The Influence of Torrefaction Temperature on Hydrophobic Properties of Waste Biomass from Food Processing
Abstract
1. Introduction
2. Materials and Methods
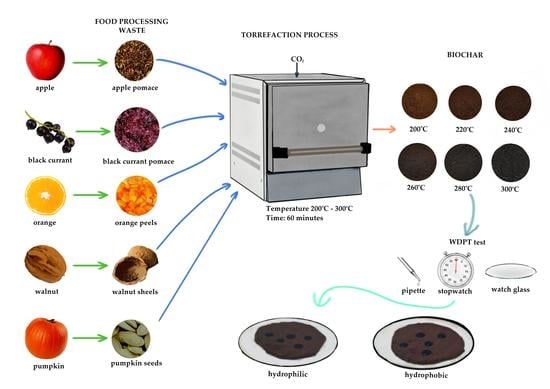
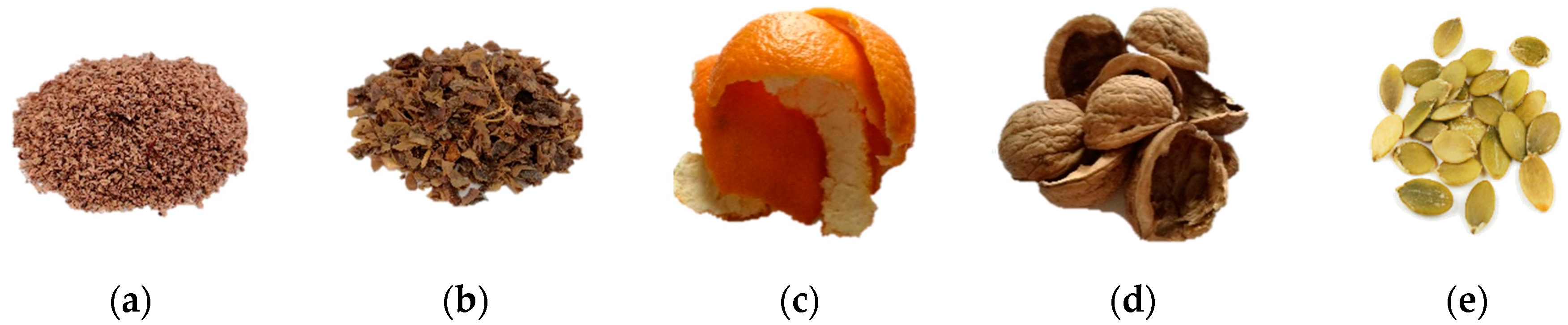
2.1. Materials Used in the Research
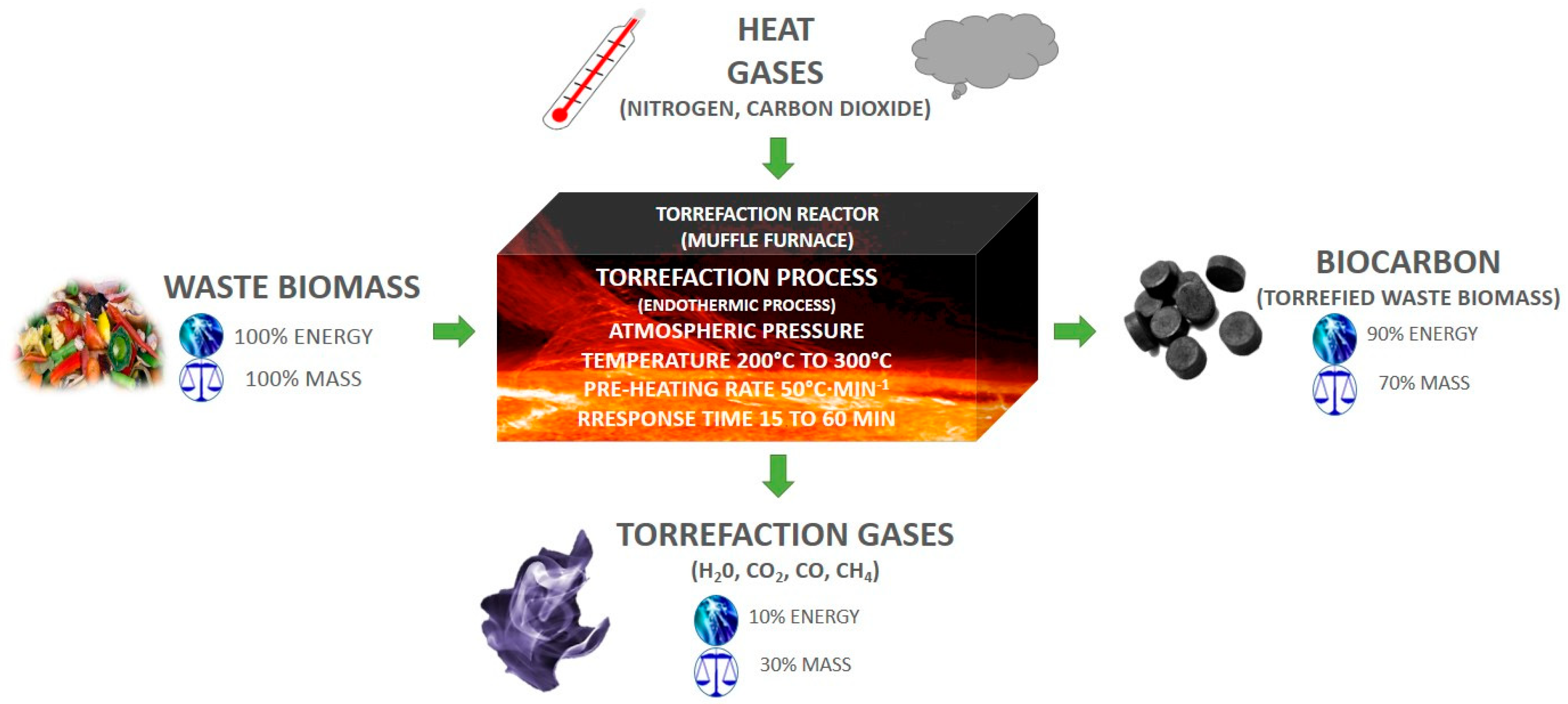
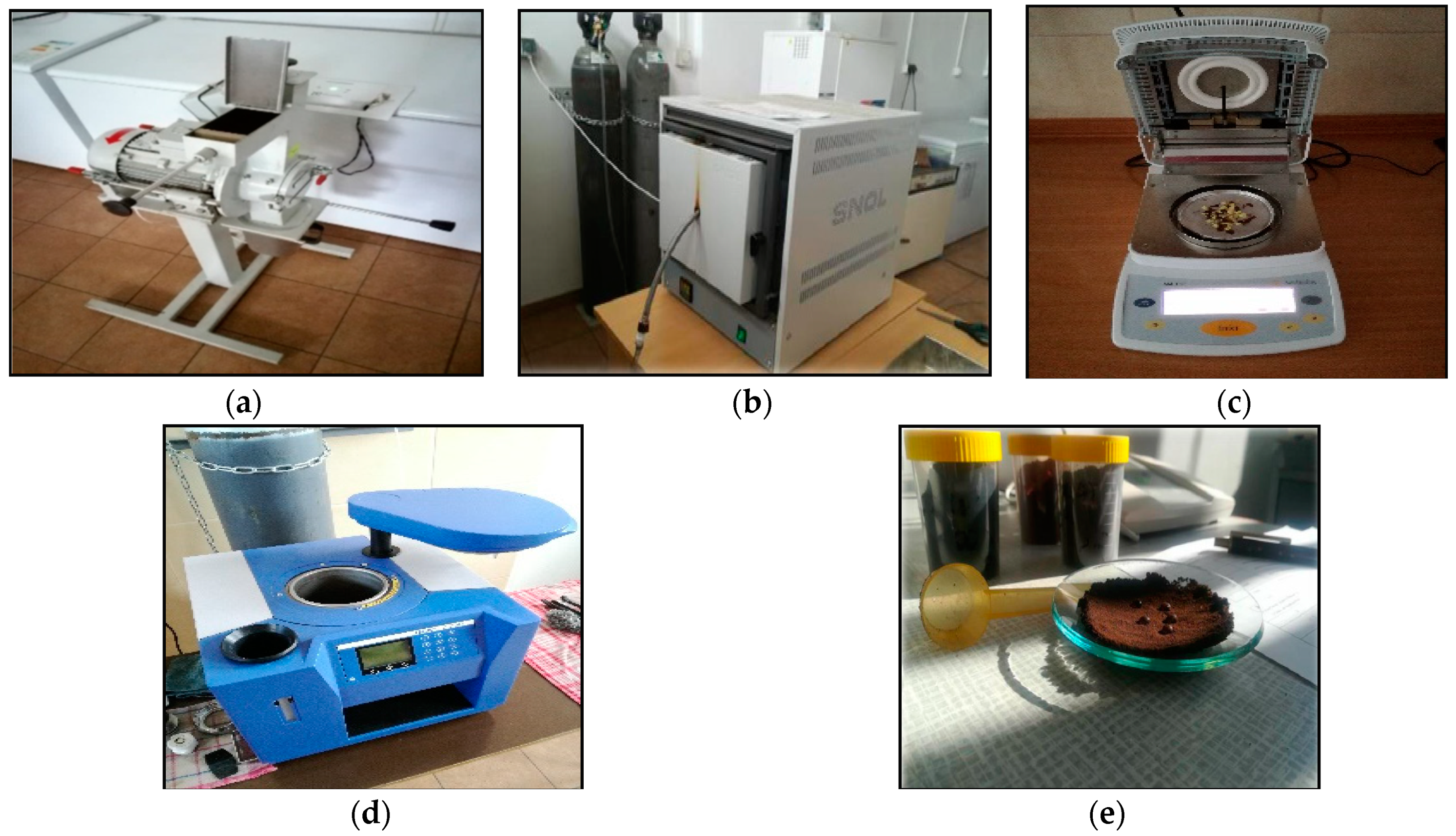
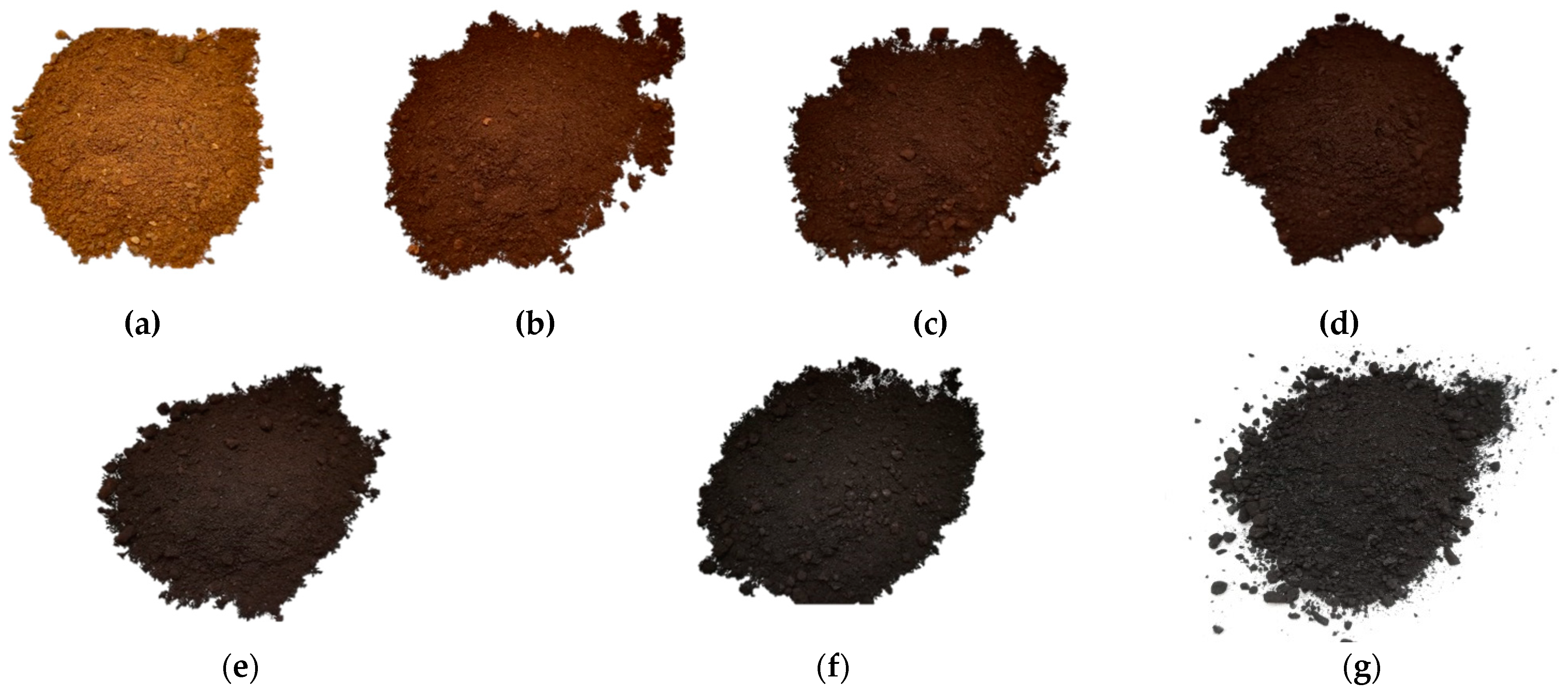
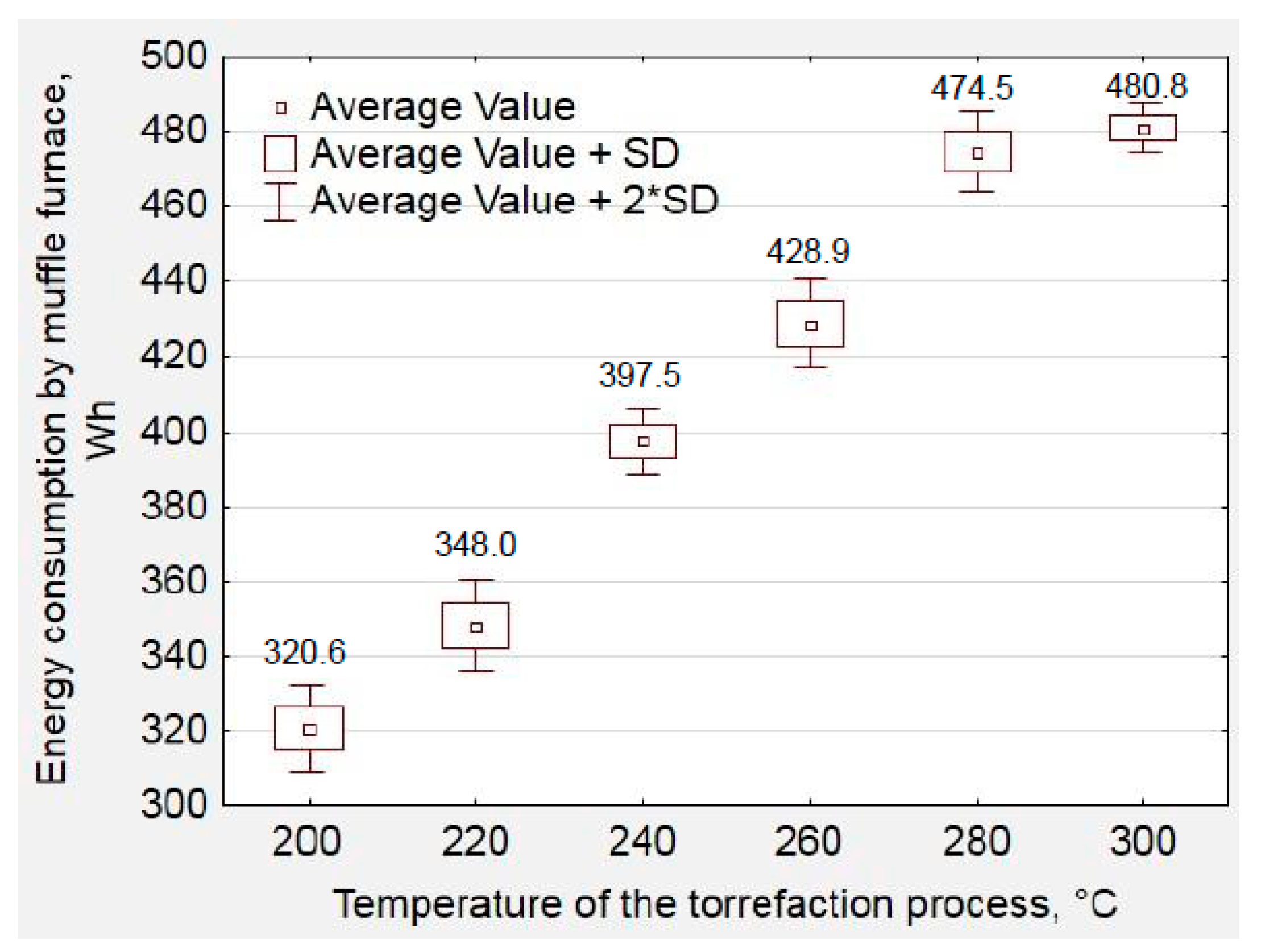
2.2. Samples Preparation and Torrefaction Procedure
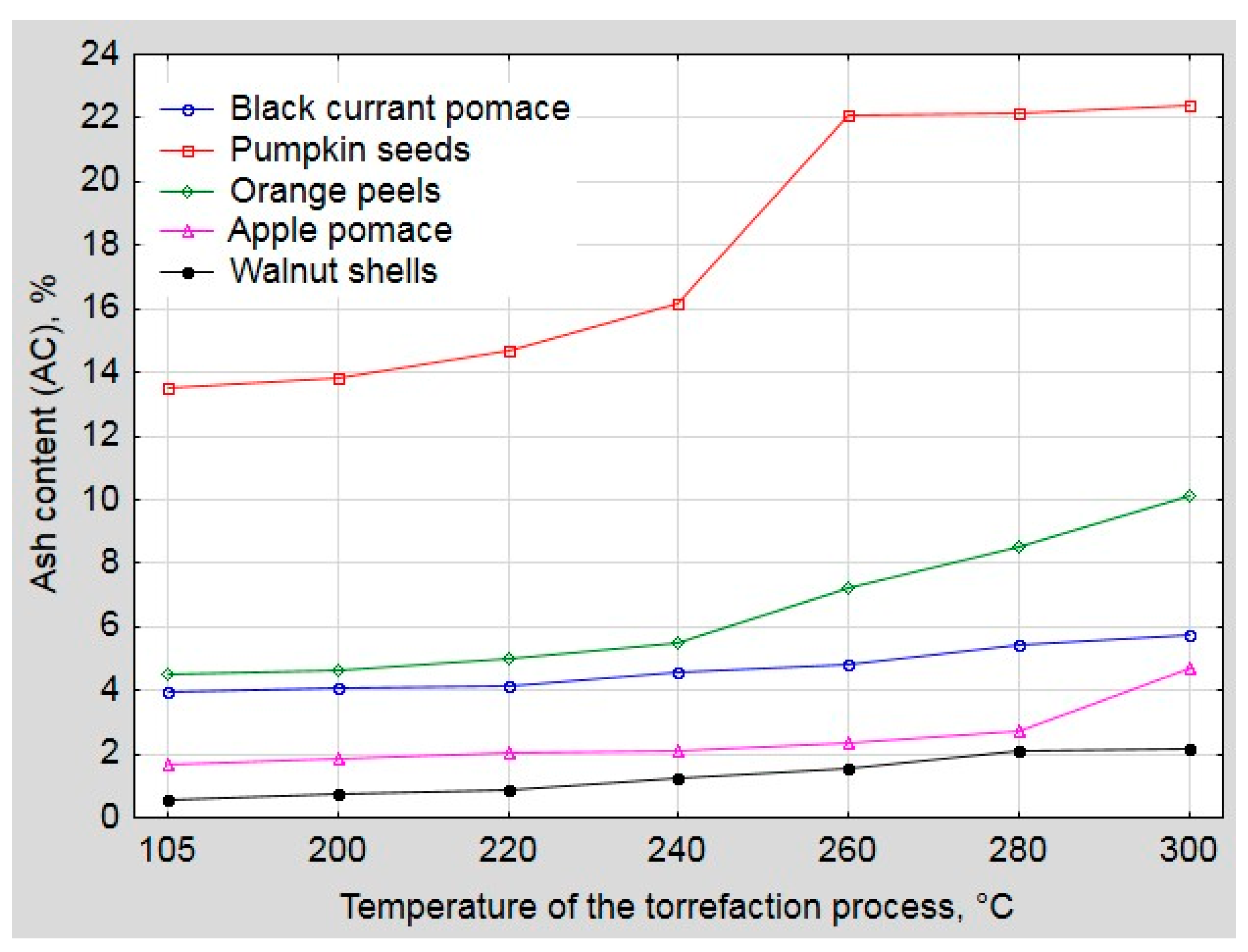
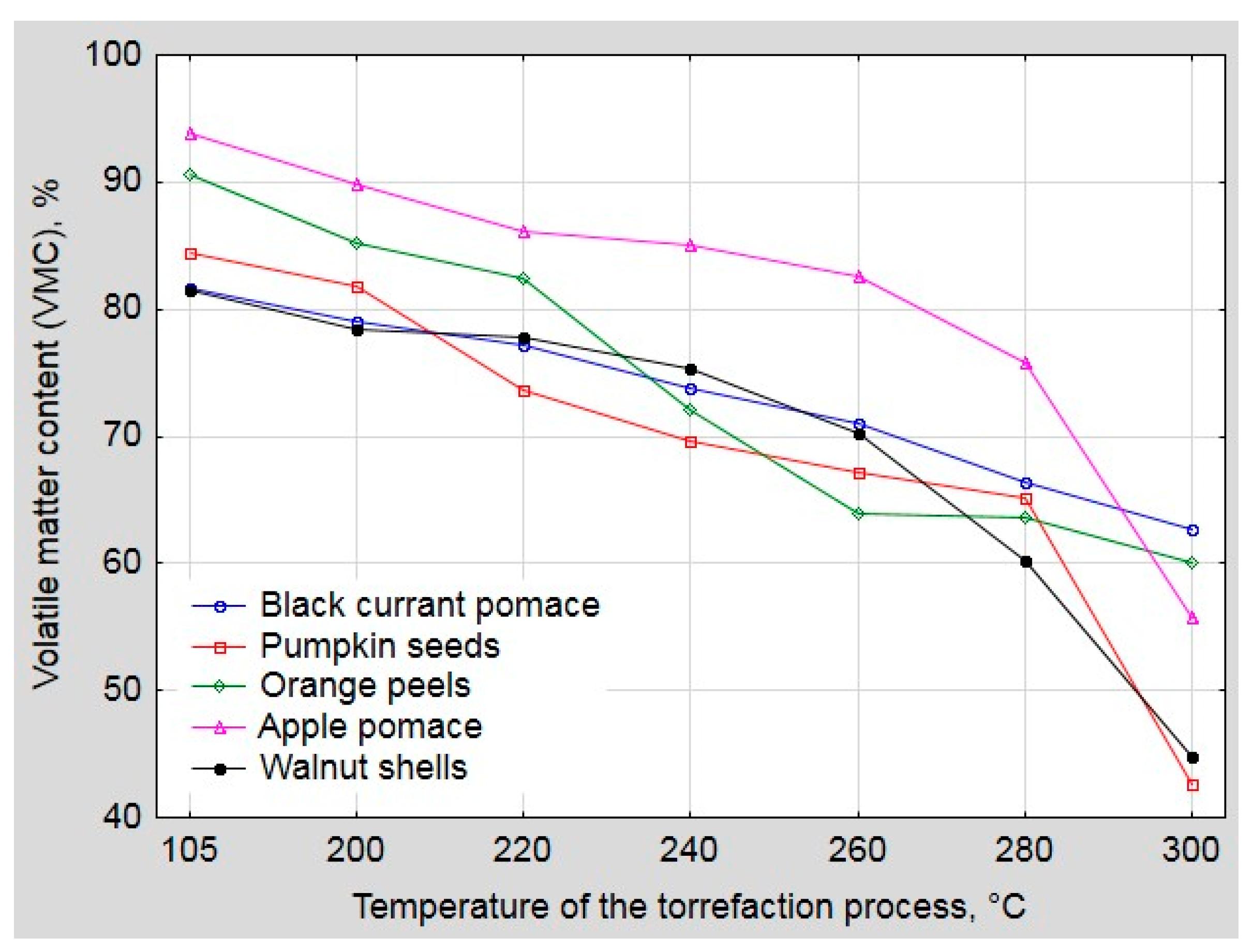
2.3. Proximate Analysis
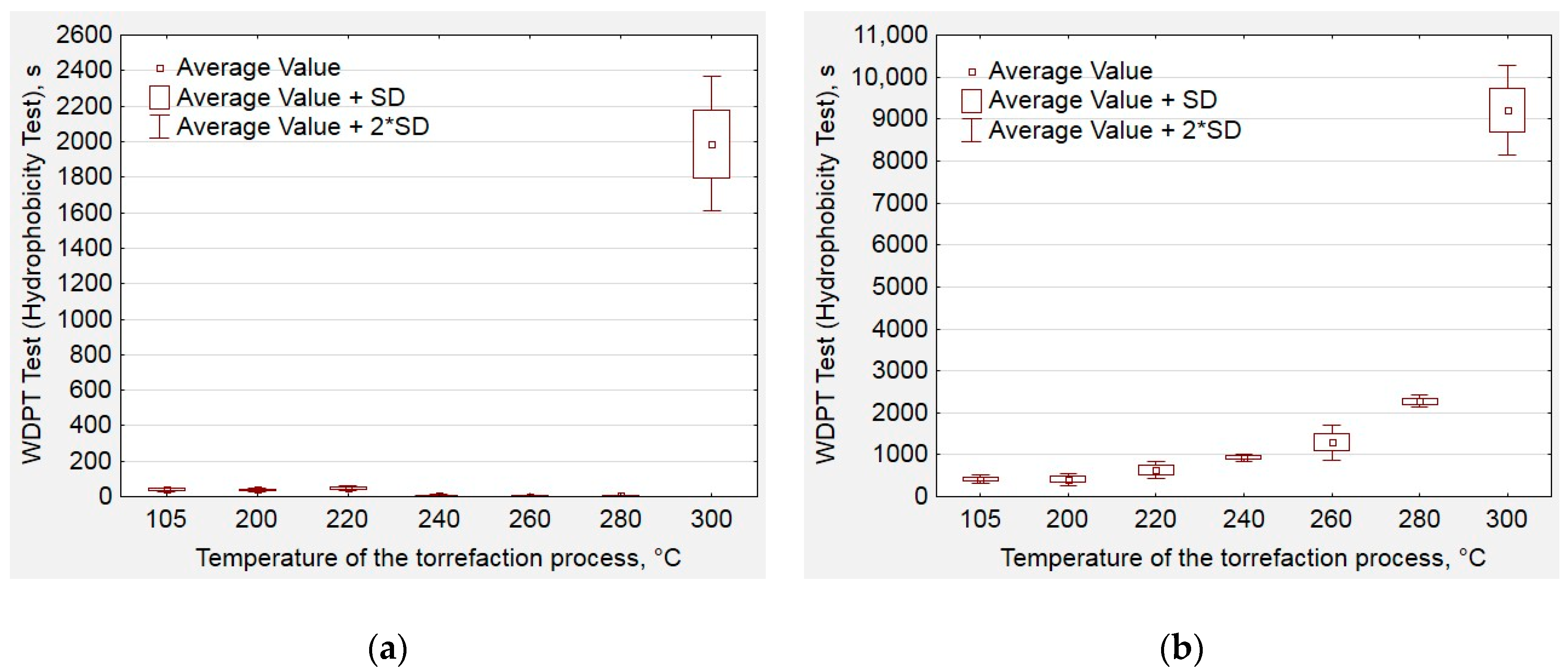
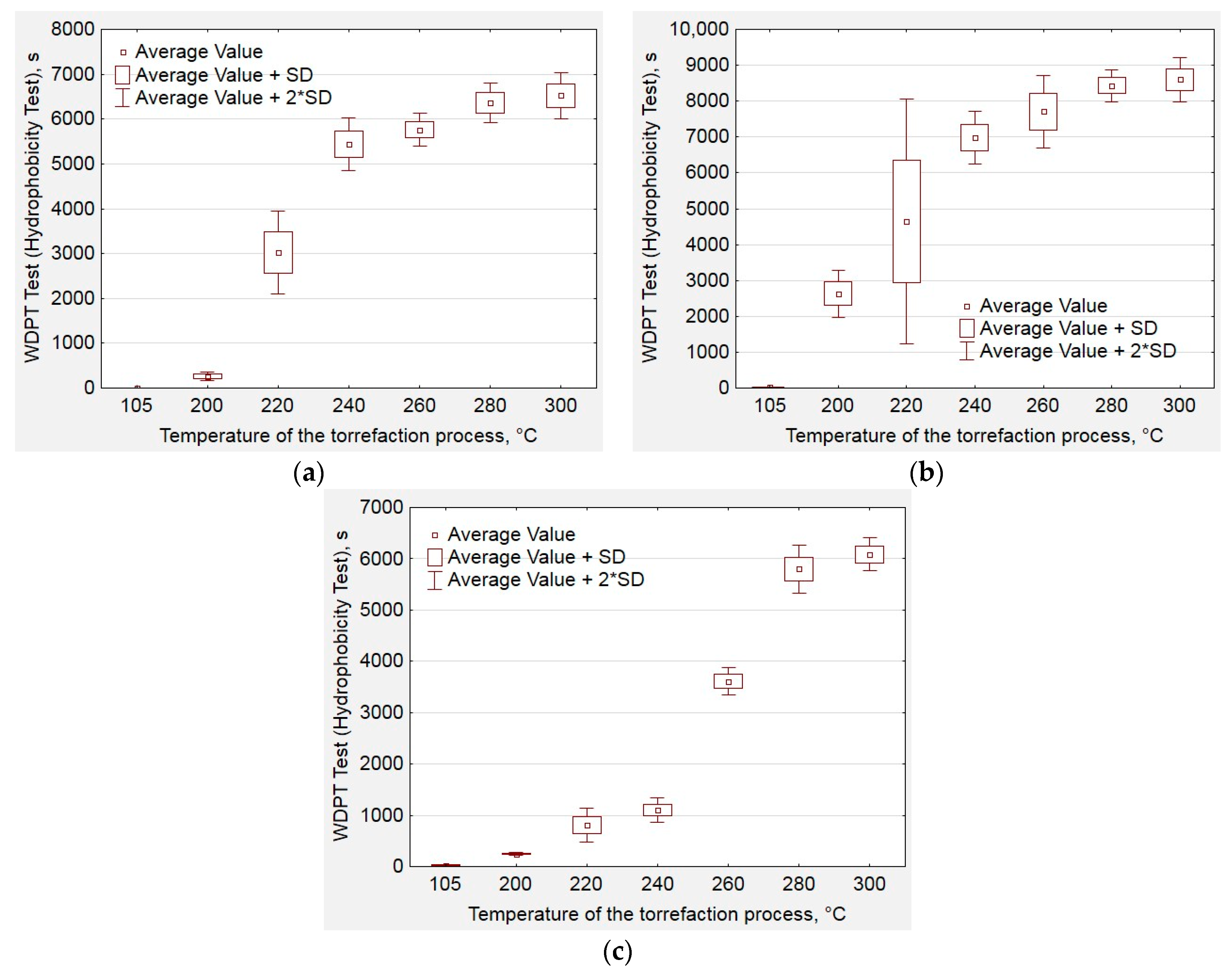
2.4. Hydrophobic Properties Analysis
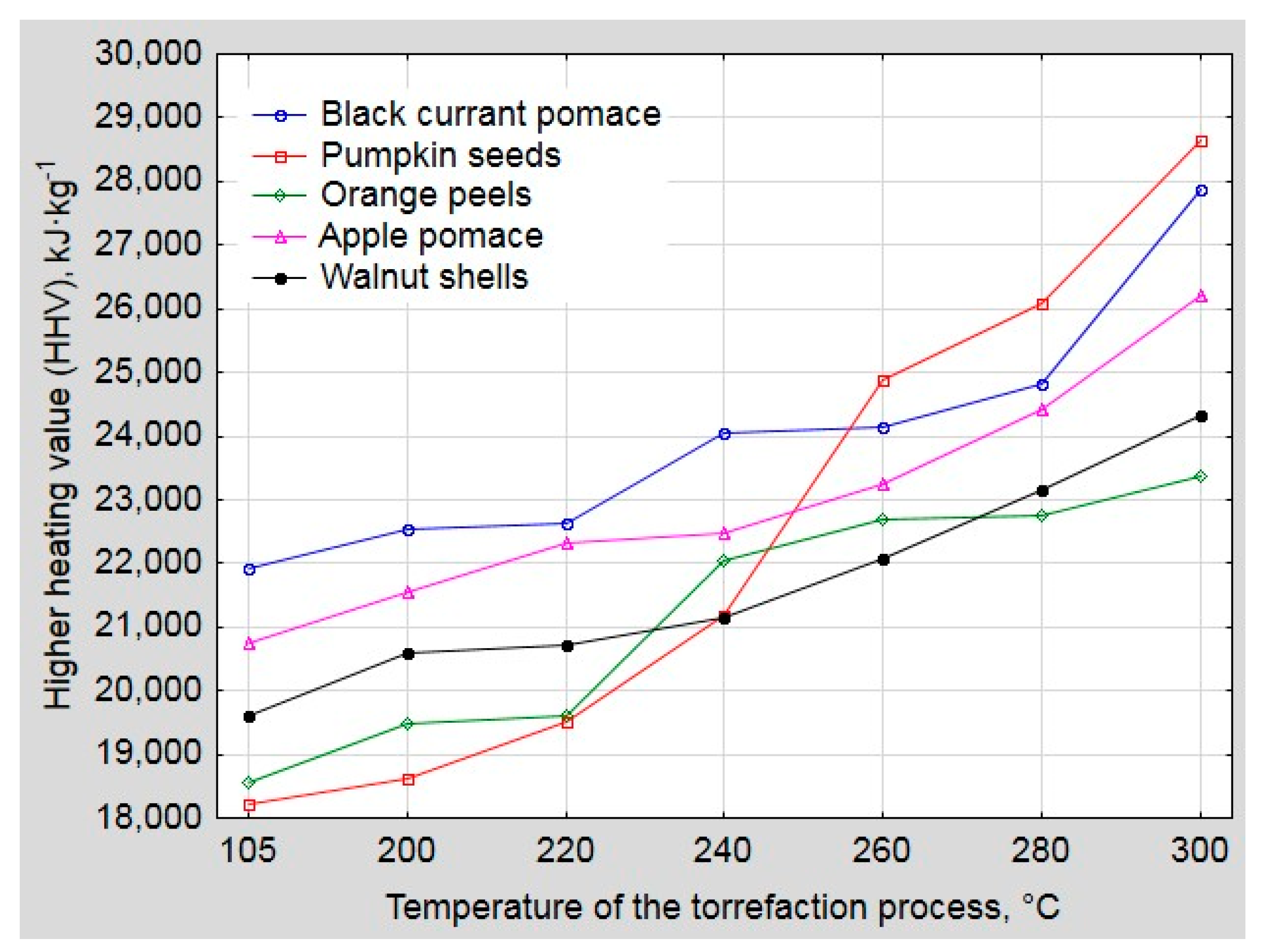
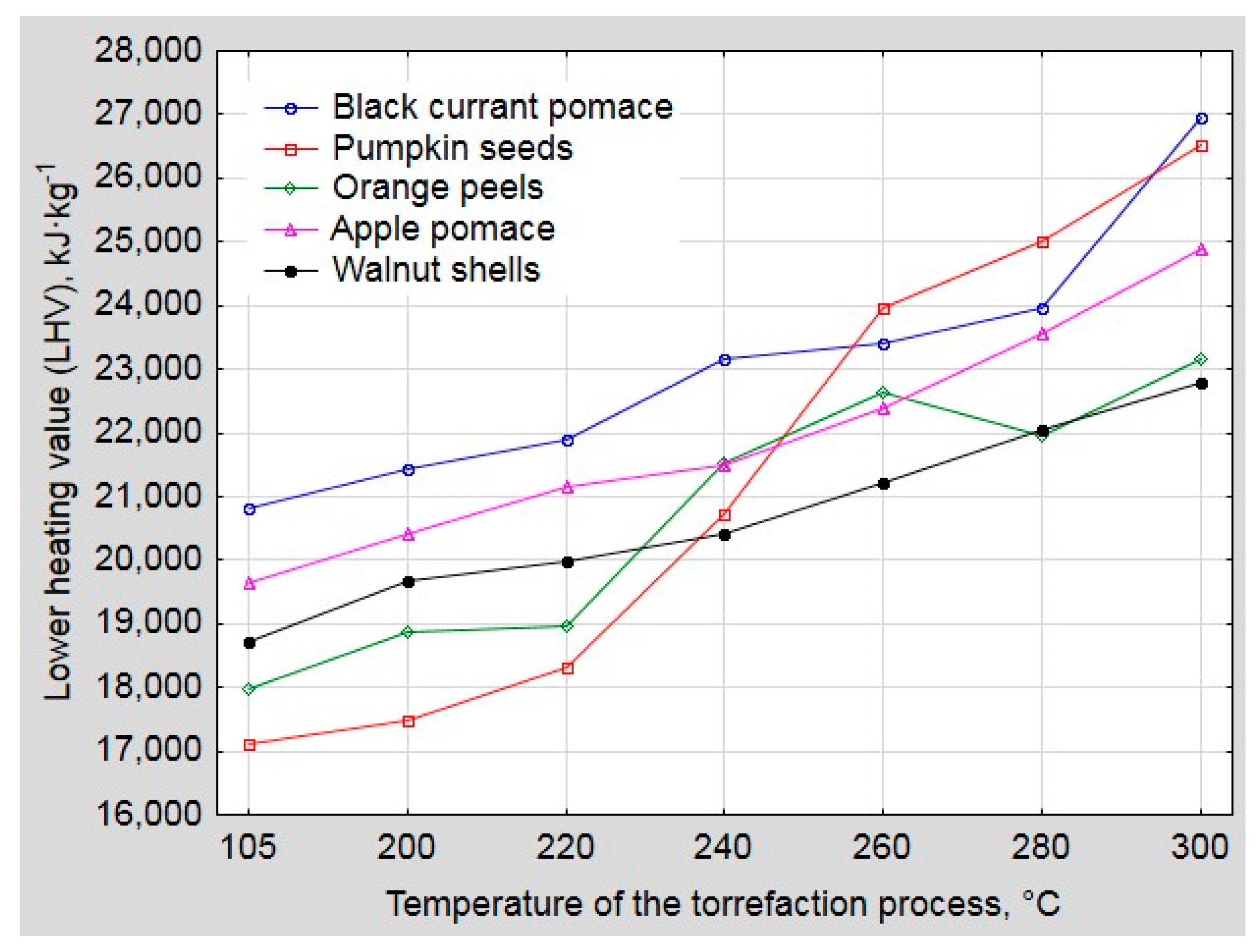
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AC | ash content |
| EC | European Commission |
| EU | European Union |
| HHV | higher heating value |
| LHV | lower heating value |
| LC | Lantana camara |
| LL | Leucaena Leucocephala |
| MC | moisture content |
| MP | Mimosa pigra |
| OPF | oil palm frond |
| RES | renewable energy sources |
| VMC | volatile matter content |
| WDPT | water drop penetration time |
References
- Caineng, Z.; Qun, Z.; Guosheng, Z.; Bo, X. Energy Revolution: From a Fossil Energy Era to a New Energy Era. Nat. Gas Ind. 2016, 3, 1–11. [Google Scholar] [CrossRef]
- Filipovici, A.; Tucu, D.; Białowiec, A.; Bukowski, P.; Crisan, G.C.; Lica, S.; Pulka, J.; Dyjakon, A.; Dębowski, M. Effect of Temperature and Heating Rate on the Char Yield in Sorghum and Straw Slow Pyrolysis. Rev. Chem. 2017, 68, 576–580. [Google Scholar]
- Ociepa-Kubicka, A. Wykorzystanie Biomasy w Przedsiębiorstwach Energetycznych. Proc. Ecopole 2015, 9, 279–286. [Google Scholar]
- Wit, M.; Faaij, A. European Biomass Resource Potential and Costs. Biomass Bioenergy 2010, 34, 188–202. [Google Scholar] [CrossRef]
