Bio-Based Chemicals from Renewable Biomass for Integrated Biorefineries
Abstract
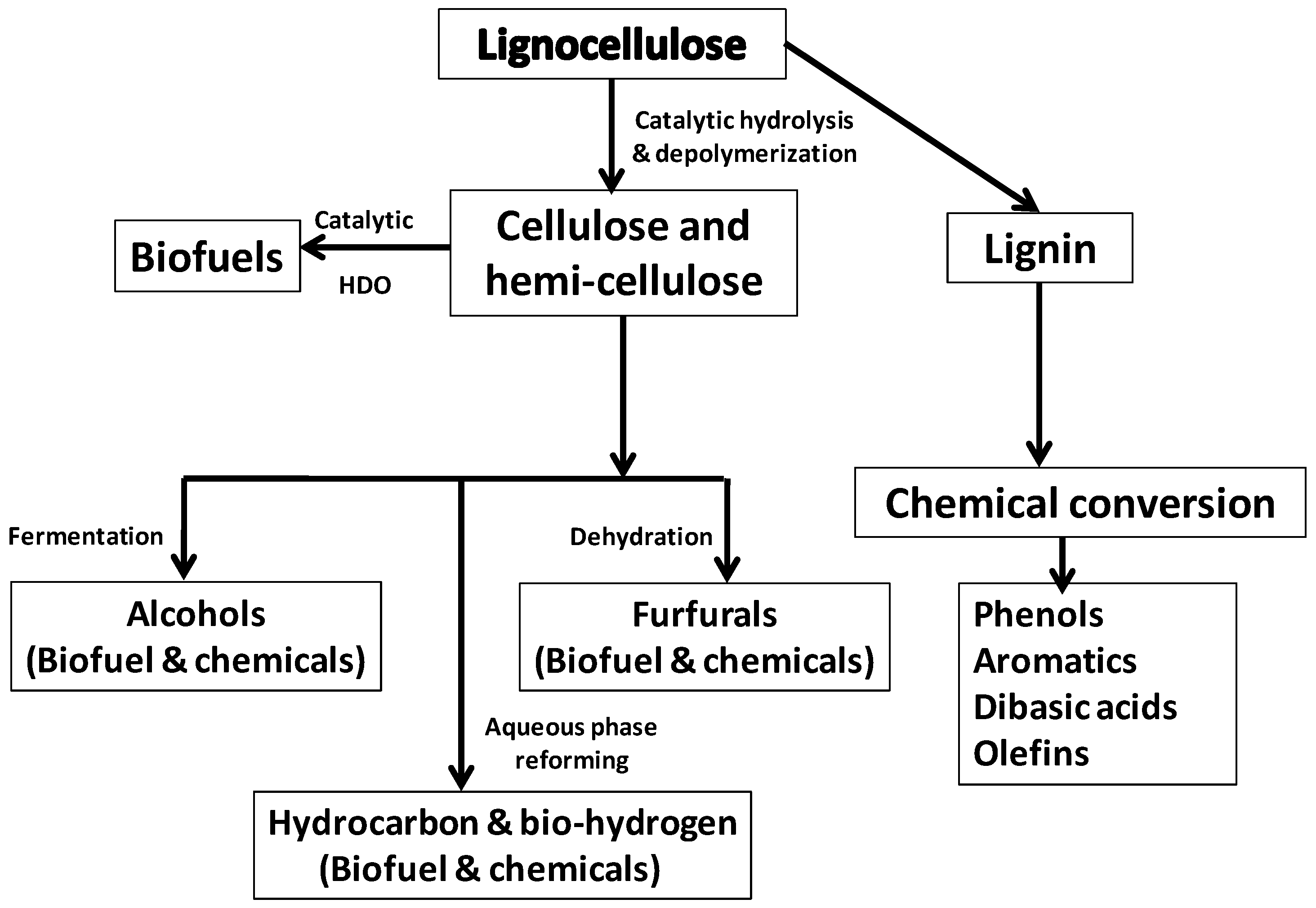
