Studies of FeSe2 Cathode Materials for Mg–Li Hybrid Batteries
Abstract
1. Introduction
2. Experimental Section
2.1. Synthesis of FeSe2
2.2. Preparation of Electrolyte
2.3. Material Characterization
2.4. Cells Assembling and Electrochemical Measurements
2.5. Preparation of Ex Situ XRD Samples
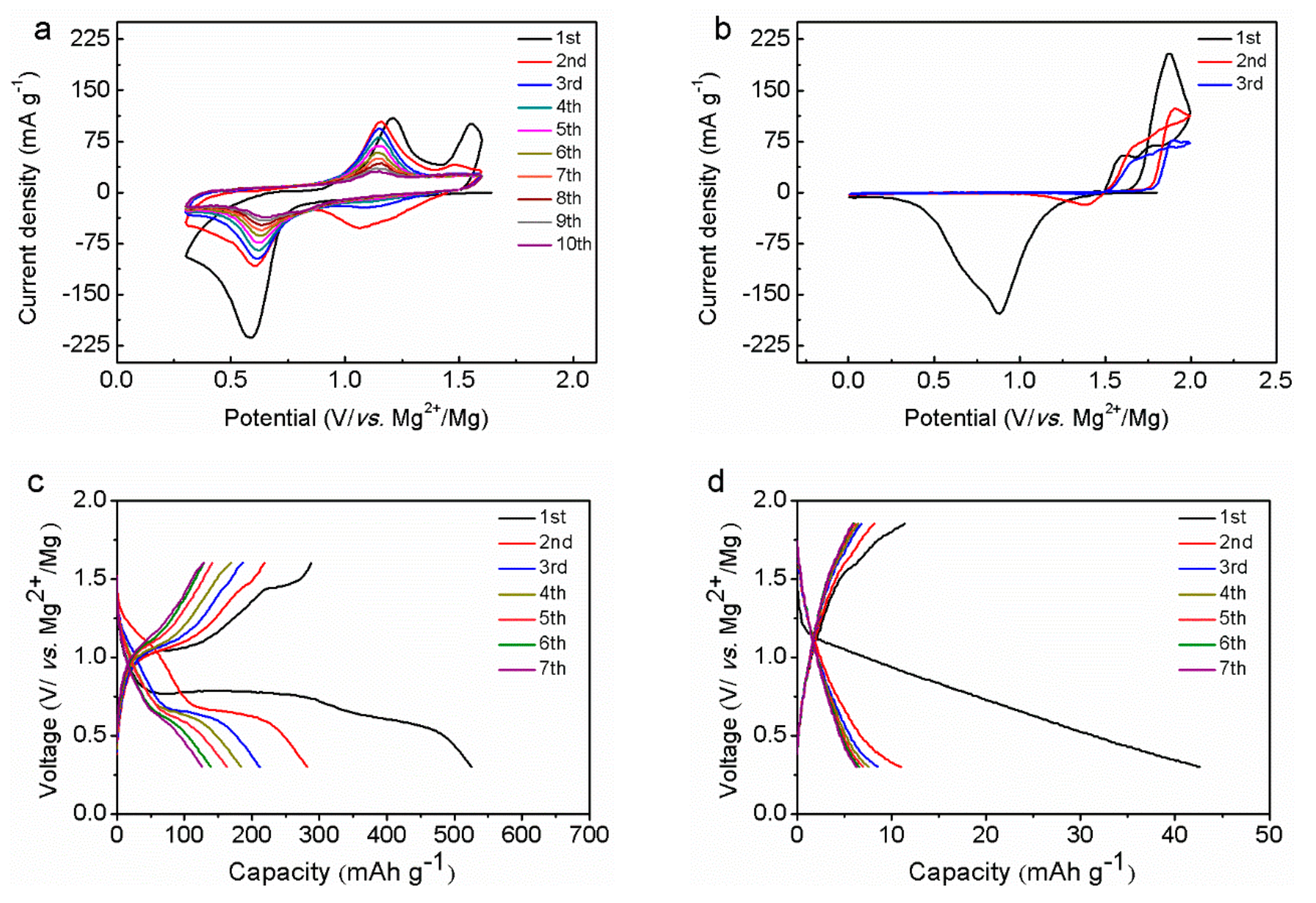
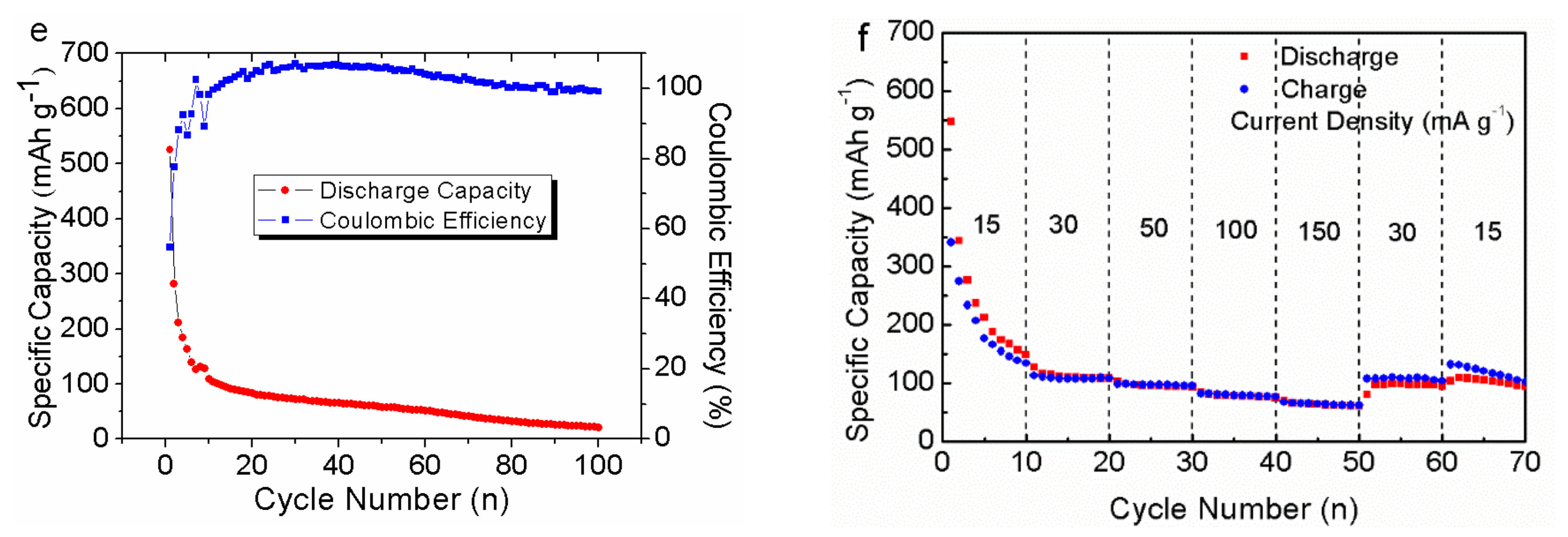
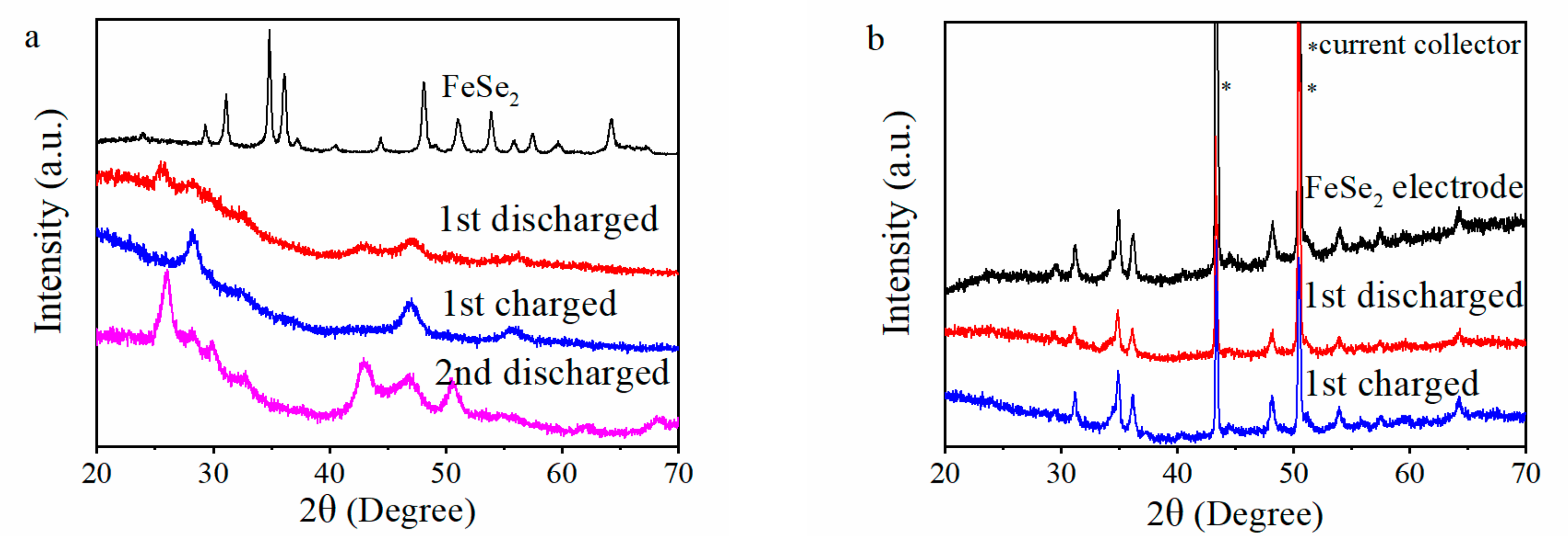
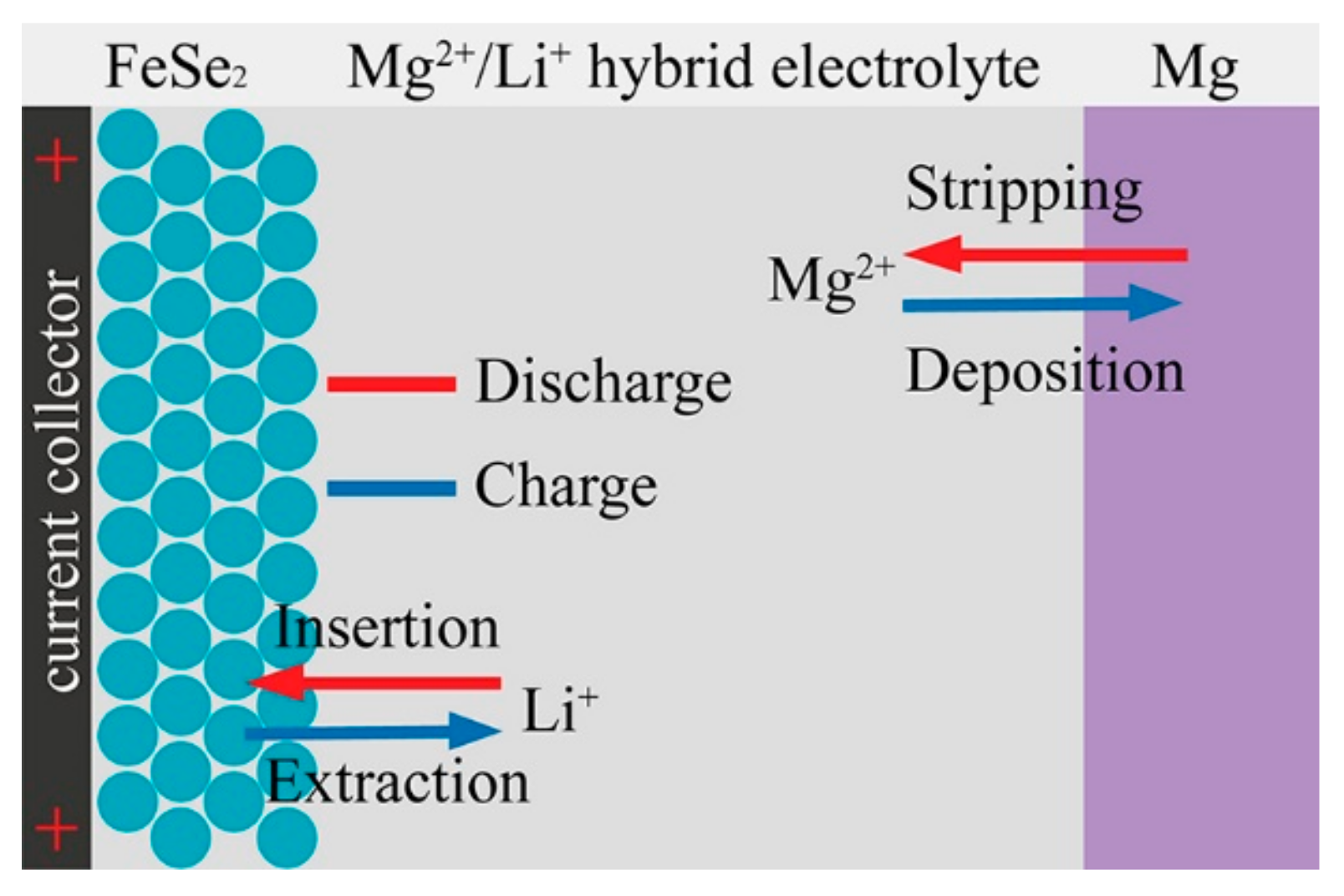
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yoo, H.D.; Shterenberg, I.; Gofer, Y.; Gershinsky, G.; Pour, N.; Aurbach, D. Mg rechargeable batteries: An on-going challenge. Energy Environ. Sci. 2013, 6, 2265–2279. [Google Scholar] [CrossRef]
- Mao, M.; Gao, T.; Hou, S.; Wang, F.; Chen, J.; Wei, Z.; Fan, X.; Ji, X.; Ma, J.; Wang, C. High energy density rechargeable Mg battery enabled by a displacement reaction. Nano Lett. 2019, 19, 6665–6672. [Google Scholar] [CrossRef] [PubMed]
- Kuganathan, N.; Gkanas, E.I.; Chroneos, A. Mg6MnO8 as a Magnesium-Ion Battery Material: Defects, Dopants and Mg-Ion Transport. Energies 2019, 12, 3213. [Google Scholar] [CrossRef]
- Aurbach, D.; Cohen, Y.; Moshkovich, M. The study of reversible magnesium deposition by in situ scanning tunneling microscopy. Electrochem. Solid State Lett. 2001, 4, A113–A116. [Google Scholar] [CrossRef]
- Muldoon, J.; Bucur, C.B.; Gregory, T. Quest for nonaqueous multivalent secondary batteries: Magnesium and beyond. Chem. Rev. 2014, 114, 11683–11720. [Google Scholar] [CrossRef] [PubMed]
- Ponrouch, A.; Rosa Palacin, M. Post-Li batteries: Promises and challenges. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2019, 377, 20180297. [Google Scholar] [CrossRef]
- Levi, M.D.; Lancry, E.; Gizbar, H.; Lu, Z.; Levi, E.; Gofer, Y.; Aurbach, D. Kinetic and thermodynamic studies of Mg2+ and Li+ ion insertion into the Mo6S8 Chevrel phase. J. Electrochem. Soc. 2004, 151, A1044–A1051. [Google Scholar] [CrossRef]