- European Commission. Estimates of European Food Waste Levels, Fusions Eu Project; European Commission: Stockholm, Sweden, 2016; ISBN 978-91-88319-01-2. [Google Scholar]
- Białecka, B. Gospodarka Odpadami z Przemysłu Rolno-Spożywczego w Województwie Śląskim. Problemy Ekol. 2008, 12, 28–32. [Google Scholar]
- Alvarez, J.G.; Moya, R.; Puente-Urbina, A.; Rodriguez-Zuniga, A. Thermogravimetric, Volatilization Rate, and Differential Scanning Calorimetry Analyses of Biomass of Tropical Plantation Species of Costa Rica Torrefied at Different Temperatures and Times. Energies 2018, 11, 696–720. [Google Scholar] [CrossRef]
- Dincer, I.; Midilli, A.; Kucuk, H. Progress in Sustainable Energy Technologies: Generating Renewable Energy; Springer International Publishing: Berlin, Germany, 2014; ISBN 978-3319078953. [Google Scholar]
- Tabil, L.; Adapa, P.; Kashaninejad, M. Biomass Feedstock Pre-Processing-Part 2: Densification. In Biofuel’s Engineering Process Technology; InTech: Rijeka, Croatia, 2011; ISBN 978-953-307-480-1. [Google Scholar]
- Singh, R.; Krishna, B.B.; Kumar, J.; Bhaskar, T. Opportunities for Utilization of Non-Conventional Energy Sources for Biomass Pretreatment. Bioresour. Technol. 2016, 199, 398–407. [Google Scholar] [CrossRef]
- Qiaoming, L.; Chmely, S.C.; Abdoulmoumine, N. Biomass Treatment Strategies for Thermochemical Conversion. Energy Fuels 2017, 31, 3525–3536. [Google Scholar] [CrossRef]
- Nguyen, C.V.; Lewis, D.; Chen, W.H.; Huang, H.W.; Al-Othman, Z.A.; Yamauchi, Y.; Wu, K.C.W. Combined treatments for producing 5-hydroxymethylfurfural (HMF) from lignocellulosic biomass. Catal. Today 2016, 278, 344–349. [Google Scholar] [CrossRef]
- Matsagar, B.M.; Hossain, S.A.; Islam, T.; Alamri, H.R.; Alothman, Z.A.; Yamauchi, Y.; Dhepe, P.L.; Wu, K.C.W. Direct Production of Furfural in One-pot Fashion from Raw Biomass Using Bronsted Acidic Ionic Liquids. Sci. Rep. 2017, 7, 13508. [Google Scholar] [CrossRef]
