Review of Core/Shell Quantum Dots Technology Integrated into Building’s Glazing
Abstract
1. Introduction
2. Characteristics of Core/Shell QDs
2.1. Types of Core/Shell QDs Based on Band Gap
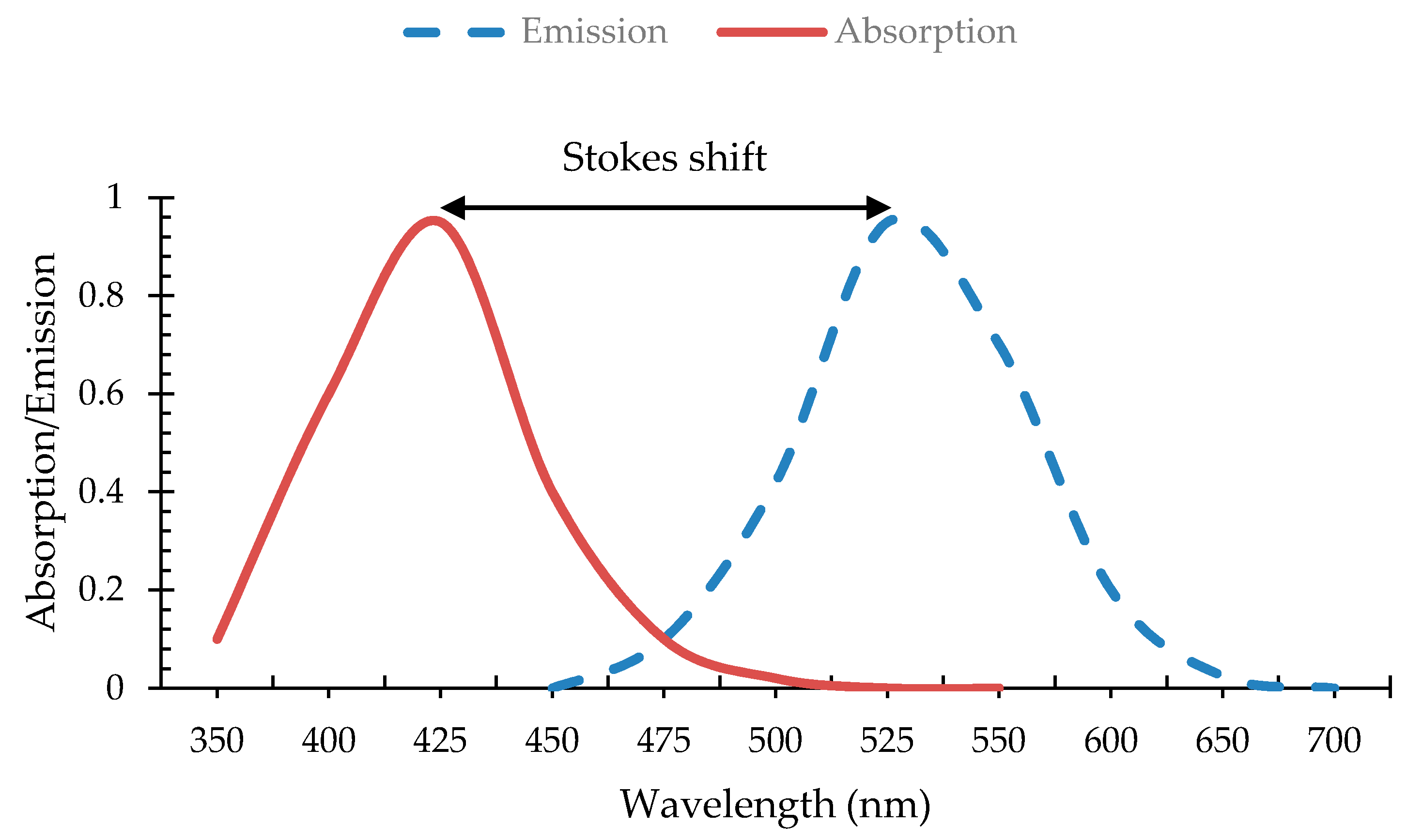
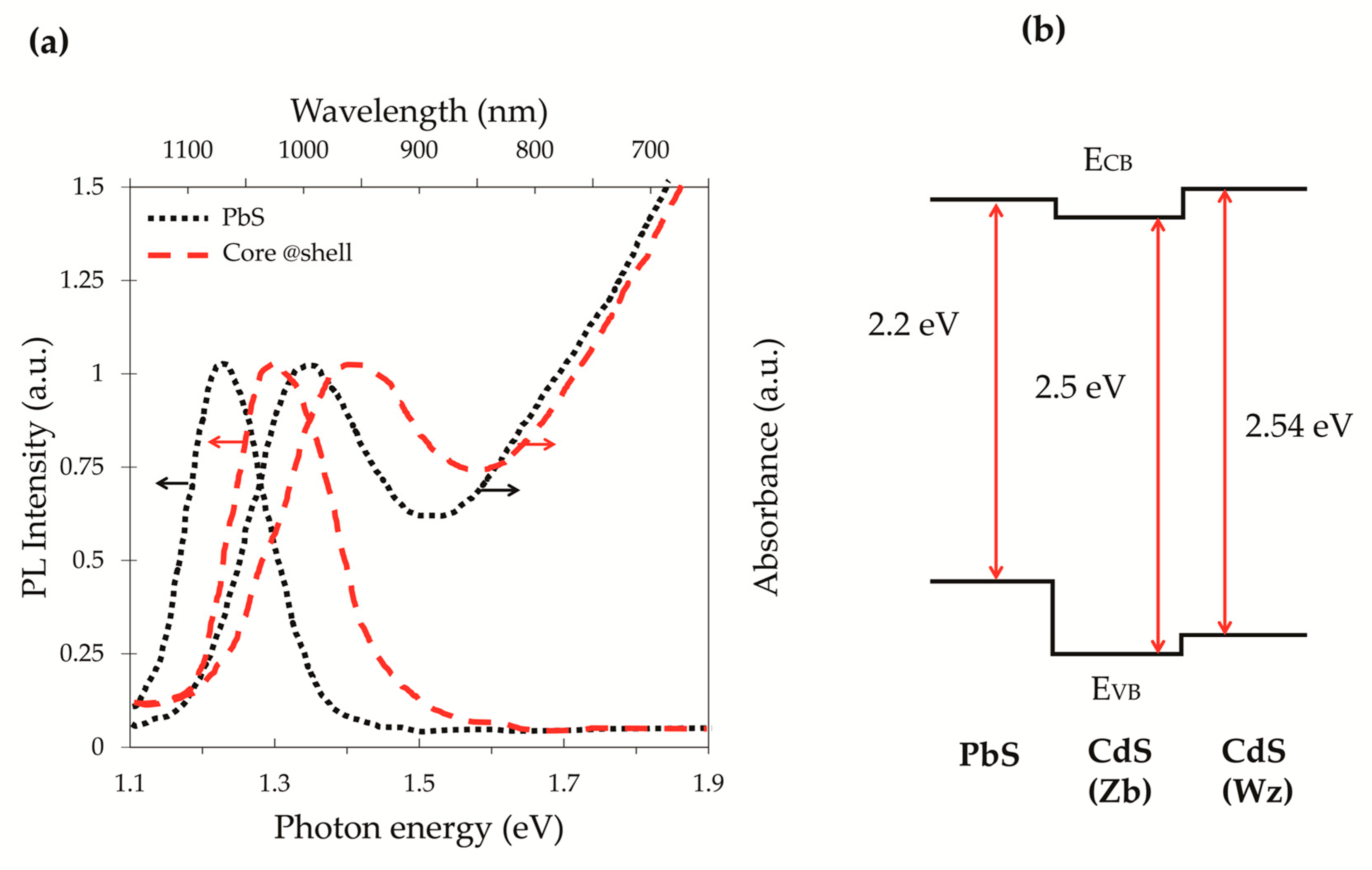
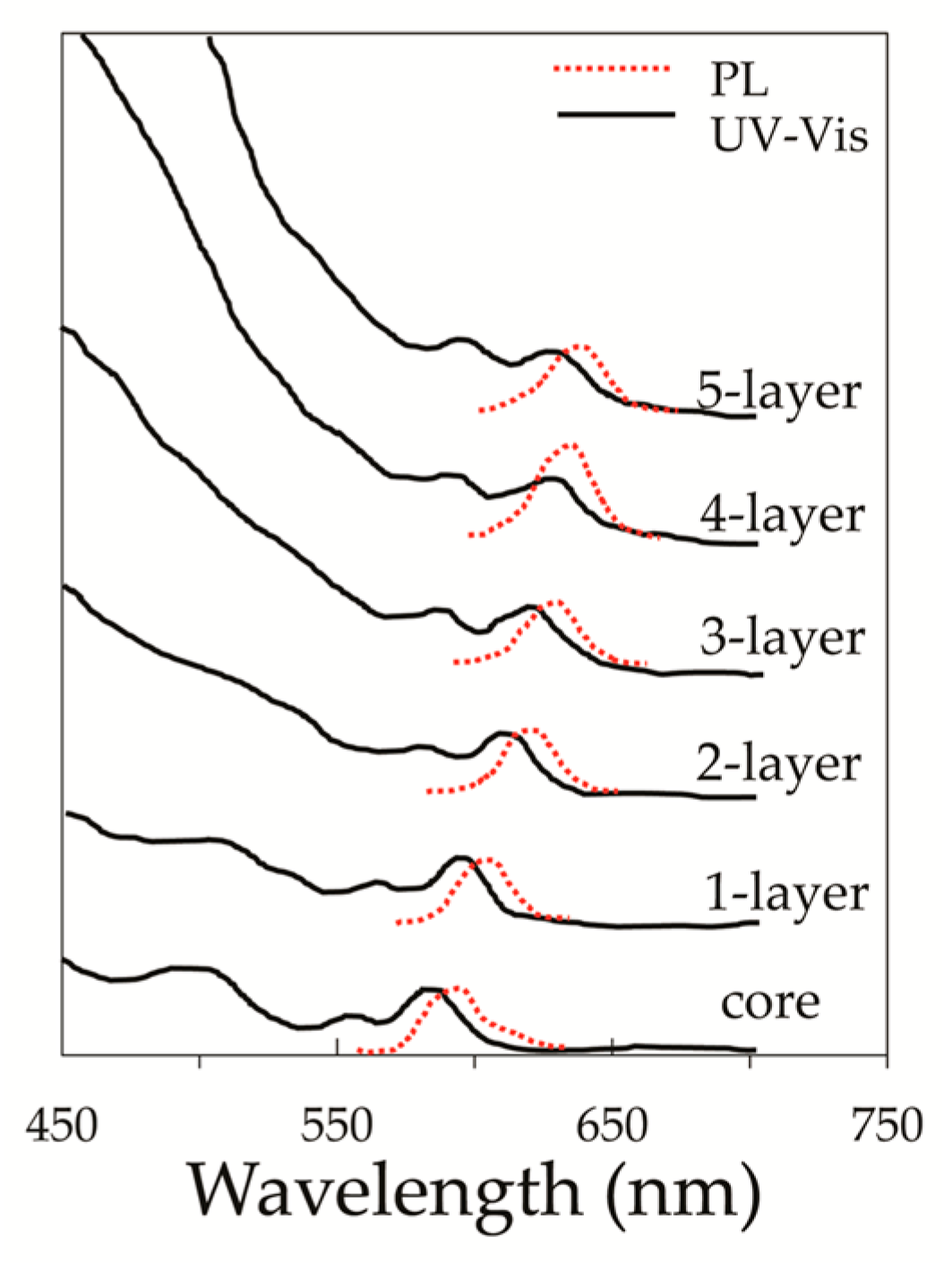
2.2. Stocks Shift and Absorption and Emission Properties of Core/Shell QDs
3. QDs as Concentrators
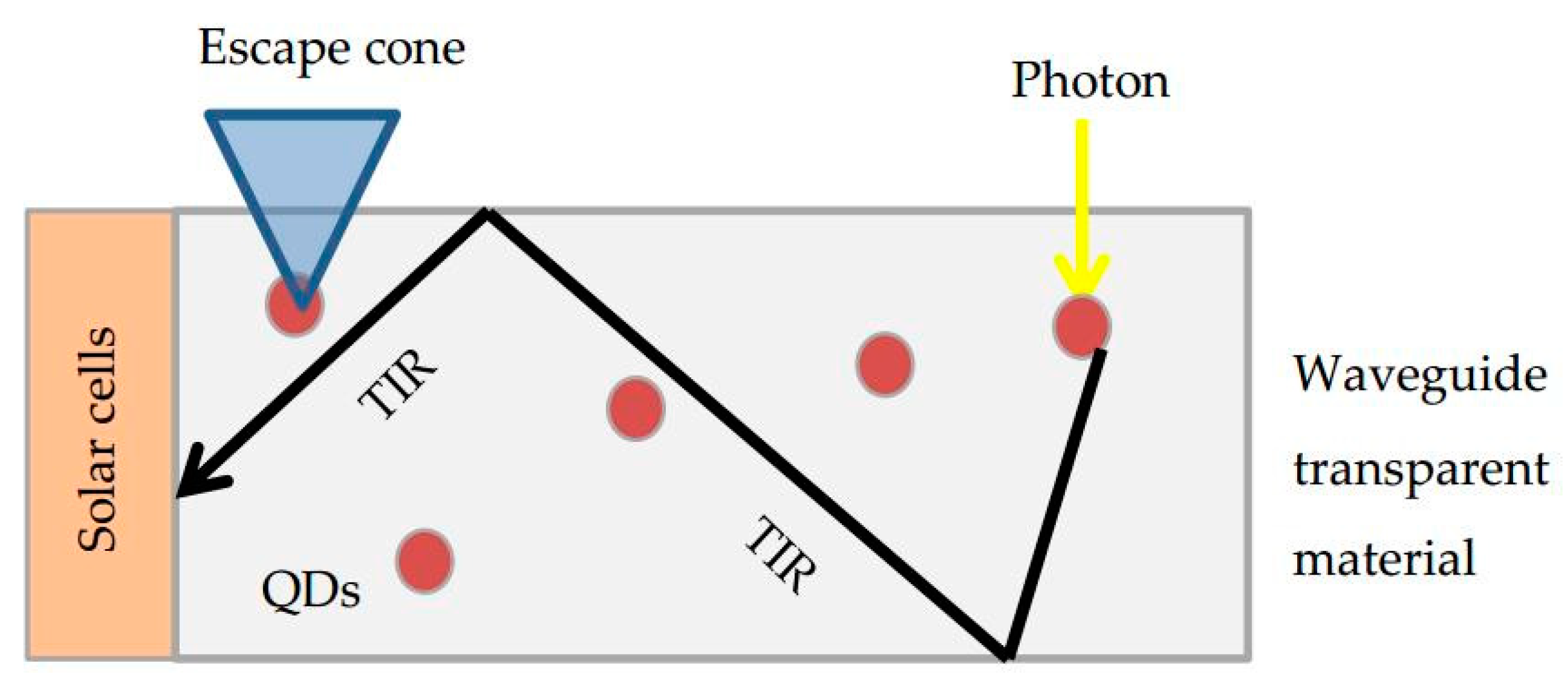
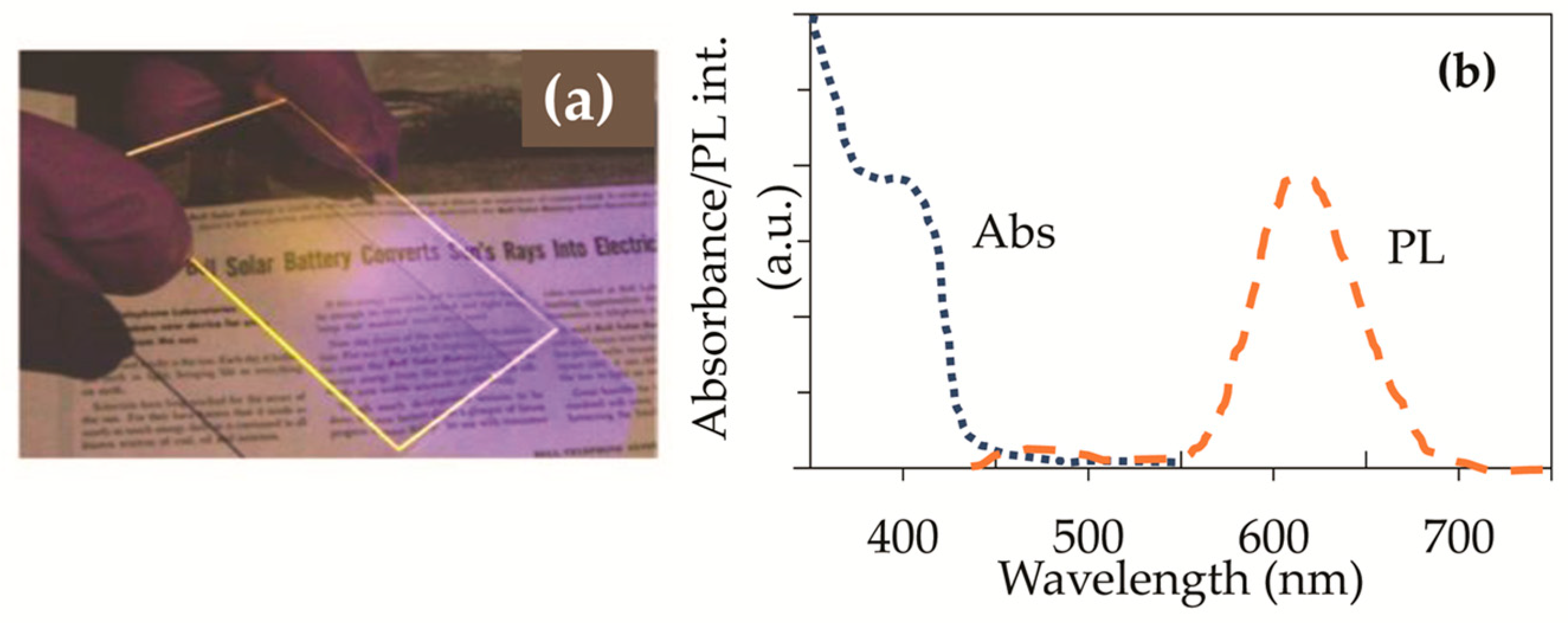
3.1. Luminescent Solar Concentrators Based on Quantum Dots (QD-LSC)
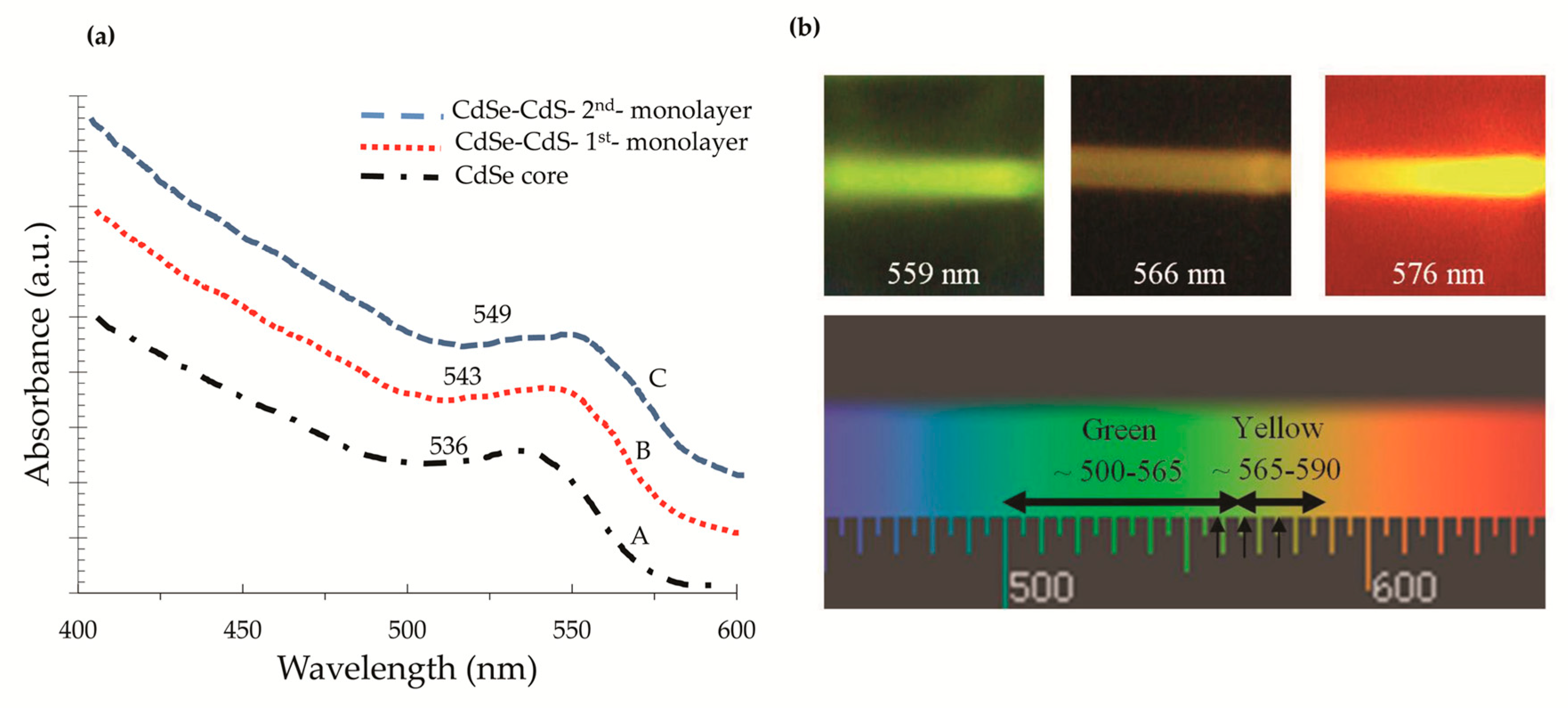
3.1.1. LSC with Type I core/shell QDs
3.1.2. LSC with Type II and Inverse Type II Core/Shell QDs
3.1.3. LSC with Type I-III-VI2 Core/Shell QDs
4. Building Integrated Applications of QDs
5. Conclusions
- Increasing the shell thickness and reducing the core size helps to enhance the optical properties of all types of core-shell QDs by widening their absorption and emission spectrum;
- Type II, inverse Type II, and type I-III-VI2 are preferred over type I because of their NIR emissions that can reach up to 800 nm; which can assist in utilizing the full solar spectrum;
- Improving the properties of core-shell QDs, such as having large stokes shift, better stability and QY, and wider absorption could be achieved via adding a number of ions to any type of the core-shell QDs;
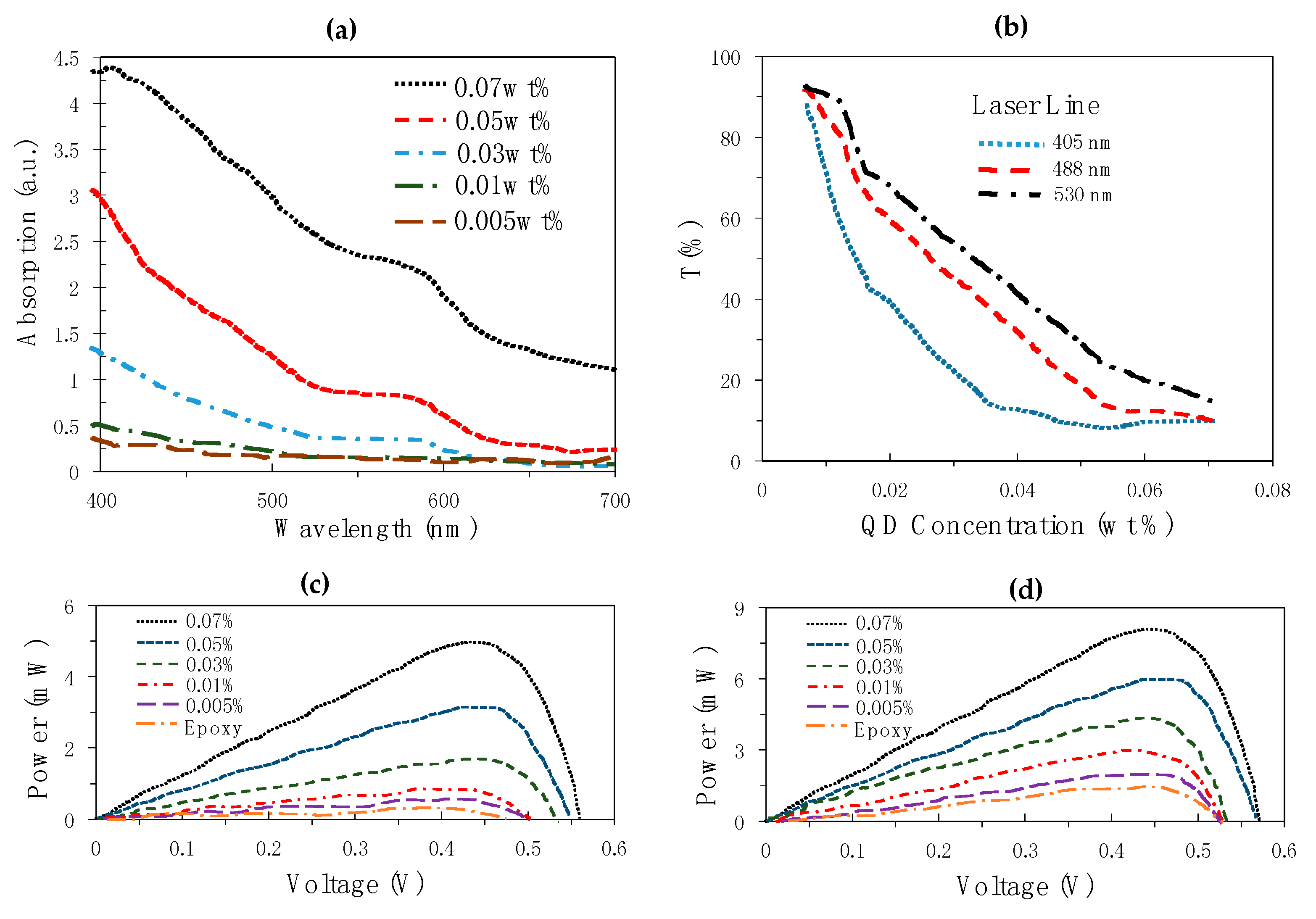
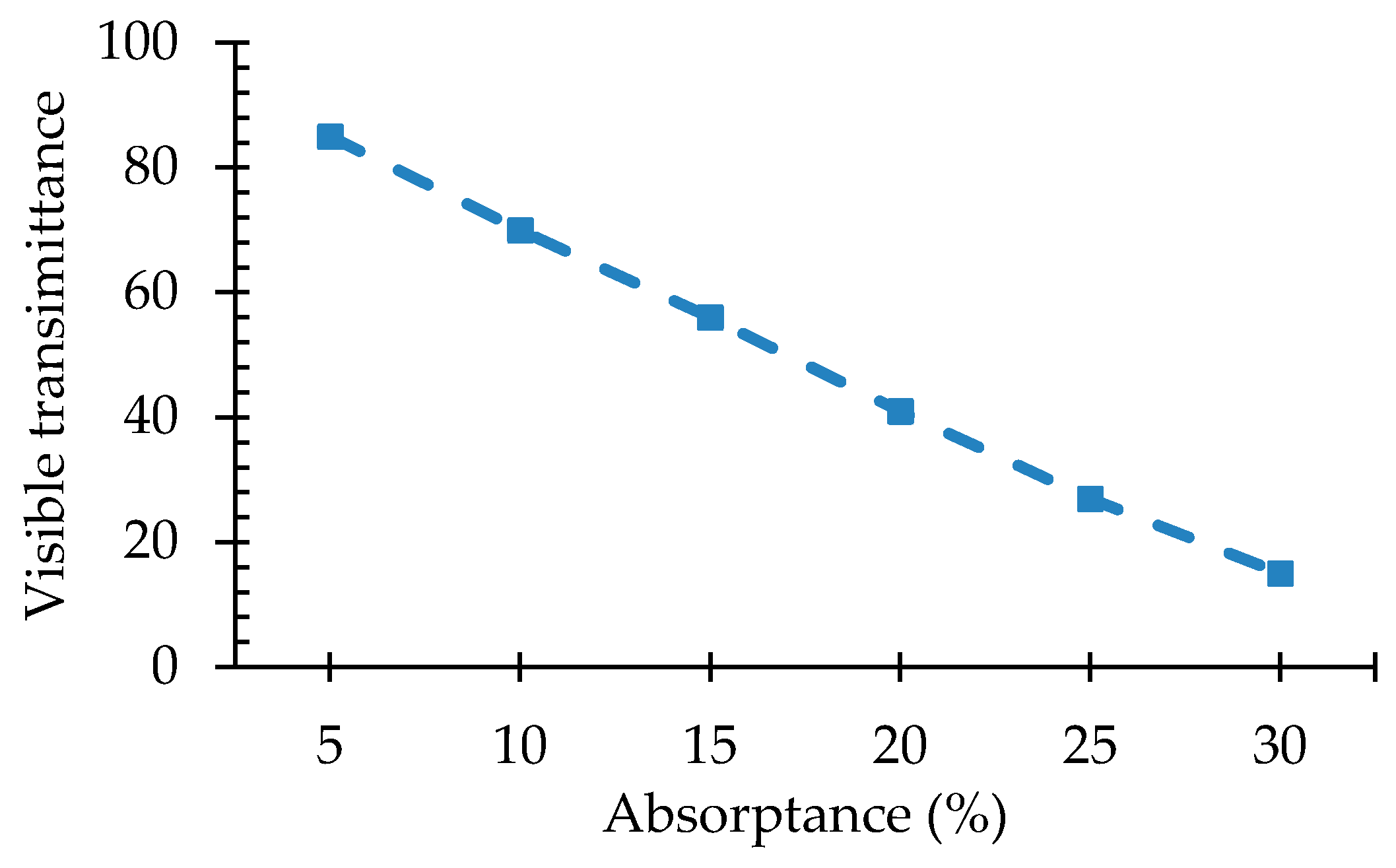
- While increasing the concentration has the advantage of enhancing the power production of the LSC technology, it also leads to some disadvantages: It reduces the number of photons reaching the edge of the system, it reduces the transparency of the material and it increases the chances of re-absorption thus leading to energy losses;
- While the inverse Type II has the advantage of being an excellent emitter of NIR emissions, it requires high concentrations to absorb a wide range of the solar spectrum. This high concentration causes re-absorptions of photons, reduces the electricity production of the system, and reduces the transparency of the LSC;
- Type I-II-VI2 are non-toxic materials that have low manufacture cost, neutral-density filter, high coverage of the solar spectrum, and low recombination of photons; which make them very suitable to enhance the power performance, the visibility, and the esthetic appearance on buildings’ facades (windows/skylights);
- It is crucial to consider both the optical characteristics and the electrical output when optimizing the size and the concentration of the QD in the QD-LSC technology.
Author Contributions
Funding
Conflicts of Interest
Nomenclature
| QDs | Quantum dots |
| UV | Ultraviolet radiations |
| Vis | Visible light |
| NIR | Near infrared radiations |
| TIR | Total internal reflection |
| BIPV | Building integrated photovoltaic |
| QY | Quantum yield |
| PL | The photoluminescence |
| LSC | Luminescent solar concentrators |
| QDs-LSC | Quantum dots luminescent solar concentrators |
| Si PV | Silicon photovoltaic |
| QDSC | Quantum dots solar concentrators |
| EQE | The external quantum efficiency of the solar cells |
| PCE | The power conversion efficiency of the solar cells |
| CRI | The color rendering index |
| CCT | Correlated color temperature |