- Aurbach, D.; Lu, Z.; Schechter, A.; Gofer, Y.; Gizbar, H.; Turgeman, R.; Cohen, Y.; Moshkovich, M.; Levi, E. Prototype systems for rechargeable magnesium batteries. Nature 2000, 407, 724–727. [Google Scholar] [CrossRef]
- Saha, P.; Jampani, P.H.; Datta, M.K.; Hong, D.; Gattu, B.; Patel, P.; Kadakia, K.S.; Manivannan, A.; Kumta, P.N. A rapid solid-state synthesis of electrochemically active Chevrel phases (Mo6T8; T = S, Se) for rechargeable magnesium batteries. Nano Res. 2017, 10, 4415–4435. [Google Scholar] [CrossRef]
- Liu, B.; Luo, T.; Mu, G.; Wang, X.; Chen, D.; Shen, G. Rechargeable Mg-ion batteries based on WSe2 nanowire cathodes. ACS Nano 2013, 7, 8051–8058. [Google Scholar] [CrossRef]
- Liu, Y.; Jiao, L.; Wu, Q.; Zhao, Y.; Cao, K.; Liu, H.; Wang, Y.; Yuan, H. Synthesis of rGO-supported layered MoS2 for high-performance rechargeable Mg batteries. Nanoscale 2013, 5, 9562–9567. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, D.; Li, X.; Shen, J.; Chen, Z.; Cao, S.-a.; Li, T.; Xu, F. a-MoS3@CNT nanowire cathode for rechargeable Mg batteries: A pseudocapacitive approach for efficient Mg-storage. Nanoscale 2019, 11, 16043–16051. [Google Scholar] [CrossRef] [PubMed]
- Juran, T.R.; Smeu, M. TiSe2 cathode for beyond Li-ion batteries. J. Power Sources 2019, 436, 226813. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, Y.; Li, X.; Shen, J.; Chen, Z.; Cao, S.-a.; Li, T.; Xu, F. CoSe2 hollow microspheres, nano-polyhedra and nanorods as pseudocapacitive Mg-storage materials with fast solid-state Mg2+ diffusion kinetics. Nanoscale 2019, 11, 23173–23181. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Rong, Z.; Malik, R.; Canepa, P.; Jain, A.; Ceder, G.; Persson, K.A. Spinel compounds as multivalent battery cathodes: A systematic evaluation based on ab initio calculations. Energ. Environ. Sci. 2015, 8, 964–974. [Google Scholar] [CrossRef]
- Kim, C.; Phillips, P.J.; Key, B.; Yi, T.; Nordlund, D.; Yu, Y.-S.; Bayliss, R.D.; Han, S.-D.; He, M.; Zhang, Z.; et al. Direct observation of reversible magnesium ion intercalation into a spinel oxide host. Adv. Mater. 2015, 27, 3377–3384. [Google Scholar] [CrossRef]