- Dutta, S.; Kim, J.; Ide, Y.; Kim, J.H.; Hossain, S.A.; Bando, Y.; Yamauchi, Y.; Wu, K.C.W. 3D network of cellulose-based energy storage devices and related emerging applications. Mater. Horiz. 2017, 4, 522–545. [Google Scholar] [CrossRef]
- Stępień, P.; Pulka, J.; Białowiec, A. Organic Waste Torrefaction–A Review: Reactor Systems, and the Biochar Properties. In Pyrolysis; IntechOpen: London, UK, 2017; ISBN 978-953-51-3312-4. [Google Scholar]
- Phanphanich, M.; Mani, S. Impact of Torrefaction on the Grindability and Fuel Characteristics of Forest Biomass. Bioresour. Technol. 2011, 102, 1246–1253. [Google Scholar] [CrossRef] [PubMed]
- Van der Stelt, M.J.C.; Gerhauser, H.; Kiel, J.H.A.; Ptasinski, K. Biomass Upgrading by Torrefaction for the Production of Biofuels: A Review. Biomass Bioenergy 2011, 35, 3748–3762. [Google Scholar] [CrossRef]
- Bajcar, M.; Zaguła, G.; Saletnik, B.; Tarapatskyy, M.; Puchalski, C. Relationship between Torrefaction Parameters and Physicochemical Properties of Torrefied Products Obtained from Selected Plant Biomass. Energies 2018, 11, 2919–2931. [Google Scholar] [CrossRef]
- Alvarez, A.; Gutierrez, G.; Matos, M.; Pizarro, C.; Bueno, J.L. Torrefaction of Short Rotation Coppice of Poplar under Oxidative and Non-Oxidative Atmospheres. Proceedings 2018, 2, 1479–1483. [Google Scholar] [CrossRef]
- Nunes, L.J.R.; Matias, J.C.O.; Catalão, J.P.S. A Review on Torrefied Biomass Pellets as a Sustainable Alternative to Coal in Power Generation. Renew. Sustain. Energy Rev. 2014, 40, 153–160. [Google Scholar] [CrossRef]
- Chen, W.; Peng, J.; Bi, X. A State of the Art Review of Biomass Torrefaction, Densification and Application. Renew. Sustain. Energy Rev. 2015, 44, 847–866. [Google Scholar] [CrossRef]
- Basu, P. Biomass Gasification, Pyrolysis and Torrefaction; Chapter 4; Academic Press: Cambridge, MA, USA, 2013; ISBN 978-0-12-396488-5. [Google Scholar]
