Abstract
This work aims to shed light on the use of two biochars, obtained from the pyrolysis at 550 °C of heavy-metal-contaminated Jatropha curcas L. roots, as heterogeneous catalysts for glycerol esterification using residual glycerine. To do this, glycerine from biodiesel production was purified. In a first step, H3PO4 or H2SO4 was used to remove non-glycerol organic matter. The glycerol-rich phase was then extracted with ethanol or propanol, which increased the glycerol content from 43.2% to up to 100%. Subsequently, the esterification of both purified glycerine and commercial USP glycerine was assayed with acetic acid (AA) or with acetic anhydride (AH) at 9:1 molar ratio to glycerol using Amberlyst-15 as catalyst. Different reaction times (from 1.5 to 3 h) and temperatures (100–115 °C when using AA and 80–135 °C when using AH) were assessed. Results revealed that the most suitable conditions were 80 °C and 1.5 h reaction time using AH, achieving 100% yield and selectivity towards triacetylglycerol (TAG) almost with both glycerines. Finally, the performance and reuse of the two heterogeneous biocatalysts was assessed. Under these conditions, one of the biocatalysts also achieved 100% TAG yield.
1. Introduction
The increasing production of biodiesel has resulted in an oversupply of its by-product, glycerine [1]. Roughly 1 kg glycerine (crude glycerol) is produced from every 10 kg biodiesel produced by transesterification [2]. As 42 billion liters biodiesel will be produced in 2020, according to projections, it is estimated that 4.2 billion liters glycerine will be available that year [1].
Glycerine or crude glycerol obtained from biodiesel production is composed of not only glycerol (40–70% wt.) but also methanol, water, salts, and soap as well as other triglycerides that have not entirely reacted (di- and mono-glycerides). The most commonly used methods for glycerine purification are based on simple distillation. The main hindrances of these purification methods are the high energy required for glycerol vaporization and the low process yield [3,4]. A sequential physicochemical purification method based on acidification driven phase separation, polar solvent extraction, and subsequent adsorption with activated carbon has been proposed, reaching 93.3% (wt.) [2] and 95.7% (wt.) [3] glycerol purity when using sulfuric acid and phosphoric acid, respectively, in the acidification stage, and ethanol and propanol as solvent, respectively, for glycerol extraction.
Glycerol is a polar molecule that is not suitable for blending with fuels due to its low solubility in hydrocarbons. However, glycerol is regarded as a good raw material to produce oxygenated additives in fuels because of its high oxygen content (roughly 52% wt.) [5], hence the need to convert glycerol into miscible compounds with biodiesel and petroleum fuels.
Fuel additives are chemical substances that added in small quantities, help to clean some parts of the engine such as the carburettor, the valve inlet, or the injector inlet. In addition, they prevent incomplete combustion and protect the different parts of the engine from corrosion, resulting in better acceleration and engine performance, fuel consumption enhancement and greenhouse gas emissions reduction [6,7].
The most common oxygenated additives are those that contain alcohols, ethers, or esters functional groups. The transformation of glycerol into oxygenated compounds can be carried out through etherification, esterification, transesterification, and acetylation, rendering the corresponding glycerol ethers, esters, and acetals. The products obtained from the esterification of glycerol can be used as oxygenated additives for vehicle engines [8,9].
Acetic acid (AA) is the most commonly reagent used in the glycerol esterification reaction [8,9,10]. Monoacetylglycerol or monoacetin (MAG), diacetylglycerol or diacetin (DAG) and triacetylglycerol or triacetin (TAG) are consecutively obtained in this three-step reaction. Among them, TAG is regarded as the most effective oxygenated additive, improving diesel and biodiesel viscosity and cold properties, increasing diesel cetane number and reducing noxious gas emission [5,9,10,11,12]. What is more, TAG is an octane booster for gasoline [13]. Therefore, TAG is an oxygenated additive suitable for gasoline, biodiesel, and diesel. However, glycerol esterification with AA is thermodynamically resistant because of its positive Gibbs free energy [1,10], thus rendering a mixture of these three compounds.
