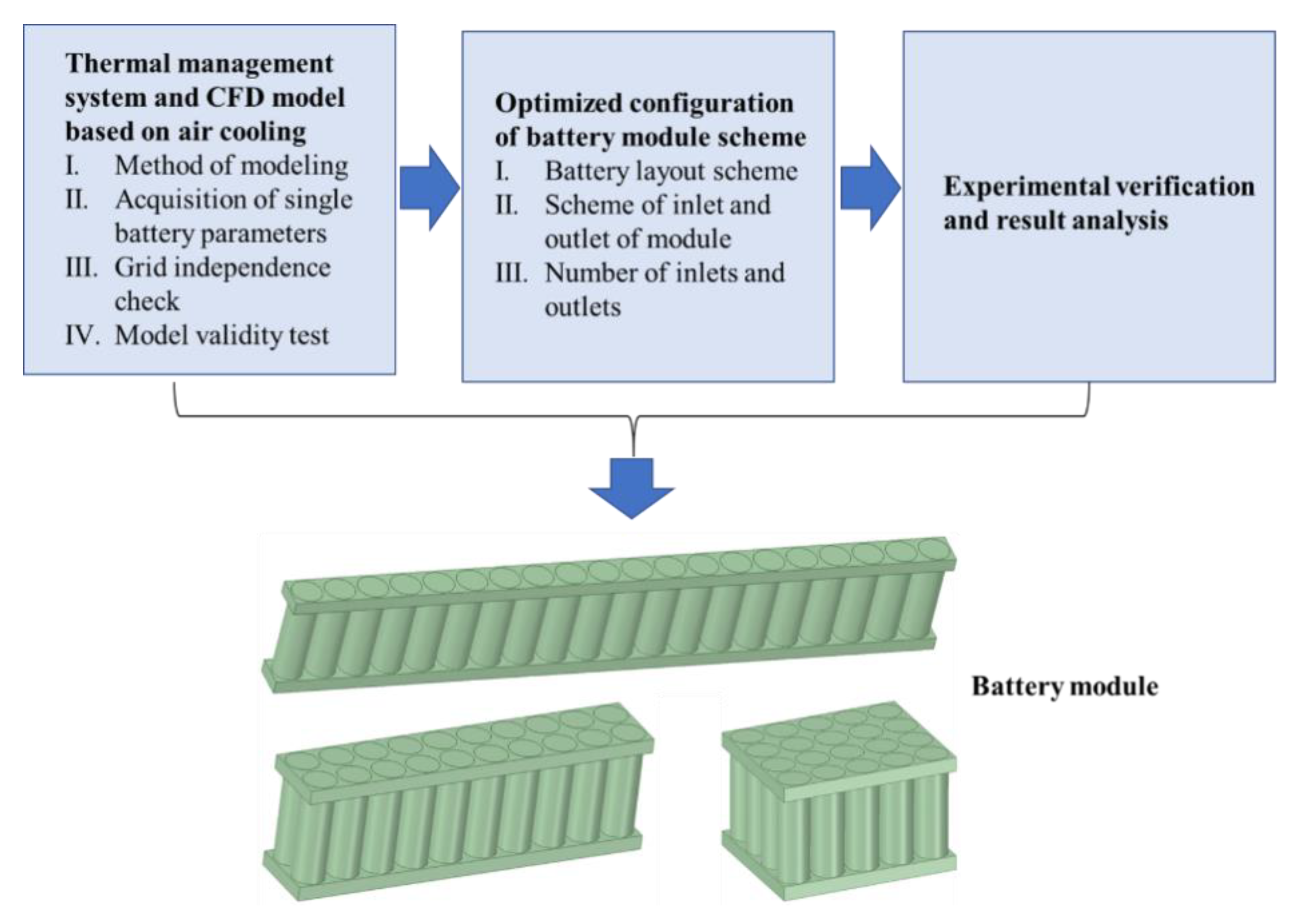
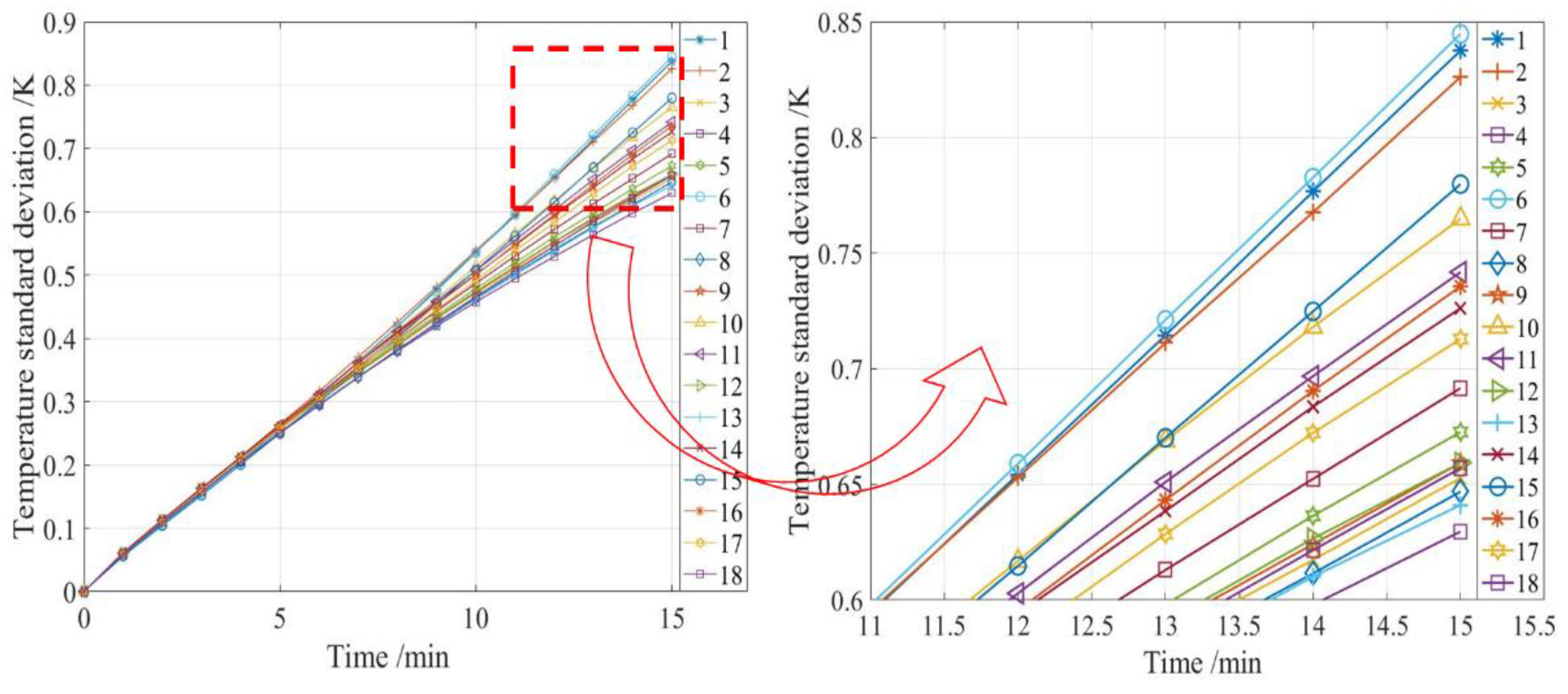
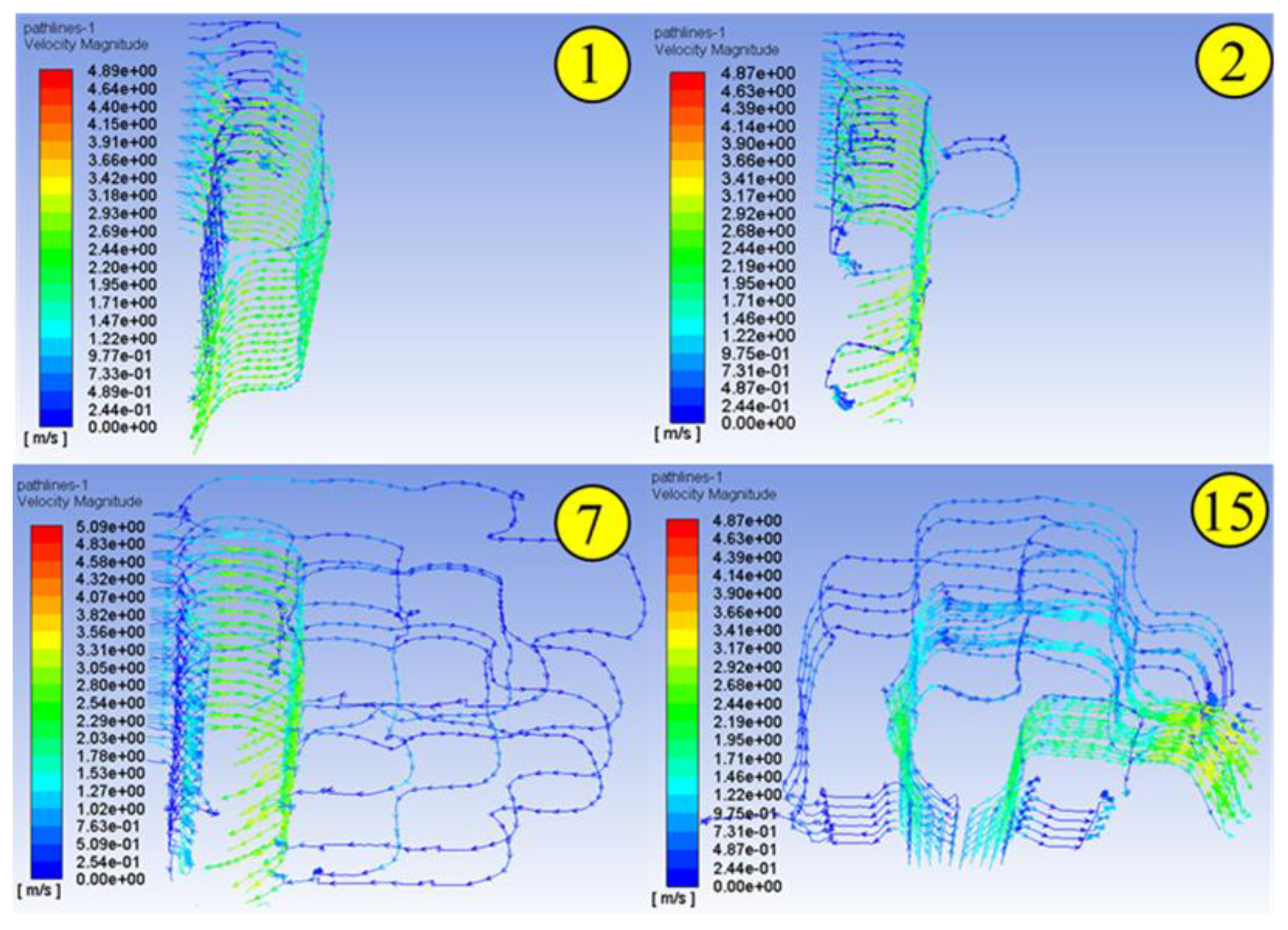
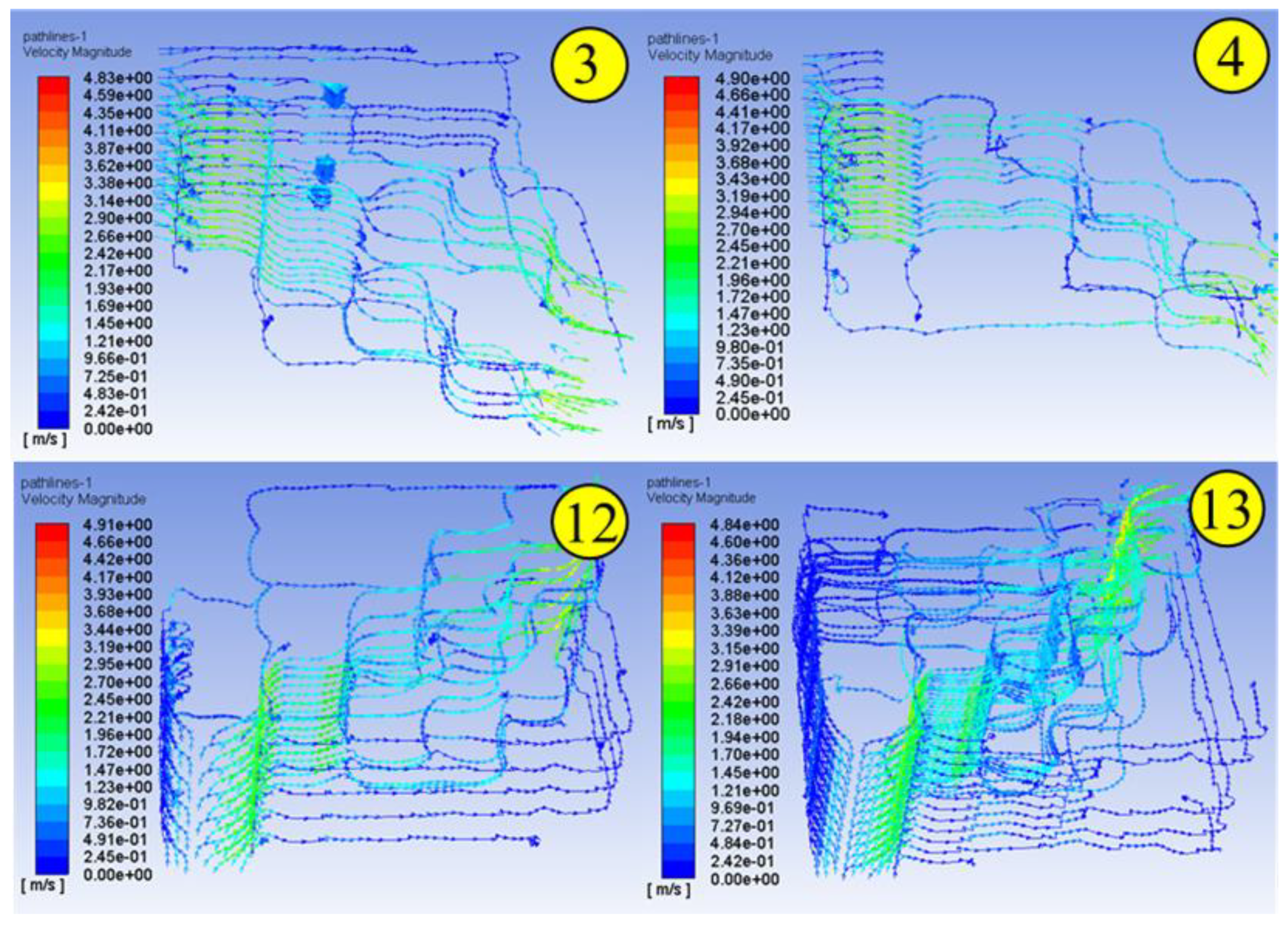
1. Introduction
The performance of the lithium-ion battery (LIB) is at the core of the driving system of an electric vehicle, and thus it significantly affects the driving range and service life of electric vehicles [
1,
2]. Among the many factors that determine battery performance, the influence of temperature on the battery should not be underestimated [
3]. Battery heating is an inevitable phenomenon and a complicated problem [
4]. Reasonable control of temperature changes in battery operation depends on an efficient battery thermal management system (BTMS) [
5,
6]. Essentially, batteries work to convert chemical energy into electrical energy for the machine to use, so the heat generated is waste energy, which reduces the energy conversion efficiency of the battery. A certain amount of heat yield is beneficial to ensure the normal chemical reaction temperature of the battery and promote the charging and discharging process of the battery. However, if an abnormal amount of heat is generated or the heat does not dissipate in time, the battery suffers overheating, which may potentially cause the active material on the electrodes to peel off and promote electrolyte degradation, that is, cause harm to the battery itself [
7,
8,
9]. These changes are irreversible and can cause permanent damage to the battery. In addition, because the battery is hot, there are safety risks. An overheated battery can short-circuit due to damage to the internal materials, such as the diaphragm, which sets off a chain reaction that can lead to a fire or even an explosion [
10]. Without effective control and management, the harmful effects of a single battery can spread throughout the entire battery pack, and thermal runaway will be amplified, causing the battery pack to be out of control. Therefore, it is very necessary for electric vehicles to design a practical and efficient BTMS [
11]. Excellent thermal management benefits the performance of the battery pack [
12].