- Bajcar, M.; Puchalski, C.; Saletnik, B.; Zaguła, G.; Fabisiak, A.; Malecka, K. Optymalizacja Punktu Temperaturowego i Czasu Trwania Procesu Toryfikacji Wybranych Produktów Odpadowych Rolniczej Produkcji Roślinnej; Wydawnictwo Uniwersytetu Rzeszowskiego: Rzeszów, Poland, 2015; ISBN 978-83-7996-216-7. [Google Scholar]
- Bergman, P.C.A.; Boersma, A.R.; Zwart, R.W.R.; Kiel, J.H.A. Torrefaction for Biomass Co-Firing in Existing Coal-Fired Power Stations. ECN BIOMASS 2005, ECN-C—05-013. Available online: https://publicaties.ecn.nl/PdfFetch.aspx?nr=ECN-C--05-013 (accessed on 10 October 2019).
- Wang, S.; Dai, G.; Ru, B.; Zhao, Y.; Wang, X.; Zhou, J.; Luo, Z.; Cen, K. Effects of Torrefaction on Hemicellulose Structural Characteristics and Pyrolysis Behaviours. Bioresour. Technol. 2016, 218, 1106–1114. [Google Scholar] [CrossRef]
- Prins, M.J.; Ptasinski, K.J.; Janssen, F.J.J.G. Torrefaction of Wood. Part 2: Analysis of Products. J. Anal. Appl. Pyrolysis 2006, 77, 35–40. [Google Scholar] [CrossRef]
- Satpathy, S.K.; Tabil, L.G.; Meda, V.; Naik, S.N.; Prasad, R. Torrefaction of Wheat and Barley Straw after Microwave Heating. Fuel 2014, 124, 269–278. [Google Scholar] [CrossRef]
- Uslu, A.; Faaij, A.P.C.; Bergman, P.C.A. Pre-Treatment Technologies and their Effect on International Bioenergy Supply Chain Logistics. Techno-Economic Evaluation of Torrefaction, Fast Pyrolysis and Palletisation. Energy 2008, 33, 1206–1223. [Google Scholar] [CrossRef]
- Bates, R.B.; Ghoniem, A.F. Modeling Kinetics-Transport Interactions during Biomass Torrefaction: The Effects of Temperature, Particle Size and Moisture Content. Fuel 2014, 137, 216–229. [Google Scholar] [CrossRef]
- Pimchuai, A.; Dutta, A.; Basu, P. Torrefaction of Agricultural Residues to Enhance Combustible Properties. Energy Fuels 2010, 24, 4638–4645. [Google Scholar] [CrossRef]
- Tumuluru, J.S.; Sokhansanj, S.; Wright, C.T.; Boardman, R.D.; Hess, R.J. Review on Biomass Torrefaction Process and Product Properties and Design of Moving Bed Torrefaction System Model Development. In Proceedings of the ASABE Annual International Meeting, Louisville, KY, USA, 7–10 August 2011. [Google Scholar]