Acetic anhydride (AH) is a strong acylating agent that renders the esterification reaction more efficiently than carboxylic acids such AA. The use of acetic anhydride as reagent results in a higher selectivity towards TAG because this reaction is highly exothermic with negative Gibbs free energy, thus favoring the production of TAG [10]. During the three-step esterification with acetic anhydride, an AA molecule is obtained from each acetic anhydride molecule that reacts, which can react in turn with glycerol or with the produced MAG and DAG, thus increasing the glycerol conversion and the selectivity towards TAG [14]. However, AH is used in narcotic production so many countries have enlisted it as contraband and even prohibited it [1], which is a strong hindrance for industrial applications. On the contrary, AA is cheaper and readily available.
There are various heterogeneous, acid catalysts for glycerol esterification, mainly ion-exchange organic resins, such as Amberlyst-15, zeolites, and silica-supported heteropoly acids, which are comprehensively reviewed elsewhere [1]. Among them, the acid exchange resin Amberlyst-15 is the most commonly catalyst used for the synthesis of oxygenated fuel additives since it provides higher selectivity towards DAG and TAG [9]. Nevertheless, the low thermal stability of Amberlyst-15 (<140 °C) is a hindrance for its applicability in catalysis. In addition, the presence of water, which is produced during esterification, decreases its catalytic activity due to the highly hygroscopic nature of this resin [12,15].
Because of these limitations, researchers have put efforts to obtain new alternative catalysts. Recent studies have shown the potential of sulfonated carbon catalysts for glycerol esterification [16,17,18]. However, to the best of our knowledge, there are not available literature on the production of natural catalysts without submitting them to chemical treatment. In this sense, plants used in the phytoremediation of metal-contaminated areas are regarded as residues. Due to its content in carbon and metals, a potential application of this residual biomass could be the production of acid, heterogeneous biocatalysts by pyrolysis [19]. One of the plants that has been widely used for the phytoremediation of heavy-metal-contaminated soils is Jatropha curcas L. This plant belongs to the Euphorbiaceae family, requires little water to grow and can survive on marginal, eroded, and depleted lands. The biochar obtained from the pyrolysis of the contaminated J. curcas L. roots has been found to have graphite structure by Raman spectroscopy [19], hence its potential as biocatalyst.
The aim of this work was to assess the performance of two heterogeneous biocatalysts, obtained from the pyrolysis at 550 °C of heavy-metal-contaminated J. curcas L. roots, in the esterification of purified residual glycerine to obtain TAG. To do this, a sequential physicochemical purification method was firstly optimized to achieve glycerine with high glycerol purity from glycerine obtained as by-product from biodiesel production from waste cooking oil. Then, the esterification of commercial USP glycerine with either AA or AH was optimized using a commercial heterogeneous catalyst (Amberlyst-15). Finally, the performance of the two biocatalysts in the esterification of both USP glycerine and purified glycerine was tested under the most suitable conditions found with Amberlyst-15.
2. Materials and Methods
2.1. Residual Glycerine
Crude glycerol was obtained as by-product in a previous work on the production of biodiesel from waste cooking oil using an oscillatory flow reactor [20].
2.2. Purification of Residual Glycerine
Purification of crude glycerol was performed using a combination of chemical and physical treatments with solvent extraction, as described elsewhere [2,3]. A factorial design was carried out using two acids (H2SO4 and H3PO4) at three pH values (1, 2 and 4), and 2 polar solvents (ethanol and propanol) at 3 glycerine to solvent ratios (3:1, 1:1, 1:3). Briefly, 300 g glycerine (initial pH = 9) were acidified to the desired pH with H2SO4 or H3PO4 and let stand for 12 h for phase separation by decantation. The glycerol-rich layer was then neutralized with 5 M NaOH, centrifuged for salt removal and extracted with ethanol or propanol, the solvent being subsequently separated from purified crude glycerol using a rotary evaporator. Finally, the purified glycerine was let stand along with 0.2 g activated carbon for 24 h to remove its color and potential traces of some fatty acids and other impurities. This activated carbon was previously dried at 105 °C for 30 min to eliminate moisture, and removed from purified glycerine by filtering on a Whatman WH 1440 filter. All the experiments were performed in duplicate.