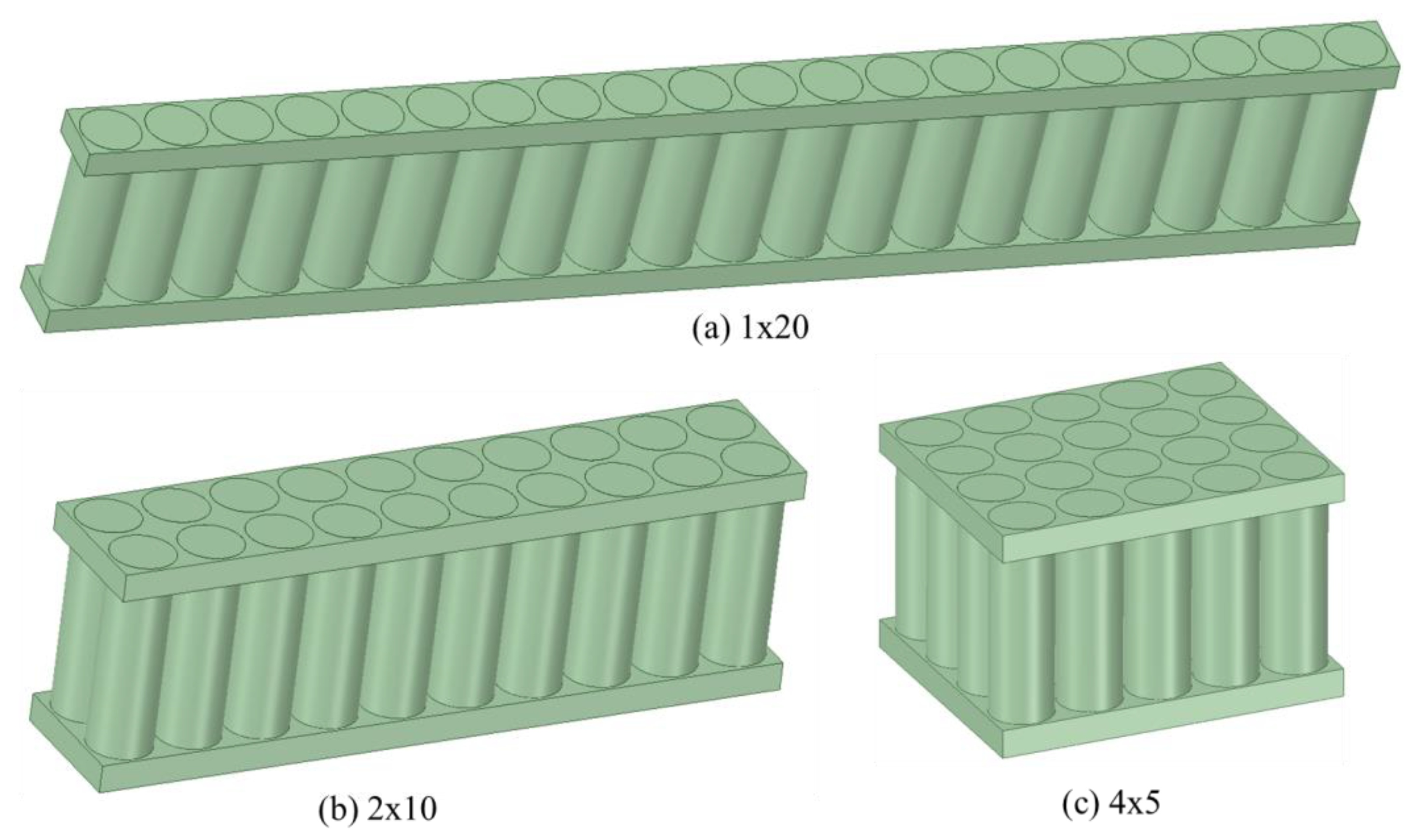
There are three main types of LIBs used in electric vehicles (EVs): (1) prismatic; (2) pouch; and (3) cylindrical batteries.
Figure 1 shows these three types of batteries. The prismatic battery can be designed according to the needs of customers. This makes the prismatic battery suitable for almost all kinds of electric cars. This high adaptability also results in prismatic batteries that vary in size, nominal voltage, and other parameters, which makes it difficult to form an industry standard. Pouch batteries are manufactured and packed by superposition. Compared with prismatic batteries, the aluminum alloy shell of the pouch battery pack is replaced with a lighter aluminum plastic film package, which improves the energy density of the whole battery pack. However, a big drawback of soft-pack batteries is their poor consistency, which means they need more sophisticated control and monitoring systems. After years of development, the cylindrical battery has obtained a high degree of standardization, which makes it easy to achieve a unified industry standard. In addition, the cylindrical battery has inherent advantages in regard to heat dissipation, and a good heat dissipation space is formed between the cylinders when packing.
Among these types of battery, the cylindrical battery is the most discussed and well-studied and it is the first mass-produced commercial battery., Thus, much of the research has focused on cylindrical batteries. Most of the studies are based on three different ways of heat dissipation: AC, liquid-cooling (LC), and phase-change material (PCM) cooling. Wang detailed the three cooling methods in his research [
13]. AC is widely used for its simplicity, low cost and there is no hidden danger of battery damage. Wang verified the effectiveness of forced-air cooling in ensuring the battery pack operates within the normal temperature range (no more than 40 °C) when the discharge of the current rate of a single battery is set at no more than 3C. Similarly, based on the AC method, Mahamud et al. [
14] studied the influence of reciprocating airflow in the BTMS of cylindrical battery packs. This method reduced the battery temperature by about 4 °C (72%) and the maximum temperature (MaxT) by about 1.5 °C. LC is a cooling method that uses a liquid material with a large specific heat capacity (SHC), such as water, to flow through the surface of the battery and take away the generated heat. This method requires the battery system to be highly sealed, but its strong heat transfer capacity leads to a better cooling effect than that of AC [
15]. Wang et al. [
16] proved that several factors, such as fluid flow, flow direction, etc. determine the cooling effect at a certain degree. They designed a BTMS based on a hot silicon plate and used experiments and simulations to explore and verify the significant influence of liquid flow, flow direction and the number of cooling channels on the cooling capacity of the BTMS. PCM cooling, which uses the process of absorbing heat during the phase change to balance the heat that the battery generates, is costly but offers a pollution-free, high-return solution. By comparing cooling performance under different conditions, Kizilel et al. [
17] proved that the PCM cooling method is superior to AC in regard to economy, effectiveness, and safety. Through simulation and experiment, Huang et al. [
18] proved that the cooling system of thermal-assisted expanded graphite is superior to the AC system in actual and extreme conditions. In addition, Wang [
19] and Wang [
20] greatly improved the performance of the cooling system without changing the cooling mode, by optimizing the battery pack structure. Yang et al. [
21] found the specific parameters of the battery pack through optimization analysis. They pointed out that the cooling system works best when the height of the battery pack is 34 mm and the width is 32 mm.
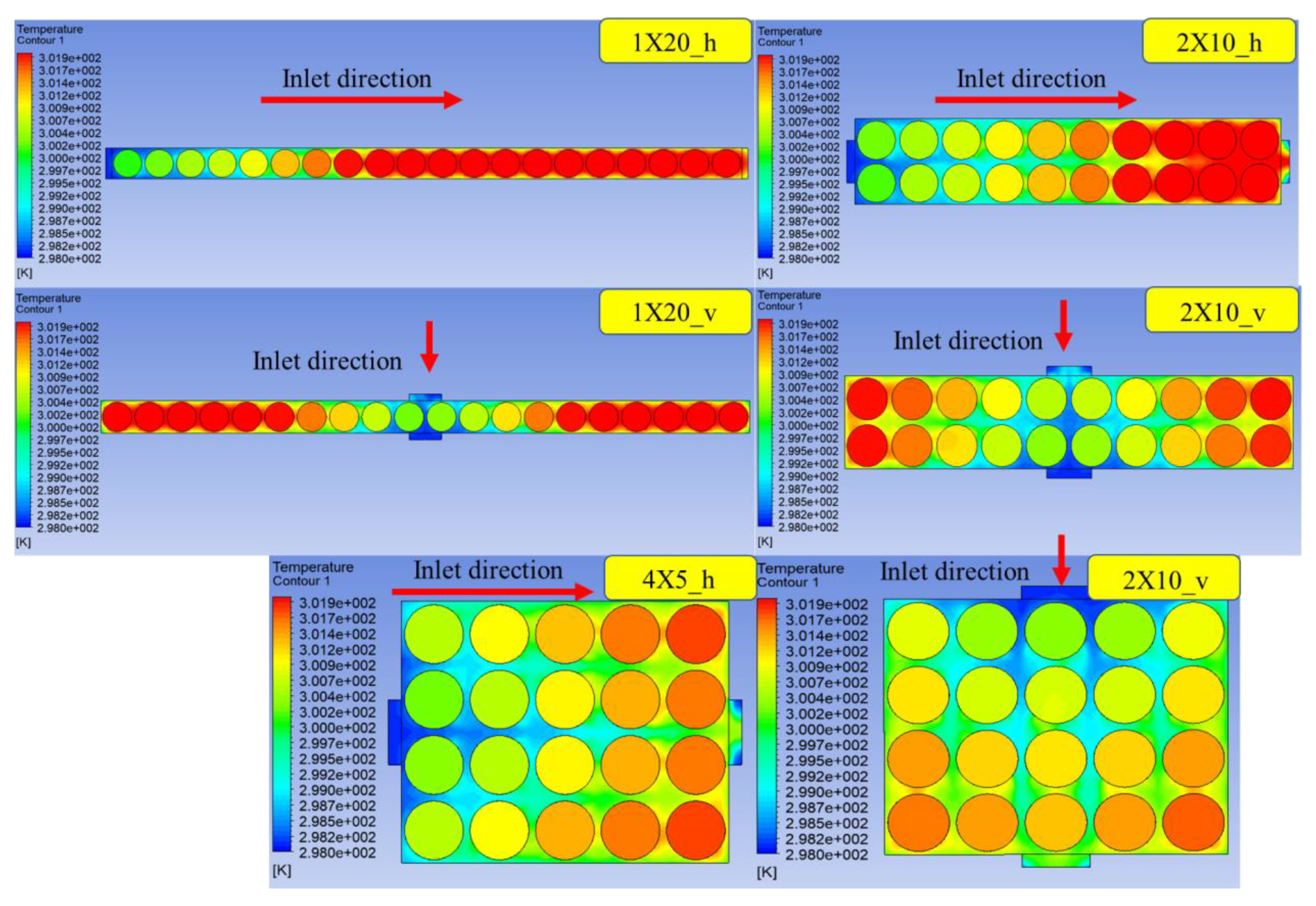
In this study, a cylindrical BTMS based on AC is proposed. The battery pack consists of 20 cylindrical battery modules of type 18650 batteries. First, the dynamic model was built and the validity of the model was verified. Then, the BTMS of the cylindrical battery pack was optimized for the comparative analysis of different battery configurations with different cell layouts, different positions of the air inlet and outlet in the AC system and the number of inlets and outlets. Finally, the accuracy and effectiveness of the whole process were verified experimentally, which provided an ideal design for the BTMS of the cylindrical battery in the future.