- Hakkou, M.; Petrissans, M.; Gerardin, P.; Zoulalian, A. Investigation of the Reasons for Fungal Durability of Heat-Treated Beech Wood. Polym. Degrad. Stabil. 2006, 91, 393–397. [Google Scholar] [CrossRef]
- Carneiro, R.J.M.; Godina, R.; Oliveira, M.J.C.; Ribeiro, N.L.J. Future Perspectives of Biomass Torrefaction: Review of the Current State-Of-The-Art and Research Development. Sustainability 2018, 10, 2323. [Google Scholar] [CrossRef]
- Prins, M.J.; Ptasinski, K.J.; Janssen, F.J.J.G. More Efficient Biomass Gasification via Torrefaction. Energy 2006, 31, 3458–3470. [Google Scholar] [CrossRef]
- Peng, J.H.; Bi, H.T.; Lim, C.J.; Sokhansanj, S. Study on Density, Hardness, and Moisture Uptake of Torrefied Wood Pellets. Energy Fuels 2013, 27, 967–974. [Google Scholar] [CrossRef]
- Yan, W.; Acharjee, T.C.; Coronella, C.J.; Vasquez, V.R. Thermal Pretreatment of Lignocellulosic Biomass. Environ. Prog. Sustain. Energy 2009, 28, 435–440. [Google Scholar] [CrossRef]
- Strandberg, M.; Olofsson, I.; Pommer, L.; Wiklund-Lindstrom, S.; Aberg, K.; Nordin, A. Effects of Temperature and Residence Time on Continuous Torrefaction of Spruce Wood. Fuel Process. Technol. 2015, 134, 387–398. [Google Scholar] [CrossRef]
- Kinney, T.J.; Masiello, C.A.; Dugan, B.; Hackaday, W.C.; Dean, M.R.; Zygourakis, K.; Barnes, R.T. Hydrologic Properties of Biochars Produced at Different Temperatures. Biomass Bioenergy 2012, 41, 34–43. [Google Scholar] [CrossRef]
- Chen, D.; Zheng, Z.; Fu, K.; Zeng, Z.; Wang, J.; Lu, M. Torrefaction of Biomass Stalk and its Effect on the Yield and Quality of Pyrolysis Products. Fuel 2015, 159, 27–32. [Google Scholar] [CrossRef]
- Acharjee, T.C.; Coronella, C.J.; Vasquez, C.R. Effect of Thermal Pretreatment on Equilibrium Moisture Content of Lignocellulosic Biomass. Bioresour. Technol. 2011, 102, 4849–4854. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, A.; Nogueiro, D.; Pizarro, C.; Matos, M.; Bueno, J.L. Non-Oxidative Torrefaction of Biomass to Enhance its Fuel Properties. Energy 2018, 158, 1–8. [Google Scholar] [CrossRef]