Once the optimal conditions were found, 12 more replicas were performed under these conditions, the resulting glycerol being put together, analyzed, and stored for subsequent oxygenated fuel additives production trials.
2.3. Biocatalysts Production
The dried and sieved roots of Jatropha curcas L. plants that were previously used for the phytoremediation of 2 mining soils [19] were subjected to pyrolysis under inert nitrogen atmosphere at 550 °C to obtain 2 biocatalysts. This temperature was selected because it has proven to be the most suitable to obtain biochar with graphite structure from J. curcas L. heavy-metal-contaminated roots [19]. Biocatalyst 1 was obtained from roots containing 1118, 16.4, 44.7 and 22.4 mg kg−1 Fe, Cu, Zn, and Pb, respectively, while the roots from which Biocatalyst 2 was obtained contained 1841, 22.4, 124.1 and 57.1 mg kg−1 Fe, Cu, Zn, and Pb, respectively.
To do this, 0.5 g roots were placed in a 25 × 300 quartz tube and introduced in a Carbolite Tube Furnace MTF 12/38/250 equipped with a Eurotherm 2416CG temperature controller. The outlet of the quartz tube was connected to two bubblers submerged in ice to condense the flue gases (bio-oils), an extractor hood eliminating the uncondensed gases. The N2 flow was set to 6 dm3 h−1, and the heating rate to 30 °C min−1 until reaching 550 °C, temperature that was maintained for 2 h. The resulting biochars, with graphite-like structure [19], were used as acid heterogeneous biocatalysts for the synthesis of oxygenated fuel additives.
2.4. Production of Oxygenated Fuel Additives
Glycerol esterifications were performed in a 2-dm3 bath reactor equipped with a temperature controller and a water-cooled condenser, the stirring speed being set at 500 rpm. Reaction time (up to 3 h) and temperature (from 100 to 115 °C when using AA and from 80 to 135 °C when using AH, the maximum temperatures limited by their boiling temperatures), were the experimental parameters assessed. The AA/glycerol or AH/glycerol molar ratio, and the catalyst loading, were set to 9:1 and 1.76% (wt.), respectively, based on other authors’ results on the acetylation of glycerol with AA over Amberlyst-15 [21] and acidic mesoporous silica [12]. These esterifications were performed using USP glycerine (≥99% glycerol) and Amberlyst-15 as catalyst. After reaction time, the AA or AH excess was removed by rotary evaporation, and the oxygenated additives were separated from the catalyst by vacuum filtration.
Once the optimal conditions were found, esterification of both purified glycerine and commercial USP glycerine was performed with the 2 biocatalysts (at three loading levels relative to mixture weight, namely 1.76%, 0.88% and 0.44%, respectively) and Amberlyst-15 (1.76% wt.) under these conditions. All the experiments were carried out in duplicate.
2.5. Catalysts Deactivation
Catalyst active sites deactivation frequently limits industrial applications of heterogeneous catalysts. For that reason, 5 consecutives glycerol esterifications (using USP glycerine) with AH over Amberlyst-15 and Biocatalyst 2, respectively, were performed to assess the number of reactions they can catalyze without affecting TAG yield and selectivity. Furthermore, an esterification of USP glycerine with AH without catalyst was performed for further comparison. These experiments were performed in duplicate under the conditions that maximized selectivity towards TAG.
2.6. Analytical Methods
Glycerol, MAG, DAG and TAG concentrations in samples were analyzed by GC-MS using a Thermo Scientific TRACE 1300 gas chromatograph coupled to a Thermo Scientific TSQ 8000 mass spectrometer. The GC-MS column was a Zebron ZB-1ms column (30 m × 0.25 mm × 0.25 µm), and 1,4-butanediol the internal standard. Standards of USP glycerine, MAG, DAG, and TAG were used as references. The retention times were 2.53 min for 1,4 butanediol, 2.75 min for glycerol, 4.86 for MAG, 8.23 and 8.30 for DAG isomers, and 10.18 min for TAG.