3. Heat Dissipation Model of Computational Fluid Dynamics
There are four main sources of heat production in working batteries, namely, reaction heat (RH), side reaction heat (SRH), joule heat (JH) and polarization heat (PH). The total calculation of the heat generation is shown in Equation (1).
where
represents the power of the total heat,
represents the power of RH,
represents the power of SRH,
represents the power of JH, and
represents the power of PH.
RH refers to the heat generated by the chemical reaction in the electrodes in charging and discharging. Generally, the charging process of LIB absorbs energy to reduce ambient temperature and in reverse, its discharging process releases heat [
22]. This results in a side reaction, that is, heat is generated by a series of chemical reactions other than the main chemical reaction, such as the partial decomposition of electrolyte at high temperature and self-discharge caused by the change in electrode material structure. These side reactions are intensified in the period before the battery fails. However, during the life of the battery, the side reactions are so weak that the heat of the side reaction is usually ignored. JH is the work done by the current on the internal resistance (IR). This part of the heat can be calculated by Joule’s law as shown in Equation (2):
where
is IR, and
I refers to the current on
.
PH refers to the heat generated when the positive and negative electrode potential deviates from the equilibrium potential. When polarization occurs, the voltage difference between the battery’s open circuit voltage and the terminal voltage generate PH. Generally, it is assumed that there is a polarization IR
, and the heating power is calculated by Joule’s law, as shown in Equation (3),
3.1. Acquisition of Battery’s Thermodynamic Parameters
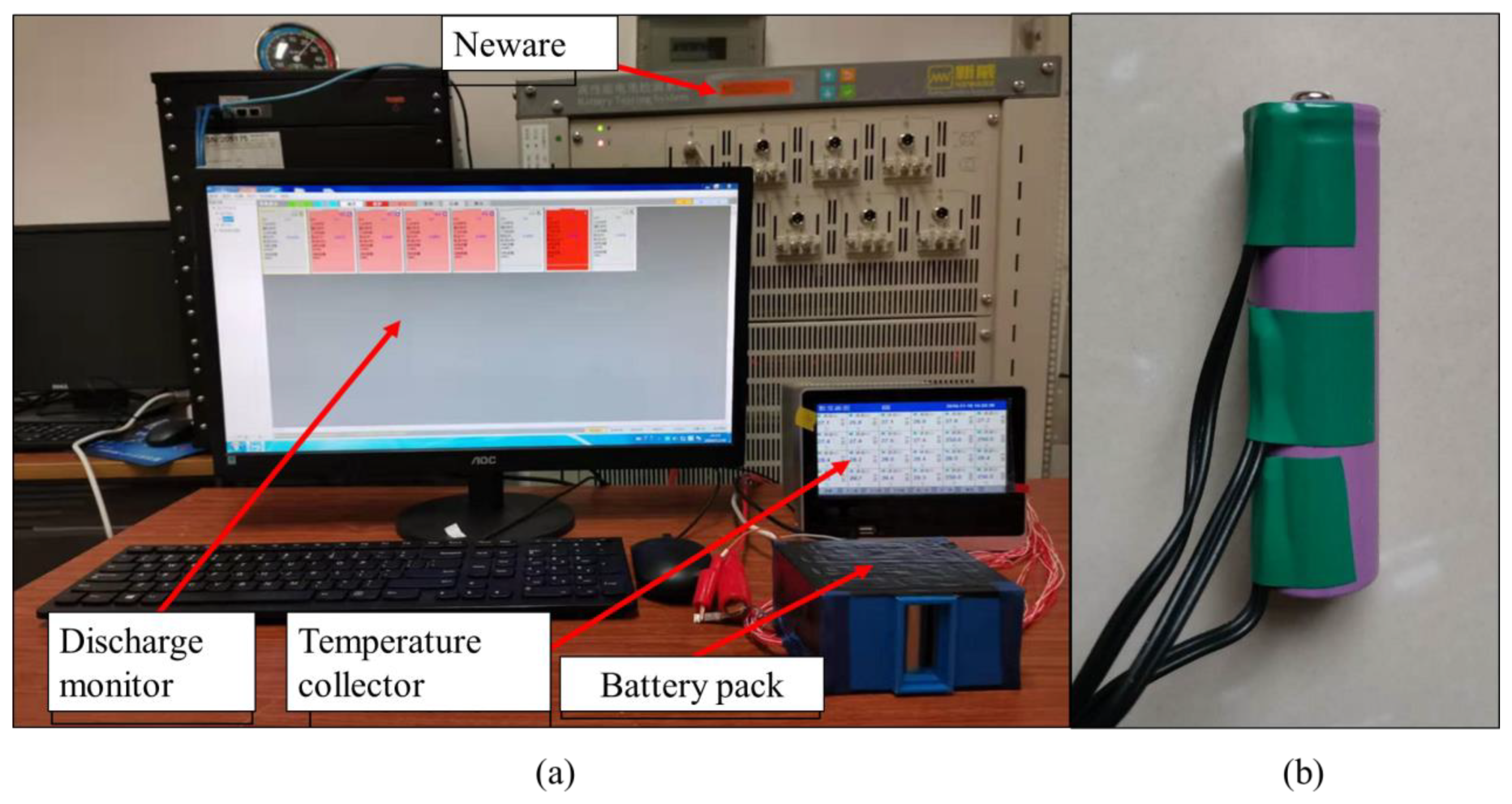
Before the heat dissipation model is built, the battery parameters need to be determined. These parameters include the IR, SHC, heat yield and thermal conductivity (TC) of the single battery. In order to obtain these parameters, it is necessary to conduct charge and discharge experiments on a single battery. The equipment generates cycles of charge and discharge, and the measurement of the parameters of current, voltage and temperature. The Arbin machine battery testing system was adopted, which can charge/discharge batteries at a set constant current or voltage value and record the respective current, voltage, capacity, and impedance per minute simultaneously. Besides the Arbin machine, several temperature sensors and a miniature blower were needed. The equipment is shown in
Figure 3a and each battery is equipped with three temperature sensors, as shown in
Figure 3b. For determining the measurement error, every single cell is installed with three temperature sensors that are attached at the cathode, middle and anode of the cell. The temperature utilized in this study is the average value.
3.1.1. Internal DC Resistance
The battery resistance refers to the direct current (DC) resistance of the battery, which consists of the static resistance and polarization resistance. The hybrid pulse power characterization test (HPPC) method was used to measure the DC resistance of the battery. The HPPC method is designed to test the resistance of the battery under specific temperatures and state of charge (SOC).
3.1.2. Specific Heat Capacity
The type 18650 battery is not homogeneous, so, the SHC
refers to the equivalent SHC of a single battery. Assuming that the equivalent SHC of the battery is
, according to the definition of SHC, it is described by Equation (4):
It is known that the value of the heat generated (i.e., temperature rise) by the battery is directly proportional to the equivalent SHC of the battery. In fact, the SHC is computed by the law of conservation of energy (COE). In an adiabatic environment, the value of the heat generation of a battery equals its stored heat. The accumulation of heat storage will lead to the rise in battery temperature. By measuring the temperature rise in a specific period time, the SHC of the battery can be calculated according to the above formula. In the actual experiment, in order to reduce the experimental error, the SHC of the battery under different discharge ratios (i.e., different value of heat generation) was measured and the SHC of the battery was calculated by linear regression. The experimental procedures have been described in detail in [
23].
3.1.3. Heat Generation of Single-Cell and Thermal Conductivity
On this basis, Bernardi et al. (1984) provided a methodology to calculate the heat production rate of batteries, which is expressed by Equation (5).
where
is the power of the heat generation,
I is current in circuit,
T is the real-time temperature of the battery,
E refers to the open circuit voltage of battery, and
U refers to the average terminal voltage of battery.