- Baronti, S.; Vaccari, F.P.; Miglietta, F.; Calzolari, C.; Lugato, C.; Orlandini, S.; Pini, R.; Zulian, C.; Genesio, L. Impact of Biochar Application on Plant Water Relations in Vitis Vinifera (L.). Eur. J. Agron. 2014, 53, 38–44. [Google Scholar] [CrossRef]
- Doerr, S.H. On Standardizing the “Water Drop Penetration Time” and the “Molarity of An Ethanol Droplet” Techniques to Classify Soil Hydrophobicity: A Case Study Using Medium Textured Soils. Earth Surf. Process. Landf. 1998, 23, 663–668. [Google Scholar] [CrossRef]
- PN-EN ISO 18134-2:2017-03E–Solid Biofuels. Determination of Moisture Content–Oven Dry Method–Part 2: Total Moisture–Simplified Method; European Committee for Standardization: Brussels, Belgium, 2017. [Google Scholar]
- PN-EN ISO 18125:2017-07. Solid Biofuels-Determination of Calorific Value; European Committee for Standardization: Brussels, Belgium, 2017. [Google Scholar]
- Dyjakon, A. The Influence of the Use of Windrowers in Baler Machinery on the Energy Balance during Pruned Biomass Harvesting in the Apple Orchard. Energies 2018, 11, 3236. [Google Scholar] [CrossRef]
- PN ISO 1171:2010–Solid Mineral Fuels. Determination of Ash; European Committee for Standardization: Brussels, Belgium, 2010. [Google Scholar]
- PN-EN ISO 18123:2016-01–Solid Fuels. Determination of Volatile Content by Gravimetric Method; European Committee for Standardization: Brussels, Belgium, 2016. [Google Scholar]
- Guatam, R.; Ashwath, N. Hydrophobicity of 43 Potting Media: Its Implications for Raising Seedlings in Revegetation Programs. J. Hydrol. 2012, 430–431, 111–117. [Google Scholar] [CrossRef]
- Bisdom, E.B.A.; Dekker, L.W.; Schoute, J.F.T. Water Repellency of Sieve Fractions from Sandy Soils and Relationships with Organic Material and Soil Structure. Geoderma 1993, 56, 105–118. [Google Scholar] [CrossRef]
