Abstract
A logical-informational model of energy resource-efficient chemical technology for the utilization of hydrogen sulfide and low molecular alkanethiols, which are toxic and difficult to remove sulfur components of residual fuel (fuel oil), is proposed. Based on the IDEF1 methodology and existing knowledge about the technological processes of the demercaptanization of various hydrocarbon raw materials (oils, gas condensates), a scheme for the production of organic sulfur compounds from sulfur waste extracted from fuel oil has been modeled. For a sufficiently complete removal of hydrogen sulfide and low molecular weight alkanethiols, energy- and resource-saving stages of the technological process have been developed, which are implemented by ultrasonic and/or magnetic treatment of fuel oil. It is proposed to use the combined action of two alternative methods of processing fuel oil to increase the efficiency of cleaning fuel oil from sulfur components. For the first time, an approach has been developed to utilize unwanted sulfuric impurities contained in fuel oil by involving electric and microwave synthesis in green technological processes, to obtain practically useful organic sulfur compounds with biological activity. It is shown that the use of one-electron oxidant thiols and hydrogen sulfide in organic media leads to the synthesis of organic disulfides and elemental sulfur. Indirect (with the use of mediators) electrosynthesis contributes to the cyclic conduct of the technological process, an increase in efficiency and a decrease in energy consumption compared to the direct (on electrodes) initiation of sulfur components.
1. Introduction
A significant increase in environmental pollution, negative climate change due to the disruption of the heat balance in the atmosphere and the unstable situation in the global energy market form number of serious factors that give rise to the need to improve energy efficiency in the global economy. The efficient use of fuel and energy resources in the industry through the introduction of modern versatile energy-saving techniques and technological processes forms the basis of a modern energy strategy. The introduction of energy-saving technologies allows us to reduce the volume of the production of energy saving by an amount equivalent to the volume of saved energy. Often, this approach to energy conservation is even more cost-efficient and environmentally friendly than the creation of new energy facilities to meet the growing human needs for energy [1,2].
Currently, there is a noticeable increase in the production and consumption of energy in the world, which creates favorable conditions for accelerating scientific and technological progress and improving the well-being of society. At the same time, the increase in energy consumption is directly related to the growth in the development of non-renewable energy sources, and, first of all, to the consumption of hydrocarbon raw materials, which constitute the fuel basis of modern energy, despite the limited reserves. It follows that, along with the introduction of energy-saving technologies, an important direction for solving energy problems is the use of alternative sources of fossil fuels, as well as renewable energy sources (solar, geothermal, wind, biomass and ocean energy) [3].
Energy-saving technologies are used in all stages of the energy life cycle, from the stage of extracting fossil fuel from the Earth and its preparation, converting the chemical energy of the fuel into electrical energy through mechanical energy (or directly into electrical energy), and up to the stage of energy consumption. The success of the implementation of energy- and resource-efficient technologies at each of these stages depends on their perfection, which determines the technical and economic potential and ways of implementation [4,5].
For the efficient and rational use of fuel and energy resources, along with energy-saving technologies, great importance is attached to the implementation of legal, organizational, scientific, industrial, technical and economic measures, and environmental safety measures. In many cases, these areas of energy conservation are interconnected, and their joint application allows one to achieve better results than separately. So, the environmental requirement of minimizing the amount of production waste causes a decrease in energy costs for their neutralization, increasing the efficiency and the level of resource conservation of modern technologies. The use of more advanced technologies for the production of industrial products helps in reducing the number of stages of production, units of production equipment and direct energy operating costs. The technical requirement for reducing by-products formed in production and reducing the concentration of undesirable impurities in the mixture of target compounds contributes to the solution of environmental problems, and contributes to the reduction of energy costs associated with the high-quality cleaning of finished products [6].
Indeed, at present, along with the problem of energy conservation, the problem of developing and implementing resource-saving (low-and waste-free) and environmentally efficient technologies in industrial enterprises is becoming increasingly important. Solving these problems is considered by states as a strategic direction that ensures the rational use of natural resources, environmental protection and production of products with the minimum possible consumption of fuel, raw materials, materials, air, water, and other material resources. Resource-saving technologies include the use of secondary resources and waste utilization, as well as energy recovery through the use of cyclic technological processes and a closed system, which saves natural resources and significantly reduces environmental pollution [7].
In recent years, the oil and gas industry has developed and successfully applied a number of advanced technological processes that increase energy and resource-saving production efficiency. Some of these processes for the extraction of unwanted, often harmful and hazardous, components from various hydrocarbon fractions, with subsequent utilization in the synthesis of practically useful compounds, are energetically low-cost. The emergence of such processes is caused by the tightening of regulatory requirements for the environmental characteristics of petroleum products, which has happened due to the need to compensate for the increasing load on the environment from human activities and the rate of industrial growth [8].
This situation can be convincingly illustrated by the example of hydrogen sulfide, which is known to be a toxic and corrosive substance, the content of which in the residual products of the secondary processing of high-sulfur raw materials often exceeds the established regulatory requirements [9,10]. For example, in the process of atmospheric distillation of high-sulfur gas condensate, the residue of atmospheric distillation (straight-run fuel oil) is obtained, containing the sulfur waste hydrogen sulfide and thiols, which are used as the main components of commercial fuel oil for heating. Among the known methods, the most widely used methods for reducing the content of sulfurous waste in fuel oil are the following: blowing off with inert or hydrocarbon gases, the use of chemical absorbers, or ozonolysis [11,12]. Chemical reagents, which are used as various organic and inorganic compounds, are widely used to solve the problem of neutralizing hydrogen sulfide [13]. At the same time, a critical analysis of the global experience in the field of technologies for the removal or neutralization of hydrogen sulfide (low molecular weight thiols) from residual fuel oil in industry has made it possible to establish that their significant consumption and toxicity, and the high cost of absorbers, are their significant disadvantages. Moreover, physical processes (stripping, stripping of hydrogen sulfide with air or inert gas) require additional energy costs.
For the compliance of the quality of fuel oil with the regulatory requirements in terms of the content of hydrogen sulfide and low molecular weight thiols, it is necessary to develop new effective methods for their removal. Recently, reagent-free low-energy wave effects have been used to improve the quality of oil products, as well as to increase the efficiency of oil preparation and processing. For these aims the magnetic and ultrasonic processing of hydrocarbon raw materials are increasingly used [14].
For the ultra-deep desulfurization of fuels, the oxidative processes of the conversion of sulfur compounds into sulfones are intensified using ultrasound. Ultrasonic methods were developed to reduce the content of sulfur compounds in oil, mixtures of oil products, residual fuels and oil waste [15,16]. Electromagnetic fields allow us to intensify various oil refining processes. With the atmospheric distillation of gas condensate, pretreated in a magnetic field, the quality of products increases due to a decrease in the yield of light distillates, an increase in the octane number of the gasoline fraction, a decrease in the viscosity of the diesel fraction and the ability of the residue to form form coke [17,18,19].
Earlier, we first proposed a method of fuel oil desulfurization based on the effect of ultrasound and a magnetic fields on residual fuel oil. It was found that a combination of two types of treatment is the most effective way to remove sulfurous waste from fuel oil. Varying the frequency of ultrasound, the magnitude of the magnetic induction and the duration of the experiment made it possible to select the optimal conditions for the process of desulfurization of the oil dispersed system [20].
Modern technologies are considered green as they cover various aspects that help to reduce the human impact on the environment, and create ways of sustainable development through the use of new and innovative production methods [21,22,23]. The use of sulfur waste in technological processes (production of technical gas sulfur from hydrogen sulfide by the Claus method, alkyldisulfides during demercaptanization of petroleum fractions) as a feedstock for the petrochemical synthesis of practically significant organic sulfur derivatives corresponds to the concept of Green Chemistry. Electrochemical and microwave synthesis methods are environmentally friendly, significantly reduce energy costs, and use renewable reagents and less toxic solvents [24,25]. Organic electrosynthesis is characterized by the ability to regulate the composition and yield of reaction products, which is achieved by varying the values of the potential (current) and leads to the selective activation of a certain functional group or fragment of a molecule that are capable of redox transformations. The technical attractiveness of the microwave activation of substances is due to a significant reduction in the reaction time (by a factor of 10–100) and a significant increase in the yield of reaction products.
These two modern synthetic approaches (initiation of reactions using electric current and microwave irradiation) are referred to as express processes, and they also determine the energy saving of certain technology by using electrolyzers and microwave reactors [26,27,28,29]. They are profitable due to a significant increase in the efficiency of the corresponding technology by reducing the cost of labor, time and materials, thus improving the quality and functional properties of the final products. Consequently, the electric and microwave activation of hydrogen sulfide and thiols, based on the principles of Green Chemistry, is advisable for use in the technology of producing organic sulfur derivatives under relatively mild conditions [30].
