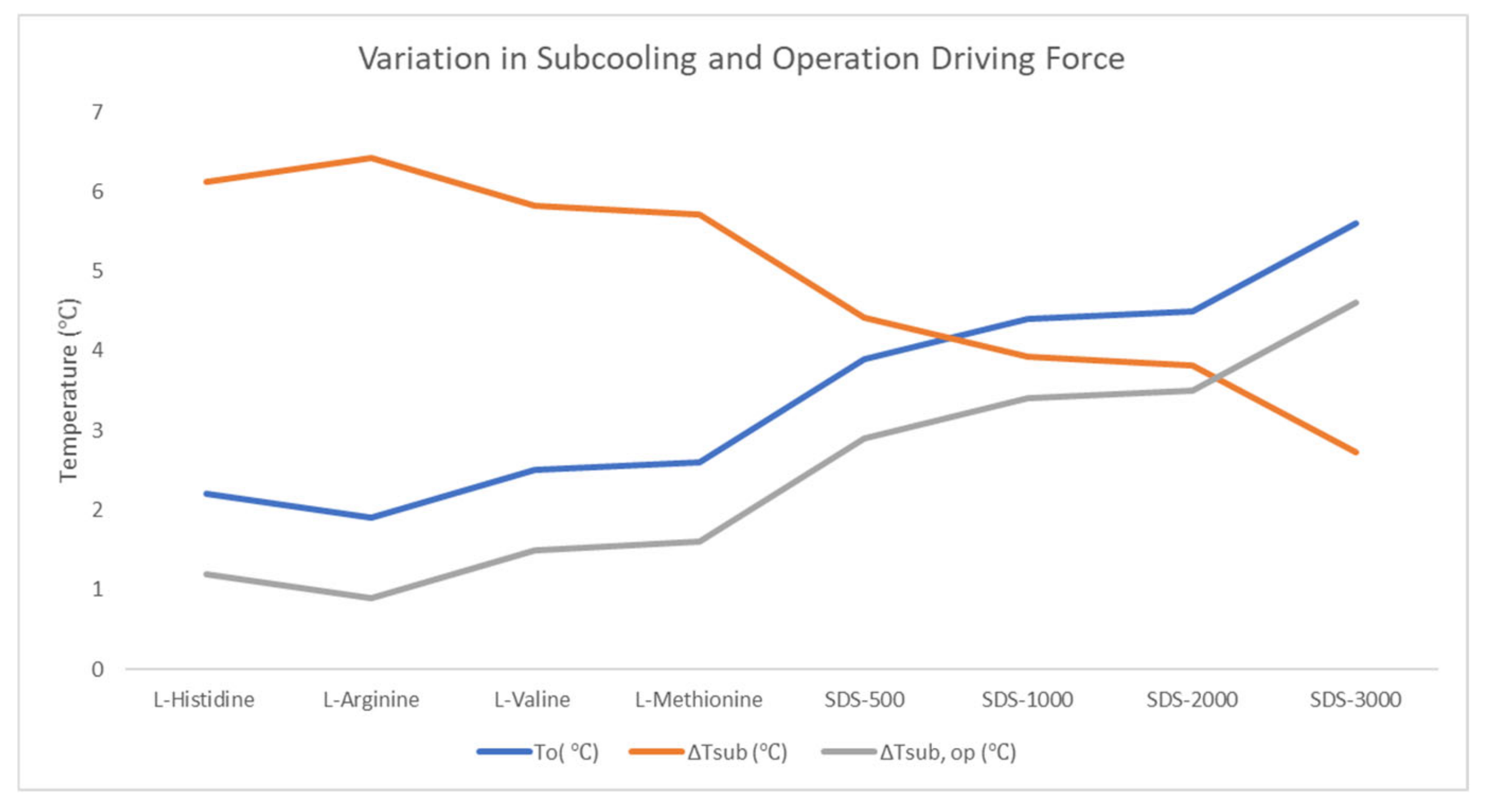








| To (°C) | ΔTsub (°C) | ΔTsub, op (°C) | |
|---|---|---|---|
| L-Valine | 2.5 | 5.82 | 1.5 |
| L-Methionine | 2.6 | 5.72 | 1.6 |
| L-Histidine | 2.2 | 6.12 | 1.2 |
| L-Arginine | 1.9 | 6.42 | 0.9 |
| SDS-500 | 3.9 | 4.42 | 2.9 |
| SDS-1000 | 4.4 | 3.92 | 3.4 |
| SDS-2000 | 4.5 | 3.82 | 3.5 |
| SDS-3000 | 5.6 | 2.72 | 4.6 |
| Induction Time (to) (in Mins) | |||||
|---|---|---|---|---|---|
| 20 mol% CO2 and 80 mol% N2 | 30 mol% CO2 and 70 mol% N2 | ||||
| Component | Description | Sand A | Sand B | Sand A | Sand B |
| Amino Acid | Valine | 114 | 115 | 80 | 37 |
| Methionine | 156 | 58 | 157 | 15 | |
| Histidine | N/A | N/A | N/A | N/A | |
| Arginine | N/A | N/A | N/A | N/A | |
| SDS | SDS-500 | 243 | N/A | N/A | N/A |
| SDS-1000 | 125 | 14 | 170 | 49 | |
| SDS-2000 | 114 | 10 | 120 | 32 | |
| SDS-3000 | 89 | 7 | 89 | 9 | |
| Peq (Gas A) | 56.84 | (bars) | Pi-Peq (Gas A) | 63.16 | (bar) | ||
| Pi | 120 (bars) | Peq (Gas B) | 41.45 | (bar) | Pi-Peq (Gas B) | 78.55 | (bar) |
| 0.11 | ΔP (bar) | Δfeedgas × 10−3 | ΔCO2 × 10−3 | ΔP(bar) | Δfeedgas × 10−3 | ΔCO2 × 10−3 | |
| For Gas A | 20% CO2-Sand A | 20% CO2-Sand B | |||||
| Water | 4.1 | 9.04 | 9.01 | 4.7 | 10.2 | 10.0 | |
| SDS-500 | 20.2 | 23.8 | 14.1 | 7.2 | 10.6 | 9.03 | |
| SDS-1000 | 20.8 | 26.1 | 18.9 | 21.1 | 24.7 | 14.6 | |
| SDS-2000 | 20.6 | 25.2 | 16.8 | 20.9 | 24.5 | 14.3 | |
| SDS-3000 | 21.0 | 25.3 | 16.2 | 20.8 | 24.9 | 15.5 | |
| Valine | 16.9 | 20.6 | 13.1 | 15.1 | 19.1 | 13.3 | |
| Methionine | 18.7 | 22.4 | 13.9 | 20.0 | 23.7 | 14.3 | |
| Histidine | 4.6 | 7.55 | 7.36 | 4.3 | 7.62 | 7.52 | |
| Arginine | 4.9 | 7.74 | 7.33 | 5.2 | 8.31 | 7.89 | |
| 30% CO2-Sand A | 30% CO2-Sand B | ||||||
