Using Chou’s 5-Step Rule to Evaluate the Stability of Tautomers: Susceptibility of 2-[(Phenylimino)-methyl]-cyclohexane-1,3-diones to Tautomerization Based on the Calculated Gibbs Free Energies
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Raczyńska, E.D.; Kosińska, W.; Ośmiałowski, B.; Gawinecki, R. Tautomeric equilibria in relation to Pi-electron delocalization. Chem. Rev. 2005, 105, 3561–3612. [Google Scholar] [CrossRef]
- Dobosz, R.; Gawinecki, R. Effect of benzoannulation on tautomeric preferences of 4,6-di(pyridin-2-yl)cyclohexane-1,3-dione. J. Mol. Model. 2013, 19, 3397–3402. [Google Scholar] [CrossRef] [Green Version]
- Ośmiałowski, B.; Dobosz, R. The influence of secondary interactions on complex stability and double proton transfer reaction in 2-[1H]-pyridone/2-hydroxypyridine dimers. J. Mol. Model. 2011, 17, 2491–2500. [Google Scholar] [CrossRef] [PubMed]
- Schnell, J.R.; Chou, J.J. Structure and mechanism of the M2 proton channel of influenza A virus. Nature 2008, 451, 591–595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berardi, M.J.; Shih, W.M.; Harrison, S.C.; Chou, J.J. Mitochondrial uncoupling protein 2 structure determined by NMR molecular fragment searching. Nature 2011, 476, 109–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- OuYang, B.; Xie, S.; Berardi, M.J.; Zhao, X.M.; Dev, J.; Yu, W.; Sun, B.; Chou, J.J. Unusual architecture of the p7 channel from hepatitis C virus. Nature 2013, 498, 521–525. [Google Scholar] [CrossRef] [PubMed]
- Oxenoid, K.; Dong, Y.; Cao, C.; Cui, T.; Sancak, Y.; Markhard, A.L.; Grabarek, Z.; Kong, L.; Liu, Z.; Ouyang, B.; et al. Architecture of the Mitochondrial Calcium Uniporter. Nature 2016, 533, 269–273. [Google Scholar] [CrossRef] [Green Version]
- Dev, J.; Park, D.; Fu, Q.; Chen, J.; Ha, H.J.; Ghantous, F.; Herrmann, T.; Chang, W.; Liu, Z.; Frey, G.; et al. Structural Basis for Membrane Anchoring of HIV-1 Envelope Spike. Science 2016, 353, 172–175. [Google Scholar] [CrossRef] [Green Version]
- Chou, K.C. Review: Structural bioinformatics and its impact to biomedical science. Curr. Med. Chem. 2004, 11, 2105–2134. [Google Scholar] [CrossRef]
- Chou, K.C. Impacts of bioinformatics to medicinal chemistry. Med. Chem. 2015, 11, 218–234. [Google Scholar] [CrossRef]
- Kolehmainen, E.; Ośmiałowski, B.; Nissinen, M.; Kauppinen, R.; Gawinecki, R. Substituent and temperature controlled tautomerism of 2-phenacylpyridine: Hydrogen bond as a configurational lock of (Z) 2-(2-hydroxy-2-phenylvinyl)-pyridine. J. Chem. Soc. Perkin Trans. 2000, 2, 2185–2191. [Google Scholar] [CrossRef]
- Kolehmainen, E.; Ośmiałowski, B.; Krygowski, T.M.; Kauppinen, R.; Nissinen, M.; Gawinecki, R. Substituent and temperature controlled tautomerism: Multinuclear magnetic resonance, X-ray, and theoretical studies on 2-phenacylquinolines. J. Chem. Soc. Perkin Trans. 2000, 2, 1259–1266. [Google Scholar] [CrossRef]
- Gawinecki, R.; Kolehmainen, E.; Loghmani-Khouzani, H.; Ośmiałowski, B.; Lovász, T.; Rosa, P. Effect of π-electron delocalization on tautomeric equilibria. Benzoannulated 2-phenacylpyridines. Eur. J. Org. Chem. 2006, 2817–2824. [Google Scholar] [CrossRef]