In Equation (5), the first item
on the right side of equation is the formula to calculate the power of RH, which equals the
in Equation (1). The second item refers to summary of JH and PH and it shows the voltage attribution. The voltage drops
in the open circuit voltage and terminal voltage are attributed to internal DC resistance and their relationship is represented by Equation (6).
make up the internal DC resistance, which can be expressed by
. So, the heat production Equation (5) can be expressed by Equation (7),
On the premise that the equivalent SHC is known (calculated in
Section 3.1.2), the heat production in a certain period of time can be solved according to the definition of the SHC [
23]. In this experiment, the heat dissipation effect of the battery within 15 min is discussed, so the heat generation of the battery should also be the heat generation within 15 min. The discharging current rate is set at 2C. Twenty single batteries were selected for heat yield measurement. The specific operational steps are as follows:
Take 20 type 18650 batteries and fully discharge them, and then hold them for 30 min at room temperature before charging them at 0.75C.
Record the current, voltage, temperature and corresponding time value.
For each cell, the temperatures were recorded. During battery discharge, oxidation occurs in the negative electrode, and lithium is separated from the carbon rod and releases energy, which causes the temperature of the negative electrode to rise. At the same time, there is a reduction at the positive pole. Lithium ions precipitate at the positive electrode and absorb a certain amount of energy, so the temperature of the positive pole decreases. Therefore, during the whole discharge process, the temperature gradually decreases from the negative electrode to the positive electrode. Thus, it is assumed that the temperature of the positive pole is transferred from the negative electrode and the equivalent TC of the whole battery is calculated based on the battery temperature distribution. The formula to calculate TC is illustrated in Equation (8):
where
is TC,
is conducted heat, and
is the distance from the negative pole to the positive pole.
refers to the temperature difference (TD) between the two poles. In fact, the conductivity of the battery is anisotropic, which means the TC is different in the surface and the thickness directions. However, the TC in the surface direction is much less than that in the thickness direction and it is ignored in this model [
23,
24].
In order to avoid contingency and remove outliers, 20 type 18650 batteries were tested under the same conditions. After calculating the average value of the 20 batteries, the thermodynamic parameters are listed in
Table 1.
3.2. Modeling Method
A three-dimensional model was built in SpaceClaim18.2. The meshing operation and numerical simulation were processed in ansys18.2. In order not to consider the energy consumption too much, the fluid inlet speed is set to 1 m/s and it is maintained by a fan in all designs. This is the typical forced convection mode. The method of forced convection improves the heat dissipation effect by increasing the heat transfer rate per unit time. Compared with natural convection, it consumes more energy but performs very well with respect to thermal management. The Reynolds number can be calculated by Equation (9). In these models, the distance between the battery and the wall is 10 mm. The gap in the battery center is 20 mm. The fluid cross section with the largest characteristic length exists in a rectangular plane of 2 × 490 mm. The max Reynolds number here is 406, which determines the laminar flow type in the simulation.
where
and
are fluid density and dynamic viscosity coefficient, and
,
are the characteristic velocities and characteristic length of the flow field.
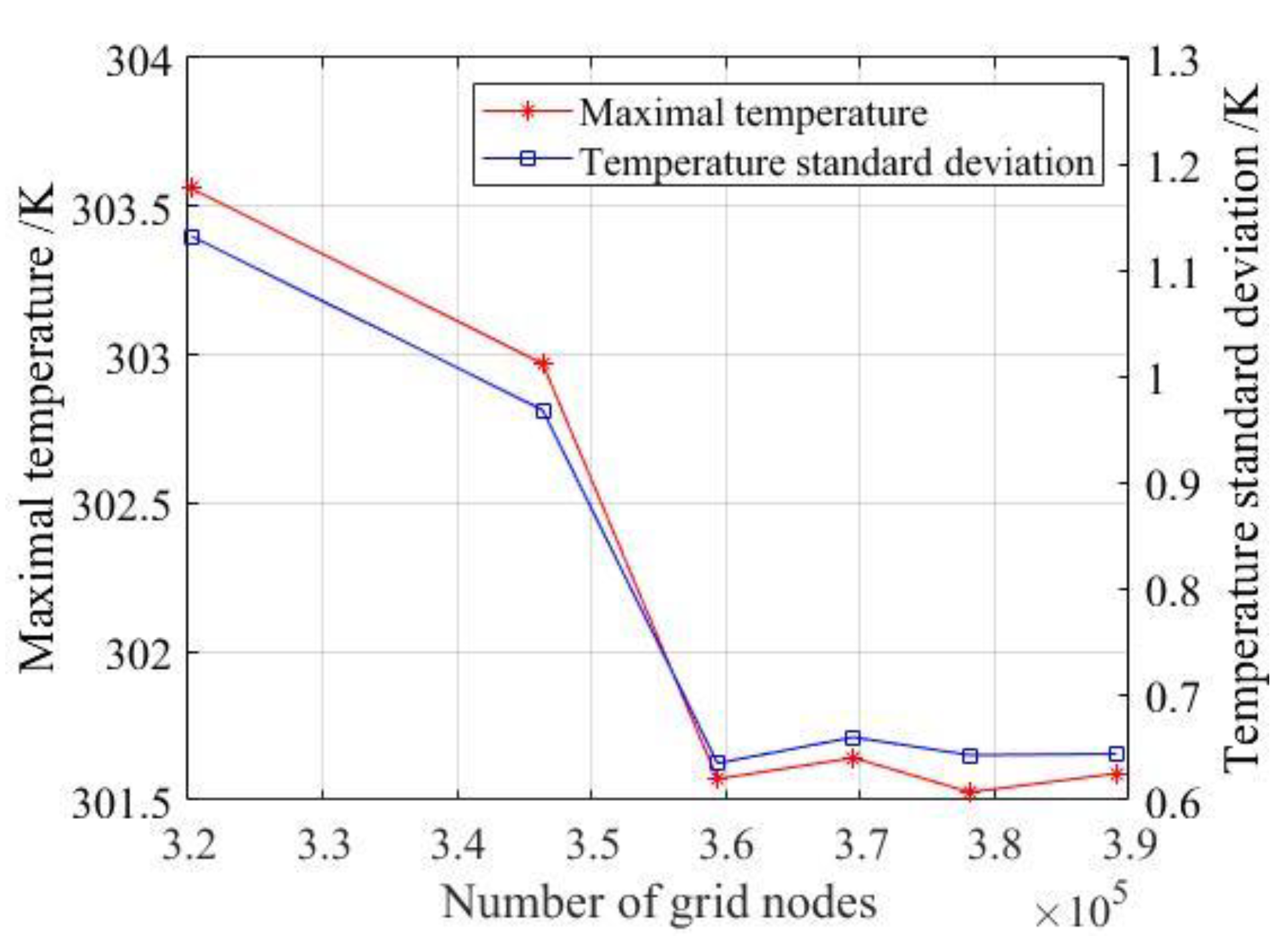
The results of the grid independence test are shown in
Figure 4, which ensure that the subsequent simulation process is reliable. In
Figure 4, once the number of grids reaches 359,370–389,048, the MaxT of the model remains constant, that is, when the number of grids is more than 359,370, the size of the nodes will not affect the calculation results and the grid will pass the independence test. In order to save computing costs, the number of grids is selected as 359,370.
To simulate the flow model in ANSYS and compare the results, some assumptions need to made, including:
Steady-state flow from the inlet
Constant thermal and physical properties
No energy within flow
Heat loss of radiation and natural convection is neglected
Gravity is not taken into consideration.
In the process of battery heat generation, the energy of the battery conforms to the law of COE [
25,
26], that is, it satisfies Equation (10),
where
refers to density of battery;
is equivalent to SHC;
refers to the battery temperature;
,
, and
represent the heat conductivity coefficient (HCC) in the
x,
y, and
z directions, respectively; and
is a volumetric heat source of battery, which is the same as the heat generation rate.
The energy conservation equation (ECE) for coolant [
8] is expressed by Equation (11):
The coolant in this study is air, so the variable refers to the density of air; and are the SHC and TC of the air, respectively.
The velocity of air is about 1 m/s whose Mach number is far less than 0.3. So, the continuous equation of coolant (which here refers to air) is as shown in Equation (12)
where
is the velocity vector.
The momentum conservation equation (MCE) is shown in Equation (13).
where
and
are the static pressure and dynamic viscosity of the air, respectively.