Hydrogen sulfide and low molecular weight thiols can act as effective and available sulfurous reagents for sulfiding organic compounds, which has showbn potential in petrochemical synthesis. It is advisable to involve alicyclic and aromatic hydrocarbons, as well as their condensed analogues, which are part of hydrocarbon feedstock, in the synthesis of practically useful organic sulfur derivatives. These compounds are widely used in agriculture and chemical industry as additives to motor oils and fuels, stabilizers of rubbers, plastics and polymers, constituents of pesticides and herbicides, bleaching agents and food flavorings. However, biologically active sulfur-containing organic compounds are of particular interest for the production of medical drugs with a wide spectrum of action [31,32].
Thus, the development and application of energy- and resource-saving technologies with a high efficiency of use of fuel and energy resources, due to the attractiveness of alternative methods of energy production (ultrasound, magnetic treatment, electric current, microwaves), is undoubtedly an area of research of great interest. The implementation in industry of a new and efficient production of organic sulfur compounds based on toxic sulfur impurities and hydrocarbons is preceded by the choice of a rational technical solution, which is made possible by obtaining scientifically based experimental results and the engineering of the proposed chemical–technological process. Modern methodologies and computer tools for information modeling (IDEF1) will make it possible to develop and build a logical-informational model, which is the first stage in studying the principles of the production system for the subsequent creation of energy resource-efficient chemical technology [33,34,35].
2. Experimental Methods and Materials
2.1. Ultrasonic and Magnetic Treatment of Fuel Oil
Low-energy wave actions were carried out using a constant magnetic field (induction 0.1–0.4 Tl, speed 0.1–0.4 m/s) and ultrasound (oscillation frequency 50 kHz, power 125 W) on a flow-through installation. The treatment with a magnetic field was carried out in a magnetic tunnel [20], and that with ultrasonic on laboratory apparatus of the Luk-0.125/50-O and Volna UZTA-0.4/22-014 models (LLC Center of Ultrasonic Technologies, Biysk, Russia).
The concentration of hydrogen sulfide (alkanethiols) in the starting fuel oil was preliminarily determined by potentiometric titration. Then, the change in the content of sulfur components in the fuel oil was recorded when passing through a flow-through installation using wave effects. In an independent experiment, it was found that when the studied fuel oil passes through the installation (without wave effects), the loss of hydrogen sulfide is 11% of the mass, relative to the initial concentration of H2S. The content of hydrogen sulfide in fuel oil was determined according to the Interstate standard P 53716-2009, and the concentration of thiols was fixed by the method of cyclic voltammetry after extraction with organic solvents. The average particle size of the dispersed phase of the oil system (fuel oil) was determined by the photoelectrocolorimetric method.
2.2. Electrosynthesis of Organic Sulfur Compounds
The supporting electrolyte 0.15 M n-Bu4NClO4 (99%, Acros) was twice recrystallized from EtOH and dried in a vacuum (48 h) at 50 °C. The purification of methylene chloride (chemically pure) was carried out according to the well-known method [36]. Commercial reagents cycloalkanes, and their condensed analogs ethanethiol (98%, Aldrich) and hexane (95%, Alfa Aesar), were used without additional purification. Hydrogen sulfide was obtained according to the technique from [37].
Electrochemical studies done by the method of cyclic voltammetry under electrolysis conditions and the recording of cyclic voltammograms (CV) were performed using an IPC Pro (Russia) potentiostat according to a well-known technique [38]. Working electrode—stationary disk Pt-electrode (d = 2 mm) or glassy carbon electrode; auxiliary—Pt-electrode (plate, S = 70 mm2); reference electrode—Ag/AgCl (KCl sat.) with watertight diaphragm. The scan rate was v = 0.2 V/s. Electrodes were thoroughly polished and rinsed before and between the measurements. The electrosynthesis of the mixture (organic substrate and hydrogen sulfide) was carried out (90 min) at 25 °C under anaerobic conditions (argon), in dichloromethane, in an undivided three-electrode cell (V = 2 mL). Hydrogen sulfide was added as a saturated solution in dichloromethane (20 μL) every 30 min. The concentration of H2S was determined by the gravimetrical method in the reaction with Pb(CH3COO)2. The electrolysis potential was varied depending on the method of H2S activation, as follows: 1.90 V (direct electrochemical oxidation); 1.3 V (electrocatalytic oxidation with o-benzoquinone); 1.0V; 1.2 V (in the presence of mediators). For preparative large-scale electrosynthesis (180 min) in dichloromethane, a three-electrode electrolyzer (V = 100 mL) and platinum electrodes (plates, S = 300 mm2) were used. The rate of intake H2S was 2–3 mL/min, which provided the necessary concentration of H2S in the electrochemical cell. Electrolysis was carried out in a mixture of CH2Cl2/substrate (3:1) in an aerobic environment. The current density was maintained within 5–10 mA/cm2. Then the reaction mixture was saturated with sulfurous waste extracted from fuel oil. The molar ratio of cycloalkane (condensed analog)/thiol/hydrogen sulfide was 1:2:5. After the end of electrolysis, the reaction mixture was degassed in a flow of argon (v = 10 h−1) to remove hydrogen sulfide for 30 min. After electrosynthesis, the supporting electrolyte was precipitated with hexane. The synthesized organic sulfur derivatives were isolated by three-stage extraction with hexane, then the extract was concentrated in a vacuum and their contents were controlled by various methods of physicochemical analysis.
2.3. Microwave Synthesis of Organic Sulfur Derivatives
The synthesis of organic sulfur compounds was carried out in a laboratory microwave reactor CEM Focused Microwave Synthesis System, model Discover (USA), under controlled conditions by coupling the reactor with IBM. Reaction mixtures of substrates (hydrocarbons) and sulfurous reagents (hydrogen sulfide, thiols) were placed in glass vessels with a volume of 5–10 mL. The values of the microwave irradiation power, synthesis duration, limiting pressure and heating temperature of the reaction mixture were set directly on the touch panel of the reactor control. The irradiation power was varied in the range 100–300 W, the synthesis duration was 10–40 min. The organic sulfur derivatives obtained in the course of microwave synthesis were isolated from the reaction mixture by three-stage extraction with hexane, then the extract was concentrated in a vacuum and their contents were controlled by various methods of physicochemical analysis.
2.4. Identification of the Obtained Organic Sulfur Compounds
To identify the obtained organic sulfur compounds, we used the methods of IR spectroscopy, UV spectrophotometry, gas chromatography, gas chromatography-mass spectrometry, electrochemistry (cyclic voltammetry), and X-ray fluorescence analysis.
The IR spectra of electrolysis products were recorded on an FSM-1201 IR Fourier spectrometer (Russia) in KBr pellets in the range from 400 to 4000 cm−1. In the IR spectra, stretching vibrations of bonds were recorded, as follows: S–S (507–520 cm−1), C–S (690–710 cm−1), and S–H (2550–2600 cm−1) [39]. Electronic absorption spectra for the analysis of the reactions of hydrogen sulfide (thiols) with substrates, as well as for the identification of inorganic polysulfanes and elemental sulfur, were recorded on an SF-103 spectrophotometer (190 ÷ 1100 nm) in quartz cells (l = 10 mm) at 298 K. Hydrocarbons (C = 0.1 × 10−3 M) were dissolved in deaerated CH2Cl2. Chromatographic analysis of the mixture of reaction products was carried out on a Kristalluks-4000M gas chromatograph (OOO NPF Meta-Khrom) in isothermal mode; the carrier gas was helium, with an Agilent capillary column (100 m × 0.25 mm) and tc = 80 °C. Detector—flame ionization, td = 250 °C, stationary phase—HP-1. The reaction product mixture was analyzed by gas chromatography–mass spectrometry on a GCMS-QP2010 Ultra instrument (Shimadzu, Japan) with a detector (ionization method: electron impact, 70 eV). The capillary column was SPB-1 SULFUR (l = 30 m, d = 0.32 mm), with tmax = 320 °C. The carrier gas was helium. The column temperature was programmed in the range of 30 to 280 °C. The total sulfur concentration in the mixture of the reaction products was determined by the X-ray fluorescence method on an ASE-1 spectrometer (Russia). 13C and 1H NMR spectra were recorded on a Bruker AM-300 spectrometer (USA) with an operating frequency of 75.47 MHz (13C), 300 MHz (1H). Carbon spectra were obtained in modes with broadband decoupling by protons and modulation with respect to the C–H spin–spin coupling constant. Quantitative measurements were carried out on the basis of 13C NMR spectra generated with a delay between pulses of 11 s duration. The concentration of the investigated homogeneous solutions of oil residues in the organic solvents was C = 10 wt.%. The recording temperature of the spectra was 35 °C. The content of paraffins and naphthenes was determined by 1H and 13C NMR spectroscopy in solutions of oil residues in deuterochloroform. The analysis was carried out on the basis of the values of the sum of the integral intensities of the characteristic signals of carbon atoms in the 13C NMR spectra and protons in the 1H NMR spectra of linear alkanes and isoalkanes, and the sum of these parameters for polycyclic naphthenes. The content of resins by 13C NMR spectra was established based on the analysis of solutions of oil residues in petroleum ether, and asphaltenes in deuterobenzene. The content of aromatic hydrocarbons in the solutions of oil residues in deuterochloroform was determined by the subtraction of signals of carbon atoms and protons of resins and asphaltenes.
