1. Introduction
Due to its versatility, hydrogen has been recently gaining traction as energy storage solution. Even though it can be produced from a variety of energy sources, its most attractive feature is the possibility of producing it from renewable energy resources and using it in various applications, either directly or after converting it into other chemical products both for energy consumption and further chemical processing.
Hydrogen is converted into electrical energy with high efficiencies in fuel cells. However, at ambient conditions (25 °C and 1 bar), hydrogen has low density of only 0.0813 g L
−1, which requires either a high pressure storage, e.g., 700 bar for automotive application, which increases the density to 40 g L
−1 and the corresponding volumetric energy density of 5.6 MJ L
−1, or liquid state storage, for many practical applications [
1,
2]. In both cases, hydrogen undergoes thermodynamic transformations, which can increase the overall storage and transportation costs significantly. Hydrogen can also be stored in carbon nanotubes, metallic hydrides, or complex hydrides under more moderate temperature and pressure conditions, but with only limited gravimetric density [
2,
3].
Another solution is to store and transport hydrogen indirectly in the chemical bonds of other chemicals, thereby increasing volumetric energy density and, consequently, transportability. Methanol and ethanol are some of the most common examples of such hydrogen rich alcohols, where hydrogen is chemically bonded with carbon, also known as carbon-based fuels [
4,
5,
6]. An issue that makes carbon-based fuels less appealing is the involvement of carbon dioxide, both in the synthesis process and in the power conversion. CO
2 has to be supplied to the chemical plant and released during the power production process. This means that two different cycles have to be closed: the hydrogen cycle and the carbon dioxide cycle. While hydrogen can be made entirely renewable by relying on renewable energy sources, such as wind and solar for the hydrogen production via water electrolysis, for the carbon cycle to close, the carbon dioxide should either come from carbon-capture or from biomass sources [
4].
An interesting alternative to this is the carbonless molecule of ammonia. In the case of ammonia, the gas involved is nitrogen, which means that the additional loop that has to be closed is the N
2 cycle. Nitrogen can be separated from air to feed the chemical process, usually the Haber–Bosh process, and released back to the atmosphere during energy consumption, making the overall system completely carbon-free [
7]. Recently, alternative green ammonia synthesis methods, including electrochemical synthesis at lower temperature and pressure conditions, are being investigated [
7,
8,
9].
Therefore, one of the advantages of the ammonia over other carbon-based fuels is the absence of CO
2 emissions at the point of use. For carbon-based fuels on the other hand, even when the carbon cycle is closed, CO
2 is still emitted locally and the power generation is not carbon-free. However, concerning local emissions, if NH
3 is used in combustion engines, the productions of NO
x should be considered [
7], an issue that is significantly alleviated or even eliminated when using NH
3 in fuel cell systems. Moreover, the availability of nitrogen is much higher than carbon dioxide. If, for instance, atmospheric air is considered as a source for both gases, nitrogen is available at high concentrations of around 79%, while carbon dioxide is in the range of hundreds of ppm (416 ppm as of June 2020 [
10]). However, it is worth mentioning that some sites, including biogas plants, bioethanol plants, and emission-intensive industries, can be used as sources of high concentration of CO
2 to produce carbon-based fuels, such as methanol through the power-to-X scheme [
4]. The concentration of N
2 and CO
2 in the feedstock is directly related not only to the separation cost, both in terms of energy and economics, but also to the availability of the quantities necessary to feed the respective fuel synthesis plants.
NH
3 has a high hydrogen concentration of 75 mol% and is liquid at a relatively low pressure of 10 bar, with high energy density of 15.6 MJ L
−1 compared to liquid hydrogen, which has an energy density of 9.1 MJ L
−1 at cryogenic temperature or compressed hydrogen, 5.6 MJ L
−1 at 70 MPa [
11]. Moreover, ammonia as a well-known fertilizer with a mature production technology, is one of the most produced chemicals worldwide, which can count on a well-established distribution network [
11]. However, its application in the energy sector is not significant and has only been investigated more recently as a fuel in several combustion-based energy systems. Experiences are reported in power systems based on gas turbine technology and on internal combustion engines [
7,
11]. In general, combustion of ammonia suffers from low flame speed and high resistance to auto-ignition, which calls for pre-mixing with other fuels as combustion promoters, e.g., hydrogen, that can be obtained with a partial decomposition of ammonia itself [
11].
An alternative solution is the use of ammonia in fuel cells [
12]. Fuel cells are electrochemical devices that directly transform the chemical energy of fuels into electricity without the typical emissions of combustion-based cycles. Ammonia can be coupled with fuel cells directly in solid oxide fuel cells (SOFC) and direct ammonia fuel cells (DAFC) or via a pre-decomposition into nitrogen and hydrogen in polymer electrolyte membrane fuel cells (PEMFC) [
13,
14]. Due to the peculiarity of fuel cells, the presence of nitrogen in the fuel stream only acts as a diluent of hydrogen and does neither generate any pollutant nor cause significant performance decay, allowing all fuel cells to operate with a mixture of nitrogen and hydrogen.
