Metallic Organic Framework-Derived Fe, N, S co-doped Carbon as a Robust Catalyst for the Oxygen Reduction Reaction in Microbial Fuel Cells
Abstract
1. Introduction
2. Experimental
2.1. Sample Preparation
2.2. Characterization of ORR Activity
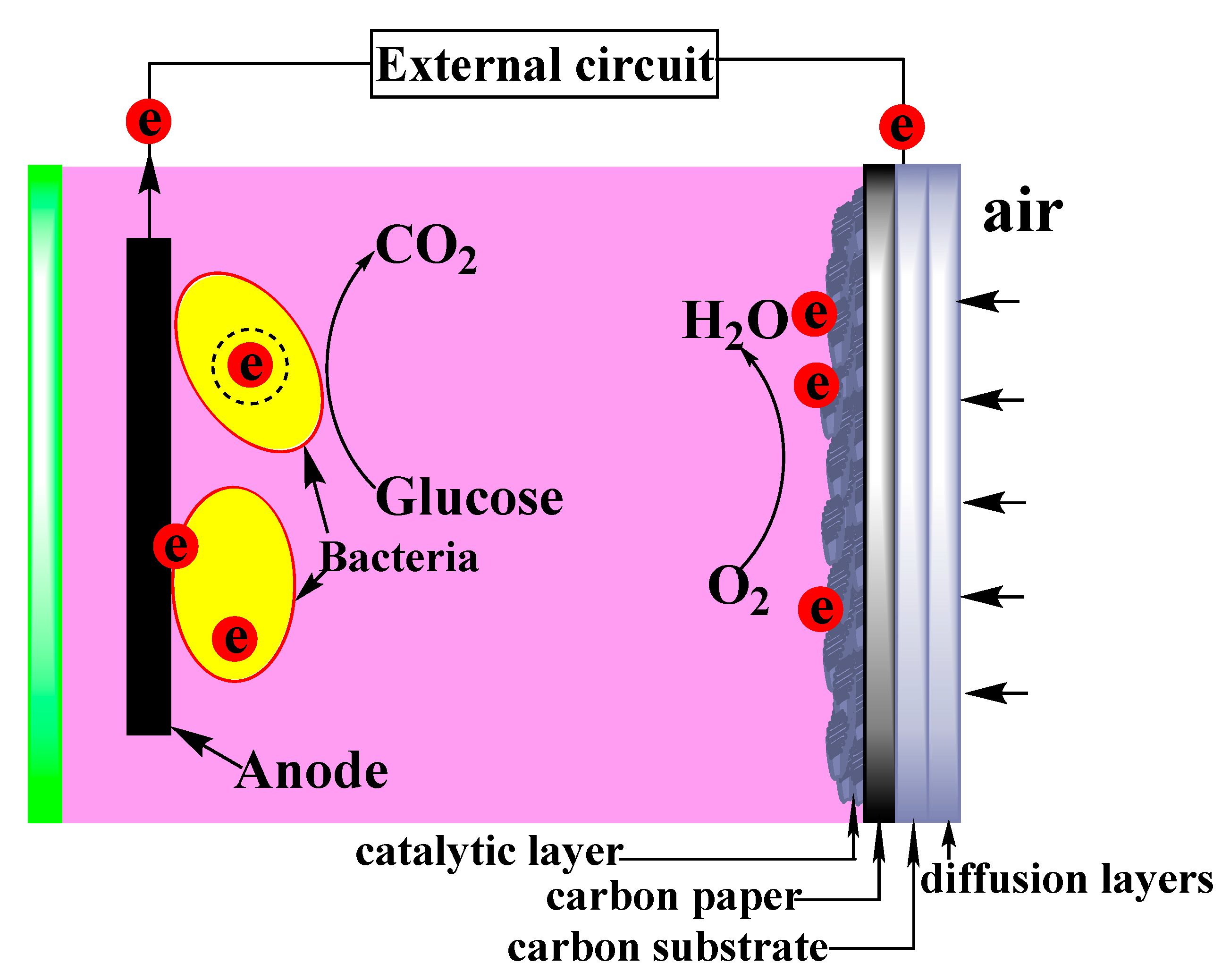
2.3. Construction of MFC Equipment
2.3.1. Air Cathode Composition
2.3.2. Construction and Operation of MFC
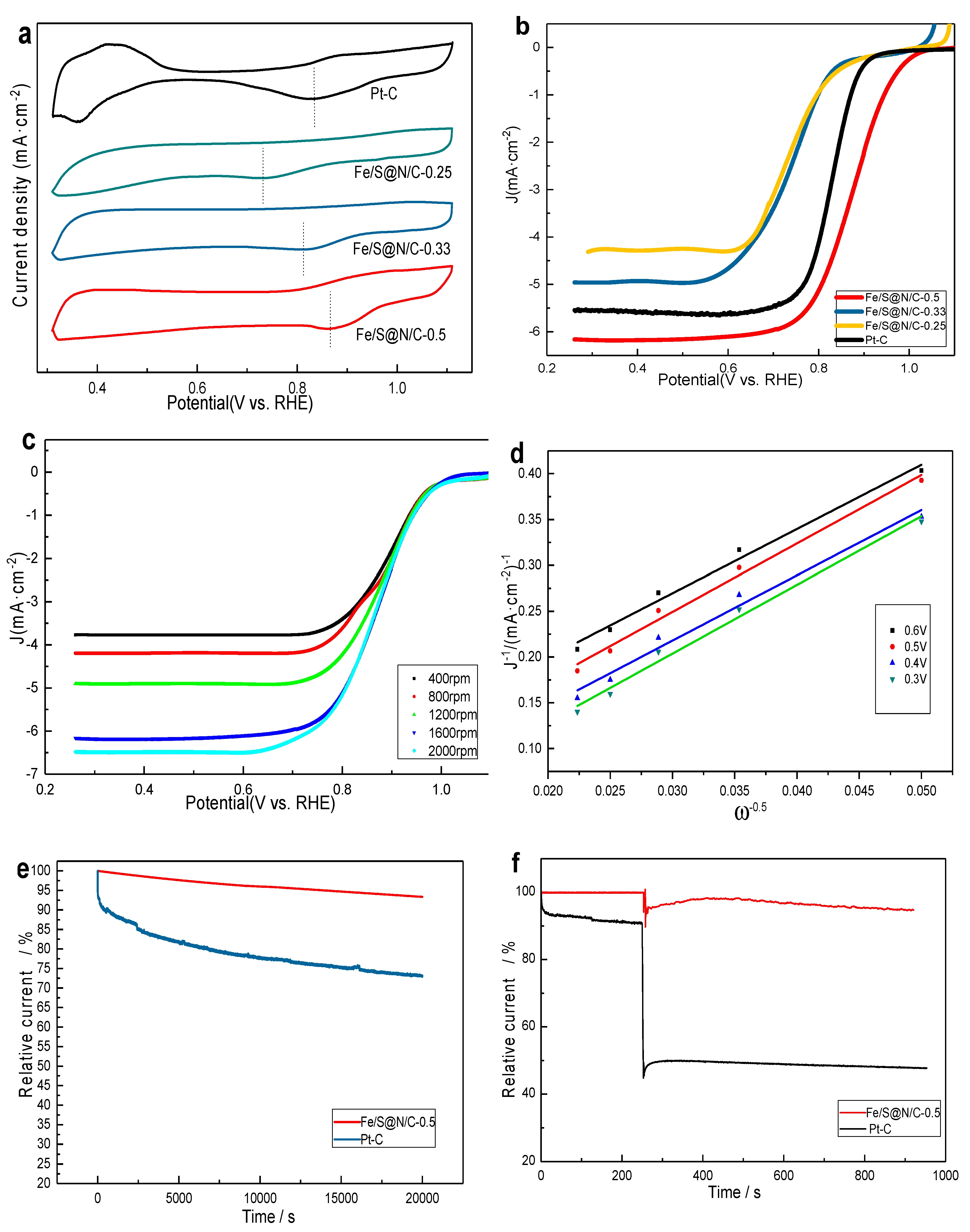
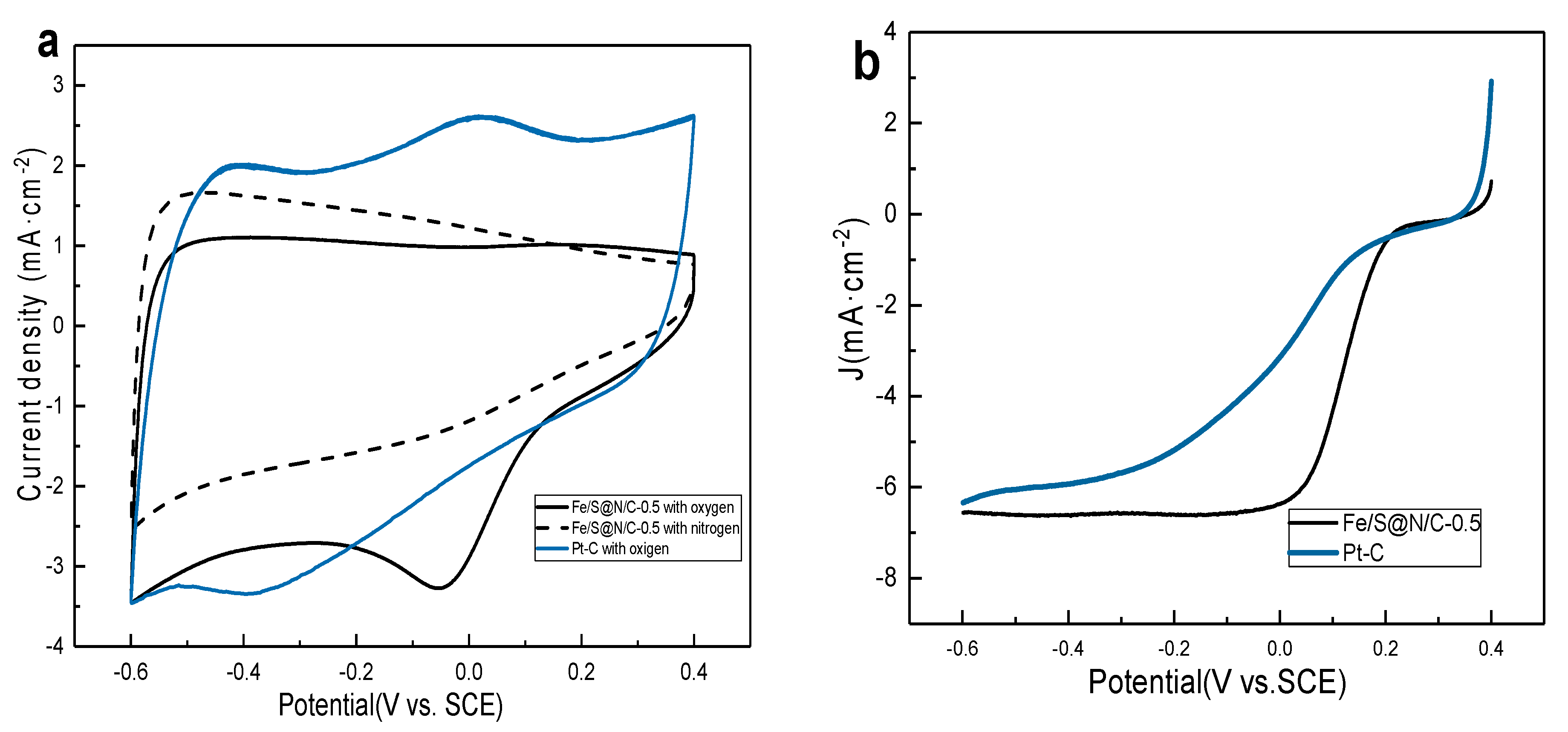
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Guo, S.J.; Zhang, S.; Sun, S.S. Tuning Nanoparticle Catalysis for the Oxygen Reduction Reaction. Angew. Chem. Int. Ed. 2013, 52, 8526–8544. [Google Scholar] [CrossRef] [PubMed]
- Jaouen, F.; Proietti, E.; Lefèvre, M.; Chenitz, R.; Dodelet, J.-P.; Wu, G.; Chung, H.T.; Johnstonb, C.M.; Zelenay, P. Recent advances in non-precious metal catalysis for oxygen-reduction reaction in polymer electrolyte fuel cells. Energy Environ. Sci. 2011, 4, 114–130. [Google Scholar] [CrossRef]
- Hara, M.; Kitano, M.; Hosono, H. Ru-loaded C12A7: E’ electride as a catalyst for ammonia synthesis. ACS Catal. 2017, 7, 2313–2324. [Google Scholar] [CrossRef]
- Kitano, M.; Kanbara, S.; Inoue, Y.; Kuganathan, N.; Sushko, P.V.; Yokoyama, T.; Hara, M.; Hosono, H. Electride support boosts nitrogen dissociation over ruthenium catalyst and shifts the bottleneck in ammoia synthesis. Nat. Commun. 2015, 6, 6731. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.Z.; Dong, S.J. Recent progress in graphene-based nanomaterials as advanced electrocatalysts towards oxygen reduction reaction. Nanoscale 2013, 5, 1753–1767. [Google Scholar] [CrossRef]
- Zhang, C.; Mahmood, N.; Yin, H.; Liu, F.; Hou, Y. Synthesis of Phosphorus-Doped Graphene and its Multifunctional Applications for Oxygen Reduction Reaction and Lithium Ion Batteries. Adv. Mater. 2013, 25, 4932–4937. [Google Scholar] [CrossRef]
- Liang, J.; Zheng, Y.; Chen, J.; Liu, J.; Hulicova-Jurcakova, D.; Jaroniec, M.; Qiao, S.Z. Facile Oxygen Reduction on a Three-Dimensionally Ordered Macroporous Graphitic C3N4/Carbon Composite Electrocatalyst. Angew. Chem. Int. Ed. 2012, 51, 3892–3896. [Google Scholar] [CrossRef]
- Lefevre, M.; Proietti, E.; Jaouen, F.; Dodelet, J.-P. Iron-Based Catalysts with Improved Oxygen Reduction Activity in Polymer Electrolyte Fuel Cells. Science 2009, 324, 71–74. [Google Scholar] [CrossRef]
