1. Introduction
Fossil fuels such as furnace oil, coal, and natural gas provide 80% of the world’s increasing energy demand and will continue to provide 60% of the world’s energy needs by 2040 [
1]. Although fossil fuel based power plants are well developed and deliver smooth operations relative to the alternative technologies such as renewables, nuclear, and carbon free systems, they have devastating drawbacks in terms of global warming, ozone depletion, and air quality [
2]. Among the greenhouse gases from fossil fuel power plants, carbon dioxide (CO
2) affects the environment the most. Considering the present situation of the increasing global warming, it is very critical to limit the amount of carbon dioxide to the environment. In order to restrict CO
2 emissions, three technologies have been proposed for carbon capture and storage (CCS), namely pre-combustion, oxy-combustion, and post-combustion [
3]. In pre-combustion, fuel is converted into carbon dioxide and hydrogen mixture (CO
2+H
2) and the carbon dioxide is removed from hydrogen and is sent to compression unit. Hydrogen is combusted in the presence of air for power production [
4,
5]. In post-combustion, flue gases from power plants are passed through selective adsorbents and absorbents to remove carbon dioxide, which is then stored or sequestrated [
6,
7]. The CO
2 emissions can be avoided in pre- and post-combustion technologies; however, NO
x would still produce due to nitrogen presence in the air. In oxy-combustion, fuel is burnt in the presence of oxygen, the products include only water vapors, and carbon dioxide, considering one-step reaction, can then easily be separated by condensing the water vapors. By implementing oxy-fuel combustion technology, removal of 98% carbon dioxide from the exhaust is possible [
8,
9].
Oxy-combustion is an emerging technology, which can be implemented in existing plants with some modifications and new plants [
10,
11]. The combustion between pure oxygen and fuel results in very high temperature that the current equipment cannot withstand. Consequently, recirculation of carrier gas is required in order to reduce the maximum temperature in the equipment, and use of carbon dioxide as the carrier gas is the economic solution [
12,
13]. Though oxy-fuel combustion is a very promising technique for CCS, lower adiabatic flame temperature, delayed ignition, flame stability, changes in reaction kinetics, radiative heat transfer and transport properties, and lower burning rate in O
2/CO
2 environment are some of the major unresolved challenges [
14,
15,
16].
Comprehensive studies have been carried out in order to evaluate potential of oxy-fuel combustion application in the existing systems [
10,
17,
18]. Andersen et al. [
19] studied chemical kinetics of methane combustion under oxy-fuel conditions. They concluded that oxy-fuel combustion characteristics are different than those of air-combustion. In their study, Westbrook and Dryer (WD) two-step, and Jones and Lindstedt (JL) four-step reaction kinetics models under O
2/CO
2 conditions were modified. Their modified mechanisms were validated against experimental data, and they found that CO prediction was improved as compared to the original models. Zhen et al. [
15] performed experiments for methane combustion in N
2 and CO
2 environments, and it was observed that lift-off co-axial velocity was reduced significantly under a CO
2 environment, which indicated weaker flame stability. Zhao et al. [
20] simulated laminar combustion in porous media, and their results showed better flame propagation velocities and stability as compared to an open space chamber. Kirchen et al. [
10] studied ion transport membranes (ITM) for oxygen separation and oxy-fuel combustion. The ITM reactors are also termed as high temperature membrane reactors (HTMR). They concluded that oxygen permeation could be increased by using reactive gas such as methane instead of an inert gas; this would reduce oxygen separation penalties. Habib et al. [