Even though it is possible to design a system were ammonia is decomposed and then the fuel stream is fed to any fuel cell unit, such a coupling should consider the presence of ammonia in the decomposed gas, which may poison the membrane electrode assembly (MEA) of certain fuel cell types. For instance, PEMFCs are extremely sensitive to ammonia contamination, where NH
3 poisons the Pt/C anode catalyst and reacts with the acidic Nafion membrane [
15,
16]. According to ISO14687-2, the maximum level of concentration of ammonia in hydrogen used in the PEM fuel cell vehicle is 0.1 ppm [
13]. To reach the ISO target, it is necessary to introduce a purification technology after ammonia decomposition [
13]. A concept design of a PEMFC-based system is proposed in [
17], where the decomposition reactor is heated electrically, reaching 99.5% conversion of ammonia. However, to the knowledge of the authors, only one experience that couples the ammonia decomposition reactor and PEM fuel cells has been reported, where 500–1000 ppm of ammonia impurity from the ammonia cracking chamber is eliminated in a selective ammonia oxidation (SAO) reactor before the produced hydrogen can be used in a PEMFC [
18]. The introduction of an additional gas purification phase increases the cost of the system and introduces additional energy losses. In general, the lower the tolerance of the end use device to ammonia, the higher the cost of the clean-up.
Another promising fuel cell technology is the high temperature PEM fuel cell (HT-PEMFC). In an HT-PEMFC, the traditional Nafion membrane, used in the low temperature PEM fuel cell technology, is substituted with a phosphoric acid-doped polybenzimidazole (PBI) membrane that operates in the temperature range 120–200 °C [
19]. The higher operating temperatures increase the tolerance to impurities, for example, CO tolerance increases up to 3% compared to only few ppm in low temperature PEM fuel cells [
19]. Due to this higher tolerance to impurities, HT-PEMFCs have been extensively studied in conjunction with methanol reformer, where the effects of the different resulting impurities, namely CO, CO
2, and CH
3OH have been reported with satisfactory tolerances for single cells and stacks [