- Yun, J.; Smith, V.H.; Pate, R.C. Managing nutrients and system operations for biofuel production from freshwater macroalgae. Algal Res. 2015, 11, 13–21. [Google Scholar] [CrossRef]
- Quek, S.B.; Cheng, L.; Cord-Ruwisch, R. Microbial fuel cell biosensor for rapid assessment of assimilable organic carbon under marine conditions. Water Res. 2015, 77, 64–71. [Google Scholar] [CrossRef]
- Liew, K.B.; Daud, W.R.W.; Ghasemi, M. Non-Pt catalyst as oxygen reduction reaction in microbial fuel cells: A review. Int. J. Hydrog. Energy 2014, 39, 4870–4883. [Google Scholar]
- Aspear, S.; Kawai, H. Density functional theory-based analysis on O2 molecular interaction with the tri-s-triazine-based graphitic carbon nitride. Surf. Sci. 2012, 606, 892–901. [Google Scholar] [CrossRef]
- Zhu, C.Z.; Fu, S.F.; Song, J.H.; Shi, Q.R.; Su, D.; Mark, H.; Engelhard, X.L. Self-Assembled Fe-N-Doped Carbon Nanotube Aerogels with Single-Atom Catalyst Feature as High-Efficiency Oxygen Reduction Electrocatalysts. Small 2017, 13, 1603407. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhao, Z.; Liang, K.; Zhou, X.; Shen, C.; Liu, Y.; Wang, X.; Xu, A. Synthesis of nanoporous structured iron carbide/Fe–N–carbon composites for efficient oxygen reduction reaction in Zn–air batteries. J. Mater. Chem. A 2016, 4, 19037–19044. [Google Scholar] [CrossRef]
- Deng, D.H.; Yu, L.; Chen, X.Q.; Wang, G.X.; Jin, L.; Pan, X.L.; Deng, J.; Sun, G.Q.; Bao, X.H. Iron Encapsulated within Pod-like Carbon Nanotubes for Oxygen Reduction Reaction. Angew. Chem. 2013, 52, 371–375. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; An, L.; Jiang, N.; Li, B.; Hua, S.; Fu, Y.; Liu, J.; Hao, W.; Xia, D. A highly active and durable iron/cobalt alloy catalyst encapsulated in N-doped graphitic carbon nano. J. Mater. Chem. A 2018, 6, 5962–5970. [Google Scholar]
- Bracamonte, V.; Melchionna, M.; Stopin, A.; Giulani, A. Carboxylated, Fe-Filled Multiwalled Carbon Nanotubes as Versatile Catalysts for O2− Reduction and H2− Evolution Reactions at Physiological pH. Chem. Eur. J. 2015, 21, 12769–12777. [Google Scholar] [CrossRef]
- Li, J.Z.; Chen, M.J.; David, A.; Wang, M.Y. Atomically dispersed manganese catalysts for oxygen reduction in proton-exchange membrane fuel cells. Nat. Catal. 2018, 1, 935–945. [Google Scholar] [CrossRef]