3.3. Model Validity Test
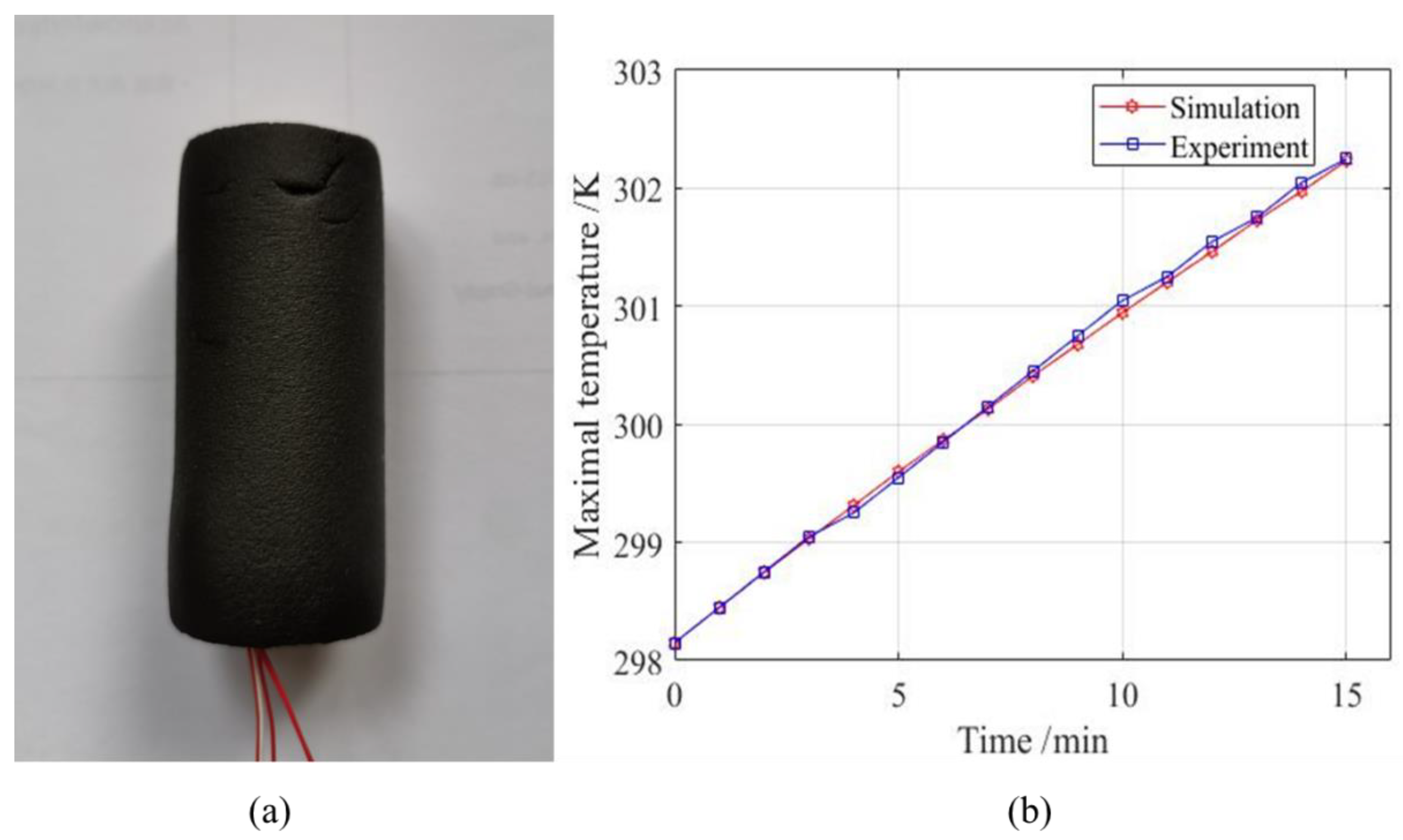
To ensure that the model has practical value, it is necessary to test the validity of the model. A validity test involved a pre-experiment and a pre-experimental simulation and then the results are compared to show whether the model is effective and can be used for further study. A single battery was selected for testing and it was covered by a piece of insulated cotton. The covered single battery used to simulate adiabatic condition is shown in
Figure 5a. Then, the battery was continuously discharged to collect the temperature change data for the battery during discharge. The result is shown in
Figure 5b. The temperature measured by the experiment was almost the same as that measured by the simulation.
5. Experimental Validation
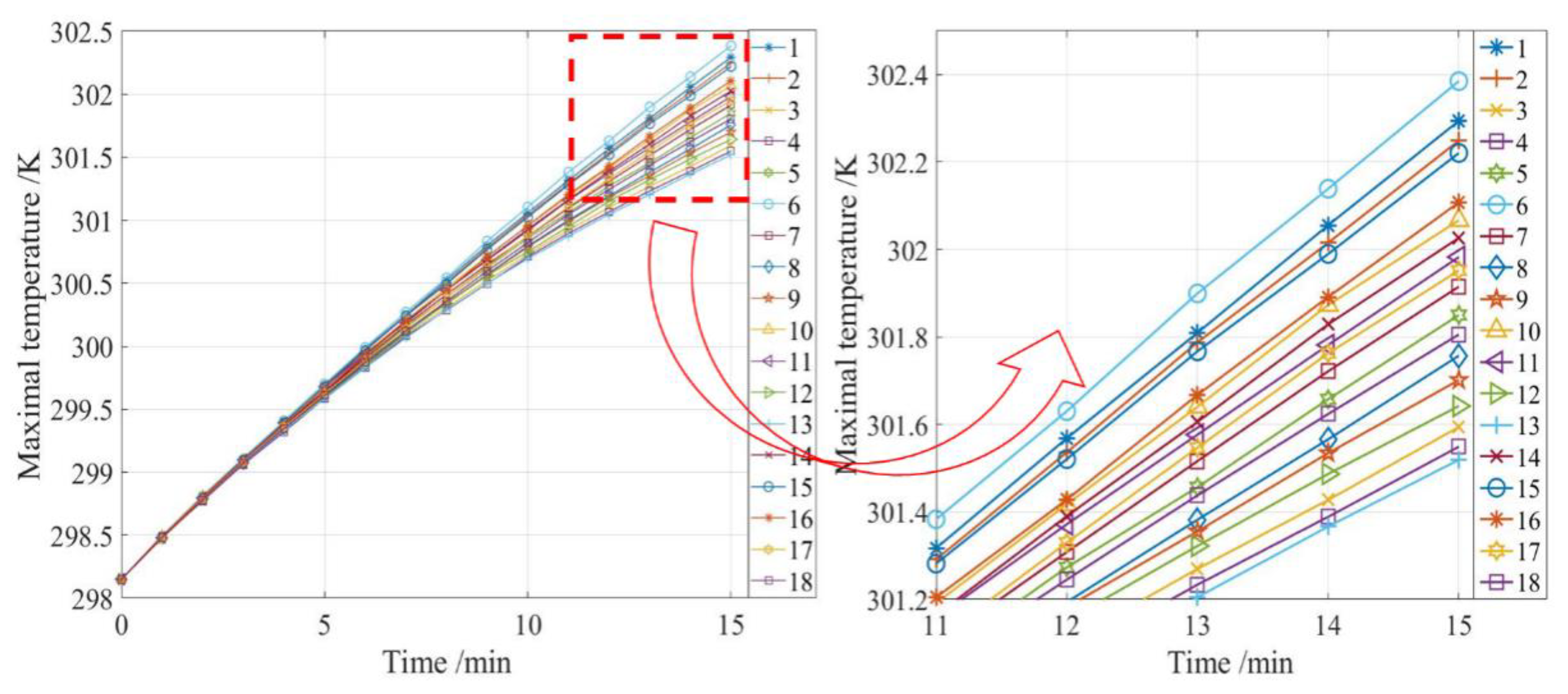
As we know from the previous section, the optimal arrangement of the inlet and outlet in the one-in-one-out model is scheme 13, and the optimal arrangement of the inlet and outlet in the one-in-two-out mode is scheme 24. This is because, under these two configurations, the fluid flow state in the battery pack is most suitable for dissipating the heat in the battery pack, which can result in good heat dissipation of the battery system when working. However, the simulation results need to be verified by experiments. Therefore, discharge tests were conducted on scheme 13 and scheme 24. The 20 batteries are arranged in a 4 × 5 arrangement and connected in a series of four and a parallel of five. To ensure a constant discharge current of 2 A, the discharge current of 8 A was set to constantly discharge for 15 min. In the experiment, the temperature of 20 batteries was collected in real-time, and a temperature sensor was installed on the side of each battery. The experimental set up is shown in
Figure 3.