20,
21,
22]. Moreover, steam methane reforming and HT-PEMFC systems are also available in the literature [
23,
24,
25].
However, despite the popularity of ammonia as a chemical commodity, and its potential as a storage for renewable hydrogen, its use in HT-PEMFCs has been largely ignored. While it is expected that traces of NH
3 will react with the PA-based electrolyte, the tolerance of an HT-PEMFC to NH
3 is only reported in [
26], where a general tolerance to percentage of NH
3 is reported from an internal report. The development of HT-PEMFC technology and the strong interest in the use of ammonia as a fuel opens the possibility for the development of ammonia-fed HT-PEM fuel cell systems.
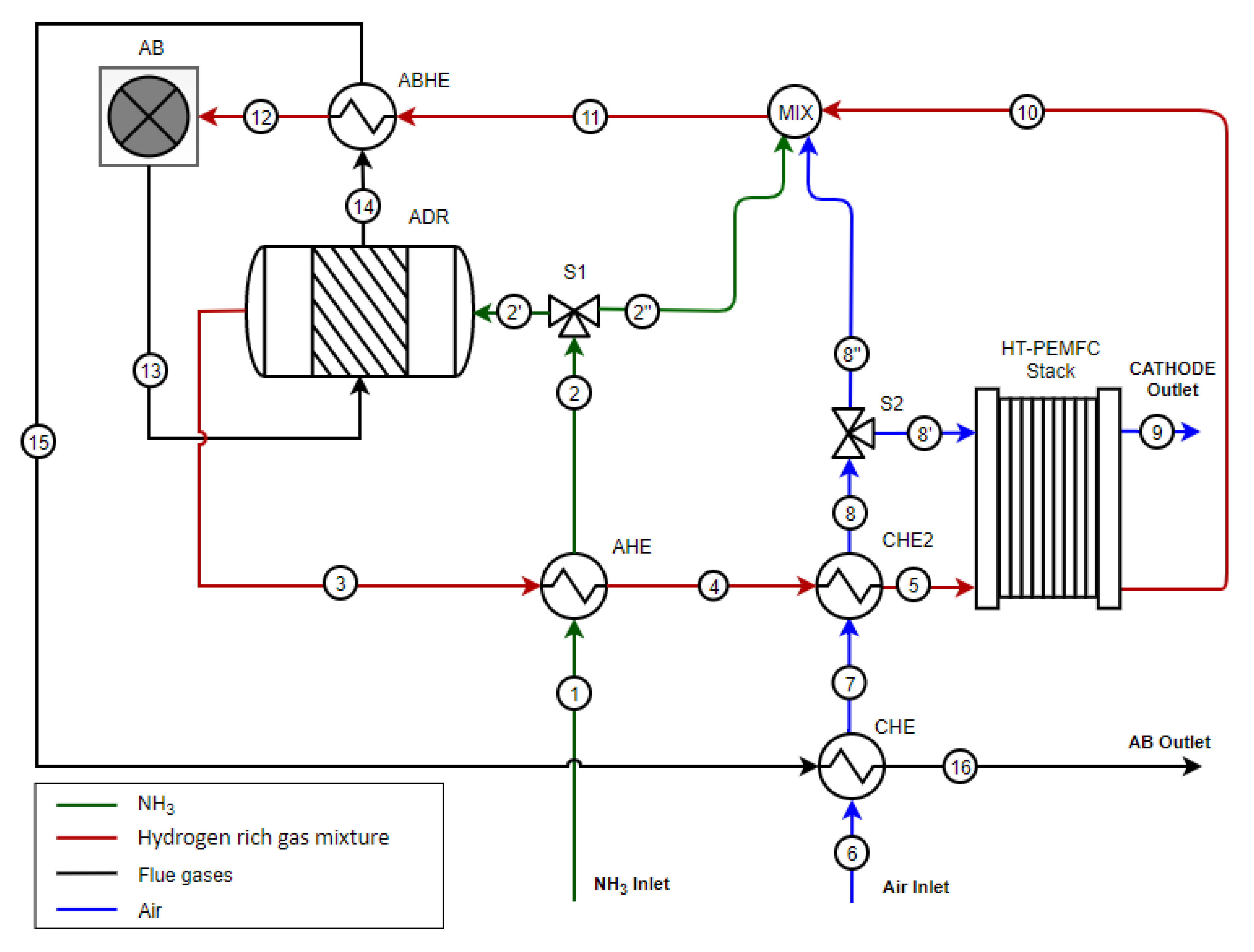
Therefore, this study presents for the first time in literature, a system study of an HT-PEMFC stack coupled with the ammonia decomposition unit. An integrated system design has been developed and thermodynamic studies of the system model were performed. A complete ammonia decomposition process is assumed, and hence, the