- Anna, L.; Manuela, B.; Claudio, T.; Lucia, N. Selective Electrocatalytic H2O2 Generation by Cobalt@N-Doped Graphitic Carbon Core–Shell Nanohybrids. ChemSusChem 2019, 12, 1664–1672. [Google Scholar]
- Wang, Q.; Zhou, Z.Y.; Lai, Y.J.; You, Y.; Liu, J.G.; Wu, X.L.; Terefe, E.; Chen, C.; Song, L.; Rauf, M.; et al. Phenylenediamine-Based FeNx/C Catalyst with High Activity for Oxygen Reduction in Acid Medium and Its Active-Site Probing. Am. Chem. Soc. 2014, 136, 10882–10885. [Google Scholar] [CrossRef]
- Chung, H.T.; Cullen, D.A.; Higgins, D. Direct atomic-level insight into the active sites of a high-performance PGM-free ORR catalyst. Science 2017, 357, 479–484. [Google Scholar] [CrossRef] [PubMed]
- Perez-Alonso, F.J.; Dominguez, C.; Al-Thabaiti, S.A. Evidences of the presence of different types of active sites for the oxygen reduction reaction with Fe/N/C based catalysts. J. Power Sources 2016, 327, 204–211. [Google Scholar] [CrossRef]
- Ingale, P.; Sakthivel, M.; Drillet, J.F. Test of Diethylmethylammonium Trifluoromethanesulfonate Ionic Liquid as Electrolyte in Electrically Rechargeable Zn/Air Battery. J. Electrochem. Soc. 2017, 164, H5224–H5229. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, D.; Wang, C.; Guo, J.; Yan, F. Rational Design of Fe1–xS/Fe3O4/Nitrogen and Sulfur Doped Porous Carbon with Enhanced Oxygen Reduction Reaction Catalytic Activity. Adv. Mater. Interfaces 2018. [Google Scholar] [CrossRef]
- Liu, B.H.; Li, Z.P. Current status and progress of direct borohydride fuel cell technology development. J. Power Sources 2009, 187, 291–297. [Google Scholar] [CrossRef]
- Gorlin, Y.; Lassalle-Kaiser, B.; Benck, J.D.; Gul, S.; Webb, S.M.; Yachandra, V.K.; Yano, J.; Jaramillo, T.F. In-Situ X-Ray Absorption Spectroscopy Investigation of a Bifunctional Manganese Oxide Catalyst with High Activity for Electrochemical Water Oxidation and Oxygen Reduction. J. Am. Chem. Soc. 2013, 135, 8525–8534. [Google Scholar] [CrossRef] [PubMed]
- Pampel, J.; Fellinger, T.-P. Opening of Bottleneck Pores for the Improvement of Nitrogen Doped Carbon Electrocatalysts. Adv. Energy Mater. 2016, 6, 1502389. [Google Scholar] [CrossRef]
- Schaber, P.; Colson, J.; Higgins, S.; Thieln, D.; Anspach, B.; Brauer, J. Thermal de-composition (pyrolysis) of urea in an open reaction vessel. Thermochim. Acta 2004, 424, 131–142. [Google Scholar] [CrossRef]
