Effects of Additional Xylanase on Saccharification and Ethanol Fermentation of Ammonia-Pretreated Corn Stover and Rice Straw
Abstract
1. Introduction
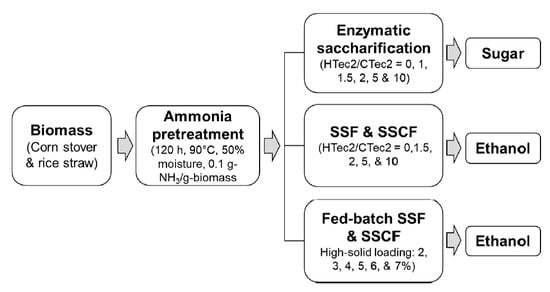
2. Materials and Methods
2.1. Feedstock
2.2. Enzymes
2.3. Pretreatment
2.4. Enzymatic Digestibility
2.5. Simultaneous Saccharification and Fermentation (SSF) and the Simultaneous Saccharification and Co-Fermentation (SSCF)
2.6. Compositional Analysis
3. Results and Discussion
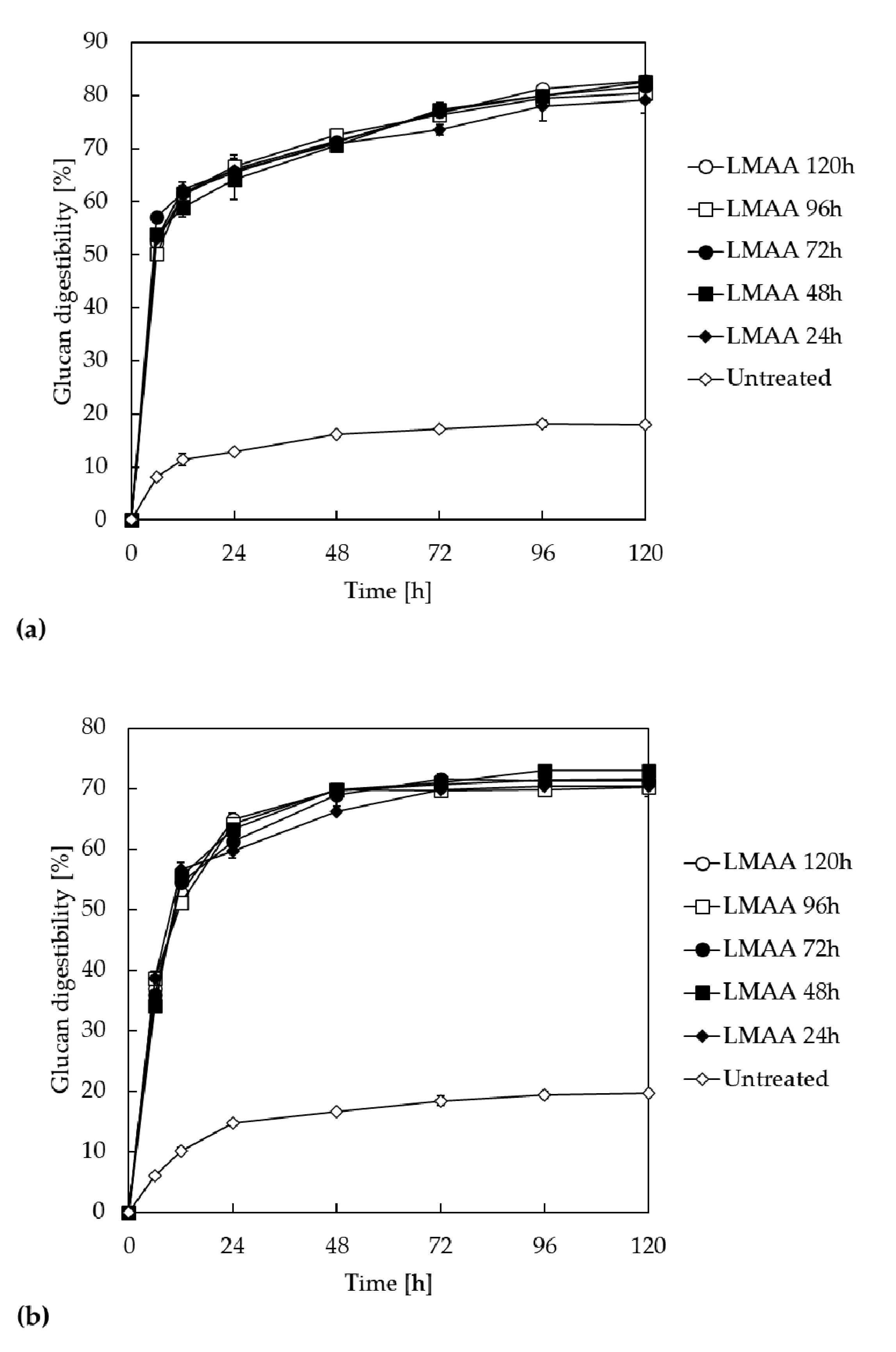
3.1. Enzymatic Saccharification of LMAA-Treated Corn Stover and Rice Straw
3.2. Effects of LMAA Pretreatment on Chemical Compositions of Corn Stover and Rice Straw
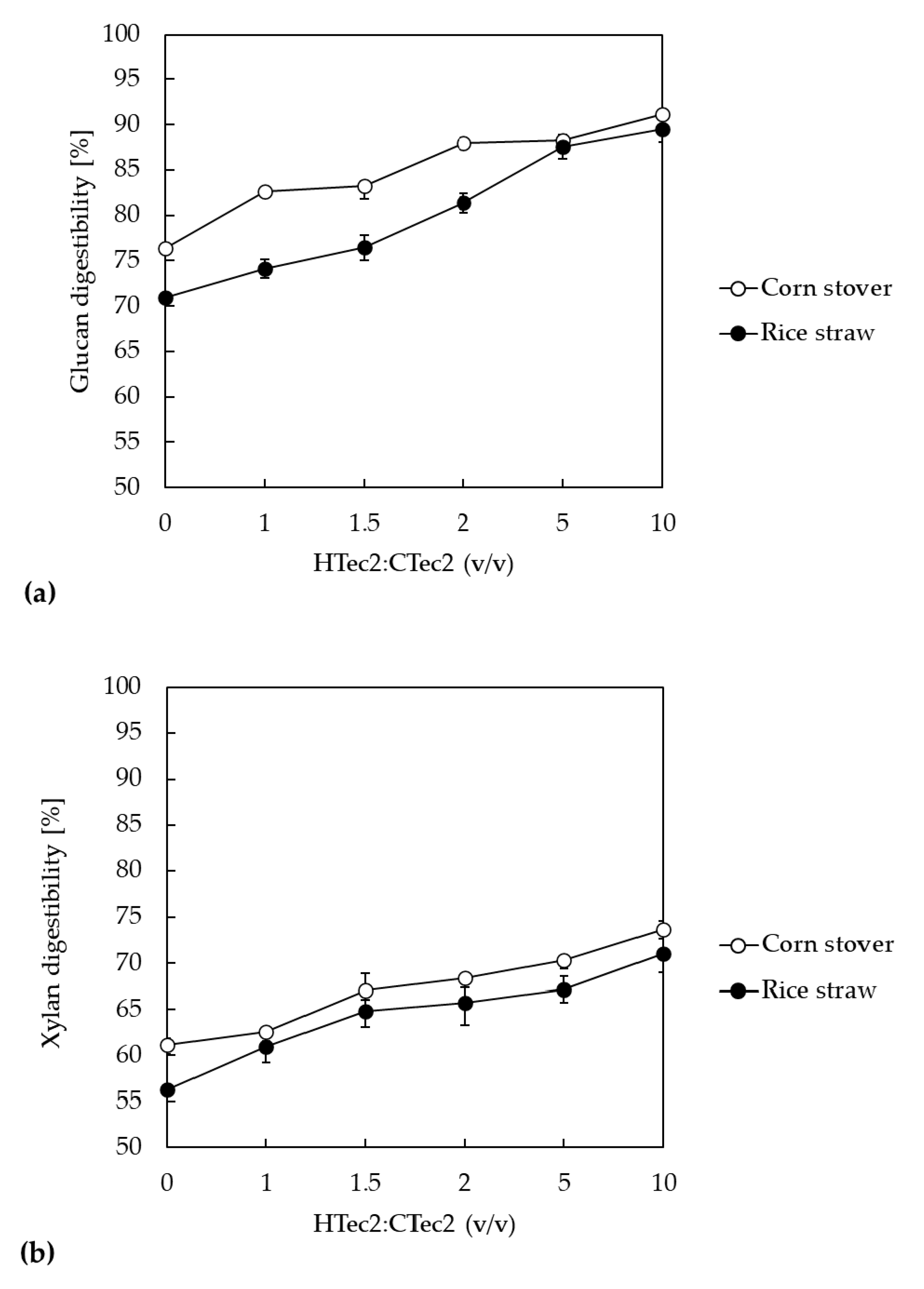
3.3. Effect of Additional Xylanase on the Enzymatic Hydrolysis of LMAA-Treated Biomass
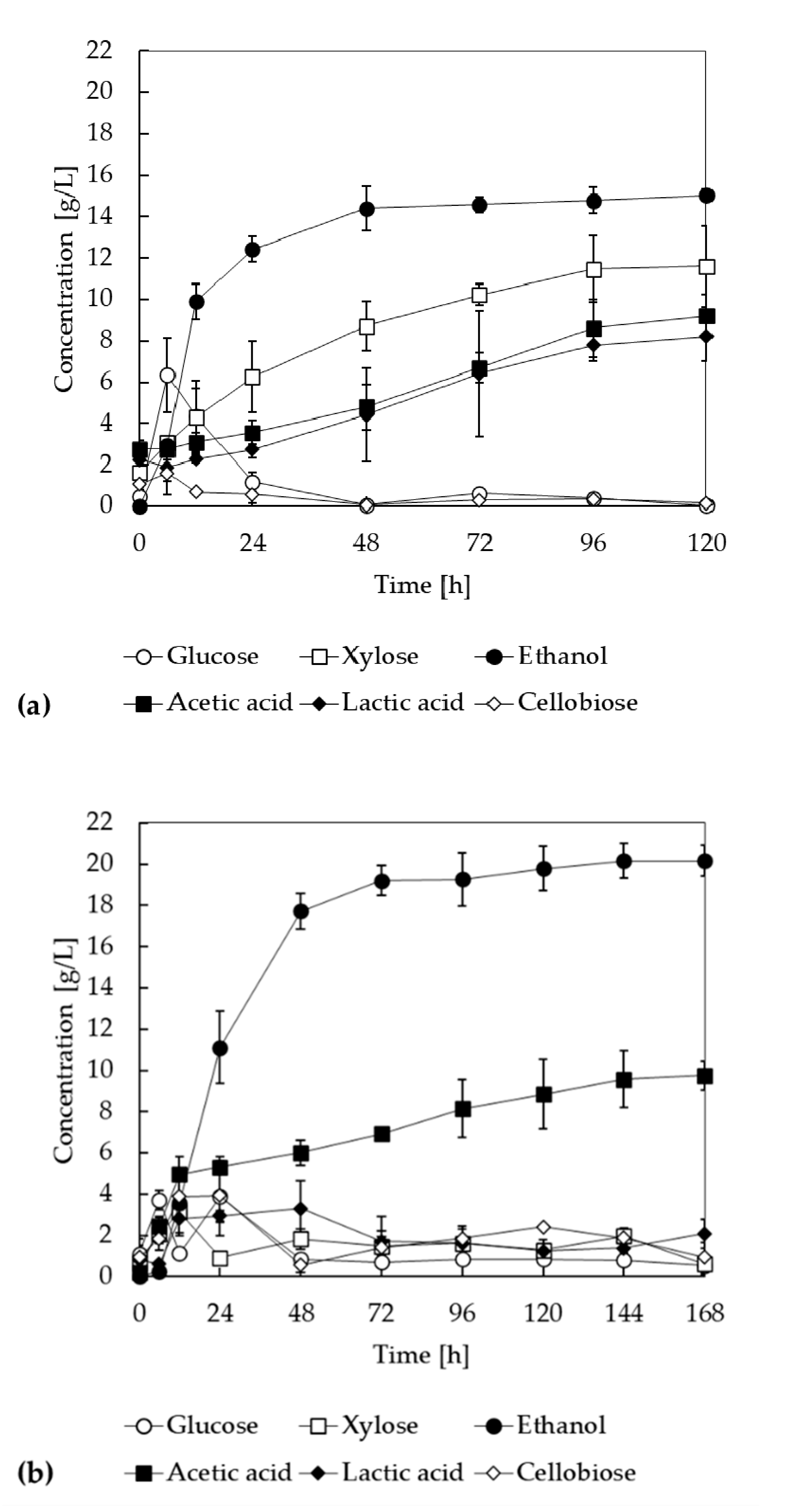
3.4. SSF and SSCF of LMAA-Treated Biomass
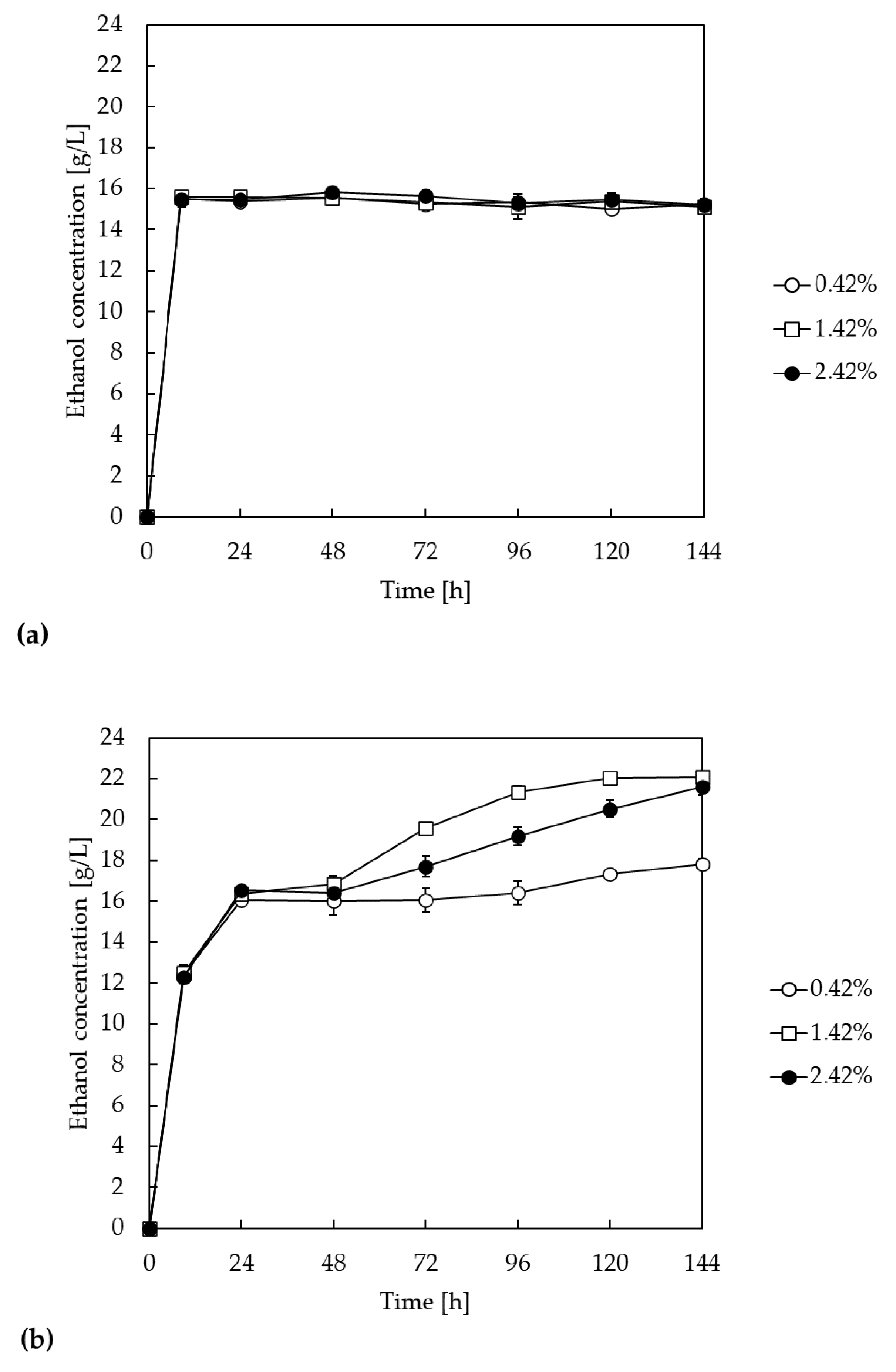
3.5. Effect of Different Xylanase Dosages on Ethanol Productions in SSF and SSCF Processes Using LMAA-Treated Corn Stover
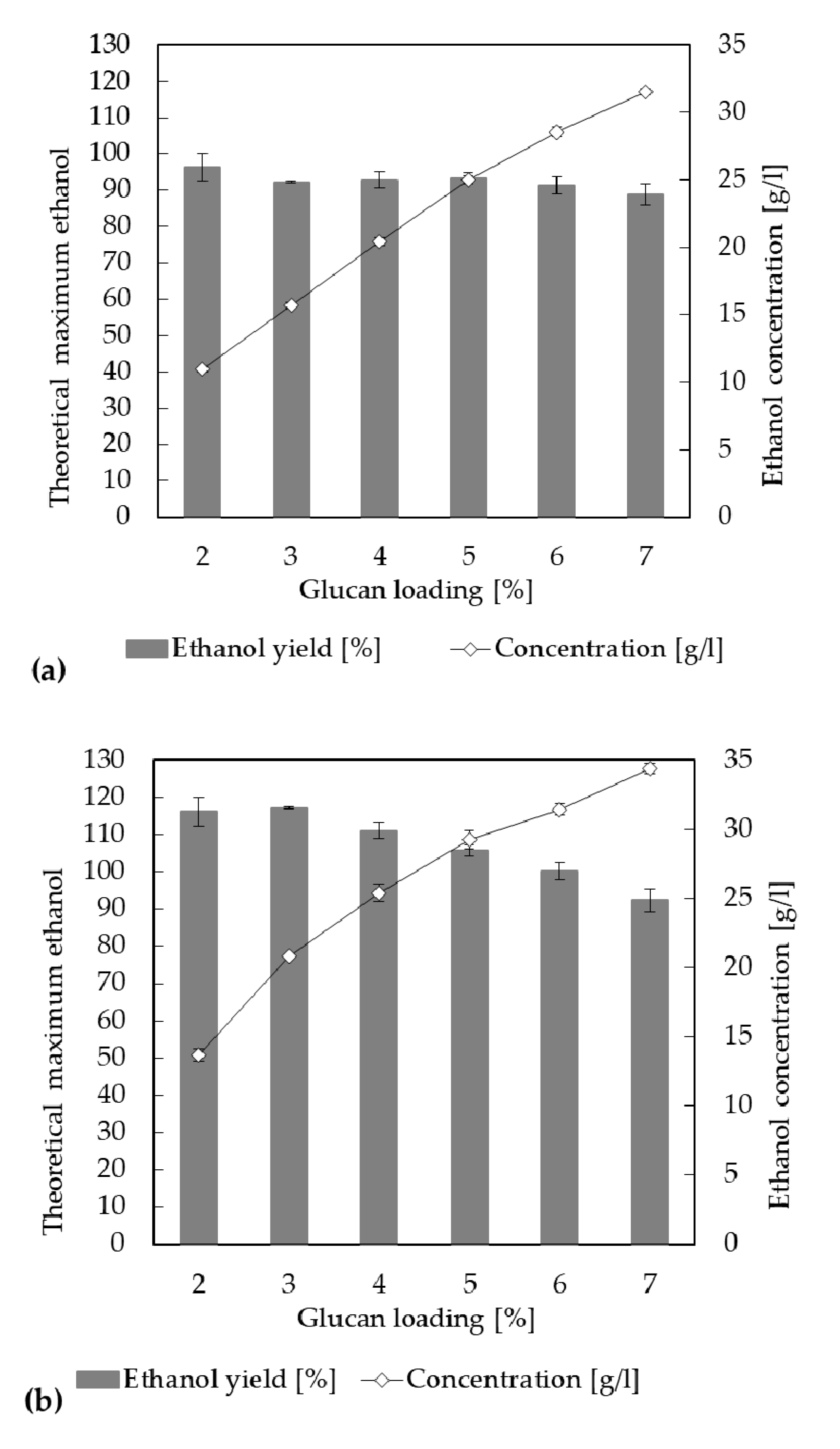
3.6. High-Solid Fermentation Using Fed-Batch SSF and SSCF
