Biogas Upgrading Via Dry Reforming Over a Ni-Sn/CeO2-Al2O3 Catalyst: Influence of the Biogas Source
Abstract
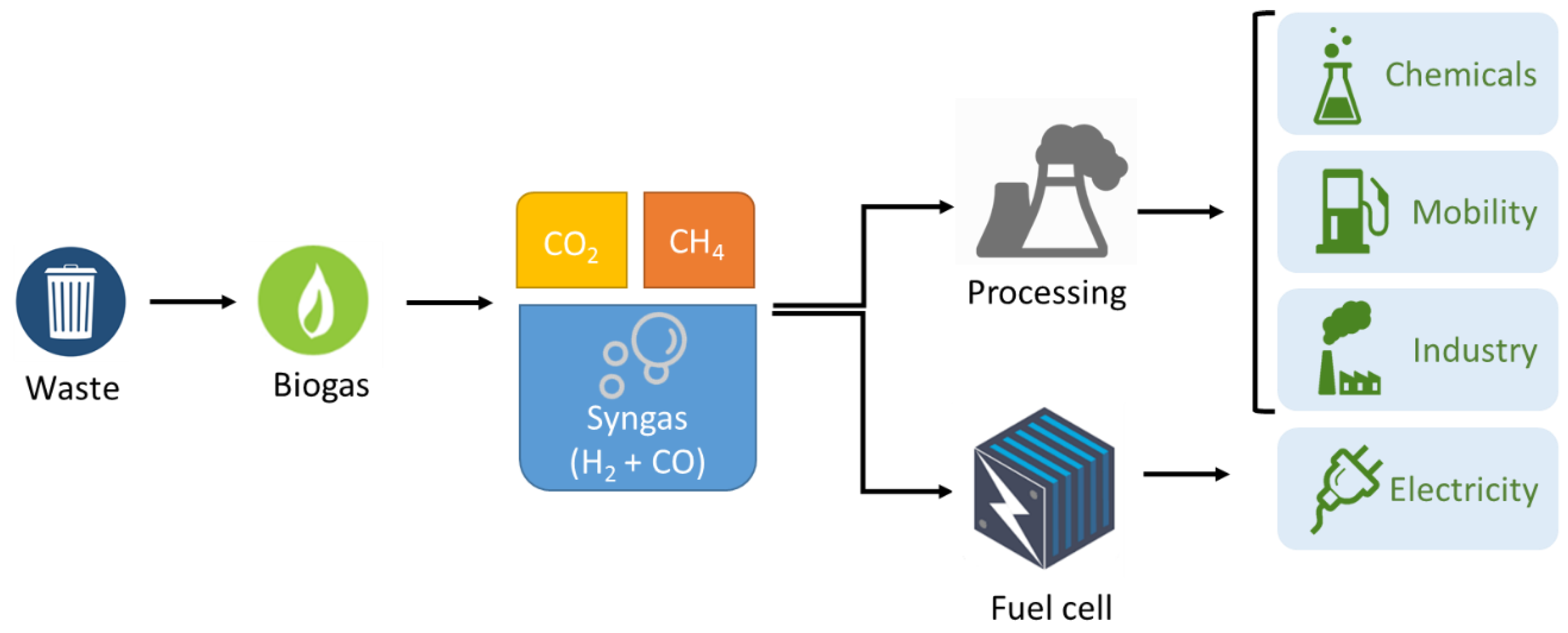
1. Introduction
2. Materials and Methods
2.1. Catalyst Preparation
2.2. Catalyst Characterisation
2.3. Catalytic Activity
3. Results and Discussion
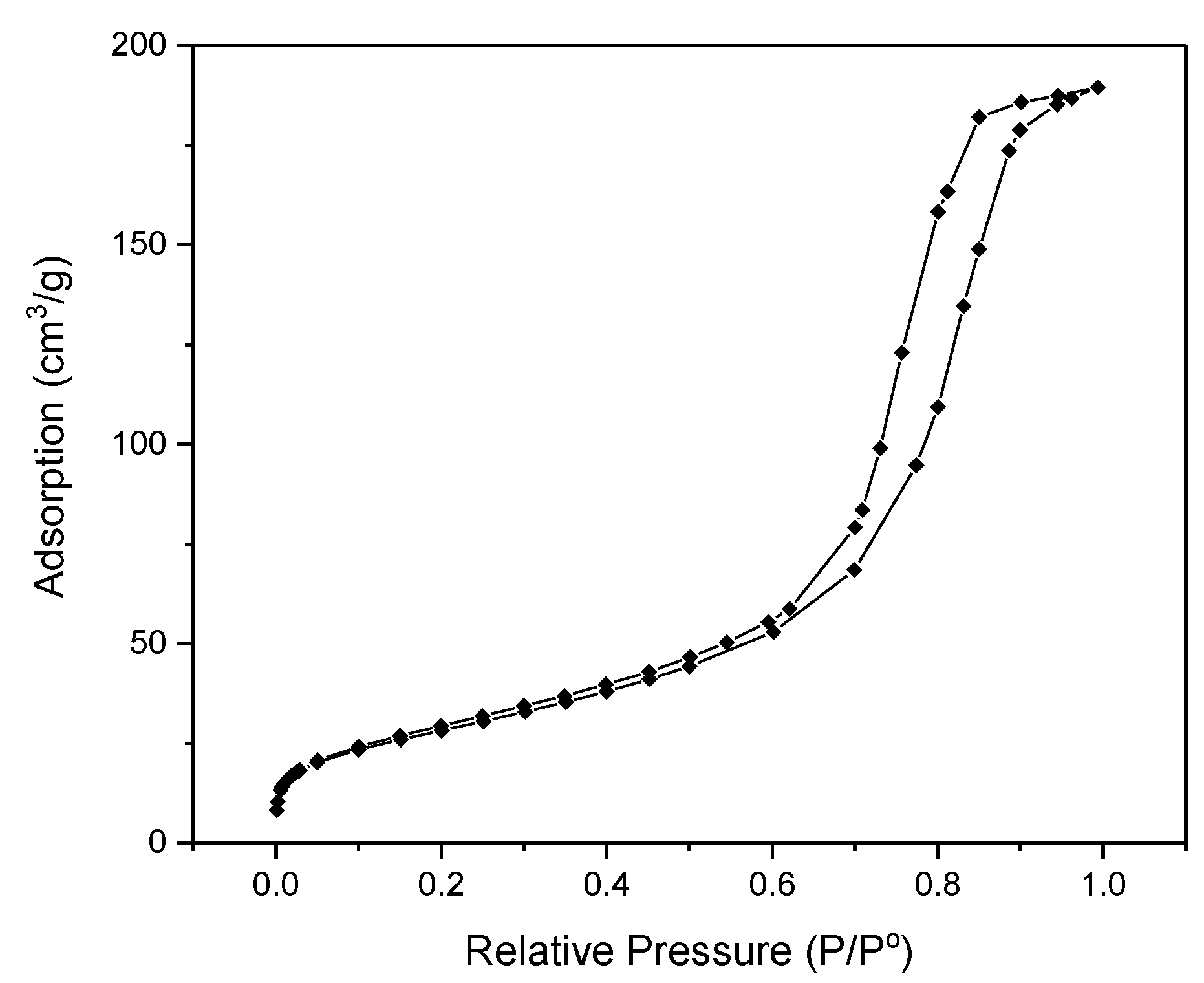
3.1. Physicochemical Properties
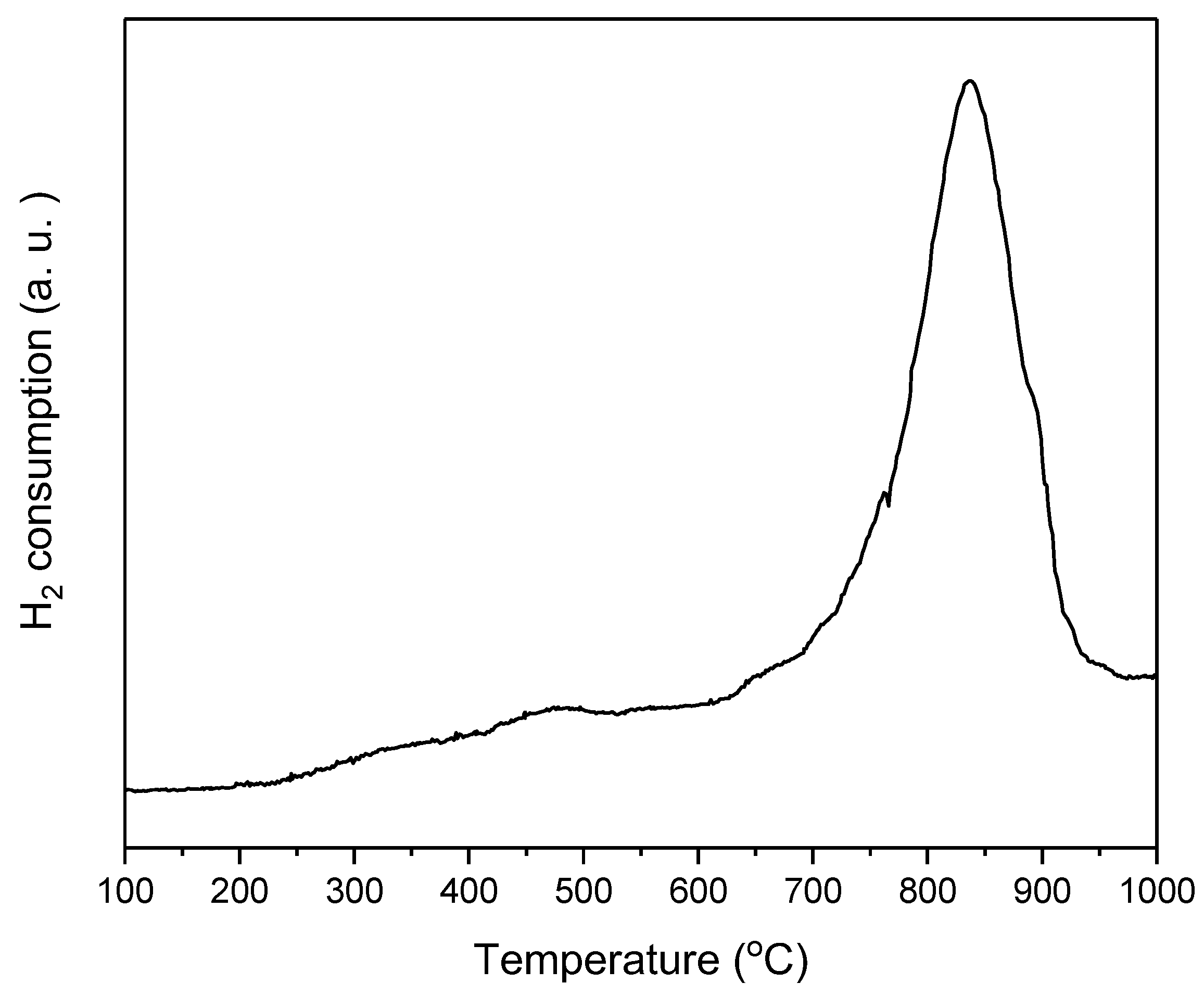
3.2. Reducibility: H2-TPR
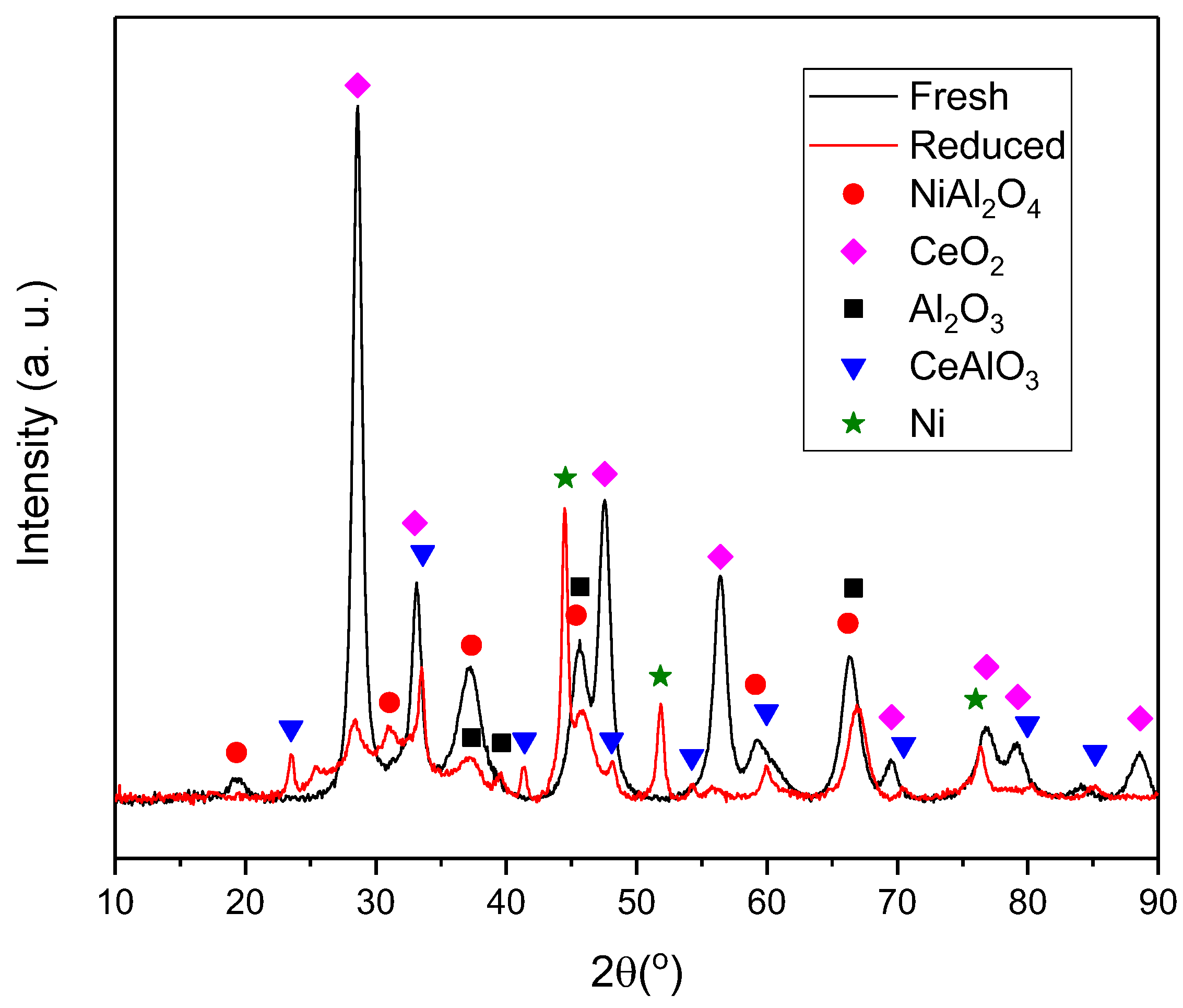
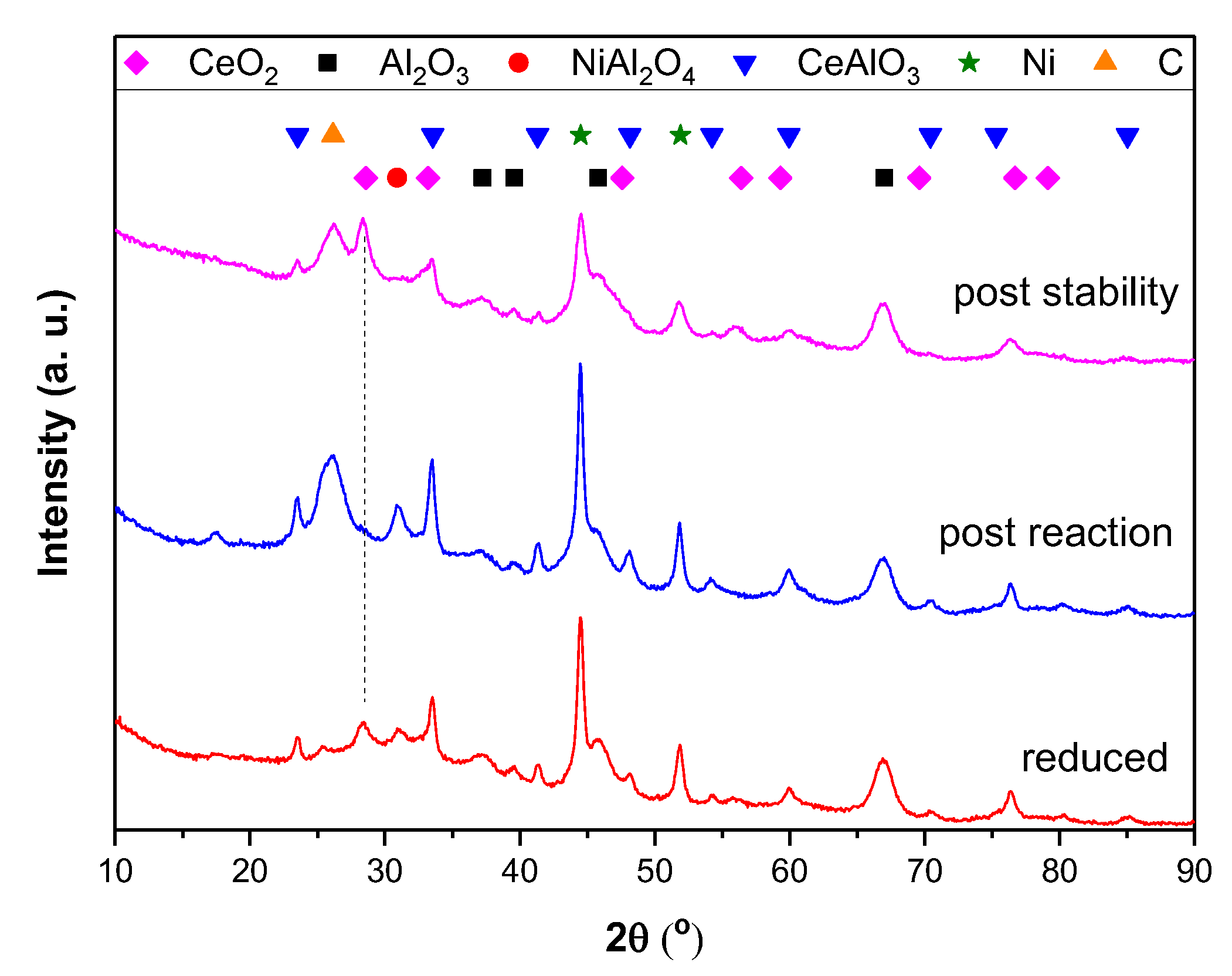
3.3. XRD
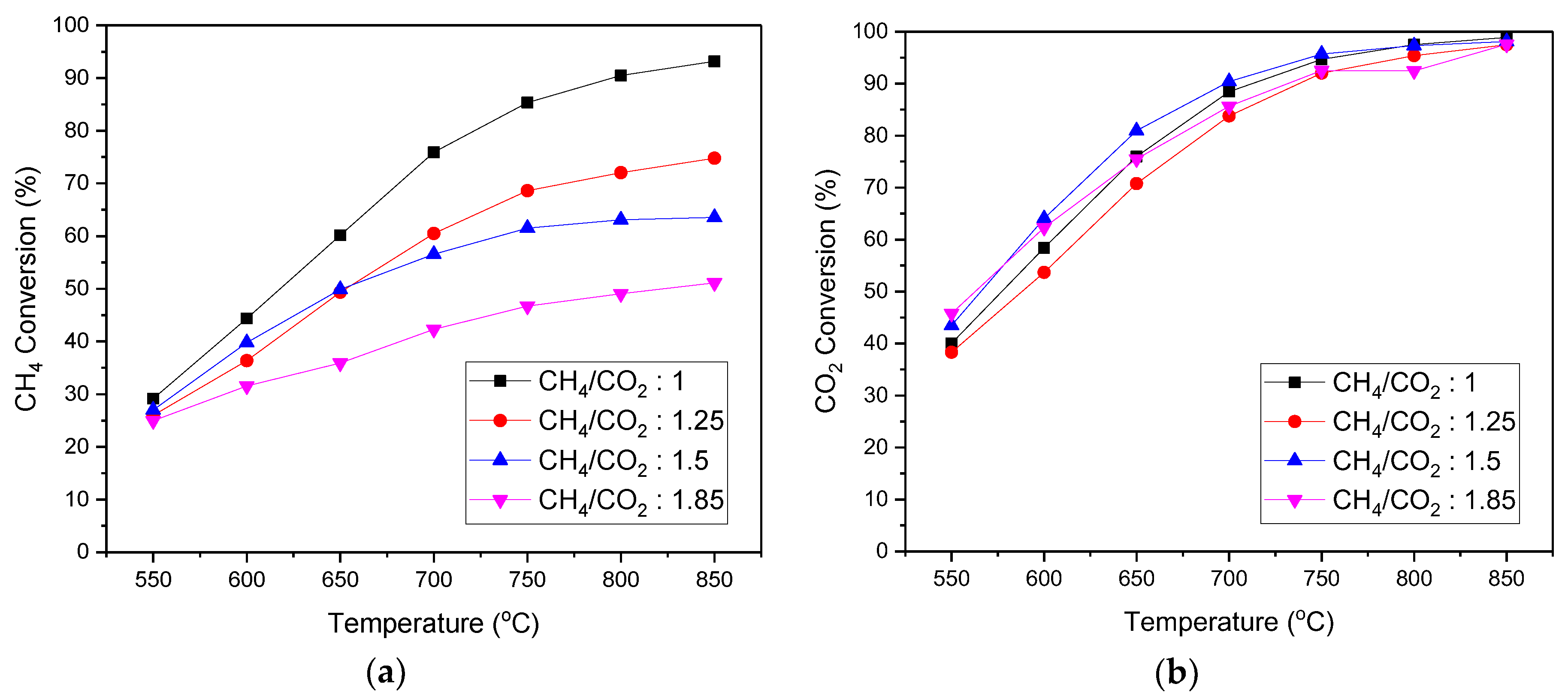
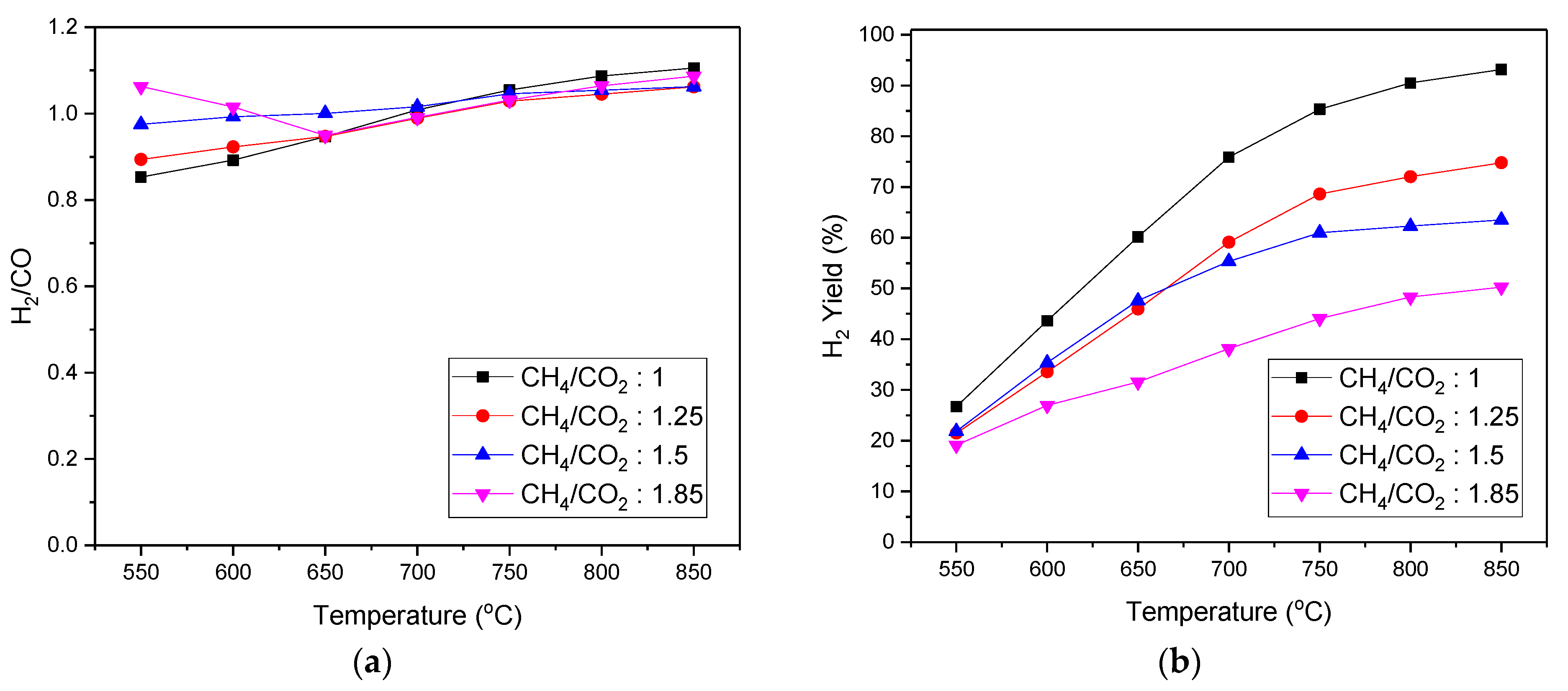
3.4. Catalyst Performance
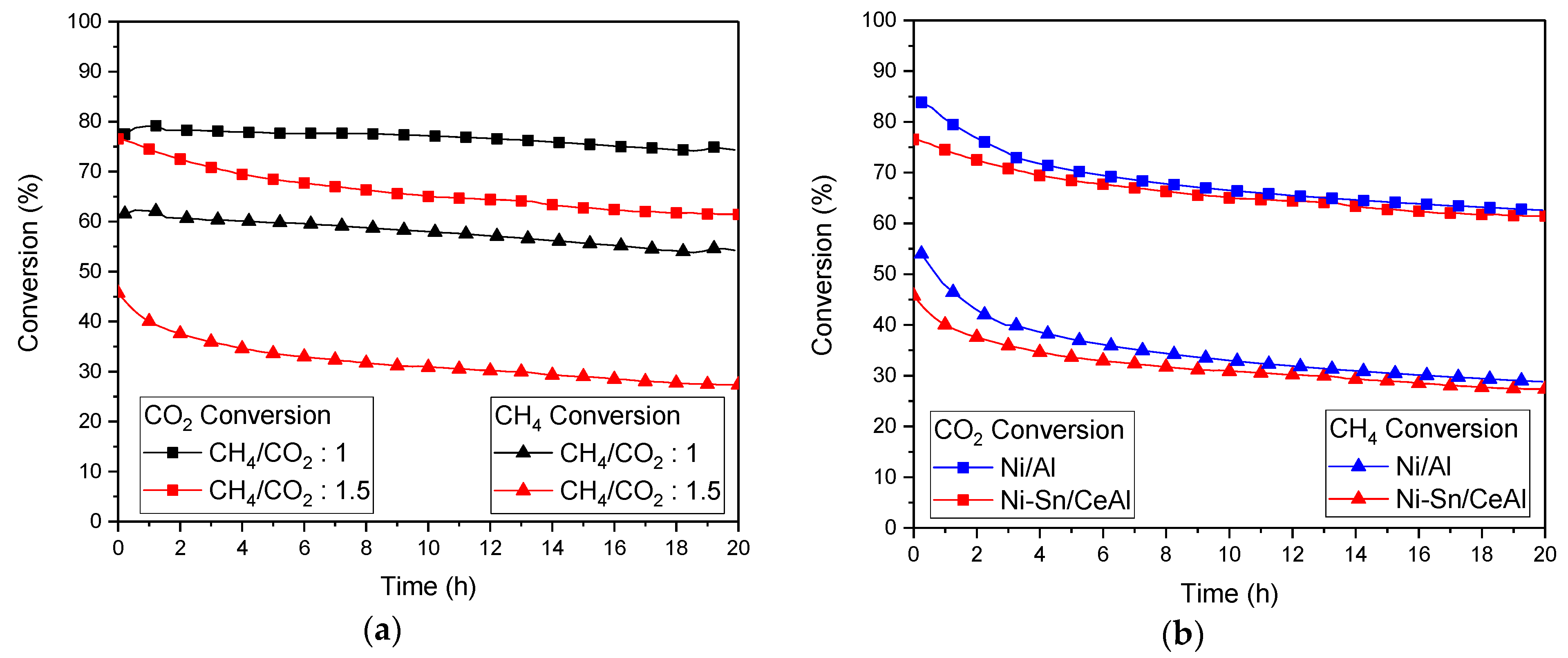
3.5. Stability Test
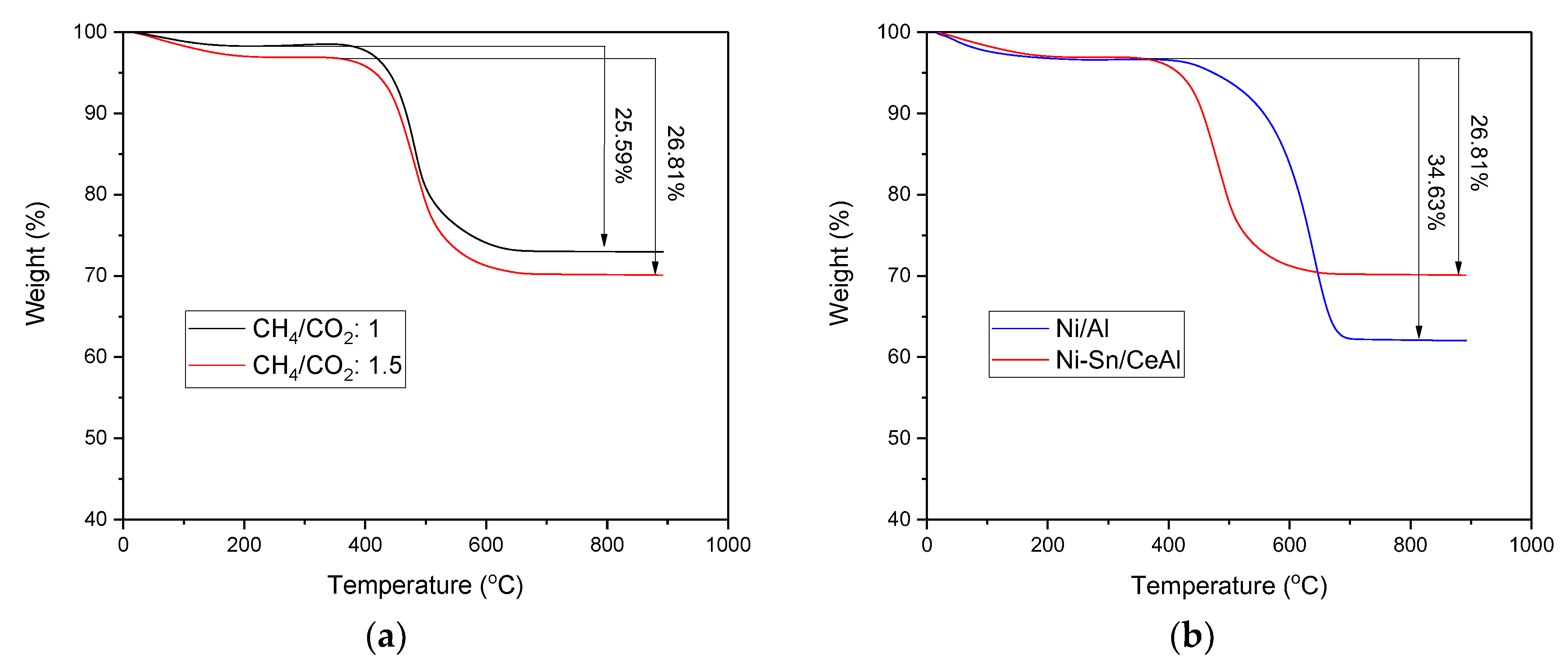
3.6. Study of Carbonaceous Deposits
3.7. Post Reaction Characterisation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- International Energy Agency. CO2 Emissions from Fuel Combustion–Highlights; International Energy Agency: Paris, France, 2018. [Google Scholar]
- Boucher, O.; Friedlingstein, P.; Collins, B.; Shine, K.P. The indirect global warming potential and global temperature change potential due to methane oxidation. Environ. Res. Lett. 2009, 4, 044007. [Google Scholar] [CrossRef]