- Olszowska, K.; Pang, J.; Wrobel, P.S.; Zhao, L.; Ta, H.Q.; Liu, Z.; Trzebicka, B.; Bachmatiuk, A.; Rummeli, M.H. Three-dimensional nanostructured graphene: Synthesis and energy, environmental and biomedical applications. Synth. Met. 2017, 234, 53–85. [Google Scholar] [CrossRef]
- Fei, H.L.; Yang, Y.; Fan, X.J.; Wang, G.; Ruan, G.D.; Tour, J.M. Tungsten-Based Porous Thin-Films for Electrocatalytic Hydrogen Generation—Electronic Supplementary Information. J. Mater. Chem. A 2015, 3, 5798–5804. [Google Scholar] [CrossRef]
- Stoerzinger, K.A.; Hong, W.T.; Azimi, G.; Giordano, L.; Lee, Y.-L.; Crumlin, E.J.; Biegalski, M.D.; Bluhm, H.; Varanasi, K.K.; Shao-Horn, Y. Reactivity of Perovskites with Water: Role of Hydroxylation in Wetting and Implications for Oxygen Electrocatalysis. J. Phys. Chem. C 2015, 119, 18504–18512. [Google Scholar] [CrossRef]
- Liu, S.; Yang, Z.; Li, M.; Liu, L.; Wang, Y.; Lv, W.; Qin, Z.; Zhao, X.; Zhu, P.; Wang, G. FeS-decorated hierarchical porous N, S-dual-doped carbon derived from silica-ionogel as an efficient catalyst for oxygen reduction reaction in alkaline media. Electrhochim. Acta 2018, 265, 221–231. [Google Scholar] [CrossRef]
- Ma, X.X.; Su, Y.; He, X. Fe9S10-decorated N, S co-doped graphene as a new and efficient electrocatalyst for oxygen reduction and oxygen evolution reactions. Catal. Sci. Technol. 2017, 7, 1181–1192. [Google Scholar] [CrossRef]
- Lalande, G.; Côté, R.; Tamizhmani, G.; Guay, D.; Dodelet, J.P.; Dignard-Bailey, L.; Weng, L.T.; Bertrand, P. Physical, chemical and electrochemical characterization of heat-treated tetracarboxylic cobalt phthalocyanine adsorbed on carbon black as electrocatalyst for oxygen reduction in polymer electrolyte fuel cells. Electrochim. Acta 1995, 40, 2635–2646. [Google Scholar] [CrossRef]
- Morris, J.M.; Jin, S.; Wang, J.; Zhu, C.; Urynowicz, M.A. Lead dioxide as an alternative catalyst to platinum in microbial fuel cells. Electrochem. Commun. 2007, 9, 1730–1734. [Google Scholar] [CrossRef]
- Valipour, A.; Ayyaru, S.; Ahn, Y. Application of graphene-based nanomaterials as novel cathode catalysts for improving power generation in single chamber microbial fuel cells. J. Power Sources 2016, 327, 548–556. [Google Scholar] [CrossRef]