- Gómez-Sánchez, A.; Paredes-León, R.; Cámpora, J. 1H and 13C NMR spectra and isomerism of 3-aminoacroleins. Magn. Reson. Chem. 1998, 36, 154–162. [Google Scholar] [CrossRef]
- Weinstein, J.; Wyman, M. A Study of β-Amino-α, β-unsaturated Ketones. J. Org. Chem. 1958, 23, 1618–1622. [Google Scholar] [CrossRef]
- Dudek, G.O.; Volpp, G.P. Nuclear Magnetic Resonance Studies of Keto-Enol Equilibria. V. Isomerization in Aliphatic Schiff Bases. J. Am. Chem. Soc. 1963, 85, 2697–2702. [Google Scholar] [CrossRef]
- Dąbrowski, J.; Kamieńska-Trela, K. Infrared spectra and structure of substituted unsaturated carbonyl compounds—III Enamino ketones with tertiary amino group. Spectrochim. Acta 1966, 22, 211–220. [Google Scholar] [CrossRef]
- Dąbrowski, J.; Dąbrowska, U. Infrarotspektren und Struktur substituierter ungesättigter Carbonylverbindungen, VII. Enaminoketone mit starrer s-cis-und s-trans-Konformation und sekundärer Aminogruppe. Chem. Ber. 1968, 101, 2365–2374. [Google Scholar] [CrossRef]
- Kania, L.; Kamieńska-Trela, K.; Wilanowski, M. Structural increments in UV spectra of conjugated carbonyl compounds: Part, I. The α-alkyl substituted enaminones. J. Mol. Struct. 1983, 102, 1–17. [Google Scholar] [CrossRef]
- Brown, N.M.D.; Nonhebel, D.C. NMR spectra of intramolecularly hydrogen-bonded compounds—II: Schiff bases of β-diketones and o-hydroxycarbonyl compounds. Tetrahedron 1968, 24, 5655–5664. [Google Scholar] [CrossRef]
- Dąbrowski, J.; Kamieńska-Trela, K. Electronic spectra of .alpha. beta-unsaturated carbonyl compounds. I. An evaluation of increments characteristic of changes in configuration (cis/trans) and conformation (s-cis/s-trans) based on direct observation of the isomerization of enamino aldehydes and ketones. J. Am. Chem. Soc. 1976, 98, 2826–2834. [Google Scholar] [CrossRef]
- Czerwińska, E.; Kozerski, L.; Boksa, J. Structural studies by 1H and 13C d.n.m.r.: I-barrier to trans-cis isomerization in aliphatic enamino ketones of the type R-CO-CH=CH-NHR1. Org. Magn. Reson. 1976, 8, 345–349. [Google Scholar] [CrossRef]
- Kozerski, L.; Von Philipsborn, W. 15N chemical shifts as a conformational probe in enaminones A variable temperature study at natural isotope abundance. Org. Magn. Reson. 1981, 17, 306–310. [Google Scholar] [CrossRef]
- Kashima, Ch.; Yamamoto, M.; Sugiyama, N. Ultraviolet spectral study of β-amino-enones. J. Chem. Soc. C 1970, 111–114. [Google Scholar] [CrossRef]
- Kashima, C.; Aoyama, H.; Yamamoto, Y.; Nishio, T. Nuclear magnetic resonance spectral study of β-aminoenones. J. Chem. Soc. Perkin Trans. 1975, 2, 665–670. [Google Scholar] [CrossRef]
- Zhou, J.-C. NMR of enaminones. Part 8—1H, 13C and 17O NMR spectra of primary and secondary 1,2-disubstituted enaminones: Configuration, conformation and intramolecular hydrogen bonding. Magn. Reson. Chem. 1998, 36, 565–572. [Google Scholar] [CrossRef]
- Fustero, S.; De la Torre, M.G.; Jofré, V.; Carlón, R.P.; Navarro, A.; Fuentes, A.S. Synthesis and reactivity of new β-enamino acid derivatives: a simple and general approach to β-enamino esters and thioesters. J. Org. Chem. 1998, 63, 8825–8836. [Google Scholar] [CrossRef]