- Ahmed, S.; Lee, S.H.D.; Ferrandon, M.S. Catalytic steam reforming of biogas–effects of feed composition and operating conditions. Int. J. Hydrogen Energy 2015, 40, 1005–1015. [Google Scholar] [CrossRef]
- Mota, N.; Alvarez-Galvan, C.; Navarro, R.; Fierro, J. Biogas as a source of renewable syngas production: Advances and challenges. Biofuels 2011, 2, 325–343. [Google Scholar] [CrossRef]
- Le Saché, E.; Santos, J.L.; Smith, T.J.; Centeno, M.A.; Arellano-Garcia, H.; Odriozola, J.A.; Reina, T.R. Multicomponent Ni-CeO2 nanocatalysts for syngas production from CO2/CH4 mixtures. J. CO2 Util. 2018, 25, 68–78. [Google Scholar] [CrossRef]
- Charisiou, N.D.; Baklavaridis, A.; Papadakis, V.G.; Goula, M.A. Synthesis gas production via the biogas reforming reaction over Ni/MgO–Al2O3 and Ni/CaO–Al2O3 catalysts. Waste Biomass Valoriz. 2016, 7, 725–736. [Google Scholar] [CrossRef]
- Bonura, G.; Cannilla, C.; Frusteri, F. Ceria–gadolinia supported nicu catalyst: A suitable system for dry reforming of biogas to feed a solid oxide fuel cell (SOFC). Appl. Catal. B 2012, 121–122, 135–147. [Google Scholar] [CrossRef]
- Lanzini, A.; Leone, P. Experimental investigation of direct internal reforming of biogas in solid oxide fuel cells. Int. J. Hydrogen Energy 2010, 35, 2463–2476. [Google Scholar] [CrossRef]