| Water | 5.2 | 4.77 | 4.71 | 4.8 | 5.49 | 5.31 | |
| SDS-500 | 3.7 | 13.2 | 11.1 | 6.3 | 3.75 | 3.45 | |
| SDS-1000 | 27.8 | 33.0 | 23.7 | 29.0 | 30.5 | 23.3 | |
| SDS-2000 | 28.9 | 32.6 | 29.1 | 28.9 | 30.7 | 23.9 | |
| SDS-3000 | 28.7 | 33.1 | 31.1 | 29.3 | 31.9 | 26.2 | |
| Valine | 26.9 | 32.1 | 31.5 | 28.4 | 30.5 | 24.7 | |
| Methionine | 27.0 | 30.9 | 28.9 | 27.3 | 30.2 | 26.2 | |
| Histidine | 3.8 | 12.4 | 10.2 | 5.5 | 7.6 | 7.19 | |
| Arginine | 6.7 | 8.5 | 7.9 | 6.0 | 9.9 | 8.2 | |
| Additive | 20% CO2, Sand A | 20% CO2, Sand B | 30% CO2, Sand A | 30% CO2, Sand B |
|---|---|---|---|---|
| None | 0.50 | 0.55 | 0.37 | 0.43 |
| SDS, 500 ppm | 0.65 | 0.41 | 0.35 | 0.26 |
| SDS, 1000 ppm | 0.85 | 0.65 | 0.58 | 0.73 |
| SDS, 2000 ppm | 0.77 | 0.65 | 0.88 | 0.75 |
| SDS, 3000 ppm | 0.73 | 0.69 | 0.93 | 0.76 |
| Valine | 0.59 | 0.59 | 0.93 | 0.76 |
| Methionine | 0.64 | 0.65 | 0.88 | 0.78 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pandey, J.S.; Daas, Y.J.; Karcz, A.P.; von Solms, N. Enhanced Hydrate-Based Geological CO2 Capture and Sequestration as a Mitigation Strategy to Address Climate Change. Energies 2020, 13, 5661. https://doi.org/10.3390/en13215661
Pandey JS, Daas YJ, Karcz AP, von Solms N. Enhanced Hydrate-Based Geological CO2 Capture and Sequestration as a Mitigation Strategy to Address Climate Change. Energies. 2020; 13(21):5661. https://doi.org/10.3390/en13215661
Chicago/Turabian StylePandey, Jyoti Shanker, Yousef Jouljamal Daas, Adam Paul Karcz, and Nicolas von Solms. 2020. "Enhanced Hydrate-Based Geological CO2 Capture and Sequestration as a Mitigation Strategy to Address Climate Change" Energies 13, no. 21: 5661. https://doi.org/10.3390/en13215661