Thermodynamics and Kinetics of CO2/CH4 Adsorption on Shale from China: Measurements and Modeling
Abstract
1. Introduction
2. Materials and Methods
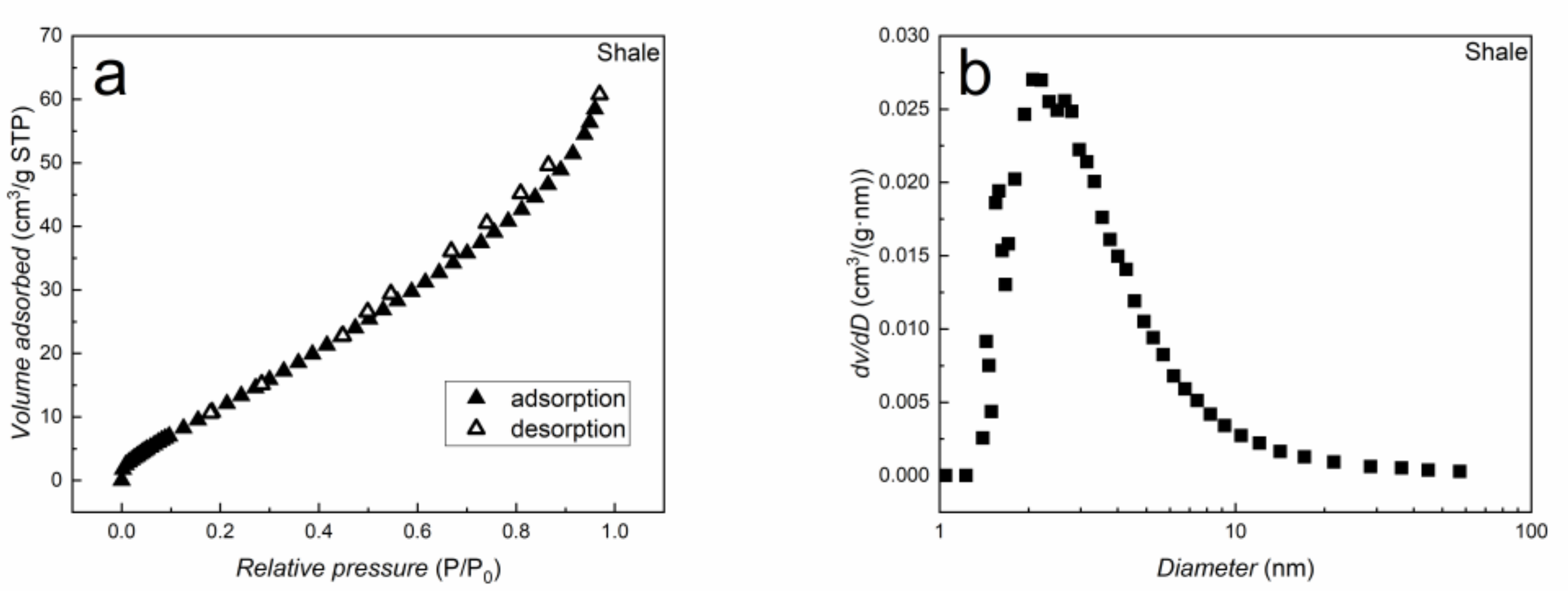
2.1. Materials
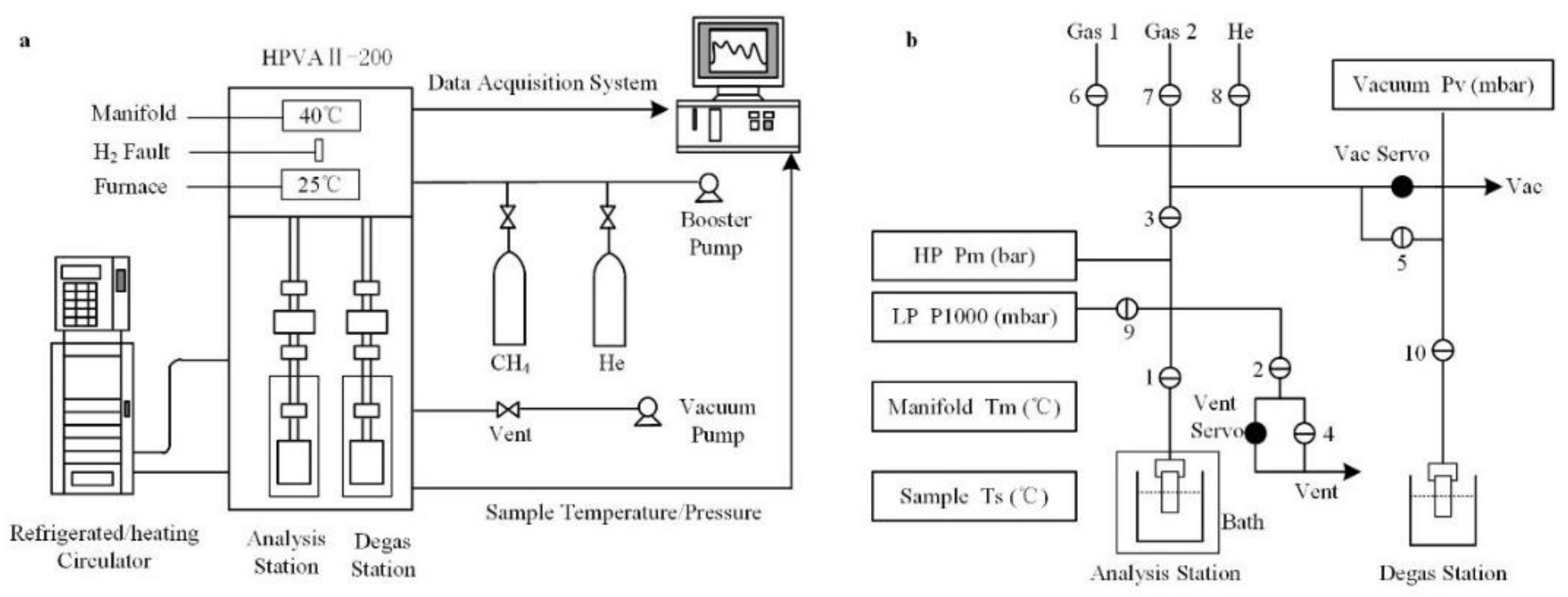
2.2. Adsorption
- (1)
- First, the shale was crushed and sieved before use, and a powder of 0.18–0.25 mm in grain size was obtained. This was dried for 8 h at 105 °C to exclude the effect of moisture on the weight measurement.
