Heat Transfer in Latent High-Temperature Thermal Energy Storage Systems—Experimental Investigation
Abstract
1. Introduction
2. Materials and Methods
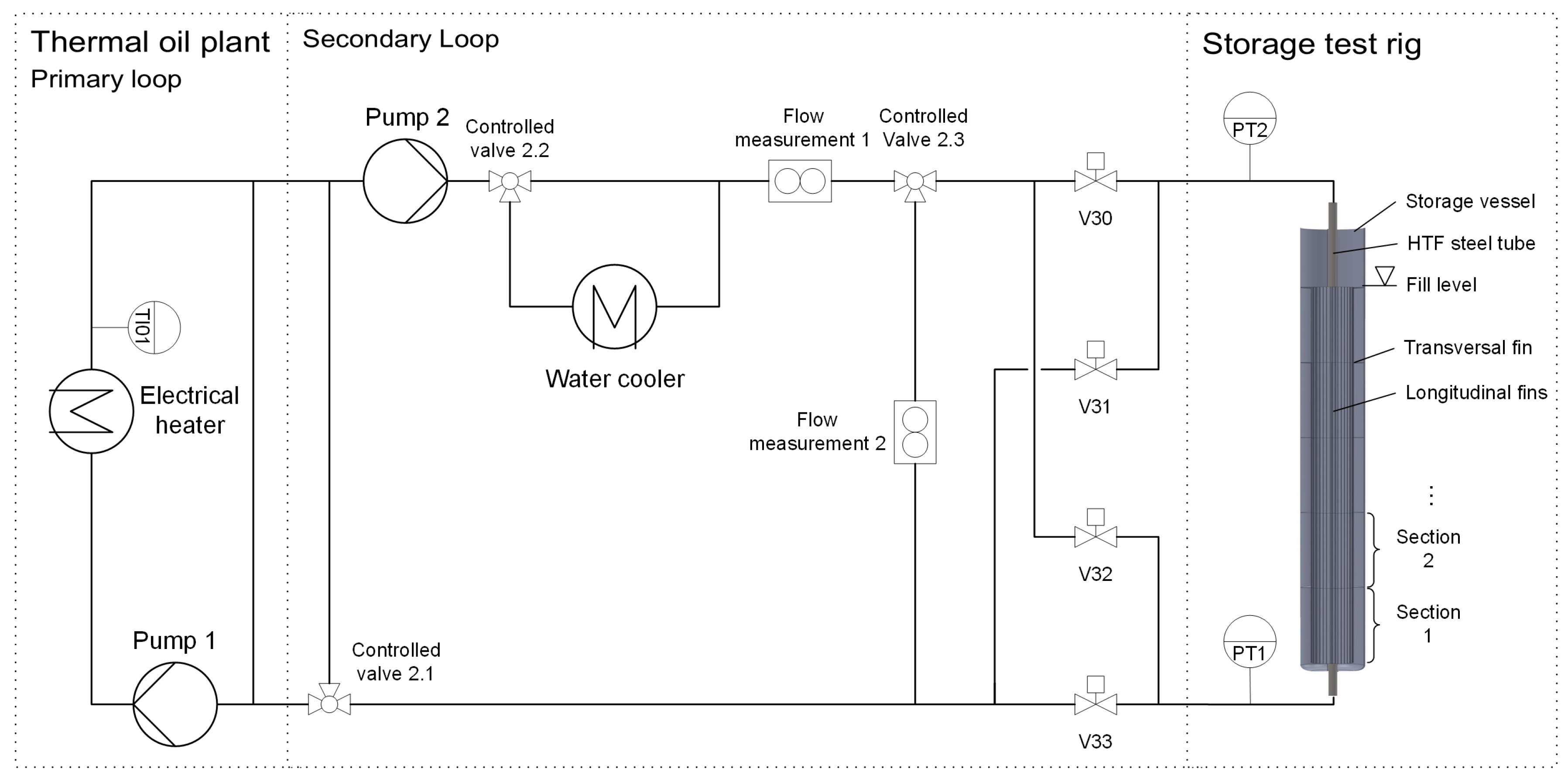
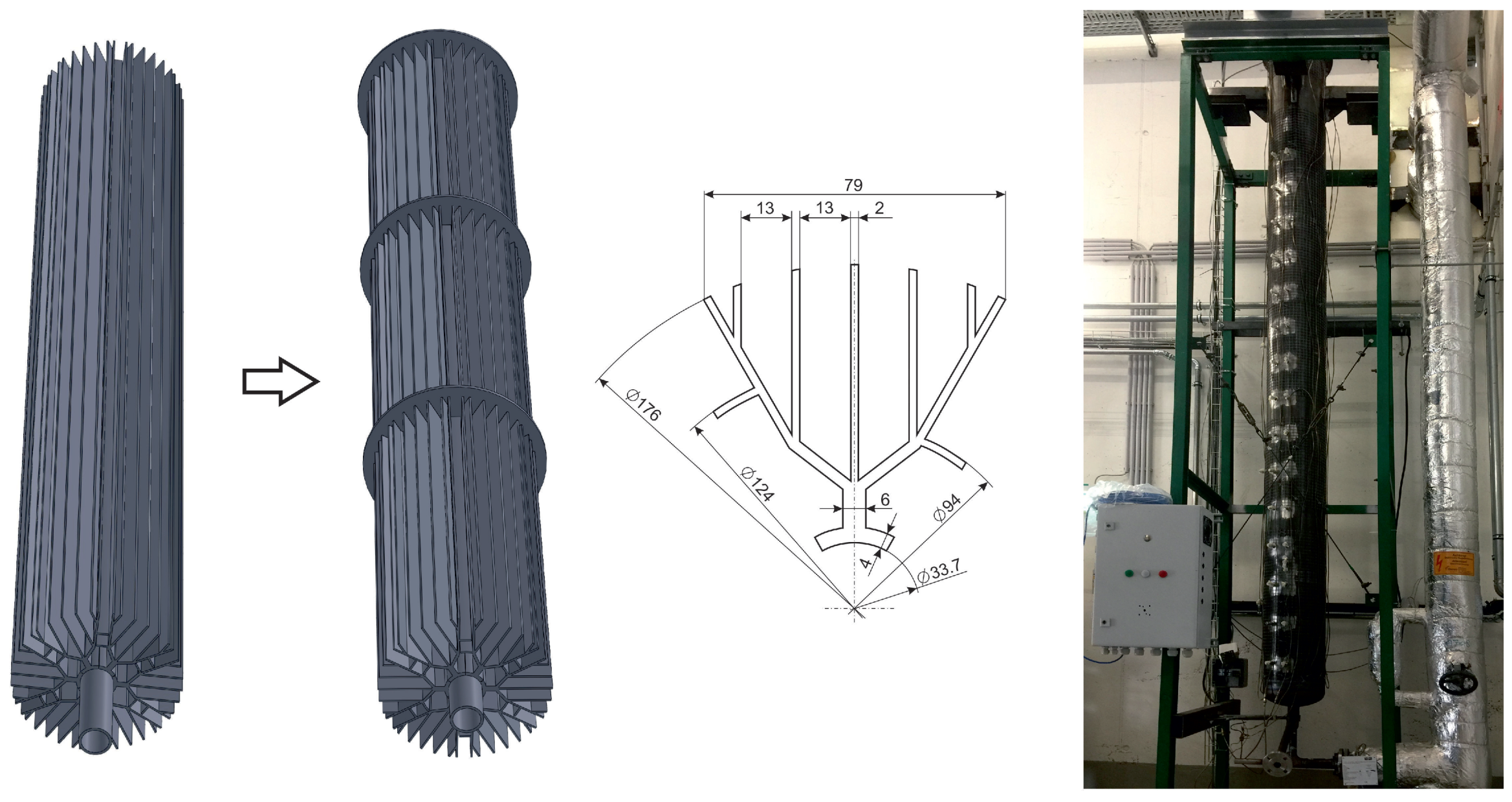
2.1. Test Rig and Testing Point Positions