2.5. Quantum Chemical Calculations and Computer Forecasting
Quantum chemical calculations were performed using the Gaussian 03 computer program, the Hartree–Fock methods (6-31G (d, p) basis) and the density functional (BLYP functional, 6-31G (d, p) basis). The effect of organic solvent was taken into account on the basis of the polarized continuum model (PCM). The energy effects of reactions (ΔH) were calculated as the difference between the total energies of the final and initial structures. Computer prediction of the potential biological activity of the obtained organic sulfur derivatives was carried out using the PASS program.
3. Discussion of Results
The work presents the results of experimental and theoretical engineering of a new energy-efficient technological process for purifying fuel oil from sulfur waste, based on its treatment with ultrasound and the action of a constant magnetic field. The process differs from traditional approaches to the removal of hydrogen sulfide (H2S) and thiols (RSH, where R = Alk) from hydrocarbons by using modern and alternative energy-saving methods, does not require the use of special chemical reagents (expensive catalysts, solid adsorbents, liquid absorbents) to remove thiols or transfer to less aggressive compounds, and excludes the creation of special high-temperature conditions. The advantage of the proposed approach to the disposal of toxic waste is the directed electric and microwave synthesis of practically useful organic sulfur compounds based on sulfur components extracted from fuel oil.
The analysis of fuel oil produced from stable gas condensate at the Astrakhan gas processing plant and selected as an object of study was carried out using standard methods for analyzing oil products and modern spectral methods of physical and chemical analysis. The contents in the oil product of low molecular weight sulfur-containing impurities, hydrogen sulfide, methylmercaptan and ethylmercaptan, were 21.3, 10.7 and 81.9 mg/m3, respectively.
It was found that the concentration of total sulfur reached 2.86% of the mass. 1H, 13C NMR data made it possible to estimate the group hydrocarbon composition of fuel oil, in which paraffinonaphthenic hydrocarbons and resins predominate. The presence of paramagnetic centers (0.69 × 1018 spin/g) in fuel oil, according to EPR spectroscopy data, indicates its high sensitivity to wave effects (magnetic field and ultrasound). Using photocolorimetric analysis, the average particle size of the dispersed phase (235 nm) of the oil dispersed system was determined, which undergoes destruction under the action of ultrasound or ordered orientation in a constant magnetic field.
Low-energy wave effects in the dynamic mode have a significant effect on the properties of the oil dispersed system, in particular on the evacuation of hydrogen sulfide and low molecular weight thiols (RSH) from fuel oil. One of the key characteristics of petroleum products that determines the indicators of further processing is the dispersed composition, characterized by the average particle size of the dispersed phase. For the analyzed fuel oil, this figure is 113 nm. It was found that the dispersed composition of fuel oil is significantly influenced by the effect of a constant magnetic field (0–0.23 T) in the dynamic mode. The data obtained are shown in Figure 1.
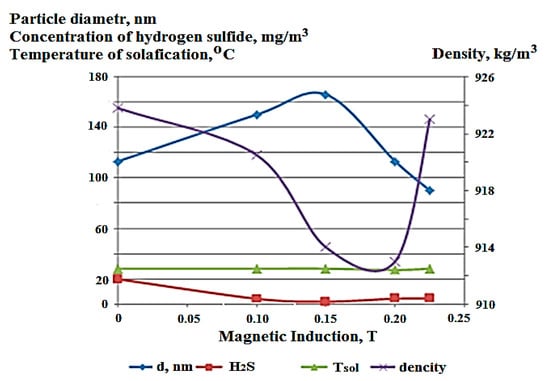
Figure 1.
Change in the properties of fuel oil (average particle size of the dispersed phase of fuel oil, hydrogen sulfide content, density and pour point) depending on the value of magnetic induction.
Under the influence of the magnetic treatment of fuel oil, along with a change in the particle diameter of the dispersed phase, an antibate decrease in density occurs, associated with the release of hydrogen sulfide and low molecular weight thiols. With an increase in magnetic induction in the range of 0 to 0.15 Tl, the content of hydrogen sulfide decreases by about 10 times, and then slightly increases. In a certain induction interval, an increase in the size of particles of the dispersed phase of fuel oil–gas bubbles occurs, which further leads to the coagulation and subsequent degassing of the oil system. This state corresponds to the minimum density value and a noticeable decrease in the H2S content. The pour point of fuel oil practically was not changed (within the error of the method). Variation in the dispersion of fuel oil under the influence of a magnetic field is associated with the presence of paramagnetic centers in the oil system, which are concentrated in high-molecular compounds, such as resins and asphaltenes.
In this work, to intensify the process of extracting hydrogen sulfide and low molecular weight thiols dissolved in fuel oil, it is proposed to use ultrasonic and magnetic treatment. When comparing the effects of ultrasound with a frequency in the range of 22–40 kHz on the fuel oil flow, an increase in the release of hydrogen sulfide with increasing frequency was observed. A comparison of the effectiveness of the influence of wave methods on the flow of fuel oil at a temperature of 60 °C gave the following results: ultrasound—frequency 40 kHz, constant magnetic field—induction 0.3 T, with a combined exposure to ultrasound and magnetic field using the specified parameters. A comparison of the effectiveness of the influence of wave methods on the flow of fuel oil at a temperature of 60 °C gave the following results: ultrasound—frequency 40 kHz, constant magnetic field—induction 0.3 T. With the combined action of ultrasound and a magnetic field, the specified parameters must be used. When exposed to ultrasound or a magnetic field, the content of dissolved gaseous sulfur components in the fuel oil decreases 1.4–2.8 times compared to the data obtained before processing. The most promising approach is a combination of ultrasonic and magnetic treatment, since the content of hydrogen sulfide and low molecular weight thiols in fuel oil is significantly reduced 9–10 times. This dependence is shown in Figure 2.
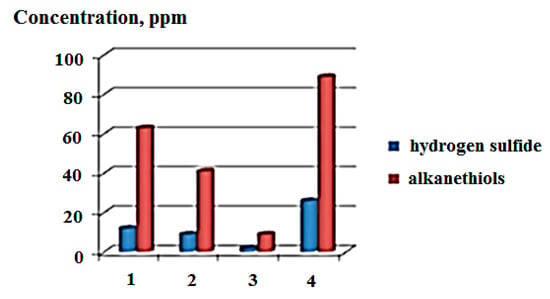
Figure 2.
Dependence of the concentration of hydrogen sulfide and alkanethiols extracted from fuel oil on the type of physical impact: 1—ultrasound, 2—constant magnetic field, 3—combination of ultrasound and magnetic field, 4—before processing.
Based on the data obtained, it was confirmed that under low-energy wave impacts, the structure of the studied oil dispersed systems changes, as a result of which the efficiency of the release of toxic and corrosive sulfur components from fuel oil increases. Low-energy wave impacts transform the structure of fuel oil, as a result of which the processes of the enlargement and coagulation of gas bubbles of hydrogen sulfide and low molecular weight thiols are intensified, which contributes to their effective removal. Further, gaseous sulfur-containing impurities were proposed to be utilized by absorption with organic solvents (acetonitrile, methylene chloride) and used as raw materials in the chemical, electrochemical and microwave synthesis of practically useful organic sulfur derivatives based on alicyclic compounds and their structural analogs (bicyclic condensed hydrocarbons).
Previous results on the use of redox, microwave and chemical activation of hydrogen sulfide (alkanethiols) showed a significant increase in their reactivity when in interaction with organic compounds in acetonitrile (dichloromethane) [40,41,42,43,44]. An important difference between the methods developed by us for the synthesis of organic sulfur derivatives is the mild process conditions compared to traditional approaches (thermolysis, radiolysis, photolysis, catalysis by metals or their complexes) required for the radical initiation of hydrocarbon reactions with hydrogen sulfide and thiols [45,46,47,48,49,50]. Within the framework of this study, methods for the synthesis of sulfur derivatives based on alicyclic, aromatic and bicyclic condensed hydrocarbons were developed, and the reactivities of structural analogs cycloalkanes (C5–C8), cycloalkenes (C5, C6), benzene, naphthalene and tetralin have been compared.
To involve the hydrogen sulfide and alkanethiols contained in fuel oil in the synthesis of organic sulfur derivatives, a scientifically grounded method for the experimental and theoretical engineering of an environmentally safe and efficient technological process has been proposed, consisting of the following stages.
Stage 1. Development of methods for activating hydrogen sulfide (electrochemical, microwave and chemical). Experimental research using an electrochemical measurement system, in a microwave reactor and in a laboratory setup.