corresponding high ammonia cracking temperatures, where full ammonia conversion is expected to take place, are used in the model. Consequently, the experiments were also done only for the dilution effect of nitrogen in the feed-gas and ammonia slip was ignored.
5. Conclusions
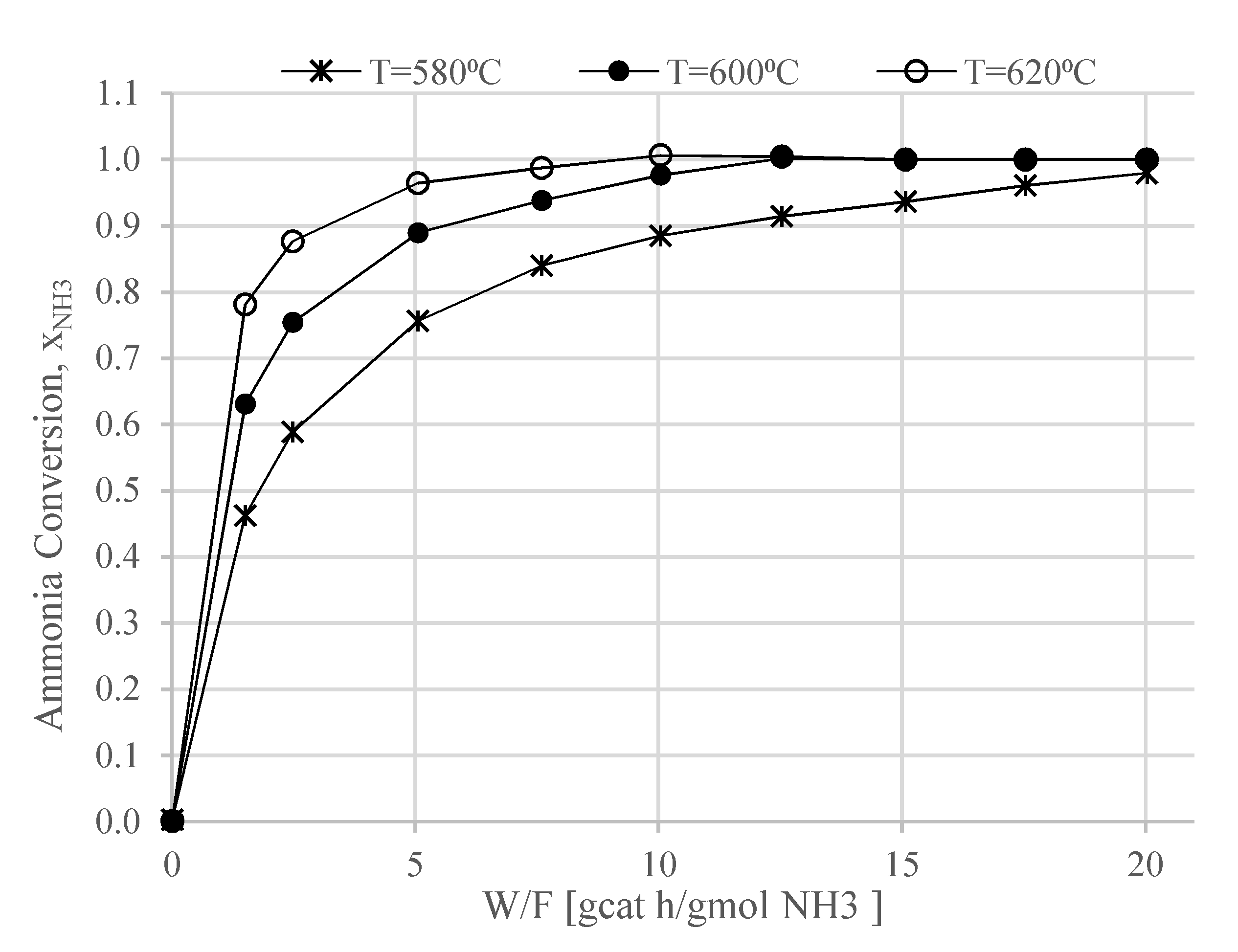
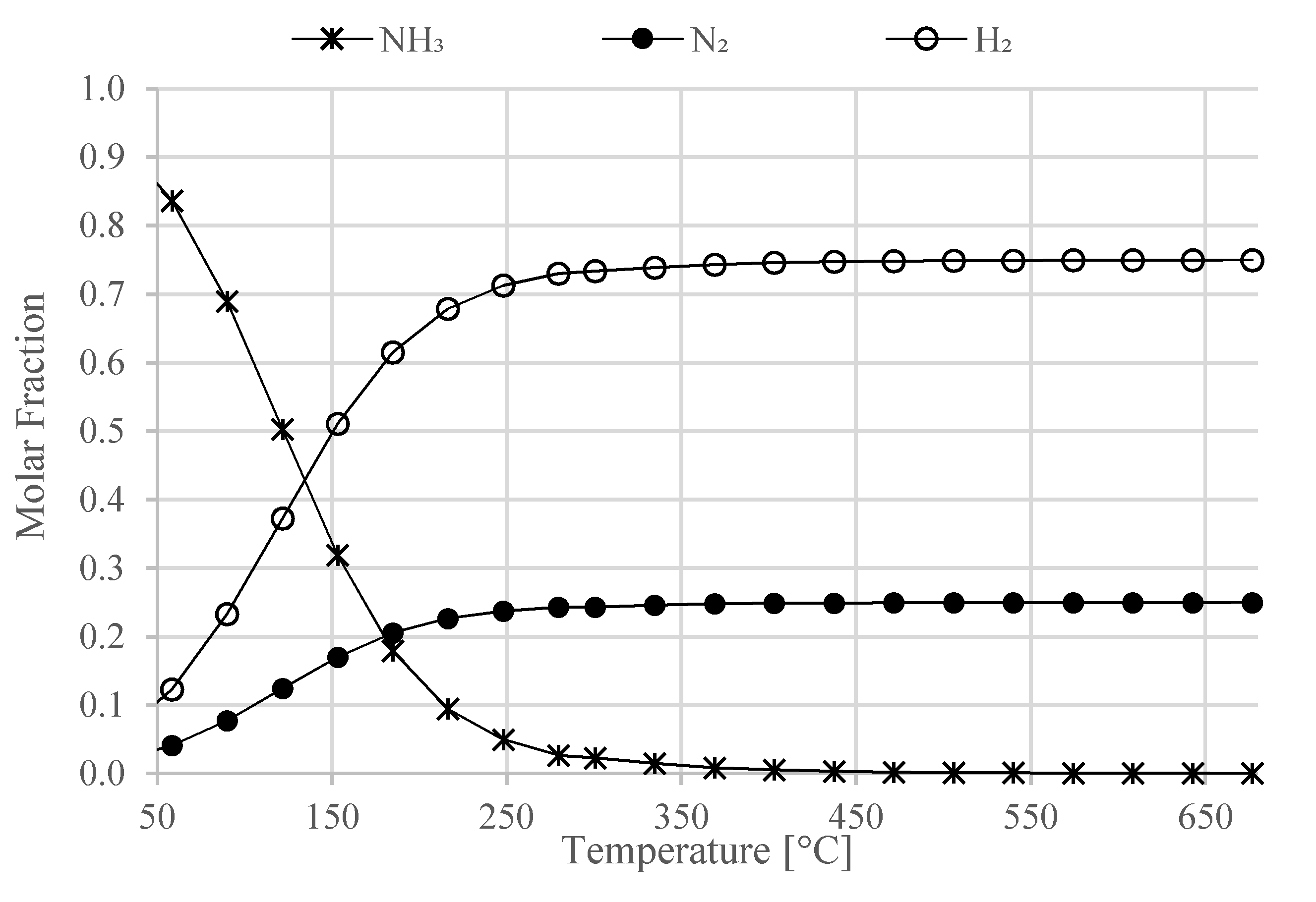
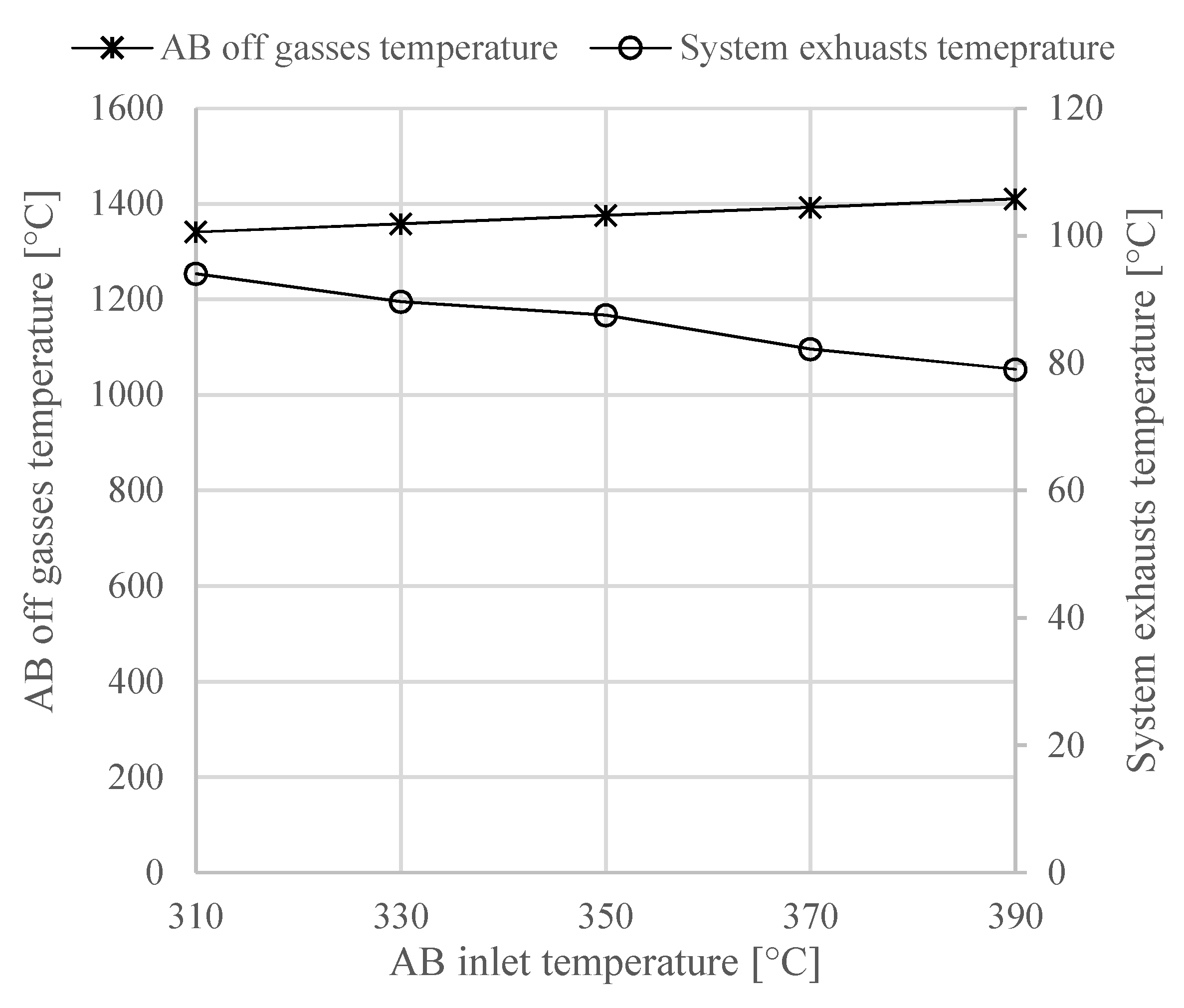
In this work, a design concept of a heat-integrated ammonia-fueled HT-PEMFC system is presented. Zero-dimensional models of the reactors and heat exchangers were developed, including those of the afterburner and the ammonia decomposition reactor. The study can be considered as a first attempt to demonstrate the thermodynamic feasibility of such a system. According to the chemical equilibrium studies in this work, the ammonia decomposition reactor can fully convert the ammonia into hydrogen and nitrogen. However, the process is endothermic and high operating temperatures of above 600 °C need to be ensured in the decomposition reactor, which in the proposed concept, is provided by the afterburner.
