Impact of Nanoadditives on the Performance and Combustion Characteristics of Neat Jatropha Biodiesel
Abstract
1. Introduction
- Pre-treatment of jatropha oil and production of jatropha biodiesel
- Amalgamation of nanoparticle additives into jatropha biodiesel
- Measurement of physico-chemical properties of various fuel blends
- Engine testing using nanoparticle-J100 blend and assessment of combustion, emission and performance characteristics
- Comparison of results with and without nanoparticles and recommendations
2. Materials and Methods
2.1. FFA Determination and Pre-Treatment (Acid Esterification) of JCO
- JCO was poured into a flask, placed on to a hot plate and heated up to 60 °C
- Methanol with a ratio of 60% (w:w) of methanol to oil, was taken into another beaker. After that 1% H2SO4 was added to the methanol beaker. The methanol and H2SO4 mixture was stirred for approximately 5 min before adding the solution to the 60 °C heated JCO. Once mixed, the solution was left on the hotplate stirrer for about 90 min at a temperature of 55 ± 5 °C
- The esterified solution was then poured into a separation funnel and left for 2 hours. The top layer was waste water and methanol, the bottom layer was esterified JCO
2.2. Transesterification
2.3. Addition of Nanoparticles to Neat Jatropha Biodiesel
- 1000 ppm of Triton-X100 was added to J100
- Either 50 ppm or 100 ppm of CeO2/Al2O3 nanoparticles were added to J100 mixture to produce four samples: J100C50 and J100C100 with CeO2 additives; J100A50 and J100A100 with Al2O3 additives (Figure 1)
- Biodiesel-nanoparticles mixtures were placed in an ultrasonicator (frequency at 40 kHz and water at 45 °C) for a duration of 45 min. After that the samples were left for 72 h at room temperature to see the stability of the mixture
