3.1. Enzymatic Hydrolysis
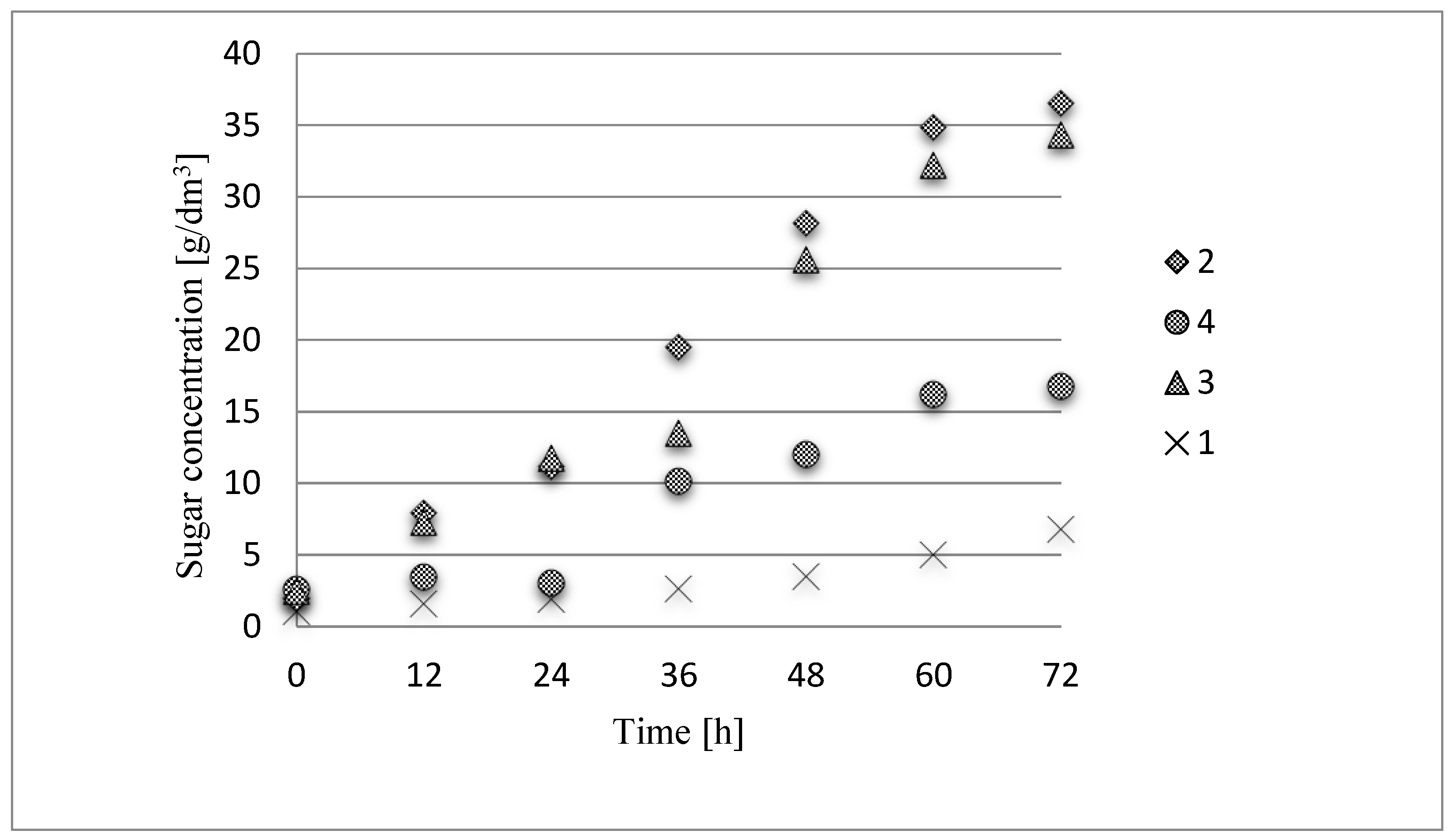
After 72 h, the obtained concentration of released sugars as a result of enzymatic hydrolysis of the native substrate (control sample) was 6.78 g/dm
3 of hydrolysate. As a result of the series of experiments utilizing triticale straw treated with 1-ethyl-3-methylimidazolium acetate a considerable increase in the susceptibility of the substrate to enzymatic hydrolysis was observed. Almost a six-fold higher content of reducing sugars in comparison to the control sample obtained after a 72-hour enzymatic hydrolysis proves the validity of treating the raw material with ionic liquid. The highest reducing sugar concentration obtained after enzymatic hydrolysis was 36.54 g/dm
3 (
Figure 1).
Comparable results were also obtained by Świątek and Lewandowska [
18], who subjected rapeseed straw to hydrolysis. The efficiency of the saccharification process of the material in the native form was 16.0% of the theoretical efficiency from glucose. In their study, Perez et al. [
19] focused on the optimization of wheat hydrolysis using heat treatment. The efficiency of biodegradation of this material prior to its pretreatment was 13.0% of the theoretical glucose value. Fu and Mazza [
20] in a study of pretreatment of triticale straw subjected the material to enzymatic hydrolysis after its purification using different concentrations of 1-ethyl-3-methylimidazolium acetate. The hydrolysis efficiency was measured based on concentration of the obtained reducing sugars, depending on the amount of the ionic liquid used. The highest values (81%) were observed for the sample, where the addition of ionic liquid in the solution was 50%.