- Edwards, W.G.H.; Petrov, V. Some heterocyclic structures derived from acenaphthene. J. Chem. Soc. 1954, 2853–2860. [Google Scholar] [CrossRef]
- Dąbrowski, J. Infra-red spectra and structure of substituted unsaturated carbonyl compounds—I: Enamino Ketones with primary amino group. Spectrochim. Acta 1963, 19, 475–496. [Google Scholar] [CrossRef]
- Ogawa, K.; Harada, J. Aggregation controlled proton tautomerization in salicylideneanilines. J. Mol. Struct. 2003, 647, 211–216. [Google Scholar] [CrossRef]
- Buemi, G.; Zuccarello, F.; Venuvanalingam, P.; Ramalingam, M. Ab initio study of tautomerism and hydrogen bonding of β-carbonylamine in the gas phase and in water solution. Theor. Chem. Acc. 2000, 104, 226–234. [Google Scholar] [CrossRef]
- Rybarczyk-Pirek, A.; Grabowski, S.J.; Małecka, M.; Nawrot-Modranka, J. Crystal and molecular structures of new chromone derivatives as empirical evidence of intramolecular proton transfer reaction; ab initio studies on intramolecular H-bonds in enaminones. J. Phys. Chem. A 2003, 106, 11956–11962. [Google Scholar] [CrossRef]
- Zabatyuk, R.I.; Volovenko, Y.M.; Shishkin, O.V.; Gorb, L.; Leszczyński, J. Aromaticity-controlled tautomerism and resonance-assisted hydrogen bonding in heterocyclic enaminone−iminoenol systems. J. Org. Chem. 2007, 72, 725–735. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.D.; Schmidt, G.M.J. Photochromy and thermochromy of anils. J. Phys. Chem. 1962, 66, 2442–2446. [Google Scholar] [CrossRef]
- Alarcón, S.H.; Olivieri, A.C.; Labadie, G.R.; Cravero, R.M.; Gonzáles-Sierra, M. Tautomerism of representative aromatic α-hydroxy carbaldehyde anils as studied by spectroscopic methods and AM1 calculations. Synthesis of 10-hydroxyphenanthrene-9-carbaldehyde. Tetrahedron 1995, 51, 4619–4626. [Google Scholar] [CrossRef]
- Vargas, V.; Amigo, L. A study of the tautomers of N-salicylidene-p-X-aniline compounds in methanol. J. Chem. Soc. Perkin Trans. 2001, 2, 1124–1129. [Google Scholar] [CrossRef]
- Dziembowska, T.; Jagodzińska, E.; Rozwadowski, Z.; Kotfica, M. Solvent effect on intramolecular proton transfer equilibrium in some N-(R-salicylidene)-alkylamines. J. Mol. Struct. 2001, 598, 229–234. [Google Scholar] [CrossRef]
- Fernández, G.J.M.; del Rio-Portilla, F.; Quiroz-García, N.; Toscano, R.A.; Salcedo, R. The structures of some ortho-hydroxy Schiff base ligands. J. Mol. Struct. 2001, 561, 197–207. [Google Scholar] [CrossRef]
- Sitkowski, J.; Stefaniak, L.; Dziembowska, T.; Grech, E.; Jagodzińska, E.; Webb, G.A. A multinuclear NMR study of proton transfer processes in Schiff bases. J. Mol. Struct. 1996, 381, 177–180. [Google Scholar] [CrossRef]
- Salman, S.R.; Lindon, J.C.; Farrant, R.D.; Carpenter, T.A. Tautomerism in 2-hydroxy-1-naphthaldehyde Schiff bases in solution and the solid state investigated using 13C NMR spectroscopy. Magn. Res. Chem. 1993, 31, 991–994. [Google Scholar] [CrossRef]
- Galić, N.; Cimerman, Z.; Tomiśić, V. Tautomeric and protonation equilibria of Schiff bases of salicylaldehyde with aminopyridines. Anal. Chim. Acta 1997, 343, 135–143. [Google Scholar] [CrossRef]