- Santoro, C.; Serov, A.; Narvaez Villarrubia, C.W.; Stariha, S.; Babanova, S.; Schuler, A.J.; Artyushk, K.; Atanassov, P. Double-chamber microbial fuel cell with a non-platinum-group metal Fe–N–C cathode catalyst. ChemSusChem 2015, 8, 828–834. [Google Scholar] [CrossRef]
- Feng, L.; Yan, Y.; Chen, Y.; Wang, L. Nitrogen-Doped Carbon Nanotubes as Efficient and Durable Metal-Free Cathodic Catalysts for Oxygen Reduction in Microbial Fuel Cells. Energy Environ. Sci. 2011, 4, 1892–1899. [Google Scholar] [CrossRef]
- Santoro, C.; Li, B.; Cristiani, P.; Squadrito, G. Power generation of microbial fuel cells (MFCs) with low cathodic platinum loading. Int. J. Hydrog. Energy 2013, 38, 692–700. [Google Scholar] [CrossRef]
- Kannan, M.V.; Kumar, G.G. Current status, key challenges and its solutions in the design and development of graphene based ORR catalysts for the microbial fuel cell applications. Biosens. Bioelectron. 2016, 77, 1208–1220. [Google Scholar] [CrossRef]
- Rabaey, K.; Verstraete, W. Microbial fuel cells: Novel biotechnology for energy generation. Trends Biotechnol. 2005, 23, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Tian, C.; Fu, Y.; Yang, Y.; Yin, J.; Wang, L.; Fu, H. Nitrogen-doped porous graphitic carbon as an excellent electrode material for advanced supercapacitors. Chem. A Eur. J. 2014, 20, 564–574. [Google Scholar] [CrossRef] [PubMed]
- Bi, L.; Ci, S.; Cai, P.; Li, H.; Wen, Z. One-step pyrolysis route to three dimensional nitrogen-doped porous carbon as anode materials for microbial fuel cells. Appl. Surf. Sci. 2018, 427, 10–16. [Google Scholar] [CrossRef]
- Cheng, H.; Scott, K. Investigation of non-platinum cathode catalysts for direct borohydride fuel cells. J. Electroanal. Chem. 2006, 596, 117–123. [Google Scholar] [CrossRef]
- Cheng, S.; Liu, H.; Logan, B.E. Increased performance of single-chamber microbial fuel cells using an improved cathode structure. Electrochem. Commun. 2006, 8, 489–494. [Google Scholar] [CrossRef]
- Santoro, C.; Serov, A.; Villarrubia, C.W.N.; Stariha, S.; Babanova, S.; Artyushkova, K.; Schuler, A.J.; Atanassov, P. High catalytic activity and pollutantsresistivity using Fe-AAPyr cathode catalyst for microbial fuel cell application. Sci. Rep. 2015, 5, 16596. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Wang, Y.; Wang, K.; Liang, Y.; Wu, D.; Tsiakaras, P.; Song, S. A novel sulfur-nitrogen dual doped ordered mesoporous carbon electrocatalyst for efficient oxygen reduction reaction. Appl. Catal. B Environ. 2016, 189, 11. [Google Scholar] [CrossRef]