In comparison, in the sample of straw not subjected to treatment to only 15.4% of the total amount of reducing sugars was obtained. The straw sample after pretreatment using 2% sulfuric acid (purification temperature: 160 °C, purification time: 20 min) added to the aqueous solution of the material was also subjected to enzymatic hydrolysis. The yield of reducing sugars in the sample was 47%. To obtain simple sugars for the production of bioethanol, Ang et al. [
21] utilized rice husks. The highest concentration of reducing sugars—42.1%—was obtained in the sample with rice husks purified via the use of 1-ethyl-3-methylimidazolium acetate. 1-Ethyl-3-methylimidazolium acetate was also used for the pretreatment of barley straw by Sáez et al. [
22]. These authors focused on the influence of time and temperature of pretreatment on the reducing sugar content measured after enzymatic hydrolysis. The highest glucose content was found in samples of barley straw pretreated with the ionic liquid at 110 °C. The glucose in those samples was 400 mg/100 g of biomass after 30 min and 600 mg/100 g of biomass after 60 min of the pretreatment. Liu and Chen [
4] conducted a study using (1-butyl-3-methylimidazolium chloride) ionic liquid where they subjected wheat straw to treatment. These authors found that the pretreatment with ionic liquid influences the depolymerization of cellulose, thus increasing its susceptibility to enzymatic hydrolysis. The efficiency of enzymatic hydrolysis was highest (70.37%) in the wheat straw sample which was incubated with ionic liquid for 10 min. Li et al. [
23] attempted saccharification of wheat straw, which was previously treated using 1-ethyl-3-methylimidazolium phosphate and 1-ethyl-3-methylimidazolium acetate. The reducing sugar content measured after 12 h hydrolysis of wheat straw samples, previously purified using 1-ethyl-3-methylimidazolium phosphate was 4.8 mg/cm
3, and in the sample of the material purified with 1-ethyl-3-methylimidazolium acetate the level of reducing sugars amounted to 3.2 mg/cm
3. As a result of the conducted experiments, a minimum of two fold higher values for the triticale straw after 12 h enzymatic hydrolysis were obtained. The pretreatment of lignocellulose raw materials using ionic liquid is an innovative solution, thus far not used at an industrial scale. The study results confirm the favorable effect of this catalyst on the yield of reducing sugars in the hydrolysis process in comparison to samples without pretreatment. The use of this type of treatment at an industrial scale would only be possible if the efficiency of the method at least equaled the pretreatments. For these reasons an experiment which compared the yield of reducing sugars after enzymatic hydrolysis process of rye straw subjected to pretreatment with ionic liquid and using sulfate method (chemical pretreatment) was conducted. The content of reducing sugars measured after enzymatic hydrolysis in the sample of triticale straw purified using the sulfate method was 34.35 g/dm
3, and the sample of this material purified with ionic liquid contained 36.54 g/dm
3 reducing sugars. The mean values of the enzymatic hydrolysis products in these materials indicate that both methods of pretreatment have similar significance and both have a positive impact on the enhancement of the reducing sugar production process. However, pretreatment with ionic liquid is more favorable due to its lack of toxicity toward the environment. In the work of Chrzanowska et al. [
24] the influence of imidazolium liquids on the efficiency of wastewater treatment and enzymatic activity of activated sludge microorganisms were examined. During the experiments it was observed that after a certain amount of time the activated sludge microorganisms adapted to the environment containing ionic liquid, and their composition did not differ significantly from the initial composition. Moreover, the rates of the nitrification processes and biochemical reactions were not reduced, which indicates the possibility of an efficient treatment of wastewater containing ionic liquids. The study published by Grabińska-Sota [
25], also concerning the biodegradability of imidazolium ionic liquid confirm that the ionic liquid was subjected to quite considerable biological decomposition within the activated sludge, which most likely stemmed from the biodegradation of these compounds by microorganisms. The literature also contains studies where the lignocellulose material was subjected to pretreatment using sulfuric acid, sodium hydroxide or calcium hydroxide prior to enzymatic hydrolysis. Chen et al. [
26] used 2% NaOH for the pretreatment of corn straw, which was incubated in the solution for 1 h at 80 °C. The use of such pretreatment allowed them to increase the content of reducing sugars determined after enzymatic hydrolysis as compared to the native material. The sugar content was 89.5 g/dm
3, whereas the glucose content in the sample was 56.7 g/dm
3, and the efficiency of hydrolysis was 83.3%. Sun and Cheng [
27] performed enzymatic hydrolysis of rye straw and increased its susceptibility to saccharification via purification in a solution of H
2SO
4. The content of reducing sugars depended on the concentration of H
2SO
4 used to purify the rye straw. In the sample of rye straw purified with 1.5% H
2SO
4, the content of reducing sugars was 159.7 mg/g after 30 min and 197 mg/g after 90 min of pretreatment. In the sample of the material pretreated with 0.6 % H
2SO
4, the content of reducing sugars was 125 mg/g after 30 min and 136 mg/g after 90 min of pretreatment.