- Nazir, H.; Yildiz, M.; Yilmaz, H.; Tahir, M.N.; Ülkü, D.J. Intramolecular hydrogen bonding and tautomerism in Schiff bases. Structure of N-(2-pyridil)-2-oxo-1-naphthylidenemethylamine. J. Mol. Struct. 2000, 524, 241–250. [Google Scholar] [CrossRef]
- Becker, R.S.; Richey, W.F. Photochromic anils. Mechanisms and products of photoreactions and thermal reactions. J. Am. Chem. Soc. 1967, 89, 1298–1302. [Google Scholar] [CrossRef]
- Hansen, P.E.; Sitkowski, J.; Stefaniak, L.; Rozwadowski, Z.; Dziembowska, T. One-bond deuterium isotope effects on 15N chemical shifts in Schiff bases. Ber. Bunsenges. Phys. Chem. 1998, 102, 410–413. [Google Scholar] [CrossRef]
- Dziembowska, T. Resonance assisted intramolecular hydrogen bond in Schiff bases. Pol. J. Chem. 1998, 72, 193–202. [Google Scholar]
- Król-Starzomska, I.; Rospenk, M.; Rozwadowski, Z.; Dziembowska, T. UV-visible absorption spectroscopic studies of intramolecular proton transfer in N-(R-salicylidene)-alkylamines. Pol. J. Chem. 2000, 74, 1441–1446. [Google Scholar]
- Zhuo, J.-C. NMR of enaminones. part 6—17O and 13C NMR study of tautomerization in Schiff bases. Magn. Reson. Chem. 1999, 37, 259–268. [Google Scholar] [CrossRef]
- Antonov, L.; Fabian, W.M.F.; Nedeltcheva, D.; Kamounah, F.S. Tautomerism of 2-hydroxynaphthaldehyde Schiff bases. J. Chem. Soc. Perkin Trans. 2000, 2, 1173–1179. [Google Scholar] [CrossRef]
- Dudek, G.O.; Dudek, E.P. Spectroscopic studies of keto-enol equilibria. IX. N15-Substituted anilides. J. Am. Chem. Soc. 1966, 88, 2407–2412. [Google Scholar] [CrossRef]
- Joshi, H.; Kamounah, F.S.; van der Zwan, G.; Gooijer, C.; Antonov, L. Temperature dependent absorption spectroscopy of some tautomeric azo dyes and Schiff bases. J. Chem. Soc. Perkin Trans. 2001, 2, 2303–2308. [Google Scholar] [CrossRef]
- Gawinecki, R.; Kuczek, A.; Kolehmainen, E.; Ośmiałowski, B.; Krygowski, T.M.; Kauppinen, R. Influence of the bond fixation in benzo-annulated N-salicylideneanilines and their orto-COX derivatives (X = CH3, NH2, OCH3) on tautomeric equilibria in solution. J. Org. Chem. 2007, 72, 5598–5607. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, K.; Kasahara, Y.; Ohtani, Y.; Harada, J. Crystal structure change for the thermochromy of N-salicylideneanilines. The first observation by X-ray diffraction. J. Am. Chem. Soc. 1998, 120, 7107–7408. [Google Scholar] [CrossRef]
- Dobosz, R.; Kolehmainen, E.; Valkonen, A.; Ośmiałowski, B.; Gawinecki, R. Tautomeric preferences of phthalones and related compounds. Tetrahedron 2007, 63, 9172–9178. [Google Scholar] [CrossRef]
- Rogers, N.A.J.; Smith, H. 2-Acylcyclohexane-1,3-diones. Part II. 2-Formyl-, 2-propionyl-, 2-isobutyryl-, and 2-phenylcarbamoyl-cyclohexane1,3-dione, and their conversion into phenathridines. J. Chem. Soc. 1955, 341–346. [Google Scholar] [CrossRef]
- Kettmann, V.; Lokaj, J.; Milata, V.; Marko, M.; Štvrtecká, M. 2-(Phenylaminomethylidene)-cyclohexane-1,3-dione. Acta Cryst. C 2004, 60, o252–o254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zacharias, G.; Wolfbeis, O.S.; Junek, H. Über Anilinomethylenverbindungen der Cyclohexandione. Monatshefte für Chemie 1974, 105, 1283–1291. [Google Scholar] [CrossRef]
