Effects of Salinity on Formation Behavior of Methane Hydrate in Montmorillonite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Procedure
3. Results and Discussion
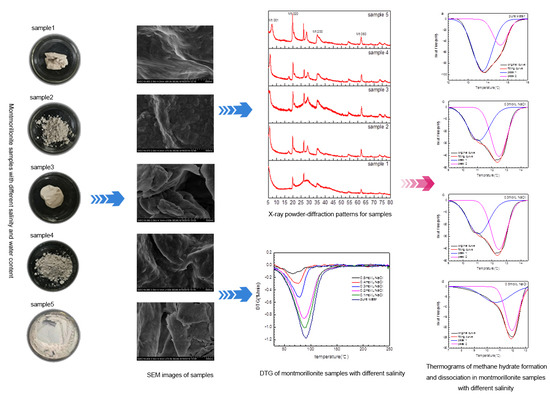
3.1. Water Content in Montmorillonite Samples
3.2. Crystal Characterization of Montmorillonite Samples
3.3. Phase Behavior of Methane Hydrate in Montmorillonite Samples
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, L.T.; Sun, C.Y.; Chen, G.J.; Nie, Y.Q. Thermodynamics Model of Predicting Gas Hydrate in Porous Media Based on Reaction-Adsorption Two-Step Formation Mechanism. Ind. Eng. Chem. Res. 2010, 49, 3936–3943. [Google Scholar] [CrossRef]
- Li, X.-S.; Zhang, Y.; Li, G.; Chen, Z.-Y.; Yan, K.-F.; Li, Q.-P. Gas hydrate equilibrium dissociation conditions in porous media using two thermodynamic approaches. J. Chem. Thermodyn. 2008, 40, 1464–1474. [Google Scholar] [CrossRef]
- Llamedo, M.; Anderson, R.; Tohidi, B. Thermodynamic prediction of clathrate hydrate dissociation conditions in mesoporous media. Am. Miner. 2004, 89, 1264–1270. [Google Scholar] [CrossRef]
- Peddireddy, S.; Lee, S.Y.; Lee, J.W. Variable contact angle model for gas hydrate equilibrium in porous media. AIChE J. 2006, 52, 1228–1234. [Google Scholar] [CrossRef]
- Anderson, R.; Llamedo, M.; Tohidi, B.; Burgass, R.W. Experimental measurement of methane and carbon dioxide clathrate hydrate equilibria in mesoporous silica. J. Phys. Chem. B 2003, 107, 3507–3514. [Google Scholar] [CrossRef]
- Kang, S.-P.; Lee, J.-W.; Ryu, H.-J. Phase behavior of methane and carbon dioxide hydrates in meso- and macro-sized porous media. Fluid Phase Equilibria 2008, 274, 68–72. [Google Scholar] [CrossRef]
- Turner, D.J.; Cherry, R.S.; Sloan, E.D. Sensitivity of methane hydrate phase equilibria to sediment pore size. Fluid Phase Equilibria 2005, 228–229, 505–510. [Google Scholar] [CrossRef]
- Uchida, T.; Ebinuma, T.; Ishizaki, T. Dissociation condition measurements of methane hydrate in confined small pores of porous glass. J. Phys. Chem. B 1999, 103, 3659–3662. [Google Scholar] [CrossRef]
- Zhang, W.; Wilder, J.W.; Smith, D.H. Interpretation of ethane hydrate equilibrium data for porous media involving hydrate-ice equilibria. AIChE J. 2002, 48, 2324–2331. [Google Scholar] [CrossRef]
- Li, X.-S.; Xu, C.-G.; Zhang, Y.; Ruan, X.-K.; Li, G.; Wang, Y. Investigation into gas production from natural gas hydrate: A review. Appl. Energy 2016, 172, 286–322. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Chen, M.; Chen, Z.; Yan, W. Clay mineral distribution in surface sediments of the South China Sea and its significance for in sediment sources and transport. Chin. J. Oceanol. Limnol. 2010, 28, 407–415. [Google Scholar] [CrossRef]
- Liu, Z.; Colin, C.; Li, X.; Zhao, Y.; Tuo, S.; Chen, Z.; Siringan, F.P.; Liu, J.T.; Huang, C.-Y.; You, C.-F.; et al. Clay mineral distribution in surface sediments of the northeastern South China Sea and surrounding fluvial drainage basins: Source and transport. Mar. Geol. 2010, 277, 48–60. [Google Scholar] [CrossRef]
- Brindley, G.W. Bentonites—Geology, Mineralogy, Properties and Uses; Elsevier: Amsterdam, The Netherlands, 1979; Volume 15, pp. 78–79. [Google Scholar]
- Guggenheim, S.; van Groos, A.F.K. New gas-hydrate phase: Synthesis and stability of clay-methane hydrate intercalate. Geology 2003, 31, 653–656. [Google Scholar] [CrossRef]