- Shiratori, Y.; Ijichi, T.; Oshima, T.; Sasaki, K. Internal reforming sofc running on biogas. Int. J. Hydrogen Energy 2010, 35, 7905–7912. [Google Scholar] [CrossRef]
- Fuerte, A.; Valenzuela, R.X.; Escudero, M.J.; Daza, L. Study of a sofc with a bimetallic cu–co–ceria anode directly fuelled with simulated biogas mixtures. Int. J. Hydrogen Energy 2014, 39, 4060–4066. [Google Scholar] [CrossRef]
- Le Saché, E.; Pastor-Pérez, L.; Watson, D.; Sepúlveda-Escribano, A.; Reina, T.R. Ni stabilised on inorganic complex structures: Superior catalysts for chemical co2 recycling via dry reforming of methane. Appl. Catal. B 2018, 236, 458–465. [Google Scholar] [CrossRef]
- Pakhare, D.; Spivey, J. A review of dry (CO2) reforming of methane over noble metal catalysts. Chem. Soc. Rev. 2014, 43, 7813–7837. [Google Scholar] [CrossRef]
- Shah, Y.T.; Gardner, T.H. Dry reforming of hydrocarbon feedstocks. Catal. Rev. Sci. Eng. 2014, 56, 476–536. [Google Scholar] [CrossRef]
- Charisiou, N.D.; Siakavelas, G.; Papageridis, K.N.; Baklavaridis, A.; Tzounis, L.; Avraam, D.G.; Goula, M.A. Syngas production via the biogas dry reforming reaction over nickel supported on modified with CeO2 and/or La2O3 alumina catalysts. J. Nat. Gas Sci. Eng. 2016, 31, 164–183. [Google Scholar] [CrossRef]