The main limitation of the use of ionic liquid at an industrial scale is its high price. The increasing interest of the industry in these solvents, their wide and easy use and lack of toxicity for the environment constitutes the basis for the statement that the price of ionic liquid may be reduced in the future [
28]. Another method for the reduction of the cost of industrial use of ionic liquids is the development of an efficient method for their recycling and reuse in another process. Based on the above statements, a study was implemented concerning purification of the ionic liquid-1-ethyl-3-methylimidazolium acetate after the purification process of rye straw and its reuse for the pretreatment of a new portion of the lignocellulose material. The efficiency of rye straw pretreatment using recycled ionic liquid was evaluated comparing the yield of reducing sugars after enzymatic hydrolysis of the material. The majority of the cellulose fraction of the biomass is retrieved from the ionic liquid via addition of a so called non-solvent. Water can be such a non-solvent for imidazolium ionic liquids, which, by precipitating cellulose from the solution, creates one phase with the ionic liquid. The cellulose from such a mixture was removed by filtration or centrifugationn. The water remaining in the solution was removed via lyophilization. As a result of lyophilization, a yellow-brown ionic liquid was obtained, with a color similar to that of a pure ionic liquid. The mean content of reducing sugars obtained after enzymatic hydrolysis of rye straw purified with recycled ionic liquid was 16.77 g/dm
3, that is approx. 52% lower than the mean content of reducing sugars obtained through hydrolysis of rye straw purified with the pure ionic liquid. Xu et al. [
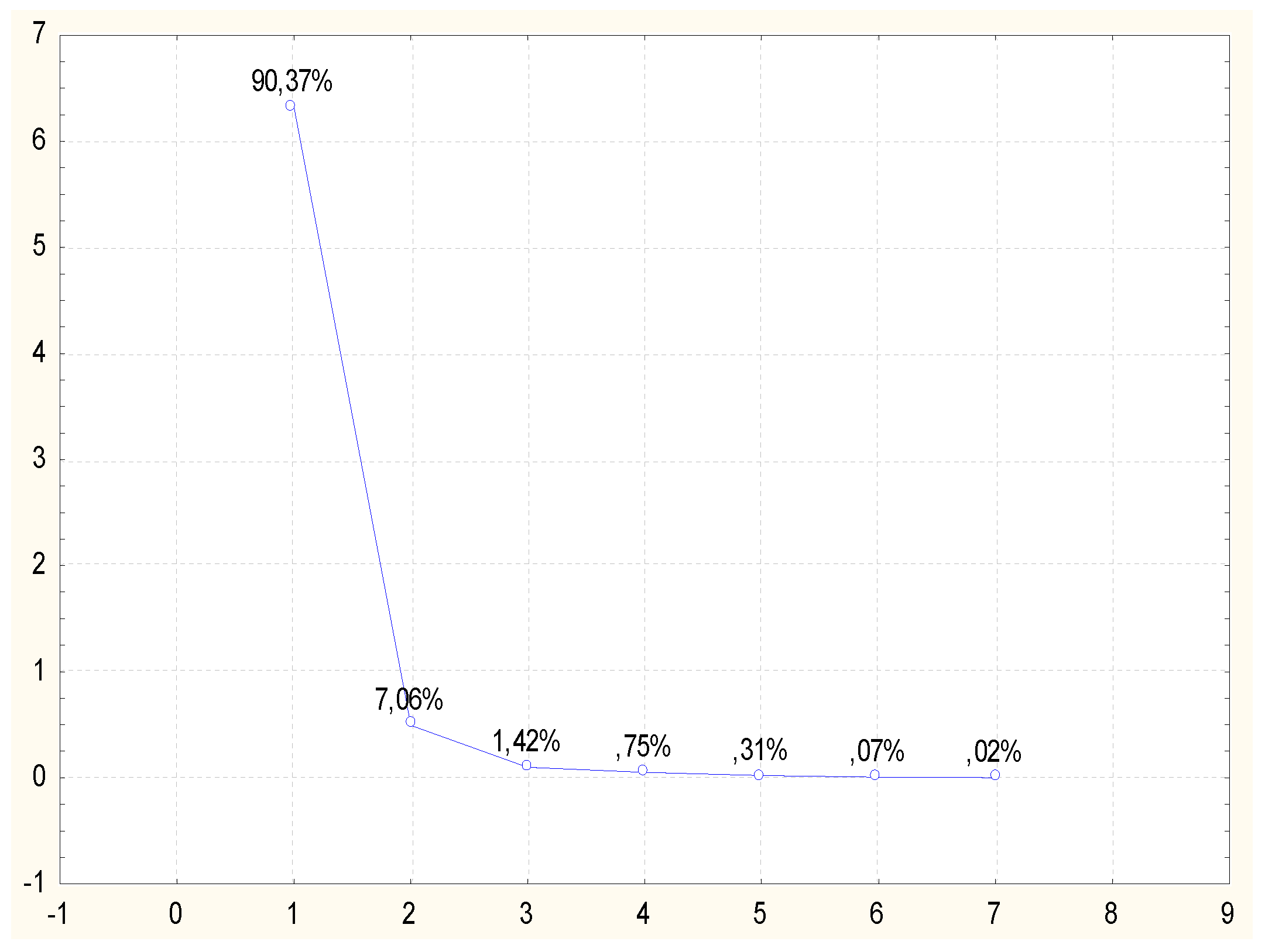
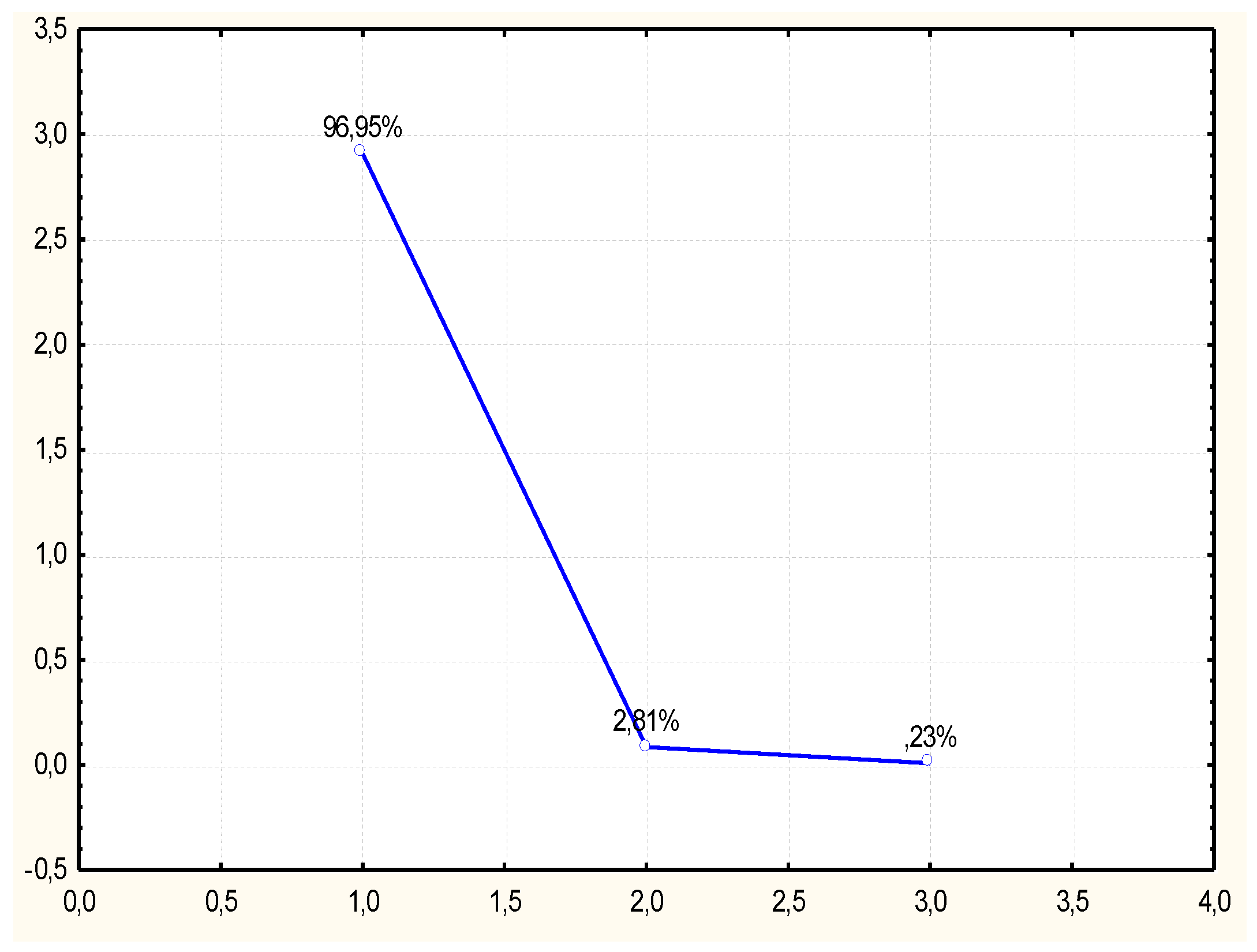