3. Results and Discussion
3.1. Glycerine Purification
Residual glycerine from the production of waste cooking oil-derived biodiesel contained 43.2 ± 0.5% (wt.) glycerol. The rest was inorganic salts (mainly sodium salts because NaOH was the used catalyst for the transesterification), MONG (matter organic non-glycerol), water, soluble soaps from free fatty acids of the starting waste cooking oil and methyl esters.
When glycerine was acidified with H3PO4, three phases were found after decantation: an upper layer containing MONG, and intermediate glycerol-rich phase, and an inorganic salts phase on the bottom. However, only 2 phases were found when working with H2SO4 because the inorganic salts layer was not observed, which agrees with the results of Manosak et al. [3] but contradictory with those reported by Kongjao et al. [2]. This could be explained by fact that NaH2PO4 is insoluble in glycerol and poorly soluble in water, while NaHSO4 is soluble in water and thus can dissolve in the water contained in the glycerol-rich phase.
The use of H3PO4 led to higher glycerol purity than when using H2SO4 under the same conditions (Table 1). This can be related to the removal of the aforementioned inorganic salts layer. The most suitable conditions were: acidification to pH 2 with H3PO4 followed by extraction of glycerol from the glycerol-rich phase with propanol at 1:1 (v/v) propanol:glycerine ratio and adsorption with 0.2 g activated carbon per g glycerine. Under these conditions, pure glycerol was obtained, improving the results obtained by the aforementioned authors [2,3]. A mass balance was performed with the 12 additional replicas carried out under these conditions (Figure 1). The yield of the process, defined as g glycerol per g crude glycerol and expressed in percentage, was 24.5%. Taking into account that residual glycerine contained 43.2 ± 0.5% (wt.) glycerol, the glycerol recovery was 56.7%. However, the purity of the resulting glycerol when combining the glycerol obtained in the 12 replicas was 97%.

Table 1.
Glycerol purity reached in each assayed condition for glycerine purification.
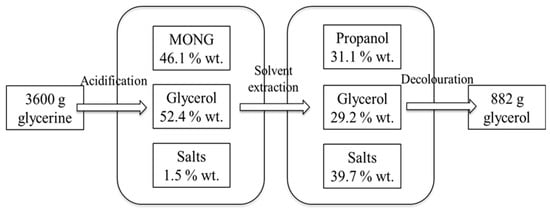
Figure 1.
Mass balance of the crude glycerol purification under the most suitable conditions.
3.2. Synthesis of Oxygenated Fuel Additives over Amberlyst-15
Optimal AA/glycerol molar ratio for DAG production is 6:1, beyond which DAG is converted into TAG [1]. Previous research has reported that the optimal temperature and AA/glycerol molar ratio for glycerol esterification over Amberlyst-15 are 110 °C and 9:1, respectively [21]. Various authors reached 97% glycerol conversion using this AA to glycerol molar ratio, but the selectivities towards TAG were low: 13% [9] and 43% [21]. Therefore, the effect of temperature on the USP glycerine esterification with AA and AH over Amberlyst-15 was firstly screened. The selected range of temperatures was from 100 °C to just-below the boiling point of each acetylation agent (Table 2), reaction time being 3 h.

Table 2.
DAG and TAG yields obtained in the USP glycerine esterification over Amberlyst-15 under different conditions.