- Tran, K.-Q.; Luo, X.; Seisenbaeva, G.; Jirjis, R. Stump torrefaction for bioenergy application. Appl. Energy 2012, 112, 539–546. [Google Scholar] [CrossRef]
- Berther, M.A.; Commandre, J.M.; Rouau, X.; Gontard, N.; Angellier-Coussy, H. Torrefaction Treatment of Lignocellulosic Fibres for Improving Fibre, Matrix Adhesion in a Biocomposite. Mater. Des. 2016, 92, 223–232. [Google Scholar] [CrossRef]
- Świechowski, K.; Liszewski, M.; Bąbelewski, P.; Koziel, J.A.; Białowiec, A. Oxytree Pruned Biomass Torrefaction: Mathematical Models of the Influence of Temperature and Residence Time on Fuel Properties Improvement. Materials 2019, 12, 2228. [Google Scholar] [CrossRef]
- Matali, S.; Rahman, N.A.; Idris, S.S.; Yaacob, N.; Alias, A.B. Lignocellulosic Biomass Solid Fuel Properties Enhancement Via Torrefaction. Proc. Eng. 2016, 148, 671–678. [Google Scholar] [CrossRef]
- Medic, D.; Darr, M.; Shah, A.; Potter, B.; Zimmerman, J. Effects of Torrefaction Process Parameters on Biomass Feedstock Upgrading. Fuel 2012, 91, 147–154. [Google Scholar] [CrossRef]
- Mundike, J.; Collard, F.X.; Görgens, J.F. Torrefaction of Invasive Alien Plantsl Influence of Heating Rate and Other Conversion Parameters on Mass Yield And Higher Heating Value. Bioresour. Technol. 2016, 209, 90–99. [Google Scholar] [CrossRef]
- Wang, Z.; Lim, C.J.; Grace, J.R. Biomass Torrefaction in a Slot-Rectangular Spouted Bed Reactor. Particuology 2018, 42, 154–162. [Google Scholar] [CrossRef]
- Matali, S.; Rahman, N.A.; Idris, S.S.; Yaacob, N. Enhancement of Energy Properties of Leucaena Leucocephala Pellets Via Torrefaction and its Non-Isothermal Decomposition Kinetics. Int. J. Eng. Technol. 2018, 7, 306–310. [Google Scholar]
- Sokhansanj, S.; Peng, J.H.; Bi, X.T.; Lim, C.J.; Wang, L.; Lam, P.S.; Hoi, J.P.; Melin, S.; Tumuluru, C.T.; Wright, C.T. Optimum Torrefaction and Pelletization of Biomass Feedstock. In Symposium on Thermal and Catalytic Science for Biofuels and Biobased Products; Iowa State University: Ames, IA, USA, 2010; pp. 21–23. [Google Scholar]