1. Introduction
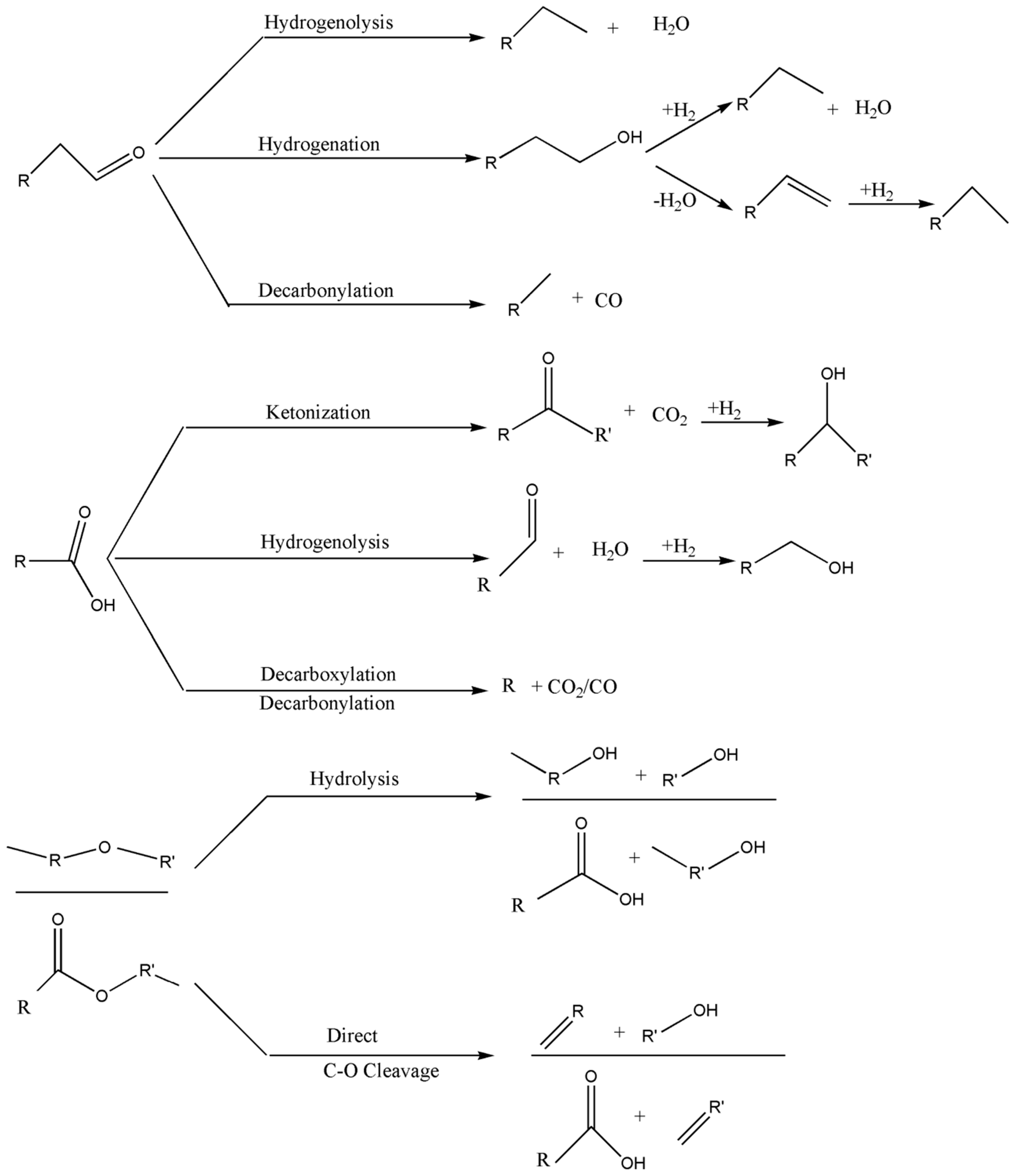
2. Common Reactions Involved in Biomass Processing