- Donovan, P.M.; Scott, L.T. Elaboration of Diaryl Ketones into Naphthalenes Fused on Two or Four Sides: A Naphthoannulation Procedure. J. Am. Chem. Soc. 2004, 126, 3108–3112. [Google Scholar] [CrossRef]
- Zhang, M.; An, H.-Y.; Zhao, B.-G.; Xu, J.-H. Aromatic annulation strategy for naphthalenes fused at 1,2-and 3,4-positions with two heterocycles. Org. Biomol. Chem. 2006, 4, 33–35. [Google Scholar] [CrossRef]
- Panda, K.; Venkatesh, Ch.; Ila, H.; Junjappa, H. Efficient Routes to Acenaphthylene-Fused Polycyclic Arenes/Heteroarenes and Heterocyclic Fluoranthene Analogues. Eur. J. Org. Chem. 2005, 2045–2055. [Google Scholar] [CrossRef]
- Chou, K.C. Some remarks on protein attribute prediction and pseudo amino acid composition. J. Theor. Biol. 2011, 273, 236–247. [Google Scholar] [CrossRef]
- Chou, K.C. Progresses in predicting post-translational modification. Int. J. Pept. Res. Ther. 2019, in press. [Google Scholar] [CrossRef]
- Chou, K.C. Advance in predicting subcellular localization of multi-label proteins and its implication for developing multi-target drugs. Curr. Med. Chem. 2019, 26, 4918–4943. [Google Scholar] [CrossRef] [PubMed]
- Chou, K.C. Impacts of pseudo amino acid components and 5-steps rule to proteomics and proteome analysis. Curr. Top. Med. Chem. 2019, 19, 2283–2300. [Google Scholar] [CrossRef] [PubMed]
- Chou, K.C. An insightful recollection for predicting protein subcellular locations in multi-label systems. Genomics 2019, in press. [Google Scholar] [CrossRef]
- Chou, K.C. Proposing Pseudo Amino Acid Components is an Important Milestone for Proteome and Genome Analyses. Int. J. Pept. Res. Ther. 2019, in press. [Google Scholar] [CrossRef]
- Chou, C.K. Recent Progresses in Predicting Protein Subcellular Localization with Artificial Intelligence (AI) Tools Developed Via the 5-Steps Rule. Jacobs J. Gastroenterol. Hepatol. 2019, 2, 1–21. [Google Scholar]
- Chou, K.C. An insightful recollection since the distorted key theory was born about 23 years ago. Genomics 2019, in press. [Google Scholar] [CrossRef]
- Chou, K.C. Artificial intelligence (AI) tools constructed via the 5-steps rule for predicting post-translational modifications. Trends Artif. Inttell. 2019, 3, 60–74. [Google Scholar] [CrossRef]
- Chou, K.C. Distorted Key Theory and Its Implication for Drug Development. Curr. Proteom. [CrossRef]
- Chou, K.C. An insightful recollection since the birth of Gordon Life Science Institute about 17 years ago. Adv. Sci. Eng. Res. 2019, 4, 31–36. [Google Scholar] [CrossRef]
- Chou, K.C. Gordon Life Science Institute: Its philosophy, achievements, and perspective. Ann. Cancer Ther. Pharmacol. 2019, 2, 1–26. [Google Scholar]
- Eistert, B.; Eifler, W.; Goth, H. Versuche in der Reihe des 3-Hydroxy-1-oxo-phenalens und des 1.2.3-Trioxo-2.3-dihydro-phenalens. Chem. Ber. 1968, 101, 2162–2175. [Google Scholar] [CrossRef]
- Wolfbeis, O.S.; Ziegler, E. Zur Reaktivität von C=N-Doppelbindungssystemen, X Synthesen von kondensierten Heterocyclen. Zeitschrift für Naturforschung B 1976, 31, 1519–1525. [Google Scholar] [CrossRef]