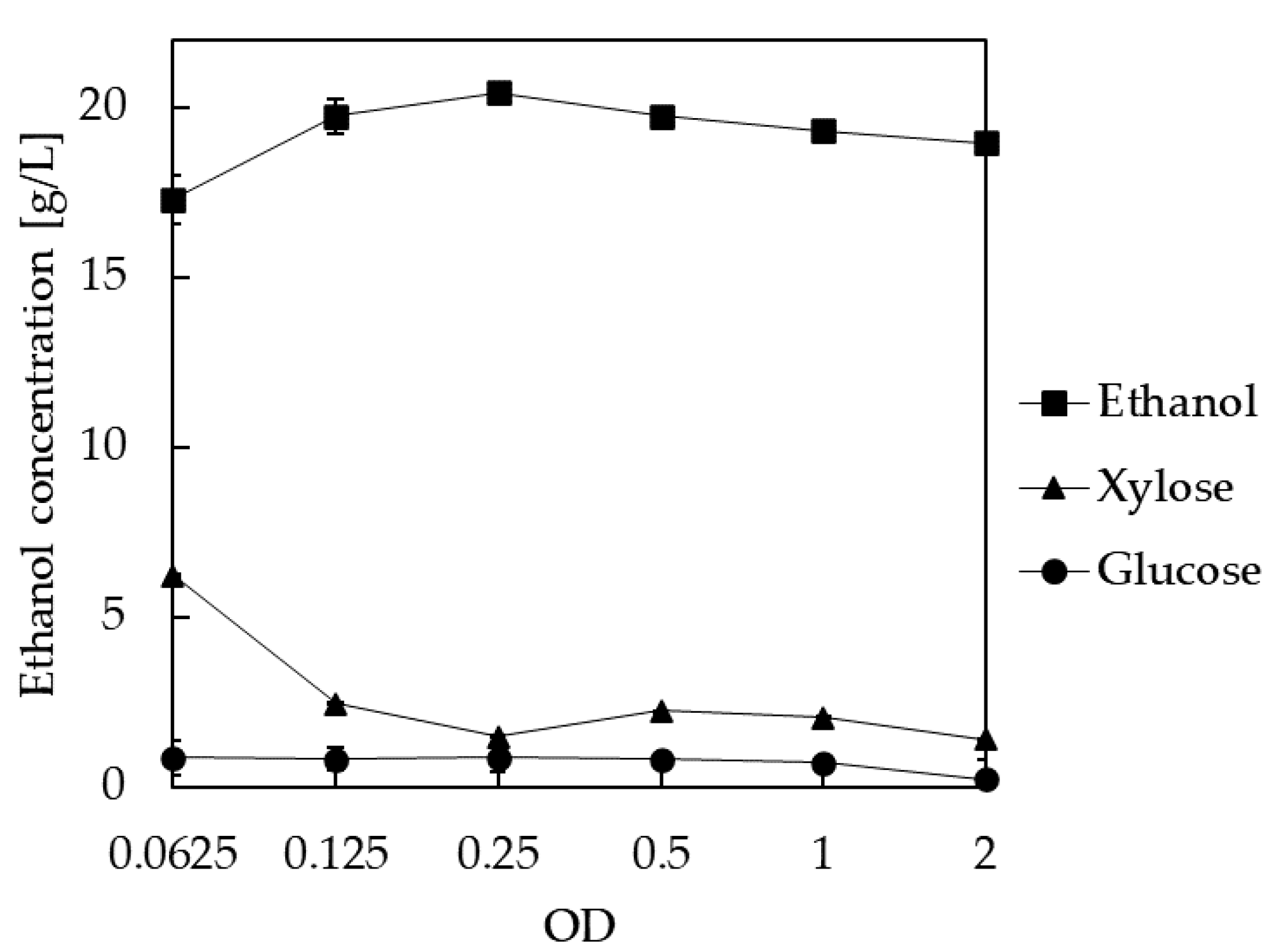
3.7. Selection of Inoculum Size for Ethanol Production in SSCF
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Uihlein, A.; Schebek, L. Environmental impacts of a lignocellulose feedstock biorefinery system: An assessment. Biomass Bioenergy 2009, 33, 793–802. [Google Scholar] [CrossRef]
- Sarkar, N.; Ghosh, S.K.; Bannerjee, S.; Aikat, K. Bioethanol production from agricultural wastes: An overview. Renew. Energy 2012, 37, 19–27. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, H.; Pang, S.; Su, C.; Lv, M.; An, N.; Wang, K.; Cai, D.; Qin, P. Importance of redefinition of corn stover harvest time to enhancing non-food bio-ethanol production. Renew. Energy 2020, 146, 1444–1450. [Google Scholar] [CrossRef]
- Luo, W.; Wang, J.; Liu, X.B.; Li, H.; Pan, H.; Gu, Q.; Yu, X. A facile and efficient pretreatment of corncob for bioproduction of butanol. Bioresour. Technol. 2013, 140, 86–89. [Google Scholar] [CrossRef]
- Liu, X.; Yu, X. Enhancement of butanol production: From biocatalysis to bioelectrocatalysis. J. Am. Chem. Soc. 2020, 5, 867–878. [Google Scholar] [CrossRef]
