Life Cycle Assessment of Synthetic Natural Gas Production from Different CO2 Sources: A Cradle-to-Gate Study
Abstract
1. Introduction
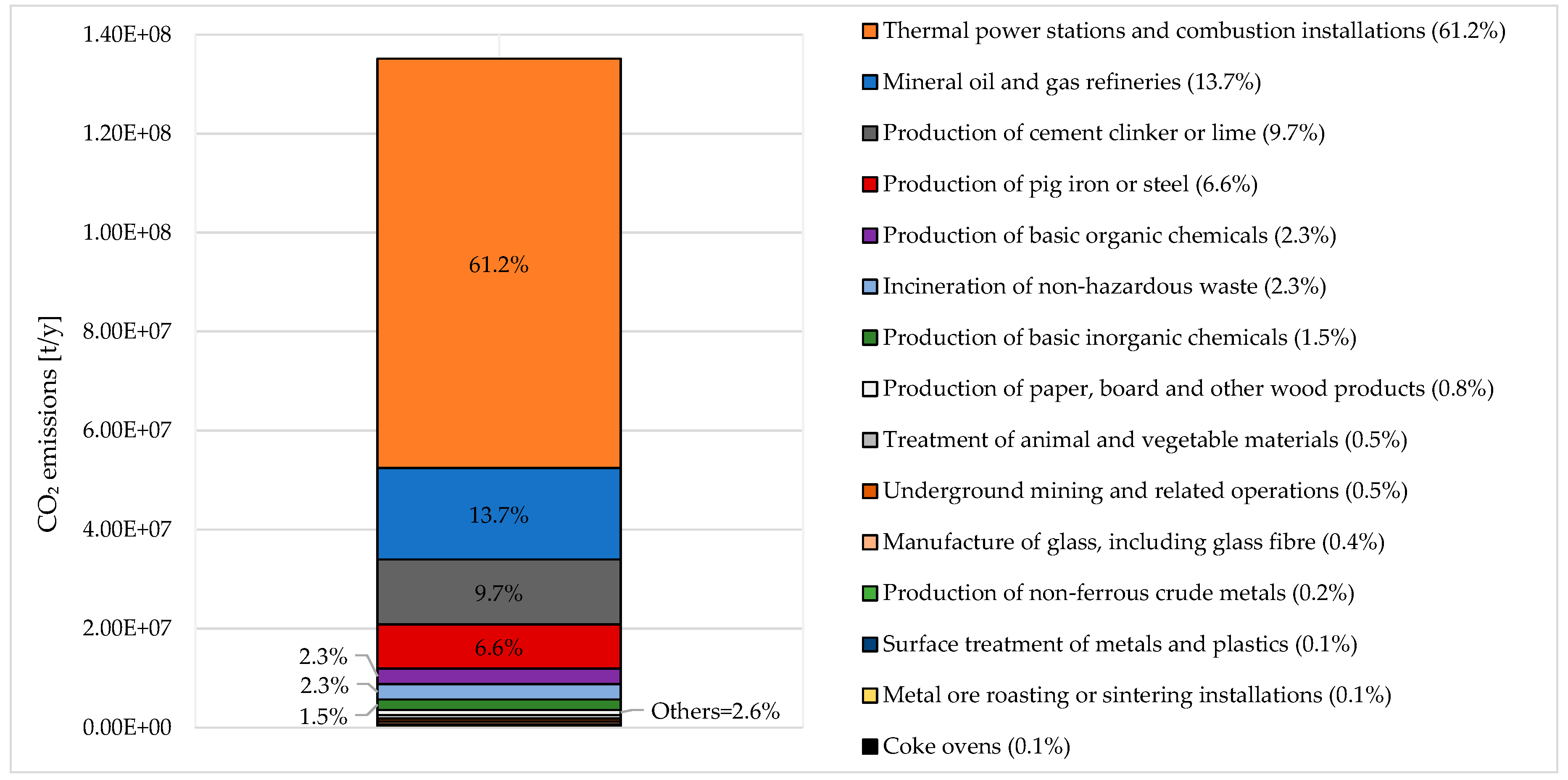
Carbon Dioxide Potential Sources and Italian Scenario
2. Materials and Methods
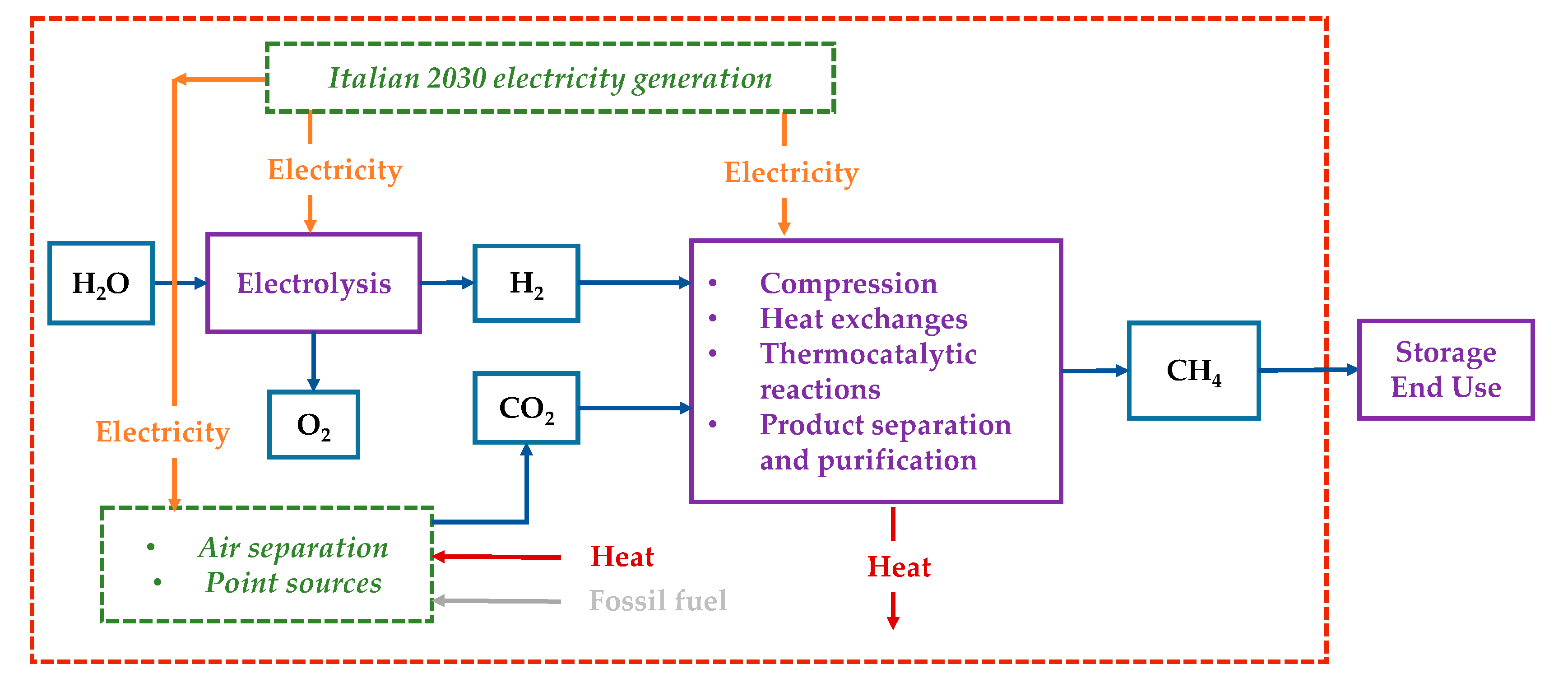
2.1. Assumptions and Study Boundaries
2.2. Data Sources
2.3. Handling Multi-Functionality
- with DAC since the only product is the captured CO2 itself;
- CO2 is considered a waste and not a co-product [40].