- (2)
- Then, the powder was weighed and placed into the sample cylinder, which was subsequently attached to the degas station and evacuated overnight at 105 °C to remove the adsorbed moisture and other gases.
- (3)
- Next, the cooled cylinder was moved to the analysis station and the manifold was cleaned to avoid contamination by gases in the manifold.
- (4)
- Finally, the adsorption of gases (CO2 or CH4) experiment was carried out automatically by the HPVA II-200. The experimental data were recorded, and the adsorption isotherms were derived.
2.3. Adsorbed Amount Calculations
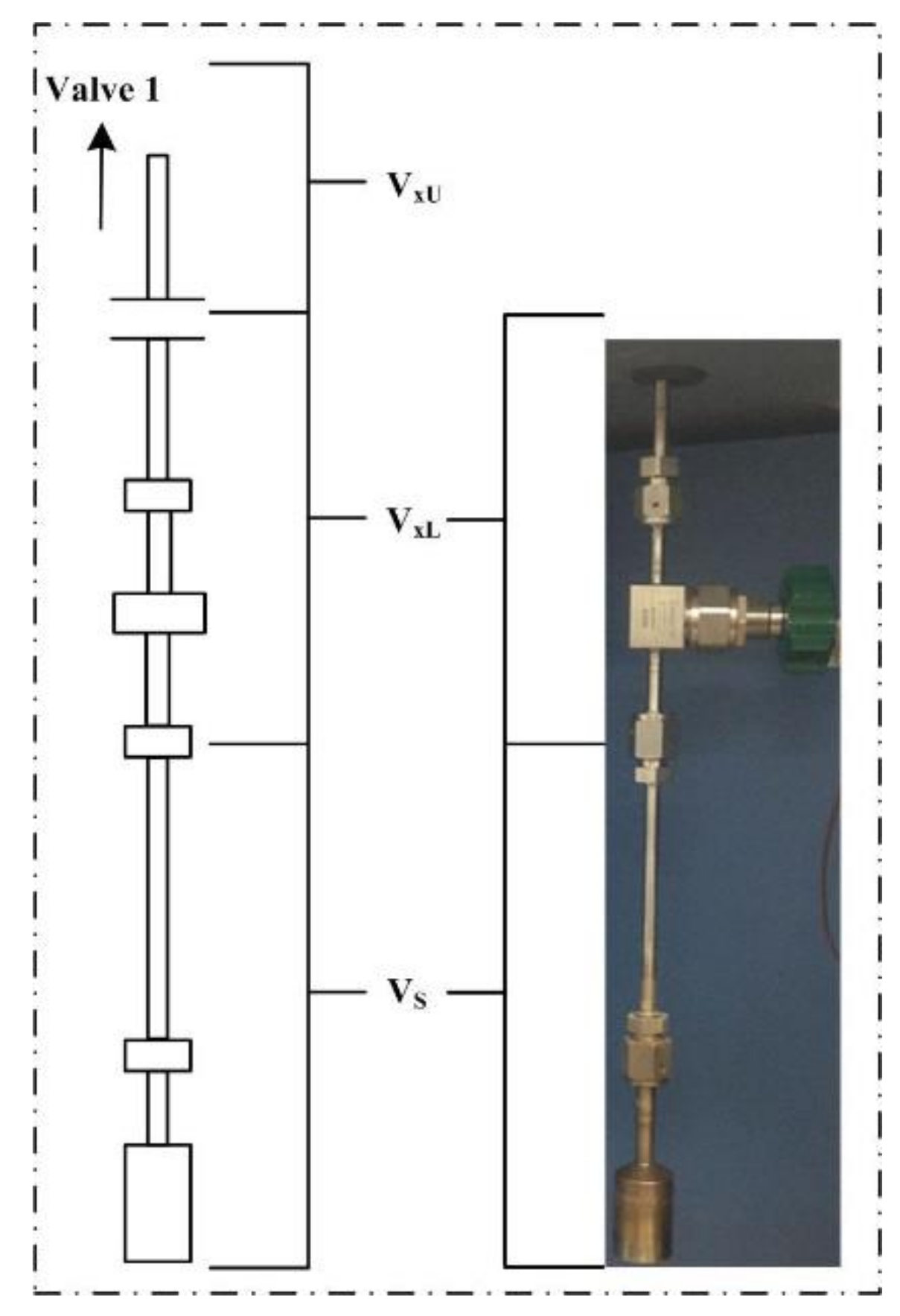
2.3.1. Free Space