21] used ITM for oxygen separation and porous plates for CO
2 induction, in a combined ITM-porous plate reactor. It was analyzed that uniform temperature profiles could be achieved by the use of porous membranes that can enhance oxygen permeation and reactor performance. Habib et al. [
22] studied oxy-fuel combustion characteristics in a porous plate reactor using methane as the fuel. They varied different parameters like oxidizer ratio (OR) and equivalence ratio (
Φ) to study the impact on flame length, flow field, reaction rates, methane depletion, oxygen permeation, and maximum outlet temperature. The advantages of porous plate reactor are as follows:
Porous plate permeation is uniform, which helps in controlling oxidizer permeation and local oxy-fuel ratio;
Controlled permeation rates can avoid hotspots inside the reactor, which would allow less expensive material for reactor;
Permeation rate can be controlled through porosity, material, and geometrical characteristics, which would help in designing for various industrial applications;
Currently, permeation rates of HTMR are insufficient and porous plates can mimic HTMR conditions with higher permeation rates for future development [
16].
However, for retrofitting purposes comparison with an N2 environment in which radiative heat transfer effects, hydrodynamics, reactor geometry and transport properties are accounted for is insufficient. In the present work, performance analysis for methane combustion in a porous plate reactor under O2/CO2 and O2/N2 environments has been made. For an O2/N2 environment, nitrogen is used as a carrier gas with the same fraction as that of air and is termed as air-combustion. The air-combustion characteristics inside the reactor have been evaluated. For the O2/CO2 environment, CO2 is used as carrier gas, and for the same adiabatic flame temperature as that of air-combustion, the oxidizer ratio has been evaluated by zero dimension analysis. For oxy-fuel combustion, the effects of oxidizer ratios, mass flow rates, and reactor height have been analyzed and compared with air-combustion (base case). In the end, parameters were highlighted that need to be modified to get the same performance as that O2/N2 conditions.
3. Results and Discussion
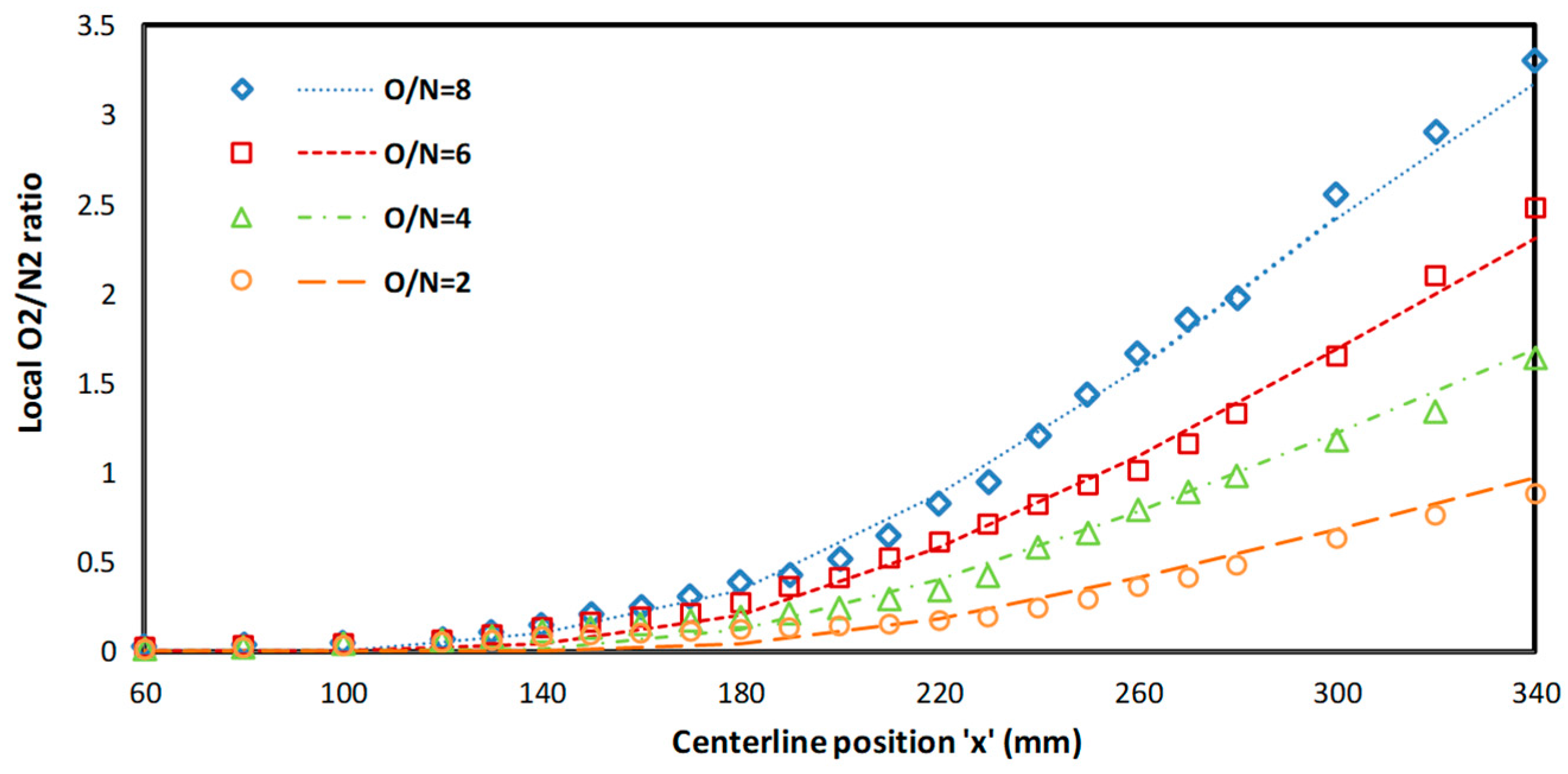
The CFD model for the porous plate reactor was validated in two steps, i.e., with non-reactive measurements from the porous plate reactor [
22] and with reactive measurements from the gas turbine oxy-fuel combustor [
25]. The local O/N ratio from the experimental data was compared with the CFD results as shown in
Figure 3 and was found in good agreement. For the reactive case validation, separate geometry was made and operating conditions were set similar to Nemitallah and Habib [
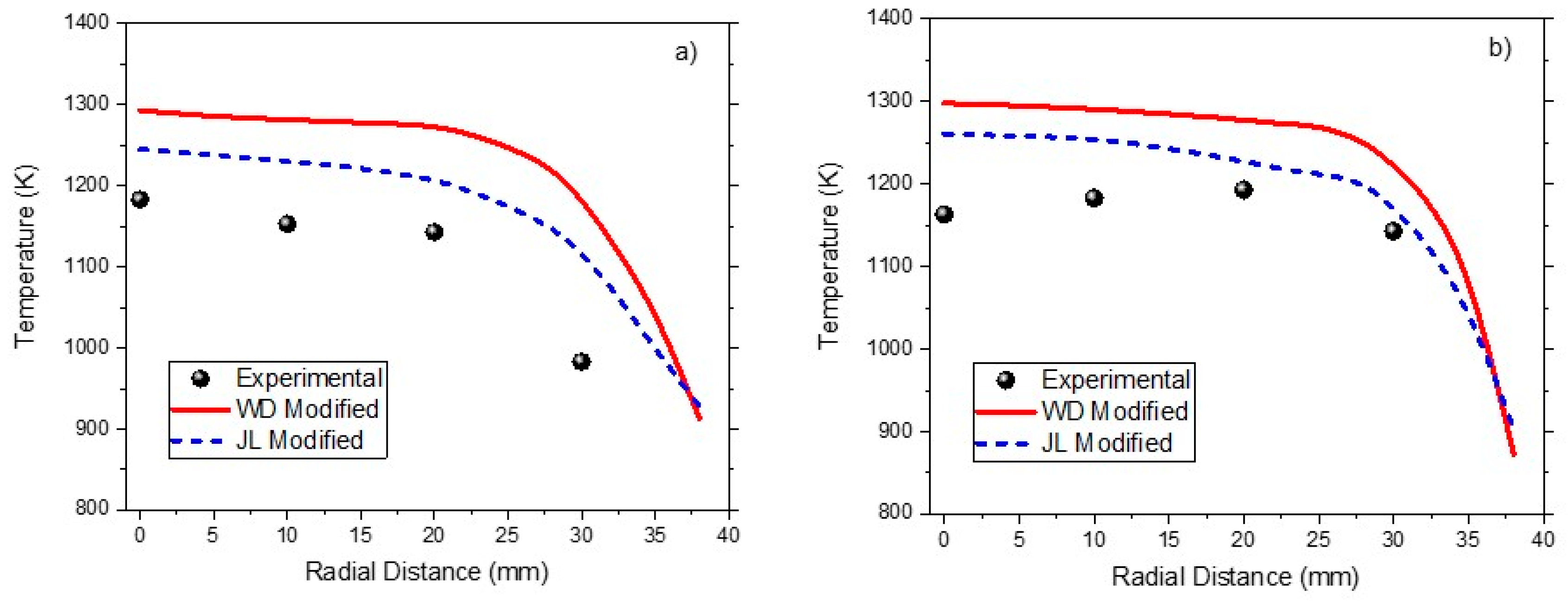
25]. Numerical results with a modified WD two-step and JL four-step reaction kinetics model were compared with oxy-fuel combustor data as shown in
Figure 4a,b. It is evident that the four-step model better predicts the temperature profile as compared to the two-step model. The error with the two-step model is less than 12% except for one point, for which it is 20.3%. The error with the four-step model is less than 9%, except for one point, for which it is 13.5%. The deviations could be due to the accuracy of the kinetics model, turbulence model, and radiation model, 2D approximation, discretization, and uncertainties in experimental data. The deviations with the two-step mechanism are in acceptable range and thus used for numerical experiments to save computational efforts.
3.1. Adiabatic Flame Temperature
The adiabatic flame temperature was found to be 2826 K for the air-combustion case that would be the maximum outlet temperature, which can be achieved in the reactor. For oxy-combustion, the oxidizer ratio OR was varied to achieve same adiabatic flame temperature as that of air-combustion and was found to be 0.243.