| Zb | A zinc blende phase CdS |
| Wz | A wurtzite CdS shell |
| ECB | Energy in the conduction band |
| EVB | Energy in the valence band |
| Eg | Energy gap |
| eV | Unit of energy |
| a.u. | Unit of absorbance |
| T | Transmission |
| QE | Quantum efficiency |
References
- Carmody, J.; Selkowitz, S.; Lee, E.; Arasteh, D.; Willmert, T. Window System for High-Performance Buildings, 1st ed.; W. W. Norton & Company: New York, NY, USA, 2004. [Google Scholar]
- Selkowitz, S.; Aschehoug, O.; Lee, E.S. Advanced interactive facades-critical elements for future green buildings? In Proceedings of the GreenBuild, the Annual USGBC International Conference and Expo, Pittsburgh, PA, USA, 12–14 November 2003. [Google Scholar]
- Debije, M.G.; Verbunt, P.P.C. Thirty years of luminescent solar concentrator research: Solar energy for the built environment. Adv. Energy Mater. 2012, 2, 12–35. [Google Scholar] [CrossRef]
- Shcherbatyuk, G.V.; Inman, R.H.; Wang, C.; Winston, R.; Ghosh, S. Viability of using near infrared PbS quantum dots as active materials in luminescent solar concentrators. Appl. Phys. Lett. 2010, 96, 191901. [Google Scholar] [CrossRef]
- Regulacio, M.D.; Han, M.-Y. Composition-tunable alloyed B1semiconductor nanocrystals. Acc. Chem. Res. 2010, 43, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Benetti, D.; Jin, L.; Zhou, Y.; Rosei, F.; Vomiero, A. Absorption Enhancement in “Giant” Core/Alloyed-Shell Quantum Dots for Luminescent Solar Concentrator. Small 2016, 12, 5354–5365. [Google Scholar] [CrossRef] [PubMed]
- Shao, H.; Bai, X.; Cui, H.; Pan, G.; Jing, P.; Qu, S.; Zhu, J.; Zhai, Y.; Dong, B.; Song, H. White light emission in Bi3+/Mn2+ ion co-doped CsPbCl3 perovskite nanocrystals. Nanoscale 2018, 10, 1023–1029. [Google Scholar] [CrossRef] [PubMed]
- Renuga, V.; Manikandan, A. Influence of Mn2+ ions on both core/shell of CuInS2/ZnS nanocrystals. Mater. Res. Bull. 2018, 98, 265–274. [Google Scholar]
- Levchuk, I.; Würth, C.; Krause, F.; Osvet, A.; Batentschuk, M.; Resch-Genger, U.; Kolbeck, C.; Herre, P.; Steinrück, H.P.; Peukert, W. Industrially scalable and cost-effective Mn 2+ doped Zn x Cd 1− x S/ZnS nanocrystals with 70% photoluminescence quantum yield, as efficient down-shifting materials in photovoltaics. Energy Environ. Sci. 2016, 9, 1083–1094. [Google Scholar] [CrossRef]
- Goetzberger, A.; Wittwer, V. Fluorescent planar collector-concentrators: A review. Sol. Cells 1981, 4, 3–23. [Google Scholar] [CrossRef]
- Wittwer, V.; Heidler, K.; Zastrow, A.; Goetzberger, A. Theory of fluorescent planar concentrators and experimental results. J. Lumin. 1981, 24, 873–876. [Google Scholar] [CrossRef]
- Bradshaw, L.R.; Knowles, K.E.; McDowall, S.; Gamelin, D.R. Nanocrystals for luminescent solar concentrators. Nano Lett. 2015, 15, 1315–1323. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wang, Q.; Shi, X.; Hornak, L.A.; Wu, N. Detection of mercury (II) by quantum dot/DNA/gold nanoparticle ensemble based nanosensor via nanometal surface energy transfer. Anal. Chem. 2011, 83, 7061–7065. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Liang, H.; Gonfa, B.A.; Chaker, M.; Ozaki, T.; Tijssen, P.; Vidal, F.; Ma, D. Investigating photoinduced charge transfer in double-and single-emission PbS@ CdS core@ shell quantum dots. Nanoscale 2014, 6, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Kemp, K.W.; Hoogland, S.; Jeong, K.S.; Liu, H.; Levina, L.; Furukawa, M.; Wang, X.; Debnath, R.; Cha, D. Colloidal-quantum-dot photovoltaics using atomic-ligand passivation. Nat. Mater. 2011, 10, 765. [Google Scholar] [CrossRef] [PubMed]