- This latter approach allows the comparison among different CO2 sources. It was adopted in the present work since CO2 is nowadays still considered a waste rather than a co-product.
- The electrolysis process instead is multi-functional since it produces hydrogen and oxygen. In the present work, three approaches were implemented:
- 100-0 allocation (base-case scenario): all the electrolysis process burdens were attributed to hydrogen. For this reason, this was the most precautionary case, and it was assumed as a base-case scenario.
- Mass allocation: in the electrolysis process, 7.94 kg O2/kg H2 was produced. Therefore, 89% of the burdens were attributed to oxygen and only 11% to hydrogen.
- Economic allocation: in the absence of reliable forecasts of chemical market prices for 2030, the oxygen and hydrogen market prices were estimated based on the average Producer Price Index (PPI) variation between December 2009 and December 2019 [42] (Table 4). The price of a chemical in a year could be calculated from its PPI, knowing its price and PPI in a reference year (see Equation (1)). Since chemical prices fluctuate greatly, we chose an average price between the years 2009 and 2019. The economic allocation was applied considering 1.21 $/kg H2 and 0.25 $/kg O2. Some considerations based on different oxygen/hydrogen price ratio would be drawn, even if results sensitivity analysis on the market prices was out of the paper scope.
3. Results and Discussion
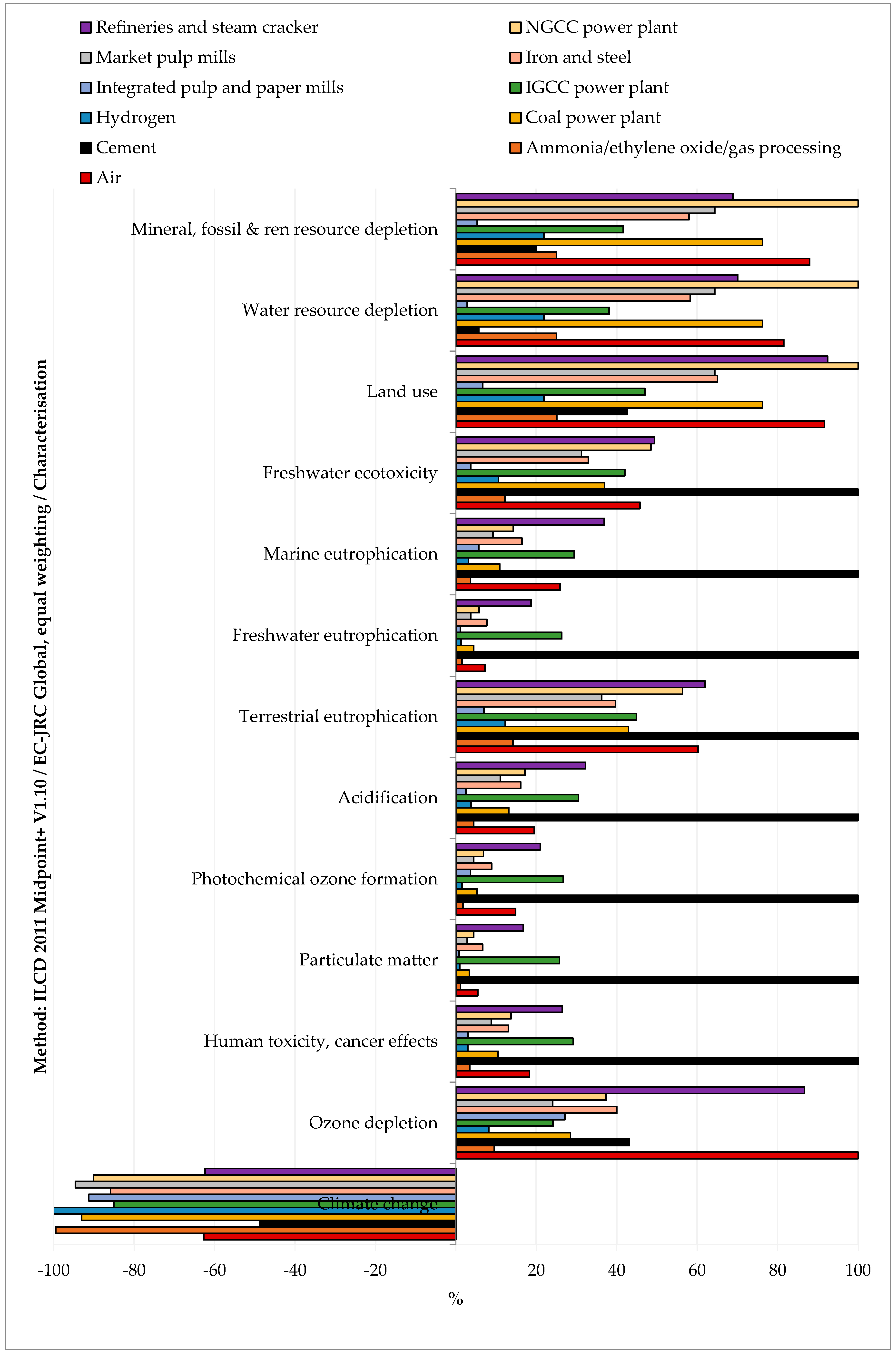
3.1. Impacts of CO2 Separation from Various Industrial Sources
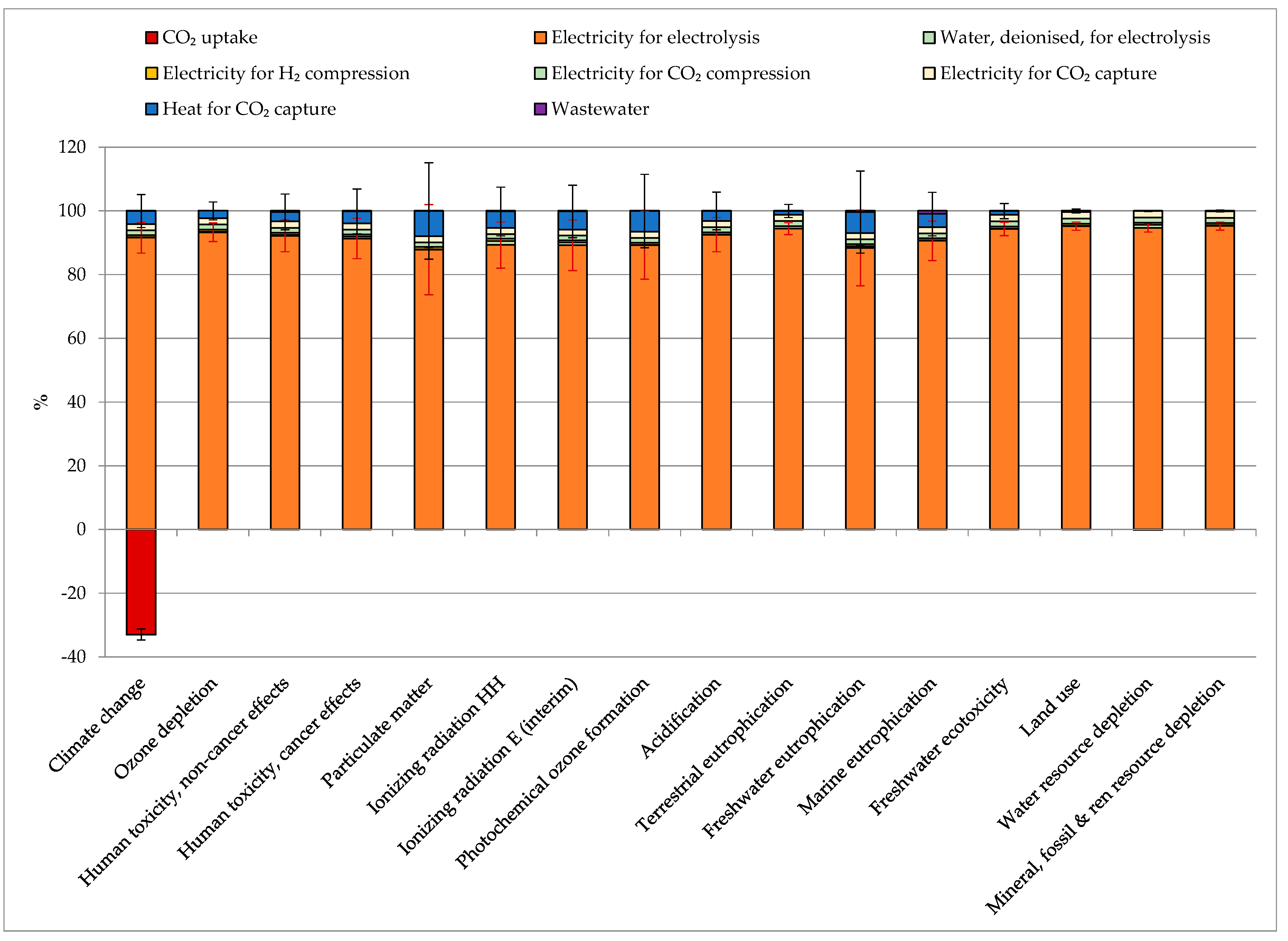
3.2. Base-Case Results
3.2.1. Contribution Analysis
3.2.2. Comparison among the CO2 Sources
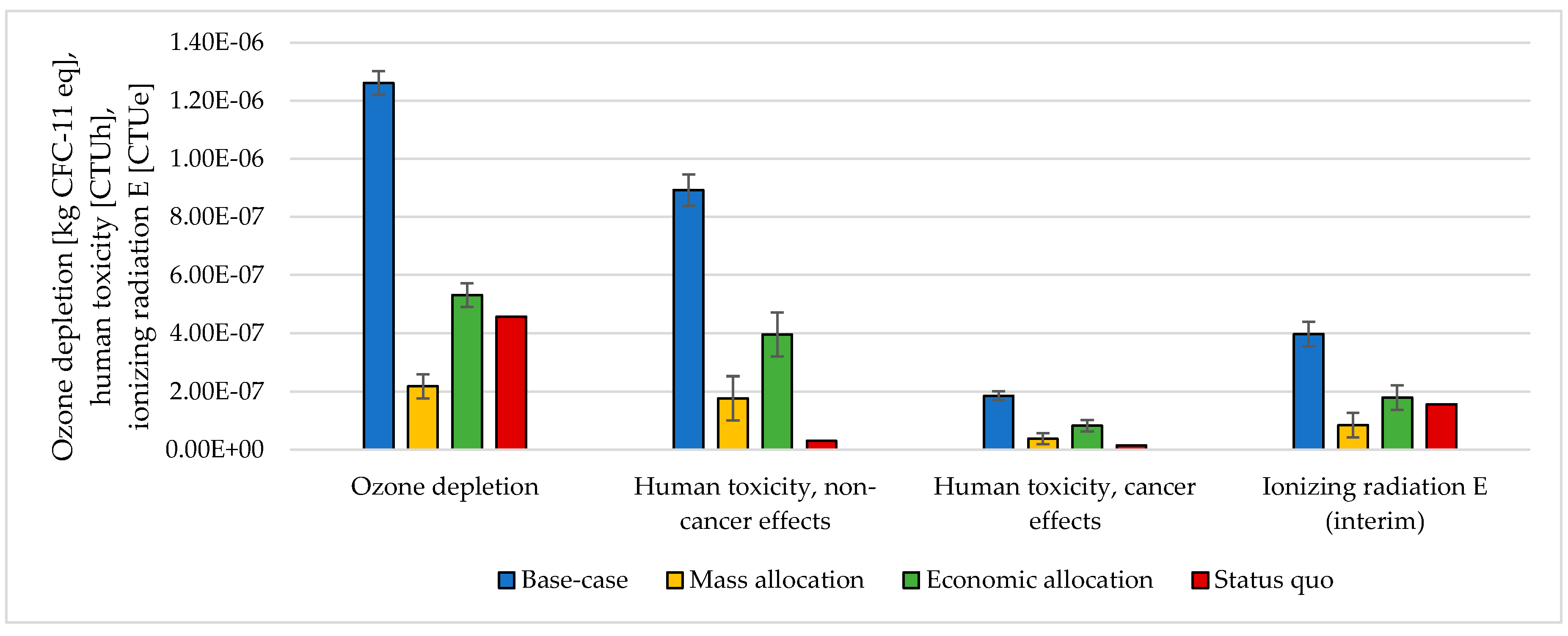
3.3. Handling Multi-Functionality in the Electrolysis Process
4. Conclusions and Outlook
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Nomenclature
| AEL | Alkaline Electrolysis |
| CC | Combined Cycle |
| CCU | Carbon Capture Utilization |
| CSP | Concentrated Solar Power |
| DAC | Direct Air Capture |
| DME | Dimethyl Ether |