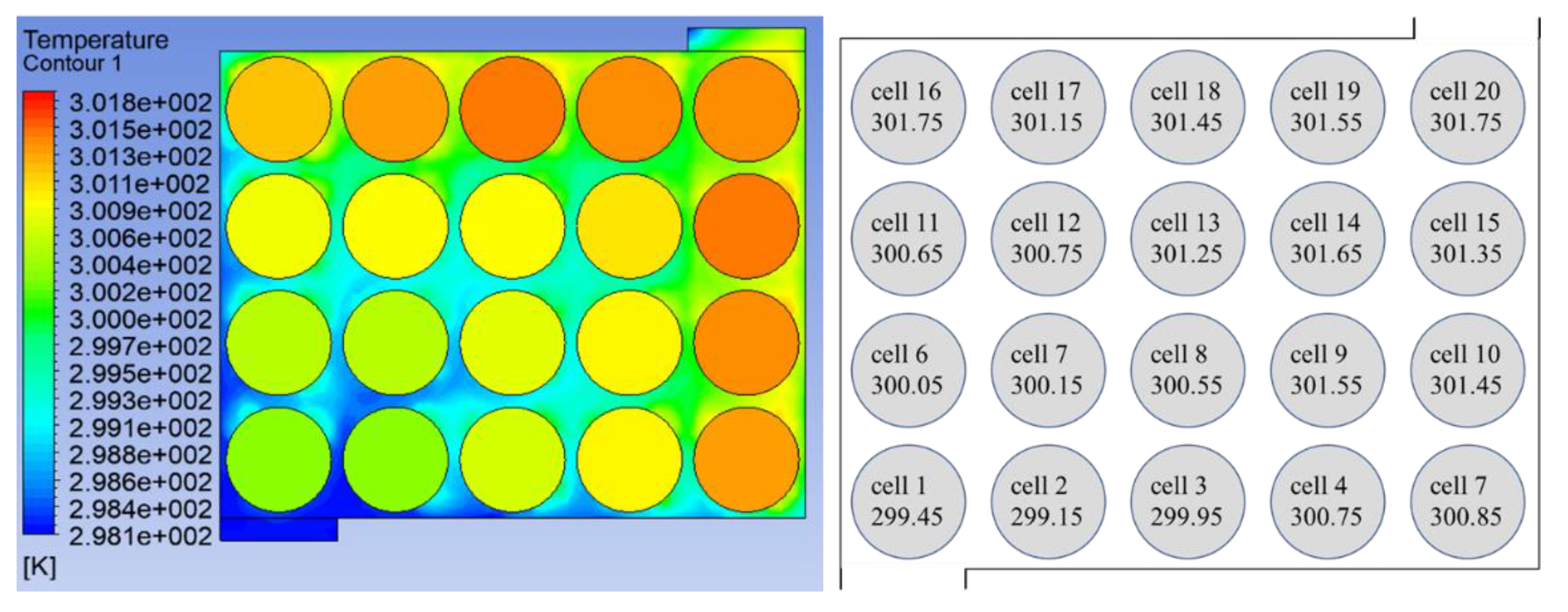
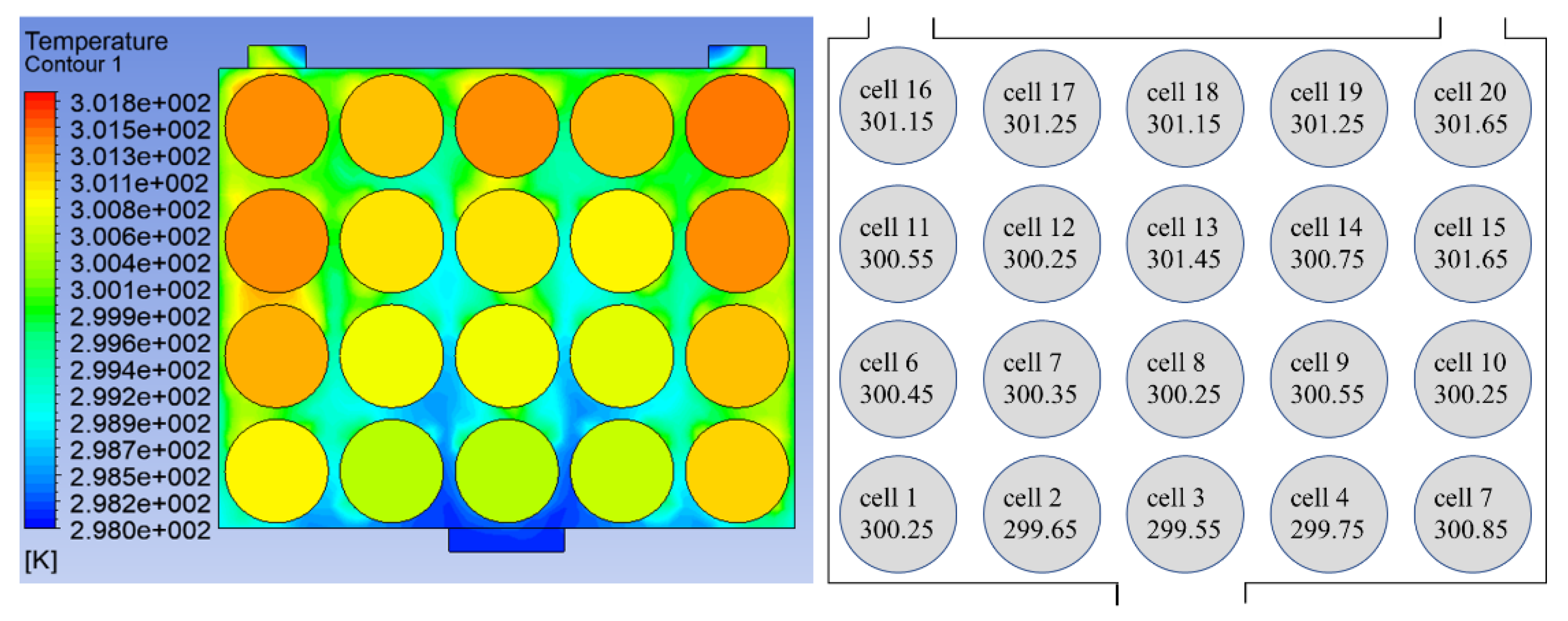
The temperature data of two battery packs were collected in the experiment. The temperature at the end of discharge (i.e., the MaxT of the battery) was marked on the single battery according to the battery layout in the experiment. The results are shown in
Figure 22 and
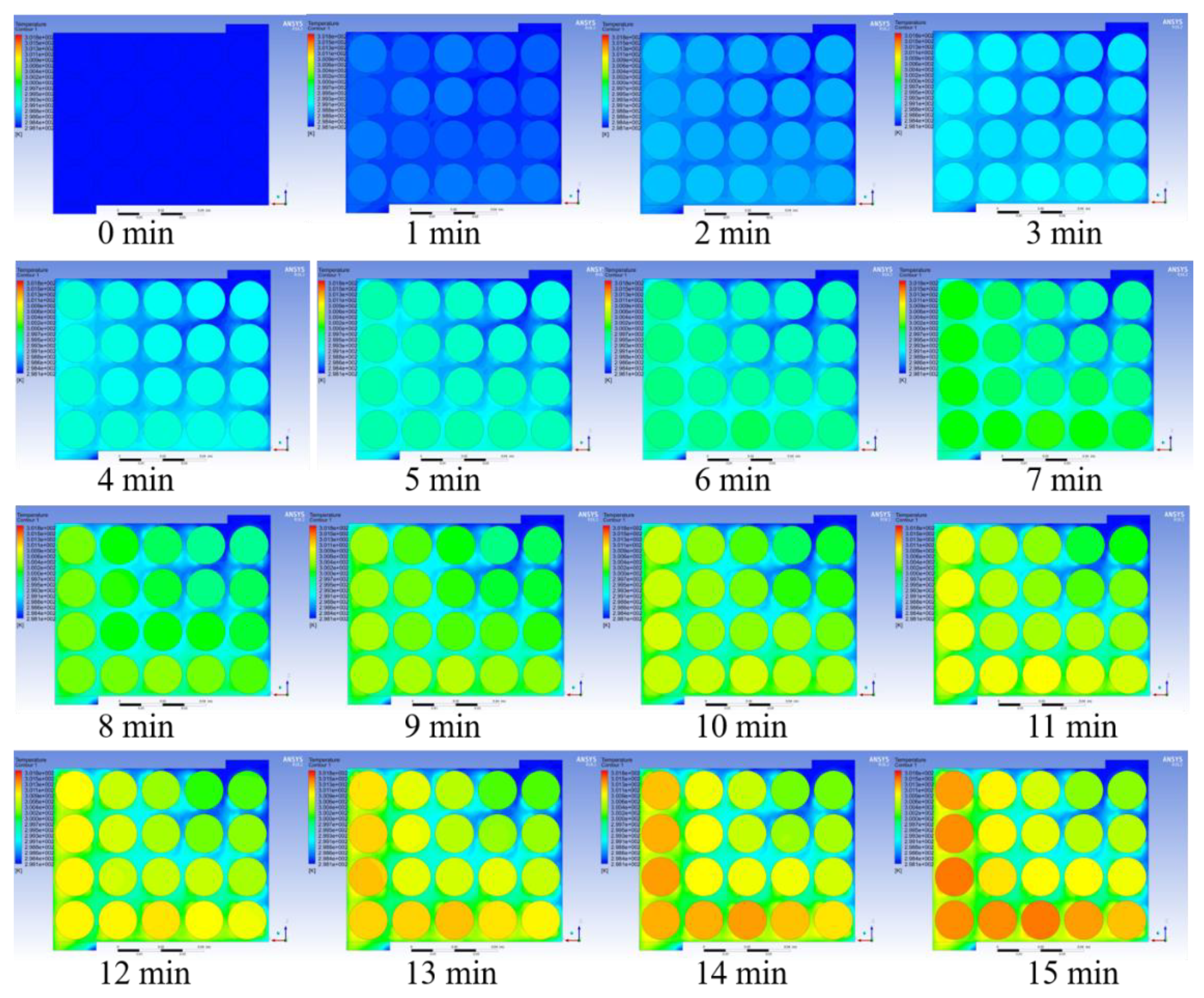
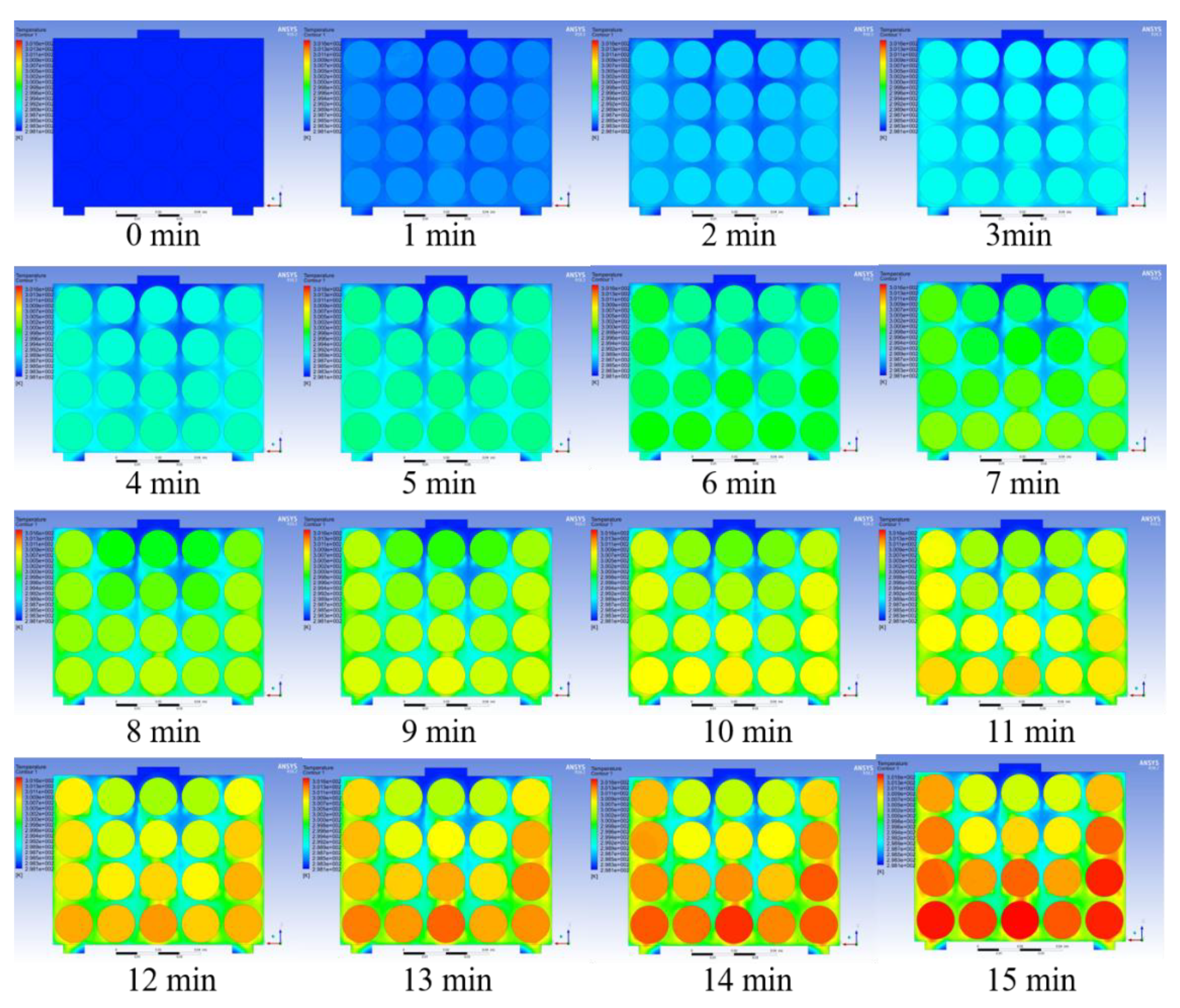
Figure 23, respectively. Under the premise of good enough performance of the BTMS, lower cost should also be a focus. The transient change process in the temperature cloud map is shown in
Figure 24 and
Figure 25.
Figure 22 and
Figure 23 show the comparison of the battery temperature during the simulation and the experiment. In
Figure 22, the error in MaxT between the simulation and experiment is 0.017% and in