- Nozik, A.J.; Beard, M.C.; Luther, J.M.; Law, M.; Ellingson, R.J.; Johnson, J.C. Semiconductor quantum dots and quantum dot arrays and applications of multiple exciton generation to third-generation photovoltaic solar cells. Chem. Rev. 2010, 110, 6873–6890. [Google Scholar] [CrossRef] [PubMed]
- Sambur, J.B.; Novet, T.; Parkinson, B.A. Multiple exciton collection in a sensitized photovoltaic system. Science 2010, 330, 63–66. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Chaker, M.; Ma, D. Effect of CdS shell thickness on the optical properties of water-soluble, amphiphilic polymer-encapsulated PbS/CdS core/shell quantum dots. J. Mater. Chem. 2011, 21, 17483–17491. [Google Scholar] [CrossRef]
- Lambert, K.; Geyter, B.D.; Moreels, I.; Hens, Z. PbTe|CdTe core|shell particles by cation exchange, a HR-TEM study. Chem. Mater. 2009, 21, 778–780. [Google Scholar] [CrossRef]
- Huang, K.; Demadrille, R.; Silly, M.G.; Sirotti, F.; Reiss, P.; Renault, O. Internal structure of InP/ZnS nanocrystals unraveled by high-resolution soft X-ray photoelectron spectroscopy. ACS Nano 2010, 4, 4799–4805. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Bai, J.; Gu, X.; Chang, C.; Shen, H.; Zhang, Q.; Li, F.; Chen, Z.; Li, Q. Efficient light-emitting diodes based on reverse type-I quantum dots. Opt. Mater. Express 2017, 7, 4395–4407. [Google Scholar] [CrossRef]
- Vasudevan, D.; Gaddam, R.R.; Trinchi, A.; Cole, I. Core-shell quantum dots: Properties and Applications. J. Alloys Compd. 2015, 636, 395–404. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, S.-N.; Li, J.-J.; Zhao, J.-W. The effect of core size on the fluorescence emission properties of CdTe@ CdS core@ shell quantum dots. J. Lumin. 2018, 199, 216–224. [Google Scholar] [CrossRef]
- Chuang, C.-H.; Lo, S.S.; Scholes, G.D.; Burda, C. Charge separation and recombination in CdTe/CdSe core/shell nanocrystals as a function of shell coverage: Probing the onset of the quasi type-II regime. J. Phys. Chem. Lett. 2010, 1, 2530–2535. [Google Scholar] [CrossRef]
- Dabbousi, B.O.; Rodriguez-Viejo, J.; Mikulec, F.V.; Heine, J.R.; Mattoussi, H.; Ober, R.; Jensen, K.F.; Bawendi, M.G. (CdSe) ZnS core-shell quantum dots: Synthesis and characterization of a size series of highly luminescent nanocrystallites. J. Phys. Chem. B 1997, 101, 9463–9475. [Google Scholar] [CrossRef]
- Pal, B.N.; Ghosh, Y.; Brovelli, S.; Laocharoensuk, R.; Klimov, V.I.; Hollingsworth, J.A.; Htoon, H. ‘Giant’CdSe/CdS core/shell nanocrystal quantum dots as efficient electroluminescent materials: Strong influence of shell thickness on light-emitting diode performance. Nano Lett. 2011, 12, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Basu, K.; Zhang, H.; Zhao, H.; Bhattacharya, S.; Navarro-Pardo, F.; Datta, P.K.; Jin, L.; Sun, S.; Vetrone, F.; Rosei, F. Highly stable photoelectrochemical cells for hydrogen production using a SnO 2–TiO 2/quantum dot heterostructured photoanode. Nanoscale 2018, 10, 15273–15284. [Google Scholar] [CrossRef] [PubMed]