| EU | European Union |
| FD | Fossil Depletion |
| FT | Fischer–Tropsch |
| GHG | Greenhouse Gases |
| GWI | Global Warming Impact |
| IGCC | Integrated Gasification Combined Cycle |
| ILCD | International Reference Life Cycle Data System |
| LCA | Life Cycle Assessment |
| MU | Multi-Functionality |
| NG | Natural Gas |
| NGCC | Natural Gas Combined Cycle |
| PEMEL | Proton Exchange Membrane Electrolysis |
| PPI | Producer Price Index |
| PtH | Power to Hydrogen |
| PtG | Power to Gas |
| RES | Renewable Energy Sources |
| SNG | Synthetic Natural Gas |
References
- Lewandowska-Bernat, A.; Desideri, U. Opportunities of power-to-gas technology. Energy Procedia 2017, 105, 4569–4574. [Google Scholar] [CrossRef]
- Bujnicki, J.; Dykstra, P.; Fortunato, E.; Heuer, R.-D.; Keskitalo, C.; Nurse, P. Novel Carbon Capture and Utilisation Technologies; Publication Office European Union: Luxembourg, 2018. [Google Scholar] [CrossRef]
- Pérez-Fortes, M.; Tzimas, E. Techno-Economic and Environmental Evaluation of CO2 Utilisation for Fuel Production; Synthesis of methanol and formic acid; EUR 27629 EN; JRC Science Hub: ZG Petten, The Netherlands, 2016. [Google Scholar] [CrossRef]
- Matzen, M.; Demirel, Y. Methanol and dimethyl ether from renewable hydrogen and carbon dioxide: Alternative fuels production and life-cycle assessment. J. Clean. Prod. 2016, 139, 1068–1077. [Google Scholar] [CrossRef]
- Matzen, M.; Alhajji, M.; Demirel, Y. Technoeconomics and sustainability of renewable methanol and ammonia productions using wind power-based hydrogen. J. Adv. Chem. Eng. 2015, 5. [Google Scholar] [CrossRef]
- Van Der Giesen, C.; Kleijn, R.; Kramer, G.J. Energy and climate impacts of producing synthetic hydrocarbon fuels from CO2. Environ. Sci. Technol. 2014, 48, 7111–7121. [Google Scholar] [CrossRef]
- Hoppe, W.; Bringezu, S.; Thonemann, N. Comparison of global warming potential between conventionally produced and CO2-based natural gas used in transport versus chemical production. J. Clean. Prod. 2016, 121, 231–237. [Google Scholar] [CrossRef]