- Bayram, E.; Yilmaz, G.; Mukerjee, S. A solution-based procedure for synthesis of nitrogen doped graphene as an efficient electrocatalyst for oxygen reduction reactions in acidic and alkaline electrolytes. Appl. Catal. B Environ. 2016, 192, 26–34. [Google Scholar] [CrossRef]
- Guerrini, E.; Grattieri, M.; Faggianelli, A.; Cristiani, P.; Trasatti, S. PTFE effect on the electrocatalysis of the oxygen reduction reaction in membraneless microbial fuel cells. Bioelectrochemistry 2015, 106, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Watson, V.J.; Delgado, C.N.; Logan, B.E. Influence of Chemical and Physical Properties of Activated Carbon Powders on Oxygen Reduction and Microbial Fuel Cell Performance. Environ. Sci. Technol. 2013, 47, 6704–6710. [Google Scholar] [CrossRef] [PubMed]
- Freguia, S.; The, E.H.; Boon, N.; Leung, K.M.; Keller, J.; Rabaey, K. Microbial fuel cells operating on mixed fatty acids. Bioresour. Technol. 2010, 101, 1233–1238. [Google Scholar] [CrossRef] [PubMed]
- Subbaraman, R.; Tripkovic, D.; Chancg, K.C.; Strmcnik, D.; Paulikas, A.P.; Hirunsit, P.; Chan, M.; Greeley, J.; Stamenkovic, V.; Markovic, N.M. Trends in activity for the water electrolyser reactions on 3d M(Ni,Co,Fe,Mn) hydr(oxy)oxide catalyst. Nat. Mater. 2012, 11, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Malik, D.J.; Trochimczuk, A.W.; Jyo, A.; Tylus, W. Synthesis and Characterization of nanostructured carbons with controlled porosity prepared from sulfonated divinylbiphenyl copolymers. Carbon 2008, 46, 310–319. [Google Scholar] [CrossRef]
- Li, Q.; Chen, W.; Xiao, H.; Gong, Y.; Li, Z.; Zheng, L.; Yan, W.; Cheong, W.; Shen, R.; Fu, N.; et al. Fe Isolated Single Atoms on S, N Codoped Carbon by Copolymer Pyrolysis Strategy for Highly Efficient Oxygen Reduction Reaction. Adv. Master. 2018, 30, 1800588. [Google Scholar] [CrossRef] [PubMed]
- Men, B.; Sun, Y.; Liu, J.; Tang, Y.; Chen, Y.; Wan, P.; Pan, J. Synergistically Enhanced Electrocatalytic Activity of Sandwich-like N-Doped Graphene/Carbon Nanosheets Decorated by Fe and S for Oxygen Reduction Reaction. ACS Appl. Master. Interfaces 2016, 30, 19533–19541. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wu, G.; Cullen, D.A.; More, K.L.; Mack, N.H.; Chung, H.T.; Zelenay, P. Phosphate-Tolerant Oxygen Reduction Catalysts. ACS Catal. 2014, 4, 3193–3200. [Google Scholar] [CrossRef]