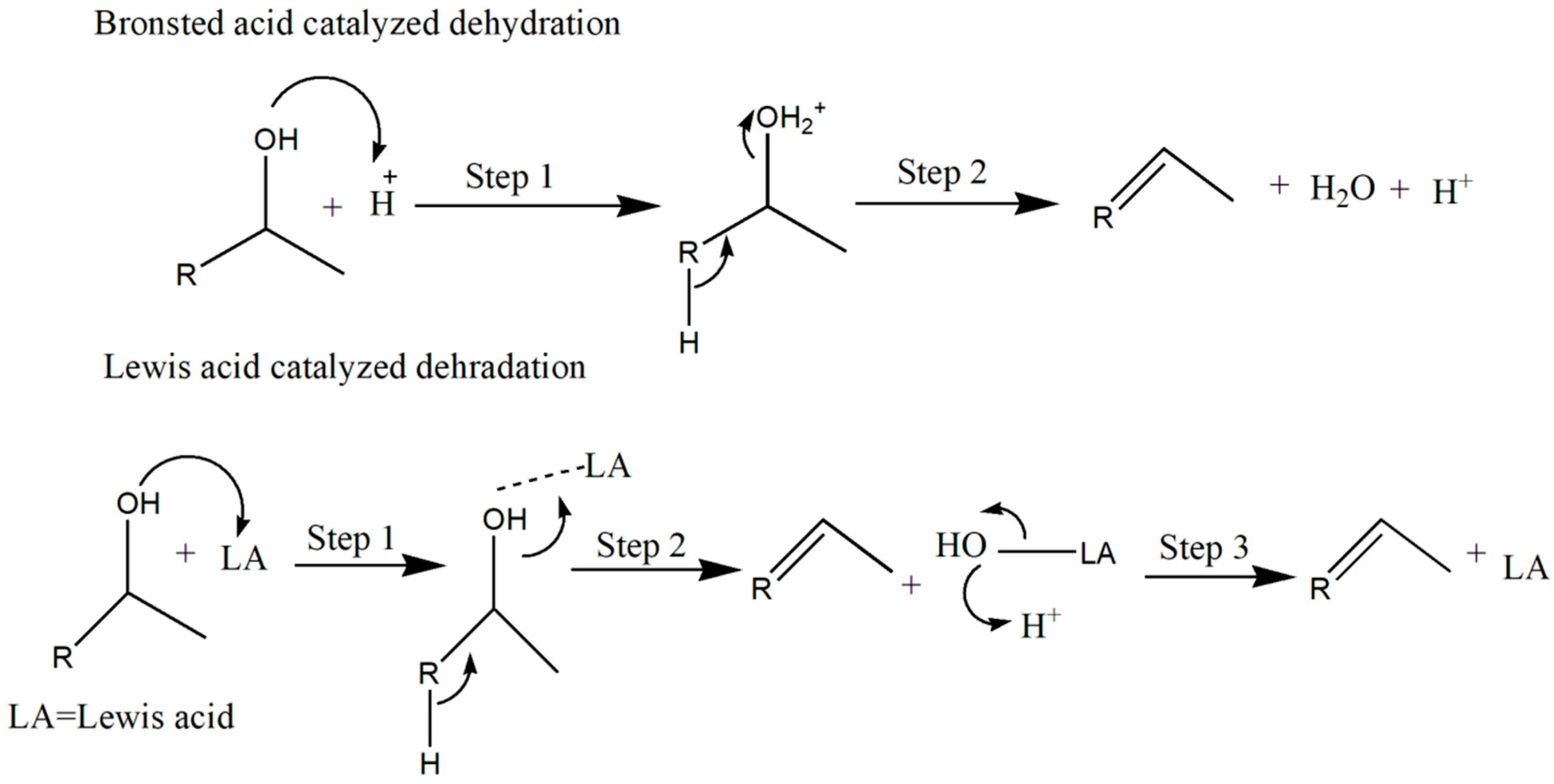
2.1. Dehydration
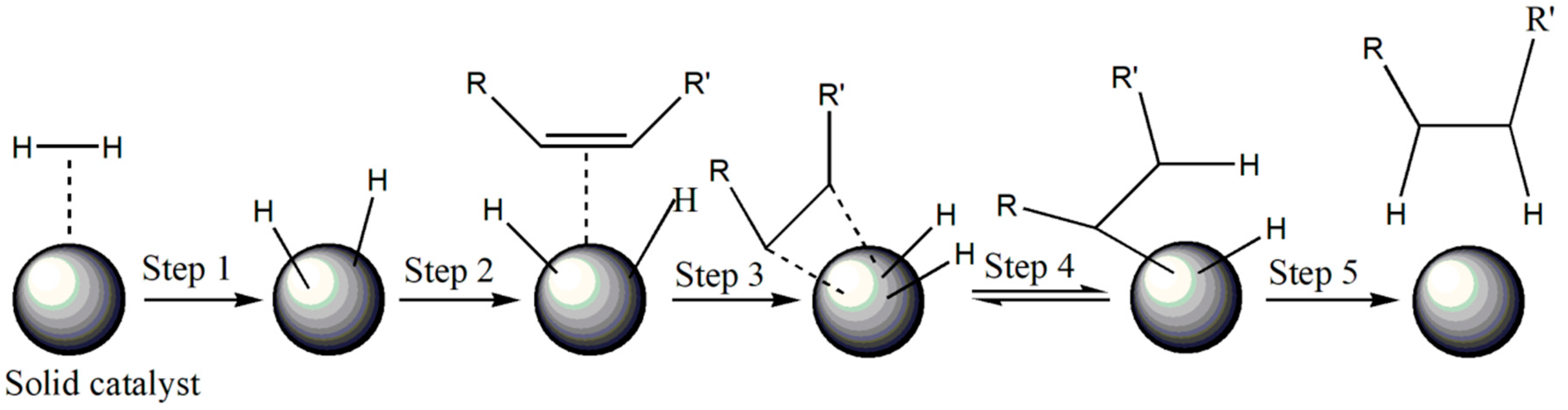