2.3.2. Adsorbed Amount of Gas
2.4. Adsorption Rate Calculations
3. Modeling
3.1. Langmuir + k Model
3.2. Ono–Kondo Lattice Model
4. Results and Discussion
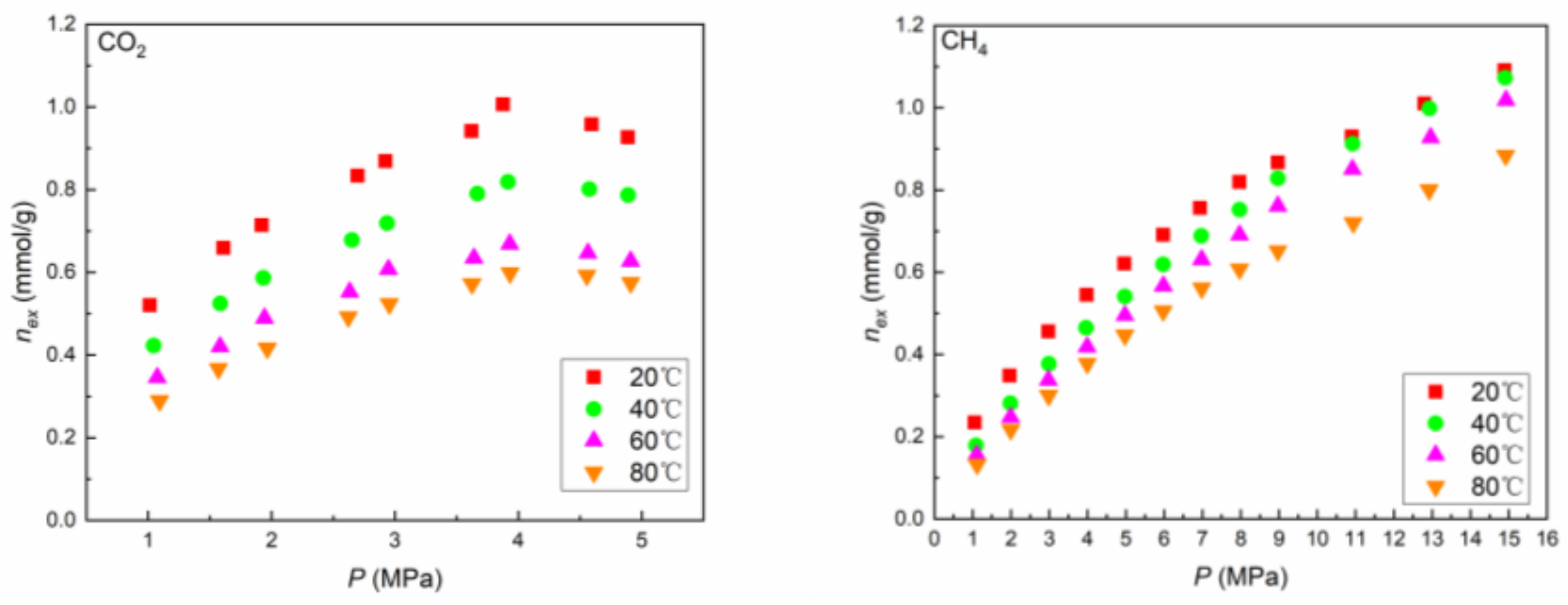
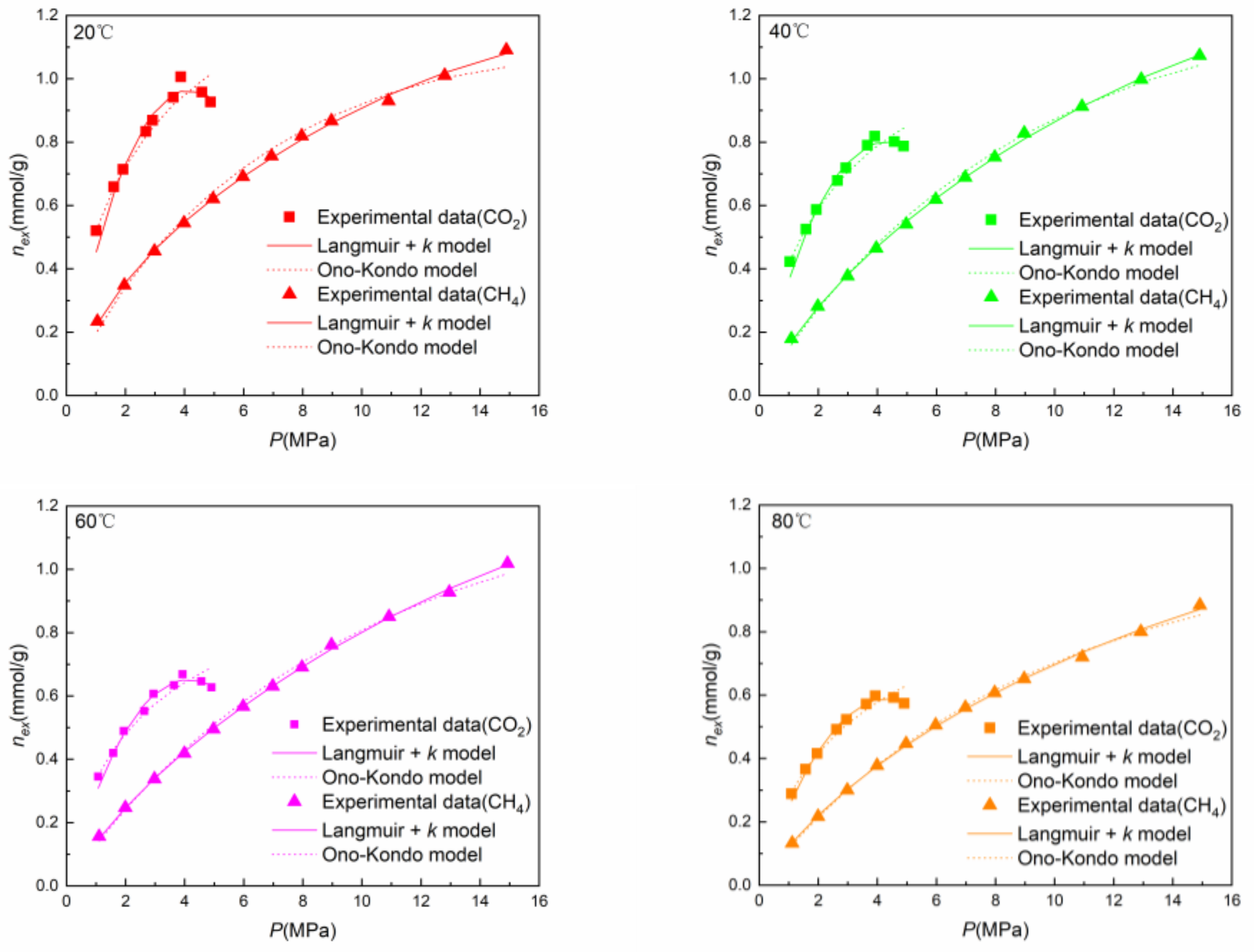
4.1. Adsorption Capacity
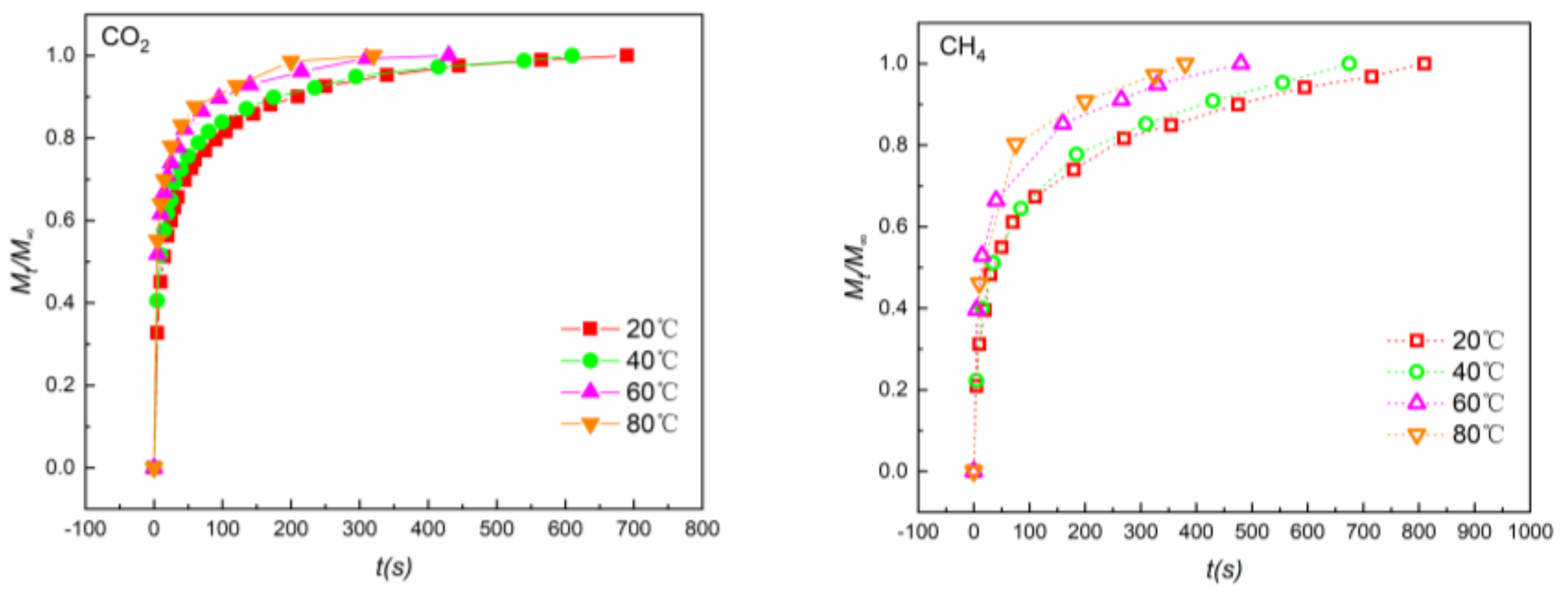