3.2. Air-Combustion
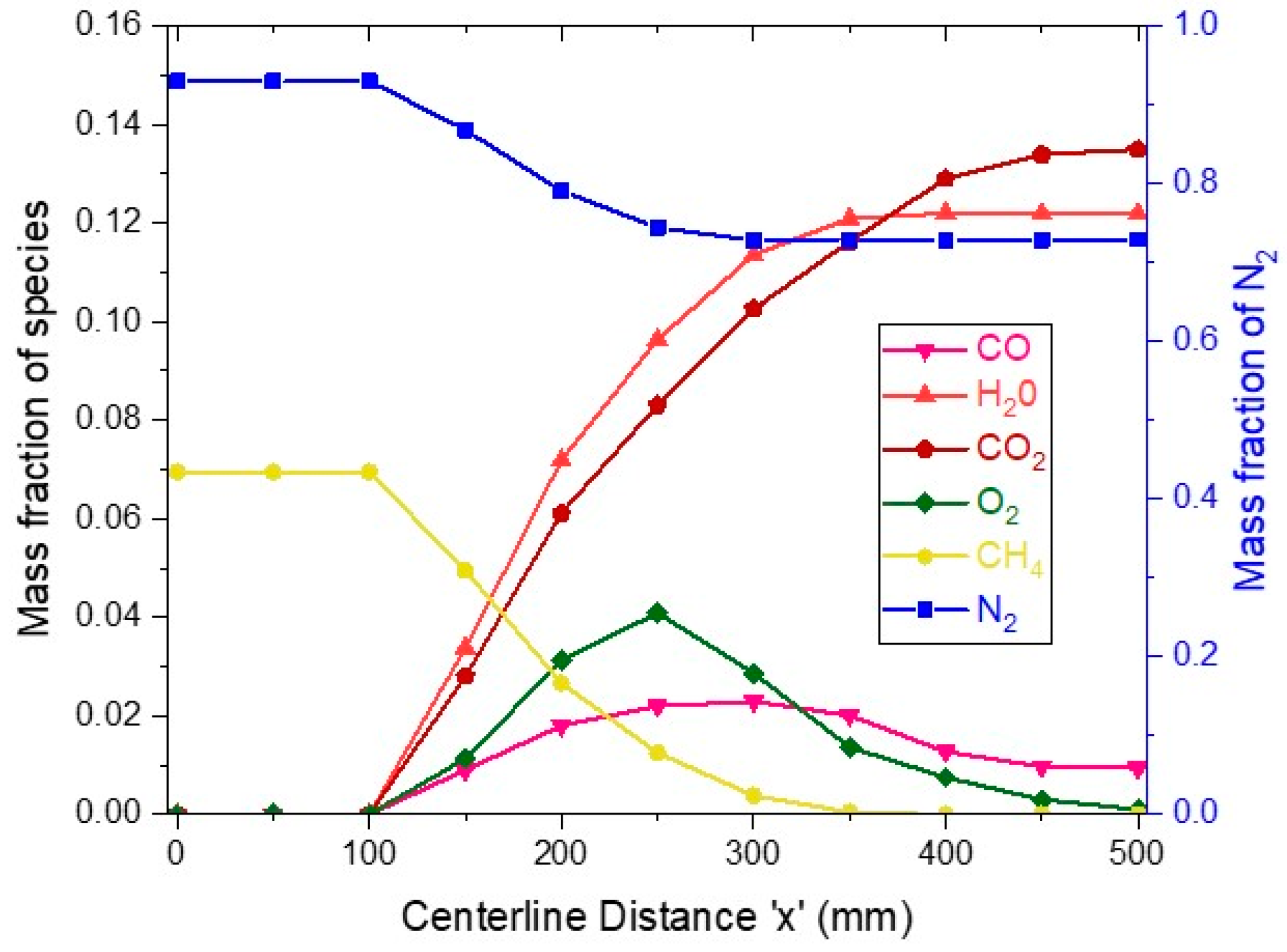
The average mass fraction variation of species in the reaction chamber is presented in
Figure 5. The total reactor length is 500 mm, and porous plates start from
x =125 mm and end at
x = 275 mm. The methane mass fraction at the inlet is around 0.07, but after
x =125 mm, methane starts to deplete as the reaction starts and diminishes at the end of porous plates. Complete methane is converted into carbon dioxide and carbon monoxide, while oxygen concentration begins to rise from zero at the start of the porous plate from where it enters the reaction chamber; the oxygen concentration rises initially and then reduces as the reaction is going on and all the oxygen is consumed. At start, water vapors mass fraction is zero and begins to increase as the reaction takes place and continues to rise until completion of reaction and water vapors mass fraction reaches a constant value. The mass fraction of carbon dioxide starts to rise as the reaction begins; it reaches a maximum value of around 0.02 and then starts to drop as some of the carbon monoxide converts to carbon dioxide. Finally, the nitrogen mass fraction has the constant value at the start but when the porous plate region starts, the oxygen induction from the porous plate into the reaction chamber reduces the overall nitrogen mass fraction.
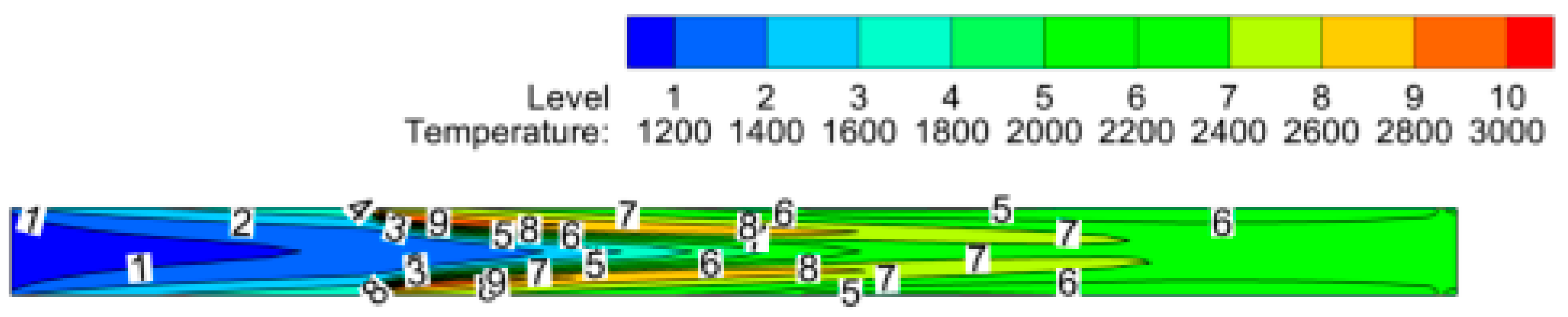
The maximum temperature in the reactor reaches 2900 K, and the outlet temperature at the exit of the reactor is 2235 K. The temperature variation inside the reactor is shown in
Figure 6. The species enters the reactor at 1173 K, and the temperature of the upstream flow rises before the reaction starts, as shown by temperature level 2, i.e., 1400K; this is due to the radiative heat transfer. The region near the top and bottom porous walls has the highest temperature; this is where the combustion begins and the maximum temperature is reached. The centerline temperature is lower than the temperature near the top and bottom walls, so mass weighted average temperature variation is more accurate for study instead of centerline temperature variation.