2.2. Hydrogenation
2.3. Hydrodeoxygenation (HDO)
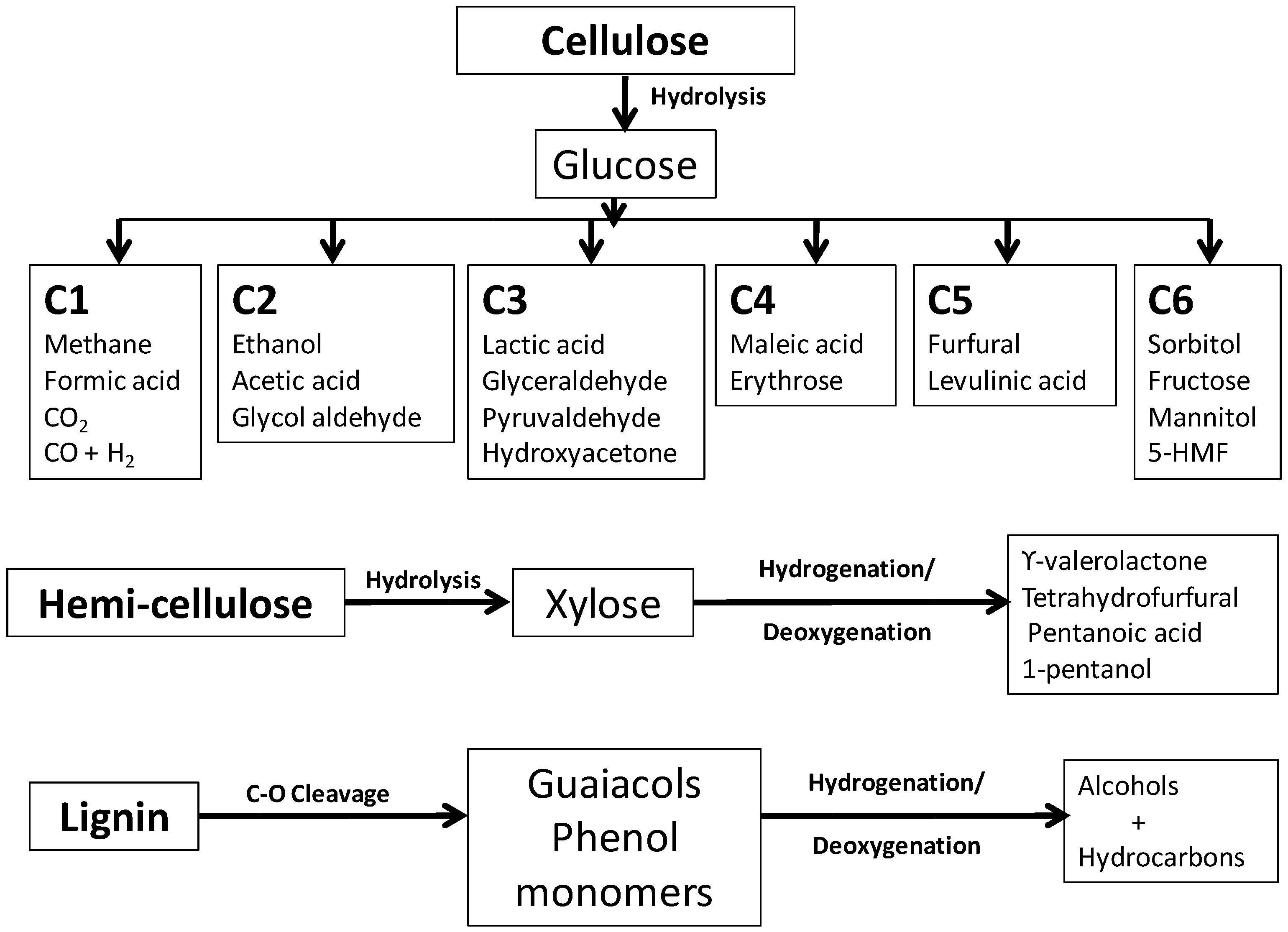
3. Sugars as Feedstocks