29] subjected eucalyptus samples to purification and enzymatic hydrolysis. The purification was conducted using pure and recycled ionic liquids (AMIMOAc and EMIMOAc). The ionic liquid recycling was conducted by vacuum drying. These authors demonstrated that both pretreatment with pure ionic liquid and recycled ionic liquid influences the change of the structure of cellulose in eucalyptus and improves the yield of reducing sugars after enzymatic hydrolysis. In order to attempt a classification of the types of treatments of lignocellulose materials in terms of obtaining the highest concentration of reducing sugars after enzymatic hydrolysis process, the obtained results were analyzed using the Principal Components Analysis (PCA) method. The active variables consisted in the reducing sugars concentration values determined at specific time intervals. The grouping variables were three types of pretreatment used prior to the hydrolysis process: treatment using ionic liquid, using recycled ionic liquid, sulfate method treatment and sample with the native material, i.e., not subjected to any pretreatment. Results of reducing sugars determined in three repetitions, for each time interval, were used for the PCA analysis conducted in the present study. The PCA analysis was conducted based on a correlation matrix. From the set of the analyzed data four factors were obtained with values >1, which characterized samples after pretreatment in the sense of their similarities and differences. They explained a total of 97.43% of the total variability, and 90.37 % of the variability could be explained by the single main component Z1. The second distinguished component Z2 explained 7.06%, and the Z3 component slightly over 1 %. Data dimension reduction was conducted based on a screening plot (
Figure 2). The moment of graph flattening is visible after the second component, thus the subsequent analyses were conducted on this basis, referring solely to the influence of components Z1 and Z2.