3.3. Effect of Oxidizer Ratio for Oxy-Combustion
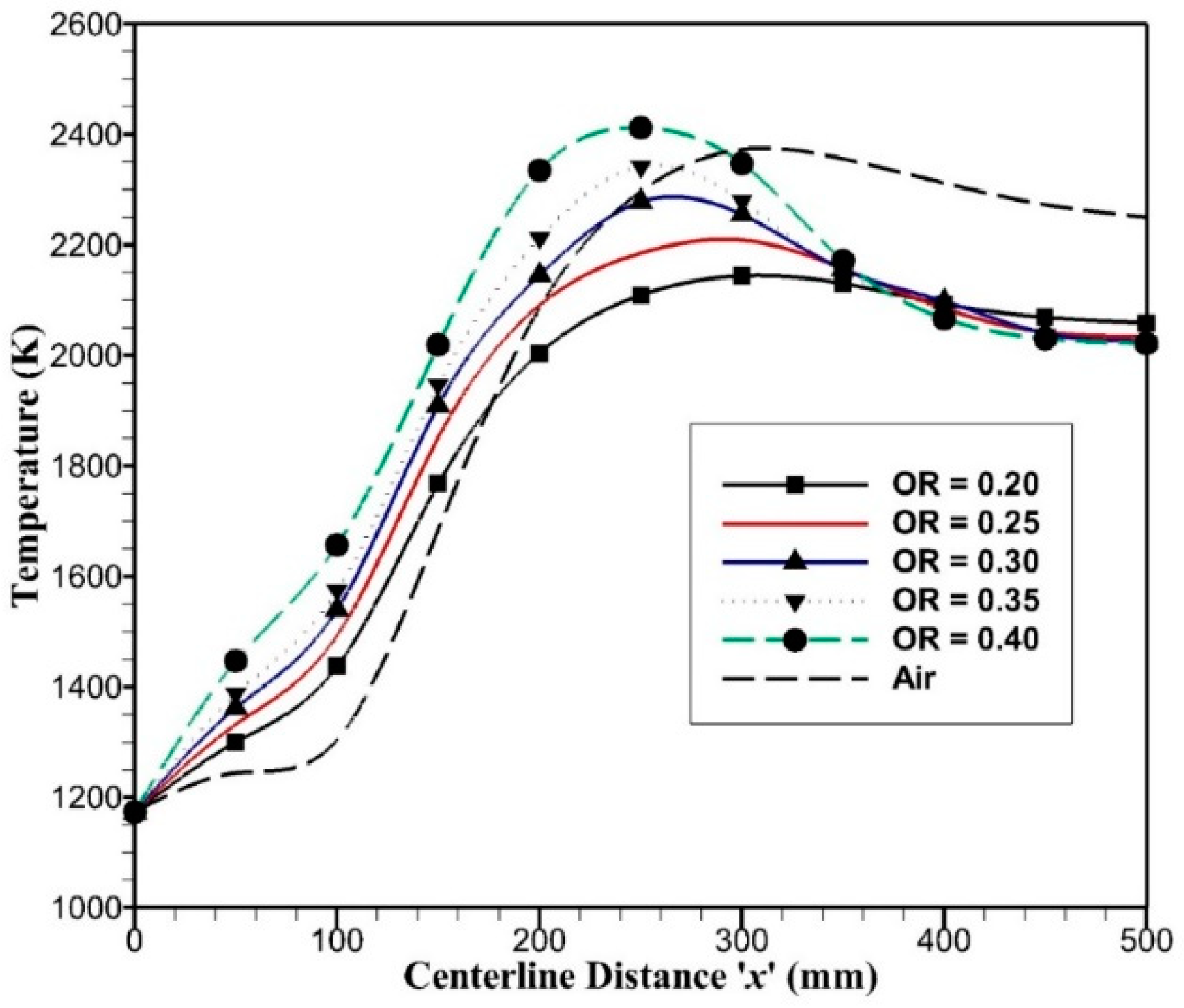
The mass weighted average temperature variation along the ‘x’ axis has been plotted for oxy-combustion with OR ranging from 0.20 to 0.40, and temperature profiles are compared with the air-combustion case, as shown in
Figure 7. For oxy-combustion, temperature rises sharply as the combustion occurs at the start of porous plates, i.e.,
x = 125 mm, where oxygen enters the reactor. The high temperature from the combustion causes upstream temperature to rise due to radiative heat transfer. As the flow temperature reaches the maximum value, it begins to decrease with an outlet temperature in the range of 2000–2050 K due to radiative cooling. The OR = 0.40 has the maximum temperature as compared to OR = 0.20, as it contains less carrier gas, i.e., carbon dioxide. Like in oxy-combustion, in the air-combustion case, temperature rises very slowly; this is because of the different transport properties of CO
2 and N
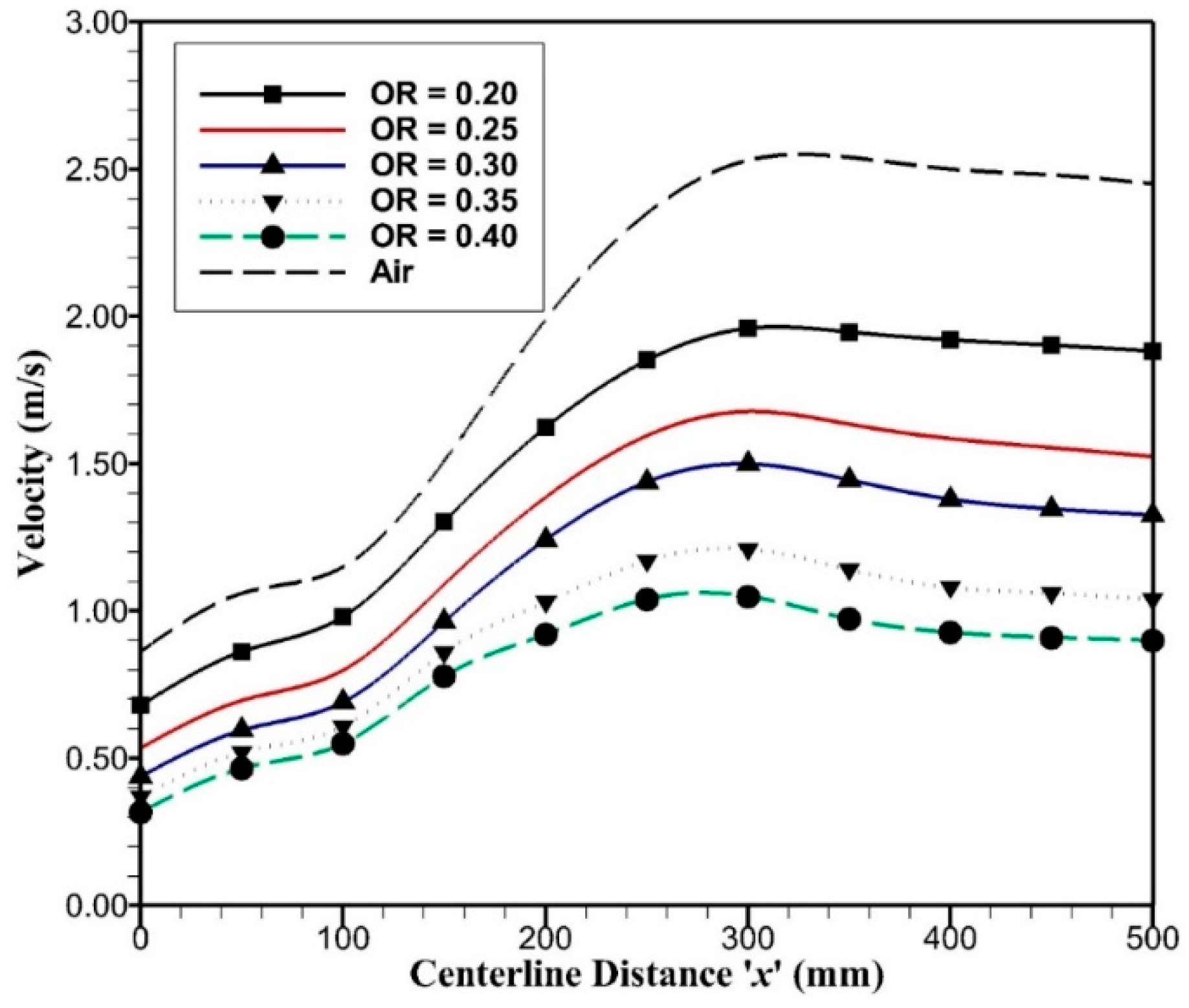
2 for convective and radiative heat transfer. Since OR = 0.243 is comparable in terms of adiabatic temperature with air-combustion and is close to OR = 0.25, the reactor outlet temperatures have a significant difference of around 200 K with air-combustion. This is due to fact that air-combustion and oxy-combustion with OR = 0.25 have comparable mass flow rates, but the density of carbon dioxide is higher than nitrogen by 1.57 times that keeps a low-velocity field in oxy-combustion and high-velocity field in air-combustion as shown in
Figure 8. Inlet velocity for oxy-combustion varies from 0.32 to 0.68 m/s and inlet velocity for air-combustion is around 0.86 m/s. As the oxygen permeates through porous plates, velocity rises due to added mass flow rates and outlet velocity reaches to 2.45 m/s for air-combustion and 0.9 to 1.9 m/s for oxy-combustion. Higher velocity enhances convection heat transfer and reduces radiation effects on upstream flow, which is quite evident in
Figure 7; hence, it raises the outlet temperature in the air-combustion case.