- Wolfbeis, O.S.; Ziegler, E.; Knierzinger, A.; Wipfler, H.; Trummer, I. Eine breit anwendbare Synthese fluoreszierender kondensierter α-Pyrone. Monatshefte für Chemie 1980, 111, 93–112. [Google Scholar] [CrossRef]
- Facchetti, A.; Streitwieser, A. Ion pair first and second acidities of some β-diketones and aggregation of their lithium and cesium enediolates in THF. J. Org. Chem. 2004, 69, 8345–8355. [Google Scholar] [CrossRef] [PubMed]
- Van Tinh, D.; Fischer, M.; Stadlbauer, W. Ring closure reactions of cyclic 2-arylaminomethylene-1,3-diones. J. Heterocycl. Chem. 1996, 33, 905–910. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 Revision, B.01; Gaussian, Inc.: Wallingford, UK, 2016. [Google Scholar]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar] [CrossRef] [Green Version]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef]
- Weigend, F. Accurate Coulomb-fitting basis sets for H to Rn. Phys. Chem. Chem. Phys. 2006, 8, 1057–1065. [Google Scholar] [CrossRef]
- Barone, V.; Cossi, M. Quantum calculation of molecular energies and energy gradients in solution by a conductor solvent model. J. Phys. Chem. A 1998, 102, 1995–2001. [Google Scholar] [CrossRef]
- Cossi, M.; Rega, N.; Scalmani, G.; Barone, V. Energies, structures, and electronic properties of molecules in solution with the C-PCM solvation model. J. Comp. Chem. 2003, 24, 669–681. [Google Scholar] [CrossRef] [PubMed]
- Woliński, K.; Hilton, J.F.; Pulay, P. Efficient Implementation of the Gauge-Independent Atomic Orbital Method for NMR Chemical Shift Calculations. J. Am. Chem. Soc. 1990, 112, 8251–8260. [Google Scholar] [CrossRef]
- Dobosz, R. Susceptibility of 2-[(Phenylimino)-methyl]-cyclohexane-1,3-diones to tautomerization. RepOD 2019. [Google Scholar] [CrossRef]
- Lloris, M.E.; Abramovitch, R.A.; Marquet, J.; Moreno-Mañas, M. Reactions of copper(II) β-diketonates under free radical conditions. II. Diazonium salts as aryl radicals source in the arylation of β-diketones. Tetrahedron 1992, 48, 6909–6916. [Google Scholar] [CrossRef]
- Olah, G.A.; Grant, J.L.; Westerman, P.W. Stable carbocations. CLXXVIII. Carbon-13 nuclear magnetic resonance spectroscopic study of protonated and diprotonated acyclic and cyclic diketones in fluorosulfuric acid-antimony pentafluoride-sulfur dioxide solution. J. Org. Chem. 1975, 40, 2102–2108. [Google Scholar] [CrossRef]
- Gawinecki, R.; Kolehmainen, E.; Dobosz, R.; Ośmiałowski, B. (1Z,3Z)-3-[Quinolin-2(1H)-ylidene]-1-(quinolin-2-yl) prop-1-en-2-ol: An unexpected most stable tautomer of 1,3-bis(quinolin-2-yl) acetone. J. Mol. Struct. 2009, 930, 78–82. [Google Scholar] [CrossRef]
- Dobosz, R.; Gawinecki, R.; Ośmiałowski, B. DFT studies on tautomeric preferences of 1-(pyridin-2-yl)-4-(quinolin-2-yl) butane-2,3-dione in the gas phase and in solution. Struct. Chem. 2010, 21, 1283–1287. [Google Scholar] [CrossRef] [Green Version]
- Borowski, P.; Gawinecki, R.; Miłaczewska, A.; Skotnicka, A.; Woliński, K.; Brzyska, A. Instability of 2,2-di(pyridin-2-yl) acetic acid. Tautomerization versus decarboxylation. J. Mol. Model. 2011, 17, 857–868. [Google Scholar] [CrossRef] [Green Version]