MAG was not detected in the reaction product under any of the assayed conditions, and the glycerol conversion (into DAG + TAG) was always 100%. As it can observed, the selectivity towards TAG was higher when using AH instead of AA. However, our TAG yields using AA as acylation agent were higher than those reached by the aforementioned authors in the glycerol esterification with AA over Amberlyst-15 using the same AA/glycerol molar ratio [9,22] and than those obtained by other authors who assayed 4:1 AA/glycerol molar ratio, achieving 100% glycerol conversion and 24% TAG selectivity in the glycerol esterification over Amberlyst-15 at 120 °C [14]. Several authors have assayed other catalysts, but their performance was lower than that obtained with Amberlyst-15. The glycerol esterification over sulfonic acid-modified SBA-15 catalyst under similar conditions (125 °C, 9:1 AA to glycerol molar ratio, 4 h reaction time) achieved 90% glycerol conversion and 85% combined selectivity towards DAG and TAG [12]. More recently, Lewatit catalyst (3% wt.) reached 73.4% glycerol conversion using 7:1 AA/glycerol molar ratio at 100 °C for 100 min [13], and solid acid catalyst synthetized from crude glycerol via partial carbonization and sulfonation in a single step reached a maximum of 88% combined selectivity towards DAG and TAG at 2% (wt.) catalyst dosage, 110 °C, 3:1 AA/glycerol molar ratio and 180 min reaction time [11].
USP glycerine esterification with AH over Amberlyst-15 achieved roughly full glycerol conversion into TAG under all the temperatures assayed. This fact is because one molecule of AA is obtained from each molecule AH during esterification with AH, which in turn replaces another free -OH group of glycerol, thus increasing the glycerol conversion rate and selectivity towards TAG when using AH instead of AA under the same conditions. The esterification with AA leads to the formation of a molecule of water per molecule of reacting AA, which causes thermodynamic limitations and hinders AA consumption. Water can also deactivate acid solid catalysts, such as Amberlyst-15, because its high affinity to their active sites. In this sense, Zhou et al. [21] found that only 74% AA was consumed at the end of the esterification, so 100% TAG selectivity cannot be reached when esterifying glycerol with AA. This limitation could be overcome by removing water from reaction medium as the reaction proceeds [12]. Therefore, conditions for glycerol esterification with AH can be milderer. As observed in Table 2, the reduction, firstly, of temperature to 80 °C and, secondly, of reaction time to 90 min did not affect the TAG yield, being close to 100%. It has been reported that Amberlyst-15 acid resin yields 100% selectivity towards TAG after 80 min of reaction and 4:1 AH/glycerol molar ratio at 60 °C [14] but these conditions could not been replicated in this work because of mixing problems when working at these low temperature and AH/glycerol molar ratio. Therefore, the purified glycerine (97% glycerol content) was esterified over Amberlyst-15 using 9:1 AA/glycerol molar ratio at 80 °C for 90 min. Under these conditions, DAG, and TAG yields were 5.12 ± 1.4 and 91.9 ± 3.5, respectively, achieving 100% glycerol conversion (Table 3).

Table 3.
DAG and TAG yields obtained in glycerol esterification with AH at 80 °C for 90 min over Amberlyst-15 and biocatalysts.
3.3. Synthesis of Oxygenated Fuel Additives over Biocatalysts
The optimal Amberlyst-15 dosage for oxygenated fuel additives production is 1.76% (wt.), as described elsewhere [21]. However, the dosage of the new biocatalysts is obviously unknown. As can be observed in Table 4, both composition and structure (BET surface area, average pore diameter and total pore volume) of Amberlyst-15 are different from those of the biochars. For this reason, three level of biocatalysts weight percentages in relation to the reaction mixture were assayed, namely 1.76%, 0.88% and 0.44% (Table 3), in the USP glycerine esterification with 9:1 AA/glycerol molar ratio at 80 °C for 90 min.

Table 4.
Main characteristics of the heterogeneous catalysts.
Complete glycerol conversion into DAG and TAG was obtained from USP glycerine at the three catalyst dosages for both biocatalysts, the selectivity towards TAG being higher for Biocatalyst 2 (Table 3). MAG was not detected in any reaction product. Biocatalyst 2 was obtained from more heavy-metal-contaminated roots than Biocatalyst 1. The higher metals content and, consequently, the higher acidic character that these metals provide to the carbon-based (graphite structure) Biocatalyst 2 could be responsible for the better catalytic action of this biocatalyst. The optimal Biocatalyst 2 dosage for TAG production was 0.88%, reaching roughly 100% TAG selectivity and yield.