- Ben-Iwo, J.; Manovic, V.; Longhurst, P. Biomass resources and biofuels potential for the production of transportation fuels in Nigeria. Renew. Sustain. Energy Rev. 2016, 63, 172–192. [Google Scholar] [CrossRef]
- Sjostrom, E. Wood Chemistry Fundamentals and Applications, 2nd ed.; Academic Press: New York, NY, USA, 1993. [Google Scholar]
- Yu, B.; Chen, H.Z. Effect of the ash on enzymatic hydrolysis of steam-exploded rice straw. Bioresour. Technol. 2010, 101, 9114–9119. [Google Scholar] [CrossRef]
- Palonen, H. Role of Lignin in the Enzymatic Hydrolysis of Lignocellulose. Ph.D. Thesis, Aalto University, Espoo, Finland, April 2004. [Google Scholar]
- Naik, S.N.; Goud, V.V.; Rout, P.K.; Dalai, A.K. Production of first and second generation biofuels: A comprehensive review. Renew. Sustain. Energy Rev. 2010, 14, 578–597. [Google Scholar] [CrossRef]
- Limayem, A.; Ricke, S.C. Lignocellulosic biomass for bioethanol production: Current perspectives, potential issues and future prospects. Prog. Energy Combust. Sci. 2012, 38, 449–467. [Google Scholar] [CrossRef]
- Tu, W.C.; Hallett, J.P. Recent advances in the pretreatment of lignocellulosic biomass. Curr. Opin. Green. Sustain. 2019, 20, 11–17. [Google Scholar] [CrossRef]