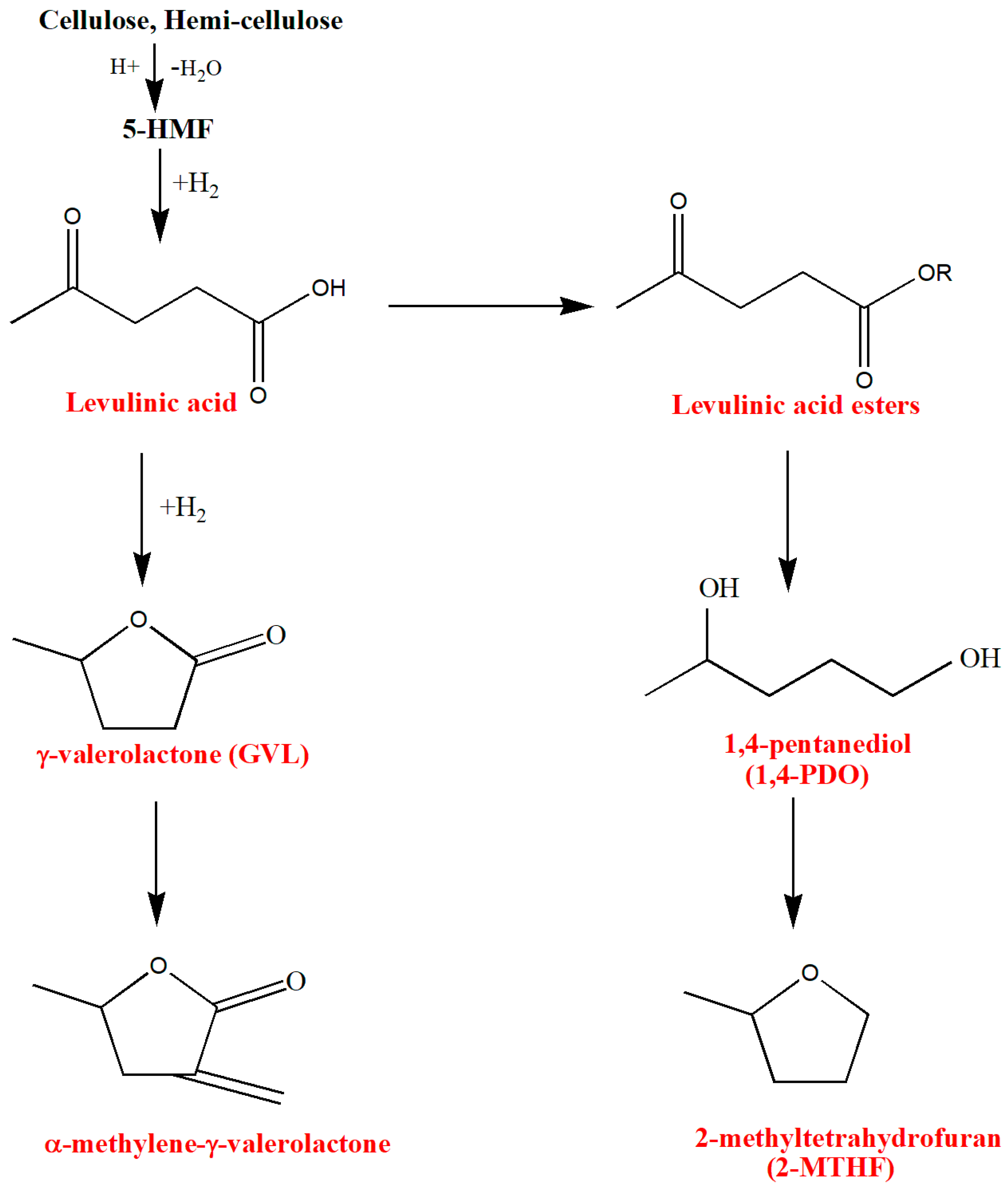
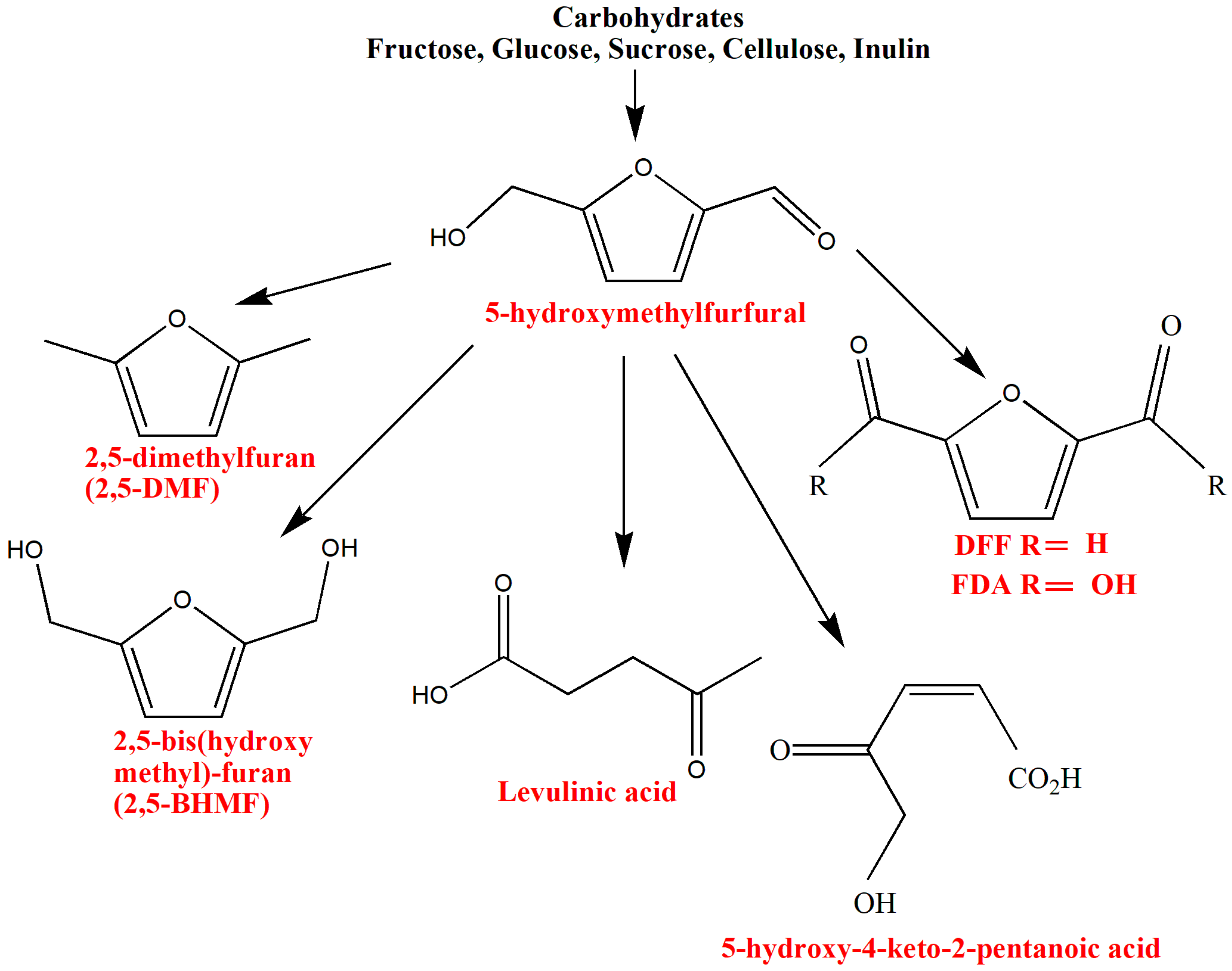
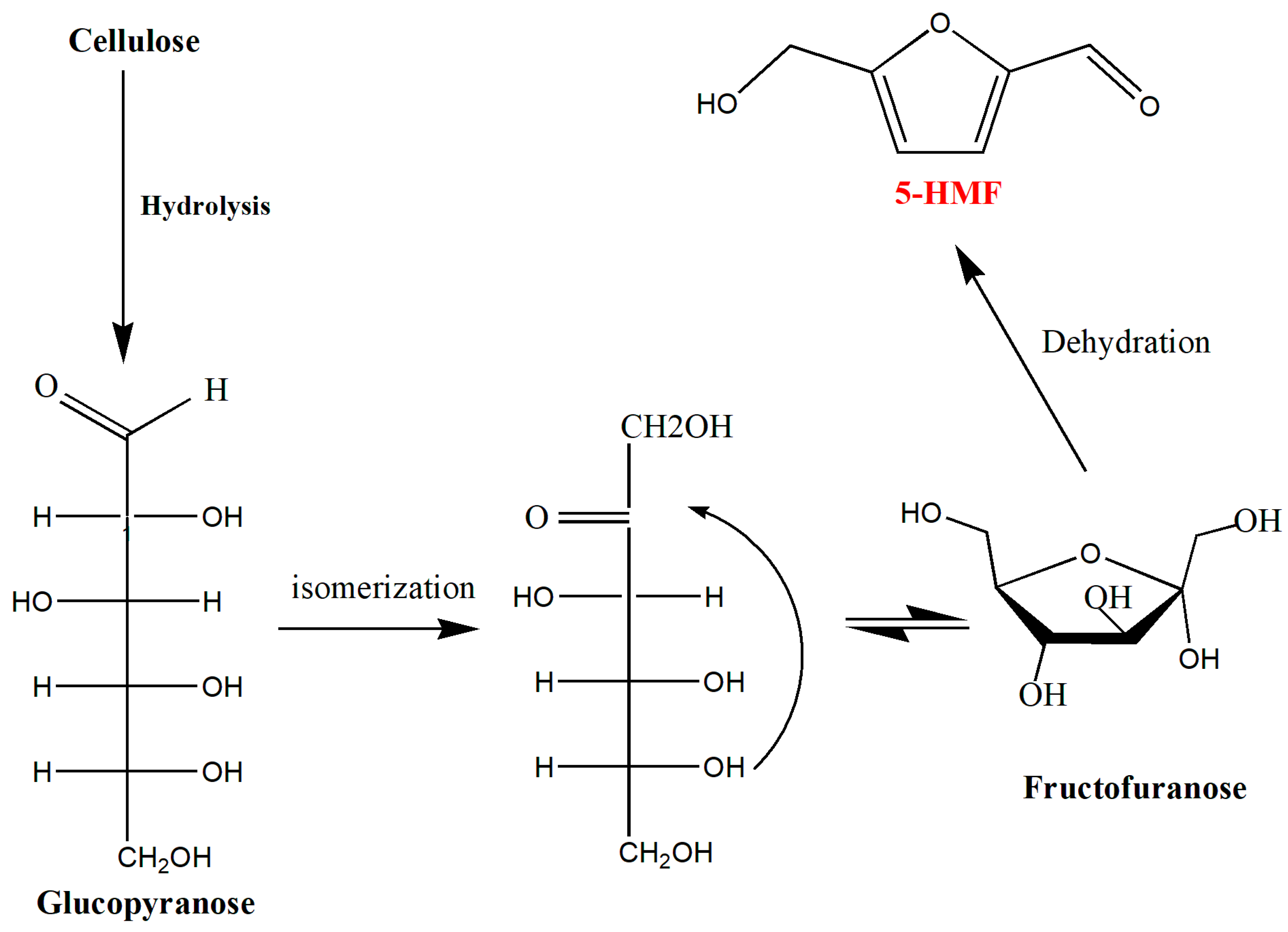