- Kurt, M.; Chinna Babu, P.; Sundaraganesan, N.; Cinar, M.; Karabacak, M. Molecular structure, vibrational, UV and NBO analysis of 4-chloro-7-nitrobenzofurazan by DFT calculations. Spectrochim. Acta Part A 2011, 79, 1162–1170. [Google Scholar] [CrossRef]
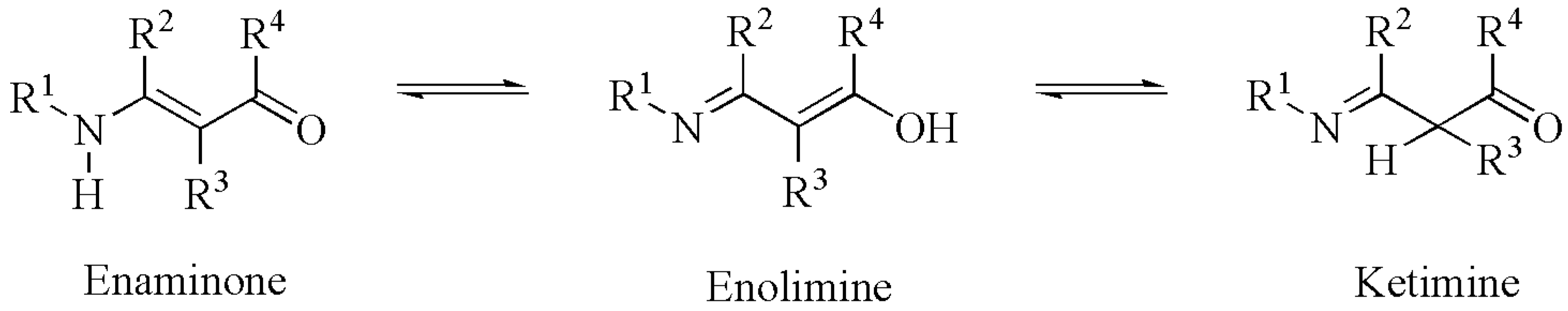
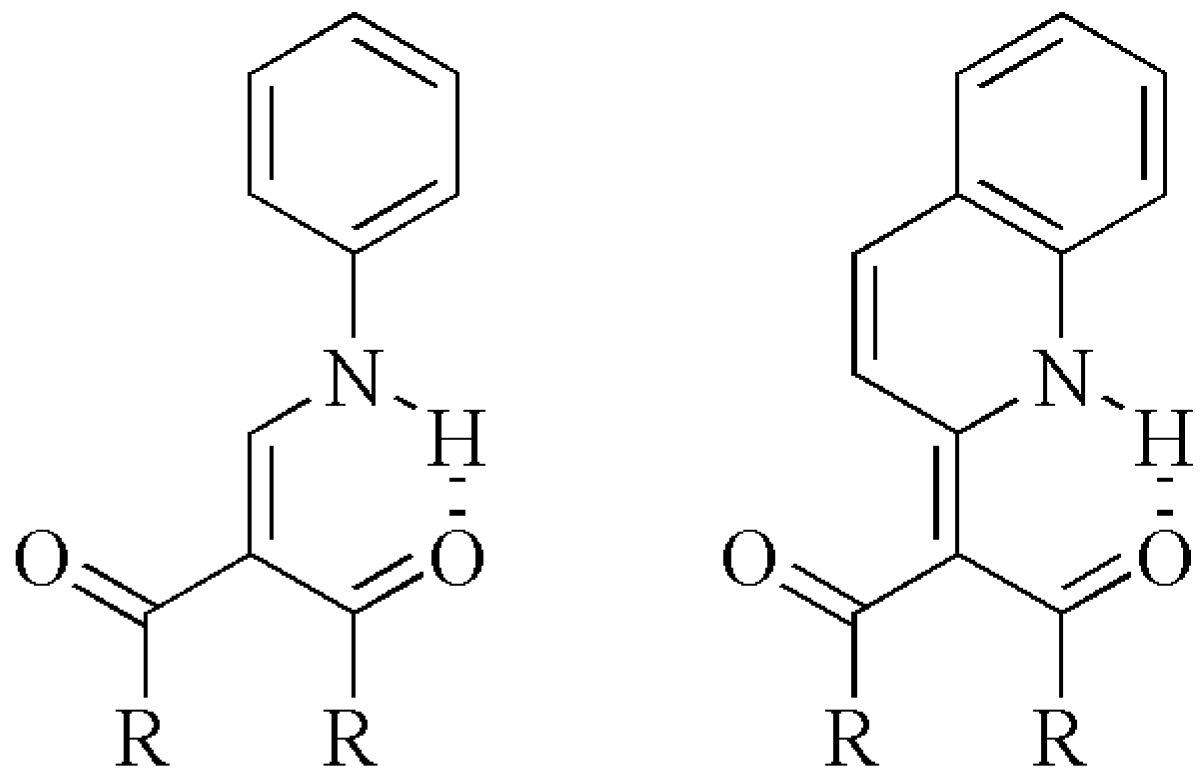
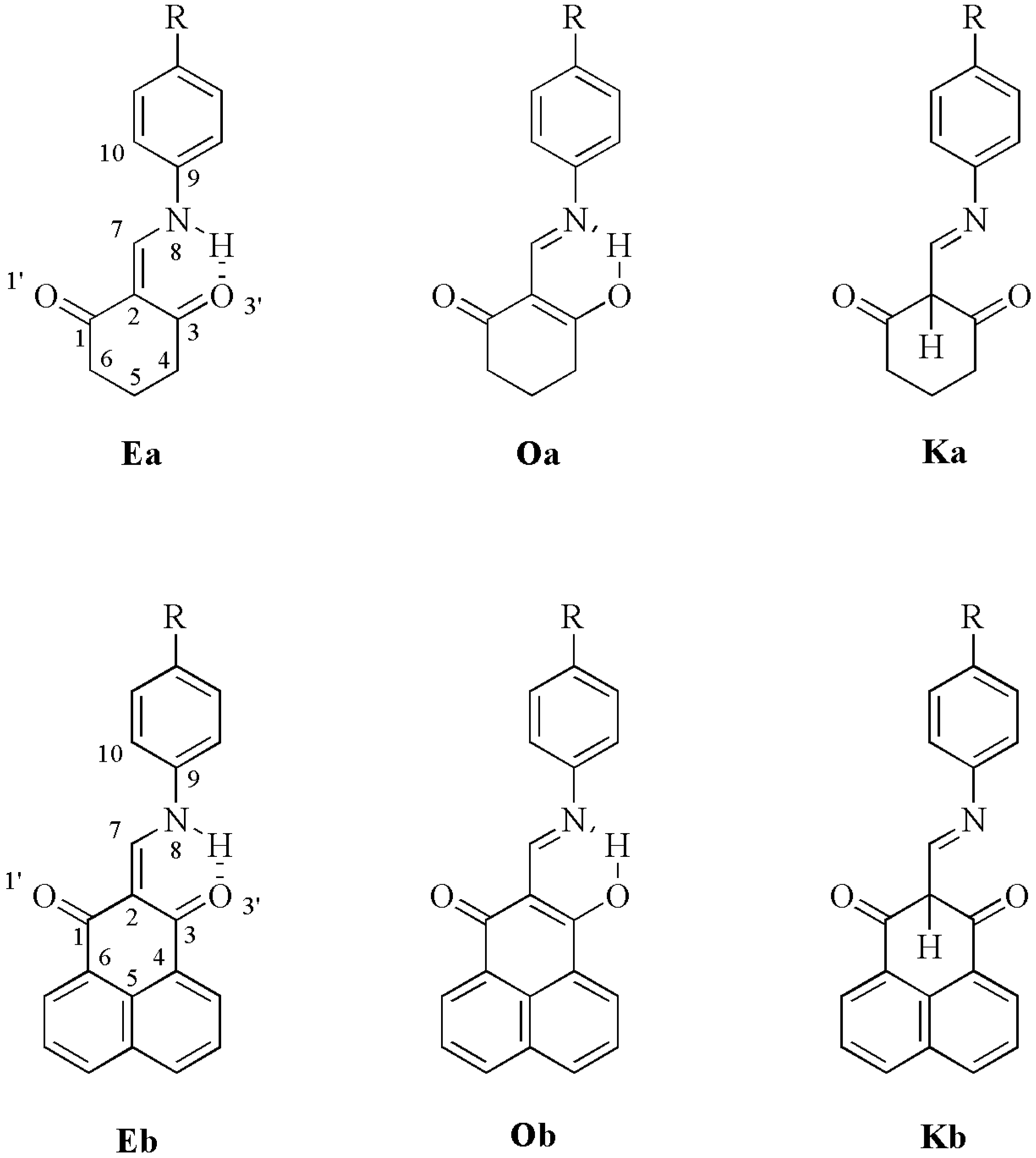

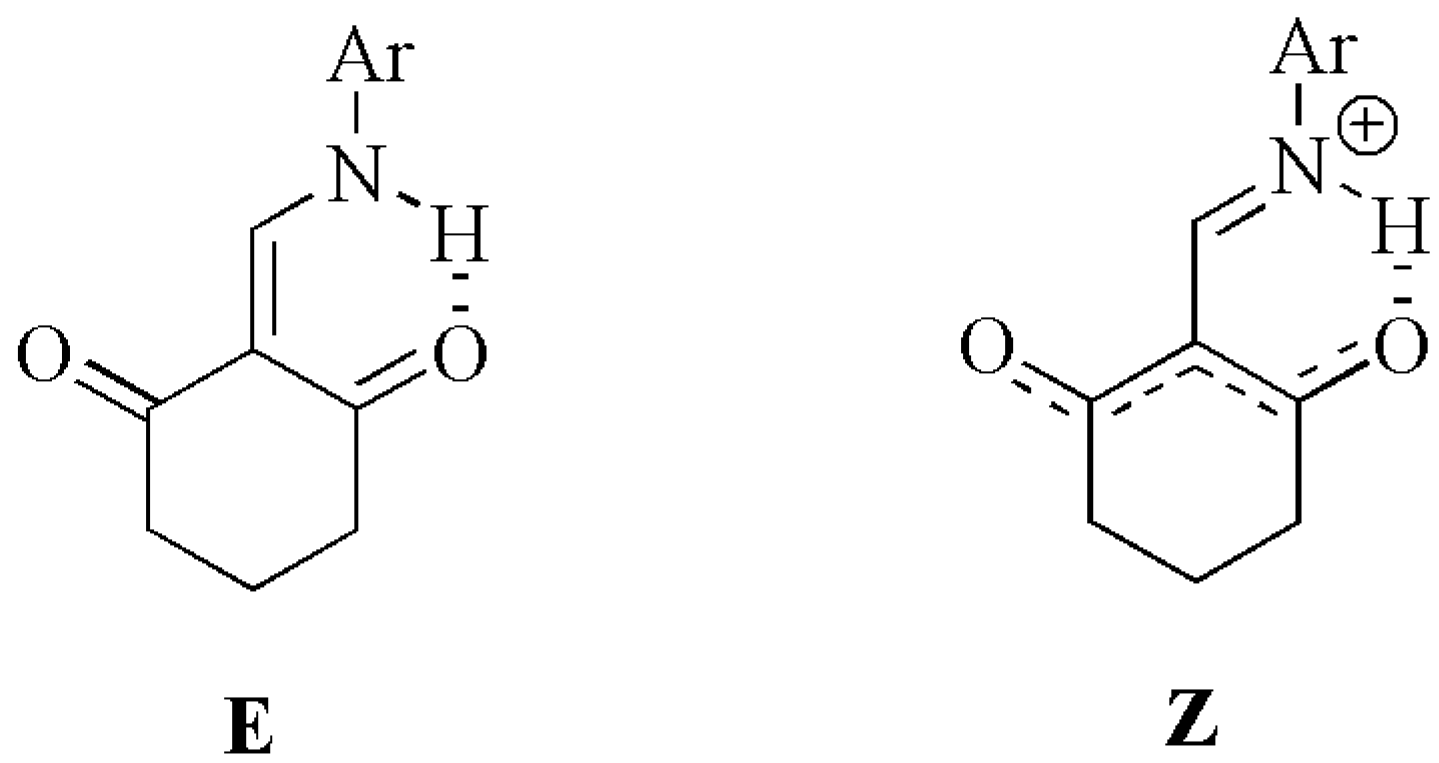
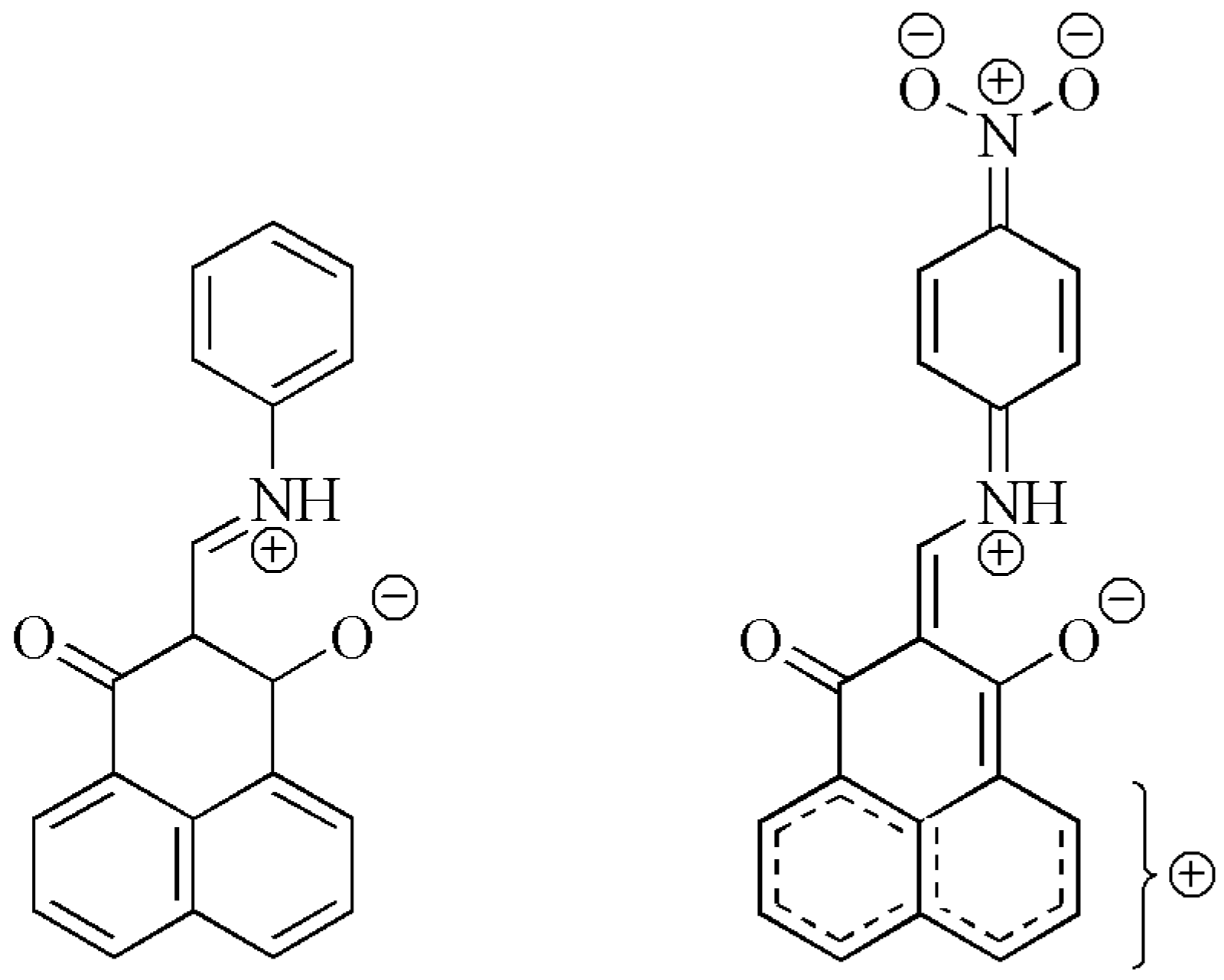
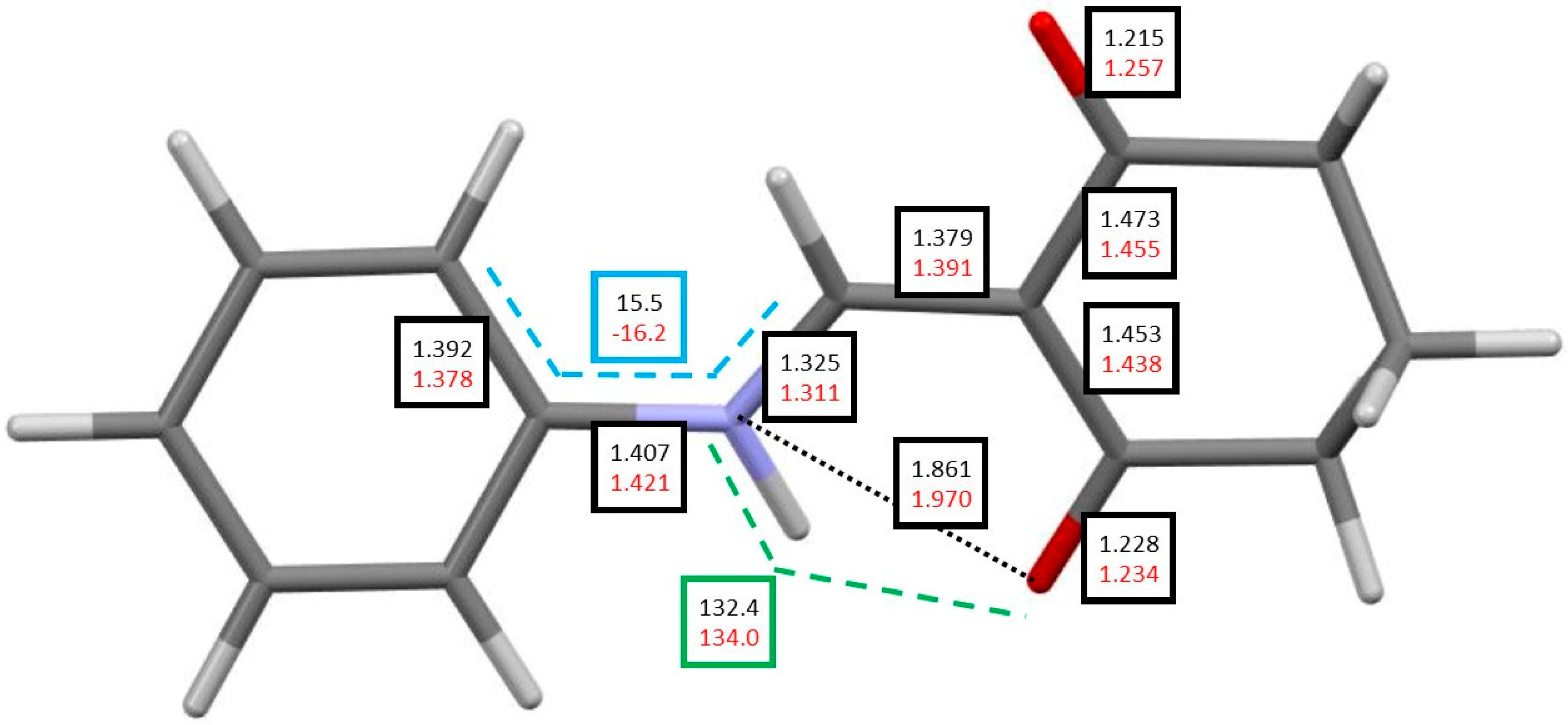
| H8 | H7 | C2 | C7 | C1 | C3 | ||
|---|---|---|---|---|---|---|---|
| 1Ea | exp. | 12.97d | 8.55d | 109.61 | 151.07 | 191.81 | 196.62 |
| calc. | 12.35 | 8.61 | 113.98 | 151.98 | 195.71 | 202.93 | |
| 1Eb | exp. | 13.54d | 8.90d | 108.86 | 152.74 | 181.44 | 183.9 |
| calc. | 12.98 | 9.10 | 113.44 | 153.69 | 183.23 | 188.12 | |
| 2Ea | exp. | 12.93d | 8.61d | 109.73 | 150.88 | 196.64 | 200.25 |
| calc. | 12.30 | 8.72 | 114.12 | 151.69 | 195.88 | 203.20 | |
| 2Eb | exp. | 13.50d | 8.96d | 109.03 | 152.78 | no | no |
| calc. | 12.94 | 9.20 | 113.53 | 153.25 | 183.39 | 188.33 | |
| 3Ea | exp. | 12.76d | 8.58d | 109.78 | 150.64 | no | no |
| calc. | 12.32 | 8.76 | 114.29 | 151.46 | 196.03 | 203.42 | |
| 3Eb | exp. | 13.49d | 9.00d | 109.24 | 153.01 | no | no |
| calc. | 12.96 | 9.25 | 113.74 | 153.19 | 183.47 | 188.50 | |
| 4Ea | exp. | 12.60d | 8.47d | 109.96 | 150.46 | 195.56 | 199.67 |
| calc. | 12.30 | 8.65 | 114.45 | 151.21 | 196.03 | 203.78 | |
| 4Eb | exp. | 13.42d | 8.96d | no | 153.18 | no | no |
| calc. | 12.95 | 9.13 | 113.88 | 152.81 | 183.45 | 188.73 | |
| 5Ea | exp. | 12.98d | 8.47d | 111.17 | 149.59 | 195.82 | 200.26 |
| calc. | 12.46 | 8.80 | 115.34 | 149.93 | 196.43 | 204.79 | |
| 5Eb | exp. | 10.80bs | 8.55bs | no | 150.11 | no | no |
| calc. | 13.12 | 9.28 | 114.83 | 151.53 | 183.70 | 189.42 |
| Form | Grel | Form | Grel | ||
|---|---|---|---|---|---|
| Vacuum | DMSO | Vacuum | DMSO | ||
| 1Ea | 0.0 | 0.0 | 1Eb | 0.0 | 0.0 |
| 1Oa | 5.5 | 6.6 | 1Ob | 4.9 | 6.0 |
| 1Ka | 20.0 | 18.2 | 1Kb | 22.3 | 20.9 |
| 2Ea | 0.0 | 0.0 | 2Eb | 0.0 | 0.0 |
| 2Oa | 5.9 | 7.0 | 2Ob | 5.2 | 7.4 |
| 2Ka | 20.5 | 18.7 | 2Kb | 21.6 | 22.3 |
| 3Ea | 0.0 | 0.0 | 3Eb | 0.0 | 0.0 |
| 3Oa | 5.4 | 7.0 | 3Ob | 5.1 | 6.1 |
| 3Ka | 19.7 | 18.7 | 3Kb | 22.2 | 20.8 |
| 4Ea | 0.0 | 0.0 | 4Eb | 0.0 | 0.0 |
| 4Oa | 5.3 | 6.4 | 4Ob | 4.6 | 5.6 |
| 4Ka | 19.4 | 18.1 | 4Kb | 21.4 | 20.1 |
| 5Ea | 0.0 | 0.0 | 5Eb | 0.0 | 0.0 |
| 5Oa | 5.6 | 6.4 | 5Ob | 5.2 | 5.7 |
| 5Ka | 19.2 | 17.8 | 5Kb | 21.4 | 20.1 |
| 1Ea | 3Ea | 5Ea | 1Eb | 3Eb | 5Eb | |
|---|---|---|---|---|---|---|
| N8–C7 | 1.322 1.317 | 1.325 1.320 | 1.333 1.329 | 1.321 1.315 | 1.324 1.318 | 1.332 1.327 |
| N8–C9 | 1.409 1.413 | 1.407 1.411 | 1.396 1.397 | 1.409 1.413 | 1.406 1.411 | 1.495 1.398 |
| C7–C2 | 1.382 1.388 | 1.379 1.385 | 1.372 1.377 | 1.382 1.390 | 1.379 1.387 | 1.372 1.379 |
| C2–C3 | 1.451 1.449 | 1.453 1.451 | 1.458 1.457 | 1.447 1.444 | 1.449 1.446 | 1.454 1.452 |
| C3–O3′ | 1.229 1.234 | 1.228 1.232 | 1.226 1.230 | 1.233 1.237 | 1.232 1.236 | 1.231 1.233 |
| C2–C1 | 1.470 1.464 | 1.473 1.466 | 1.478 1.472 | 1.465 1.458 | 1.467 1.461 | 1.472 1.466 |
| C1–O1′ | 1.215 1.223 | 1.215 1.222 | 1.213 1.220 | 1.219 1.226 | 1.219 1.226 | 1.217 1.223 |