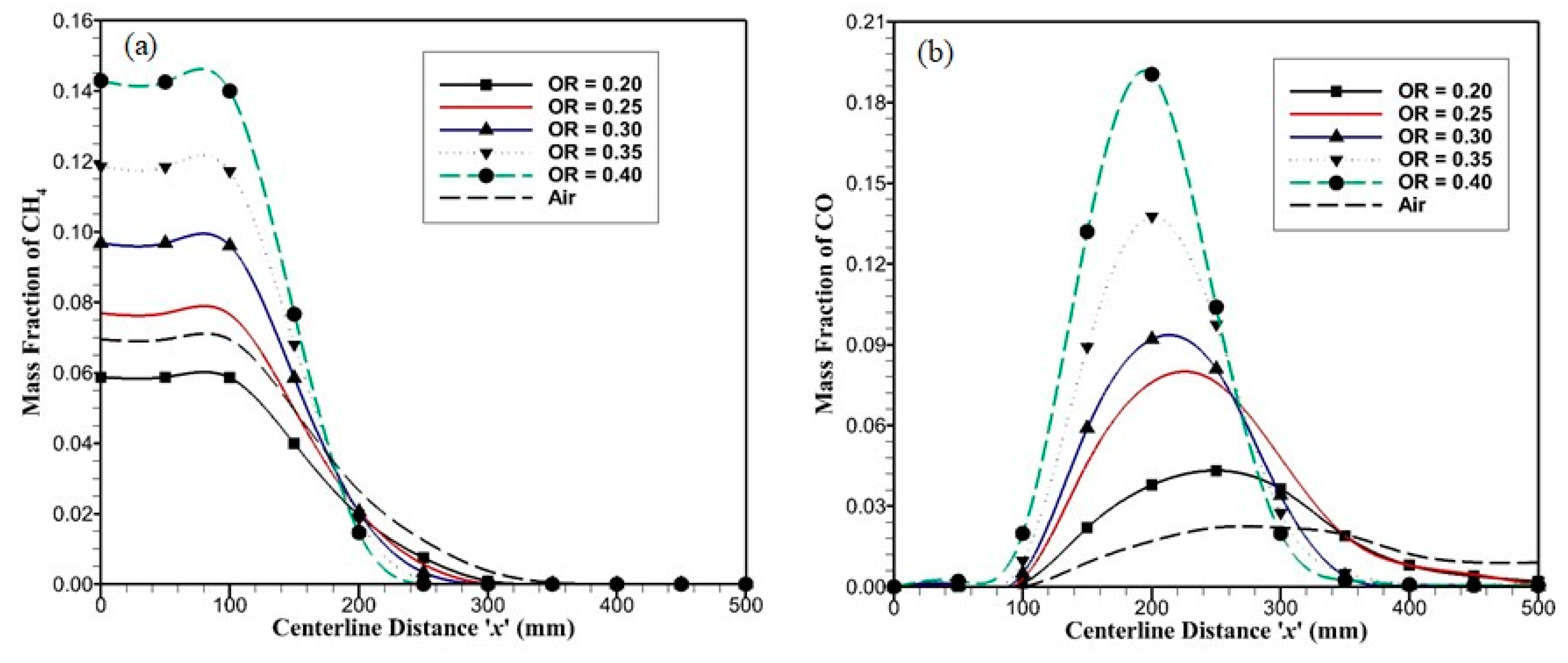
Figure 9a shows species mass fraction variation of methane for OR = 0.20–0.40 along the axial direction. As the OR is varied from 0.20 to 0.40, methane depletion rate increases due to a reduced velocity field in the reaction zone; which causes the residence time and temperature of species at certain point to increase, and combustion occurs faster. Due to this, complete methane depletion occurs at
x = 225 mm for OR = 0.40 and at
x = 325 mm for OR = 0.20. A higher methane depletion rate and less carrier gas result in higher maximum temperature in the reactor. For air-combustion, the methane depletion rate is lower due to the increased velocity field, and the reduced residence time in the reaction zone, methane diminishes completely at
x = 375 mm, i.e., after porous plates
x > 275 mm.
Figure 9b shows species mass fraction variation of carbon monoxide along the axial direction. In air-combustion, carbon monoxide is produced only as the result of methane combustion; while in oxy-combustion, carbon monoxide is produced by methane combustion and from the disintegration of carbon dioxide at higher temperatures, which is present as a carrier gas. The carbon monoxide mass fraction at the outlet for air-combustion is lower. For oxy-combustion, higher oxidizer ratios lead to higher temperature at which carbon dioxide disintegrates into carbon monoxide and oxygen before the combustion starts, i.e.,
x < 125 mm. These factors result in peak shift for maximum carbon monoxide from
x = 250 mm for OR = 0.20 to
x = 200 mm for OR = 0.40. As the carbon monoxide reaches the maximum value, carbon monoxide starts to convert into carbon dioxide. Since for oxy-combustion cases with different ORs, the velocity field is low as compared to air-combustion, all of the carbon monoxide converts into carbo dioxide before the reactor outlet. The CO conversion rate is faster in higher oxidizer ratios. From these results, it is evident that convective effects need to be enhanced by raising the velocity field in the reactor for oxy-combustion cases to meet outlet temperature for the air-combustion case. This can be accomplished by either increasing flow rates or reducing the reactor height h.
3.4. Effect of Mass Flow Rate for Oxy-Combustion
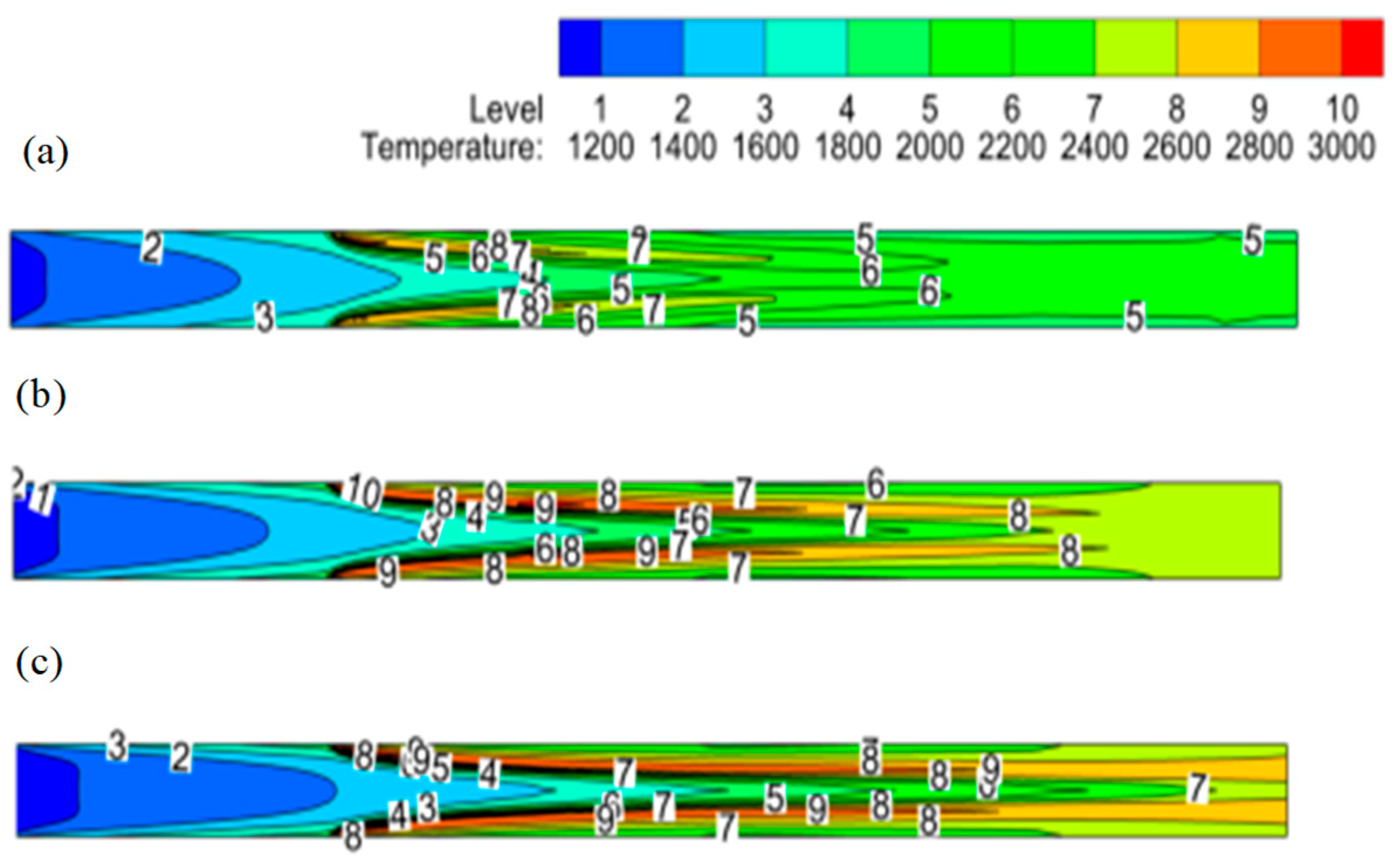
The temperature contours at inlet flow rates (1×, 2×, 3×) are shown in
Figure 10. It is noticeable from these contours that mass flow rate increment causes convective heat transfer to dominate over radiative heat transfer and upstream flow becomes cooler for higher inlet flow rates as shown by blue region. The flame length also increases as the flow rates are doubled and tripled. In addition, with higher flow rates, the flame shifts towards the reactor center due to a higher oxygen influx from porous plates.