- Wang, S.; Lu, G.Q. Role of CeO2 in Ni/CeO2–Al2O3 catalysts for carbon dioxide reforming of methane. Appl. Catal. B 1998, 19, 267–277. [Google Scholar] [CrossRef]
- Stroud, T.; Smith, T.J.; le Saché, E.; Santos, J.L.; Centeno, M.A.; Arellano-Garcia, H.; Odriozola, J.A.; Reina, T.R. Chemical CO2 recycling via dry and bi reforming of methane using Ni-Sn/Al2O3 and Ni-Sn/CeO2-Al2O3 catalysts. Appl. Catal. B 2018, 224, 125–135. [Google Scholar] [CrossRef]
- San-José-Alonso, D.; Juan-Juan, J.; Illán-Gómez, M.J.; Román-Martínez, M.C. Ni, CO and bimetallic Ni–CO catalysts for the dry reforming of methane. Appl. Catal. A 2009, 371, 54–59. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, H.; Dalai, A.K. Development of stable bimetallic catalysts for carbon dioxide reforming of methane. J. Catal. 2007, 249, 300–310. [Google Scholar] [CrossRef]
- Crisafulli, C.; Scirè, S.; Minicò, S.; Solarino, L. Ni–Ru bimetallic catalysts for the CO2 reforming of methane. Appl. Catal. A 2002, 225, 1–9. [Google Scholar] [CrossRef]
- Crisafulli, C.; Scirè, S.; Maggiore, R.; Minicò, S.; Galvagno, S. CO2 reforming of methane over Ni–Ru and Ni–Pd bimetallic catalysts. Catal. Lett. 1999, 59, 21–26. [Google Scholar] [CrossRef]