2.4. Characterisation of Fuel Samples
2.5. Engine Testing
3. Results and Discussion
3.1. Nanoparticles Addition and Fuel Characteristics
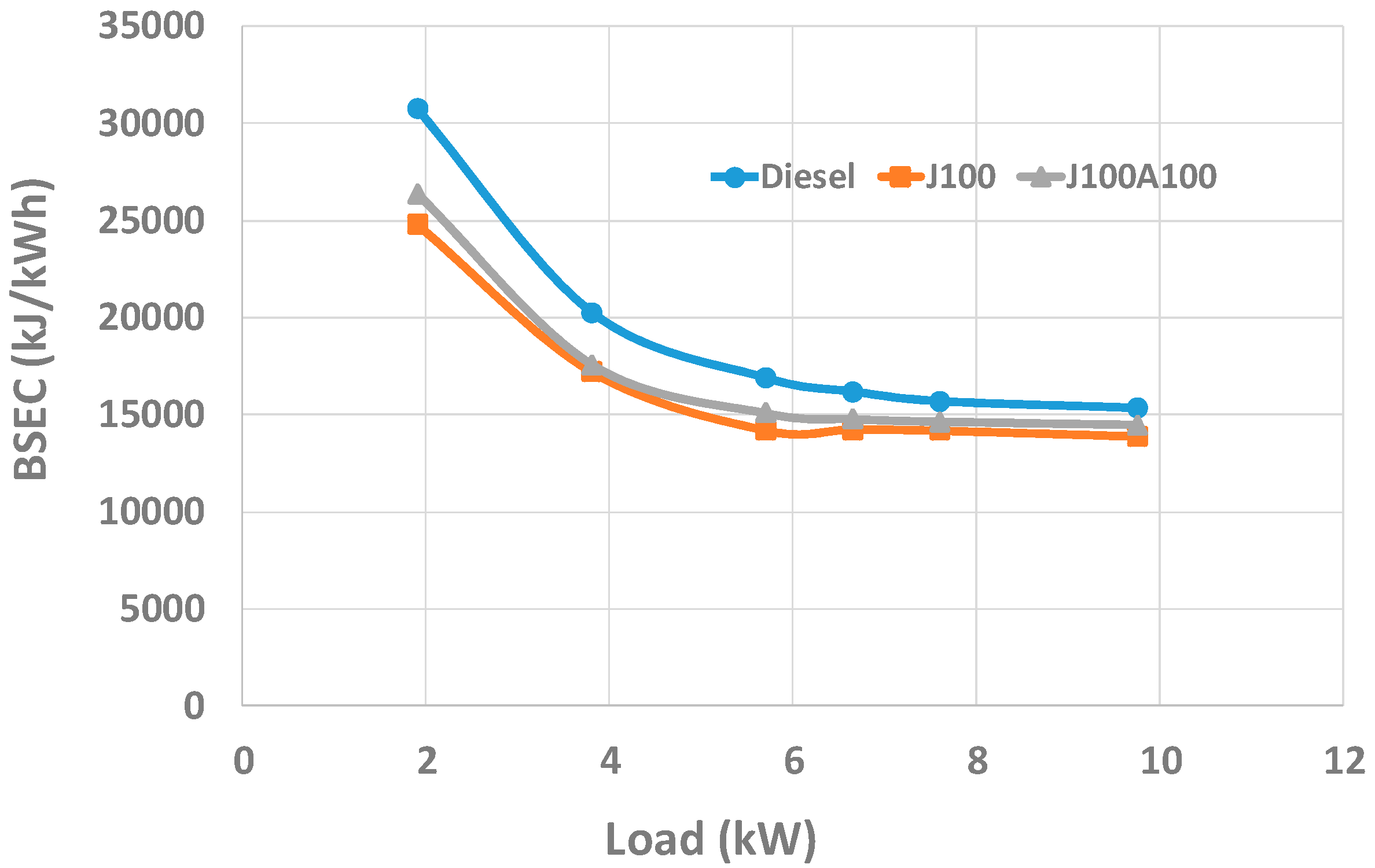
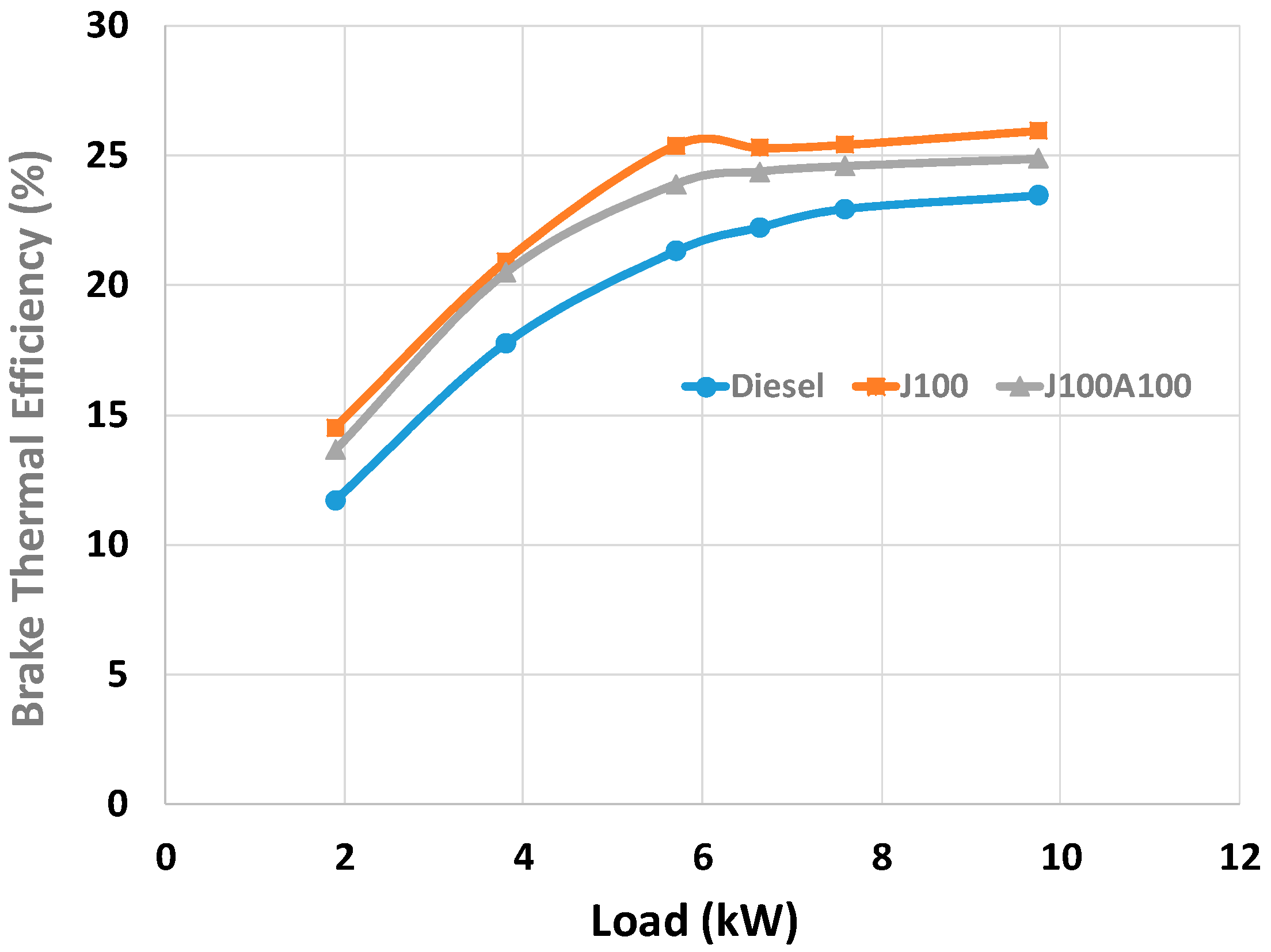
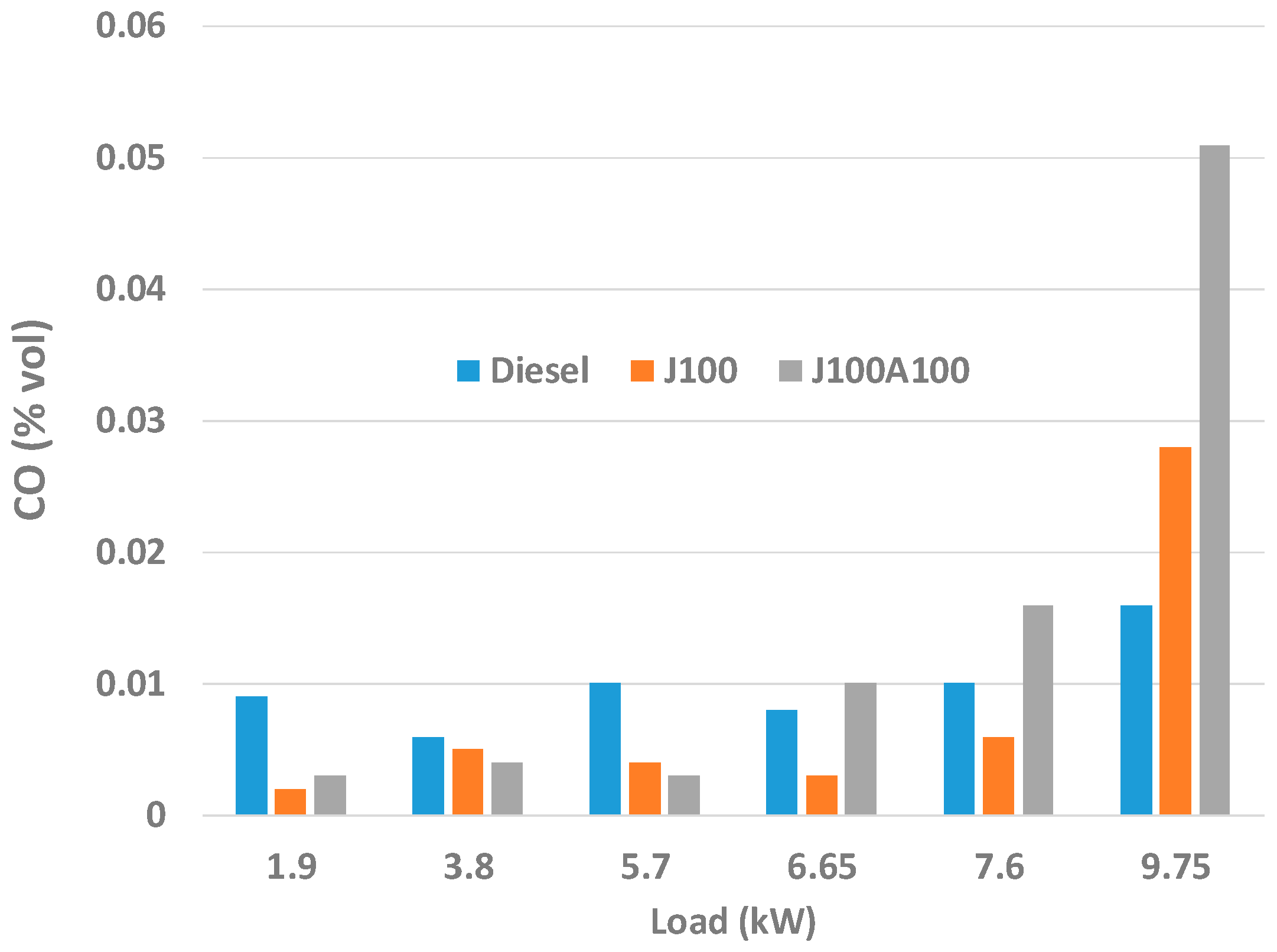
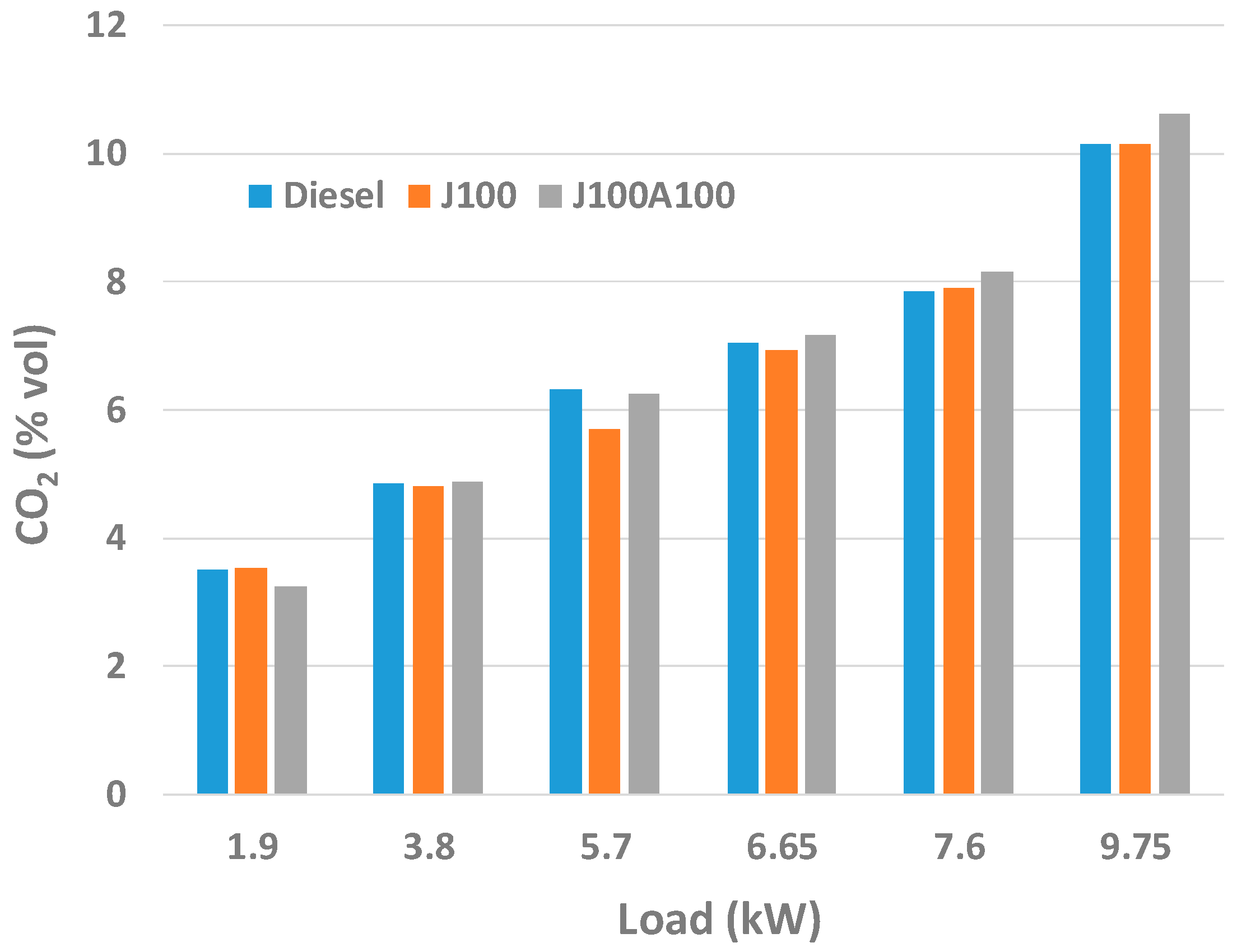
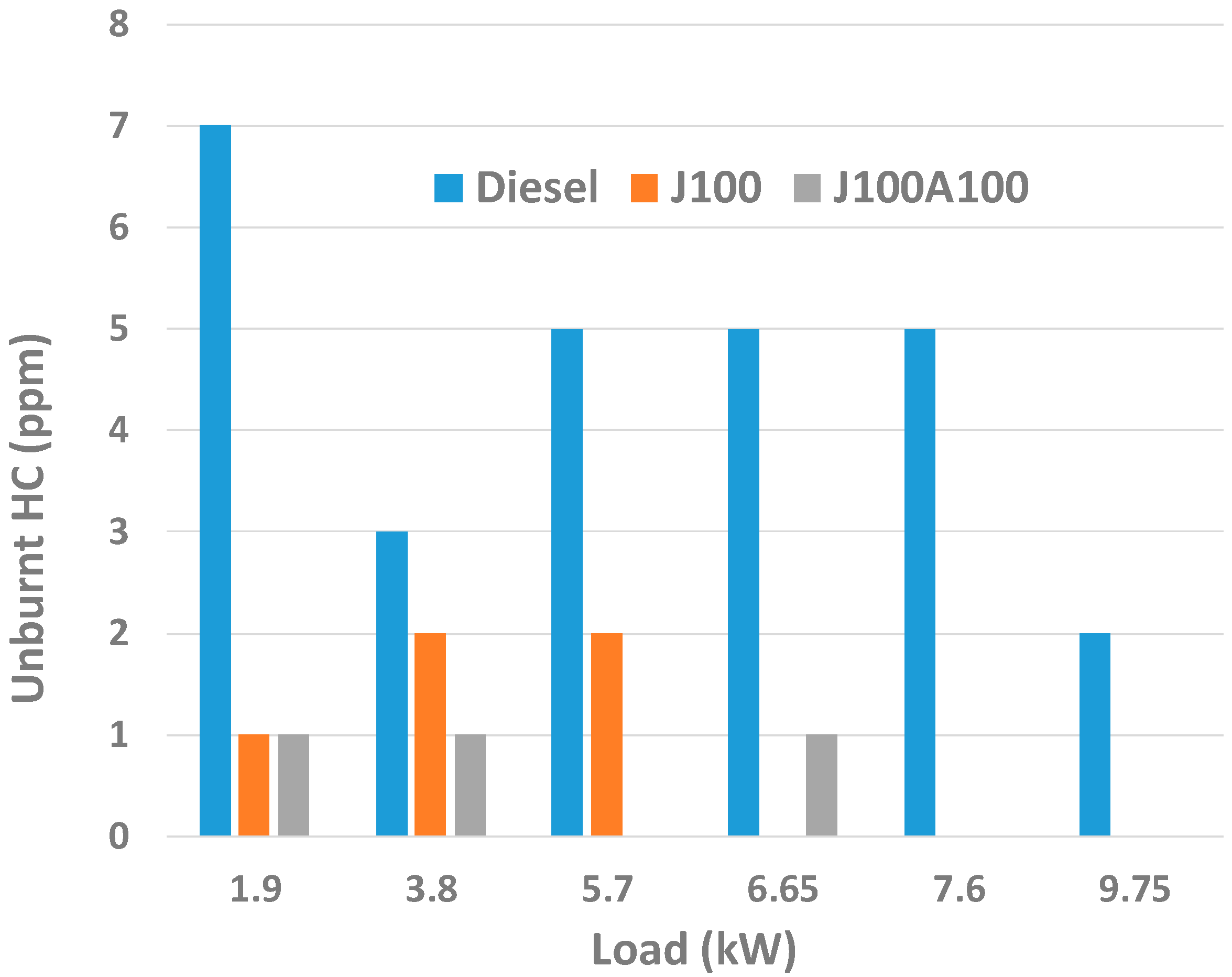
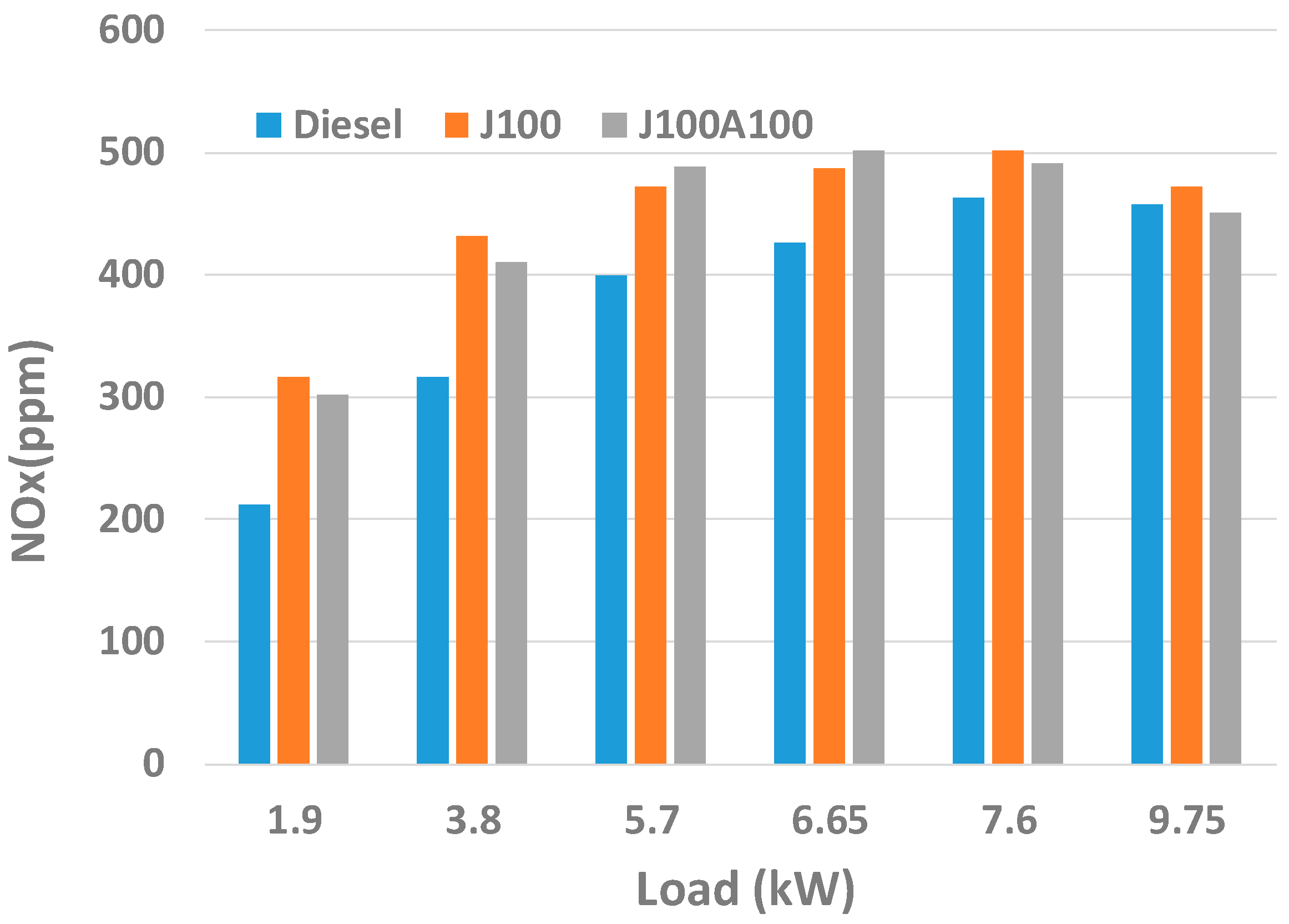
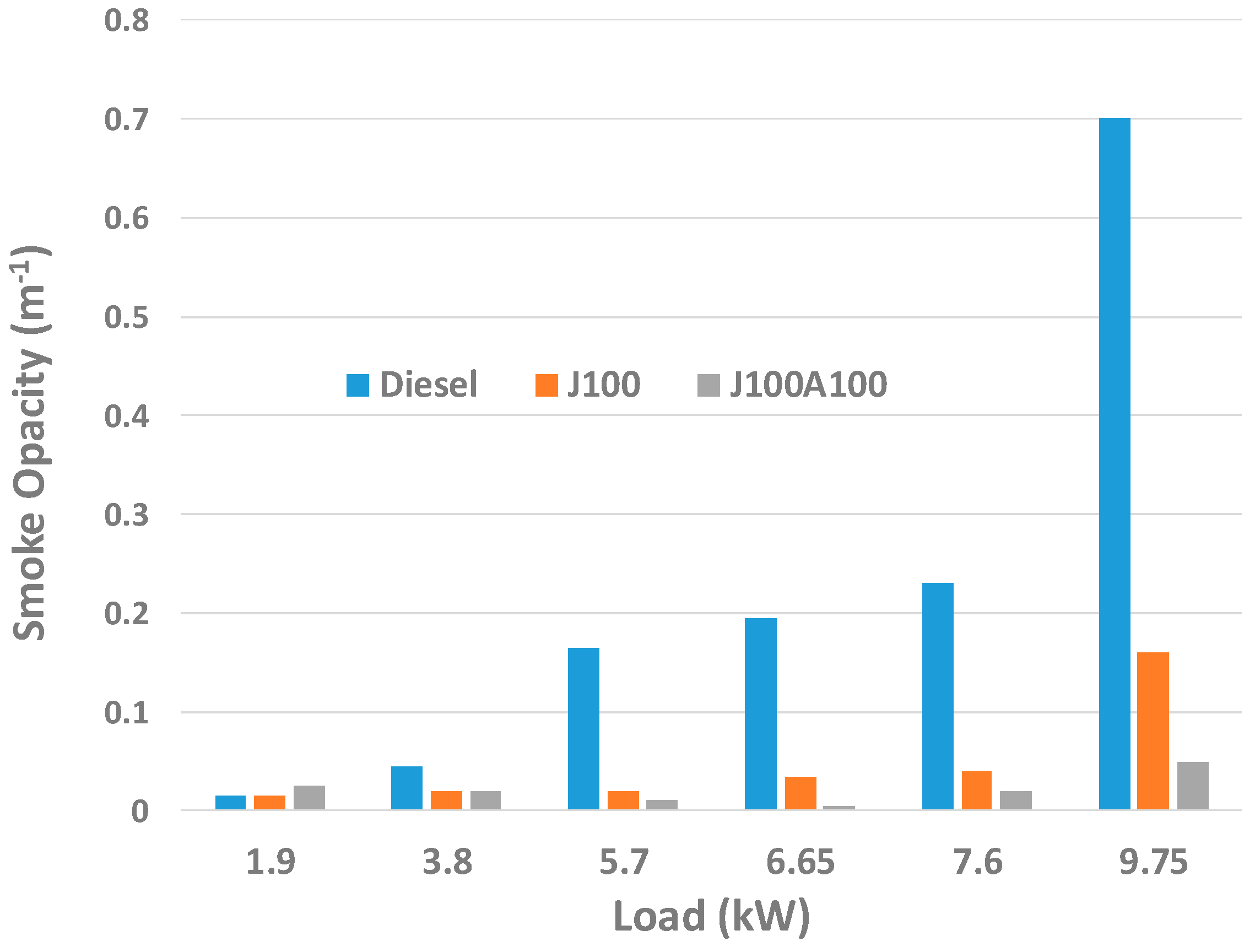
3.2. Engine Performance and Emission Characteristics
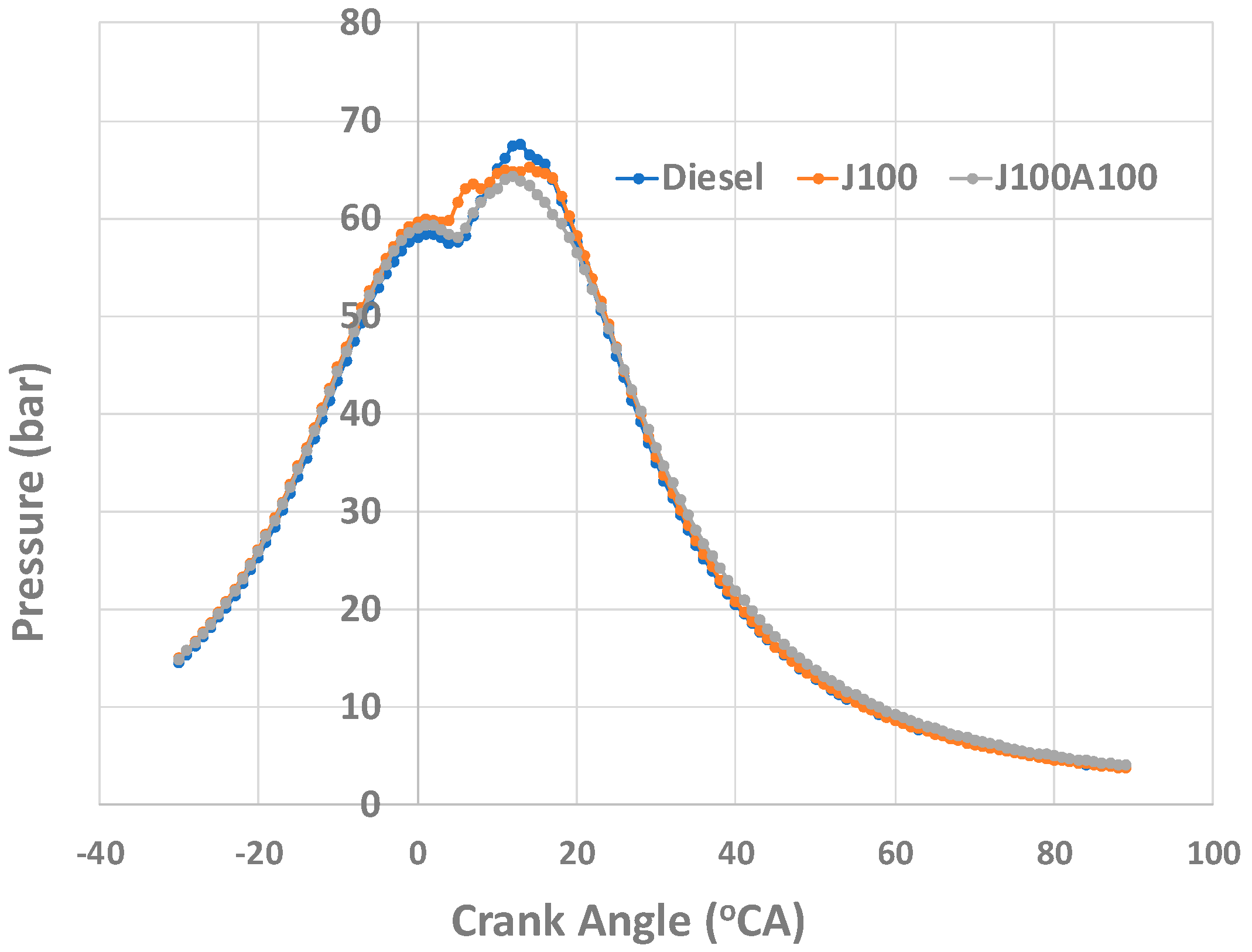