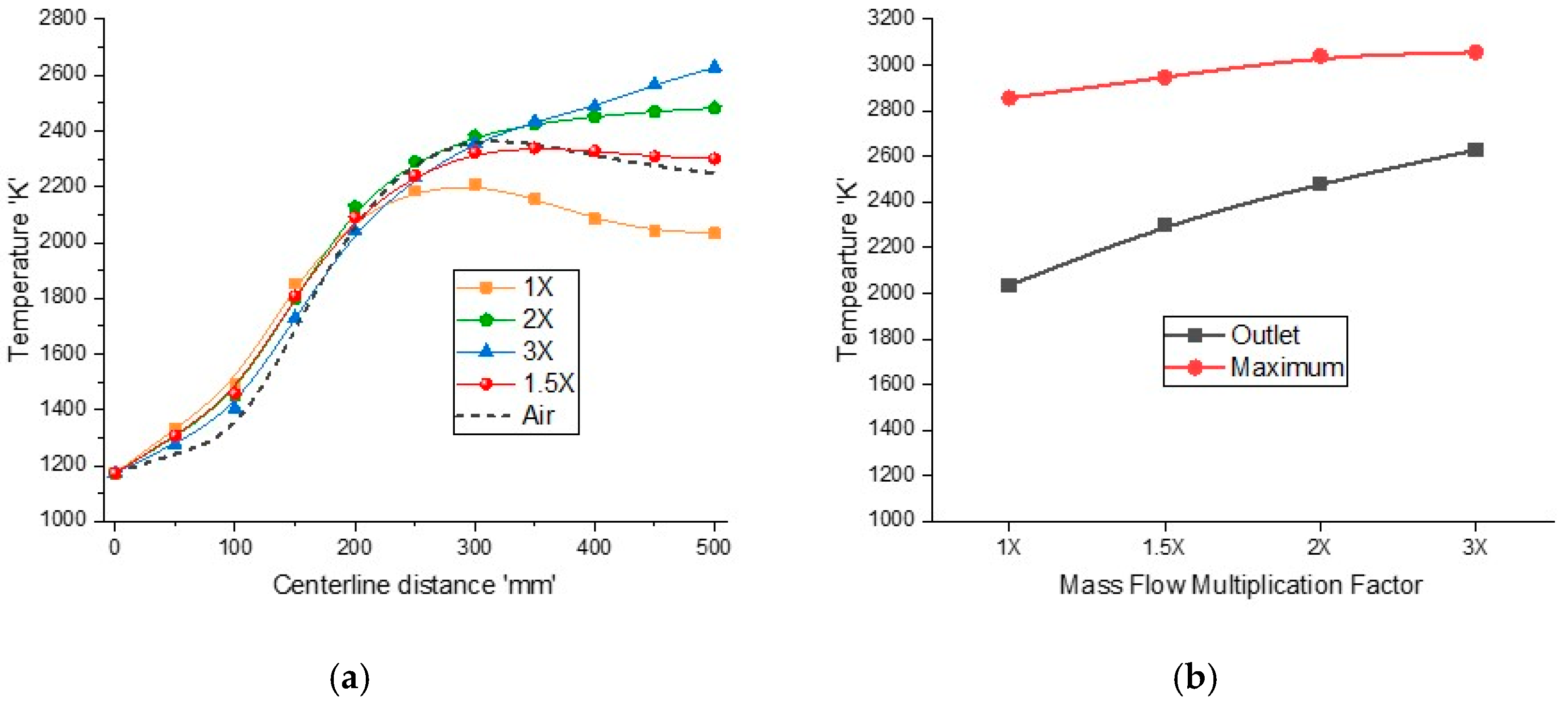
The mass weighted average temperature variations for different inlet flow rates with fixed OR = 0.25 are shown in
Figure 11a. The maximum temperature in the reactor and outlet temperature for different inlet flow conditions are shown in
Figure 11b. As the mass flow rate increases, the sweep flow rate increases, enhancing the velocity field and convection heat transfer, which result in higher temperatures at the reactor outlet. The outlet temperature increases from 2034 K to around 2628 K, and the maximum temperature rises from 2855 K to 3056 K when the inlet flow rates are multiplied by a factor of 3. For 1.5× flow rates, the temperature increases to a maximum value and then slightly decreases to 2301 K, which is comparable to air-combustion with 2250 K at the outlet. However, the maximum temperature for 1.5× flow rates is lower than air-combustion due to radiative cooling, which is beneficial for reactor design. For 2× flow rates, the temperature continues to rise and maintains a constant value of around 2480 K and for 3× flow rates and the temperature continues to rise until the end and does not maintain a constant value or decrease, which reflects that the reaction is not complete by the end of the reactor.