- Bakhoda, S.; Assadi, M.K.; Ahmadi_Kandjani, S.; Al_Kayiem, H.H.; Bhat, A.H. Application of Quantum Dot nanocrystal in Luminescent solar concentrators. In Proceedings of the 3rd International Conference on Mechanical, Manufacturing and Process Plant Engineering (ICMMPE 2017), Batu Ferringhi, Penang, Malaysia, 22–23 November 2017; p. 012022. [Google Scholar]
- Ramanery, F.P.; Mansur, A.A.P.; Mansur, H.S. Synthesis and characterization of water-dispersed CdSe/CdS core-shell quantum dots prepared via layer-by-layer method capped with carboxylic-functionalized poly (vinyl alcohol). Mater. Res. 2014, 17, 133–140. [Google Scholar] [CrossRef]
- Pan, Z.; Zhang, H.; Cheng, K.; Hou, Y.; Hua, J.; Zhong, X. Highly efficient inverted type-I CdS/CdSe core/shell structure QD-sensitized solar cells. ACS Nano 2012, 6, 3982–3991. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Park, J.; Kim, T.; Jang, E.; Jun, S.; Jang, H.; Kim, B.; Kim, S.W. Reverse Type-I ZnSe/InP/ZnS Core/Shell/Shell Nanocrystals: Cadmium-Free Quantum Dots for Visible Luminescence. Small 2011, 7, 70–73. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Fisher, B.; Eisler, H.-J.; Bawendi, M. Type-II quantum dots: CdTe/CdSe (core/shell) and CdSe/ZnTe (core/shell) heterostructures. J. Am. Chem. Soc. 2003, 125, 11466–11467. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Song, N.; Liu, Z.; Zhu, H.; Rodríguez-Córdoba, W.; Lian, T. Interfacial charge separation and recombination in InP and quasi-type II InP/CdS core/shell quantum dot-molecular acceptor complexes. J. Phys. Chem. A 2013, 117, 7561–7570. [Google Scholar] [CrossRef] [PubMed]
- Reiss, P.; Protiere, M.; Li, L. Core/shell semiconductor nanocrystals. Small 2009, 5, 154–168. [Google Scholar] [CrossRef] [PubMed]
- Berends, A.C.; Rabouw, F.T.; Spoor, F.C.M.; Bladt, E.; Grozema, F.C.; Houtepen, A.J.; Siebbeles, L.D.A.; de Mello Donegá, C. Radiative and nonradiative recombination in CuInS2 nanocrystals and CuInS2-based core/shell nanocrystals. J. Phys. Chem. Lett. 2016, 7, 3503–3509. [Google Scholar] [CrossRef] [PubMed]
- Rice, W.D.; McDaniel, H.; Klimov, V.I.; Crooker, S.A. Magneto-optical properties of CuInS2 nanocrystals. J. Phys. Chem. Lett. 2014, 5, 4105–4109. [Google Scholar] [CrossRef] [PubMed]
- Meinardi, F.; McDaniel, H.; Carulli, F.; Colombo, A.; Velizhanin, K.A.; Makarov, N.S.; Simonutti, R.; Klimov, V.I.; Brovelli, S. Highly efficient large-area colourless luminescent solar concentrators using heavy-metal-free colloidal quantum dots. Nat. Nanotechnol. 2015, 10, 878–885. [Google Scholar] [CrossRef] [PubMed]
- Schuster, M.; Sisterhenn, P.; Graf, L.; Wellmann, P.J. Processing and Characterization of Vacuum-Free CuInSe2 Thin Films from Nanoparticle-Precursors using Novel Temperature Treatment Techniques. J. Nanopart. Res. 2018, 2, 4. [Google Scholar]
- Erickson, C.S. Doped Quantum Dot Luminescent Solar Concentrators. Master’s Thesis, Western Washington University, Bellingham, WA, USA, 2014. [Google Scholar]
- Tanabe, K. A review of ultrahigh efficiency III-V semiconductor compound solar cells: Multijunction tandem, lower dimensional, photonic up/down conversion and plasmonic nanometallic structures. Energies 2009, 2, 504–530. [Google Scholar] [CrossRef]