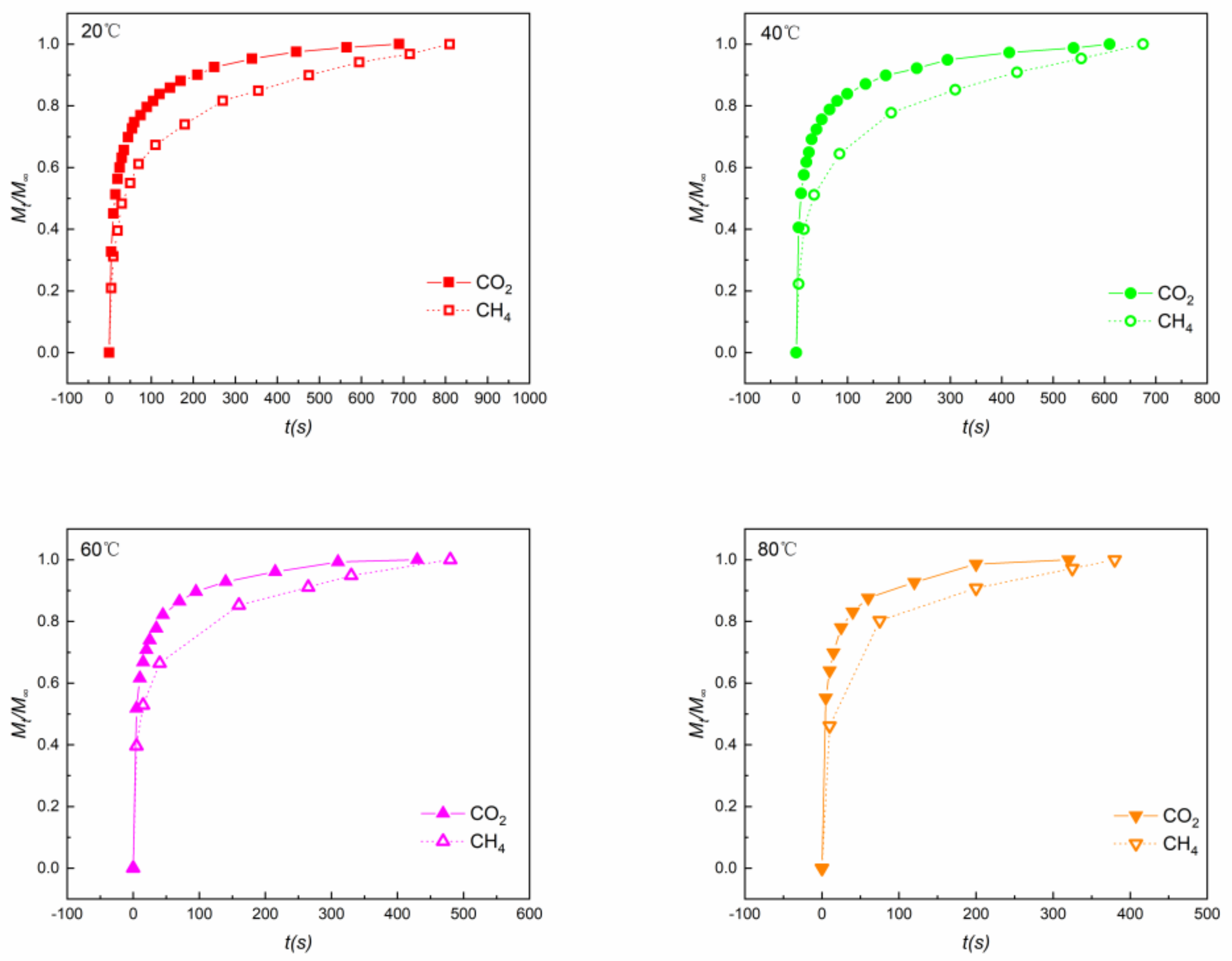
4.2. Adsorption Rates
4.3. Thermodynamic Models
5. Conclusions
- (1)
- The excess adsorbed amount of CH4 increased with increasing pressure across the complete range of experimental pressures applied. By contrast, a maximum excess adsorbed amount of CO2 was observed at approximately 4 MPa.