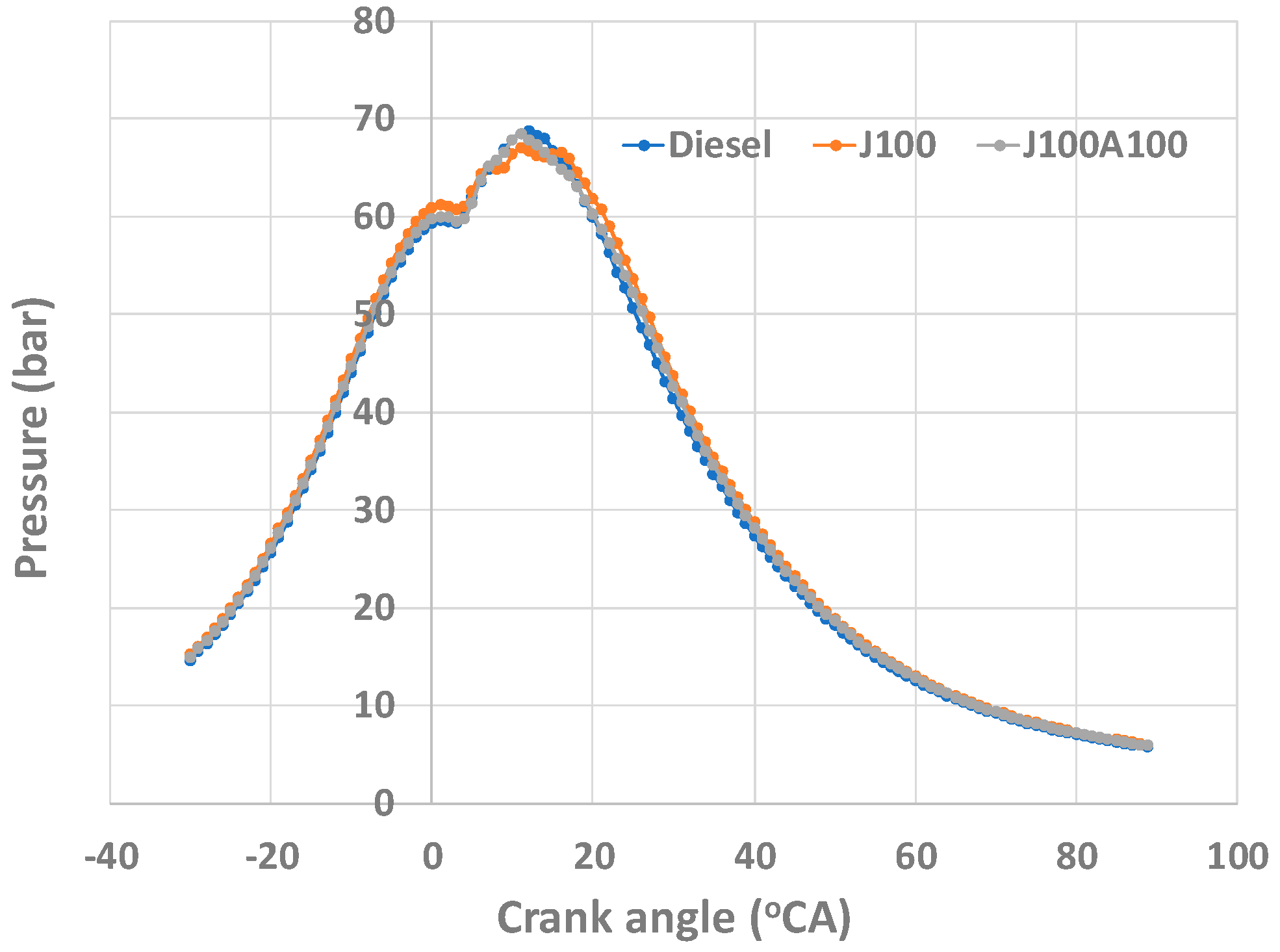
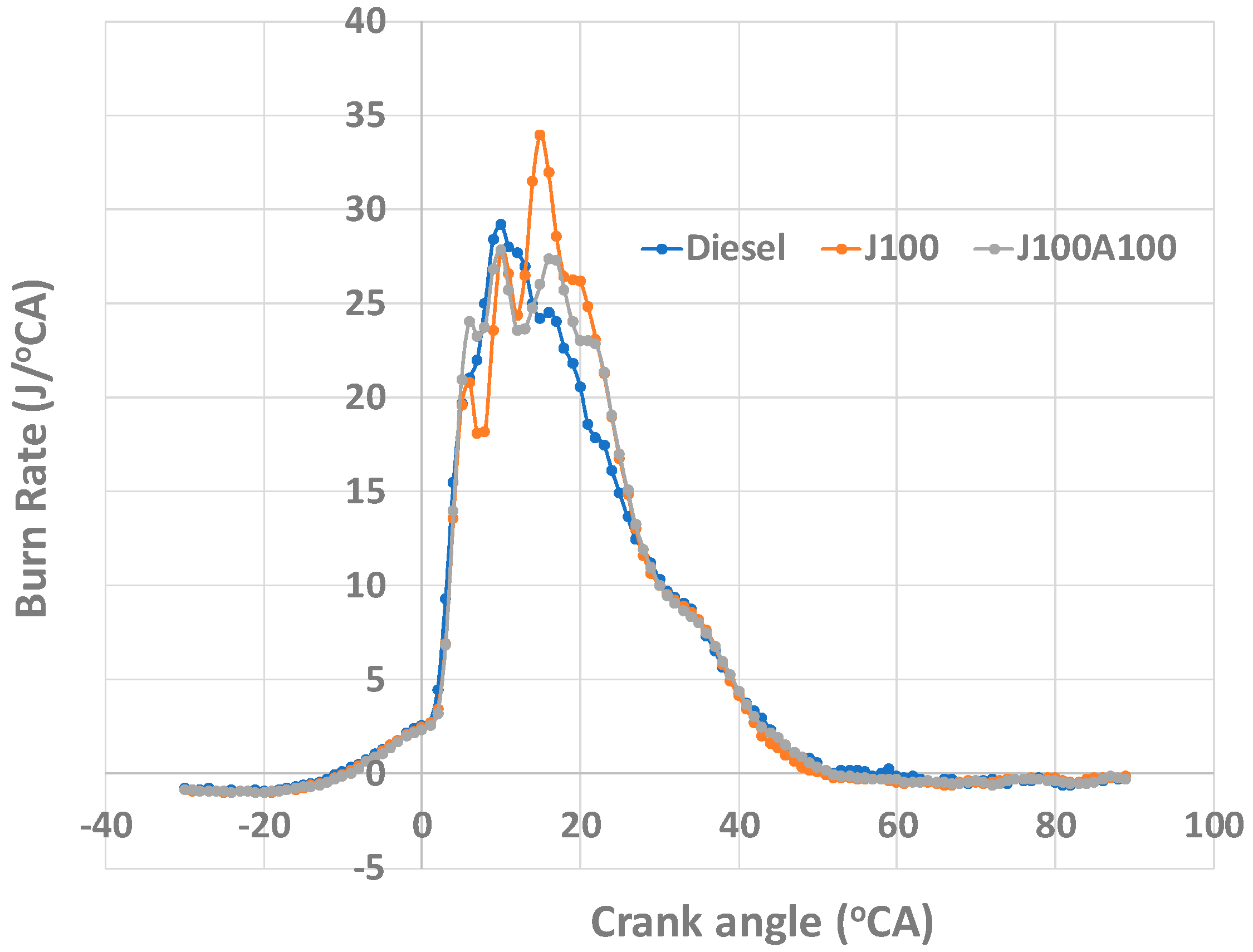
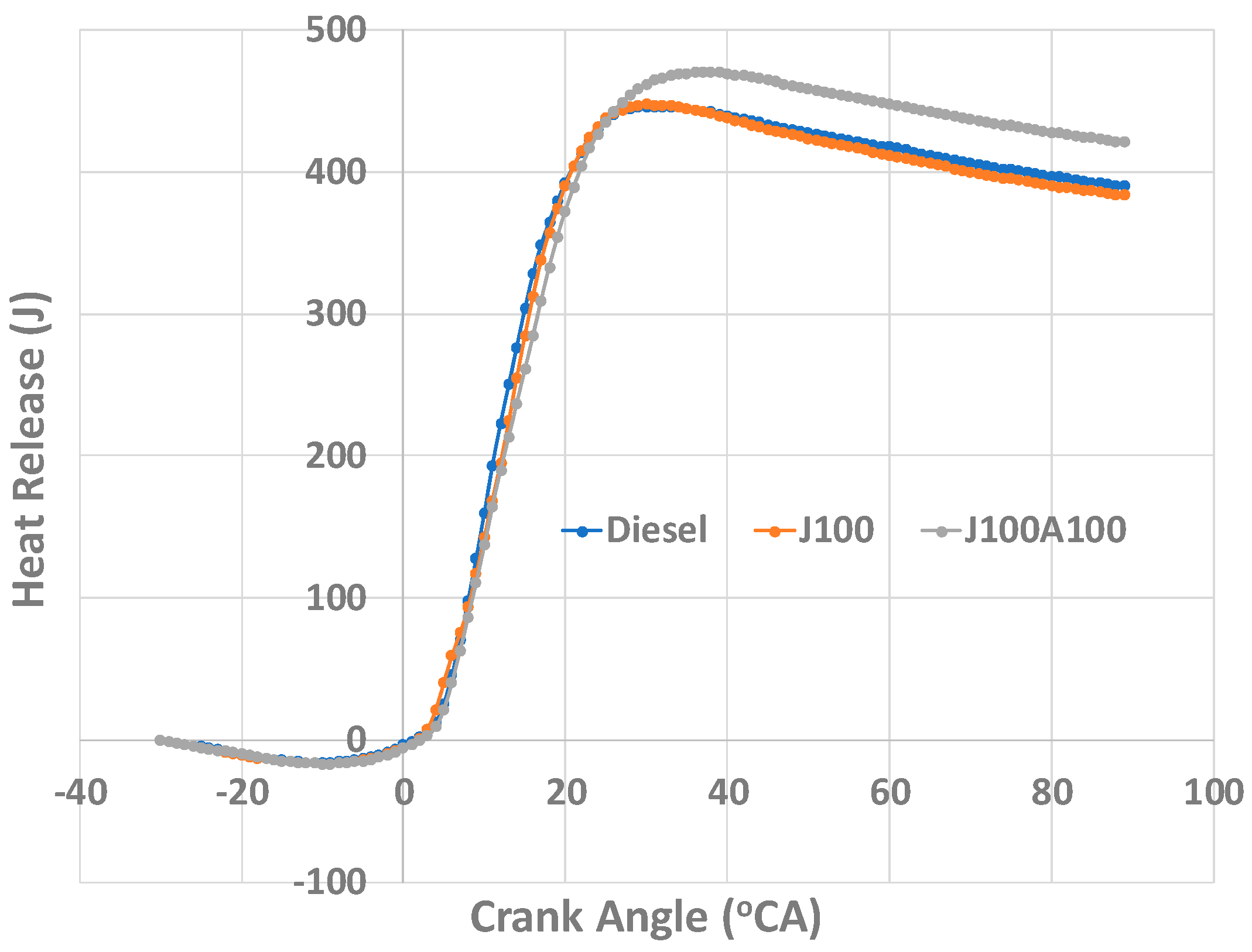
3.3. Combustion Characteristics
4. Conclusions and Recommendations
- (1)
- At full load, the BSEC values of J100A100 blend was found to be 4% higher and 6% lower than the corresponding values obtained for J100 and neat fossil diesel fuels respectively. On the other hand, the BSFC value of J100A100 blend was found to be higher than fossil diesel; however, on average, an improvement of 3% in BTE was observed for J100A100 fuel when compared to fossil diesel.