2.2. Parameter Sets
2.3. Phase-Change Material
2.3.1. Modeling the Phase Change
2.3.2. Sodium Nitrate
2.4. Heat Transmission from the HTF into the PCM
3. Results
3.1. Temperature Distribution in the Storage with the Combination of Transversal and Longitudinal Fins
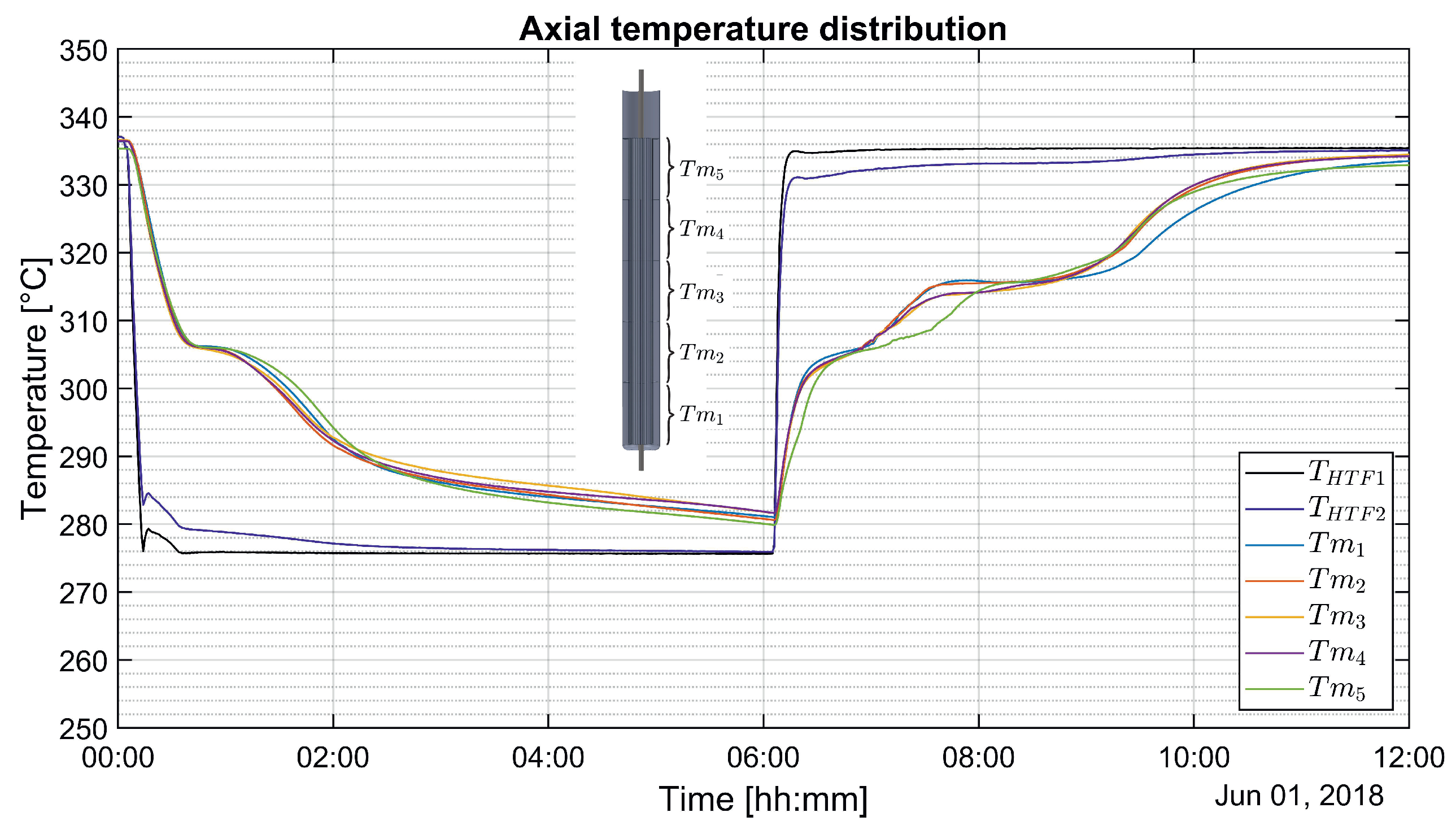
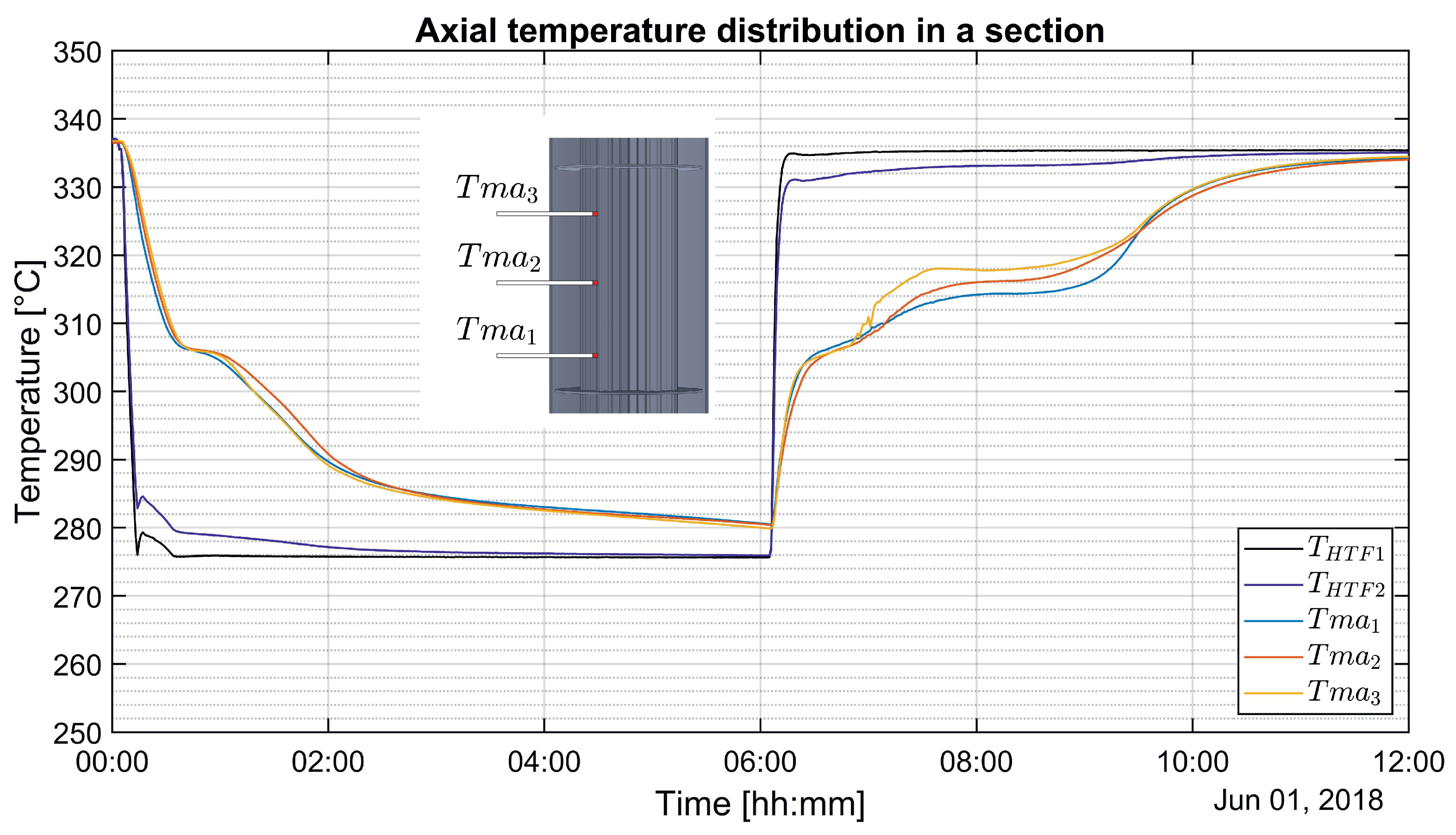
3.2. Axial Temperature Distribution in a Vertical Section
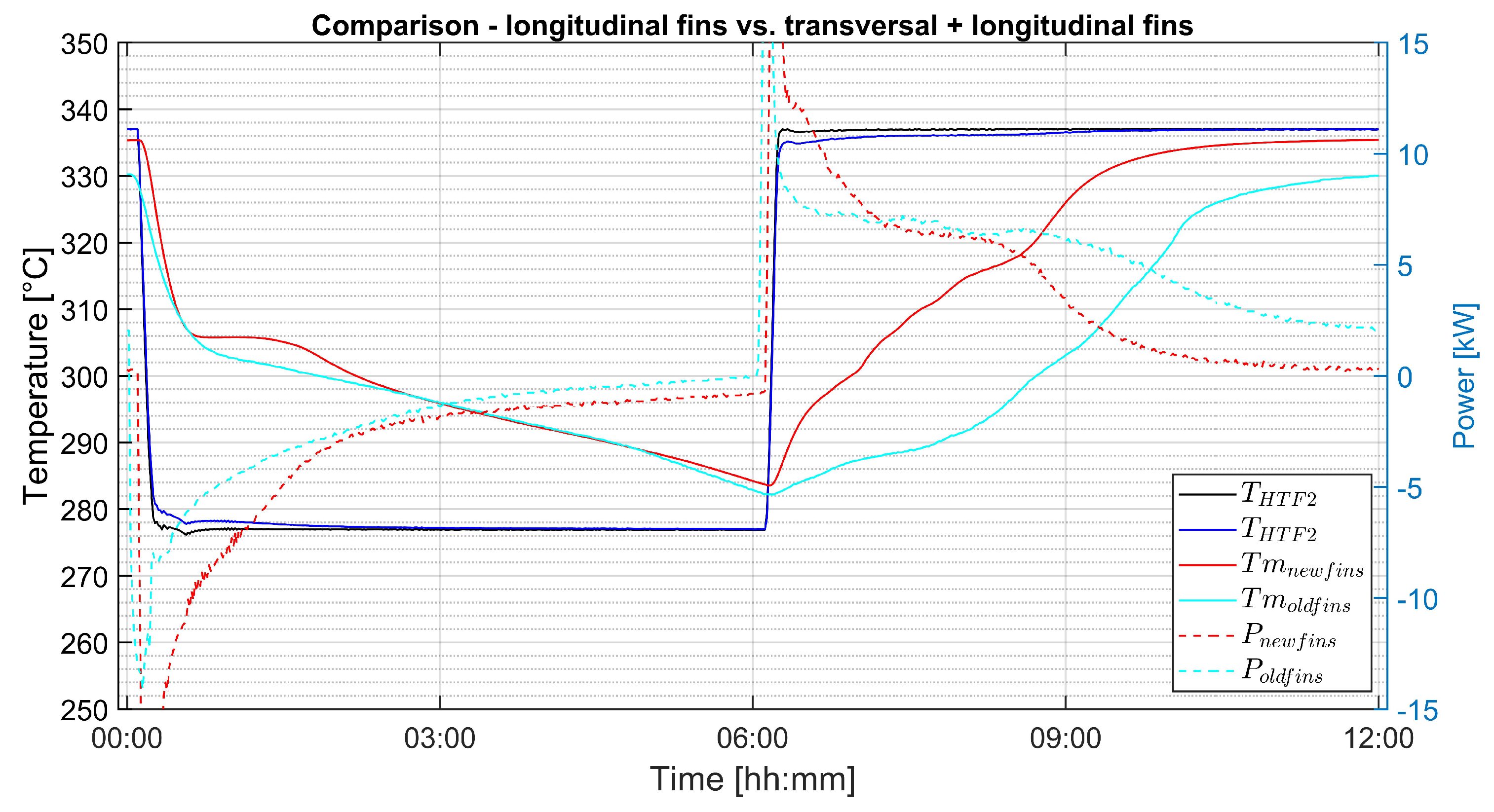
3.3. Comparison of Different fin Geometries
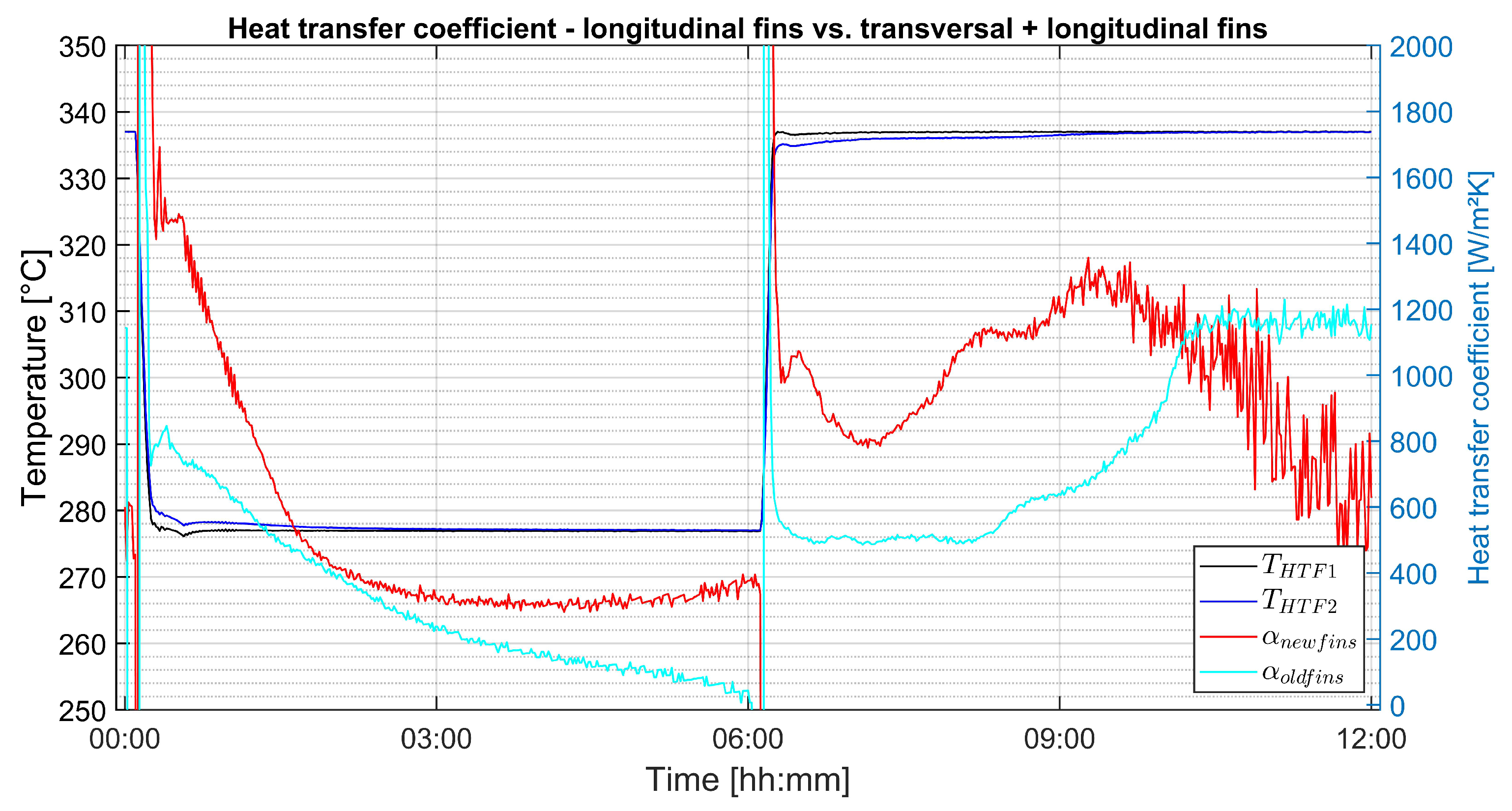
3.4. Apparent Heat-Transfer Coefficient
3.5. Energy Balance—Capacities and Losses
4. Discussion
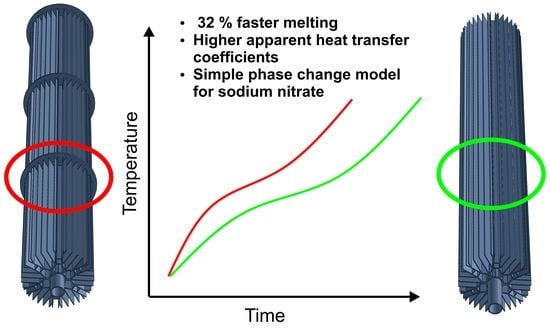
- The combination of transversal and longitudinal fins leads to global axially symmetric melting.
- The combination of transversal and longitudinal fins leads to 32% faster melting compared to plain longitudinal fins.
- Apparent heat-transfer coefficients between 400 and 1500 could be determined
- A simple method for the enthalpy function of sodium nitrate in the melting range is presented, based on an apparent specific heat capacity function.