- Liu, M.; Jain, A.; Rong, Z.; Qu, X.; Canepa, P.; Malik, R.; Ceder, G.; Persson, K.A. Evaluation of sulfur spinel compounds for multivalent battery cathode applications. Energ. Environ. Sci. 2016, 9, 3201–3209. [Google Scholar] [CrossRef]
- Attias, R.; Salama, M.; Hirsch, B.; Gofer, Y.; Aurbach, D. Solvent effects on the reversible intercalation of magnesium-ions into V2O5 electrodes. Chemelectrochem 2018, 5, 3514–3524. [Google Scholar] [CrossRef]
- Mukherjee, A.; Sa, N.; Phillips, P.J.; Burrell, A.; Vaughey, J.; Klie, R.F. Direct investigation of Mg intercalation into the orthorhombic V2O5 cathode using atomic-resolution transmission electron microscopy. Chem. Mater. 2017, 29, 2218–2226. [Google Scholar] [CrossRef]
- Barnes, T.A.; Wan, L.F.; Kent, P.R.C.; Prendergast, D. Hybrid DFT investigation of the energetics of Mg ion diffusion in alpha-MoO3. Phys. Chem. Chem. Phys. 2018, 20, 24877–24884. [Google Scholar] [CrossRef]
- Wang, Y.; Xue, X.; Liu, P.; Wang, C.; Yi, X.; Hu, Y.; Ma, L.; Zhu, G.; Chen, R.; Chen, T.; et al. Atomic substitution enabled synthesis of vacancy-rich two-dimensional black TiO2-x nanoflakes for high-performance rechargeable magnesium batteries. ACS Nano 2018, 12, 12492–12502. [Google Scholar] [CrossRef] [PubMed]
- Koketsu, T.; Ma, J.; Morgan, B.J.; Body, M.; Legein, C.; Dachraoui, W.; Giannini, M.; Demortiere, A.; Salanne, M.; Dardoize, F.; et al. Reversible magnesium and aluminium ions insertion in cation-deficient anatase TiO2. Nat. Mater. 2017, 16, 1142–1146. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Nuli, Y.; Yang, J.; Yilinuer, T.; Wang, J. MgFeSiO4 prepared via a molten salt method as a new cathode material for rechargeable magnesium batteries. Chin. Sci. Bull. 2011, 56, 386–390. [Google Scholar] [CrossRef]
- Feng, Z.; Yang, J.; NuLi, Y.; Wang, J. Sol-gel synthesis of Mg1.03Mn0.97SiO4 and its electrochemical intercalation behavior. J. Power Sources 2008, 184, 604–609. [Google Scholar] [CrossRef]