- Titiloye, J.O.; Skipper, N.T. Molecular dynamics simulation of methane in sodium montmorillonite clay hydrates at elevated pressures and temperatures. Mol. Phys. 2001, 99, 899–906. [Google Scholar] [CrossRef]
- Yan, K.; Li, X.; Xu, C.; Lv, Q.; Ruan, X. Molecular dynamics simulation of the intercalation behaviors of methane hydrate in montmorillonite. J. Mol. Model. 2014, 20, 2311. [Google Scholar] [CrossRef]
- Yan, K.; Li, X.; Chen, Z.; Zhang, Y.; Xu, C.; Xia, Z. Methane hydrate formation and dissociation behaviors in montmorillonite. Chin. J. Oceanol. Limnol. 2019, 27, 1212–1218. [Google Scholar] [CrossRef]
- DeJong, P.H.K.; Wilson, J.E.; Neilson, G.W.; Buckingham, A.D. Hydrophobic hydration of methane. Mol. Phys. 1997, 91, 99–103. [Google Scholar] [CrossRef]
- Radhika, I.; Burla, S.K.; Kandadai, S.; Ch, E.; Prasad, P.S.R.; Babu, D.S. Influence Of Sediment Structural Properties And Their Dynamics In The Formation And Dissociation Of Methane Hydrates. Mater. Today Proc. 2018, 5, 17572–17578. [Google Scholar] [CrossRef]
- Martos-Villa, R.; Guggenheim, S.; Pilar Mata, M.; Ignacio Sainz-Diaz, C.; Nieto, F. Interaction of methane hydrate complexes with smectites: Experimental results compared to molecular models. Am. Miner. 2014, 99, 401–414. [Google Scholar] [CrossRef]
- Wu, Z.; Li, Y.; Sun, X.; Li, M.; Jia, R. Experimental study on the gas phase permeability of montmorillonite sediments in the presence of hydrates. Mar. Pet. Geol. 2018, 91, 373–380. [Google Scholar] [CrossRef]
- Zhang, Q.; Wu, Q.; Zhang, H.; Zhang, B.-y.; Xia, T. Effect of montmorillonite on hydrate-based methane separation from mine gas. J. Cent. South Univ. 2018, 25, 38–50. [Google Scholar] [CrossRef]
- Uchida, T.; Takeya, S.; Chuvilin, E.M.; Ohmura, R.; Nagao, J.; Yakushev, V.S.; Istomin, V.A.; Minagawa, H.; Ebinuma, T.; Narita, H. Decomposition of methane hydrates in sand, sandstone, clays, and glass beads. J. Geophys. Res. Solid Earth 2004, 109. [Google Scholar] [CrossRef] [Green Version]
- Yan, K.; Li, X.; Chen, Z.; Xu, C.; Zhang, Y.; Xia, Z. The Formation of CH4 hydrate in the slit nanopore between the smectite basal surfaces by molecular dynamics simulation. Energy Fuels 2018, 32, 6467–6474. [Google Scholar] [CrossRef]
- Nguyen, N.N.; Nguyen, A.V. The dual effect of sodium halides on the formation of methane gas hydrate. Fuel 2015, 156, 87–95. [Google Scholar] [CrossRef]
- Park, S.H.; Sposito, G. Do montmorillonite surfaces promote methane hydrate formation? Monte Carlo and molecular dynamics simulations. J. Phys. Chem. B 2003, 107, 2281–2290. [Google Scholar] [CrossRef]
- Kotkoskie, A.U.; Al-Ubaidi, B.; Wildeman, T.R.; Sloan, E.D. Inhibition of gas hydrates in water-based drilling muds. SPE Drill. Eng. 1992, 7, 130–136. [Google Scholar] [CrossRef]
- Pusch, R.; Karnland, O. Physico chemical stability of smectite clays. Eng. Geol. 1996, 41, 73–85. [Google Scholar] [CrossRef]
- Cao, C.; Lei, H. Geochemical characteristics of pore water in shallow sediments from north continental slope of South China Sea and their significance for natural gas hydrate occurrence. Procedia Environ. Sci. 2012, 12, 1017–1023. [Google Scholar] [CrossRef] [Green Version]
- Hoareau, G.; Monnin, C.; Odonne, F. The stability of gypsum in marine sediments using the entire ODP/IODP porewater composition database. Mar. Geol. 2011, 279, 87–97. [Google Scholar] [CrossRef]