- Sternberg, A.; Bardow, A. Life cycle assessment of power-to-gas: Syngas vs methane. ACS Sustain. Chem. Eng. 2016, 4, 4156–4165. [Google Scholar] [CrossRef]
- Sternberg, A.; Bardow, A. Power-to-What?-Environmental assessment of energy storage systems. Energy Environ. Sci. 2015, 8, 389–400. [Google Scholar] [CrossRef]
- Hoppe, W.; Thonemann, N.; Bringezu, S. Life cycle assessment of carbon dioxide–based production of methane and methanol and derived polymers. J. Ind. Ecol. 2018, 22, 327–340. [Google Scholar] [CrossRef]
- Reiter, G.; Lindorfer, J. Evaluating CO2 sources for power-to-gas applications—A case study for Austria. J. CO2 Util. 2015, 10, 40–49. [Google Scholar] [CrossRef]
- Zhang, X.; Bauer, C.; Mutel, C.L.; Volkart, K. Life cycle assessment of power-to-gas: Approaches, system variations and their environmental implications. Appl. Energy 2017, 190, 326–338. [Google Scholar] [CrossRef]
- Meylan, F.D.; Moreau, V.; Erkman, S. CO2 utilization in the perspective of industrial ecology, an overview. J. CO2 Util. 2015, 12, 101–108. [Google Scholar] [CrossRef]
- Cuéllar-Franca, R.M.; Azapagic, A. Carbon capture, storage and utilisation technologies: A critical analysis and comparison of their life cycle environmental impacts. J. CO2 Util. 2015, 9, 82–102. [Google Scholar] [CrossRef]
- Thonemann, N. Environmental impacts of CO2-based chemical production: A systematic literature review and meta-analysis. Appl. Energy 2020, 263, 114599. [Google Scholar] [CrossRef]
- Von der Assen, N.; Müller, L.J.; Steingrube, A.; Voll, P.; Bardow, A. Selecting CO2 sources for CO2 utilization by environmental-merit-order curves. Environ. Sci. Technol. 2016, 50, 1093–1101. [Google Scholar] [CrossRef]
- Bargiacchi, E.; Antonelli, M.; Desideri, U. A comparative assessment of Power-to-Fuel production pathways. Energy 2019, 183, 1253–1265. [Google Scholar] [CrossRef]
- Ritchie, H.; Roser, M. CO2 and Greenhouse Gas Emissions; Our World In Data Organization: England/Wales, UK, 2017; Available online: https://outwolrdindata.org/co2-and-other-greenhouse-gas-emissions (accessed on 27 July 2020).