- Boldrin, P.; Ruiz-Trejo, E.; Mermelstein, J.; Bermúdez Menéndez, J.M.; Ramírez Reina, T.; Brandon, N.P. Strategies for carbon and sulfur tolerant solid oxide fuel cell materials, incorporating lessons from heterogeneous catalysis. Chem. Rev. 2016, 116, 13633–13684. [Google Scholar] [CrossRef]
- Guharoy, U.; Le Saché, E.; Cai, Q.; Reina, T.R.; Gu, S. Understanding the role of Ni-Sn interaction to design highly effective CO2 conversion catalysts for dry reforming of methane. J. CO2 Util. 2018, 27, 1–10. [Google Scholar] [CrossRef]
- Lau, C.S.; Tsolakis, A.; Wyszynski, M.L. Biogas upgrade to syn-gas (H2–CO) via dry and oxidative reforming. Int. J. Hydrogen Energy 2011, 36, 397–404. [Google Scholar] [CrossRef]
- Tao, K.; Shi, L.; Ma, Q.; Wang, D.; Zeng, C.; Kong, C.; Wu, M.; Chen, L.; Zhou, S.; Hu, Y.; et al. Methane reforming with carbon dioxide over mesoporous nickel–alumina composite catalyst. Chem. Eng. J. 2013, 221, 25–31. [Google Scholar] [CrossRef]
- Yang, L.; Pastor-Pérez, L.; Gu, S.; Sepúlveda-Escribano, A.; Reina, T.R. Highly efficient Ni/CeO2-Al2O3 catalysts for CO2 upgrading via reverse water-gas shift: Effect of selected transition metal promoters. Appl. Catal. B 2018, 232, 464–471. [Google Scholar] [CrossRef]