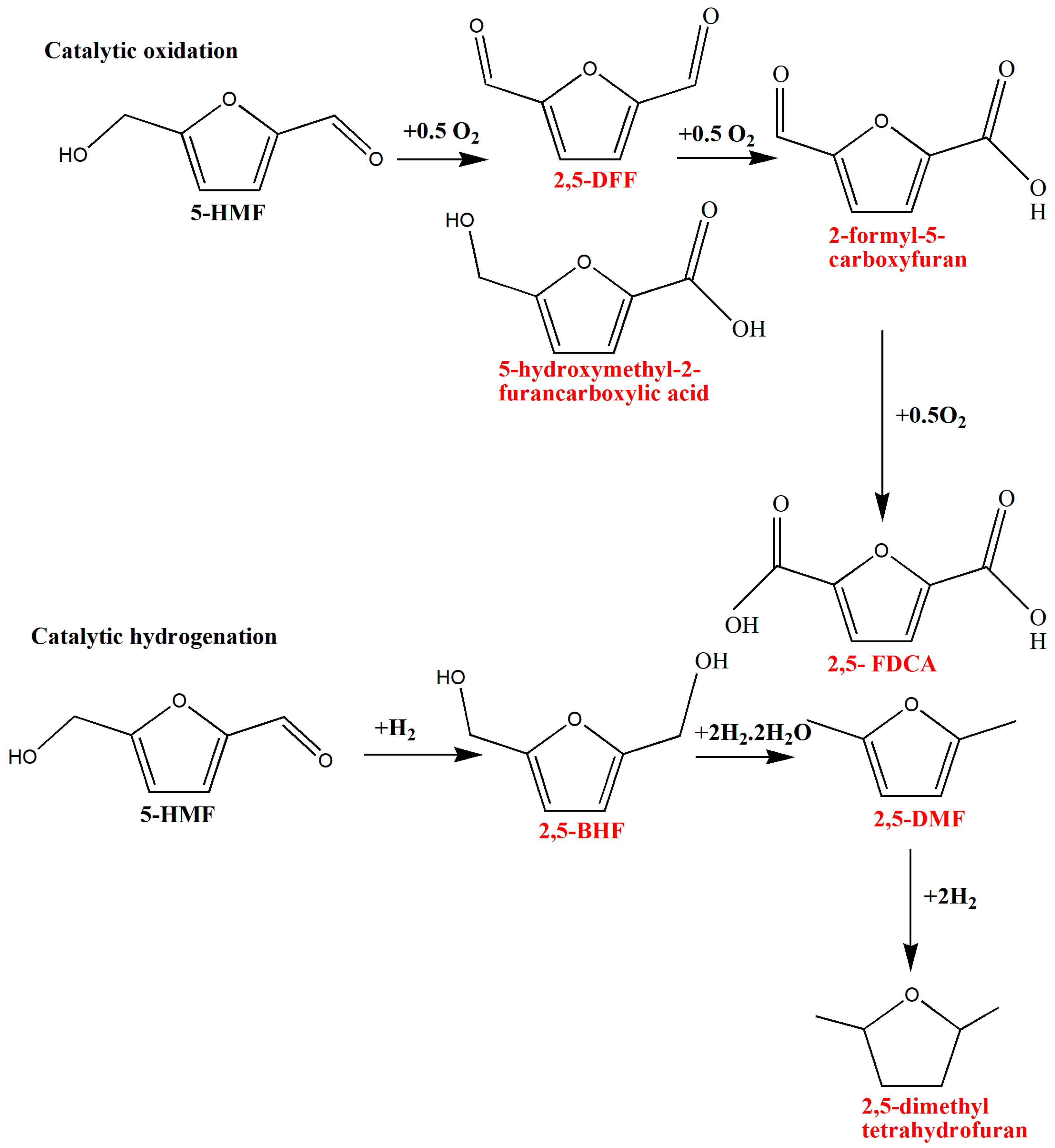
3.1. Hydroxymethylfurfural (5-HMF)
5-HMF derivatives