One-electron irreversible oxidation of hydrogen sulfide, in CH3CN (CH2Cl2) at potentials 1.6 V and 1.7 V, to an unstable radical cation promotes the formation of a thiyl radical as a result of its fragmentation with proton abstraction. The cyclic voltammogram of hydrogen sulfide oxidation is shown in Figure 3.
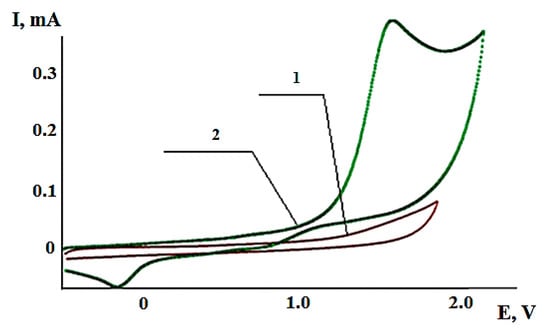
Figure 3.
Cyclic voltammogram of oxidation: 1—supporting electrolyte; 2—hydrogen sulfide (CH3CN, Pt-anode, Ag/AgCl, 0,1 n-Bu4NClO4, C(H2S) = 5 × 10−3 mol/L).
The one-electron reduction of hydrogen sulfide at different potentials, depending on the nature of the solvent (−1.6 V or −1.5 V), leads to the generation of a thiolate anion, which is oxidized on the counter electrode to a thiyl radical.
The activation of the sulfur components of fuel oil under the action of MW occured due to the homolytic decomposition of H2S and RSH molecules, with the formation of thiyl and alkylthiyl radicals, respectively.
The chemical activation of hydrogen sulfide and low molecular weight thiols is realized with the participation of one-electron oxidants (sterically hindered ortho-benzoquinones), which leads to the sequential generation of radical cation and radical intermediates. A feature of this method for activating the sulfurous waste of fuel oil is the easy regeneration of the oxidizer with atmospheric oxygen, which contributes to the cyclic process.
Stage 2. Selection of organic compounds for SH-and SR-functionalization with the participation of activated hydrogen sulfide and low molecular weight thiols under conditions of the electrochemical, microwave and chemical activation of reagents.
The investigated organic substrates are inert nonpolar hydrocarbons that are difficult make to undergo redox activation (their redox potentials are higher than those of sulfur reagents) in organic media, chemical oxidation by means of ortho-benzoquinones, and homolysis under microwave irradiation. The following hydrocarbons were chosen as substrates: cycloalkane C5–C8 I–IV; cycloalkenes C5, C6—V, VI; benzene VII; naphthalene VIII and tetralin IX. The structural formulas of the organic compounds are shown in Figure 4.
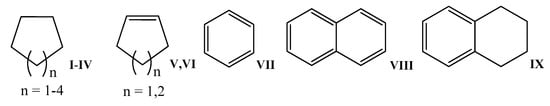
Figure 4.
Structural formulas of hydrocarbons used to obtain organic sulfur derivatives based on activated hydrogen sulfide and thiols.
Stage 3. The implementation of electro-, preparative and microwave synthesis of organic sulfur derivatives based on hydrocarbons.
For the electrosynthesis of organic sulfur compounds, a diaphragmless three-electrode electrochemical cell was used, which is a model of an industrial flow-through electrolyzer. An image of a three-electrode organic synthesis cell can be seen in Figure 5.
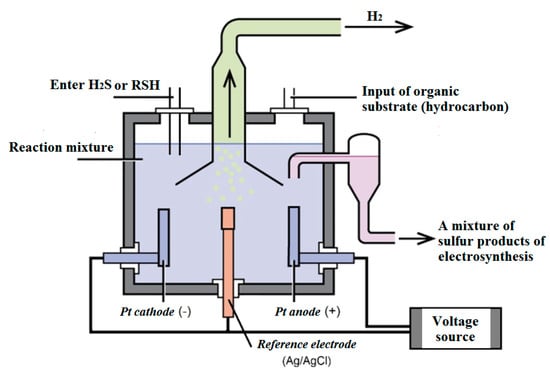
Figure 5.
Schematic diagram of a diaphragmless electrolyzer used in industry.
Flow type electrolyzers are mainly used in electrochemical production and are widely used. A diaphragmless electrolyzer with electrodes placed in it is the simplest design. It has been proposed to use platinum as an electrode material, the advantage of which is a long service life in electrolyzers. Inert platinum electrodes minimize solution contamination during electrolysis. Stirring of the solution is carried out due to the gas-lift (in the direction of movement of the gas bubbles released on the electrodes) movement of the liquid during the operation of the electrolyzer. The evolved gases are removed from the electrolyzer’s chamber by means of jet pumps. The electrolysis time and productivity of the complex is determined by the volume and concentration of the reaction mixture.
The potential for carrying out the electrochemical process at room temperature and atmospheric pressure is comparable to the values of the anodic (cathodic) potentials of hydrogen sulfide and thiols. It was found that the duration of electrosynthesis, the area of the electrodes and the structure of the substrate affect the composition, ratio and yield of the obtained target reaction products. With an increase in the reaction time of anodically activated hydrogen sulfide with cycloheptane III from 90 to 120 min, the ratio of the main electrosynthesis products (thiols—RSH, symmetric disulfides—R2S2 and symmetric trisulfides—R2S3) shifts towards the predominant formation of di-and trisulfides. The results of the synthesis at different reaction times are shown in Figure 6.
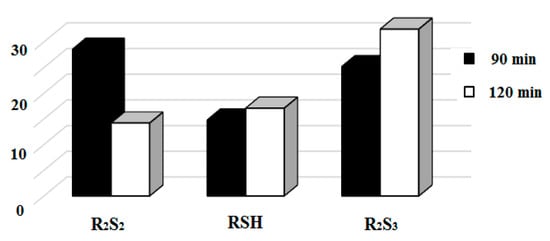
Figure 6.
Diagram of the dependence of the products yield of the reaction of hydrogen sulfide with cycloheptane, under conditions of anodic activation of the reagent, on time.
Disulfides are obtained by the oxidation of cycloalkane thiolation products at the potential of electrosynthesis. For the formation of trisulfides, elemental sulfur is required—a product of the direct electrochemical oxidation of hydrogen sulfide. Following the example of the interaction of naphthalene VIII with hydrogen sulfide under conditions of anodic initiation, the influence of the nature of the organic solvent and the time of electrosynthesis on the yield of organic sulfur derivatives, and the conversion of hydrocarbons, is considered. The values of these parameters can be seen in Table 1 and Figure 7.

Table 1.
Dependence of the yield of the target products of the reaction of naphthalene with hydgogen sulfide under conditions of anodic activation of the reagent on the nature of the solvent and the duration of electrosynthesis.
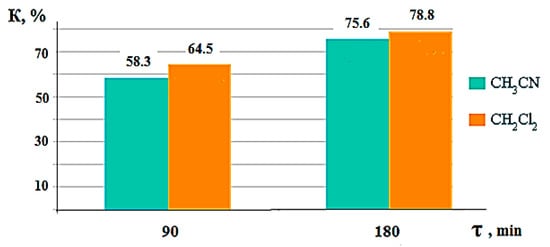
Figure 7.
Diagram of the dependence of the conversion (K) of naphthalene upon interaction with anodic activated hydrogen sulfide on the time and nature of the organic solvent.
For the execution of microwave synthesis, a reactor was used that allows one to obtain organic sulfur compounds in a solvent medium or in its absence. In contrast with electrosynthesis, the conditions of microwave irradiation contribute to a significant increase in the reaction rate, which leads to an increase in the yield of the target transformation products. Varying the power and duration of the synthesis allows one to change the spectrum and content of organic sulfur derivatives. Table 2 shows data on the yield of the products of the thiolation reaction of compounds I–IV with the participation of hydrogen sulfide, under the action of MW.

Table 2.
The yield of organic sulfur compounds formed during the microwave activation of hydrogen sulfide in the presence of substrates I–IV (microwave irradiation MW power—300 W, τ = 20 min).
The preparative synthesis of organic sulfur derivatives, with the participation of one-electron oxidants of hydrogen sulfide and alkanethiols in reactions with hydrocarbons I–IX, was carried out in a laboratory setup at room temperature and atmospheric pressure. Due to the regeneration of sterically hindered quinones by atmospheric oxygen, chemical synthesis under homogeneous catalysis conditions occurs cyclically; however, compared with electro- and microwave synthesis, the yield of the target products was significantly lower (2.5–3.0%).
All of these approaches facilitate the generation of thiyl and alkylthiyl radicals, which interact with various types of organic substrates. This is due to the structures of the initial hydrocarbons, which are characterized by the saturation (or unsaturation) of carbon–carbon bonds. Methods for activating the sulfur components of fuel oil, which are effectively involved in radical substitution reactions (with compounds I–IV, VII–IX), or addition (with compounds V, VI) with the formation of substrates of thiolation products at the first stage, are shown in the scheme in Figure 8.