- Li, P.-P.; Lang, W.-Z.; Xia, K.; Yan, X.; Guo, Y.-J. The promotion effects of Ni on the properties of Cr/Al catalysts for propane dehydrogenation reaction. Appl. Catal. A 2016, 522, 172–179. [Google Scholar] [CrossRef]
- Zou, X.; Wang, X.; Li, L.; Shen, K.; Lu, X.; Ding, W. Development of highly effective supported nickel catalysts for pre-reforming of liquefied petroleum gas under low steam to carbon molar ratios. Int. J. Hydrogen Energy 2010, 35, 12191–12200. [Google Scholar] [CrossRef]
- Luisetto, I.; Tuti, S.; Battocchio, C.; Lo Mastro, S.; Sodo, A. Ni/CeO2–Al2O3 catalysts for the dry reforming of methane: The effect of CeAlO3 content and nickel crystallite size on catalytic activity and coke resistance. Appl. Catal. A 2015, 500, 12–22. [Google Scholar] [CrossRef]
- Penkova, A.; Bobadilla, L.; Ivanova, S.; Domínguez, M.I.; Romero-Sarria, F.; Roger, A.C.; Centeno, M.A.; Odriozola, J.A. Hydrogen production by methanol steam reforming on NiSn/MgO–Al2O3 catalysts: The role of mgo addition. Appl. Catal. A 2011, 392, 184–191. [Google Scholar] [CrossRef]
- Izquierdo, U.; Barrio, V.L.; Requies, J.; Cambra, J.F.; Guemez, M.B.; Arias, P.L. Tri-reforming: A new biogas process for synthesis gas and hydrogen production. Int. J. Hydrogen Energy 2013, 38, 7623–7631. [Google Scholar] [CrossRef]