- (2)
- At low loads, J100A100 fuel gave lower amount of CO emissions. On the other hand, in almost all loads, J100A100 produced smallest amount of UHC emission due to the rich oxygen content in the nanoadditive fuel blend. At higher loads, J100A100 fuel gave improved NOx emission characteristics when compared to J100 fuel.
- (3)
- Better combustion due to nanoadditives led to least smoke opacity values when the engine was operated with J100A100 fuel. The J100A100’s combustion characteristics helped display and measure the fuel’s performance in the combustion chamber. The peak in-cylinder pressure for J100A100 fuel at 60% engine load was seen as being lowest when compared to other fuels; however, this trend was changed when the engine load was increased. At 100% load, the peak in-cylinder pressures for all three fuels were almost same.
- (4)
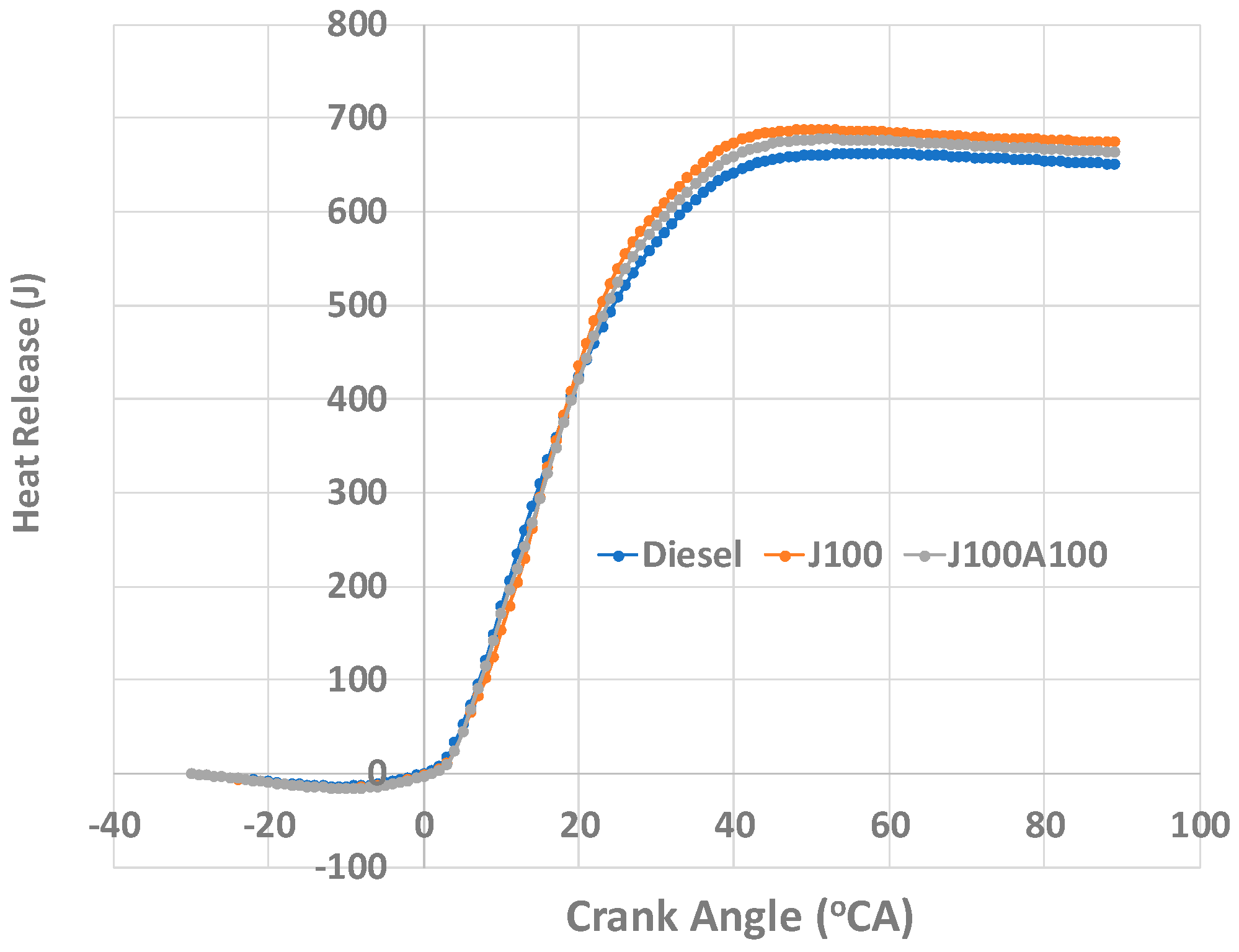
- At 60% and 100% loads, the J100A100 blend was observed as having a constant heat release rate, this was attributed to the additives ability to provide a constant burn and not to succumb to volatility. The total heat release was found to be higher at 60% load for the J100A100 fuel; however, at 100% load, this value was equal to those obtained for other fuels.
Author Contributions
Funding
Conflicts of Interest
Nomenclature
| BSEC | Brake Specific Energy Consumption |
| BSFC | Brake Specific Fuel Consumption |
| BTE | Brake Thermal Efficiency |
| FFA | Free Fatty Acid |
| GHG | Greenhouse Gas |
| HHV | Higher Heating Value |
| J100 | Neat jatropha biodiesel (100%) |
| J100A100 | Jatropha biodiesel (100%) with 100 ppm Al2O3 |
| J100A50 | Jatropha biodiesel (100%) with 50 ppm Al2O3 |
| J100C100 | Jatropha biodiesel (100%) with 100 ppm CeO2 |
| J100C50 | Jatropha biodiesel (100%) with 50 ppm CeO2 |
| JBD | Jatropha Biodiesel |
| JCO | Jatropha Curcas Oil |
| IC | Internal Combustion |
| UHC | Unburnt Hydrocarbons |
References
- Khoo, H.H.; Tan, R.B.H. Environmental impact evaluation of conventional fossil fuel production (oil and natural gas) and enhanced resource recovery with potential CO2 sequestration. Energy Fuels 2006, 20, 1914–1924. [Google Scholar] [CrossRef]
- Global Emissions by Economic Sector. Available online: https://www.epa.gov/ghgemissions/global-greenhouse-gas-emissions-data#Trends (accessed on 17 February 2019).
- Environmental Protection UK. Car Pollution. Environmental Protection UK, 2015. Available online: https://www.environmental-protection.org.uk/policy-areas/air-quality/air-pollution-and-transport/car-pollution/ (accessed on 20 November 2018).
- DAWN Biofuels: A Substitute for Petroleum. Available online: https://www.dawn.com/news/266470 (accessed on 15 November 2018).
- Conserve Energy Future. Advantages and Disadvantages of Biofuels. Conserve Energy Future. Available online: https://www.conserve-energy-future.com/advantages-and-disadvantages-of-biofuels.php (accessed on 22 December 2018).