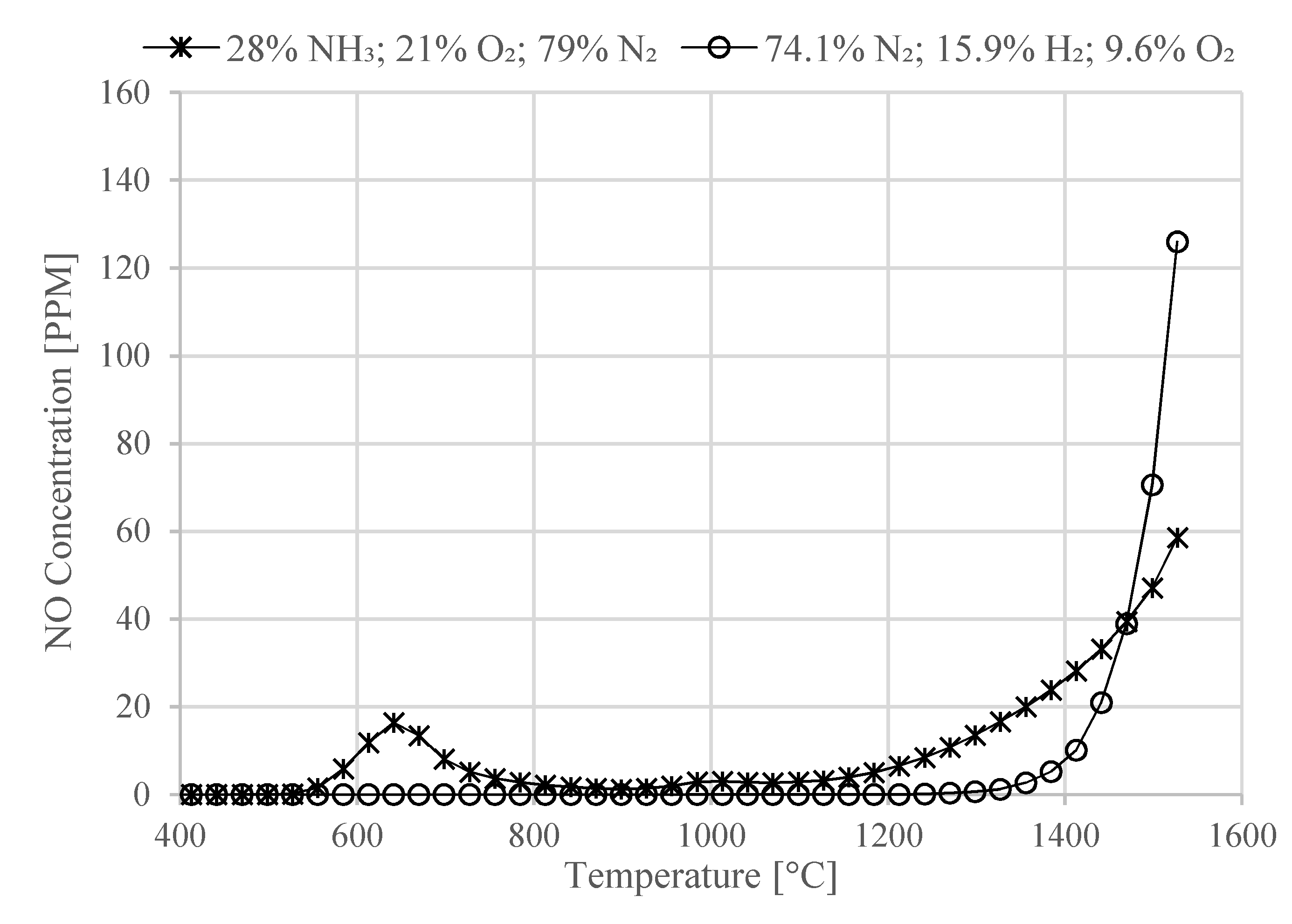
Moreover, the NOx emission of the combustion processes involved in the proposed system have been analytically investigated. The model showed that both ammonia/air combustion and H2/air combustion results in a limited amount of NO production of around 22 ppm and 4 ppm, respectively, below 1370 °C. Therefore, the afterburner temperature should be kept below this temperature in order to limit the production of NO during the combustion process. Since the combustion of ammonia is characterized by low flame speed and long ignition time, in the proposed