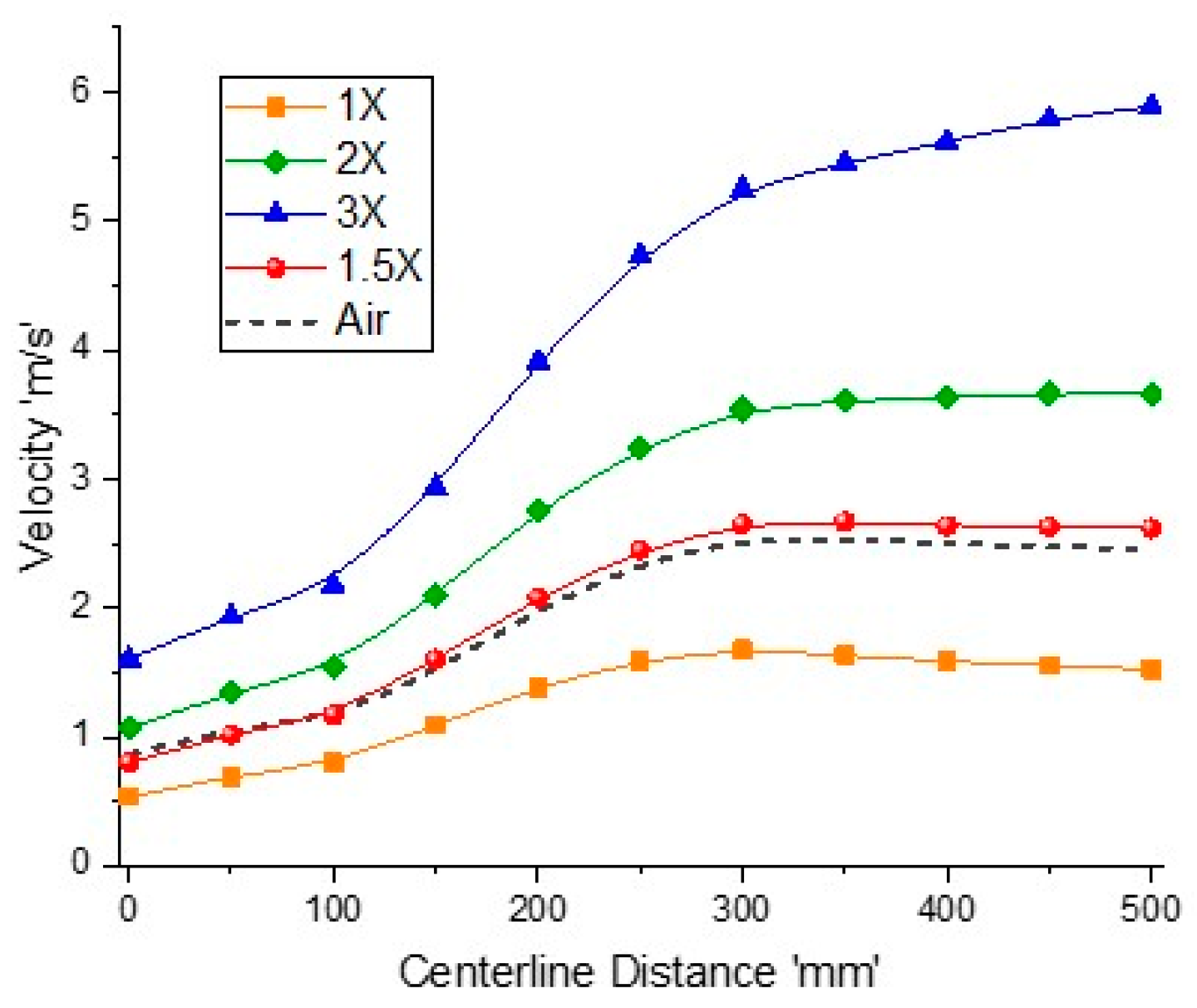
Figure 12 shows velocity variation along the reactor for different exit Reynolds numbers and air-combustion. As the mass flow rate is multiplied by a factor of 1.5, 2, and 3, the velocity rise is more than these factors. This is because at a higher mass flow rate, the outlet temperature increases, which lowers the average density, hence increasing the velocity field. This velocity variation behavior is similar to that of outlet temperature profiles. It is clear that the velocity fields for the air-combustion case and 1.5× inlet flow rates are similar to the temperature profiles. Therefore, for a comparable reactor performance to that of air-combustion, the mass flow rate should be increased by 50%; however, energy contents at the outlet will also be increased.
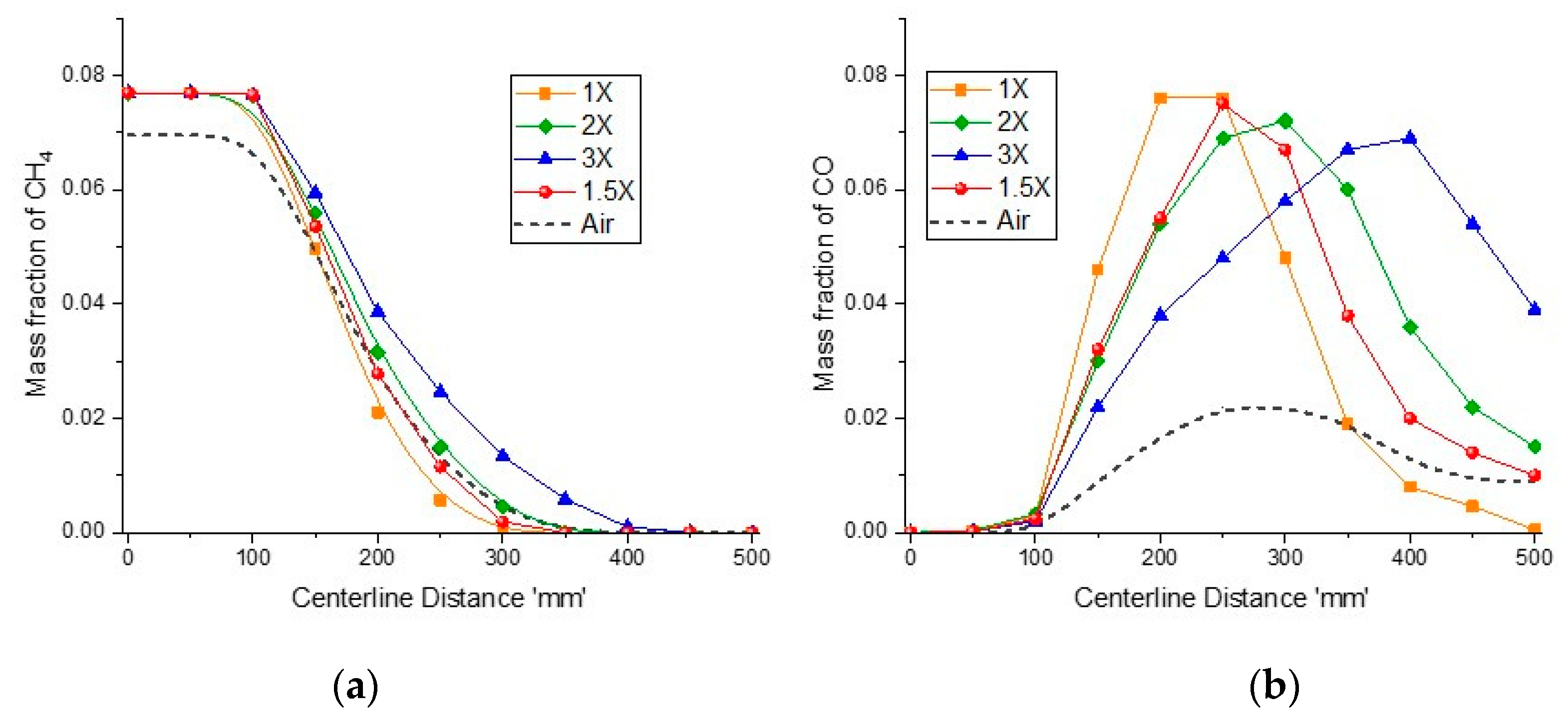
Figure 13a shows the methane depletion rate for different inlet flow rates and air-combustion. Increment in flow rates causes the velocity field inside the reactor to rise, which reduces the residence time and reaction rate. For a 1× flow rates, the reaction occurs faster, and methane diminishes at around
x = 300 mm, while for the 1.5× flow rates, the reaction rate is slower and methane fully converts into combustible products at around
x = 320 mm, which is comparable to that of the air-combustion case. For the 2× and 3× flow rates, the reaction is further slower, and methane converts completely at
x = 350 mm and
x = 410 mm, respectively.
Figure 13b shows carbon monoxide fraction variation along the reaction zone for different sweep flow rates. For higher sweep flow rates, maximum CO peak shifts towards the end of the porous plate and not all of the carbon monoxide is converted and the reaction is incomplete, while for lower sweep flow rates, CO peak occurs at the start of porous plate and all of the carbon monoxide is converted before the reactor’s exit. For the 3× flow rates, CO concentration is high as the reaction is incomplete. Since the 1.5× flow rates for oxy-combustion is comparable to that for air-combustion, they also result in similar CO mass fraction at outlet, i.e., 1% and 0.9%, respectively.