Finally, the esterification of the purified glycerine at laboratory (97% glycerol content) with AH under the optimal conditions over Biocatalyst 2 (0.88% wt.) was assayed. Complete glycerine conversion was attained as well, TAG yield and selectivity being 96.1% and 99.1%, respectively. This fact is of major importance since glycerine cannot be present in fuel additives because they cause corrosion in engines. Results illustrated that glycerine from waste cooking oil-derived biodiesel was successfully purified achieving high glycerol content at laboratory, without containing any undesirable element that could hindrance its esterification. Its purity could be ranging between technical grade and USP glycerine. Furthermore, the performance of Biocatalyst 2 was similar to that of the most common catalyst used for esterification reactions, i.e., Amberlyst-15.
3.4. Deactivation of Catalysts
The whole USP glycerine esterifications with AH achieved 100% glycerol conversion towards DAG and TAG, MAG production not being detected even in the trial without catalyst (Table 5). The performance of both Amberlyst-15 and Biocatalyst 2 lowered from the first reuse. From the second reuse on, TAG yield achieved with both catalysts drastically diminished, being even lower than that in absence of catalyst. This could imply not only the catalyst active sites are deactivated, but also the presence of a deactivated catalyst in the reaction medium is a hindrance for the reaction (Table 5). Figure 2 shows the GC-MS chromatograms of the esterification products reusing up to 4 times Biocatalyst 2 (0.88% wt.). Regarding the reaction in absence of catalyst, the increase of temperature markedly improves the selectivity towards TAG. In this sense, the glycerol esterification with 4:1 AH/glycerol molar ratio for 120 min achieved 10%, 56% and 34% MAG, DAG, and TAG selectivities, respectively, at 60 °C, while selectivities of 6% DAG and 94% TAG were obtained at 120 °C [14]. Using 9:1 AH/glycerol molar ratio at 80 °C for 90 min, 22.2% and 77.8% DAG and TAG selectivities, respectively, were achieved in the present work (Table 5).

Table 5.
Reusability of Amberlyst-15 and Biocatalyst 2 for USP glycerine esterification with 9:1 AH/glycerol molar ratio at 80 °C for 90 min.
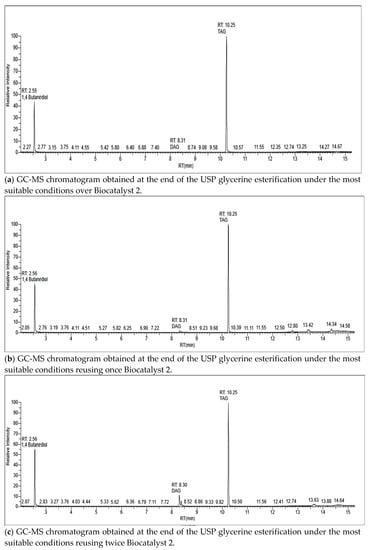
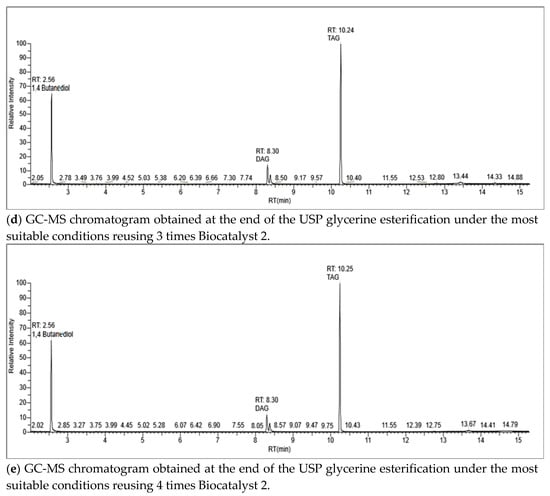
Figure 2.
GC-MS chromatograms obtained during Biocatalyst 2 reuse trials.