- European Union. Greenhouse gas emission statistics-emission inventories. Eurostat 2018, 63, 175–180. [Google Scholar]
- Center for Climate and Energy Solution. Global Emissions 2019. Available online: https://www.c2es.org/content/international-emissions/ (accessed on 27 July 2020).
- Kätelhön, A.; Meys, R.; Deutz, S.; Suh, S.; Bardow, A. Climate change mitigation potential of carbon capture and utilization in the chemical industry. Proc. Natl. Acad. Sci. USA 2019, 166, 11187–11194. [Google Scholar] [CrossRef]
- Normann, F.; Gararsdóttir, S.Ó.; Skagestad, R.; Mathisen, A.; Johnsson, F. Partial capture of carbon dioxide from industrial sources—A discussion on cost optimization and the CO2 capture rate. Energy Procedia 2017, 114, 113–121. [Google Scholar] [CrossRef]
- Naims, H. Economics of carbon dioxide capture and utilization—A supply and demand perspective. Environ. Sci. Pollut. Res. 2016. [Google Scholar] [CrossRef]
- European Environment Agency. European Pollutant Release and Tranfer Register 2017. Available online: https://prtr.eea.europa.eu/#/pollutantreleases (accessed on 27 July 2020).
- International Organization for Standardization. ISO 14040 Environmental Management-Life Cycle Assessment-Principles and Framework; International Organization for Standardization: Vernier, Geneva, Switzerland, 1997; ICS 13.020.10. [Google Scholar]
- International Organization for Standardization. ISO 14044 Environmental Management-Life Cycle Assessme-Requirements and Guidelines; International Organization for Standardization: Vernier, Geneva, Switzerland, 2006; ICS 13.020.10. [Google Scholar] [CrossRef]
- Swiss Centre for Life Cycle Inventories. Ecoinvent Data Version 3.5. Available online: https://www.ecoinvent.org/ (accessed on 3 February 2020).
- Wedema, B.P.; Bauer, C.; Hischier, R.; Mutel, C.; Nemecek, T.; Reinhard, J.; Vadenbo, C.O.; Wernet, G. Data Quality Guideline For The Ecoinvent Database Version 3; Ecoinvent Report 1 (v3); Swiss Cent Life Cycle Invent: St. Gallen, Switzerland, 2013; Volume 3. [Google Scholar]
- European Commission-Joint Research Centre-Institute for Environment and Sustainability. International Reference Life Cycle Data System (ILCD) Handbook: Specific Guide for Life Cycle Inventory Data Sets; EUR 24709 EN; Publications Office of the European Union: Luxembourg, 2010. [Google Scholar] [CrossRef]
- Aspentech. Aspen Plus. Available online: https://www.aspentech.com/en/products/engineering/aspen-plus (accessed on 30 April 2020).
- Italian Ministry of Economic Development. Proposta di Piano Nazionale Integrato per l’Energia e il Clima. 2018. Available online: https://www.mise.gov.it/images/stories/documenti/Proposta_di_Piano_Nazionale_Integrato_per_Energia_e_il_Clima_Italiano.pdf (accessed on 27 July 2020).
- Ministero dello Sviluppo Economico, Ministero dell’Ambiente e della Tutela del Territorio e del Mare. Strategia Energetica Nazionale (SEN) 2017, 308. Available online: https://www.mise.gov.it/images/stories/documenti/Testo-integrale-SEN-2017.pdf (accessed on 27 July 2020).
- Gestore dei Servizi Energetici GSE S.p.A. Direzione. Rapporto Statistico Solare Fotovoltaico 2018-Il Solare Fotovoltaico in Italia Stato di Sviluppo e Trend del Settore. 2018. Available online: https://www.gse.it/documenti_site/Documenti%20GSE/Rapporti%20statistici/Solare%20Fotovoltaico%20-%20Rapporto%20Statistico%202018.pdf (accessed on 27 July 2020).
- Bhandari, R.; Trudewind, C.A.; Zapp, P. Life cycle assessment of hydrogen production via electrolysis-A review. J. Clean. Prod. 2014, 85, 151–163. [Google Scholar] [CrossRef]
- Müller, L.J.; Kätelhön, A.; Bachmann, M.; Zimmermann, A. A guideline for life cycle assessment of carbon capture and utilization. Front. Energy Res. 2020, 8, 1–20. [Google Scholar] [CrossRef]
- Thonemann, N.; Maga, D.; Petermann, C. Handling of multi-functionality in life cycle assessments for steel mill gas based chemical production. Chemie Ingenieur Technik 2018, 90, 1576–1586. [Google Scholar] [CrossRef]
- Curran, M.A. Co-product and input allocation approaches for creating life cycle inventory data: A literature review. Int. J. Life Cycle Assess 2007, 12, 65–78. [Google Scholar]
- Jung, J.; Von Der Assen, N.; Bardow, A. Sensitivity coefficient-based uncertainty analysis for multi-functionality in LCA. Int. J. Life Cycle Assess 2014, 19, 661–676. [Google Scholar] [CrossRef]
- Pelletier, N.; Ardente, F.; Brandão, M.; De Camillis, C.; Pennington, D. Rationales for and limitations of preferred solutions for multi-functionality problems in LCA: Is increased consistency possible? Int. J. Life Cycle Assess 2015, 20, 74–86. [Google Scholar] [CrossRef]
- Von der Assen, N.; Jung, J.; Bardow, A. Life-Cycle assessment of carbon dioxide capture and utilization: Avoiding the pitfalls. Energy Environ. Sci. 2013. [Google Scholar] [CrossRef]
- Von der Assen, N.; Voll, P.; Peters, M.; Bardow, A. Life cycle assessment of CO2 capture and utilization: A tutorial review. Chem. Soc. Rev. 2014, 43, 7982–7994. [Google Scholar] [CrossRef]
- Texas A&M University Libraries. Research Guides-Bulk chemical Prices. Last. Available online: https://tamu.libguides.com/c.php?g=587308&p=4076262 (accessed on 30 April 2020).
- FRED Economic Data St.Louis Fed. Producer Price Index by Industry: Industrial Gas Manufacturing: Argon and Hydrogen. Available online: https://fred.stlouisfed.org/series/PCU325120325120C (accessed on 30 April 2020).
- Dillich, S.; Ramsden, T.; Melaina, M. Hydrogen production cost using low-cost natural gas. DOE Hydrog. Fuel Cells Progr. Rec. 2012, 3–8. Available online: https://www.hydrogen.energy.gov/pdfs/12024_h2_production_cost_natural_gas.pdf (accessed on 27 July 2020).