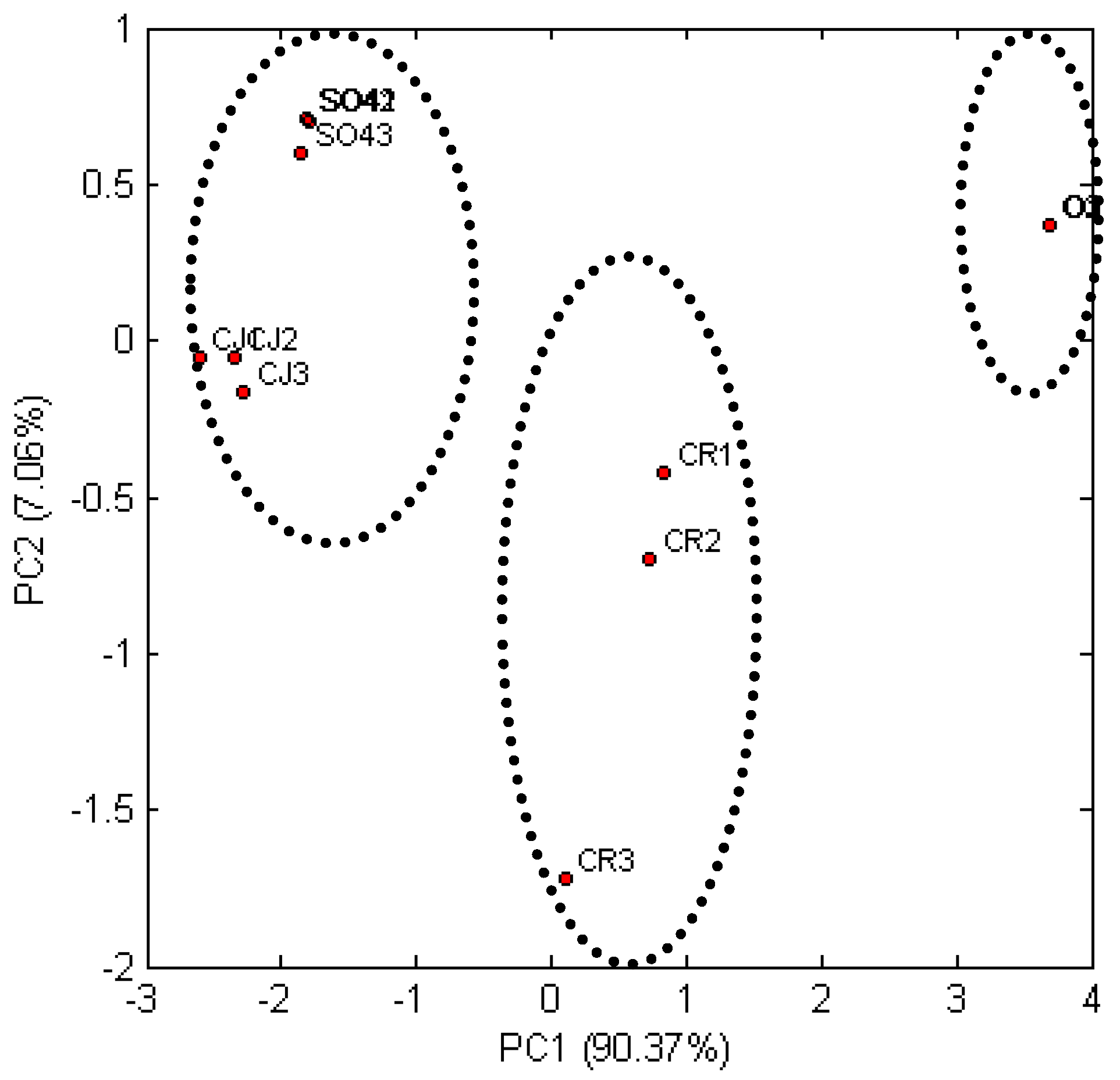
In order to investigate the influence of pretreatment of lignocellulose materials subjected to enzymatic hydrolysis for the content of reducing sugars, an observation chart (
Figure 3) was drawn up, which presents the location of the grouped variables in the new dimensional space defined by the components Z1 and Z2 determined during the analysis. The resemblance measure during the analysis was the Euclidean distance. An analysis of the chart indicated the existence of three groups. First group was characterized by the triticale straw samples subjected to pretreatment using ionic liquid (CJ1-3) and samples of the same material treated with sulfate method (SO41-3). The second group consisted of triticale straw samples purified using recycled ionic liquid (CR1-3). Third group contains triticale straw samples subjected to enzymatic hydrolysis without pretreatment. Enzymatic hydrolysis of samples from the first group had the best efficiency due to the better availability of cellulose for the enzymes. Thus, the Euclidean distance of these samples from the O sample is highest (
Figure 3).