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CSP | Concentrating solar power |
| DSC | Differential scanning calorimetry |
| HTF | Heat-transfer fluid |
| PCM | Phase-change material |
| TES | Thermal energy storage |
Nomenclature
| Latin symbols | |
| specific heat capacity ( −1 −1) | |
| equivalent specific heat capacity ( −1 −1) | |
| E | Energy ( ) |
| apparent specific heat capacity ( −1 −1) | |
| h | specific enthalpy ( −1) |
| L | Latent heat of fusion ( −1) |
| P | Power () |
| HTF mass flow ( ) | |
| T | Temperature () |
| Temperature spread () | |
| t | time () |
| Mean PCM temperature of the storage () | |
| Greek symbols | |
| Heat-transfer coefficient ( −2 −1) | |
| Liquid fraction (-) | |
| Heat conduction coefficient ( ) | |
| Subscripts | |
| a-d | Temperature subscript: Radial position of thermocouples |
| a-e | Polynomial coefficients for the equivalent specific heat capacity |
| cen | centered |
| ch | charging |
| dis | discharging |
| erf | error function |
| 1–5 | Sections 1–5 |
| fins | parameter valid for this fin type |
| HTF | Heat-transfer fluid |
| HFT1 | HTF position at the bottom/inlet of the storage |
| HFT2 | HTF position at the top/outlet of the storage |
| i | referring to the inside of the HTF tube |
| l | Liquid |
| lrv | lower range validity |
| melt | Melting temperature |
| m | Melting range |
| newfins | New fin geometry |
| oldfins | Old fin geometry |
| poly | polynomial function |
| ref | reference |
| s | Solid |
| sens | Sensible |
| storage | Storage including PCM, steel and aluminum |
| urv | upper range validity |
References
- Ioan Sarbu and Calin Sebarchievici. A Comprehensive Review of Thermal Energy Storage. Sustainability 2018, 10, 191. [Google Scholar] [CrossRef]
- IRENA. Renewable Power Generation Costs in 2017: International Renewable Energy Agency; IRENA: Abu Dhabi, UAE, 2018. [Google Scholar]
- U.S. Department of Energy. Department of Energy. DOE Global Energy Storage Database; U.S. Department of Energy: Washington, DC, USA, 2018.
- Liu, M.; Steven Tay, N.H.; Bell, S.; Belusko, M.; Jacob, R.; Will, G.; Saman, W.; Bruno, F. Review on concentrating solar power plants and new developments in high temperature thermal energy storage technologies. Renew. Sustain. Energy Rev. 2016, 53, 1411–1432. [Google Scholar] [CrossRef]
- Qiu, S.; Solomon, L.; Rinker, G. Development of an Integrated Thermal Energy Storage and Free-Piston Stirling Generator for a Concentrating Solar Power System. Energies 2017, 10, 1361. [Google Scholar] [CrossRef]
- Riahi, S.; Jovet, Y.; Saman, W.Y.; Belusko, M.; Bruno, F. Sensible and latent heat energy storage systems for concentrated solar power plants, exergy efficiency comparison. Solar Energy 2019, 180, 104–115. [Google Scholar] [CrossRef]
- De La Calle, A.; Bayon, A.; Hinkley, J.; Pye, J. System-level simulation of a novel solar power tower plant based on a sodium receiver, PCM storage and sCO2 power block. AIP Conf. Proc. 2018, 2033, 210003. [Google Scholar] [CrossRef]
- Bayon, A.; Liu, M.; Sergeev, D.; Grigore, M.; Bruno, F.; Müller, M. Novel solid–solid phase-change cascade systems for high-temperature thermal energy storage. Solar Energy 2019, 177, 274–283. [Google Scholar] [CrossRef]
- Groulx, D. The rate problem in solid-liquid phase change heat transfer: Efforts and questions towards heat exchanger design rules. In Proceedings of the 16th International Heat Transfer Conference (IHTC-16), International Heat Transfer Conferences, Beijing, China, 10–16 August 2018. [Google Scholar]
- Nazir, H.; Batool, M.; Bolivar Osorio, F.J.; Isaza-Ruiz, M.; Xu, X.; Vignarooban, K.; Phelan, P.; Inamuddin; Kannan, A.M. Recent developments in phase change materials for energy storage applications: A review. Int. J. Heat Mass Transfer 2019, 129, 491–523. [Google Scholar] [CrossRef]
- Agyenim, F.; Hewitt, N.; Eames, P.; Smyth, M. A review of materials, heat transfer and phase change problem formulation for latent heat thermal energy storage systems (LHTESS). Renew. Sustain. Energy Rev. 2010, 14, 615–628. [Google Scholar] [CrossRef]
- Sharma, S.D.; Sagara, K. Latent Heat Storage Materials and Systems: A Review. Int. J. Green Energy 2005, 2, 1–56. [Google Scholar] [CrossRef]
- Höhlein, S.; König-Haagen, A.; Brüggemann, D. Macro-Encapsulation of Inorganic Phase-Change Materials (PCM) in Metal Capsules. Materials 2018, 11, 1752. [Google Scholar] [CrossRef]
- Alam, T.E.; Dhau, J.S.; Goswami, D.Y.; Stefanakos, E. Macroencapsulation and characterization of phase change materials for latent heat thermal energy storage systems. Appl. Energy 2015, 154, 92–101. [Google Scholar] [CrossRef]