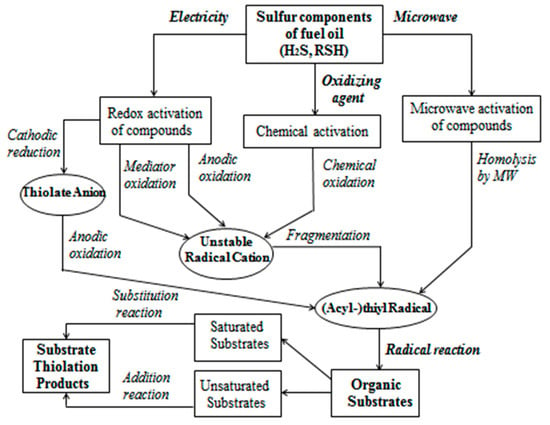
Figure 8.
Step-by-step scheme of the process of obtaining practically useful organic sulfur derivatives based on hydrocarbons I–IX by using various methods of activating the sulfurous waste of fuel oil.
Stage 4. Synthesis of inorganic sulfanes, elemental sulfur and symmetric alkyldisulfides as products of the chemical, electrochemical and microwave activation of hydrogen sulfide and low molecular weight thiols.
In industry, the oxidation of the sulfur components (H2S, RSH) of hydrocarbon feedstock (oil and gas condensate) in order to obtain elemental sulfur and disulfides is usually carried out under severe temperature conditions (Claus process), or in the presence of catalysts (MEROX process) [50].
The anodic (in the presence of one-electron oxidants) activation of sulfurous waste extracted from fuel oil leads to the production of sulfur and alkyldisulfides (with a quantitative yield of 76–78%), and at room temperature and atmospheric pressure is characterized by a high conversion of H2S (RSH).
Stage 5. Three-component electro- and microwave synthesis of organic polysulfides with the participation of hydrocarbons I–IX and the system ‘hydrogen sulfide–elemental sulfur’.
In connection with the increase in the potential biological compounds with an increase in the number of sulfur atoms in the sulfide chain, a method was developed for obtaining symmetric tri- and tetrasulfides. It was established that the growth of the sulfide chain is possible in the course of electric and microwave synthesis due to the formation of elemental sulfur during the oxidation of hydrogen sulfide. The electrochemical and microwave activation of hydrogen sulfide in the presence of sulfur leads to the formation of hydro-polysulfide radicals along with thiyl radicals. The polysulfide yield is influenced by the method of H2S activation, the ratio of hydrogen sulfide and sulfur, the structure of the starting substrate, and the duration of synthesis. It is shown in this work that the size of the alicyclic hydrocarbon affects the total yield of organic sulfur derivatives and the content of biologically active trisulfides. The results of the synthesis and the spectrum of the reaction products can be seen in Figure 9.
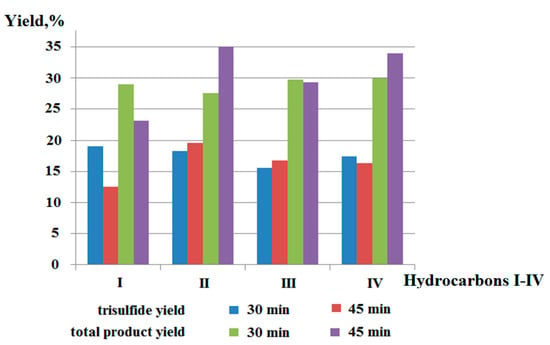
Figure 9.
Diagram of the dependence of the total yield of sulfur-containing reaction products based on cycloalkanes I–IV and trisulfide content on the duration of MW (200 V).
Stage 6. Three-component electric and microwave synthesis of asymmetric organic sulfides based on hydrocarbons I–IX, hydrogen sulfide and isomeric dibutyldisulfides.
The presence of various substituents on sulfur atoms contributes to an increase in their biological activity [51]; therefore, asymmetric sulfides have a broader spectrum of action as drugs. Isomeric dibutyl disulfides were used to initiate the reaction of a hydrocarbon with hydrogen sulfide by a radical mechanism, which made it possible to obtain a mixture of sulfur derivatives with symmetric and asymmetric structures. The yield of the target sulfur-containing reaction products depends on the hydrogen sulfide/di(t.-butyl)disulfide ratio, the structure of the substrate and disulfide, and the duration of the transformations. Information about the results of the synthesis is presented in Table 3.

Table 3.
Dependence of the yield of the products of the reaction of hydrogen sulfide, di(tert-butyl)disulfide with cycloheptane on the time of electrosynthesis (t = 25 °C, CH2Cl2, El = 1.9 V).
The total yield of the reaction products increased with an increase in the transformation time, while the content of sulphide of an asymmetric structure, expected when using this approach to electrosynthesis, remains constant over time. For cycloalkanes C5, C6 and C8, the influence of the alicyclic value on the technical and economic indicators of the electrochemical production of sulfide was studied. The synthesis efficiency characteristics are presented in Table 4.

Table 4.
Dependence of the yield (η) of unsymmetric sulfide formed in the reaction of hydrogen sulfide, di(n-butyl)disulfide with cycloalkanes C5, C6 and C8, selectivity (S), and the conversion of disulfide (K) on the size of the alicyclic (τ = 90 min, t = 25 °C, CH2Cl2, E = 1.9 V).
Based on the data in Table 4, it can be concluded that cyclooctane is most promising in the synthesis of asymmetric sulfide using dibutyl disulfide with a linear structure, the conversion of which does not depend on the size of the alicyclic.
Stage 7. Indirect electrosynthesis of organic sulfur derivatives based on sulfur components of fuel oil using mediators of various natures (organic and inorganic).
The use of electromediators in the preparation of organic sulfur derivatives makes it possible to activate hydrogen sulfide and low molecular weight thiols at lower values of electrode potential in comparison with direct electrosynthesis. A decrease in the anodic overvoltage of the electrochemical oxidation process of sulfur reagents (H2S, RSH) is possible due to the preliminary activation of the mediator at a low value of the oxidation potential. The active form of the neurotransmitter, generated electrochemically, during the oxidation of hydrogen sulfide and thiols, is regenerated to a neutral form. This stage contributes to the regeneration of the mediator and the cyclic carrying out of the process of obtaining organic sulfur compounds.
Under the conditions of mediator electrosynthesis when using the hydrogen sulfide–elemental sulfur system, it is more promising to direct the technological stream of sulfurous reagents and hydrocarbons to obtain tri- and tetrasulfides, which are characterized by high biological activity. The domination of polysulfides of higher molecular weight in the mixture of the reaction products of cycloalkanes I, II with the H2S-S8 system, in the presence of n-tetrabutylammonium bromide as an electromediator, is evidenced by the data in Table 5.

Table 5.
The results of the reactions (90 min, 25 °C, CH2Cl2) of the hydrogen sulfide—sulfur system with cycloalkanes I, II with the oxidation potential of n-tetrabutylammonium bromide (E = 1.1 V).
The anode overvoltage for this version of electrosynthesis is 0.8 V, in comparison with the direct activation of hydrogen sulfide in reactions with hydrocarbons. The obtained experimental data indicate the influence of the content of elemental sulfur in the reaction mixture on the yield of sulfur-containing reaction products, which can be seen in Table 6.

Table 6.
The yield of the obtained reaction products (90 min, 25 °C, CH2Cl2) of cyclohexane, hydrogen sulfide and S8, realized at the oxidation potential of the mediator (n-tetrabutylammonium bromide) (E = 1.1 V).
Thus, by varying the sulfur concentration, it is possible to change the yield and ratio of di-, tri- and tetrasulfides in the reactions of hydrocarbons with hydrogen sulfide. The most optimal for the synthesis of a mixture of polysulfides in the case of cyclohexane was a sulfur concentration of 0.015 M. A further increase in the sulfur content leads to the growth of the sulfide chain and the formation of polysulfides with a higher molecular weight.
Stage 8. The use of quantum chemistry to establish thermodynamically favorable directions for the formation of organic sulfur derivatives based on hydrocarbons and the sulfurous waste of fuel oil, as well as the use of computer prediction to assess the biological activity of the obtained sulfur-containing reaction products.
For a number of hydrocarbons I–IX, quantum chemical calculations of the thermal effects of the reactions of the thiolation of substrates and the dimerization of the resulting sulfur-centered radicals were performed. The calculated data made it possible to estimate the preferred position of the carbon atom for the introduction of the (alkyl-)thio group into the interaction of organic compounds with activated hydrogen sulfide and low molecular weight thiols.