- Bhatiaa, S.K.; Jagtapc, S.S.; Bedekarc, A.A.; Bhatiae, R.K.; Patelf, A.K.; Pantg, D.; Banuh, J.R.; Raoc, C.V.; Kimi, Y.G.; Yang, Y.H. Recent developments in pretreatment technologies on lignocellulosic biomass: Effect of key parameters, technological improvements, and challenges. Bioresour. Technol. 2020, 300, 122724. [Google Scholar] [CrossRef] [PubMed]
- Sainz, M.B. Commercial cellulosic ethanol: The role of plant-expressed enzymes. In Vitro Cell. Dev. Biol. Plant 2009, 45, 314–329. [Google Scholar] [CrossRef]
- Kim, H.; Kim, J.S.; Sunwoo, C.; Lee, Y.Y. Pretreatment of corn stover by aqueous ammonia. Bioresour. Technol. 2003, 90, 39–47. [Google Scholar] [CrossRef]
- Kim, T.H.; Lee, Y.Y. Pretreatment and fractionation of corn stover by ammonia recycle percolation process. Bioresour. Technol. 2005, 96, 2007–2013. [Google Scholar] [CrossRef] [PubMed]
- Yoo, C.G.; Nghiem, P.N.; Hicks, K.B.; Kim, T.H. Pretreatment of corn stover using low-moisture anhydrous ammonia (LMAA) process. Bioresour. Technol. 2011, 102, 10028–10034. [Google Scholar] [CrossRef]