| N8–H8 | 1.021 1.020 | 1.021 1.020 | 1.021 1.020 | 1.022 1.020 | 1.021 1.020 | 1.022 1.021 |
| H8···O3′ | 1.857 1.884 | 1.861 1.887 | 1.849 1.873 | 1.849 1.875 | 1.852 1.875 | 1.839 1.862 |
| N8···O3′ | 2.656 2.669 | 2.659 2.671 | 2.653 2.665 | 2.650 2.662 | 2.652 2.662 | 2.645 2.656 |
| N8H8O3′ | 132.5 133.3 | 132.4 131.1 | 133.0 133.4 | 132.6 131.4 | 132.5 131.4 | 133.2 132.0 |
| C7N8C9C10 | 19.9 22.7 | 15.5 19.9 | −2.3 −6.8 | 20.5 23.5 | 16.2 18.7 | 3.1 9.1 |
| Tautomer | Donor (i) | Type | Acceptor (j) | Type | E(2) [kJ/mol] | |
|---|---|---|---|---|---|---|
| Vacuum | DMSO | |||||
| 1Ea | N8 | LP | C7–C2 | π* | 88.85 | 95.36 |
| C7–C2 | π | C3–O3′ | π* | 39.92 | 42.07 | |
| C7–C2 | π | C1–O1′ | π* | 33.13 | 37.43 | |
| 3Ea | N8 | LP | C7–C2 | π* | 85.37 | 91.48 |
| C7–C2 | π | C3–O3′ | π* | 38.65 | 40.77 | |
| C7–C2 | π | C1–O1′ | π* | 32.30 | 36.43 | |
| 5Ea | N8 | LP | C7–C2 | π* | 76.48 | 80.29 |
| C7–C2 | π | C3–O3′ | π* | 35.82 | 37.30 | |
| C7–C2 | π | C1–O1′ | π* | 29.77 | 33.35 | |
| 1Eb | N8 | LP | C7–C2 | π* | 88.47 | 96.54 |
| C7–C2 | π | C3–O3′ | π* | 41.96 | a | |
| C7–C2 | π | C1–O1′ | π* | 35.16 | 40.08 | |
| C7–C2 | π | C3 | LP* | a | 73.06 | |
| 3Eb | N8 | LP | C7–C2 | π* | 85.00 | 93.05 |
| C7–C2 | π | C3–O3′ | π* | 40.68 | 43.63 | |
| C7–C2 | π | C1–O1′ | π* | 34.31 | 39.03 | |
| 5Eb | N8 | LP | C7–C2 | π* | 75.86 | 81.63 |
| C7–C2 | π | C3–O3′ | π* | 37.67 | 40.11 | |
| C7–C2 | π | C1–O1′ | π* | 31.68 | 35.90 | |
| N8 | O3′ | O1′ | |
|---|---|---|---|
| 1Ea | −0.514 −0.500 | −0.628 −0.660 | −0.463 −0.640 |
| 3Ea | −0.520 −0.508 | −0.623 −0.655 | −0.583 −0.636 |
| 5Ea | −0.528 −0.517 | −0.613 −0.642 | −0.572 −0.623 |
| 1Eb | −0.510 −0.493 | −0.635 −0.660 | −0.591 −0.638 |
| 3Eb | −0.516 −0.501 | −0.630 −0.656 | −0.587 −0.634 |
| 5Eb | −0.525 −0.511 | −0.622 −0.644 | −0.578 −0.623 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dobosz, R.; Mućko, J.; Gawinecki, R. Using Chou’s 5-Step Rule to Evaluate the Stability of Tautomers: Susceptibility of 2-[(Phenylimino)-methyl]-cyclohexane-1,3-diones to Tautomerization Based on the Calculated Gibbs Free Energies. Energies 2020, 13, 183. https://doi.org/10.3390/en13010183
Dobosz R, Mućko J, Gawinecki R. Using Chou’s 5-Step Rule to Evaluate the Stability of Tautomers: Susceptibility of 2-[(Phenylimino)-methyl]-cyclohexane-1,3-diones to Tautomerization Based on the Calculated Gibbs Free Energies. Energies. 2020; 13(1):183. https://doi.org/10.3390/en13010183
Chicago/Turabian StyleDobosz, Robert, Jan Mućko, and Ryszard Gawinecki. 2020. "Using Chou’s 5-Step Rule to Evaluate the Stability of Tautomers: Susceptibility of 2-[(Phenylimino)-methyl]-cyclohexane-1,3-diones to Tautomerization Based on the Calculated Gibbs Free Energies" Energies 13, no. 1: 183. https://doi.org/10.3390/en13010183
APA StyleDobosz, R., Mućko, J., & Gawinecki, R. (2020). Using Chou’s 5-Step Rule to Evaluate the Stability of Tautomers: Susceptibility of 2-[(Phenylimino)-methyl]-cyclohexane-1,3-diones to Tautomerization Based on the Calculated Gibbs Free Energies. Energies, 13(1), 183. https://doi.org/10.3390/en13010183