4. Conclusions
The best crude glycerol purification conditions were acidification with H3PO4 to pH 2 to remove MONG and inorganic salts, followed by extraction of the glycerol-rich phase with propanol (1:1 molar ratio) and decoloration with activated carbon. The resulting glycerol purity ranged between technical grade and USP glycerine.
The most suitable glycerol esterification conditions were 80 °C for 1.5 h using 9:1 acetic anhydride/glycerol molar ratio, triacetin yield being 100% over both Amberlyst-15 and Biocatalyst 2, regardless of the starting glycerine (USP glycerine or purified crude glycerol). Biocatalyst 2 achieved the same esterification performance as Amberlyst-15 under the experimental conditions at half catalyst dosage (0.88% wt. vs 1.76% wt.), which is an advantage. When comparing the performance of the two assayed biocatalysts, the one obtained from the pyrolysis of the most heavy-metal-contaminated Jatropha curcas L. roots (Biocatalyst 2) showed the best catalytic action, probably due to its higher metal content and, therefore, acidic character. Finally, both Biocatalyst 2 and Amberlyst-15 can be reused only once before complete deactivation under the assayed conditions.
Author Contributions
Conceptualization, P.Á.-M.; methodology, P.Á.-M.; formal analysis, P.Á.-M., J.F.G.-M., M.T.-G and C.-H.F.; investigation, F.J.A.-Á.; data curation, J.F.G.-M. and F.J.A.-Á.; writing—original draft preparation, J.F.G.-M.; writing—review and editing, J.F.G.-M.; supervision, P.Á.-M.; project administration, P.Á.-M.; funding acquisition, P.Á.-M. and M.T.-G.
Funding
This research was funded by European Union under grant LIFE 13-Bioseville ENV/1113.
Acknowledgments
Chao-Hui Feng would like to express her gratitude to University of Seville for the mobility grant (VIPPIT-2019-I.3) awarded under the VI Plan Propio de Investigación y Transferencia of the University of Seville.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Okoye, P.U.; Abdullah, A.Z.; Hameed, B.H. A review on recent developments and progress in the kinetics and deactivation of catalytic acetylation of glycerol—A byproduct of biodiesel. Renew. Sustain. Energy Rev. 2017, 74, 387–401. [Google Scholar] [CrossRef]
- Kongjao, S.; Damronglerd, S.; Hunsom, M. Purification of crude glycerol derived from waste used-oil methyl ester plant. Korean J. Chem. Eng. 2010, 27, 944–949. [Google Scholar] [CrossRef]
- Manosak, R.; Limpattayanate, S.; Hunsom, M. Sequential-refining of crude glycerol derived from waste used-oil methyl ester plant via a combined process of chemical and adsorption. Fuel Process. Technol. 2011, 92, 92–99. [Google Scholar] [CrossRef]
- Hájek, M.; Skopal, F. Treatment of glycerol phase formed by biodiesel production. Bioresour. Technol. 2010, 101, 3242–3245. [Google Scholar] [CrossRef] [PubMed]
- Mota, C.J.A.; da Silva, C.X.A.; Rosenbach, N.; Costa, J.; da Silva, F. Glycerin derivatives as fuel additives: The addition of glycerol/acetone ketal (solketal) in gasolines. Energy Fuels 2010, 24, 2733–2736. [Google Scholar] [CrossRef]
- Rahmat, N.; Abdullah, A.Z.; Mohamed, A.R. Recent progress on innovative and potential technologies for glycerol transformation into fuel additives: A critical review. Renew. Sustain. Energy Rev. 2010, 14, 987–1000. [Google Scholar] [CrossRef]
- Shah, P.R.; Ganesh, A. A comparative study on influence of fuel additives with edible and non-edible vegetable oil based on fuel characterization and engine characteristics of diesel engine. Appl. Therm. Eng. 2016, 102, 800–812. [Google Scholar] [CrossRef]