- (2)
- With increasing temperature, the time to reach equilibrium of both CO2 and CH4 decreased and, therefore, the adsorption rates rose for both gases. CO2 exhibited a larger adsorption rate than CH4. The effective diffusion coefficient De of CO2 (1.65 × 10−3 s−1) was almost 3 times larger than that of CH4 (0.56 × 10−3 s−1) at 40 °C. This may be attributed to the higher affinity between shale and CO2, making it easier for CO2 to diffuse into the micropores on the surface of the shale.
- (3)
- The Langmuir + k model predicted the adsorption data well, and the AADs of CO2 and CH4 were 2.12–3.10% and 0.88–1.11%, respectively. The trend of peaking at approximately 4 MPa for CO2 was accurately modeled. From the Ono–Kondo model, the AADs of CO2 and CH4 were 2.70–3.79% and 1.31–3.51%, respectively. The interaction energy εis/k between CO2 and shale in the Ono–Kondo model was 2–3 times larger than that between CH4 and shale, which is indicative of a stronger affinity between CO2 and shale than between CH4 and shale. This also may account for the relatively larger adsorption rate for CO2.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Song, Y.; Cheng, C.; Zhao, J.; Zhu, Z.; Liu, W.; Yang, M.; Xue, K. Evaluation of gas production from methane hydrates using depressurization, thermal stimulation and combined methods. Appl. Energy 2015, 145, 265–277. [Google Scholar] [CrossRef]
- Song, Y.; Wang, X.; Yang, M.; Jiang, L.; Liu, Y.; Dou, B.; Zhao, J.; Wang, S. Study of Selected Factors Affecting Hydrate-Based Carbon Dioxide Separation from Simulated Fuel Gas in Porous Media. Energy Fuels 2013, 27, 3341–3348. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, D.; Yang, M.; Song, Y. Analysis of heat transfer effects on gas production from methane hydrate by depressurization. Int. J. Heat Mass Transfer 2014, 77, 529–541. [Google Scholar] [CrossRef]
- Zhao, J.; Yu, T.; Song, Y.; Liu, D.; Liu, W.; Liu, Y.; Yang, M.; Ruan, X.; Li, Y. Numerical simulation of gas production from hydrate deposits using a single vertical well by depressurization in the Qilian Mountain permafrost, Qinghai-Tibet Plateau, China. Energy 2013, 52, 308–319. [Google Scholar] [CrossRef]
- Zhao, Y.; Song, Y.; Liu, Y.; Liang, H.; Dou, B. Visualization and Measurement of CO2 Flooding in Porous Media Using MRI. Ind. Eng. Chem. Res. 2011, 50, 4707–4715. [Google Scholar] [CrossRef]
- Yang, M.; Chong, Z.R.; Zheng, J.; Song, Y.; Linga, P. Advances in nuclear magnetic resonance (NMR) techniques for the investigation of clathrate hydrates. Renew. Sustain. Energy Rev. 2017, 74, 1346–1360. [Google Scholar] [CrossRef]
- Yang, M.; Zheng, J.; Liu, W.; Liu, Y.; Song, Y. Effects of C3H8 on hydrate formation and dissociation for integrated CO2 capture and desalination technology. Energy 2015, 93 Pt 2, 1971–1979. [Google Scholar] [CrossRef]
- Zheng, J.N.; Yang, M.J.; Liu, Y.; Wang, D.Y.; Song, Y.C. Effects of cyclopentane on CO2 hydrate formation and dissociation as a co-guest molecule for desalination. J. Chem. Thermodyn. 2017, 104, 9–15. [Google Scholar] [CrossRef]
- Howarth, R.W.; Santoro, R.; Ingraffea, A. Methane and the greenhouse-gas footprint of natural gas from shale formations. Clim. Chang. 2011, 106, 679–690. [Google Scholar] [CrossRef]
- Fathi, E.; Akkutlu, I.Y. Multi-component gas transport and adsorption effects during CO2 injection and enhanced shale gas recovery. Int. J. Coal Geol. 2014, 123, 52–61. [Google Scholar] [CrossRef]
- Godec, M.; Koperna, G.; Petrusak, R.; Oudinot, A. Potential for enhanced gas recovery and CO2 storage in the Marcellus Shale in the Eastern United States. Int. J. Coal Geol. 2013, 118, 95–104. [Google Scholar] [CrossRef]
- Li, X.; Elsworth, D. Geomechanics of CO2 enhanced shale gas recovery. J. Nat. Gas Sci. Eng. 2015, 26, 1607–1619. [Google Scholar] [CrossRef]