The use of pretreatment with ionic liquid or sulfate method has a similar effect on the concentration of reducing sugars obtained after enzymatic hydrolysis. The Z1 component allows one to distinguish the type of pretreatment used and indicates the increase of reducing sugars in the given time intervals of enzymatic hydrolysis. On the other hand, the Z2 component informs on the level of cellulose decomposition to simple sugars.
The results of the statistical tests corroborate the validity of using triticale straw pretreatment with ionic liquid and classify the type of used treatments and their influence on the formation reducing sugars in the process of enzymatic hydrolysis. Triticale straw pretreatment with ionic liquid has a significant impact on the reducing sugar content, and its increase is comparable to the increase of reducing sugars in the triticale straw sample purified using the sulfate method. In summary, triticale straw pretreatment has a significant impact on formation of reducing sugars by enzymatic hydrolysis. Weaker results are obtained in samples purified with ionic liquid after its dehydration.
3.2. Alcoholic Fermentation
Samples of triticale straw purified with ionic liquid and using the sulfate method and recycled ionic liquid were subjected to alcoholic fermentation. For comparison, a sample of triticale straw not subjected to pretreatment was also fermented (control sample).
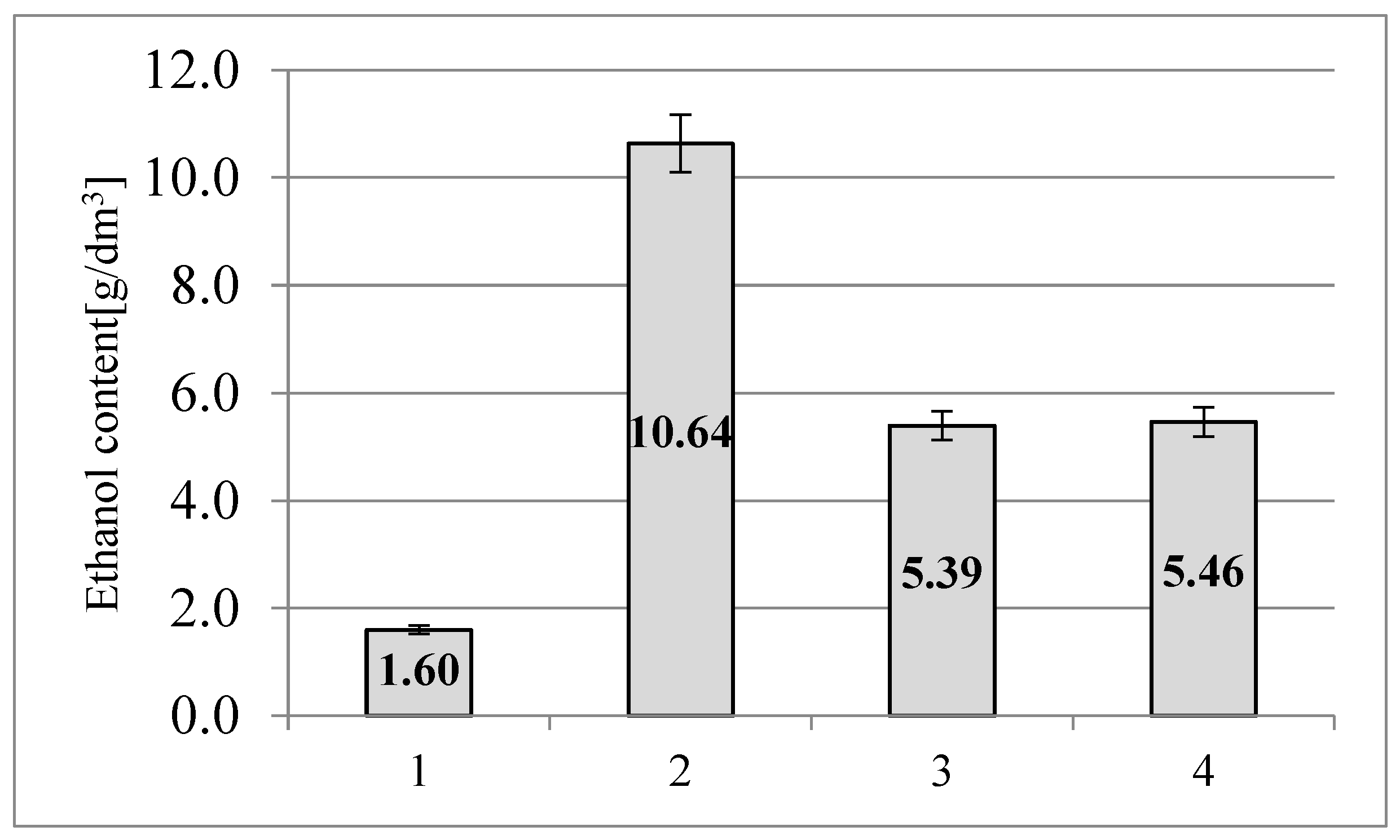
The highest concentration of ethyl alcohol (10.64 g/dm
3) was demonstrated in the sample of triticale straw purified with ionic liquid (
Figure 4). For comparison, the content of ethyl alcohol in the control sample was 1.60 g/dm
3. These differences result from the fact, that the hydrolysate of triticale straw purified with ionic liquid was characterized by a higher concentration of reducing sugars, as well as lower lignin content, which blocks the functioning of yeasts in mash.