- Mallesham, B.; Govinda Rao, B.; Reddy, B.M. Production of biofuel additives by esterification and acetalization of bioglycerol. C. R. Chim. 2016, 19, 1194–1202. [Google Scholar] [CrossRef]
- Gonçalves, V.L.C.; Pinto, B.P.; Silva, J.C.; Mota, C.J.A. Acetylation of glycerol catalyzed by different solid acids. Catal. Today 2008, 133–135, 673–677. [Google Scholar] [CrossRef]
- Okoye, P.U.; Abdullah, A.Z.; Hameed, B.H. Synthesis of oxygenated fuel additives via glycerol esterification with acetic acid over bio-derived carbon catalyst. Fuel 2017, 209, 538–544. [Google Scholar] [CrossRef]
- García, E.; Laca, M.; Pérez, E.; Garrido, A.; Peinado, J. New class of acetal derived from glycerin as a biodiesel fuel component. Energy Fuels 2008, 22, 4274–4280. [Google Scholar] [CrossRef]
- Melero, J.A.; van Grieken, R.; Morales, G.; Paniagua, M. Acidic mesoporous silica for the acetylation of glycerol: Synthesis of bioadditives to petrol fuel. Energy Fuels 2007, 21, 1782–1791. [Google Scholar] [CrossRef]
- Setyaningsih, L.; Siddiq, F.; Pramezy, A. Esterification of glycerol with acetic acid over lewatit catalyst. MATEC Web Conf. 2018, 154, 2–5. [Google Scholar] [CrossRef]
- Silva, N.; Gonçalves, V.L.C.; Mota, C.J.A. Catalytic acetylation of glycerol with acetic anhydride. Catal. Commun. 2010, 11, 1036–1039. [Google Scholar] [CrossRef]
- Liu, Y.; Lotero, E.; Goodwin, J.G. Effect of carbon chain length on esterification of carboxylic acids with methanol using acid catalysis. J. Catal. 2006, 243, 221–228. [Google Scholar] [CrossRef]
- Sánchez, J.A.; Hernández, D.L.; Moreno, J.A.; Mondragón, F.; Fernández, J.J. Alternative carbon based acid catalyst for selective esterification of glycerol to acetylglycerols. Appl. Catal. A Gen. 2011, 1–2, 55–60. [Google Scholar] [CrossRef]
- Galhardo, T.S.; Simone, N.; Gonçalves, M.; Figueiredo, F.C.A.; Mandelli, D.; Carvalho, W.A. Preparation of sulfonated carbons from rice husk and their application in catalytic conversion of glycerol. ACS Sustain. Chem. Eng. 2013, 1, 1381–1389. [Google Scholar] [CrossRef]
- Tao, M.L.; Guan, H.Y.; Wang, X.H.; Liu, Y.C.; Louh, R.F. Fabrication of sulfonated carbon catalyst from biomass waste and its use for glycerol esterification. Fuel Process. Technol. 2015, 138, 355–360. [Google Scholar] [CrossRef]
- Álvarez-Mateos, P.; Alés-Álvarez, F.-J.; García-Martín, J.F. Phytoremediation of highly contaminated mining soils by Jatropha curcas L. and production of catalytic carbons from the generated biomass. J. Environ. Manag. 2019, 231, 886–895. [Google Scholar] [CrossRef] [PubMed]
- García-Martín, J.F.; Barrios, C.C.; Alés-Álvarez, F.J.; Dominguez-Sáez, A.; Alvarez-Mateos, P. Biodiesel production from waste cooking oil in an oscillatory flow reactor. Performance as a fuel on a TDI diesel engine. Renew. Energy. 2018, 125, 546–556. [Google Scholar] [CrossRef]
- Zhou, L.; Nguyen, T.H.; Adesina, A.A. The acetylation of glycerol over amberlyst-15: Kinetic and product distribution. Fuel Process. Technol. 2012, 104, 310–318. [Google Scholar] [CrossRef]
- Álvarez-Mateos, P.; García-Martín, J.F.; Guerrero-Vacas, F.J.; del Naranjo-Calderón, M.; Barrios, C.C.; del Pérez Camino, M.; Barrios, C.C. Valorization of a high-acidity residual oil generated in the waste cooking oils recycling industries. Grasas Aceites 2019, 70, e335. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).