- Huang, Z.-D.; Masese, T.; Orikasa, Y.; Mori, T.; Minato, T.; Tassel, C.; Kobayashi, Y.; Kageyama, H.; Uchimoto, Y. MgFePO4F as a feasible cathode material for magnesium batteries. J. Mater. Chem. A 2014, 2, 11578–11582. [Google Scholar] [CrossRef]
- Rubio, S.; Liu, R.; Liu, X.; Lavela, P.; Tirado, J.L.; Li, Q.; Liang, Z.; Ortiz, G.F.; Yang, Y. Exploring the high-voltage Mg2+/Na+ co-intercalation reaction of Na3VCr(PO4)3 in Mg-ion batteries. J. Mater. Chem. A 2019, 7, 18081–18091. [Google Scholar] [CrossRef]
- Zeng, J.; Yang, Y.; Lai, S.; Huang, J.; Zhang, Y.; Wang, J.; Zhao, J. A promising high-voltage cathode material based on mesoporous Na3V2(PO4)3/C for rechargeable magnesium batteries. Chem. Eur. J. 2017, 23, 16898–16905. [Google Scholar] [CrossRef]
- Zhijun, J.; Jiawei, H.; Lujing, L.; Yi, W.; Tao, Q. Vertically aligned alpha-MnO2 nanosheets on carbon nanotubes as cathodic materials for aqueous rechargeable magnesium ion battery. Ionics 2018, 24, 3483–3491. [Google Scholar] [CrossRef]
- Zhang, R.; Yu, X.; Nam, K.-W.; Ling, C.; Arthur, T.S.; Song, W.; Knapp, A.M.; Ehrlich, S.N.; Yang, X.-Q.; Matsui, M. alpha-MnO2 as a cathode material for rechargeable Mg batteries. Electrochem. Commun. 2012, 23, 110–113. [Google Scholar] [CrossRef]
- Sun, X.; Duffort, V.; Mehdi, B.L.; Browning, N.D.; Nazar, L.F. Investigation of the mechanism of Mg insertion in birnessite in nonaqueous and aqueous rechargeable Mg-ion batteries. Chem. Mater. 2016, 28, 534–542. [Google Scholar] [CrossRef]
- Nam, K.W.; Kim, S.; Lee, S.; Salama, M.; Shterenberg, I.; Gofer, Y.; Kim, J.-S.; Yang, E.; Park, C.S.; Kim, J.-S.; et al. The high performance of crystal water containing manganese birnessite cathodes for magnesium batteries. Nano Lett. 2015, 15, 4071–4079. [Google Scholar] [CrossRef] [PubMed]
- Duffort, V.; Sun, X.; Nazar, L.F. Screening for positive electrodes for magnesium batteries: A protocol for studies at elevated temperatures. Chem. Commun. 2016, 52, 12458–12461. [Google Scholar] [CrossRef] [PubMed]