- Cayetano, R.D.; Kim, T.H. Effects of low moisture anhydrous ammonia (LMAA) Pretreatment at controlled ammoniation temperatures on enzymatic hydrolysis of corn stover. Appl. Biochem. Biotechnol. 2017, 181, 1257–1269. [Google Scholar] [CrossRef]
- Hu, J.; Arantes, V.; Saddler, J.N. The enhancement of enzymatic hydrolysis of lignocellulosic substrates by the addition of accessory enzymes such as xylanase: Is it an additive or synergistic effect? Biotechnol. Biofuels 2011, 4, 36–48. [Google Scholar] [CrossRef]
- Gupta, R.; Kim, T.H.; Lee, Y.Y. Substrate dependency and effect of xylanase supplementation on enzymatic hydrolysis of ammonia-treated biomass. Appl. Biochem. Biotechnol. 2008, 148, 59–70. [Google Scholar] [CrossRef]
- Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass; NREL/TP-510-42618; National Renewable Energy Laboratory (NREL): Golden, CO, USA, 2008. [Google Scholar]
- Ghose, T.K.; Bisaria, V.S. Measurement of hemicellulose activities; Part 1: Xylanases. Pure Appl. Chem. 1987, 59, 1739–1752. [Google Scholar] [CrossRef]