- Zitolo, A.; Goellner, V.; Armel, V.; Sougrati, M.-T.; Mineva, T.; Stievano, L.; Fonda, E.; Jaouen, F. Identification of catalytic sites for oxygen reduction in iron- and nitrogen-doped graphene materials. Nat. Mater. 2015, 14, 937–942. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.-T.; Mecheri, B.; Iannaci, A.; D’Epifanio, A.; Licoccia, S. Iron/polyindole-based electrocatalysts to enhance oxygen reduction in microbial fuel cells. Electrochim. Acta 2016, 190, 388–395. [Google Scholar] [CrossRef]
- Haolin, T.; Yan, Z.; Yin, Z.; Rui, W.; Shichang, C.; Cong, L.; Haopeng, C. Iron-embedded nitrogen doped carbon frameworks as robust catalyst for oxygen reduction reaction in microbial fuel cells. J. Power Sources 2014, 202, 550–556. [Google Scholar]
- Bruce, P.G.; Freunberger, S.A.; Hardwick, L.J.; Tarascon, J.M. Li-O2 and Li-S batteries with high energy storage. Nat. Mater. 2012, 11, 19–29. [Google Scholar] [CrossRef]
- Ferrandon, M.; Kropf, A.J.; Myers, D.J. Multitechnique Characterization of a Polyaniline–Iron–Carbon Oxygen Reduction Catalyst. J. Phys. Chem. C 2012, 116, 16001–16013. [Google Scholar] [CrossRef]
- Armel, V.; Hindocha, S.; Salles, F. Structural Descriptors of Zeolitic-Imidazolate Frameworks Are Keys to the Activity of Fe-N-C Catalysts. J. Am. Chem. Soc. 2017, 139, 453–464. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.J.; Gu, L.; Li, L. Understanding the High Activity of Fe–N–C Electrocatalysts in Oxygen Reduction: Fe/Fe3C Nanoparticles Boost the Activity of Fe–Nx. J. Am. Chem. Soc. 2016, 138, 3570–3578. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-W.; Seo, D.-H.; Ma, X.; Ceder, G.; Kang, K. Electrode Materials for rechargeable sodium-ion batteries: Potential alternatives to current lithium-ion batteries. Adv. Energy Mater. 2012, 2, 710–721. [Google Scholar] [CrossRef]
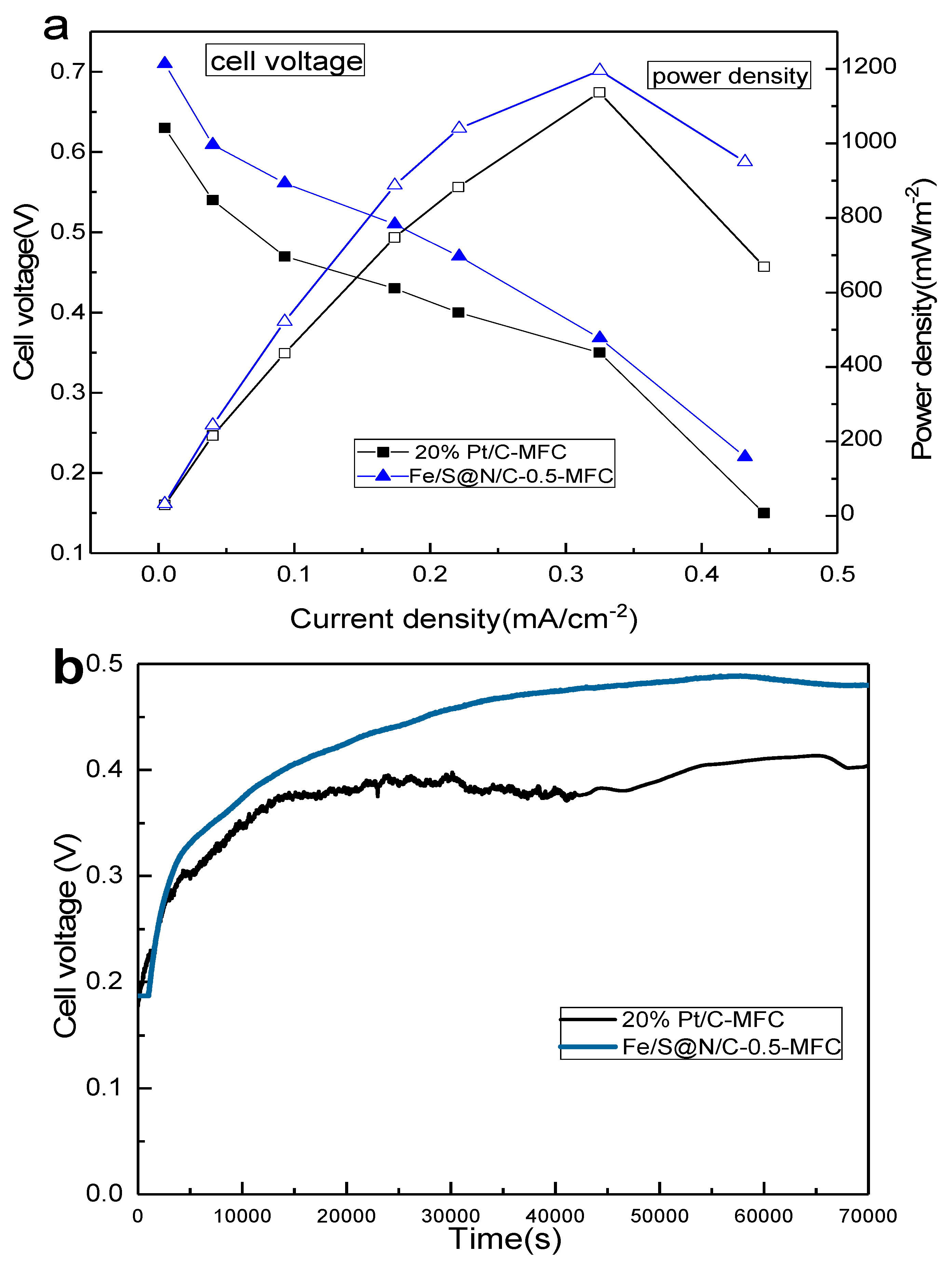
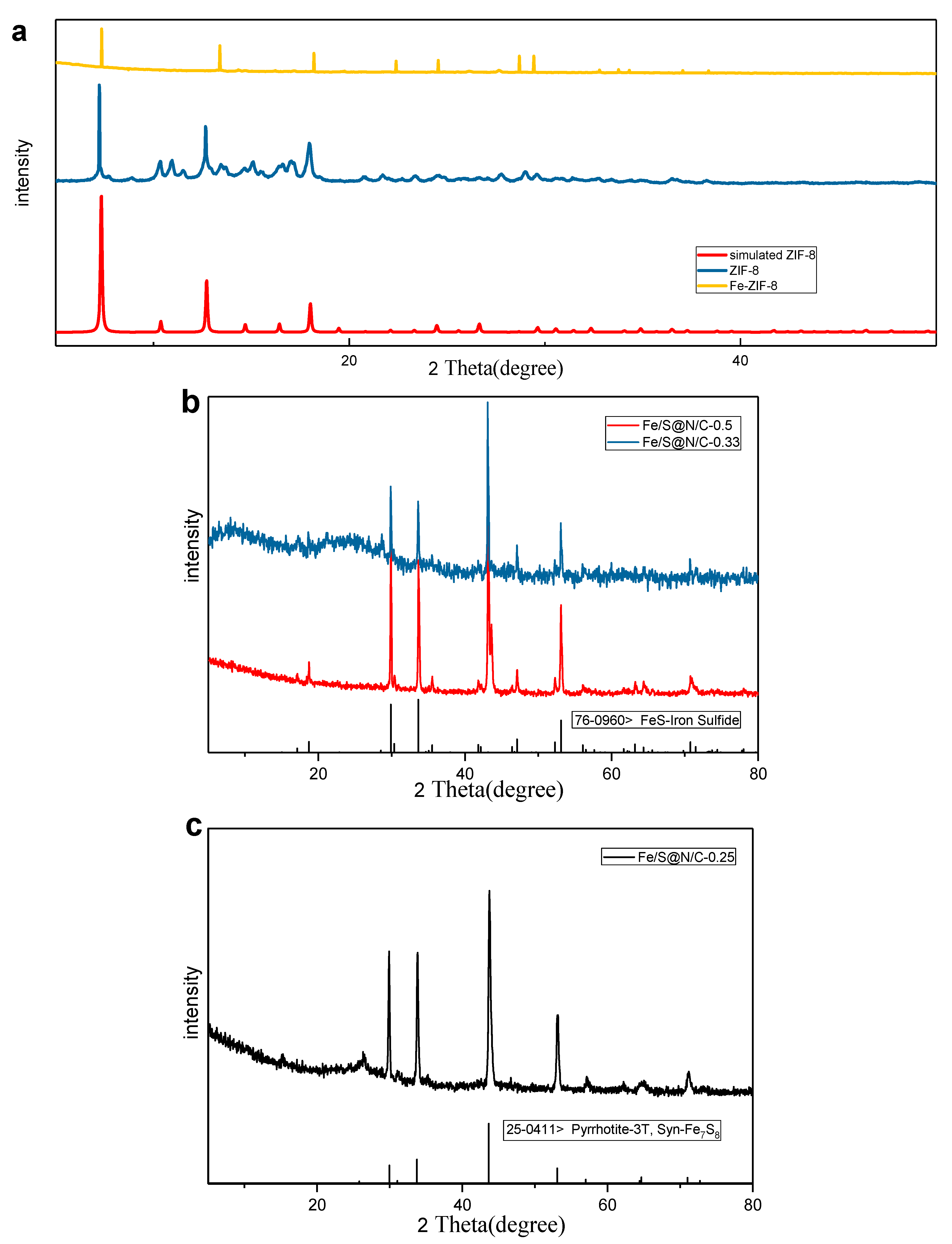
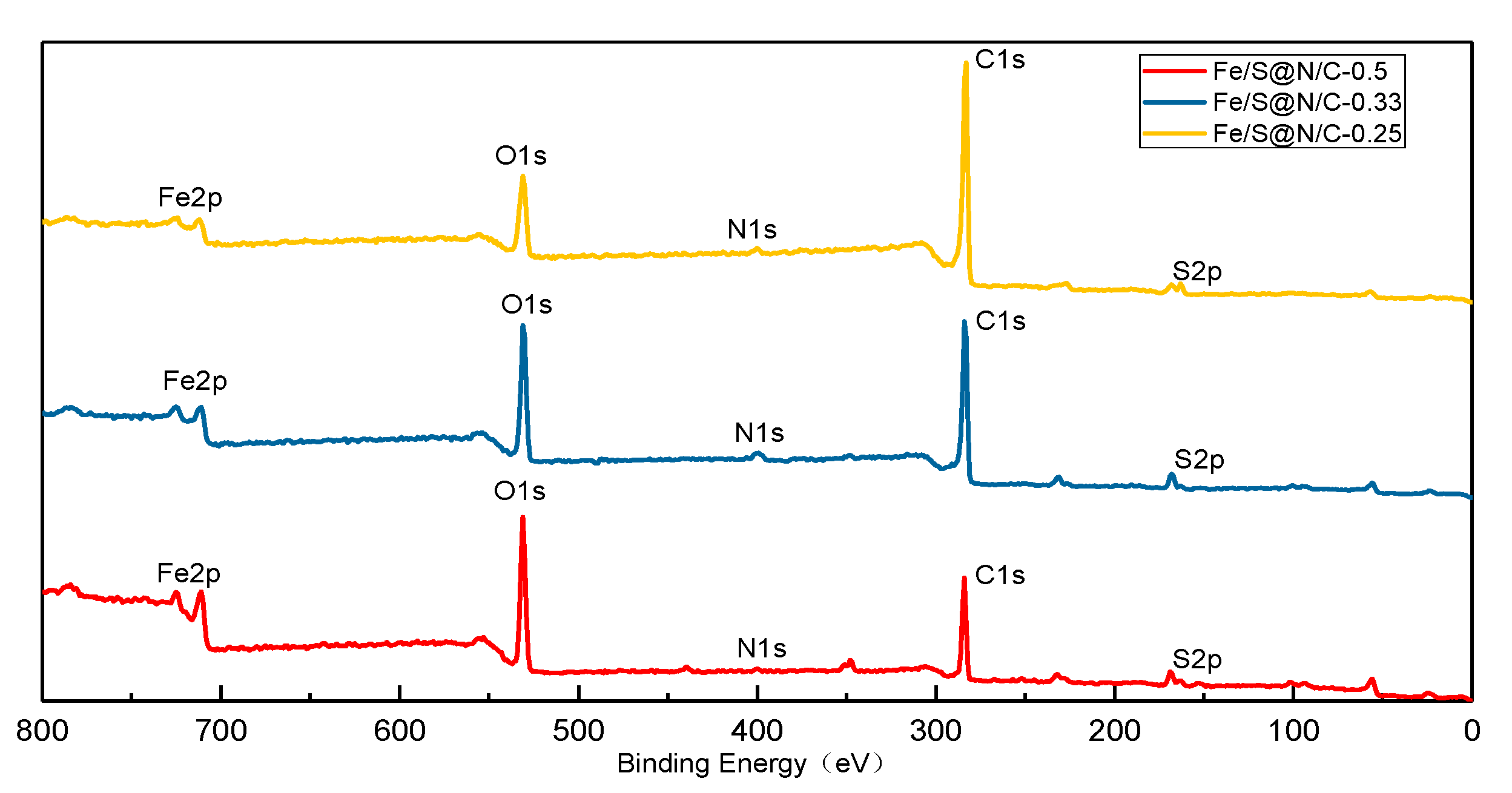
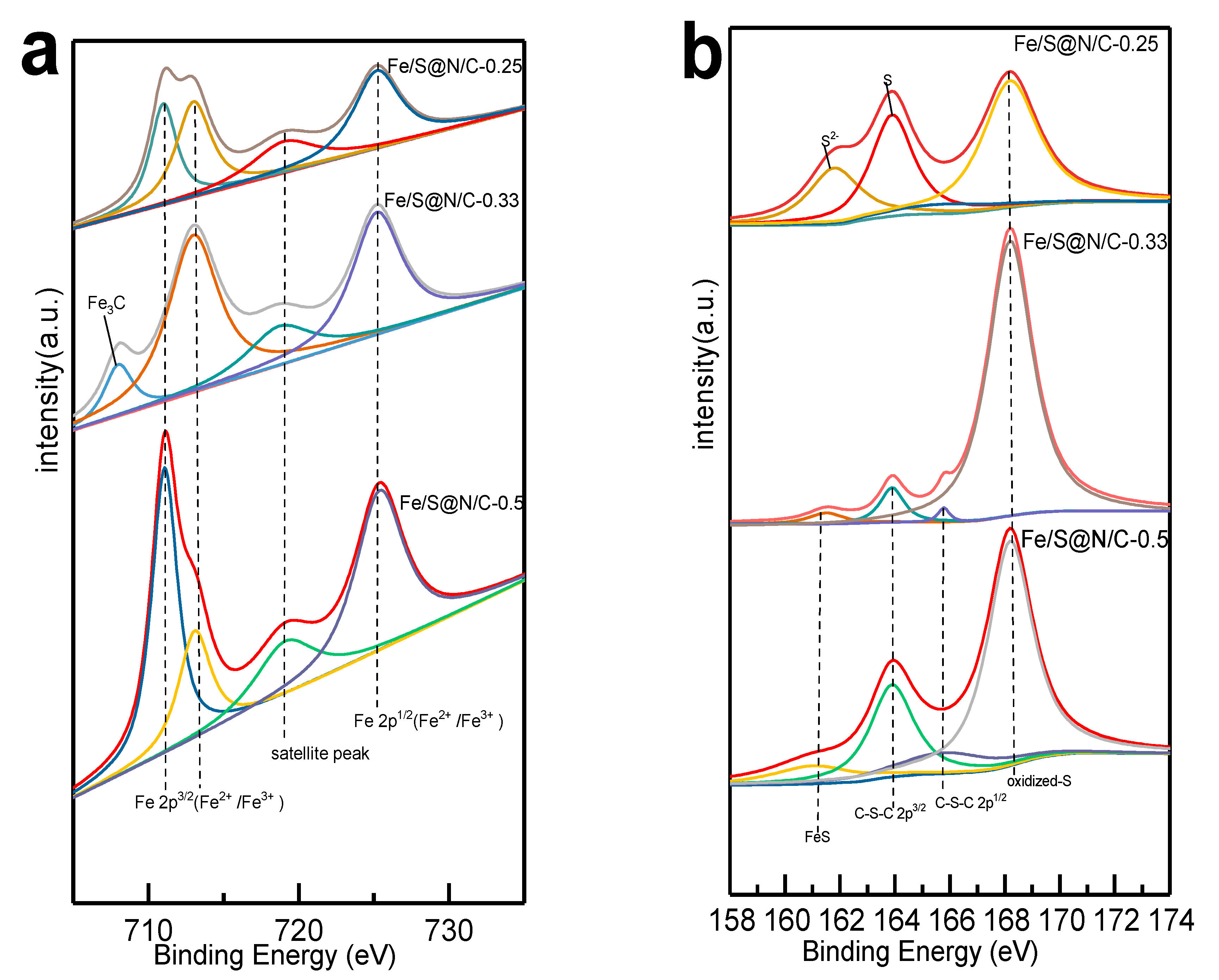
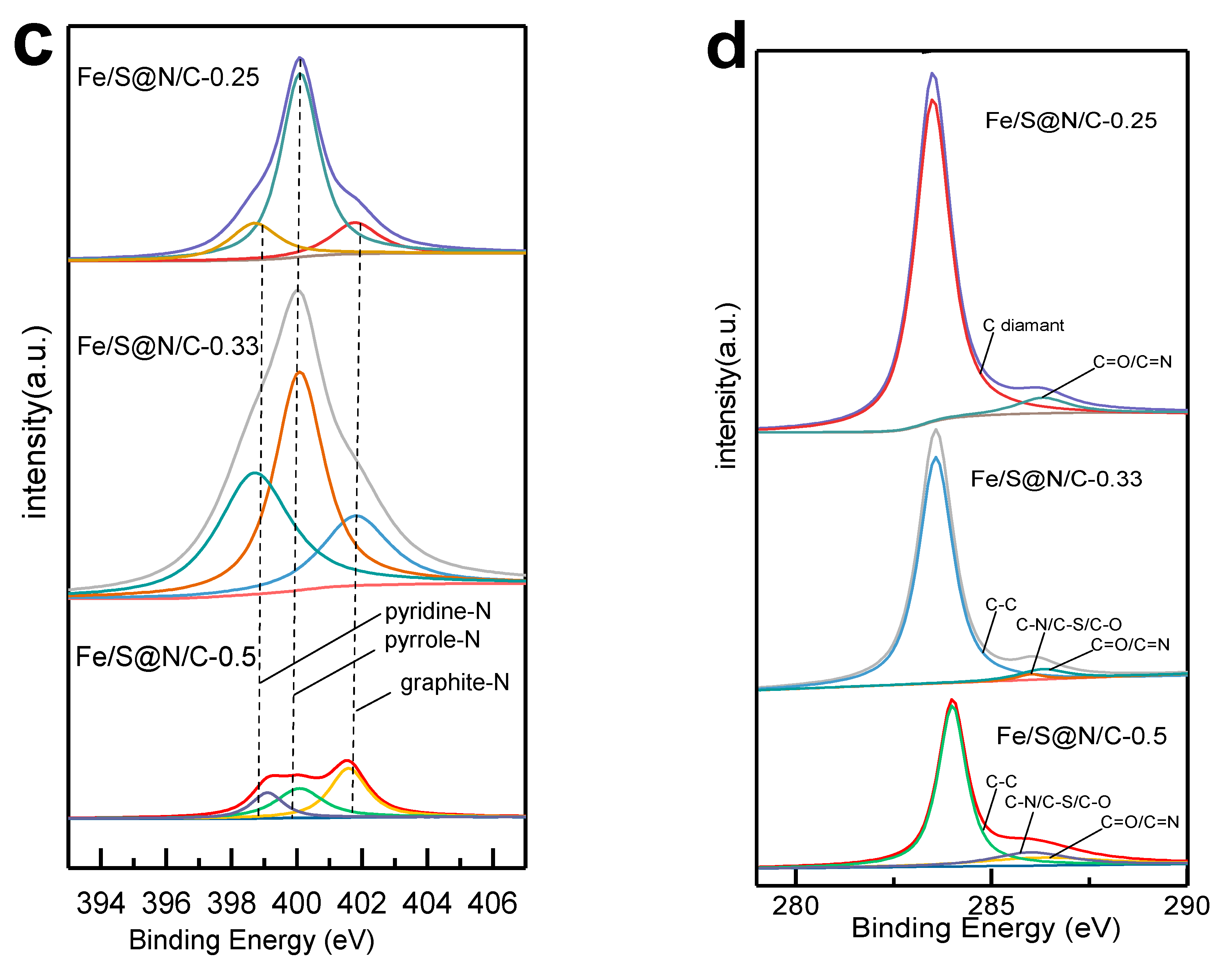
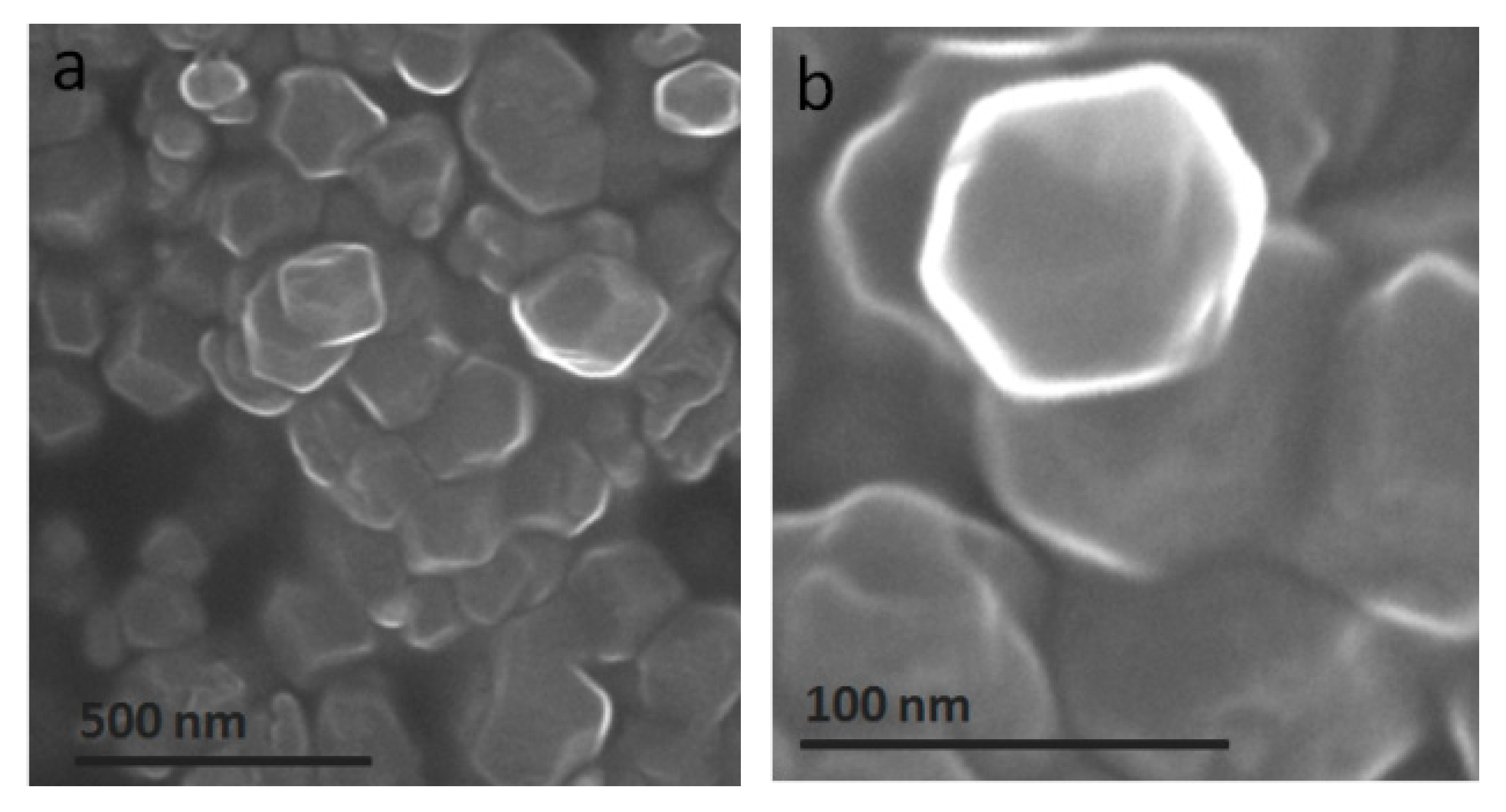

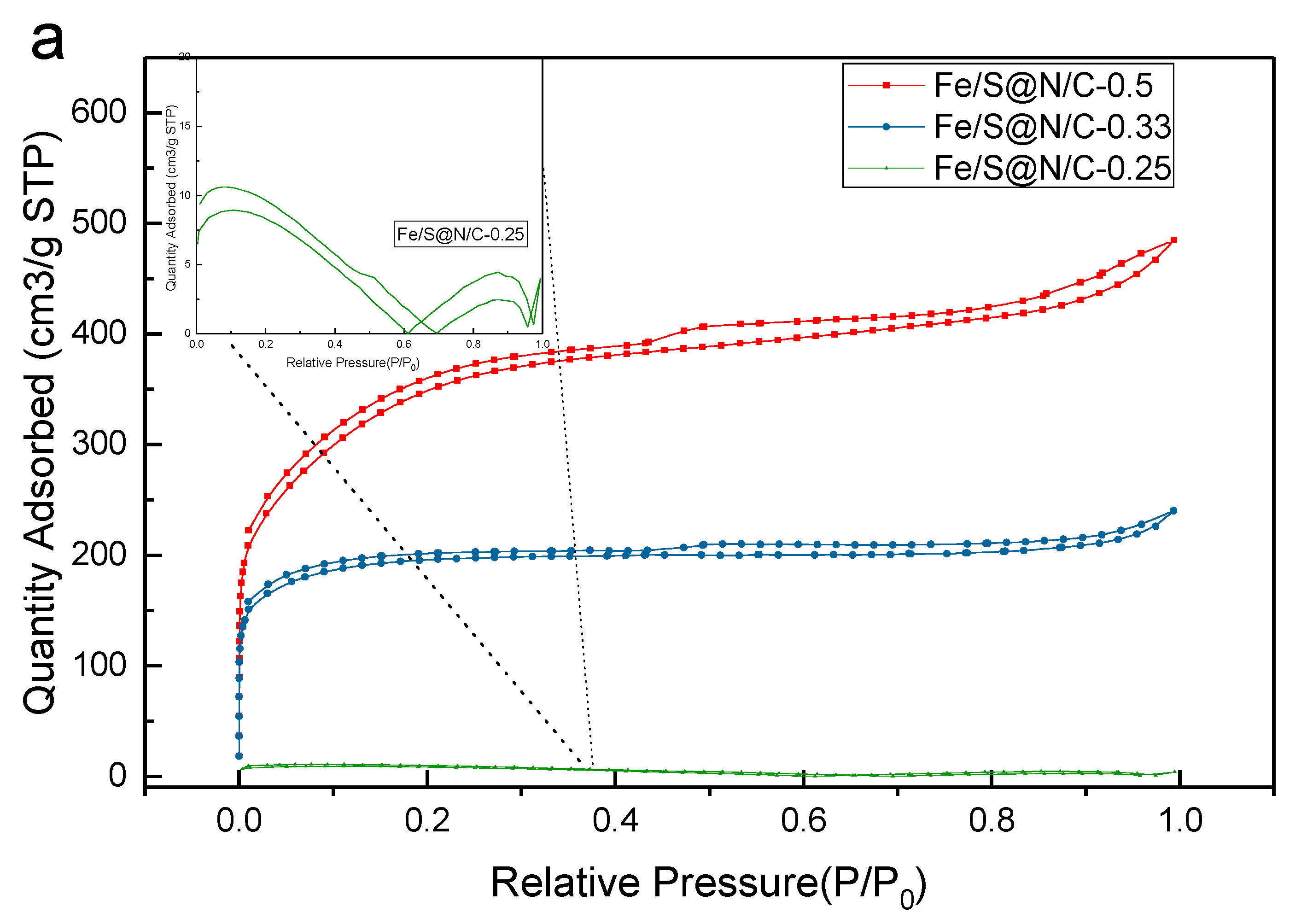
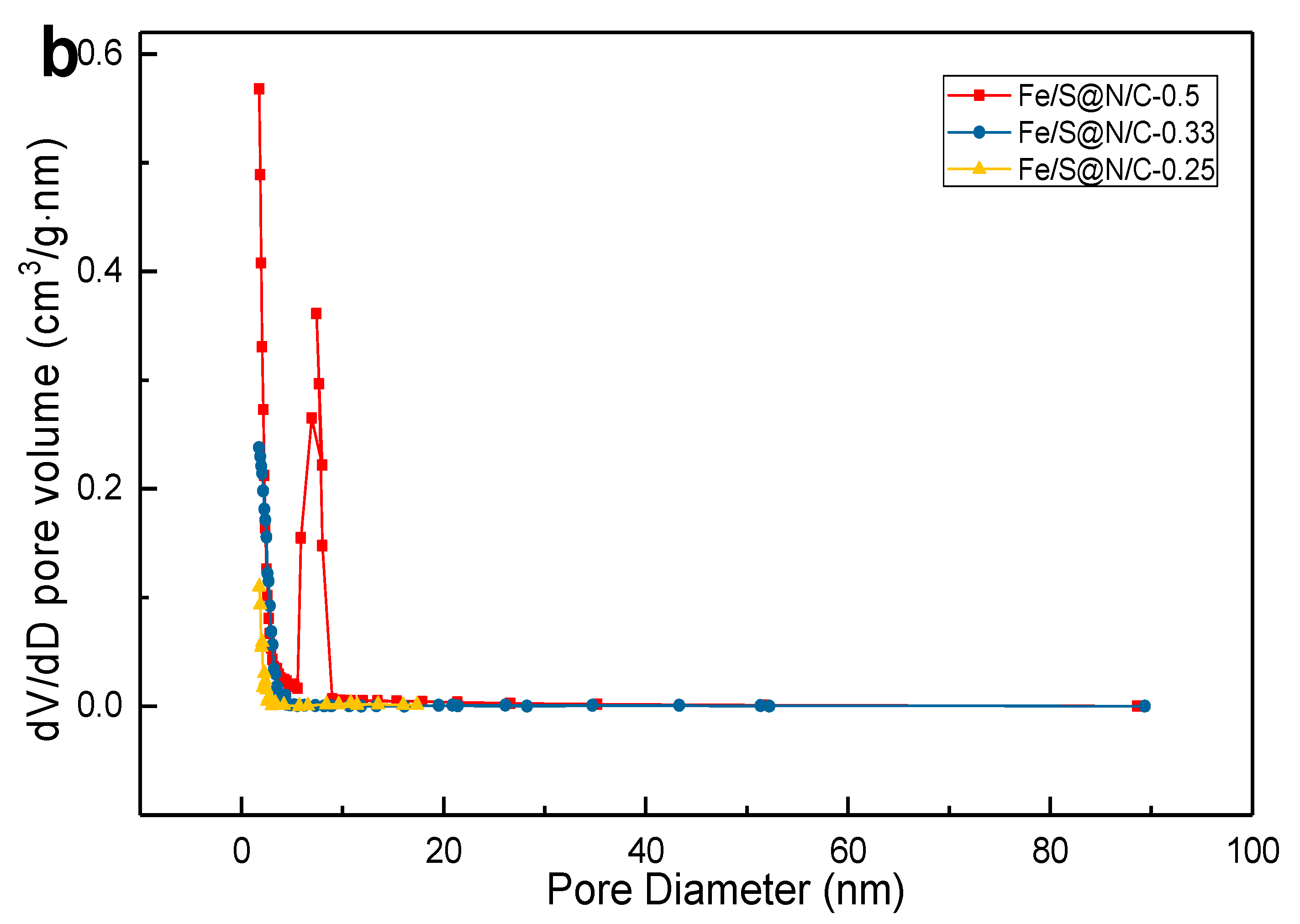