- Xiong, F.; Fan, Y.; Tan, S.; Zhou, L.; Xu, Y.; Pei, C.; An, Q.; Mai, L. Magnesium storage performance and mechanism of CuS cathode. Nano Energy 2018, 47, 210–216. [Google Scholar] [CrossRef]
- Liu, H.; Shi, X.; Xu, F.; Zhang, L.; Zhang, W.; Chen, L.; Li, Q.; Uher, C.; Day, T.; Snyder, G.J. Copper ion liquid-like thermoelectrics. Nat. Mater. 2012, 11, 422–425. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, Y.; Li, X.; Shen, J.; Chen, Z.; Cao, S.-a.; Li, T.; Xu, F. Nanosheets assembling hierarchical starfish-like Cu2-xSe as advanced cathode for rechargeable Mg batteries. Chem. Eng. J. 2020, 384, 123235. [Google Scholar] [CrossRef]
- Zhang, R.; Ling, C.; Mizuno, F. A conceptual magnesium battery with ultrahigh rate capability. Chem. Commun. 2015, 51, 1487–1490. [Google Scholar] [CrossRef]
- Pan, B.; Huang, J.; Feng, Z.; Zeng, L.; He, M.; Zhang, L.; Vaughey, J.T.; Bedzyk, M.J.; Fenter, P.; Zhang, Z.; et al. Polyanthraquinone-based organic cathode for high-performance rechargeable magnesium-ion batteries. Adv. Energy. Mater. 2016, 6, 1600140. [Google Scholar] [CrossRef]
- Pan, B.; Zhou, D.; Huang, J.; Zhang, L.; Burrell, A.K.; Vaughey, J.T.; Zhang, Z.; Liao, C. 2,5-Dimethoxy-1,4-Benzoquinone (DMBQ) as organic cathode for rechargeable magnesium-ion batteries. J. Electrochem. Soc. 2016, 163, A580–A583. [Google Scholar] [CrossRef]
- Vardar, G.; Smith, J.G.; Thomson, T.; Inagaki, K.; Naruse, J.; Hiramatsu, H.; Sleightholme, A.E.S.; Sakamoto, J.; Siegel, D.J.; Monroe, C.W. Mg/O2 battery based on the magnesium-aluminum chloride complex (MACC) electrolyte. Chem. Mater. 2016, 28, 7629–7637. [Google Scholar] [CrossRef]
- Li, W.; Cheng, S.; Wang, J.; Qiu, Y.; Zheng, Z.; Lin, H.; Nanda, S.; Ma, Q.; Xu, Y.; Ye, F.; et al. Synthesis, crystal structure, and electrochemical properties of a simple magnesium electrolyte for magnesium/sulfur batteries. Angew. Chem. Int. Ed. 2016, 55, 6406–6410. [Google Scholar] [CrossRef]