- Hoover, F.-A.; Abraham, J. Biofuels can help solve climate change, especially with a carbon tax. Int. J. Sustain. Energy 2009, 28, 171–182. [Google Scholar] [CrossRef]
- Giakoumis, E.G.; Sarakatsanis, C.K. A Comparative Assessment of Biodiesel Cetane Number Predictive Correlations Based on Fatty Acid Composition. Energies 2019, 12, 422. [Google Scholar] [CrossRef]
- Kaya, T.; Kutlar, O.A.; Taskiran, O.O. Evaluation of the Effects of Biodiesel on Emissions and Performance by Comparing the Results of the New European Drive Cycle and Worldwide Harmonized Light Vehicles Test Cycle. Energies 2018, 11, 2814. [Google Scholar] [CrossRef]
- Wan Ghazali, W.N.M.; Mamat, R.; Masjuki, H.H.; Najafi, G. Effects of biodiesel from different feedstocks on engine performance and emissions: A review. Renew. Sustain. Energy Rev. 2015, 51, 585–602. [Google Scholar] [CrossRef]
- Mofijur, M.; Hazrat, M.A.; Rasul, M.G.; Mahmudul, H.M. Comparative Evaluation of Edible and Non-edible Oil Methyl Ester Performance in a Vehicular Engine. Energy Procedia 2015, 75, 37–43. [Google Scholar] [CrossRef]
- Mofijur, M.; Atabani, A.E.; Masjuki, H.H.; Kalam, M.A.; Masum, B.M. A study on the effects of promising edible and non-edible biodiesel feedstocks on engine performance and emissions production: A comparative evaluation. Renew. Sustain. Energy Rev. 2013, 23, 391–401. [Google Scholar] [CrossRef]
- Shaafi, T.; Sairam, K.; Gopinath, A.; Kumaresan, G.; Velraj, R. Effect of dispersion of various nanoadditives on the performance and emission characteristics of a CI engine fuelled with diesel, biodiesel and blends—A review. Renew. Sustain. Energy Rev. 2015, 49, 563–573. [Google Scholar] [CrossRef]
- Saxena, V.; Kumar, N.; Saxena, V.K. A comprehensive review on combustion and stability aspects of metal nanoparticles and its additive effect on diesel and biodiesel fuelled C.I. engine. Renew. Sustain. Energy Rev. 2017, 70, 563–588. [Google Scholar] [CrossRef]
- Aalam, C.S.; Saravanan, C.G. Effects of nano metal oxide blended Mahua biodiesel on CRDI diesel engine. Ain Shams Eng. J. 2017, 8, 689–696. [Google Scholar] [CrossRef]
- Nanoparticles Increase Biofuel Performance. Available online: https://www.sciencedaily.com/releases/2011/04/110408075042.htm (accessed on 25 August 2018).
- Soutter, B.W. Nanoparticles as Fuel Additives. 2012, pp. 1–3. Available online: https://www.azonano.com/article.aspx?ArticleID=3085 (accessed on 17 February 2019).
- Arul Mozhi Selvan, V.; Anand, R.B.; Udayakumar, M. Effects of Cerium Oxide Nanoparitcle Addition in Diesel and Diesel-Biodiesel-Ethanol Blends on the performance and emission characteristics of a C1 Engine. ARPN J. Eng. Appl. Sci. 2009, 4, 1–6. [Google Scholar]
- Gan, Y.; Qiao, L. Combustion characteristics of fuel droplets with addition of nano and micron-sized aluminum particles. Combust. Flame 2011, 158, 354–368. [Google Scholar] [CrossRef]
- Sadhik Basha, J.; Anand, R.B. Role of nanoadditive blended biodiesel emulsion fuel on the working characteristics of a diesel engine. J. Renew. Sustain. Energy 2011, 3. [Google Scholar] [CrossRef]
- Saraee, H.S.; Jafarmadar, S.; Taghavifar, H.; Ashraf, S.J. Reduction of emissions and fuel consumption in a compression ignition engine using nanoparticles. Int. J. Environ. Sci. Technol. 2015, 12, 2245–2252. [Google Scholar] [CrossRef]
- Valencia, M.; López, E.; Andrade, S.; Iris, M.L.; Hurtado, N.G.; Pérez, V.R.; García, A.G.; de Lecea, C.S.M.; Bueno López, A. Evidences of the Cerium Oxide-Catalysed DPF Regeneration in a Real Diesel Engine Exhaust. Available online: https://core.ac.uk/download/pdf/32320231.pdf (accessed on 11 February 2019).
- Razek, S.M.A.; Gad, M.S.; Thabet, O.M. Effect of Aluminum Oxide Nano-Particle in Jatropha Biodiesel on Performance, Emissions and Combustion Characteristics of D I Diesel Engine. Ijraset 2017, 5, 358–372. [Google Scholar] [CrossRef]
- Ramesh, D.K.; Dhananjaya Kumar, J.L.; Hemanth Kumar, S.G.; Namith, V.; Basappa Jambagi, P.; Sharath, S. Study on effects of Alumina nanoparticles as additive with Poultry litter biodiesel on Performance, Combustion and Emission characteristic of Diesel engine. Mater. Today Proc. 2018, 5, 1114–1120. [Google Scholar] [CrossRef]
- Ghanbari, M.; Najafi, G.; Ghobadian, B.; Yusaf, T.; Carlucci, A.P.; Kiani Deh Kiani, M. Performance and emission characteristics of a CI engine using nano particles additives in biodiesel-diesel blends and modeling with GP approach. Fuel 2017, 202, 699–716. [Google Scholar] [CrossRef]
- Kumar, S.; Dinesha, P.; Bran, I. Influence of nanoparticles on the performance and emission characteristics of a biodiesel fuelled engine: An experimental analysis. Energy 2017, 140, 98–105. [Google Scholar] [CrossRef]
- Chandrasekaran, V.; Arthanarisamy, M.; Nachiappan, P.; Dhanakotti, S.; Moorthy, B. The role of nano additives for biodiesel and diesel blended transportation fuels. Transp. Res. Part D Transp. Environ. 2016, 46, 145–156. [Google Scholar] [CrossRef]
- Sadhik Basha, J. Impact of Carbon Nanotubes and Di-Ethyl Ether as additives with biodiesel emulsion fuels in a diesel engine—An experimental investigation. J. Energy Inst. 2018, 91, 289–303. [Google Scholar] [CrossRef]
- Heydari-Maleney, K.; Taghizadeh-Alisaraei, A.; Ghobadian, B.; Abbaszadeh-Mayvan, A. Analyzing and evaluation of carbon nanotubes additives to diesohol-B2 fuels on performance and emission of diesel engines. Fuel 2017, 196, 110–123. [Google Scholar] [CrossRef]
- Hoseini, S.S.; Najafi, G.; Ghobadian, B.; Mamat, R.; Ebadi, M.T.; Yusaf, T. Novel environmentally friendly fuel: The effects of nanographene oxide additives on the performance and emission characteristics of diesel engines fuelled with Ailanthus altissima biodiesel. Renew. Energy 2018, 125, 283–294. [Google Scholar] [CrossRef]
- Dhinesh, B.; Maria Ambrose Raj, Y.; Kalaiselvan, C.; KrishnaMoorthy, R. A numerical and experimental assessment of a coated diesel engine powered by high-performance nano biofuel. Energy Convers. Manag. 2018, 171, 815–824. [Google Scholar] [CrossRef]