For example, for the reaction of tetralin with hydrogen sulfide, the thermal effects were calculated for two directions of the SH-functionalization of the hydrocarbon due to the replacement of the hydrogen atom by the thio group—(1) in the alicyclic and (2) in an aromatic ring. In this regard, the formation of four isomeric thiols is possible—1,2,3,4-tetrahydronaphthalene-1-thiol (ΔH = −226.1 kJ/mol), 1,2,3,4-tetrahydronaphthalene-2-thiol (ΔH = −233.9 kJ/mol), 5,6,7,8-tetrahydronaphthalene-1-thiol (ΔH = −239.8 kJ/mol) and 5,6,7,8-tetrahydronaphthalene-2-thiol (ΔH = −232.3 kJ/mol). The approximate values of the heat effects of the reaction, calculated in accordance with the 1st consequence of Hess’s law as the sum (ΔH = ΔH1 + ΔH2, where ΔH1 is the thermal effect of the stage of detachment of a hydrogen atom from a hydrocarbon molecule upon attack by a thiyl radical, with the formation of a carbo-centered radical; ΔH2 is the thermal effect stages of the dimerization of carbocentered and thiyl radicals), confirm the possibility of the parallel formation of isomeric products of tetralin thiolation. A comparative analysis of the calculated data for the first initiating stage of the reaction showed that only in the case of the formation of 1,2,3,4-tetrahydronaphthalene-1-thiol was the value of ΔH1 < 0. Therefore, this compound should be considered as the predominant product of the tetralin thiolation reaction. For all the considered hydrocarbons I–IX, thermodynamically favorable directions of their transformations with activated forms of hydrogen sulfide and thiols have been established.
In the series of cycloalkanes I–IV, the heat effects of the SH-functionalization reaction with the participation of the thiyl radical were calculated, and the highest reactivity of cyclooctane was found, which is consistent with electrochemical data on the anodic activation of hydrogen sulfide (Table 4). The values of the heat effects of the reactions are presented in Table 7.

Table 7.
Quantum chemical calculation of the values of the heat effects of the formation reaction of cycloalkanethiols based on compounds (I–IV) and activated hydrogen sulfide (Gaussian 03, Hartree–Fock method, basis 6-31G (d, p)).
Due to the fact that polysulfides with a sulfur atom content of three to five are of the greatest interest among organic sulfur compounds obtained from hydrocarbons I–IV and the sulfurous waste of fuel oil, calculations of the thermal effects of their formation reactions were performed to assess the energy costs at these stages of the chemical engineering process. Graphical dependences of the thermal effect of formation of polysulfides on the number of sulfur atoms in their structure dependences for the heat produced by the formation of various polysulfides are shown in Figure 10.
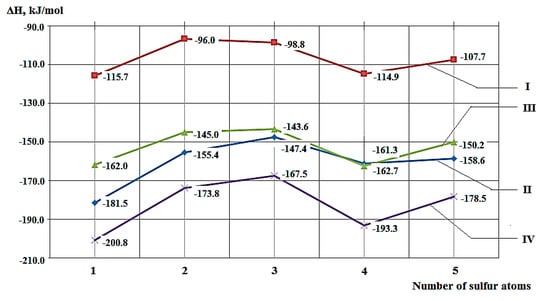
Figure 10.
Dependence of the values of the thermal effects of the formation of mono-, di-and polysulfides on the number of sulfur atoms (Gaussian 03, Hartree–Fock method, basis 6-31G (d, p)).
Based on quantum chemical calculations, it was concluded that the synthesis of tetrasulfides R2S4 is thermodynamically advantageous compared to the production of R2Sn (n = 2, 3). It is most expedient to use alicyclic C8 as a substrate, and cyclopentane is less promising.
Computer forecasting using the PASS program made it possible to determine the main types of potential biological activity of the obtained sulfur-containing compounds with a certain probability. It was found that the introduction of a thio group into hydrocarbons I–IX or a fragment of the S-S bond significantly expands the spectrum of biological activity of the parent compound, and increases the prospects for its use in the production of drugs. Thus, the product of tetralin thiolation can be used as an inhibitor of a number of enzymes—thioredoxin (68.7%), monooxygenase (77.4%), lysase (78.1%), superoxide dismutase (78.1%), transferase (78.6%), dehydrogenase (78.5%), and mucomembrane protector (75.2%). The symmetric disulfide obtained on the basis of the thio derivative of tetralin has a protoseborrheic effect (76.4%), can be used for the treatment of phobic disorders (78.2%), and has been shown to have an antitumor effect against breast cancer (84.7%).
The assessment of the biological activity was carried out for the products of thiolation of all studied hydrocarbons I–IX, and, with a high degree of probability, it turned out that their thi derivatives exhibit the most valuable pharmacological properties, and most of the obtained sulfur-containing reaction products are distinguished by antitumor activity (mainly against sarcoma).
Thus, the developed approach for the purification of fuel oil from toxic and corrosive sulfurous wastes, and the proposed methods for the synthesis of practically useful organic sulfur derivatives based on mono- and bicyclic hydrocarbons, are efficient, environmentally friendly, affordable, and characterized by low energy and material costs.
4. Description of the Logical-Information Model
4.1. Application of the IDEF1 Standard to Create a Logical-Information Model
Within the framework of this study, a method was developed for constructing a logical-information model of a chemical–technological system on the example of searching for a technological solution as the basis for the production process of organic sulfur compounds. This technological solution is one of the sections of the design documentation required to describe the technology involved in the utilization of low molecular sulfur waste from fuel oil, with the subsequent involvement of practically useful organic sulfur derivatives in the synthesis. The manufacturing of products of a given quality, ensuring environmental protection, rational use of resources and effective use of equipment, will be ensured by the formation of a logical-information model of the developed technological process, in accordance with a methodology consisting of standard operations.
The IDEF1 Standard was used to execute the scheme describing the process flows of the process of removing hydrogen sulfide and thiols from fuel oil with the involvement of alternative, energy-saving methods of processing the hydrocarbon fraction for subsequent involvement in the synthesis of organic sulfur derivatives. This software product is a reliable tool for analyzing and studying the relationships between information (or technological) flows within the activities of an enterprise with various profiles.
The purpose of using the IDEF1 Standard is to structure the available experimentally obtained information aregarding the operation of each of the technological units of the proposed scheme, and to ensure the high-quality implementation of the technology by optimizing the technological flows. The IDEF1 methodology promotes the more efficient use of the information space. The results of the analysis of information flows can be used for the strategic and tactical planning of enterprise activities and the improvement of information management. The need for such a reorganization of the flows of modern technological processes in the oil and gas profile, as a rule, arises at the initial stage of building a logical information system. The IDEF1 methodology makes it possible to clearly reveal the shortcomings of the existing classical structure of the technological flows of this particular technology. With the help of IDEF1, it is possible to study the existing information regarding various objects in the field of the enterprise.
The use of the IDEF1 methodology as a tool for constructing a visual model of the technological multistage process of recycling sulfurous wastes, with their subsequent transformation into practically useful compounds, the need for which is high enough, will allow the enterprise to complete the following tasks: (1) determine the structure and content of technological flows in the enterprise; (2) identify the problems identified as a result of the functional analysis; (3) identify technological flows that require additional management for the effective implementation of the logical-information model; (4) analysis of the relationship between existing information flows within the enterprise.
4.2. Main Advantages of the IDEF1 Methodology
The IDEF1 methodology makes it possible, on the basis of simple graphical images, to model informational relationships between various objects (technological nodes). One of the main advantages of the IDEF1 methodology is the provision of a consistent and strictly structured process for analyzing information flows within the framework of a manufacturing enterprise. A distinctive feature of IDEF1 is its widely developed modularity, which makes it possible to effectively identify and correct the existing structure of information at all stages of modeling. The IDEF1 methodology was developed as a tool for establishing the rules and mechanisms for changing objects in the information area when changing the corresponding objects in the real world [52,53].
In connection with the aforementioned advantages, the IDEF1 standard is used in this work to create a logical-informational model of the technology for removing waste (hygrogen sulfide and thiols), with their subsequent processing into widely used organic sulfur compounds.
The logical information model of an effective chemical technological process is shown in Figure 11.
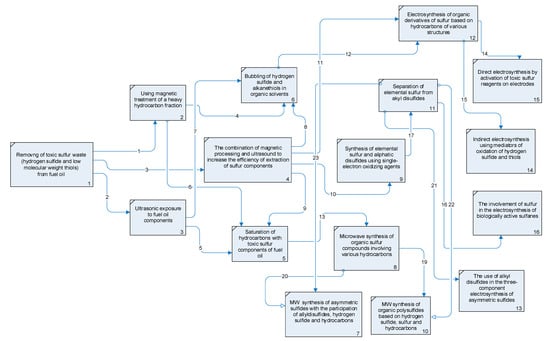
Figure 11.
IDEF1 diagram of a logical-informational model of energy- and resource-saving technology for processing the sulfurous waste of fuel oil to obtain organic sulfur derivatives.
For the effective extraction of hydrogen sulfide and thiols from fuel oil (1), the work proposes three methods—magnetic treatment (2), ultrasonic treatment (3) and their combined action (4). On the basis of experimental data, it was shown that the combination of two types of exposure (ultrasound and magnetic treatment) is more effective. The proposed logical-informational model provides the possibility of using various technological units (2–4) for the extraction of hydrogen sulfide and thiols from fuel oil, the functioning of which is possible sequentially or in parallel.