- Fan, H.; Zheng, Z.; Zhao, L.; Li, W.; Wang, J.; Dai, M.; Zhao, Y.; Xiao, J.; Wang, G.; Ding, X.; et al. Extending cycle life of Mg/S battery by activation of Mg anode/electrolyte interface through an LiCl-assisted MgCl2 solubilization mechanism. Adv. Funct. Mater. 2019, 30, 1909370. [Google Scholar] [CrossRef]
- Xu, Y.; Zhou, G.; Zhao, S.; Li, W.; Shi, F.; Li, J.; Feng, J.; Zhao, Y.; Wu, Y.; Guo, J.; et al. Improving a Mg/S Battery with YCl3 Additive and Magnesium Polysulfide. Adv. Sci. 2019, 6, 1800981. [Google Scholar] [CrossRef]
- Gao, T.; Ji, X.; Hou, S.; Fan, X.; Li, X.; Yang, C.; Han, F.; Wang, F.; Jiang, J.; Xu, K.; et al. Thermodynamics and kinetics of sulfur cathode during discharge in MgTFSI2-DME electrolyte. Adv. Mater. 2018, 30, 1704313. [Google Scholar] [CrossRef]
- Tian, H.; Gao, T.; Li, X.; Wang, X.; Luo, C.; Fan, X.; Yang, C.; Suo, L.; Ma, Z.; Han, W.; et al. High power rechargeable magnesium/iodine battery chemistry. Nat. Commun. 2017, 8, 14083. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Wang, W.; NuLi, Y.; Yang, J.; Wang, J. Effect of copper to Selenium@Microporous carbon cathode for Mg-Se batteries with nucleophilic electrolyte. Electrochim. Acta 2020, 330, 135354. [Google Scholar] [CrossRef]
- Liu, F.; Liu, Y.; Zhao, X.; Liu, K.; Yin, H.; Fan, L.-Z. Prelithiated V2C MXene: A high-performance electrode for hybrid magnesium/lithium-ion batteries by ion cointercalation. Small 2020, 16, 1906076. [Google Scholar] [CrossRef]
- Fan, X.; Gaddam, R.R.; Kumar, N.A.; Zhao, X.S. A hybrid Mg2+/Li+ battery based on interlayer-expanded MoS2/graphene cathode. Adv. Energy Mater. 2017, 7, 1700317. [Google Scholar] [CrossRef]
- Gao, T.; Han, F.; Zhu, Y.; Suo, L.; Luo, C.; Xu, K.; Wang, C. Hybrid Mg2+/Li+ battery with long cycle life and high rate capability. Adv. Energy. Mater. 2015, 5, 1401507. [Google Scholar] [CrossRef]
- Su, S.; Huang, Z.; Nuli, Y.; Tuerxun, F.; Yang, J.; Wang, J. A novel rechargeable battery with a magnesium anode, a titanium dioxide cathode, and a magnesium borohydride/tetraglyme electrolyte. Chem. Commun. 2015, 51, 2641–2644. [Google Scholar] [CrossRef]