- FRED Economic Data St.Louis Fed. Producer Price Index by Industry: Industrial Gas Manufacturing: Oxygen. Available online: https://fred.stlouisfed.org/series/PCU325120325120A (accessed on 30 April 2020).
- Wikipedia. Prices of Chemical Elements. Available online: https://en.wikipedia.org/wiki/Prices_of_chemical_elements#cite_note-cryocoolers11-26 (accessed on 30 April 2020).
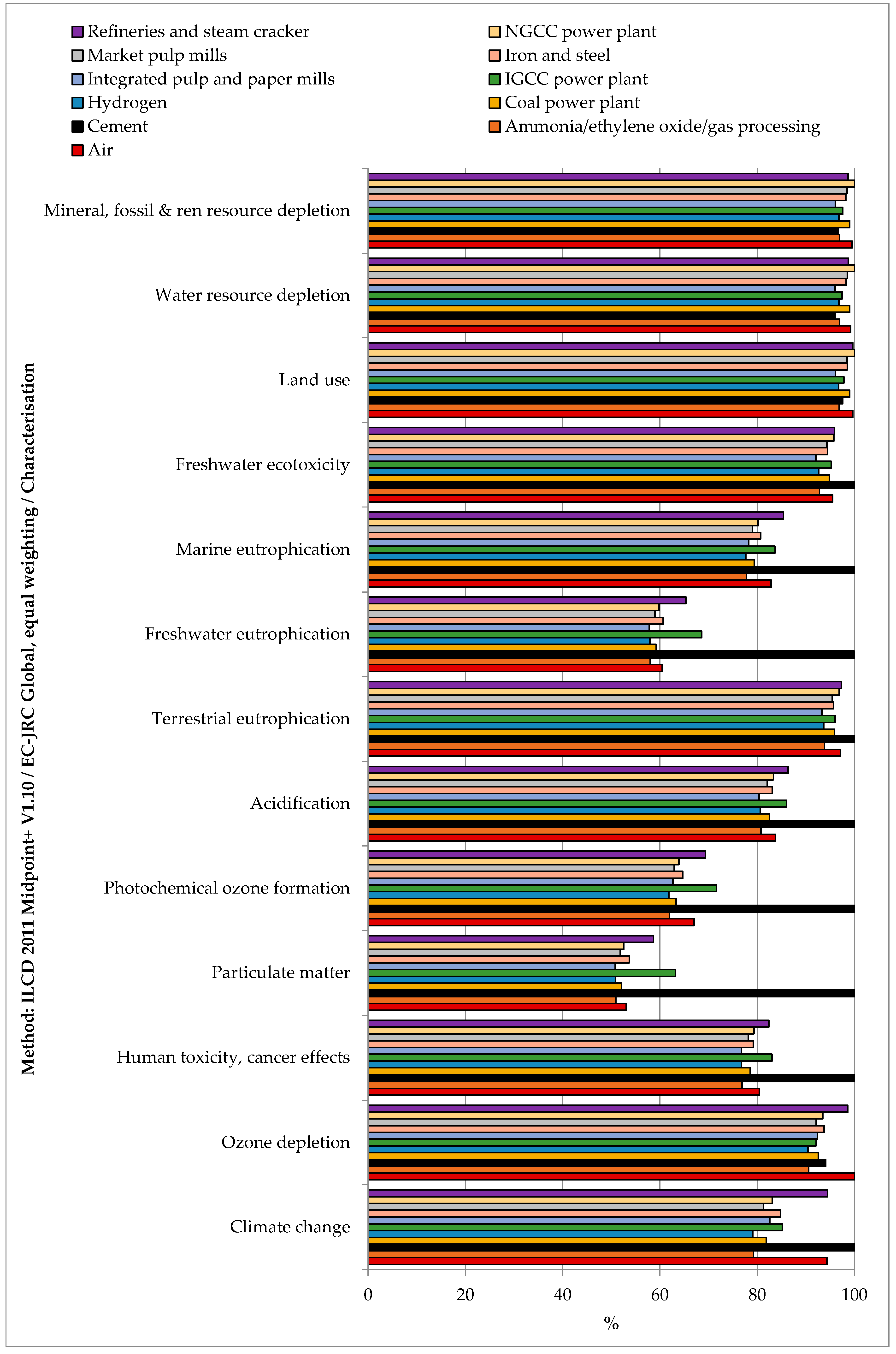
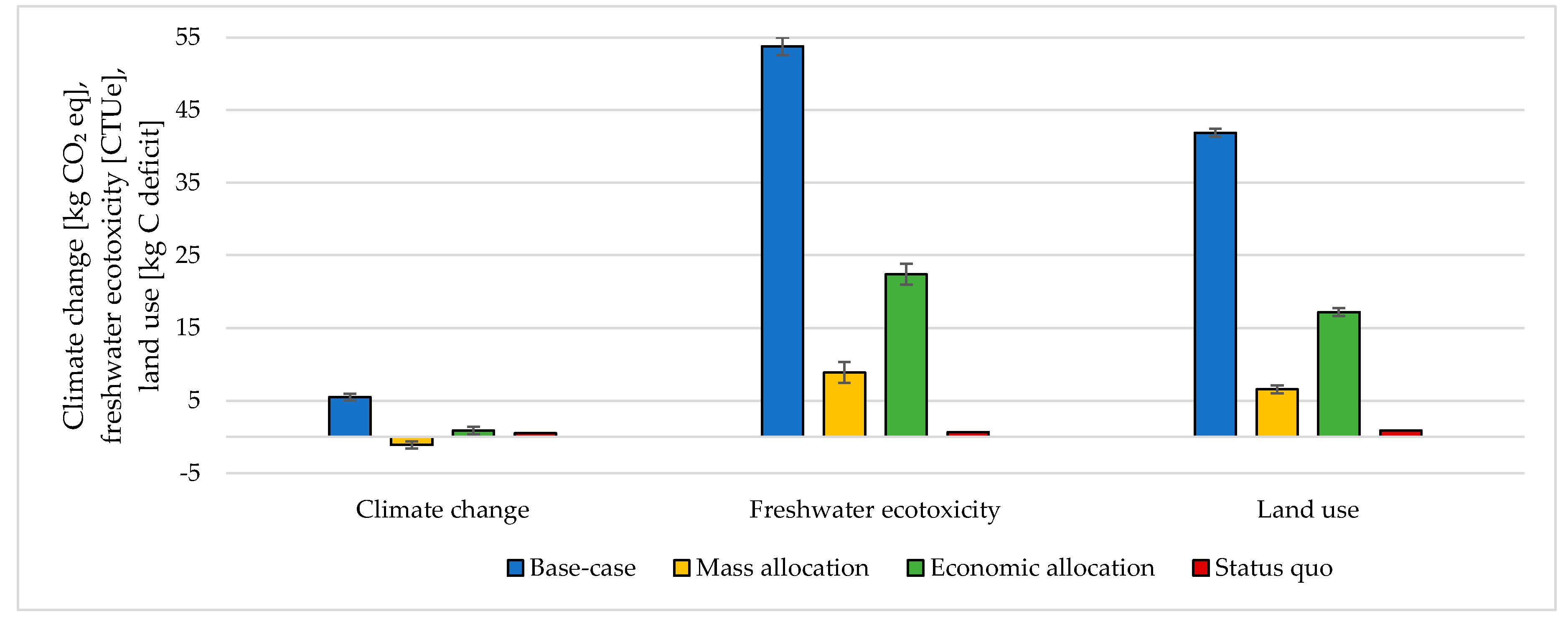
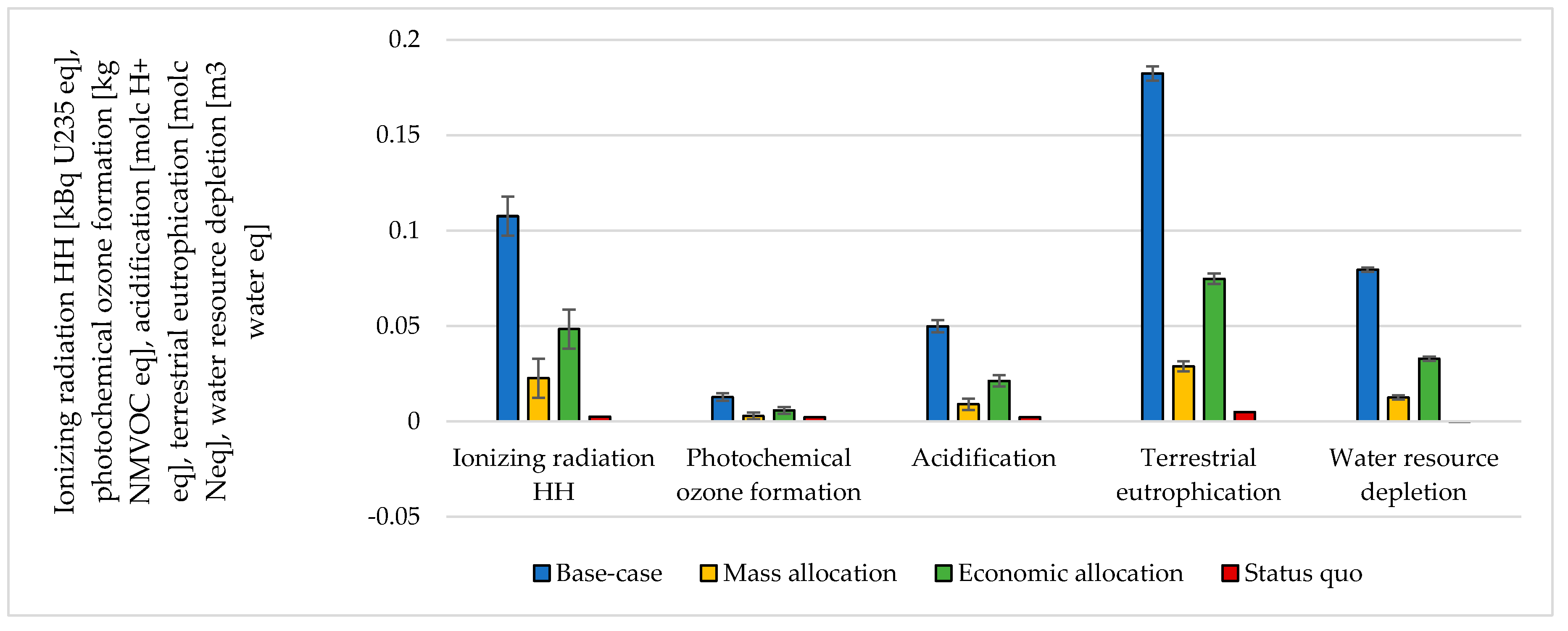
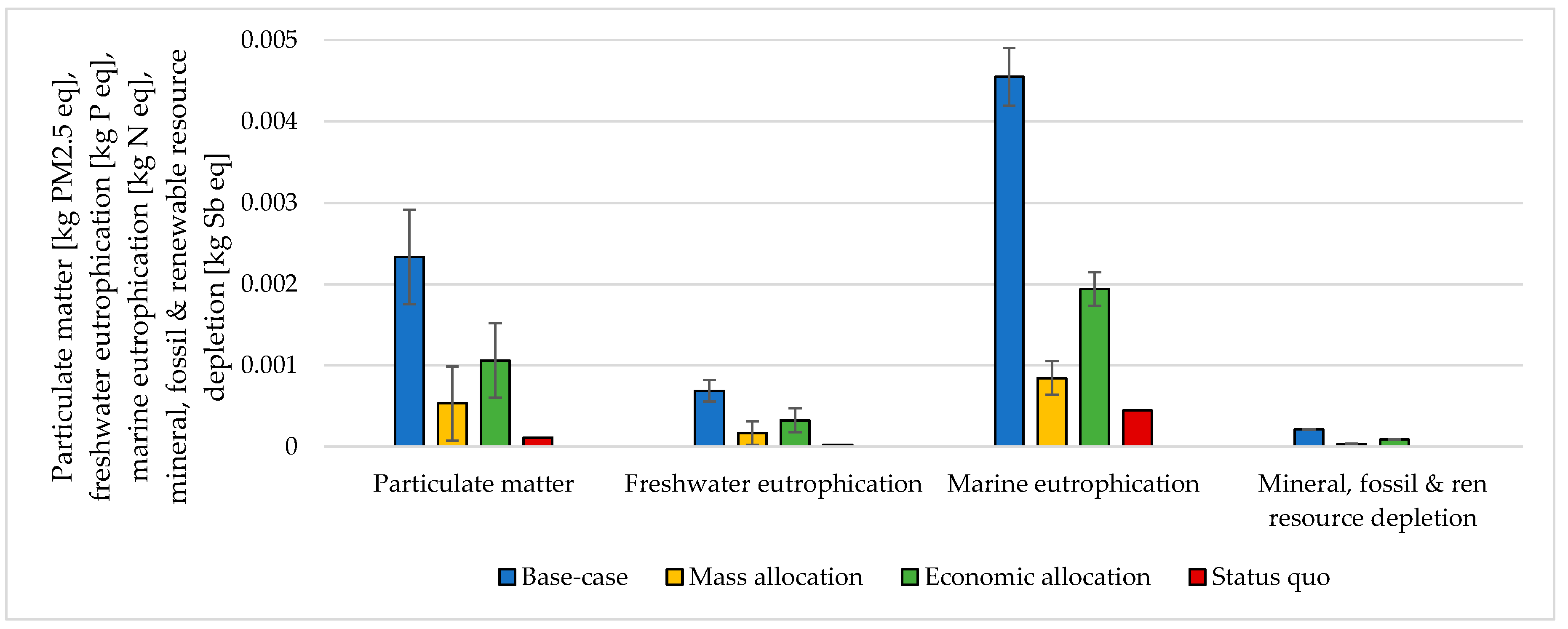
| CO2 Emitting Source | Capture Rate | CO2 Purity |
|---|---|---|
| NGCC power plant | 85–100% | ≥99.9% |
| Refineries and steam crackers | 40–50% | ≥95% |
| Coal power plant | 85–100% | ≥99.9% |
| Integrated pulp and paper mills | N.A. | N.A. |
| Market pulp mills | N.A. | N.A. |
| Iron and steel | 50% | ≥95% |
| Cement | 85–100% | ≥95% |
| IGCC power plant | 85–100% | ≥99.9% |
| Ammonia | 85–100% | ≥95% |
| Ethylene oxide | 90–99% | >98% with post-combustion; 85% with oxyfuel purposes |
| Gas processing | N.A. | N.A. |
| Hydrogen | 85–100% | ≥95% |
| Installed Technology | Installed Capacity (TWh) | Share (%) |
|---|---|---|
| Gas | 118.00 | 38.5 |
| Gas, CC | 36.09 | 11.8 |
| Gas, conventional | 9.69 | 3.2 |
| Gas, CC, 400 MW | 44.68 | 14.6 |
| Gas, Conventional, 100 MW | 27.54 | 9.0 |
| Coal | 0 | 0 |
| Oil and others | 2.00 | 0.7 |