concept, ammonia combustion is only used during the startup phase, and during normal operating conditions, the unreacted hydrogen at the anode outlet is used in the afterburner as it can sustain the system thermal requirements with a fuel cell anode stoichiometric ratio of 1.37.
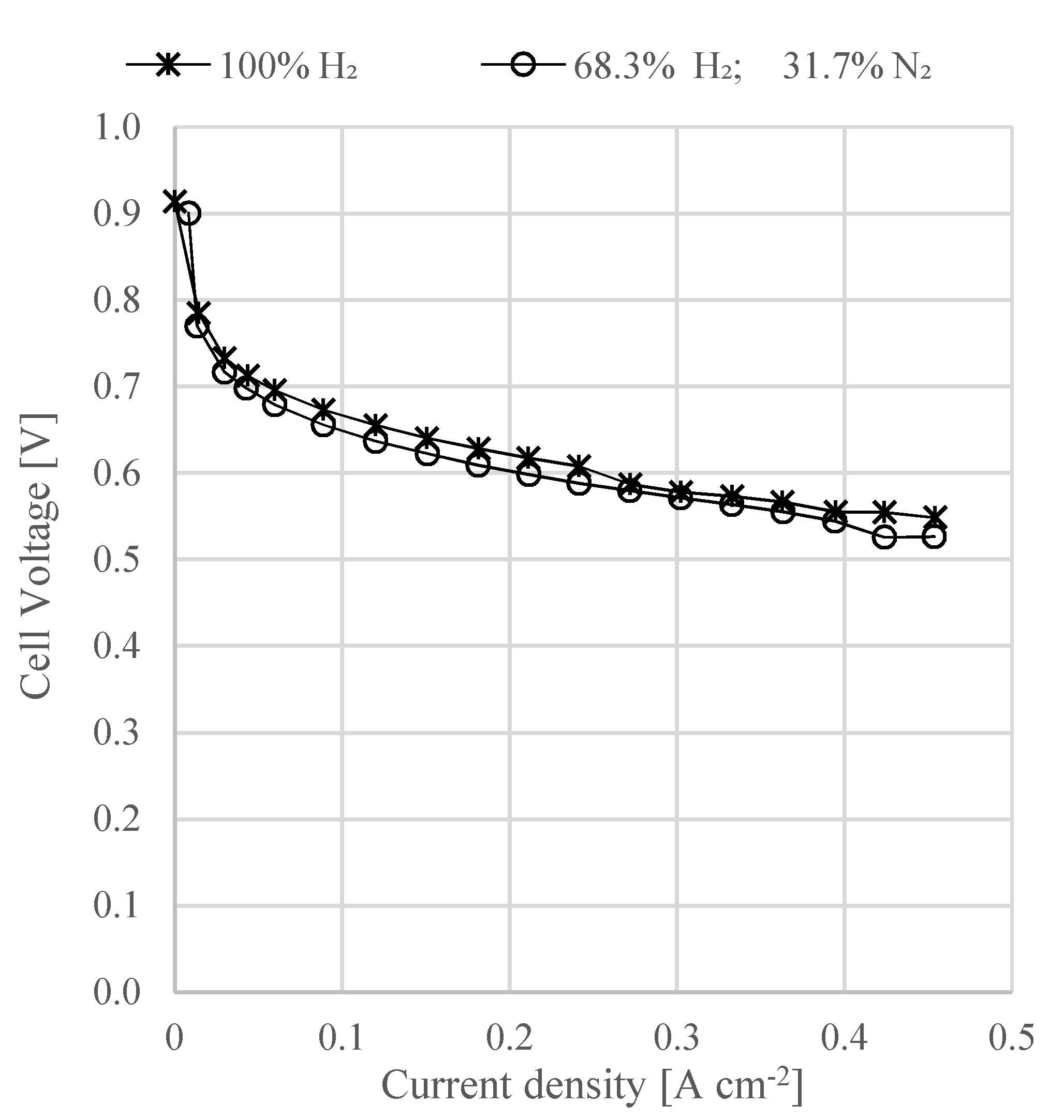
An experimental test on an HT-PEMFC short stack was also conducted to observe the cell electrical performance using a fuel mixture with a composition of hydrogen and nitrogen gas similar to the one at the outlet of the ammonia decomposition reactor. The experimental results