- Asif, M.; Rashad, M.; Ali, Z.; Ahmed, I. Synthesis of ternary metal oxides as positive electrodes for Mg-Li hybrid ion batteries. Nanoscale 2020, 12, 924–932. [Google Scholar] [CrossRef]
- Huang, H.; Zhao, G.; Zhang, N.; Sun, K. Two-dimensional Nb2O5 holey nanosheets prepared by a graphene sacrificial template method for high performance Mg2+/Li+ hybrid ion batteries. Nanoscale 2019, 11, 16222–16227. [Google Scholar] [CrossRef] [PubMed]
- Soundharrajan, V.; Sambandam, B.; Kim, S.; Mathew, V.; Jo, J.; Kim, S.; Lee, J.; Islam, S.; Kim, K.; Sun, Y.-K.; et al. Aqueous magnesium zinc hybrid battery: An advanced high-voltage and high-energy MgMn2O4 cathode. ACS Energy Lett. 2018, 3, 1998–2004. [Google Scholar] [CrossRef]
- Rashad, M.; Li, X.; Zhang, H. Magnesium/Lithium-Ion hybrid battery with high reversibility by employing NaV3O8·1.69H2O nanobelts as a positive electrode. ACS Appl. Mater. Interfaces 2018, 10, 21313–21320. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, S.; Wang, H. High performance hybrid Mg-Li ion batteries with conversion cathodes for low cost energy storage. Electrochim. Acta 2018, 265, 175–183. [Google Scholar] [CrossRef]
- Kravchyk, K.V.; Walter, M.; Kovalenko, M.V. A high-voltage concept with sodium-ion conducting beta-alumina for magnesium-sodium dual-ion batteries. Commun. Chem. 2019, 2, 84. [Google Scholar] [CrossRef]
- Miao, Q.; Nuli, Y.; Wang, N.; Yang, J.; Wang, J.; Hirano, S.-i. Effect of Mg2+/Li+ mixed electrolytes on a rechargeable hybrid battery with Li4Ti5O12 cathode and Mg anode. RSC Adv. 2016, 6, 3231–3234. [Google Scholar] [CrossRef]
- Zhou, L.; Xiong, F.; Tan, S.; An, Q.; Wang, Z.; Yang, W.; Tao, Z.; Yao, Y.; Chen, J.; Mai, L. Nickel-iron bimetallic diselenides with enhanced kinetics for high-capacity and long-life magnesium batteries. Nano Energy 2018, 54, 360–366. [Google Scholar] [CrossRef]