- Chattanathan, S.A.; Adhikari, S.; McVey, M.; Fasina, O. Hydrogen production from biogas reforming and the effect of H2S on CH4 conversion. Int. J. Hydrogen Energy 2014, 39, 19905–19911. [Google Scholar] [CrossRef]
| Biogas Source | CH4 Content (%) | CO2 Content (%) | CH4/CO2 Molar Ratio |
|---|---|---|---|
| Model Composition | 50 | 50 | 1:1 |
| Landfill Waste | 55 | 45 | 1.25 |
| Sewage Waste | 60 | 40 | 1.5 |
| Organic Waste | 65 | 35 | 1.85 |
| NiO wt % | Sn wt % | SBet (m2·g−1) | VPore (cm3·g−1) | DPore (nm) | |
|---|---|---|---|---|---|
| Ni-Sn/CeAl | 10.3 | 1.3 | 103 | 0.31 | 8.3 |
| CeAl | - | - | 130 | 0.34 | 9.6 |
| Temperature (°C) | WHSV (L·g−1h−1) | CH4 Conversion (%) | CO2 Conversion (%) | H2 Yield (%) | Reference | |
|---|---|---|---|---|---|---|
| Ni/MgO | 800 | 60 | 62.2 | 92.3 | 52.6 | [30] |
| Ni/Ce-Al2O3 | 800 | 60 | 59.3 | 77.8 | 57.1 | [30] |
| Ni/Zr-Al2O3 | 800 | 60 | 58.4 | 73.5 | 51.9 | [30] |
| Ni/Ce-Zr-Al2O3 | 800 | 60 | 64.5 | 85.6 | 57.5 | [30] |
| Rh-Ni/Ce-Al2O3 | 800 | 60 | 60.1 | 94.4 | 63.3 | [30] |
| Ni/Al2O3 | 800 | 120 | 60.0 | 83.0 | 48.0 | [14] |
| Ni/Ce-Al2O3 | 800 | 120 | 63.0 | 89.0 | 50.0 | [14] |
| Ni/La-Al2O3 | 800 | 120 | 69.0 | 94.0 | 57.0 | [14] |
| Ni/Ce-La-Al2O3 | 800 | 120 | 69.0 | 94.5 | 58.0 | [14] |
| Ni/CaO-Al2O3 | 800 | 120 | 57.9 | 75.2 | 30.5 | [6] |