- Husebo, J.; Ersland, G.; Graue, A.; Kvamme, B. Effects of salinity on hydrate stability and implications for storage of CO(2) in natural gas hydrate reservoirs. Energy Procedia 2009, 1, 3731–3738. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.-S.; Kang, J.W.; Kang, S.-P. Tuning ionic liquids for hydrate inhibition. Chem. Commun. 2011, 47, 6341–6343. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Wu, W.; Liu, Y.; Xie, Y.; Chen, X. The influence of NaCl ions on hydrate structure and thermodynamic equilibrium conditions of gas hydrates. Fluid Phase Equilibria 2012, 325, 6–10. [Google Scholar] [CrossRef]
- Lu, H.; Matsumoto, R. Experimental studies on the possible influences of composition changes of pore water on the stability conditions of methane hydrate in marine sediments. Mar. Chem. 2005, 93, 149–157. [Google Scholar] [CrossRef]
- Milkov, A.V.; Dickens, G.R.; Claypool, G.E.; Lee, Y.J.; Borowski, W.S.; Torres, M.E.; Xu, W.; Tomaru, H.; Tréhu, A.M.; Schultheiss, P. Co-existence of gas hydrate, free gas, and brine within the regional gas hydrate stability zone at Hydrate Ridge (Oregon margin): Evidence from prolonged degassing of a pressurized core. Earth Planet. Sci. Lett. 2004, 222, 829–843. [Google Scholar] [CrossRef]
- Torikai, Y.; Sato, S.; Ohashi, H. Thermodynamic properties of water in compacted sodium montmorillonite. Nucl. Technol. 1996, 115, 73–80. [Google Scholar] [CrossRef]
- Yan, K.F.; Li, X.S.; Chen, Z.Y.; Xia, Z.M.; Xu, C.G.; Zhang, Z.Q. Molecular Dynamics simulation of the crystal nucleation and growth behavior of methane hydrate in the presence of the surface and nanopores of porous sediment. Langmuir 2016, 32, 7975–7984. [Google Scholar] [CrossRef]
- Greene, R.S.B.; Posner, A.M.; Quirk, J.P. Factors affecting formation of quasi-crystals of montmorillonite. Soil Sci. Soc. Am. J. 1973, 37, 457–460. [Google Scholar] [CrossRef]
- Karaborni, S.; Smit, B.; Heidug, W.; Urai, J.; vanOort, E. The swelling of clays: Molecular simulations of the hydration of montmorillonite. Science 1996, 271, 1102–1104. [Google Scholar] [CrossRef] [Green Version]
- Benrhaiem, H.; Tessier, D.; Pons, C.H. Swelling behavior and structural and textural evolution of montmorillonites during a dessication humectation cycle 1 calcium montmorillonites. Clay Miner. 1986, 21, 9–29. [Google Scholar] [CrossRef]
- Morodome, S.; Kawamura, K. Swelling behavior of Na- and Ca-montmorillonite up to 150 degrees c by In Situ X-ray diffraction experiments. Clays Clay Miner. 2009, 57, 150–160. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Wang, J. Cation exchange, interlayer spacing, and thermal analysis of Na/Ca-montmorillonite modified with alkaline and alkaline earth metal ions. J. Therm. Anal. Calorim. 2012, 110, 1199–1206. [Google Scholar] [CrossRef]
- Turner, D.J.; Miller, K.T.; Sloan, E.D. Direct conversion of water droplets to methane hydrate in crude oil. Chem. Eng. Sci. 2009, 64, 5066–5072. [Google Scholar] [CrossRef]
- Turner, D.J.; Miller, K.T.; Sloan, E.D. Methane hydrate formation and an inward growing shell model in water-in-oil dispersions. Chem. Eng. Sci. 2009, 64, 3996–4004. [Google Scholar] [CrossRef]
- Xue, H.; Lu, S.; Fu, X. Forecasting model of solubility of CH4, CO2 and N2 in crude oil. Oil Gas Geol. 2005, 26, 444–449. [Google Scholar]