- Pei, P.; Ling, K.; He, J.; Liu, Z. Shale gas reservoir treatment by a CO2 -based technology. J. Nat. Gas Sci. Eng. 2015, 26, 1595–1606. [Google Scholar] [CrossRef]
- Gasparik, M.; Bertier, P.; Gensterblum, Y.; Ghanizadeh, A.; Krooss, B.M.; Littke, R. Geological controls on the methane storage capacity in organic-rich shales. Int. J. Coal Geol. 2014, 123, 34–51. [Google Scholar] [CrossRef]
- Liu, F.; Ellett, K.; Xiao, Y.; Rupp, J.A. Assessing the feasibility of CO2 storage in the New Albany Shale (Devonian–Mississippian) with potential enhanced gas recovery using reservoir simulation. Int. J. Greenhouse Gas Control 2013, 17, 111–126. [Google Scholar] [CrossRef]
- Sun, H.; Yao, J.; Gao, S.-H.; Fan, D.-Y.; Wang, C.-C.; Sun, Z.-X. Numerical study of CO2 enhanced natural gas recovery and sequestration in shale gas reservoirs. Int. J. Greenhouse Gas Control 2013, 19, 406–419. [Google Scholar] [CrossRef]
- Yuan, W.; Pan, Z.; Li, X.; Yang, Y.; Zhao, C.; Connell, L.D.; Li, S.; He, J. Experimental study and modelling of methane adsorption and diffusion in shale. Fuel 2014, 117, 509–519. [Google Scholar] [CrossRef]
- Gu, M.; Xian, X.; Duan, S.; Du, X. Influences of the composition and pore structure of a shale on its selective adsorption of CO2 over CH4. J. Nat. Gas Sci. Eng. 2017, 46, 296–306. [Google Scholar] [CrossRef]
- Weniger, P.; Kalkreuth, W.; Busch, A.; Krooss, B.M. High-pressure methane and carbon dioxide sorption on coal and shale samples from the Paraná Basin, Brazil. Int. J. Coal Geol. 2010, 84, 190–205. [Google Scholar] [CrossRef]
- Chareonsuppanimit, P.; Mohammad, S.A.; Robinson, R.L.; Gasem, K.A.M. High-pressure adsorption of gases on shales: Measurements and modeling. Int. J. Coal Geol. 2012, 95, 34–46. [Google Scholar] [CrossRef]
- Du, X.-D.; Gu, M.; Duan, S.; Xian, X.-F. Investigation of CO2–CH4 Displacement and Transport in Shale for Enhanced Shale Gas Recovery and CO2 Sequestration. J. Energy Resour. Technol. 2016, 139, 012909. [Google Scholar] [CrossRef]
- Busch, A.; Gensterblum, Y.; Krooss, B.M.; Littke, R. Methane and carbon dioxide adsorption–diffusion experiments on coal: Upscaling and modeling. Int. J. Coal Geol. 2004, 60, 151–168. [Google Scholar] [CrossRef]
- Terzyk, A.P.; Gauden, P.A. The simple procedure of the calculation of diffusion coefficient for adsorption on spherical and cylindrical adsorbent particles--experimental verification. J. Colloid Interface Sci. 2002, 249, 256–261. [Google Scholar] [CrossRef]
- Terzyk, A.P.; Gauden, P.A. The Simple Procedure of the Calculation of Diffusion Coefficient for Adsorption on Spherical and Cylindrical Adsorbent Particles. Sep. Sci. Technol. 2007, 36, 513–525. [Google Scholar] [CrossRef]
- Sakurovs, R.; Day, S.; Weir, S.; Duffy, G. Application of a Modified Dubinin-Radushkevich Equation to Adsorption of Gases by Coals under Supercritical Conditions. Energy Fuels 2007, 21, 992–997. [Google Scholar] [CrossRef]
- Day, S.; Duffy, G.; Sakurovs, R.; Weir, S. Effect of coal properties on CO2 sorption capacity under supercritical conditions. Int. J. Greenhouse Gas Control 2008, 2, 342–352. [Google Scholar] [CrossRef]
- Gensterblum, Y.; van Hemert, P.; Billemont, P.; Battistutta, E.; Busch, A.; Krooss, B.M.; De Weireld, G.; Wolf, K.H.A.A. European inter-laboratory comparison of high pressure CO2 sorption isotherms II: Natural coals. Int. J. Coal Geol. 2010, 84, 115–124. [Google Scholar] [CrossRef]
- Sakurovs, R.; Day, S.; Weir, S. Relationships between the Critical Properties of Gases and Their High Pressure Sorption Behavior on Coals. Energy Fuels 2010, 24, 1781–1787. [Google Scholar] [CrossRef]
- Zhang, Y.; Xing, W.; Liu, S.; Liu, Y.; Yang, M.; Zhao, J.; Song, Y. Pure methane, carbon dioxide, and nitrogen adsorption on anthracite from China over a wide range of pressures and temperatures: Experiments and modeling. RSC Adv. 2015, 5, 52612–52623. [Google Scholar] [CrossRef]