- Selig, M.; Weiss, N.; Ji, Y. Enzymatic Saccharification of Lignocellulosic Biomass; NREL/TP-510-42629; National Renewable Energy Laboratory (NREL): Golden, CO, USA, 2008. [Google Scholar]
- Dowe, N.; McMillan, J. SSF Experimental Protocols-Lignocellulosic Biomass Hydrolysis and Fermentation; NREL/TP-510-42630; National Renewable Energy Laboratory (NREL): Golden, CO, USA, 2008. [Google Scholar]
- Tomás-Pejó, E.; García-Aparicio, M.; Negro, M.J.; Oliva, J.M.; Ballesteros, M. Effect of different cellulase dosages on cell viability and ethanol production by Kluyveromy cesmarxianus in SSF processes. Bioresour. Technol. 2009, 100, 890–895. [Google Scholar] [CrossRef]
- Modenbach, A.A.; Nokes, S.E. The use of high-solids loadings in biomass pretreatment-a review. Biotechnol. Bioeng. 2012, 109, 1430–1442. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.H.; Qin, L.; Zhu, J.Q.; Li, B.Z.; Yuan, Y.J. Simultaneous saccharification and fermentation of steam-exploded corn stover at high glucan loading and high temperature. Biotechnol. Biofuels 2014, 7, 167–182. [Google Scholar] [CrossRef]