The further utilization of hydrogen sulfide and thiols into various useful sulfur-containing compounds depends on the processing method. One of them is the chemical oxidation of hydrogen sulfide and thiols in an organic medium using one-electron oxidants, sterically hindered o-benzoquinones (9). This variant leaded to the production of elemental sulfur and alkyldisulfides of a symmetric structure, which are separated for the purposeful use of alkyl disulfides and the attraction of sulfur in the three-component organic synthesis (11).
The second option for the utilization of sulfur components is their subsequent use in synthesis under the action of microwave irradiation.
This method required their preliminary mixing with hydrocarbons selected as substrates and bases, for obtaining S-functionalization products (5). From the standpoint of electrochemical and microwave activation methods, alicyclic (aromatic) hydrocarbons and their condensed analogs are more inert in the considered transformations than hydrogen sulfide and thiols.
A mixture of sulfurous waste is used in the microwave two-component synthesis of organic sulfur derivatives based on various hydrocarbons (8). The addition of elemental sulfur to a mixture of sulfur components and hydrocarbons allowed three-component synthesis under the action of microwave irradiation (10). In this case, the proposed technological unit permits the possibility of obtaining the most promising organic sulfur derivatives from the standpoint of biological activity—polysulfides with a sulfur atom chain length ≥3. Another option for carrying out a three-component synthesis is the involvement of alkyldisulfides obtained from thiols in the synthesis of sulfides of an asymmetric structure, which are distinguished by their high potential biological activity (7).
The third way to involve hydrogen sulfide and alkanethiols in the synthesis is the activation of reagents via the action of an electric current in organic solvents (12). For carrying out electrosynthesis in organic solvents, a unit for mixing hydrogen sulfide and thiols with polar aprotic solvents is proposed (6). This technique can be implemented using two approaches: the direct oxidation of hydrogen sulfide and thiols on platinum (glassy carbon) electrodes (15), and the indirect oxidation of sulfur waste using electromediators (14). Direct two-component electrosynthesis, similar to microwave synthesis, can be replaced by a three-component one, and be carried out either using elemental sulfur in order to preferentially obtain organic polysulfides (16), or in the presence of alkyl disulfides, which are important for the synthesis of asymmetric sulfides (13).
5. Conclusions
Thus, the physicochemical and technological foundations were developed for the environmentally safe and efficient production of organic sulfur compounds based on the unwanted sulfur impurities contained in fuel oil. It is shown that the use of modern energy-saving methods of wave action (ultrasound and magnetic treatment) should improve the quality of commercial fuel oil by removing hydrogen sulfide and low molecular weight thiols. It was found that the extracted sulfur components should be directed to the production of organic sulfur derivatives via various promising methods (electro-, microwave, or preparative chemical synthesis). This approach is due to the possibility of minimizing energy consumption by carrying out the process at an ordinary temperature and pressure (laboratory experiments were carried out at room temperature and atmospheric pressure) to implement the process of the thiolation of inert hydrocarbons in order to obtain biologically active sulfur-containing products with a high antitumor effect.
On the basis of a preliminary versatile experimental and theoretical study of various stages of the chemical–technological process of processing toxic sulfurous waste (hydrogen sulfide and low molecular weight thiols) into practically useful organic sulfur derivatives, a logical-informational model of the oil and gas industry was created. The choice of an effective energy-saving technology for the disposal of toxic sulfurous components of fuel oil by wave action, taking into account the established control indicators and requirements for the safety of technological modes, made it possible to determine a specific number of technological solutions that deserve the attention of specialists in this profile, and to assess the investment prospects of the proposed technological solution.
The use of a logical-informational model of the production process in the algorithm for choosing an energy resource-efficient technology, along with the optimization of technological parameters, contributed to the creation of information support for the design and operation of an installation for the utilization of the sulfurous residues of fuel oil, with their subsequent processing into organic sulfur compounds.
Author Contributions
Conceptualization, V.M. and N.B.; methodology, E.S. and N.P.; software, A.O.; validation, E.S. and N.B.; data curation, E.S.; writing—original draft preparation, E.S.; writing—review and editing, E.S. and F.I.; project administration, N.B.; funding acquisition, V.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received external funding. This work was supported by the Russian Foundation for Basic Research grants No. 18-29-24001 (research on the removal of sulfurous wastes using wave action and electrosynthesis of condensed bicyclic hydrocarbon derivatives, development of a logical-information model of the technological process), Russian Science Foundation No. 20-13-00084 (electrical, microwave and preparative synthesis of organic sulfur compounds based on alicyclic and aromatic hydrocarbons by utilizing hydrogen sulfide and low molecular weight thiols from fuel oil).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tuttle, D.P.; Baldick, R. Technological, Market and Policy Drivers of Emerging Trends in the Diffusion of Plug-in Electric Vehicles in the U.S. Electr. J. 2015, 28, 29–43. [Google Scholar] [CrossRef]
- Hallale, N. Burning bright—Trends in process integration. Chem. Eng. Prog. 2001, 97, 30–41. [Google Scholar]
- Dörr, M.; Wahren, S.; Bauernhansl, T. Methodology for Energy Efficiency on Process Level. Procedia CIRP 2013, 7, 652–657. [Google Scholar] [CrossRef]
- Popp, D.C. The effect of new technology on energy consumption. Resour. Energy Econ. 2001, 23, 215–239. [Google Scholar] [CrossRef]
- Krones, M.; Müller, E. An Approach for Reducing Energy Consumption in Factories by Providing Suitable Energy Efficiency Measures. Procedia CIRP 2014, 17, 505–510. [Google Scholar] [CrossRef][Green Version]
- Zhao, B.W. Make the chemical industry clean with green chemistry: An interview with Buxing Han. Natl. Sci. Rev. 2018, 5, 953–956. [Google Scholar] [CrossRef]
- Tekkaya, A.E. Energy saving by manufacturing technology. Procedia Manuf. 2018, 21, 392–396. [Google Scholar] [CrossRef]
- Mahinroosta, M.A. Review on Energy Efficiency Improvement methods for Oil and Gas Industries. In Proceedings of the 2nd Conference on Emerging Trends in Energy Conservation, Tehran, Iran, 2013; Available online: https://www.researchgate.net/publication/301221524 (accessed on 19 February 2013).
- Akhmadullina, A.G.; Akhmadullin, R.M. New developments and implementations in the field of hydrocarbon-stock desulfurization. Chem. Technol. Fuels Oils 2008, 44, 371–378. [Google Scholar] [CrossRef]
- Pivovarova, N.A.; Akishina, E.S.; Berberova, N.T.; Shinkar, E.V. Promising Technology For Removal And Disposal Of Hydrogen Sulfide From Fuel Oil. Izv. Vyss. Uchebnykh Zaved Khimiya Khimicheskaya Tekhnol. 2020, 63, 39–53. [Google Scholar] [CrossRef]
- Andrienko, O.; Kobotaeva, N.; Skorokhodova, T.; Marakina, E.; Orlov, V.V. A removal of sulfur-containing compounds from fuel oils using a naturally occurring iron oxyhydroxide. AIP Conf. Proc. 2019, 2101, 020009. [Google Scholar] [CrossRef]