- Sudibandriyo, M.; Mohammad, S.A.; Robinson, R.L.; Gasem, K.A.M. Ono–Kondo Model for High-Pressure Mixed-Gas Adsorption on Activated Carbons and Coals. Energy Fuels 2011, 25, 3355–3367. [Google Scholar] [CrossRef]
- Sudibandriyo, M.; Mohammad, S.A.; Robinson, R.L., Jr.; Gasem, K.A.M. Ono–Kondo lattice model for high-pressure adsorption: Pure gases. Fluid Phase Equilib. 2010, 299, 238–251. [Google Scholar] [CrossRef]
- Bakhshian, S.; Hosseini, S.A. Prediction of CO2 adsorption-induced deformation in shale nanopores. Fuel 2019, 241, 767–776. [Google Scholar] [CrossRef]
- Gasparik, M.; Rexer, T.F.T.; Aplin, A.C.; Billemont, P.; De Weireld, G.; Gensterblum, Y.; Henry, M.; Krooss, B.M.; Liu, S.; Ma, X.; et al. First international inter-laboratory comparison of high-pressure CH4, CO2 and C2H6 sorption isotherms on carbonaceous shales. Int. J. Coal Geol. 2014, 132, 131–146. [Google Scholar] [CrossRef]
- Wang, J.; Yang, Z.; Dong, M.; Gong, H.; Sang, Q.; Li, Y. Experimental and Numerical Investigation of Dynamic Gas Adsorption/Desorption–Diffusion Process in Shale. Energy Fuels 2016, 30, 10080–10091. [Google Scholar] [CrossRef]
- Wang, J.; Wang, B.E.; Li, Y.; Yang, Z.; Gong, H.; Dong, M. Measurement of dynamic adsorption–diffusion process of methane in shale. Fuel 2016, 172, 37–48. [Google Scholar] [CrossRef]
| Ultimate Analysis (Dry wt % Basis) | Proximate Analysis (wt %) | |||||
|---|---|---|---|---|---|---|
| N | C | S | H | Moisture | Ash | TOC |
| 0.732 | 24.66 | 3.001 | 2.161 | 4.04 | 57.88 | 4.91 |
| T (K) | 293 | 313 | 333 | 353 | |
|---|---|---|---|---|---|
| Vs (cm3) | CO2 | 7.29 | 8.16 | 8.97 | 9.78 |
| CH4 | 7.31 | 8.17 | 8.91 | 9.72 | |
| De (× 10−3 s−1) | 20 °C | 40 °C | 60 °C | 80 °C |
|---|---|---|---|---|
| CO2 | 1.23 | 1.65 | 2.94 | 4.16 |
| CH4 | 0.49 | 0.56 | 1.61 | 2.48 |
| T (K) | n | nL (mmol·g−1) | ρL (g·cm−3) | k (cm3·g−1) | AAD | |
|---|---|---|---|---|---|---|
| CO2 | 293.20 | 9 | 3.0430 | 0.0824 | −5.9953 | 3.10% |
| 312.79 | 9 | 3.2252 | 0.0959 | −7.5770 | 2.54% | |
| 333.11 | 9 | 5.6648 | 0.1526 | −15.5211 | 2.47% | |
| 353.35 | 9 | 8.9370 | 0.2184 | −21.9797 | 2.12% | |
| CH4 | 293.13 | 12 | 0.5244 | 0.0149 | 8.6347 | 1.11% |
| 313.14 | 12 | 0.4471 | 0.0222 | 9.9930 | 1.03% | |
| 333.32 | 12 | 0.3960 | 0.0236 | 10.3226 | 0.88% | |
| 353.33 | 12 | 0.4460 | 0.0266 | 8.5970 | 1.06% |
| T (K) | n | ρ1 (g·cm−3) | C (mmol·g−1) | εis/k (K) | AAD | |
|---|---|---|---|---|---|---|
| CO2 | 293.20 | 9 | 0.6867 | 0.9435 | −1066.2 | 3.11% |
| 312.79 | 9 | 0.6249 | 0.8418 | −1088.7 | 2.70% | |
| 333.10 | 9 | 0.6004 | 0.7022 | −1152.1 | 3.79% | |
| 353.35 | 9 | 0.5330 | 0.7196 | −1121.1 | 3.08% | |
| CH4 | 293.13 | 12 | 0.2476 | 1.7363 | −483.3 | 3.51% |
| 313.14 | 12 | 0.1944 | 2.5245 | −363.7 | 2.91% | |
| 333.32 | 12 | 0.1734 | 2.7091 | −351.4 | 2.40% | |
| 353.31 | 12 | 0.1735 | 2.1090 | −420.5 | 1.31% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chi, Y.; Zhao, C.; Lv, J.; Zhao, J.; Zhang, Y. Thermodynamics and Kinetics of CO2/CH4 Adsorption on Shale from China: Measurements and Modeling. Energies 2019, 12, 978. https://doi.org/10.3390/en12060978
Chi Y, Zhao C, Lv J, Zhao J, Zhang Y. Thermodynamics and Kinetics of CO2/CH4 Adsorption on Shale from China: Measurements and Modeling. Energies. 2019; 12(6):978. https://doi.org/10.3390/en12060978
Chicago/Turabian StyleChi, Yuan, Changzhong Zhao, Junchen Lv, Jiafei Zhao, and Yi Zhang. 2019. "Thermodynamics and Kinetics of CO2/CH4 Adsorption on Shale from China: Measurements and Modeling" Energies 12, no. 6: 978. https://doi.org/10.3390/en12060978
APA StyleChi, Y., Zhao, C., Lv, J., Zhao, J., & Zhang, Y. (2019). Thermodynamics and Kinetics of CO2/CH4 Adsorption on Shale from China: Measurements and Modeling. Energies, 12(6), 978. https://doi.org/10.3390/en12060978