- Liu, Z.H.; Chen, H.Z. Simultaneous saccharification and co-fermentation for improving the xylose utilization of steam exploded corn stover at high solid loading. Bioresour. Technol. 2016, 201, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Dada, O.; Kalil, M.S.; Yusoff, W.M.W. Effect of inoculum and substrate concentrations in anaerobic fermentation of treated rice bran to acetone, butanol and ethanol. Bacteriol. J. 2012, 2, 79–89. [Google Scholar] [CrossRef][Green Version]
- Ferchichi, M.; Crabbe, E.; Hintz, W.; Gil, G.H.; Almadid, A. Influence of culture parameters on biological hydrogen production by Clostridium Saccharoperbutylacetonicum ATCC 27021. World J. Mcrob. Biotechnol. 2005, 21, 855–866. [Google Scholar] [CrossRef]

| Component | Corn Stover | Rice Straw | |||
|---|---|---|---|---|---|
| Untreated | LMAA-Treated | Untreated | LMAA-Treated | ||
| Glucan | [%] | 31.6 ± 0.3 | 31.5 ± 0.4 | 34.7 ± 0.5 | 34.8 ± 0.3 |
| XMG a | [%] | 22.6 ± 0.9 | 22.5 ± 0.8 | 20.2 ± 0.6 | 20.1 ± 0.3 |
| AIL b | [%] | 16.2 ± 0.8 | 15.5 ± 0.6 | 15.7 ± 0.8 | 15.1 ± 0.2 |
| ASL c | [%] | 1.8 ± 0.2 | 2.5 ± 0.3 | 1.6 ± 0.3 | 2.2 ± 0.1 |
| Ash | [%] | 0.7 ± 0.2 | 0.8 ± 0.2 | 8.4 ± 0.3 | 9.2 ± 0.2 |
| Source of Variation | SS | DF | MS | F | p-Value | Fcrit |