- Jin, L.; AlOtaibi, B.; Benetti, D.; Li, S.; Zhao, H.; Mi, Z.; Vomiero, A.; Rosei, F. Near-Infrared Colloidal Quantum Dots for Efficient and Durable Photoelectrochemical Solar-Driven Hydrogen Production. Adv. Sci. 2016, 3, 1500345. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Sirigu, G.; Parisini, A.; Camellini, A.; Nicotra, G.; Rosei, F.; Morandi, V.; Zavelani-Rossi, M.; Vomiero, A. Dual emission in asymmetric “giant” PbS/CdS/CdS core/shell/shell quantum dots. Nanoscale 2016, 8, 4217–4226. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Benetti, D.; Fan, Z.; Zhao, H.; Ma, D.; Govorov, A.O.; Vomiero, A.; Rosei, F. Near infrared, highly efficient luminescent solar concentrators. Adv. Energy Mater. 2016, 6, 1501913. [Google Scholar] [CrossRef]
- Hines, M.A.; Scholes, G.D. Colloidal PbS nanocrystals with size-tunable near-infrared emission: Observation of post-synthesis self-narrowing of the particle size distribution. Adv. Mater. 2003, 15, 1844–1849. [Google Scholar] [CrossRef]
- Zhao, H.; Chaker, M.; Wu, N.; Ma, D. Towards controlled synthesis and better understanding of highly luminescent PbS/CdS core/shell quantum dots. J. Mater. Chem. 2011, 21, 8898–8904. [Google Scholar] [CrossRef]
- Batchelder, J.S.; Zewai, A.H.; Cole, T. Luminescent solar concentrators. 1: Theory of operation and techniques for performance evaluation. Appl. Opt. 1979, 18, 3090–3110. [Google Scholar] [CrossRef] [PubMed]
- Richards, B.S. Enhancing the performance of silicon solar cells via the application of passive luminescence conversion layers. Sol. Energy Mater. Sol. Cells 2006, 90, 2329–2337. [Google Scholar] [CrossRef]
- Richards, B.S.; Shalav, A.; Corkish, R.P. A low escape-cone-loss luminescent solar concentrator. In Proceedings of the 19th European Photovoltaic Solar Energy Conference, Paris, France, 7–11 June 2004; pp. 113–116. [Google Scholar]
- McIntosh, K.R.; Yamada, N.; Richards, B.S. Theoretical comparison of cylindrical and square-planar luminescent solar concentrators. Appl. Phys. B 2007, 88, 285–290. [Google Scholar] [CrossRef]
- Bornstein, J.G. Luminescent Solar Concentrator Daylighting. In Proceedings of the 28th Annual Technical Symposium, San Diego, CA, USA, 27 July–1 August 2014; p. 8. [Google Scholar]
- Earp, A.A.; Smith, G.B.; Franklin, J.; Swift, P. Optimisation of a three-colour luminescent solar concentrator daylighting system. Sol. Energy Mater. Sol. Cells 2004, 84, 411–426. [Google Scholar] [CrossRef]
- Wilson, L.R. Luminescent Solar Concentrators: A Study of Optical Properties, Re-Absorption and Device Optimisation. Ph.D. Thesis, Herior-Watt University Edingurgh, Edingurgh, UK, 2010. [Google Scholar]
- Zhang, Q.; Guo, X.; Huang, X.; Huang, S.; Li, D.; Luo, Y.; Shen, Q.; Toyoda, T.; Meng, Q. Highly efficient CdS/CdSe-sensitized solar cells controlled by the structural properties of compact porous TiO2 photoelectrodes. Phys. Chem. Chem. Phys. 2011, 13, 4659–4667. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.-Y.; Liao, J.-Y.; Qiu, K.-Q.; Kuang, D.-B.; Su, C.-Y. Dynamic study of highly efficient CdS/CdSe quantum dot-sensitized solar cells fabricated by electrodeposition. ACS Nano 2011, 5, 9494–9500. [Google Scholar] [CrossRef] [PubMed]