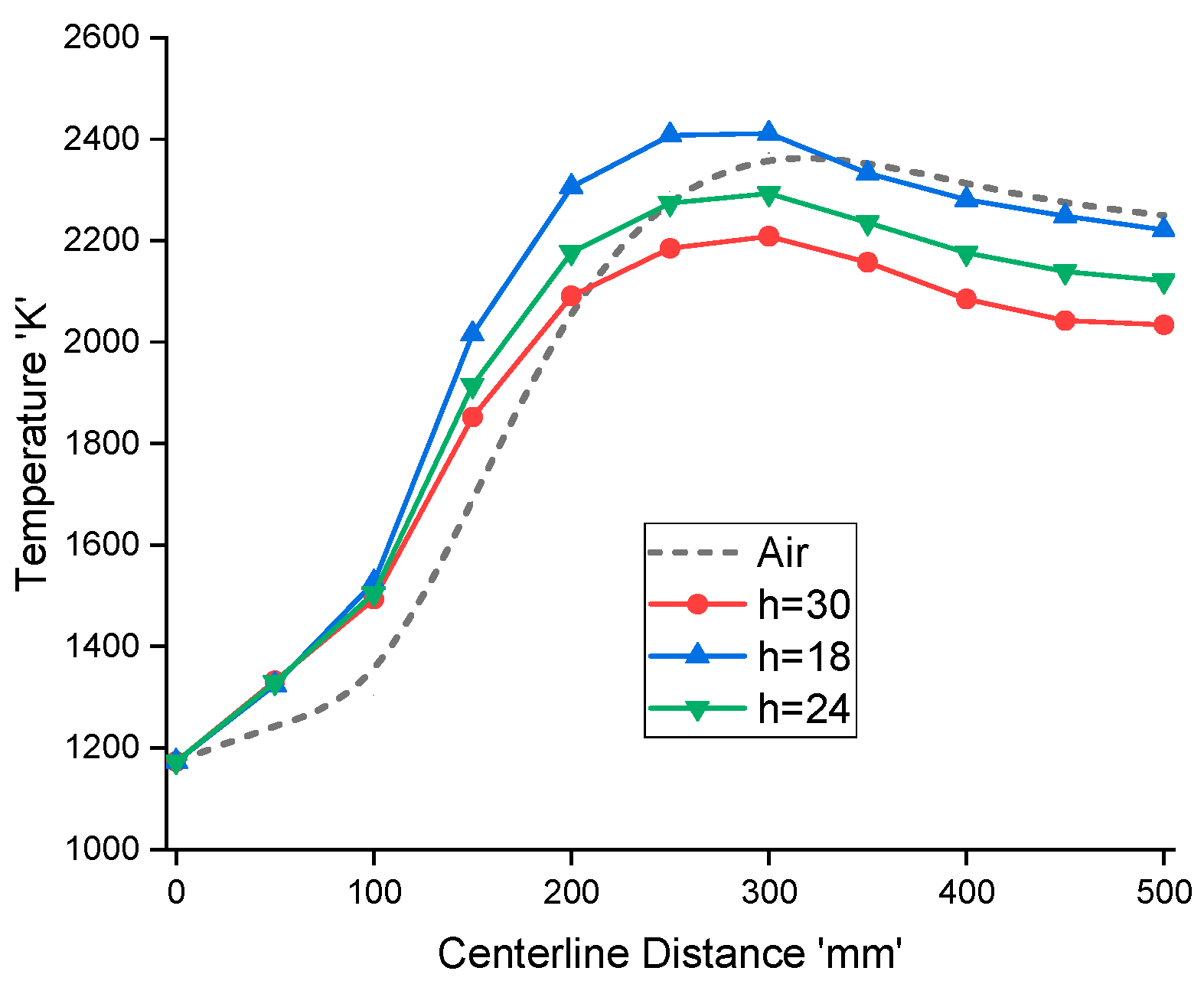
3.5. Effect of Reactor Height
Figure 14 shows the temperature variation profiles for different reactor heights ranging from h = 30 mm to 16 mm. It can be seen that as the reactor height reduces to
h = 18 mm, the outlet temperature changes from 2034 K to 2221K, which is a bit lower than that of the air-combustion case. However, the maximum temperature for
h = 18 mm is much higher than that for the air-combustion. A higher temperature at a lower reactor height causes upstream temperature to rise significantly due to increased radiative heat transfer. Higher temperatures are not desirable as it limits material selection and reactor design.
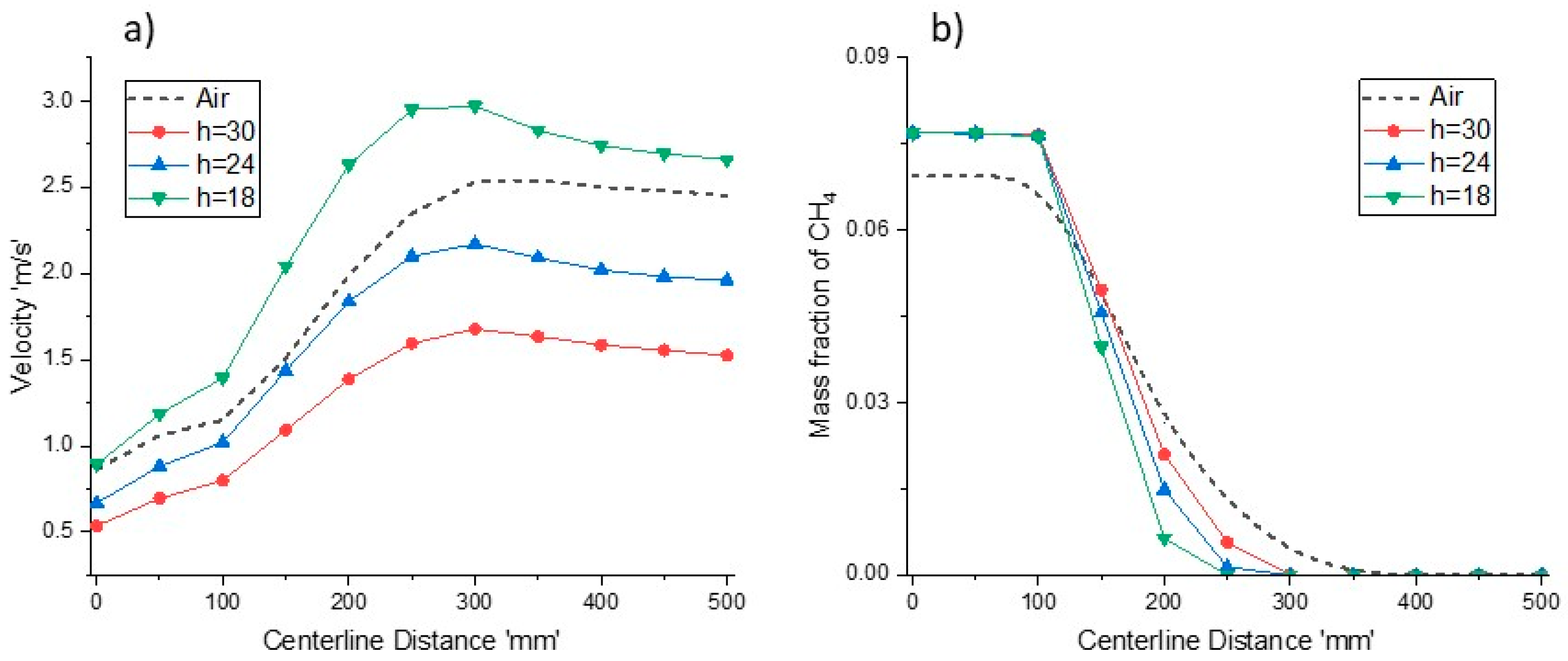
Figure 15a,b shows velocity and CH
4 mass fraction profile for different reactor heights respectively. Decrement in reactor heights lead to increased velocities, and a further increment in the velocity profile is observed as the combustion starts. The velocity of combustion products decreases as the mixture cools near the exit of the reactor. The higher temperature profile at
h = 18 mm is due to the rapid combustion, as shown by the methane conversion profile in
Figure 15b. The methane depletion rates for all cases are higher than methane combustion in an O
2/N
2 environment. Therefore, for a comparable reactor performance to that of air-combustion, the reactor height should be reduced by 40%; however, the peak temperature is much higher than in the air case, which is not desirable.