| Oil, conventional | 0.43 | 0.1 |
| Oil, cogeneration | 1.57 | 0.5 |
| Geothermic | 7.10 | 2.3 |
| Bioenergy | 15.70 | 5.1 |
| Biogas, gas engine | 11.96 | 3.9 |
| Wood chips | 3.74 | 1.2 |
| Solar | 74.50 | 24.3 |
| PV, rooftop | 30.15 | 9.8 |
| PV, ground mounted | 43.06 | 14.0 |
| CSP | 1.27 | 0.4 |
| Wind | 40.10 | 13.1 |
| Onshore, <1 MW | 10.70 | 3.5 |
| Onshore, 1–3 MW | 24.17 | 7.9 |
| Onshore, >3 MW | 3.27 | 1.1 |
| Offshore | 1.96 | 0.6 |
| Hydro | 49.30 | 16.1 |
| Hydro, Pumped storage | 1.43 | 0.5 |
| Hydro, Reservoir | 30.64 | 10.0 |
| Hydro, Run-on | 17.23 | 5.6 |
| Tot | 306.7 | 100 |
| Tot RES | 186.7 | 60.9 |
| Average Energy Demand (GJ/(t CO2)) | |||||
|---|---|---|---|---|---|
| Type of CO2 Source | CO2 Concentration | Electricity | Heat | Natural Gas | Coal |
| Air | 400 ppm | 1.29 | 4.19 | ||
| NGCC power plant | 3–4% | 1.60 | |||
| Refineries and steam cracker | 3–13% | 0.91 | 3.16 | ||
| Coal power plant | 12–15% | 1.22 | |||
| Integrated pulp and paper mills | 7–20% | 0.04 | 1.57 | ||
| Market pulp mills | 7–20% | 1.03 | |||
| Iron and steel | 17–35% | 0.87 | 0.95 | ||
| Cement | 14–33% | 0.09 | 3.35 | ||
| IGCC power plant | 1/40% | 0.61 | 0.81 | ||
| Ammonia/ethylene oxide/gas processing | ≈100% | 0.40 | 0.01 | ||
| Hydrogen | ≈100% | 0.35 | |||
| Chemical | Reference | December 2009 | December 2019 | Current (Average) | Allocation Factors | |