| Constituent | Concentration |
|---|---|
| glucose | 1 g/L |
| PBS (phosphate buffered saline) | 50 mmol/L |
| vitamin | 5 mL/L |
| minerals | 12.5 mL/L |
| Vitamin | Concentration (mg/L) | Vitamin | Concentration (mg/L) |
|---|---|---|---|
| biotin | 2 | nicotinic acid | 5 |
| folic acid | 2 | vitamin b12 | 0.1 |
| pyridoxine hydrochloride | 10 | DL-calcium pantothenate | 5 |
| thiamine hydrochloride | 5 | p-aminobenzoic acid | 5 |
| riboflavin | 5 | lipoic acid | 5 |
| Mineral | Concentration (mg/L) |
|---|---|
| NaCl sodium chloride | 1 |
| CaCl2 calcium chloride | 0.08 |
| MgSO4·7H2O magnesium sulfate heptahydrate | 6.14 |
| MgSO4·H2O magnesium sulfate monohydrate | 0.56 |
| ZnSO4·7H2O zinc sulfate heptahydrate | 0.27 |
| FeSO4·7H2O ferrous sulfate heptahydrate | 0.1 |
| CuSO4·5H2O copper pentahydrate sulfate | 0.01 |
| Samples | C (%) | N (%) | S (%) | Fe (%) |
|---|---|---|---|---|
| Fe/S@N/C-0.5 | 50.47 | 0.7 | 6.63 | 6.2 |
| Fe/S@N/C-0.33 | 64.72 | 3.59 | 4.32 | 3.87 |
| Fe/S@N/C-0.25 | 76.85 | 1.89 | 3.97 | 2.62 |
| Samples | SBET (m2/g) | SLangmuir (m2/g) | Dmicropore (nm) | Dmesopore (nm) |
|---|---|---|---|---|
| Fe/S@N/C-0.5 | 598 | 704 | 1.8 | 7.5 |
| Fe/S@N/C-0.33 | 367 | 582 | 1.8 | —— |
| Fe/S@N/C-0.25 | 209 | 311 | 1.8 | —— |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, X.; Han, W.; Ren, H.; Zhuang, Q. Metallic Organic Framework-Derived Fe, N, S co-doped Carbon as a Robust Catalyst for the Oxygen Reduction Reaction in Microbial Fuel Cells. Energies 2019, 12, 3846. https://doi.org/10.3390/en12203846
Luo X, Han W, Ren H, Zhuang Q. Metallic Organic Framework-Derived Fe, N, S co-doped Carbon as a Robust Catalyst for the Oxygen Reduction Reaction in Microbial Fuel Cells. Energies. 2019; 12(20):3846. https://doi.org/10.3390/en12203846
Chicago/Turabian StyleLuo, Xiao, Wuli Han, Han Ren, and Qingzuo Zhuang. 2019. "Metallic Organic Framework-Derived Fe, N, S co-doped Carbon as a Robust Catalyst for the Oxygen Reduction Reaction in Microbial Fuel Cells" Energies 12, no. 20: 3846. https://doi.org/10.3390/en12203846
APA StyleLuo, X., Han, W., Ren, H., & Zhuang, Q. (2019). Metallic Organic Framework-Derived Fe, N, S co-doped Carbon as a Robust Catalyst for the Oxygen Reduction Reaction in Microbial Fuel Cells. Energies, 12(20), 3846. https://doi.org/10.3390/en12203846