|---|---|---|---|---|---|---|
| Between Groups | 12.35 | 5 | 2.47 | 5.34 | 0.03 | 4.39 |
| Within Groups | 2.78 | 6 | 0.46 | |||
| Total | 15.12 | 11 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.H.; Pham, T.T.H.; Kim, T.H. Effects of Additional Xylanase on Saccharification and Ethanol Fermentation of Ammonia-Pretreated Corn Stover and Rice Straw. Energies 2020, 13, 4574. https://doi.org/10.3390/en13174574
Park SH, Pham TTH, Kim TH. Effects of Additional Xylanase on Saccharification and Ethanol Fermentation of Ammonia-Pretreated Corn Stover and Rice Straw. Energies. 2020; 13(17):4574. https://doi.org/10.3390/en13174574
Chicago/Turabian StylePark, Seung Hyeon, Thi Thu Huong Pham, and Tae Hyun Kim. 2020. "Effects of Additional Xylanase on Saccharification and Ethanol Fermentation of Ammonia-Pretreated Corn Stover and Rice Straw" Energies 13, no. 17: 4574. https://doi.org/10.3390/en13174574
APA StylePark, S. H., Pham, T. T. H., & Kim, T. H. (2020). Effects of Additional Xylanase on Saccharification and Ethanol Fermentation of Ammonia-Pretreated Corn Stover and Rice Straw. Energies, 13(17), 4574. https://doi.org/10.3390/en13174574