- Zhang, H.; Balram, A.; Tiznobaik, H.; Shin, D.; Santhanagopalan, S. Microencapsulation of molten salt in stable silica shell via a water-limited sol-gel process for high temperature thermal energy storage. Appl. Therm. Eng. 2018, 136, 268–274. [Google Scholar] [CrossRef]
- Hu, Y.; He, Y.; Zhang, Z.; Wen, D. Effect of Al2O3 nanoparticle dispersion on the specific heat capacity of a eutectic binary nitrate salt for solar power applications. Energy Convers. Manag. 2017, 142, 366–373. [Google Scholar] [CrossRef]
- Buonomo, B.; Di Pasqua, A.; Ercole, D.; Manca, O. Numerical Study of Latent Heat Thermal Energy Storage Enhancement by Nano-PCM in Aluminum Foam. Inventions 2018, 3, 76. [Google Scholar] [CrossRef]
- Martin, V.; He, B.; Setterwall, F. Direct contact PCM–water cold storage. Appl. Energy 2010, 87, 2652–2659. [Google Scholar] [CrossRef]
- Zipf, V.; Neuhäuser, A.; Willert, D.; Nitz, P.; Gschwander, S.; Platzer, W. High temperature latent heat storage with a screw heat exchanger: Design of prototype. Appl. Energy 2013, 109, 462–469. [Google Scholar] [CrossRef]
- Tay, N.; Liu, M.; Belusko, M.; Bruno, F. Review on transportable phase change material in thermal energy storage systems. Renew. Sustain. Energy Rev. 2017, 75, 264–277. [Google Scholar] [CrossRef]
- Laing, D.; Bauer, T.; Breidenbach, N.; Hachmann, B.; Johnson, M. Development of high temperature phase-change-material storages. Appl. Energy 2013, 109, 497–504. [Google Scholar] [CrossRef]
- Prötsch, A. Auslegung und Inbetriebnahme einer Latentwärmespeicherversuchsanlage. Master’s Thesis, TU Wien, Wien, Austria, 2012. [Google Scholar]
- Urschitz, G.; Walter, H.; Hameter, M. Laboratory Test Rig of a LHTES (Latent Heat Thermal Energy Storage): Construction and First Experimental Results. J. Energy Power Eng. 2014, 8, 1838–1847. [Google Scholar]
- Pizzolato, A.; Sharma, A.; Maute, K.; Sciacovelli, A.; Verda, V. Design of effective fins for fast PCM melting and solidification in shell-and-tube latent heat thermal energy storage through topology optimization. Appl. Energy 2017, 208, 210–227. [Google Scholar] [CrossRef]
- Sciacovelli, A.; Gagliardi, F.; Verda, V. Maximization of performance of a PCM latent heat storage system with innovative fins. Appl. Energy 2015, 137, 707–715. [Google Scholar] [CrossRef]
- Eslamnezhad, H.; Rahimi, A.B. Enhance heat transfer for phase-change materials in triplex tube heat exchanger with selected arrangements of fins. Appl. Therm. Eng. 2017, 113, 813–821. [Google Scholar] [CrossRef]
- Kuboth, S.; König-Haagen, A.; Brüggemann, D. Numerical Analysis of Shell-and-Tube Type Latent Thermal Energy Storage Performance with Different Arrangements of Circular Fins. Energies 2017, 10, 274. [Google Scholar] [CrossRef]
- Tay, N.; Bruno, F.; Belusko, M. Comparison of pinned and finned tubes in a phase change thermal energy storage system using CFD. Appl. Energy 2013, 104, 79–86. [Google Scholar] [CrossRef]
- Walter, H.; Beck, A.; Hameter, M. Influence of the Fin Design on the Melting and Solidification Process of NaNO3 in a Thermal Energy Storage System. J. Energy Power Eng. 2015, 9. [Google Scholar] [CrossRef]
- Koller, M.; Beck, A.; Walter, H.; Hameter, M. Comparison of Different Heat Exchanger Tube Designs used in Latent Heat Thermal Energy Storage Systems—A Numerical Study. In Comparison of Different Heat Exchanger Tube De- Signs Used in Latent Heat Thermal Energy Storage Systems—A Numerical Study; Koller, M., Beck, A., Walter, H., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; Volume 38, pp. 277–282. [Google Scholar]
- Sciacovelli, A.; Verda, V.; Colella, F. Numerical Investigation on the Thermal Performance Enhancement in a Latent Heat Thermal Storage Unit. In ASME 2012 11th Biennial Conference on Engineering Systems Design and Analysis; ASME: New York, NY, USA, 2012; p. 543. [Google Scholar]
- Qiu, S.; Solomon, L.; Fang, M. Study of Material Compatibility for a Thermal Energy Storage System with Phase Change Material. Energies 2018, 11, 572. [Google Scholar] [CrossRef]
- Urschitz, G.; Walter, H.; Brier, J. Experimental investigation on bimetallic tube compositions for the use in latent heat thermal energy storage units. Energy Convers. Manag. 2016, 125, 368–378. [Google Scholar] [CrossRef]
- Johnson, M.; Hübner, S.; Braun, M.; Martin, C.; Fiß, M.; Hachmann, B.; Schönberger, M.; Eck, M. Assembly and attachment methods for extended aluminum fins onto steel tubes for high temperature latent heat storage units. Appl. Therm. Eng. 2018, 144, 96–105. [Google Scholar] [CrossRef]
- Benlekkam, M.L.; Nehari, D.; Cheriet, N. Numerical investigation of latent heat thermal energy storage system. Case Stud. Therm. Eng. 2018. [Google Scholar] [CrossRef]