3.2. Levulinic Acid
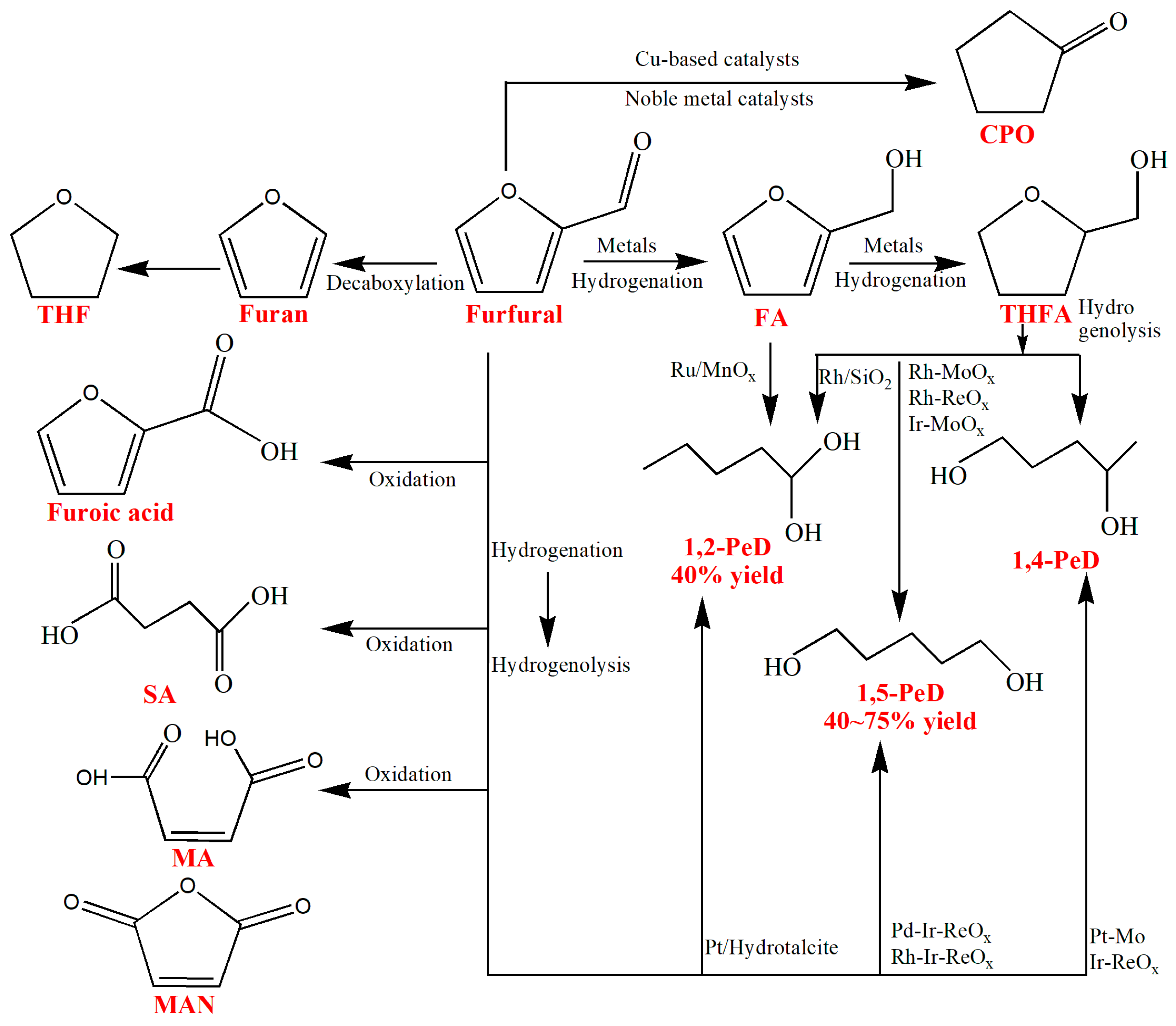
3.3. Furfural
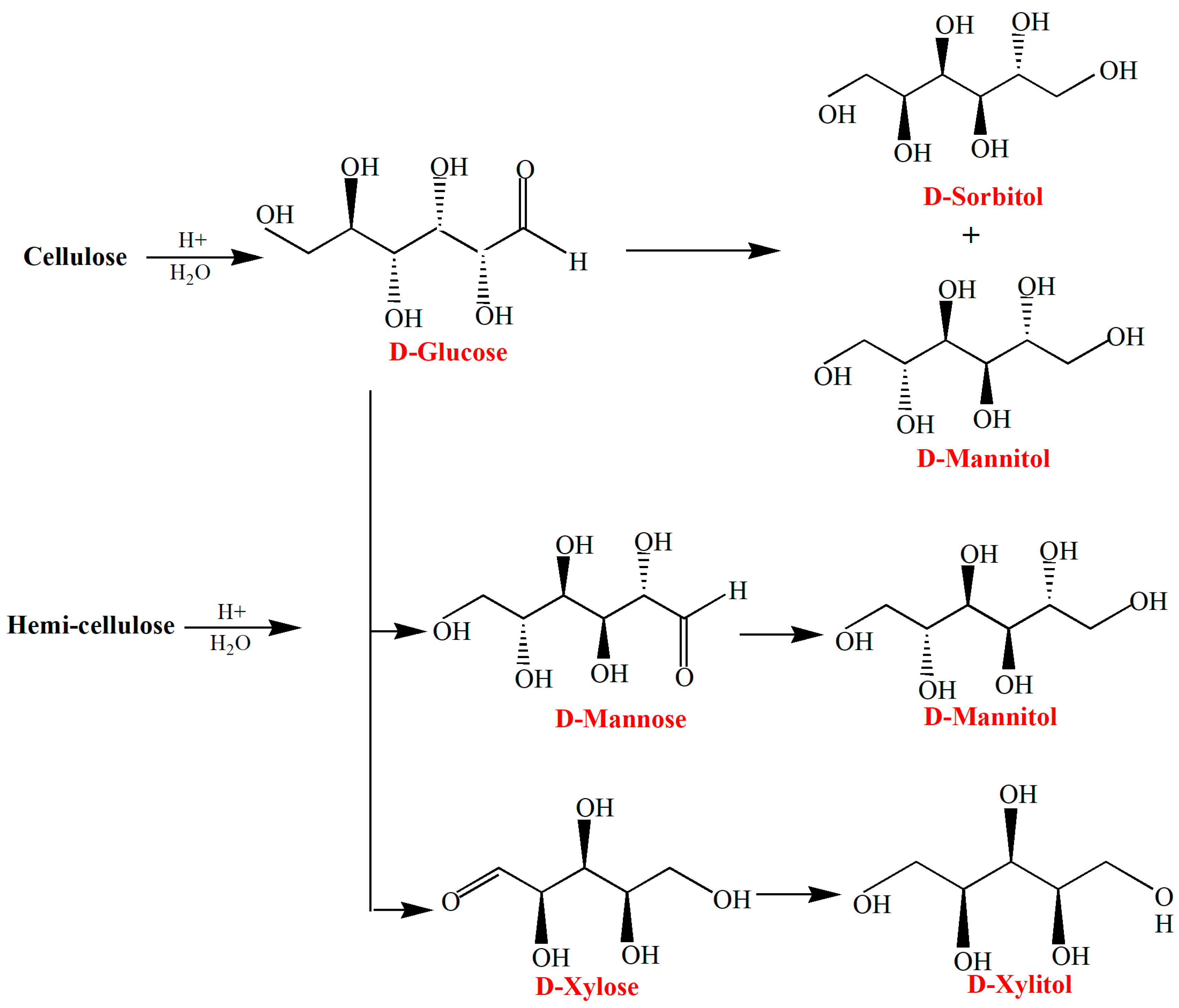