- Kongkanand, A.; Tvrdy, K.; Takechi, K.; Kuno, M.; Kamat, P.V. Quantum dot solar cells. Tuning photoresponse through size and shape control of CdSe− TiO2 architecture. J. Am. Chem. Soc. 2008, 130, 4007–4015. [Google Scholar] [CrossRef] [PubMed]
- Cossairt, B.M.; Owen, J.S. CdSe clusters: At the interface of small molecules and quantum dots. Chem. Mater. 2011, 23, 3114–3119. [Google Scholar] [CrossRef]
- Meinardi, F.; Colombo, A.; Velizhanin, K.A.; Simonutti, R.; Lorenzon, M.; Beverina, L.; Viswanatha, R.; Klimov, V.I.; Brovelli, S. Large-area luminescent solar concentrators based on ‘Stokes-shift-engineered’nanocrystals in a mass-polymerized PMMA matrix. Nat. Photonics 2014, 8, 392–399. [Google Scholar] [CrossRef]
- Chandra, S.; McCormack, S.J.; Kennedy, M.; Doran, J. Quantum dot solar concentrator: Optical transportation and doping concentration optimization. Sol. Energy 2015, 115, 552–561. [Google Scholar] [CrossRef]
- Bomm, J.; Büchtemann, A.; Chatten, A.J.; Bose, R.; Farrell, D.J.; Chan, N.L.A.; Xiao, Y.; Slooff, L.H.; Meyer, T.; Meyer, A. Fabrication and full characterization of state-of-the-art quantum dot luminescent solar concentrators. Sol. Energy Mater. Sol. Cells 2011, 95, 2087–2094. [Google Scholar] [CrossRef]
- Erickson, C.S.; Bradshaw, L.R.; McDowall, S.; Gilbertson, J.D.; Gamelin, D.R.; Patrick, D.L. Zero-reabsorption doped-nanocrystal luminescent solar concentrators. ACS Nano 2014, 8, 3461–3467. [Google Scholar] [CrossRef] [PubMed]
- Beaulac, R.; Archer, P.I.; Gamelin, D.R. Luminescence in colloidal Mn2+-doped semiconductor nanocrystals. J. Solid State Chem. 2008, 181, 1582–1589. [Google Scholar] [CrossRef]
- de Mello Donegá, C. Synthesis and properties of colloidal heteronanocrystals. Chem. Soc. Rev. 2011, 40, 1512–1546. [Google Scholar] [CrossRef] [PubMed]
- González-Pedro, V.; Sima, C.; Marzari, G.; Boix, P.P.; Giménez, S.; Shen, Q.; Dittrich, T.; Mora-Seró, I. High performance PbS Quantum Dot Sensitized Solar Cells exceeding 4% efficiency: The role of metal precursors in the electron injection and charge separation. Phys. Chem. Chem. Phys. 2013, 15, 13835–13843. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-W.; Son, D.-Y.; Ahn, T.K.; Shin, H.-W.; Kim, I.Y.; Hwang, S.-J.; Ko, M.J.; Sul, S.; Han, H.; Park, N.-G. Quantum-dot-sensitized solar cell with unprecedentedly high photocurrent. Sci. Rep. 2013, 3, 1050. [Google Scholar] [CrossRef] [PubMed]
- Pietryga, J.M.; Schaller, R.D.; Werder, D.; Stewart, M.H.; Klimov, V.I.; Hollingsworth, J.A. Pushing the band gap envelope: Mid-infrared emitting colloidal PbSe quantum dots. J. Am. Chem. Soc. 2004, 126, 11752–11753. [Google Scholar] [CrossRef] [PubMed]
- Lai, L.-H.; Protesescu, L.; Kovalenko, M.V.; Loi, M.A. Sensitized solar cells with colloidal PbS-CdS core-shell quantum dots. Phys. Chem. Chem. Phys. 2014, 16, 736–742. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Leventis, H.C.; Moon, S.J.; Chen, P.; Ito, S.; Haque, S.A.; Torres, T.; Nüesch, F.; Geiger, T.; Zakeeruddin, S.M. PbS and CdS quantum dot-sensitized solid-state solar cells: “Old concepts, new results”. Adv. Funct. Mater. 2009, 19, 2735–2742. [Google Scholar] [CrossRef]
- Wu, K.; Li, H.; Klimov, V.I. Tandem luminescent solar concentrators based on engineered quantum dots. Nat. Photonics 2018, 12, 105–110. [Google Scholar] [CrossRef]