|---|---|---|---|---|---|---|
| Hydrogen | PPI index | 105 (2012) [43] | 92.9 [43] | 89.3 [43] | 37.9% | |
| Price ($/kg) | 1.39 (2012) [44] | 1.23 | 1.18 | 1.21 | ||
| Oxygen | PPI index | 170 (2001) [45] | 230.3 [45] | 311.0 [45] | 62.1% | |
| Price ($/kg) | 0.154 (2001) [46] | 0.21 | 0.28 | 0.25 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bargiacchi, E.; Thonemann, N.; Geldermann, J.; Antonelli, M.; Desideri, U. Life Cycle Assessment of Synthetic Natural Gas Production from Different CO2 Sources: A Cradle-to-Gate Study. Energies 2020, 13, 4579. https://doi.org/10.3390/en13174579
Bargiacchi E, Thonemann N, Geldermann J, Antonelli M, Desideri U. Life Cycle Assessment of Synthetic Natural Gas Production from Different CO2 Sources: A Cradle-to-Gate Study. Energies. 2020; 13(17):4579. https://doi.org/10.3390/en13174579
Chicago/Turabian StyleBargiacchi, Eleonora, Nils Thonemann, Jutta Geldermann, Marco Antonelli, and Umberto Desideri. 2020. "Life Cycle Assessment of Synthetic Natural Gas Production from Different CO2 Sources: A Cradle-to-Gate Study" Energies 13, no. 17: 4579. https://doi.org/10.3390/en13174579
APA StyleBargiacchi, E., Thonemann, N., Geldermann, J., Antonelli, M., & Desideri, U. (2020). Life Cycle Assessment of Synthetic Natural Gas Production from Different CO2 Sources: A Cradle-to-Gate Study. Energies, 13(17), 4579. https://doi.org/10.3390/en13174579