| Ni/MgO-Al2O3 | 800 | 120 | 57.7 | 75.2 | 32.4 | [6] |
| Reformax® 250 | 750 | 18 | 64.0 | 86.0 | 28.8 | [31] |
| Ni-Sn/Ce–Al2O3 | 800 | 30 | 63.1 | 97.5 | 62.3 | This work |
| Equilibrium | 800 | - | 65.5 | 99.1 | 65.2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
le Saché, E.; Johnson, S.; Pastor-Pérez, L.; Amini Horri, B.; Reina, T.R. Biogas Upgrading Via Dry Reforming Over a Ni-Sn/CeO2-Al2O3 Catalyst: Influence of the Biogas Source. Energies 2019, 12, 1007. https://doi.org/10.3390/en12061007
le Saché E, Johnson S, Pastor-Pérez L, Amini Horri B, Reina TR. Biogas Upgrading Via Dry Reforming Over a Ni-Sn/CeO2-Al2O3 Catalyst: Influence of the Biogas Source. Energies. 2019; 12(6):1007. https://doi.org/10.3390/en12061007
Chicago/Turabian Stylele Saché, Estelle, Sarah Johnson, Laura Pastor-Pérez, Bahman Amini Horri, and Tomas R. Reina. 2019. "Biogas Upgrading Via Dry Reforming Over a Ni-Sn/CeO2-Al2O3 Catalyst: Influence of the Biogas Source" Energies 12, no. 6: 1007. https://doi.org/10.3390/en12061007
APA Stylele Saché, E., Johnson, S., Pastor-Pérez, L., Amini Horri, B., & Reina, T. R. (2019). Biogas Upgrading Via Dry Reforming Over a Ni-Sn/CeO2-Al2O3 Catalyst: Influence of the Biogas Source. Energies, 12(6), 1007. https://doi.org/10.3390/en12061007