- Purcell-Milton, F.; Gun’ko, Y.K. Quantum dots for Luminescent Solar Concentrators. J. Mater. Chem. 2012, 22, 16687–16697. [Google Scholar] [CrossRef]
- Neo, D.C.J.; Cheng, C.; Stranks, S.D.; Fairclough, S.M.; Kim, J.S.; Kirkland, A.I.; Smith, J.M.; Snaith, H.J.; Assender, H.E.; Watt, A.A.R. Influence of shell thickness and surface passivation on PbS/CdS core/shell colloidal quantum dot solar cells. Chem. Mater. 2014, 26, 4004–4013. [Google Scholar] [CrossRef]
- Liu, M.; Voznyy, O.; Sabatini, R.; Pelayo Garcı’a de Arquer, F.; Munir, R.; Balawi, A.H.; Lan, X.; Fan, F.; Walters, G.; Kirmani, A.R.; et al. Hybrid organic-inorganic inks flatten the energy landscape in colloidal quant. Nat. Mater. 2017, 16, 258. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Du, Z.; Hu, J.-S.; Pan, Z.; Shen, Q.; Sung, J.; Long, D.; Dong, H.; Sun, L.; Zhong, X.; et al. Zn-Cu-In-Se quantum dot solar cells with a certified power conversion efficiency of 11.6%. J. Am. Chem. Soc. 2016, 138, 4201–4209. [Google Scholar] [CrossRef] [PubMed]
- Bergren, M.R.; Makarov, N.S.; Ramasamy, K.; Jackson, A.; Guglielmetti, R.; McDaniel, H. High-Performance CuInS2 Quantum Dot Laminated Glass Luminescent Solar Concentrators for Windows. ACS Energy Lett. 2018, 3, 520–525. [Google Scholar] [CrossRef]
- Li, C.; Chen, W.; Wu, D.; Quan, D.; Zhou, Z.; Hao, J.; Qin, J.; Li, Y.; He, Z.; Wang, K. Large stokes shift and high efficiency luminescent solar concentrator incorporated with CuInS2/ZnS quantum dots. Sci. Rep. 2015, 5, 17777. [Google Scholar] [CrossRef] [PubMed]
- Van Sark, W. Luminescent solar concentrators—A low cost photovoltaics alternative. Renew. Energy 2013, 49, 207–210. [Google Scholar] [CrossRef]
- Rowan, B.C.; Wilson, L.R.; Richards, B.S. Advanced Material Concepts for Luminescent Solar Concentrators. IEEE J. Sel. Top. Quant. Electron. 2008, 14, 1312–1322. [Google Scholar] [CrossRef]
- Vossen, F.M.; Aarts, M.P.J.; Debije, M.G. Visual performance of red luminescent solar concentrating windows in an office environment. Energy Build. 2016, 113, 123–132. [Google Scholar] [CrossRef]
- ten Kate, O.M.; Krämer, K.W.; van der Kolk, E. Efficient luminescent solar concentrators based on self-absorption free, Tm2+ doped halides. Sol. Energy Mater. Sol. Cells 2015, 140, 115–120. [Google Scholar] [CrossRef]
- Aste, N.; Tagliabue, L.C.; Palladino, P.; Testa, D. Integration of a luminescent solar concentrator: Effects on daylight, correlated color temperature, illuminance level and color rendering index. Sol. Energy 2015, 114, 174–182. [Google Scholar] [CrossRef]
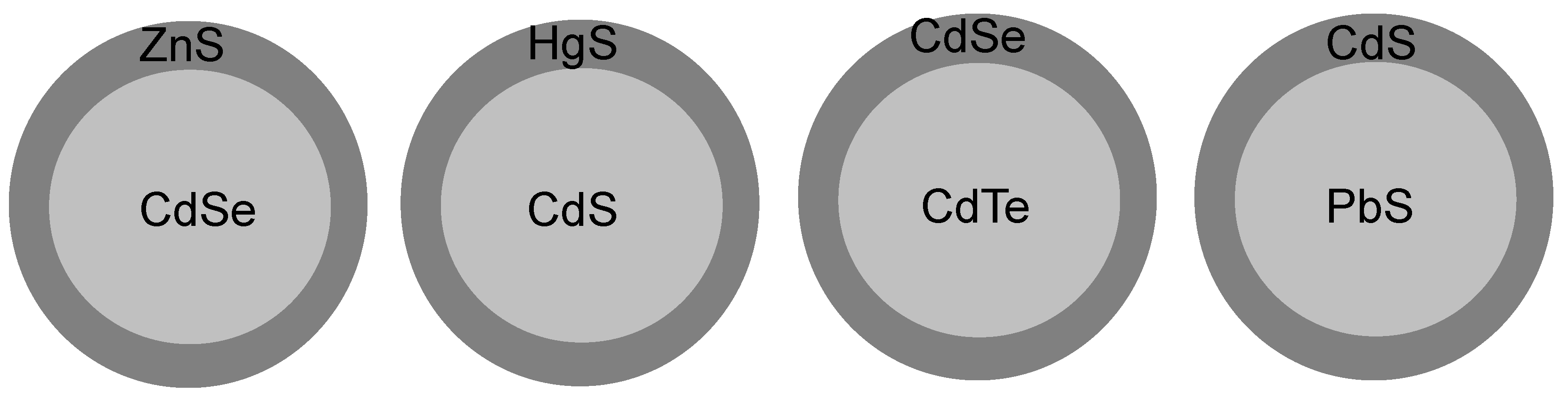
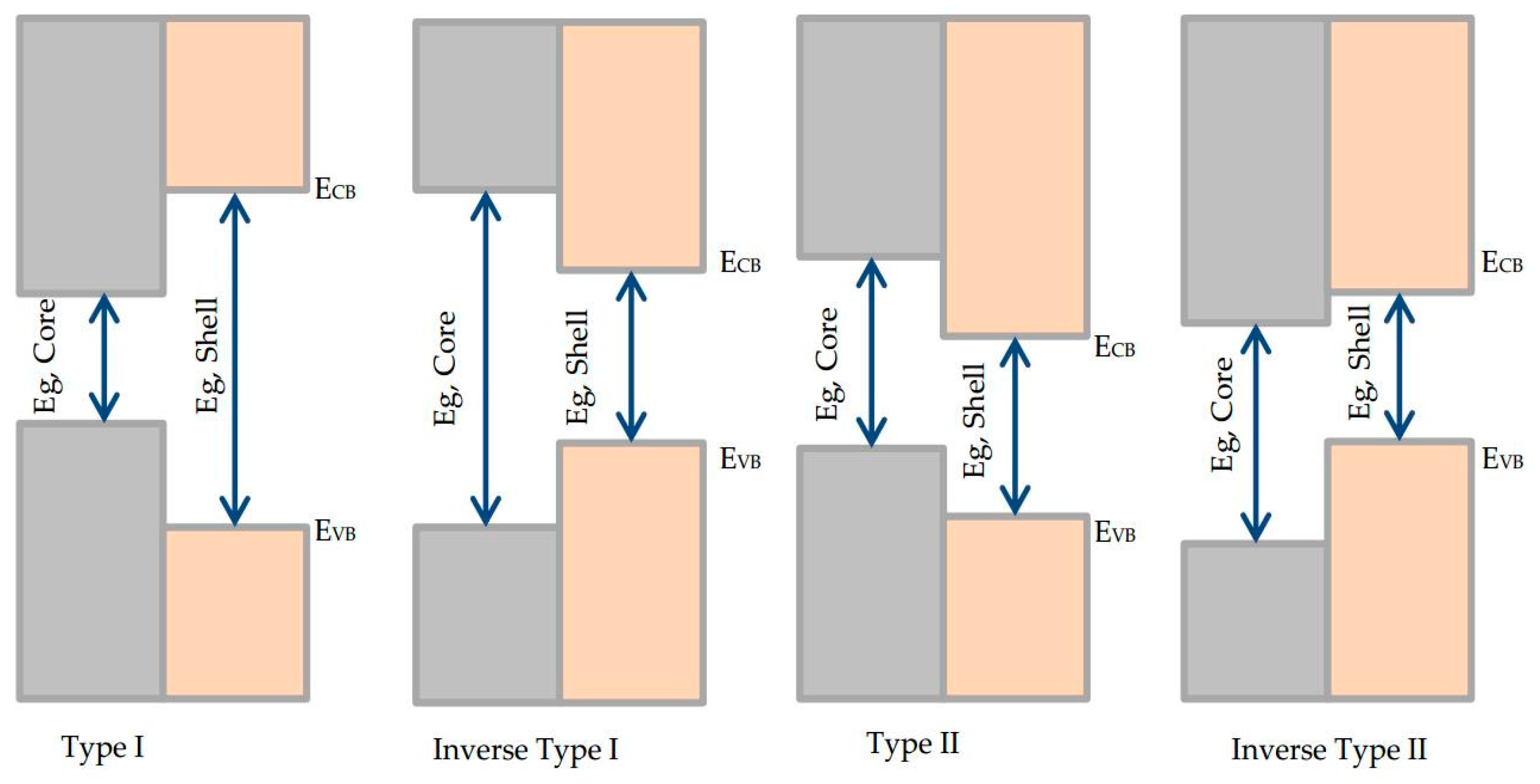




| Parameter | Type I | Inverse Type I | Type II | Inverse Type II |
|---|---|---|---|---|
| Band gap | The band gap of the core is smaller than the band gap of the shell, as well as the band gap of the core falls within the band gap of the shell [18,19,20] | The band gap of the core is greater than the band gap of the shell, as well as the band gap of the shell falls within band gap of the core [21] | Valence band edge of the core is within the band gap of the shell or conduction band edge of the shell is within the band gap of the core [22,23] | Conduction band edge of the core is within the band gap of the shell or valence band edge of the shell is within the band gap of the core [24] |