Figure 23, the error in MaxT between the simulation and experiment is 0.049%. The experimental results are close to the simulation temperature. Battery temperature is generally lower near the inlet, and the temperature starts to rise as the distance from the inlet increases. High-temperatures appear near the outlet, and the closer they are to the outlet, the higher the temperature. These phenomena are consistent with the basic predictions of heat transfer. However, the experimental results are still inconsistent with the simulation results. At the end of discharge, the battery temperature is still slightly lower than the simulation results. In addition, the influence of pressure on the temperature field was not considered in this study, which is a possible cause of temperature error.
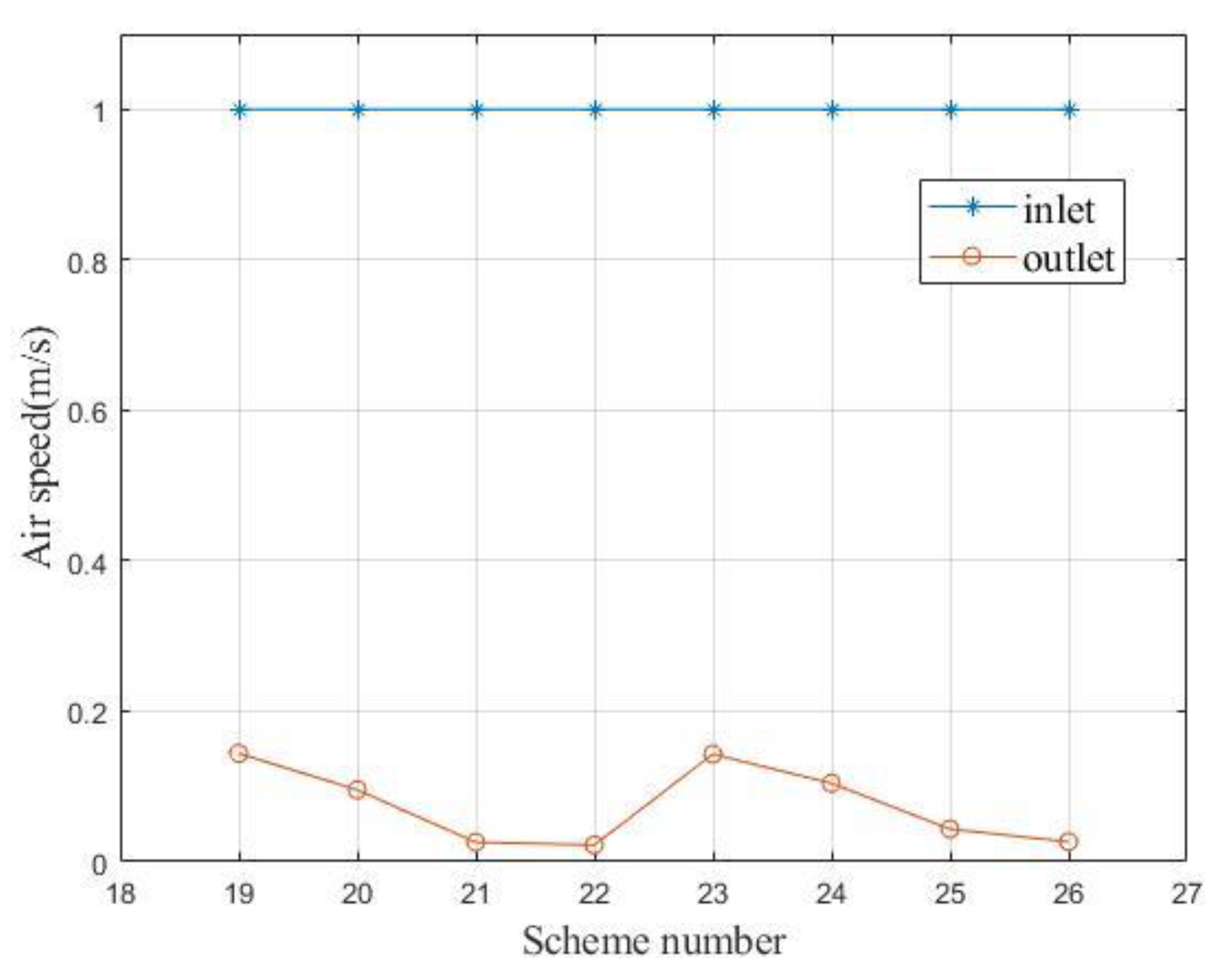
Table 2 shows a comparison of the air speed in the simulation and the experiment. Limited by the difficulty of adjustment and measurement accuracy, the forced air speed at the inlet can only be adjusted close to 1 m/s. The error in the simulation and experiment stabilized at less than 15.38%.