- Gasia, J.; Martin, M.; Solé, A.; Barreneche, C.; Cabeza, L. Phase Change Material Selection for Thermal Processes Working under Partial Load Operating Conditions in the Temperature Range between 120 °C and 200 °C. Appl. Sci. 2017, 7, 722. [Google Scholar] [CrossRef]
- Maldonado, J.; Fullana-Puig, M.; Martín, M.; Solé, A.; Fernández, Á.; de Gracia, A.; Cabeza, L. Phase Change Material Selection for Thermal Energy Storage at High Temperature Range between 210 °C and 270 °C. Energies 2018, 11, 861. [Google Scholar] [CrossRef]
- Kargar, M.R.; Baniasadi, E.; Mosharaf-Dehkordi, M. Numerical analysis of a new thermal energy storage system using phase change materials for direct steam parabolic trough solar power plants. Solar Energy 2018, 170, 594–605. [Google Scholar] [CrossRef]
- Cabeza, L.F.; Tay, N.H.S. (Eds.) High-Temperature Thermal Storage Systems Using Phase Change Materials; Academic Press: London, UK, 2018. [Google Scholar]
- Urschitz, G.; Hameter, M.; Illyés, V.; Walter, H. Experimental Investigation on a High Temperature Latent TES with Novel Fin Geometry. In Proceedings of the 13th Conference on Sustainable Development of Energy, Water and Environment System—SDEWES, Palermo, Italy, 27 September–4 October 2018; pp. 392–404. [Google Scholar]
- DIN Deutsches Institut für Normung. Industrielle Platin-Widerstandsthermometer und Platin-Temperatursensoren (IEC 60751:2008); Deutsche Fassung EN 60751:2008; DIN Deutsches Institut für Normung: Berlin, Germany, 2009. [Google Scholar]
- Bauernfeind, T. Untersuchungen zur thermischen Energiespeicherung an einem Latentwärmespeicher-Einrohr-System (LESY 2). Master’s Thesis, TU Wien, Wien, Austria, 2018. [Google Scholar]
- Rösler, F.; Brüggemann, D. Shell-and-tube type latent heat thermal energy storage: Numerical analysis and comparison with experiments. Heat Mass Transfer 2011, 47, 1027–1033. [Google Scholar] [CrossRef]
- Lager, D. Evaluation of Thermophysical Properties for Thermal Energy Storage Materials—Determining Factors, Prospects and Limitations. Ph.D. Thesis, TU Wien, Wien, Austria, 2017. [Google Scholar]
- Carling, R.E. Heat capacities of NaNO3 and KNO3 from 350 to 800 K. Thermochim. Acta 1983, 60, 265–275. [Google Scholar] [CrossRef]
- Jriri, T.; Rogez, J.; Bergman, C.; Mathieu, J.C. Thermodynamic study of the condensed phases of Thermodynamic study of the condensed phases of NaNO3, KNO3 and CsNO3 and their transitions. Thermochim. Acta 1995, 266, 147–161. [Google Scholar] [CrossRef]
- Bauer, T.; Laing, D.; Kröner, U. Sodium nitrate for high temperature latent heat storage. In Proceedings of the Thermal Energy Storage for Efficiency and Sustainability: 11th International Conference on Thermal Energy Storage, Effstock, Sweden, 14–17 July 2009. [Google Scholar]
- VDI-Gesellschaft (Ed.) VDI Heat Atlas, 2nd ed.; VDI Buch; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar] [CrossRef]
- Therminol® VP-1 Heat Transfer Fluid. Available online: https://www.therminol.com/products/Therminol-VP1 (accessed on 25 February 2019).
- Böswarth, M. Numerische Simulation des Schmelz- und Erstarrungsprozesses in einem Latentwärmespeicher mit Rippenrohren. Master’s Thesis, TU Wien, Wien, Austria, 2017. [Google Scholar]






| Parameter | Symbol | Parameter Set 1 | Parameter Set 2 |
|---|---|---|---|
| Charging time | 6 | 6 | |
| Discharging time | 6 | 6 | |
| Temperature spread | 30 | 30 | |
| PCM melting temperature | 306 | 306 | |
| HTF inlet temperature charging | 336 | 336 | |
| HTF inlet temperature discharging | 276 | 276 | |
| HTF mass flow | 1 | 3 | |
| HTF flow direction | - | to | to |
| Description | Symbol | Value |
|---|---|---|
| Melting temperature | 306 | |
| Latent heat of fusion | L | −1 |
| Solidification temperature limit | 300 | |
| Liquidation temperature limit | 312 | |
| Enthalpy reference temperature | 0 | |
| Lower range of validity | 0 | |
| Upper range of validity | 400 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scharinger-Urschitz, G.; Walter, H.; Haider, M. Heat Transfer in Latent High-Temperature Thermal Energy Storage Systems—Experimental Investigation. Energies 2019, 12, 1264. https://doi.org/10.3390/en12071264
Scharinger-Urschitz G, Walter H, Haider M. Heat Transfer in Latent High-Temperature Thermal Energy Storage Systems—Experimental Investigation. Energies. 2019; 12(7):1264. https://doi.org/10.3390/en12071264
Chicago/Turabian StyleScharinger-Urschitz, Georg, Heimo Walter, and Markus Haider. 2019. "Heat Transfer in Latent High-Temperature Thermal Energy Storage Systems—Experimental Investigation" Energies 12, no. 7: 1264. https://doi.org/10.3390/en12071264
APA StyleScharinger-Urschitz, G., Walter, H., & Haider, M. (2019). Heat Transfer in Latent High-Temperature Thermal Energy Storage Systems—Experimental Investigation. Energies, 12(7), 1264. https://doi.org/10.3390/en12071264