| Excited electrons/holes positions | Excited electrons and holes are completely confined in the core region [25] | The excited electrons and holes are completely or partially confined in the shell based on the thickness of the shell. | One charge carrier either excited electron or hole is confined to the core, while the other is mostly confined to the shell | One of the excited electrons or the holes are delocalized in the core/shell structure, and the other one is confined within the core. |
| Quantum yield (QY) | Higher QY and long-term stability [25,26] | Lower QY and poor stability | Lower QY and poor stability [27] | Relatively higher QY and fair stability |
| Stokes shift | Small | Significantly large [21] | Large [28] | Large and tunable via controlling the size of the core and thickness of the shell |
| Average absorption range | (400–500) nm [29] | (400–500) nm [30,31] | (600–800) nm [23,32] | (300–1600) nm [33] |
| Average emission range | (430–600) nm [34] | (400–700) nm [34] | (700–1000) nm [34] | (700–1000) nm [34] |
| Limitations | The shell can trap charge carriers which leads to reduced fluorescence QY | Both the excited electrons and holes may leak to the surface | One of the excited electrons or hole leak to the surface | The excited electron or hole can be absorbed leading to reduced excited decay time one carrier is mostly confined to the core, while the other is mostly confined to the shell |
| Construct/materials |
|
|
|
|
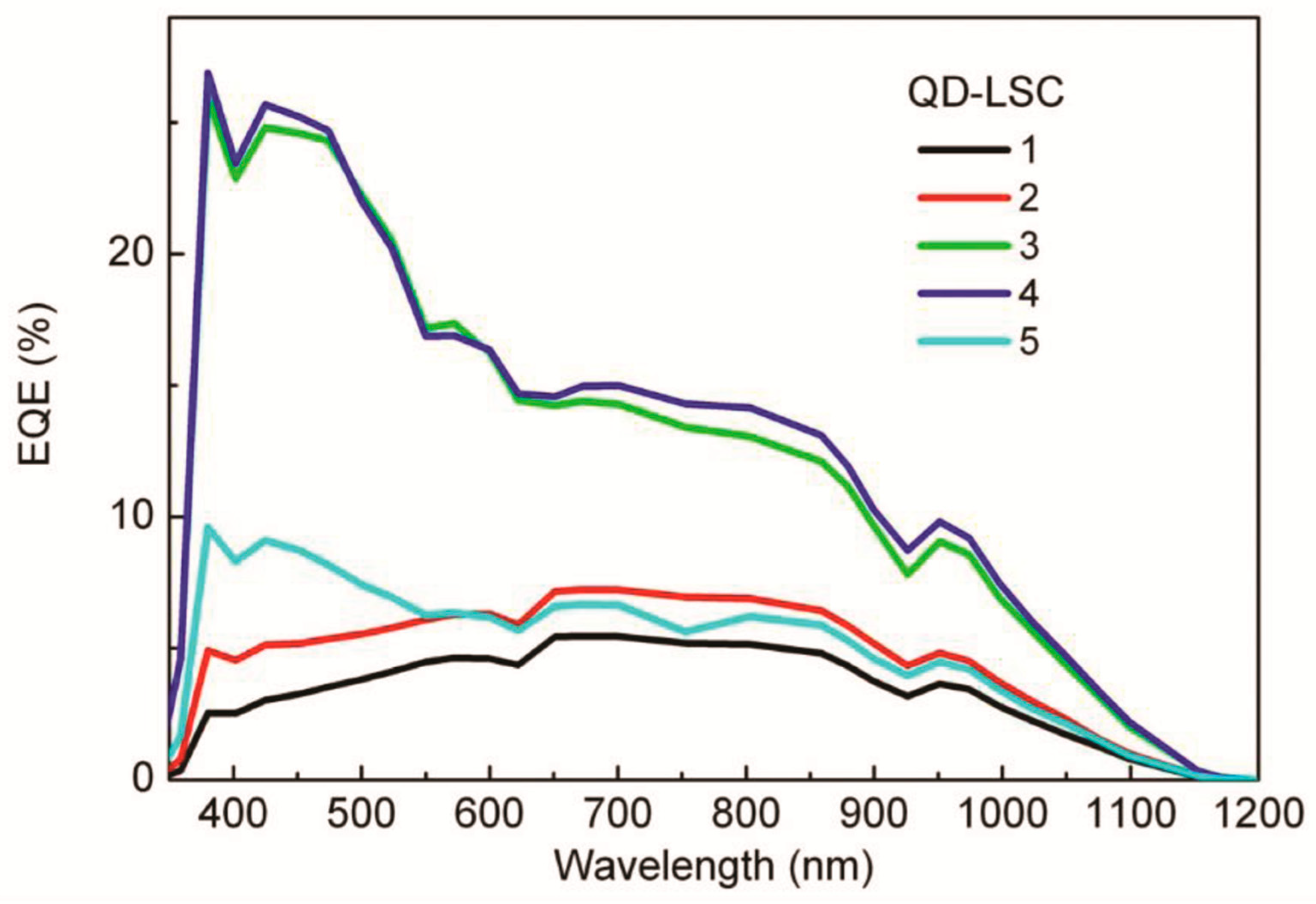
| QDs-LSC Number | Concentration QDs (µmol/L) | Concentration UV-Initiator (wt%) | QY (%) | Isc Total (mA) | Dimensions (L × W) cm (h = 4 cm) | Isc per Cell Area (mA/cm2) |
|---|---|---|---|---|---|---|
| 1 | 0.11 | 0.5 | 9 | 33.1 | 50 × 3.8 | 21.8 |
| 2 | 0.11 | 0.25 | 18.1 | 25.5 | 3.2 × 3.3 | 19.3 |
| 3 | 0.67 | 0.1 | 45.4 | 90.4 | 4.0 × 3.8 | 59.5 |
| 4 | 0.52 | 0.1 | 44.2 | 95.7 | 5.0 × 3.1 | 77.2 |
| 5 | 0.32 | 0.1 | 33.3 | 45.6 | 4.9 × 3.8 | 30.0 |
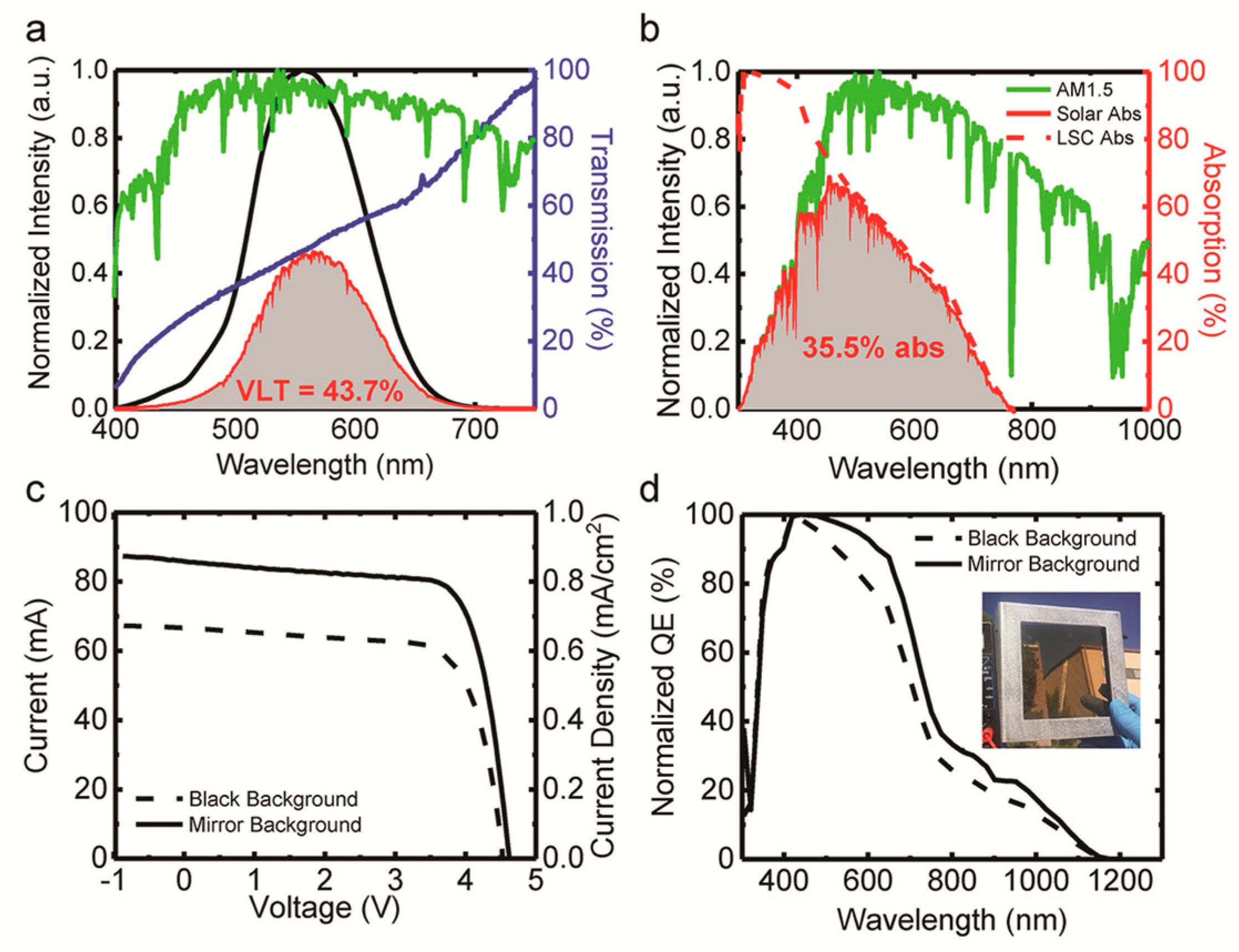
| LSC Type | Stoke Shift | Optical Efficiency (%) | Weight Ratio (%) | Photocurrent (%) | QY (%) | Spectral Range | PCE (%) |
|---|---|---|---|---|---|---|---|
| Type I | Small | 3.2 | 0.03 | 3.2 | 50–85 | UV-Vis | (1.97–3.2) [69] |
| Type II inverse II | Large | 12.6 | NA | 12.6 | 4–30 | UV-Vis-NIR | (5.6–11.28) [70,71] |
| Type I-III-VI2 | Larger | 8.1 | 0.3–0.5 | NA | 20 | UV-Vis-NIR | (3.1–11.6) [68,72] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
AbouElhamd, A.R.; Al-Sallal, K.A.; Hassan, A. Review of Core/Shell Quantum Dots Technology Integrated into Building’s Glazing. Energies 2019, 12, 1058. https://doi.org/10.3390/en12061058
AbouElhamd AR, Al-Sallal KA, Hassan A. Review of Core/Shell Quantum Dots Technology Integrated into Building’s Glazing. Energies. 2019; 12(6):1058. https://doi.org/10.3390/en12061058
Chicago/Turabian StyleAbouElhamd, Amira R., Khaled A. Al-Sallal, and Ahmed Hassan. 2019. "Review of Core/Shell Quantum Dots Technology Integrated into Building’s Glazing" Energies 12, no. 6: 1058. https://doi.org/10.3390/en12061058
APA StyleAbouElhamd, A. R., Al-Sallal, K. A., & Hassan, A. (2019). Review of Core/Shell Quantum Dots Technology Integrated into Building’s Glazing. Energies, 12(6), 1058. https://doi.org/10.3390/en12061058