The hydrolysate of triticale straw purified using the sulfate method, after fermentation was characterized by an ethanol content of 5.46 g/dm
3. Similar results were obtained for triticale straw samples subjected to pretreatment with recycled ionic liquid. The concentration of ethanol in this sample was 5.39 g/dm
3. The vitality of yeasts in both samples was lower than in the remaining hydrolysates (60% for sulfate method purified straw and 65% for recycled ionic liquid purified straw) (
Table 1).
Analysis of the count and vitality of yeasts performing the fermentation process allows us to conclude that the release of inhibitors in the sulfate method had a negative influence on the growth and vitality of the microorganisms engaged in the fermentation process. Similar conclusions were drawn by Szymanowska et al. [
30], who to produce bioethanol used potato pulp hydrolyzed using sulfuric acid and enzymes. The acidic hydrolysate contained 22 g/dm
3 reducing sugars, from which only 6 g/dm
3 ethanol was obtained as a result of fermentation. Furthermore, the authors determined that the cost of obtaining hydrolysates using amylases, cellulases and pectinases is not comparable to the efficiency of the process. The concentration of ethanol after fermentation of enzymatic hydrolysates was not higher than 2.5%, which, including the process costs such as separation and concentration of the final product becomes unprofitable. Perhaps the use of membrane distillation will contribute to reduction of the process costs in the future [
30]. The literature contains an increasing number of solutions aiming at purification of the substrate prior to fermentation process. The most commonly described methods include detoxification via using bases, reducing agents and polymers. Worth mentioning is the fact that screening of microorganisms immune to environmental stresses, selection of the culture method, microbial treatment, evolutionary engineering and genetic engineering are also among methods, which aim at increase of the efficiency of the use of lignocellulose [
31]. For comparison Saha et al. [
32] fermented wheat straw using
Saccharomyces cerevisiae yeast in two systems: SHF—separate hydrolysis and fermentation process SSF—simultaneous hydrolysis and fermentation process. The wheat straw was previously purified with sulfuric acid and enzymatically hydrolyzed to obtain reducing sugars. In addition, the authors subjected to fermentation a sample of wheat straw which apart from acid was also purified with lime. As a result of the conducted study it was determined that the ethanol content did not depend on the system of fermentation and only on the method of material purification. In the simultaneous hydrolysis and fermentation of the samples, which were subjected to pretreatment using sulfuric acid and enzymes, the ethanol content was 13 g/dm
3 and in the variant with additional lime purification 17 g/dm
3 ethanol was obtained.
The influence of pretreatment of lignocellulose material on the efficiency of alcoholic fermentation was also tested by Eisenhuber et al. [
33], who conducted their study on an industrial scale. They obtained ethanol from different types of straw (wheat, rye and corn). Prior to fermentation, they used pretreatment with high temperature (160–200 °C) for 10 and 20 min. Alcoholic fermentation was conducted using Saccharomyces cerevisiae at 30 °C for 168 h. In the sample of rye straw subjected to pretreatment in temperature 200 °C for 10 min 108 kg of ethanol was obtained, and the efficiency of fermentation amounted to 44% of theoretical value. For rye straw pretreated at 200 °C for a longer period (20 min) the content of ethanol was 169 kg, and the efficiency of fermentation was at 70% of the theoretical value level. Kádár et al. [
34] conducted simultaneous hydrolysis and fermentation of cellulose industrial waste (corrugated cardboard, cellulose sludge formed during paper production) originating from the Hungarian company Dunapack (Ujazd, Poland) and SOLKA FLOC 200 cellulose powder (International Fiber Corporation, New York, North Tonawanda, USA). In the samples fermented by S. cerevisiae, the ethanol content was 14.2 g/dm
3 for corrugated cardboard, 9.0 g/dm
3 for cellulose sludge and 16.6 g/dm
3 for SOLKA FLOC 200cellulose powder. In the present study, the ethanol content differed considerably. For these reasons, an attempt was made to demonstrate the relationship between ethanol concentration and the hydrolysate type subjected to alcoholic fermentation, for which the PCA method was used. The active variables consisted of the concentration of ethyl alcohol obtained after fermentation and the grouping variable was type of pretreatment of triticale straw, which influenced not only the reducing sugars concentration after enzymatic hydrolysis, but also the content of ethanol after fermentation. In this case, the PCA analysis was also conducted based on a correlation matrix. From the analyzed data two first coefficients with values of above >1 were generated, characterizing hydrolysate samples in terms of similarities and differences. In this case the main component Z1 explained as much as 96.95% of the total variability (
Figure 5).