- Fu, T.; Chen, Y.; Hao, J.; Wang, X.; Liu, G.; Li, Y.; Liu, Z.; Cheng, L. Facile preparation of uniform FeSe2 nanoparticles for PA/MR dual-modal imaging and photothermal cancer therapy. Nanoscale 2015, 7, 20757–20768. [Google Scholar] [CrossRef]
- Mizrahi, O.; Amir, N.; Pollak, E.; Chusid, O.; Marks, V.; Gottlieb, H.; Larush, L.; Zinigrad, E.; Aurbach, D. Electrolyte solutions with a wide electrochemical window for recharge magnesium batteries. J. Electrochem. Soc. 2008, 155, A103–A109. [Google Scholar] [CrossRef]
- Wang, H.; Wang, X.; Li, Q.; Li, H.; Xu, J.; Li, X.; Zhao, H.; Tang, Y.; Zhao, G.; Li, H.; et al. Constructing three-dimensional porous carbon framework embedded with FeSe2 nanoparticles as an anode material for rechargeable batteries. ACS Appl. Mater. Interfaces 2018, 10, 38862–38871. [Google Scholar] [CrossRef]
- Zhong, D.; Chen, J.; Zhang, J.; Luo, Y.; Li, Z.; Cheng, L.; Chen, Y.; Wang, G.; Wang, R. The yolk-shell FeSe@C nanobox with novel synthesis and its high performance for the anode of lithium-ion batteries. Mater. Res. Express 2019, 6, 085058. [Google Scholar] [CrossRef]
- Gofer, Y.; Chusid, O.; Gizbar, H.; Viestfrid, Y.; Gottlieb, H.E.; Marks, V.; Aurbach, D. Improved electrolyte solutions for rechargeable magnesium batteries. Electrochem. Solid State Lett. 2006, 9, A257–A260. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Zhang, L.; Li, N.; Zhang, X. Studies of FeSe2 Cathode Materials for Mg–Li Hybrid Batteries. Energies 2020, 13, 4375. https://doi.org/10.3390/en13174375
Zhang C, Zhang L, Li N, Zhang X. Studies of FeSe2 Cathode Materials for Mg–Li Hybrid Batteries. Energies. 2020; 13(17):4375. https://doi.org/10.3390/en13174375
Chicago/Turabian StyleZhang, Changhuan, Liran Zhang, Nianwu Li, and Xiuqin Zhang. 2020. "Studies of FeSe2 Cathode Materials for Mg–Li Hybrid Batteries" Energies 13, no. 17: 4375. https://doi.org/10.3390/en13174375
APA StyleZhang, C., Zhang, L., Li, N., & Zhang, X. (2020). Studies of FeSe2 Cathode Materials for Mg–Li Hybrid Batteries. Energies, 13(17), 4375. https://doi.org/10.3390/en13174375