- Zhong, D.L.; Yang, C.; Liu, D.P.; Wu, Z.M. Experimental investigation of methane hydrate formation on suspended water droplets. J. Cryst. Growth 2011, 327, 237–244. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.-S.; Chen, Z.-Y.; Li, G.; Wang, Y. Effects of particle and pore sizes on the formation behaviors of methane hydrate in porous silica gels. J. Nat. Gas Sci. Eng. 2016, 35, 1463–1471. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, H.-J.; Li, X.-S.; Chen, Z.-Y.; Li, G.; Zeng, Z.-Y. Dissociation Behavior of Methane Hydrate in Porous Media. Chem. J. Chin. Univ. Chin. 2010, 31, 1848–1854. [Google Scholar]
- Harris, J.D.; Rusch, A.W. Identifying hydrated salts using simultaneous thermogravimetric analysis and differential scanning calorimetry. J. Chem. Educ. 2013, 90, 235–238. [Google Scholar] [CrossRef]
- Semenov, M.E.; Manakov, A.Y.; Shitz, E.Y.; Stoporev, A.S.; Altunina, L.K.; Strelets, L.A.; Misyura, S.Y.; Nakoryakov, V.E. DSC and thermal imaging studies of methane hydrate formation and dissociation in water emulsions in crude oils. J. Therm. Anal. Calorim. 2015, 119, 757–767. [Google Scholar] [CrossRef]
- Maekawa, T. Equilibrium conditions for gas hydrates of methane and ethane mixtures in pure water and sodium chloride solution. Geochem. J. 2001, 35, 59–66. [Google Scholar] [CrossRef]
- Mignon, P.; Ugliengo, P.; Sodupe, M.; Hernandez, E.R. Ab initio molecular dynamics study of the hydration of Li+, Na+ and K+ in a montmorillonite model. Influence of isomorphic substitution. Phys. Chem. Chem. Phys. 2010, 12, 688–697. [Google Scholar] [CrossRef] [PubMed]









| Number | Sample | Weight-Loss Ratio (%) | Inflection Point (°C) |
|---|---|---|---|
| 1 | pure water | 5.85 | 90.94 |
| 2 | 0.1 mol/L NaCl | 5.01 | 89.23 |
| 3 | 0.2 mol/L NaCl | 4.28 | 86.75 |
| 4 | 0.3 mol/L NaCl | 3.92 | 78.07 |
| 5 | 0.4 mol/L NaCl | 2.07 | 76.27 |
| 6 | 0.5 mol/L NaCl | 1.41 | 65.73 |
| Number | Solution Concentration | Water Content (%) |
|---|---|---|
| 1 | pure water | 200 |
| 2 | pure water | 47 |
| 3 | 0.5 mol/L | 200 |
| 4 | 0.5 mol/L | 47 |
| 5 | dry montmorillonite | ≈ 0 |
| Number | a (Å) | b (Å) | c (Å) |
|---|---|---|---|
| 1 | 5.205 | 9.028 | 18.547 |
| 2 | 5.233 | 8.988 | 15.573 |
| 3 | 5.269 | 8.986 | 18.677 |
| 4 | 5.235 | 8.982 | 15.492 |
| 5 | 5.250 | 8.998 | 9.820 |
| Number | Peak d(001) 2θ (°) | Basal Spacing (Å) | Number of Water Molecules |
|---|---|---|---|
| 1 | - | - | - |
| 2 | 5.89 | 14.98 | 2 |
| 3 | - | - | - |
| 4 | 6.13 | 14.41 | 2 |
| 5 | 9.12 | 9.64 | 0 |
| Number | Solution Concentration | Reaction Heat (j/g) | Onset Point (°C) | Freezing Point (°C) | Conversion Rate (%) |
|---|---|---|---|---|---|
| 1 | pure water | 216.083 | 12.718 | −2.318 | 65.4 |
| 2 | 0.1 mol/L NaCl | 159.007 | 12.065 | −3.501 | 63.6 |
| 3 | 0.3 mol/L NaCl | 153.252 | 9.778 | −5.708 | 60.1 |
| 4 | 0.5 mol/L NaCl | 135.534 | 10.774 | −5.439 | 58.3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tao, Y.; Yan, K.; Li, X.; Chen, Z.; Yu, Y.; Xu, C. Effects of Salinity on Formation Behavior of Methane Hydrate in Montmorillonite. Energies 2020, 13, 231. https://doi.org/10.3390/en13010231
Tao Y, Yan K, Li X, Chen Z, Yu Y, Xu C. Effects of Salinity on Formation Behavior of Methane Hydrate in Montmorillonite. Energies. 2020; 13(1):231. https://doi.org/10.3390/en13010231
Chicago/Turabian StyleTao, Yuanqing, Kefeng Yan, Xiaosen Li, Zhaoyang Chen, Yisong Yu, and Chungang Xu. 2020. "Effects of Salinity on Formation Behavior of Methane Hydrate in Montmorillonite" Energies 13, no. 1: 231. https://doi.org/10.3390/en13010231