- Dhinesh, B.; Niruban Bharathi, R.; Isaac JoshuaRamesh Lalvani, J.; Parthasarathy, M.; Annamalai, K. An experimental analysis on the influence of fuel borne additives on the single cylinder diesel engine powered by Cymbopogon flexuosus biofuel. J. Energy Inst. 2017, 90, 634–645. [Google Scholar] [CrossRef]
- Wu, Q.; Xie, X.; Wang, Y.; Roskilly, T. Effect of carbon coated aluminum nanoparticles as additive to biodiesel-diesel blends on performance and emission characteristics of diesel engine. Appl. Energy 2018, 221, 597–604. [Google Scholar] [CrossRef]
- Prabu, A. Nanoparticles as additive in biodiesel on the working characteristics of a DI diesel engine. Ain Shams Eng. J. 2017, 9, 2343–2349. [Google Scholar] [CrossRef]
- Najafi, G. Diesel engine combustion characteristics using nano-particles in biodieseldiesel blends. Fuel 2018, 212, 668–678. [Google Scholar] [CrossRef]
- Chhetri, A.B.; Tango, M.S.; Budge, S.M.; Watts, K.C.; Islam, M.R. Non-edible plant oils as new sources for biodiesel production. Int. J. Mol. Sci. 2008, 9, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Banković-Ilić, I.B.; Stamenković, O.S.; Veljković, V.B.; Stamenkovi, O.S.; Veljkovi, V.B.; Bankovi, I.B. Biodiesel production from non-edible plant oils. Renew. Sustain. Energy Rev. 2012, 16, 3621–3647. [Google Scholar] [CrossRef]
- Hossain, A.K.; Hussain, A. Effect of Nanoparticle on Combustion and Emission Characteristics of neat Jatropha Biodiesel (Paper No 0552-1). In Proceedings of the 13th SDEWES Conference, Palermo, Italy, 30 September–4 October 2018. [Google Scholar]
- Heroor, S.H.; Bharadwaj, S.D.R. Production of Bio-fuel from Crude Neem Oil and its Performance. Int. J. Environ. Eng. Manag. 2013, 4, 425–432. [Google Scholar]
- Chongkhong, S.; Kanjaikaew, U.; Tongurai, C.; Yai, H. A Review of FFA Esterification for Biodiesel Production. 10th Int. PSU Eng. Conf. 2012, 1, 1–5. [Google Scholar]
- Patil, P.D.; Deng, S. Optimization of biodiesel production: Transesterification of edible and non-edible vegetable oils. Fuel 2009, 88, 1302–1306. [Google Scholar] [CrossRef]
- Singh, R.; Padhi, S. Characterization of jatropha oil for tha preparation of biodiesel. Nat. Prod. Radiance 2009, 8, 127–132. [Google Scholar]
- Kumar Tiwari, A.; Kumar, A.; Raheman, H. Biodiesel production from jatropha oil (Jatropha curcas) with high free fatty acids: An optimized process. Biomass Bioenergy 2007, 31, 569–575. [Google Scholar] [CrossRef]
- Ma, F.; Hanna, M.A. Biodiesel production: A review. Bioresour. Technol. 1999, 70, 1–15. [Google Scholar] [CrossRef]
- Kumar, A. Effects of Cerium Oxide Nanoparticle on Compression Ignintion Engine Performance and Emission Charachteristic when Using Water Diesel Emuslion. Master’s Thesis, Thapar University, Patiala, India, 2014. [Google Scholar]
- Enweremadu, C.C.; Rutto, H.L. Combustion, emission and engine performance characteristics of used cooking oil biodiesel—A review. Renew. Sustain. Energy Rev. 2010, 14, 2863–2873. [Google Scholar] [CrossRef]
- Dhar, A.; Agarwal, A.K. Performance, emissions and combustion characteristics of Karanja biodiesel in a transportation engine. Fuel 2014, 119, 70–80. [Google Scholar] [CrossRef]
- Bakeas, E.; Karavalakis, G.; Stournas, S. Biodiesel emissions profile in modern diesel vehicles. Part 1: Effect of biodiesel origin on the criteria emissions. Sci. Total Environ. 2011, 409, 1670–1676. [Google Scholar] [CrossRef] [PubMed]
- Qi, D.H.; Chen, H.; Geng, L.M.; Bian, Y.Z.; Ren, X.C. Performance and combustion characteristics of biodiesel-diesel-methanol blend fuelled engine. Appl. Energy 2010, 87, 1679–1686. [Google Scholar] [CrossRef]
- Kadarohman, A.; Hernani; Rohman, I.; Kusrini, R.; Astuti, R.M. Combustion characteristics of diesel fuel on one cylinder diesel engine using clove oil, eugenol, and eugenyl acetate as fuel bio-additives. Fuel 2012, 98, 73–79. [Google Scholar] [CrossRef]
- Singh, A.P.; Agarwal, A.K. Combustion characteristics of diesel HCCI engine: An experimental investigation using external mixture formation technique. Appl. Energy 2012, 99, 116–125. [Google Scholar] [CrossRef]

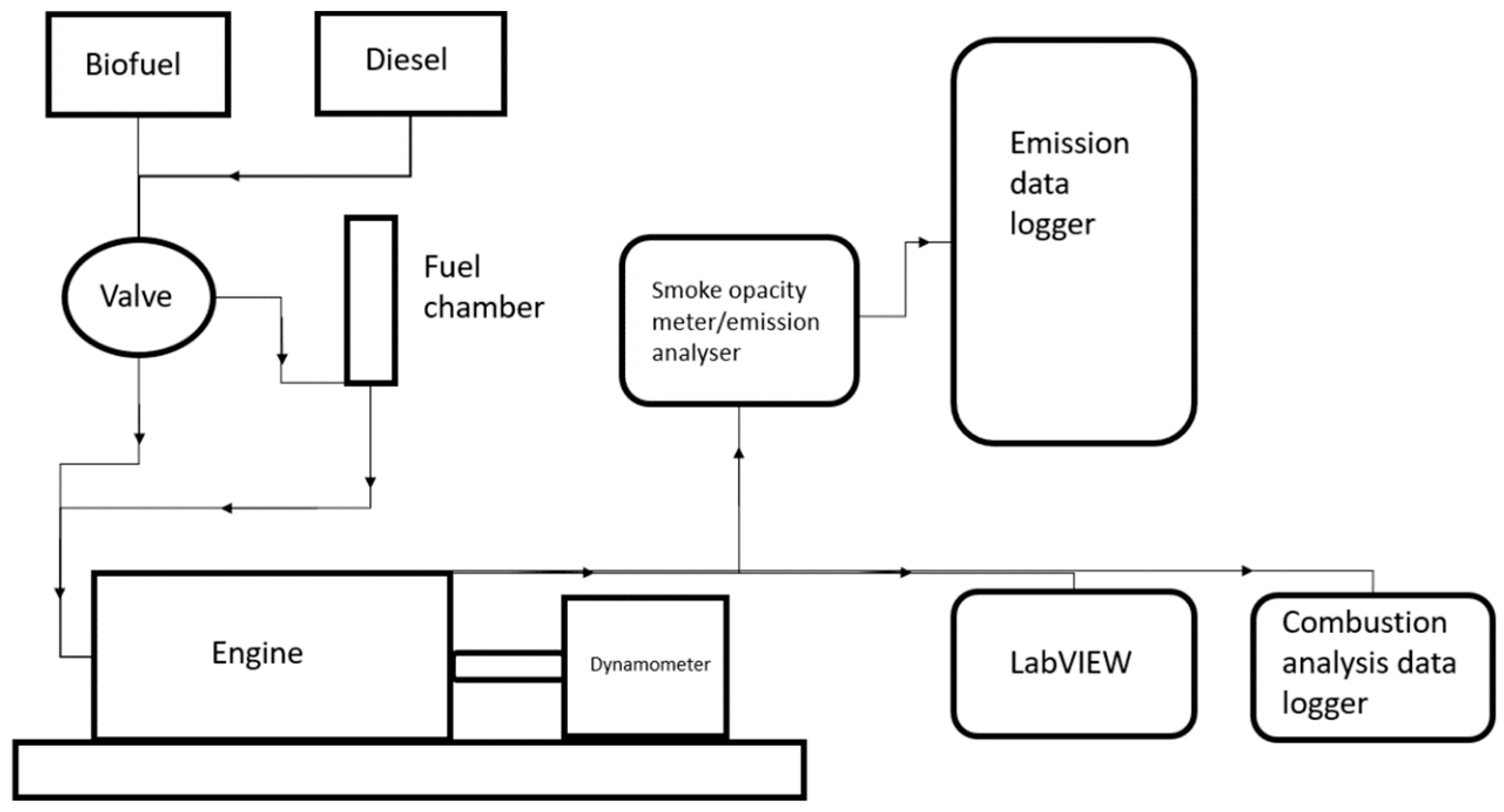


| Model/Type | LPWS Bio3 Water Cooled |
|---|---|
| No. of cylinders | 3 |
| Rated speed | 1500 rpm |
| Continuous power at rated speed | 9.9 kW |
| Type of fuel injection | Indirect injection with individual fuel injection pumps |
| Fuel pump injection timing | 20_ BTDC |
| Continuous power fuel consumption at 1500 rpm | 3.19 L/h (fossil diesel) |
| Exhaust gas flow | 41.4 L/s at full loads at 1500 rpm |
| Property | Units | Neat Diesel | Jatropha Curcas Oil (JCO) | Jatropha Biodiesel (J100) | J100C50 | J00C100 | J00A50 | J00A100 | EN 14214 Standards (Biodiesel) |
|---|---|---|---|---|---|---|---|---|---|
| Acid Value | mg KOH/g | 0.34 | 13.59 | 0.45 | 0.28 | 0.20 | 0.20 | 0.20 | <0.50 |
| Flash point | °C | 63.6 | 181.8 | 171.2 | 174.8 | 177 | 173.6 | 175.6 | >101 |
| Density | Kg/m3 | 832.3 | 922.6 | 881.6 | 880 | 879 | 877 | 878 | 860–900 |
| Viscosity at 22 °C | cSt | 3 | 75.57 | 4.73 | 5.92 | 6.23 | 6.03 | 5.95 | N/A |
| Viscosity at 40 °C | cSt | 2.13 | 37.47 | 3.37 | 3.99 | 4.08 | 4.11 | 4.07 | >3.5–5.0 |
| HHV | MJ/kg | 45.64 | 39.39 | 37.54 | 37.39 | 37.29 | 37.27 | 37.34 | N/A |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hossain, A.K.; Hussain, A. Impact of Nanoadditives on the Performance and Combustion Characteristics of Neat Jatropha Biodiesel. Energies 2019, 12, 921. https://doi.org/10.3390/en12050921
Hossain AK, Hussain A. Impact of Nanoadditives on the Performance and Combustion Characteristics of Neat Jatropha Biodiesel. Energies. 2019; 12(5):921. https://doi.org/10.3390/en12050921
Chicago/Turabian StyleHossain, Abul Kalam, and Abdul Hussain. 2019. "Impact of Nanoadditives on the Performance and Combustion Characteristics of Neat Jatropha Biodiesel" Energies 12, no. 5: 921. https://doi.org/10.3390/en12050921
APA StyleHossain, A. K., & Hussain, A. (2019). Impact of Nanoadditives on the Performance and Combustion Characteristics of Neat Jatropha Biodiesel. Energies, 12(5), 921. https://doi.org/10.3390/en12050921