- Chen, W.H.; Lin, B.J.; Colin, B.; Chang, J.S.; Petrissans, A.; Bi, X.; Petrissans, M. Hygroscopic Transformation of Woody Biomass Torrefaction for Carbon Storage. Appl. Energy 2018, 231, 768–776. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, B.; Yang, H.; Yang, Q.; Chen, H. Evolution of Functional Groups and Pore Structure During Cotton and Corn Stalks Torrefaction and its Correlation with Hydrophobicity. Fuel 2014, 137, 41–49. [Google Scholar] [CrossRef]
- Chen, D.; Gao, A.; Cen, K.; Zhang, J.; Cao, X.; Ma, Z. Investigation of Biomass Torrefaction Based on Three Major Components: Hemicellulose, Cellulose and Lignin. Energy Convers. Manag. 2018, 169, 228–237. [Google Scholar] [CrossRef]
- Wang, S.; Dai, G.; Ru, B.; Zhao, Y.; Wang, X.; Xiao, G.; Luo, Z. Influence of Torrefaction on the Characteristics and Pyrolysis Behaviour of Cellulose. Energy 2017, 120, 864–871. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dyjakon, A.; Noszczyk, T.; Smędzik, M. The Influence of Torrefaction Temperature on Hydrophobic Properties of Waste Biomass from Food Processing. Energies 2019, 12, 4609. https://doi.org/10.3390/en12244609
Dyjakon A, Noszczyk T, Smędzik M. The Influence of Torrefaction Temperature on Hydrophobic Properties of Waste Biomass from Food Processing. Energies. 2019; 12(24):4609. https://doi.org/10.3390/en12244609
Chicago/Turabian StyleDyjakon, Arkadiusz, Tomasz Noszczyk, and Martyna Smędzik. 2019. "The Influence of Torrefaction Temperature on Hydrophobic Properties of Waste Biomass from Food Processing" Energies 12, no. 24: 4609. https://doi.org/10.3390/en12244609
APA StyleDyjakon, A., Noszczyk, T., & Smędzik, M. (2019). The Influence of Torrefaction Temperature on Hydrophobic Properties of Waste Biomass from Food Processing. Energies, 12(24), 4609. https://doi.org/10.3390/en12244609