showed that in the absence of the NH3 slip in the feed gas (via complete decomposition or gas purification), a hydrogen–nitrogen gas mixture at concentrations corresponding to a typical ammonia decomposition process does not degrade the fuel cell stack performance.
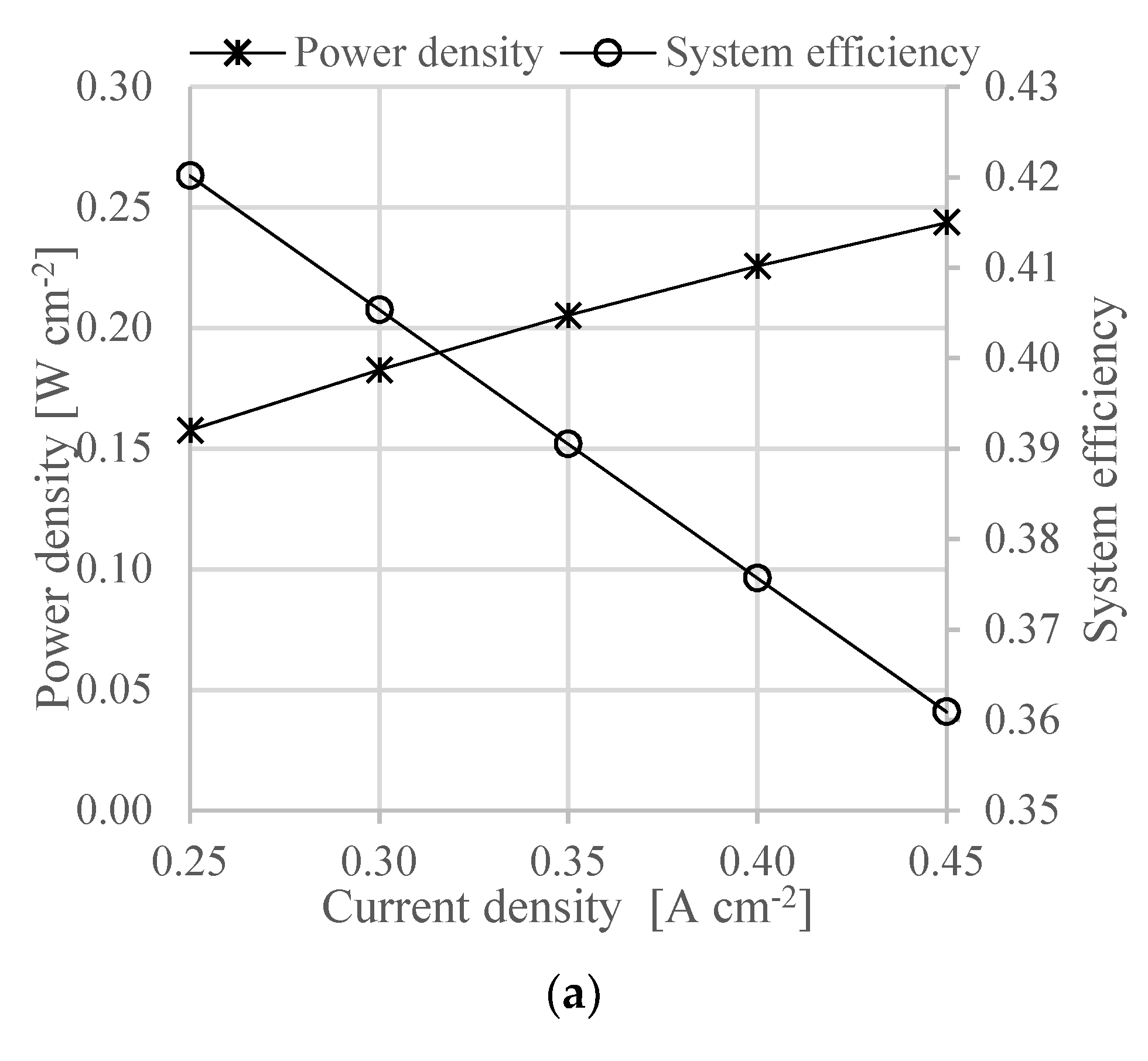
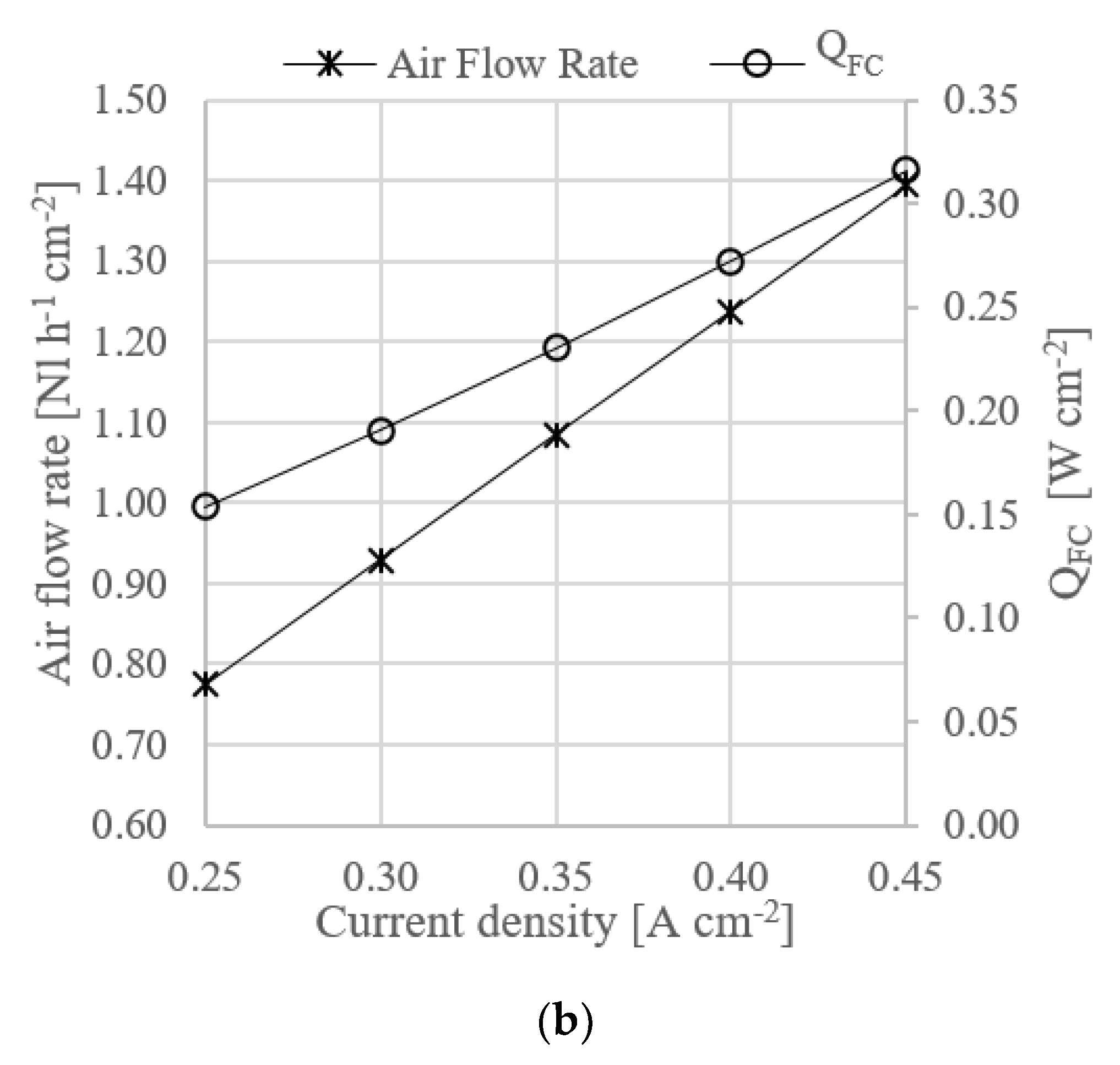
At system level, the proposed design allows to reach a total efficiency of 40.1% at a power density of 0.21 W cm−2. The heat recovery strategy allows to feed the ammonia decomposition reactor and to preheat the gas before entering the HT-PEMFC.