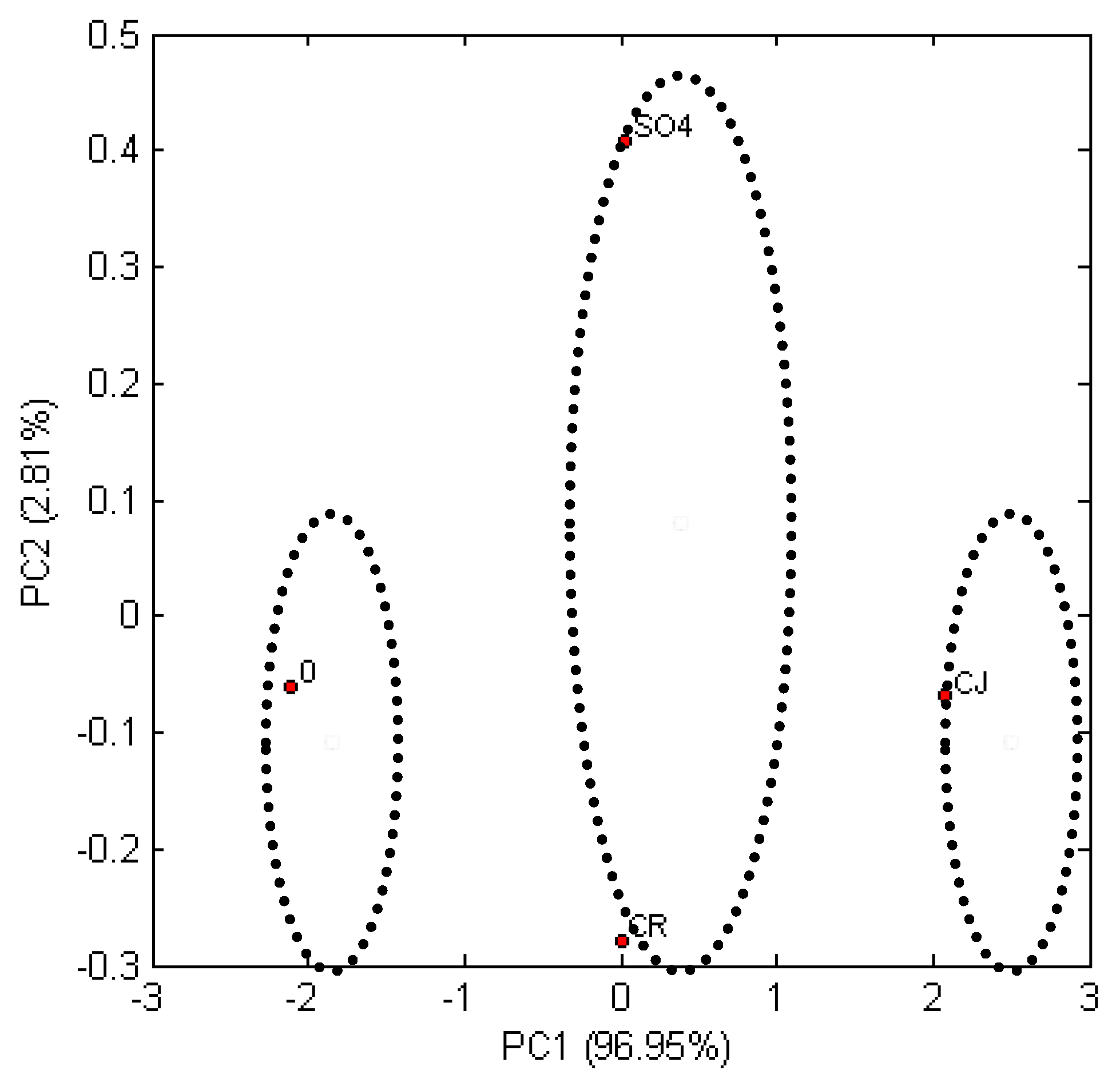
Figure 6 presents the influence of the used pretreatment of triticale straw on the ethanol concentration after fermentation. The location of grouping variables is presented in a new coordinate system, defined by the Z1 and Z2 components. The measure of resemblance in this statistical method is the Euclidean distance. The coefficient loads for the Z1 component indicated its relationship with the ethanol concentration values in the tested hydrolysates. Classification using the PCA method allowed re-establishing three groups of samples subjected to fermentation. The first group consisted of control samples of triticale straw not subjected to pretreatment, which gave the lowest ethanol concentration. The second group consisted of triticale straw samples purified with recycled ionic liquid (CR) and samples of triticale straw purified using the sulfate method (SO
4). The third group— triticale straw samples purified with ionic liquid (C)—were the samples with the highest ethanol content.
The most extreme location on the observation chart is taken by the samples marked as ‘0′ and ‘CJ’. This indicates the greatest differences between ethanol concentrations in these samples, which is linked to the use of pretreatment with ionic liquid. The highest increase of ethanol was observed for triticale straw subjected to ionic liquid treatment, the Euclidean distances of the remaining samples in relation to the control sample of triticale straw (0) are similar.
Thus, the ethanol concentration in these samples has a similar level and it is strictly related to the content of reducing sugars in the mashes prior alcoholic fermentation. The PCA analysis results demonstrated that the content of reducing sugars in the samples of triticale straw is influenced by the use of ionic liquid for the pretreatment of these materials. The results of ethanol concentration in mashes are also highest for the samples purified with pure ionic liquid than in the samples, where white bleach is used for the treatment (sulfate method).