- Ma, C.; Xiao, F.; Chen, N.; Liu, F.; Sun, X.; Dai, B. Oxidative desulfurization of a model fuel using ozone oxidation generated by dielectric barrier discharge plasma combined with Co3O4/γ-Al2O3 catalysis. RSC Adv. 2015, 5, 96945–96952. [Google Scholar] [CrossRef]
- Vetrova, T.K.; Morozov, V.A.; Dorogochinskaya, V.A.; Romanova, V.; Tonkonogov, B.P. Effectiveness of various types of absorbers of hydrogen sulfide in residual fuel oil. Chem. Technol. Fuels Oils 2012, 47, 446–448. [Google Scholar] [CrossRef]
- Pivovarova, N.A. Use of effect in processing of the hydrocarbonic raw material. Pet. Chem. 2019, 59, 559–569. [Google Scholar] [CrossRef]
- Shafeghat, A.; Ghaedian, M.; Mehrabi, M. Desulfurization of gas oil by using ultrasonic waves. J. Pet. Res. 2015, 24, 85–95. [Google Scholar]
- Mahmood, L.H.; Abid, M.F.; Mohammed, M.I. Study on ultrasound assisted desulfurization of light gas oil using inorganic liquid. Environ. Prot. Eng. 2019, 45, 5–19. [Google Scholar] [CrossRef]
- Ali, S.F.; Saadi, K.A.-N.; Nather, J.; Raed, I.; Mezher, A.; Zainab, F.; Akeel, K.; Nihad, R.; Ali, C.; Hazim, M.; et al. Effects of Magnetic Field on Fuel Consumption and Exhaust. Energy Procedia 2012, 18, 327–338. [Google Scholar]
- Ciobanu, R.; Dontu, O.; Gheorghe, G.; Avarvarei, I.; Besnea, D. System with permanent magnets used for magnetic treatment of fuel fluids. Rom. Rev. Precis. Mech. 2011, 3, 211–214. [Google Scholar]
- Loskutova, Y.V.; Yudina, N.V.; Pisareva, S.I. Effect of magnetic field on the paramagnetic, antioxidant, and viscosity characteristics of some crude oils. Pet. Chem. 2008, 48, 51–55. [Google Scholar] [CrossRef]
- Pivovarova, N.A.; Vlasova, G.V.; Akishina, E.S.; Ryzhova, M.V. Relationship between the Degree of Dispersion of Fuel Oil and the Degree of Removal of Hydrogen Sulfide from It. Pet. Chem. 2020, 60, 716–721. [Google Scholar] [CrossRef]
- Bhardwaj, M.; Neelam, N. The Advantages and Disadvantages of Green Technology. J. Basic Appl. Eng. Res. 2015, 2, 1957–1960. [Google Scholar]
- Ghanshyam, D. Advantages of green thechnology. Int. J. Res. 2015, 3, 1–5. [Google Scholar]
- Chhipa, N.; Jatakiya Viraj, P.; Gediya, P.; Patel, S. Green Chemistry: An unique relationship between waste and recycling. Int. J. Adv. Pharmac. Res. 2013, 4, 2000–2008. [Google Scholar]
- Leech, M.C.; Garcia, A.D.; Petti, A.; Dobbs, A.P.; Lam, K.G. Organic electrosynthesis: From academia to industry. React. Chem. Eng. 2020, 5, 977–990. [Google Scholar] [CrossRef]
- Pollok, D.; Waldvogel, S.R. Electro-organic synthesis–a 21st century technique. Chem. Sci. 2020. [Google Scholar] [CrossRef]
- Gawande, M.B.; Shelke, S.N.; Zbořil, R.; Varma, R.S. Microwave-Assisted Chemistry: Synthetic Applications for Rapid Assembly of Nanomaterials and Organics. Acc. Chem. Res. 2014, 47, 1338–1348. [Google Scholar] [CrossRef]
- Cardoso, D.S.P.; Šljukić, B.; Santos, D.M.F.; Sequeira, C. Organic Electrosynthesis: From Laboratorial Practice to Industrial Applications. Org. Process Res. Dev. 2017, 21, 1213–1226. [Google Scholar] [CrossRef]
- Kokel, A.; Schäfer, C.; Török, B. Microwave-Assisted Reactions in Green Chemistry. Encycl. Sustain. Sci. Technol. 2018, 1–40. [Google Scholar] [CrossRef]
- Surati, M.A.; Jauhari, S.; Desai, K.R. A brief review: Microwave assisted organic reaction. Arch. Appl. Sci. Res. 2012, 4, 645–661. [Google Scholar]
- Moiseev, I.I. Green chemistry: Development trajectory. Russ. Chem. Rev. 2013, 82, 616–623. [Google Scholar] [CrossRef]
- Lesch, J.E. The First Miracle Drugs: How the Sulfa Drugs Transformed Medicine; Oxford University Press: Oxford, UK, 2007; p. 364. [Google Scholar]
- Estevam, E.C.; Faulstich, L.; Griffin, S.; Burkholz, T.; Jacob, C. Polysulfides in Biology: From Intricate Chemistry to an Astonishing Yet Hidden Biological Activity. Curr. Org. Chem. 2015, 20, 211–217. [Google Scholar] [CrossRef]
- Bogomolov, B.B.; Bykov, E.D.; Men’Shikov, V.V.; Zubarev, A.M. Organizational and technological modeling of chemical process systems. Theor. Found. Chem. Eng. 2017, 51, 238–246. [Google Scholar] [CrossRef]
- Bogomolov, B.B.; Boldyrev, V.S.; Zubarev, A.M.; Meshalkin, V.P.; Men’Shikov, V.V. Intelligent Logical Information Algorithm for Choosing Energy-and Resource-Efficient Chemical Technologies. Theor. Found. Chem. Eng. 2019, 53, 709–718. [Google Scholar] [CrossRef]
- Meshalkin, V.P.; Khodchenko, S.M. The nature and types of engineering of energy- and resource-efficient chemical process systems. Polym. Sci. Ser. D 2017, 10, 347–352. [Google Scholar] [CrossRef]
- Gordon, A.J.; Ford, R.A. The Chemist’s Companion; A Wiley Interscience Publication: New York, NY, USA, 1972; p. 541. [Google Scholar]
- Letichevskaya, N.N.; Shinkar, E.V.; Berberova, N.T.; Okhlobystin, O.Y. Radical Cation of Hydrogen Sulfide as a Superacid. Russ J. Gen. Chem. 1996, 66, 1739–1741. [Google Scholar]
- Lund, H. A Century of Organic Electrochemistry. J. Electrochem. Soc. 2002, 149, S21–S33. [Google Scholar] [CrossRef]
- Workman, J. The Handbook of Organic Compounds; Elsevier: Amsterdam, The Netherlands, 2001. [Google Scholar]
- Berberova, N.T.; Shinkar’, E.V.; Smolyaninov, I.V.; Abdulaeva, V.F. Anodic activation of hydrogen sulfide in reaction with cyclopentane. Russ. J. Gen. Chem. 2015, 85, 998–1000. [Google Scholar] [CrossRef]
- Shinkar’, E.V.; Shvetsova, A.V.; Sediki, D.B.; Berberova, N.T. Redox activation of hydrogen sulfide in reaction with cycloheptane. Russ. J. Electrochem. 2015, 51, 1046–1053. [Google Scholar] [CrossRef]
- Berberova, N.T.; Smolyaninov, I.V.; Shinkar, E.V.; Kuzmin, V.V.; Sediki, D.B.; Shevtsova, A.V. Electrosynthesis of biologically active dicycloalkyl di- and trisulfides involving an H2S—S8 redox system. Russ. Chem. Bull. 2018, 67, 108–113. [Google Scholar] [CrossRef]
- Berberova, N.T.; Shinkar’, E.V.; Smolyaninov, I.V.; Pashchenko, K.P. Redox mediators of hydrogen sulfide oxidation in reactions with cycloalkanes. Dokl. Chem. 2015, 465, 295–298. [Google Scholar] [CrossRef]
- Shinkar’, E.V.; Kudryavtsev, D.A.; Pashchenko, K.P.; Berberova, N.T.; Okhlobystina, A.V. Thiolation of cycloalkenes C 5, C 6 by redox-activation of hydrogen sulfide. Mendeleev Commun. 2017, 27, 180–182. [Google Scholar] [CrossRef]
- Zhao, J.; Fang, H.; Han, J.; Pan, Y.; Li, G. Metal-Free Preparation of Cycloalkyl Aryl SulfidesviaDi-tert-butyl Peroxide-Promoted Oxidative C(sp3)—H Bond Thiolation of Cycloalkanes. Adv. Synth. Catal. 2014, 356, 2719–2724. [Google Scholar] [CrossRef]
- Guo, S.; He, W.; Xiang, J.-N.; Yuan, Y. Ruthenium-catalyzed direct thiolation of alkanes and ethers using arylsulfonyl chlorides as a sulfur source. Tetrahedron Lett. 2015, 56, 2159–2162. [Google Scholar] [CrossRef]
- Abbasi, M.; Nowrouzi, N.; Latifi, H. Selective synthesis of organic sulfides or disulfides by solvent exchange from aryl halides and KSCN catalyzed by NiCl2·6H2O. J. Organomet. Chem. 2016, 822, 112–117. [Google Scholar] [CrossRef]
- Grim, J.C.; Marozas, I.A.; Anseth, K.S. Thiol-ene and photo-cleavage chemistry for controlled presentation of biomolecules in hydrogels. J. Control. Release 2015, 219, 95–106. [Google Scholar] [CrossRef]
- Yang, Y.; Dong, W.; Guo, Y.; Rioux, R.M. Cu(i)-catalyzed aerobic cross-dehydrogenative coupling of terminal alkynes with thiols for the construction of alkynyl sulfides. Green Chem. 2013, 15, 3170. [Google Scholar] [CrossRef]
- De Angelis, A.R. Natural gas removal of hydrogen sulphide and mercaptans. Appl. Catal. B Environ. 2012, 37–42. [Google Scholar] [CrossRef]
- Feng, M.; Tang, B.; Liang, S.H.; Jiang, X. Sulfur Containing Scaffolds in Drugs: Synthesis and Application in Medicinal Chemistry. Curr. Top. Med. Chem. 2016, 16, 1200–1216. [Google Scholar] [CrossRef]
- Banja, V. Functional and Information Modeling of Production Using IDEF Methods. J. Mech. Eng. 2009, 55, 131–140. [Google Scholar]
- Jeong, K.-Y.; Wu, L.; Hong, J.-D. IDEF method-based simulation model design and development framework. J. Ind. Eng. Manag. 2009, 2, 337–359. [Google Scholar] [CrossRef][Green Version]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).