3.4. Sugar Alcohols
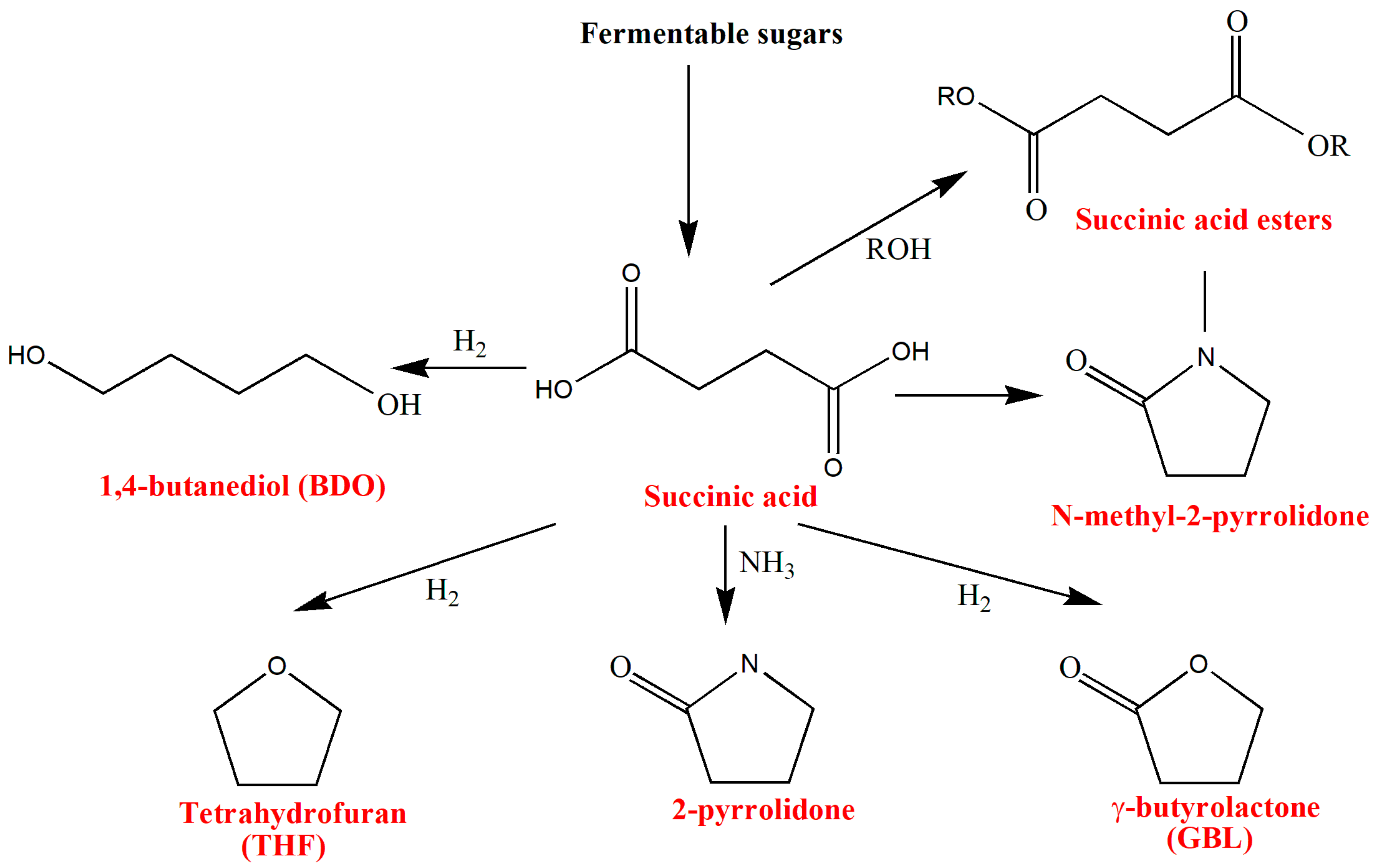
3.5. Succinic Acid
3.6. Lactic Acid (LA)
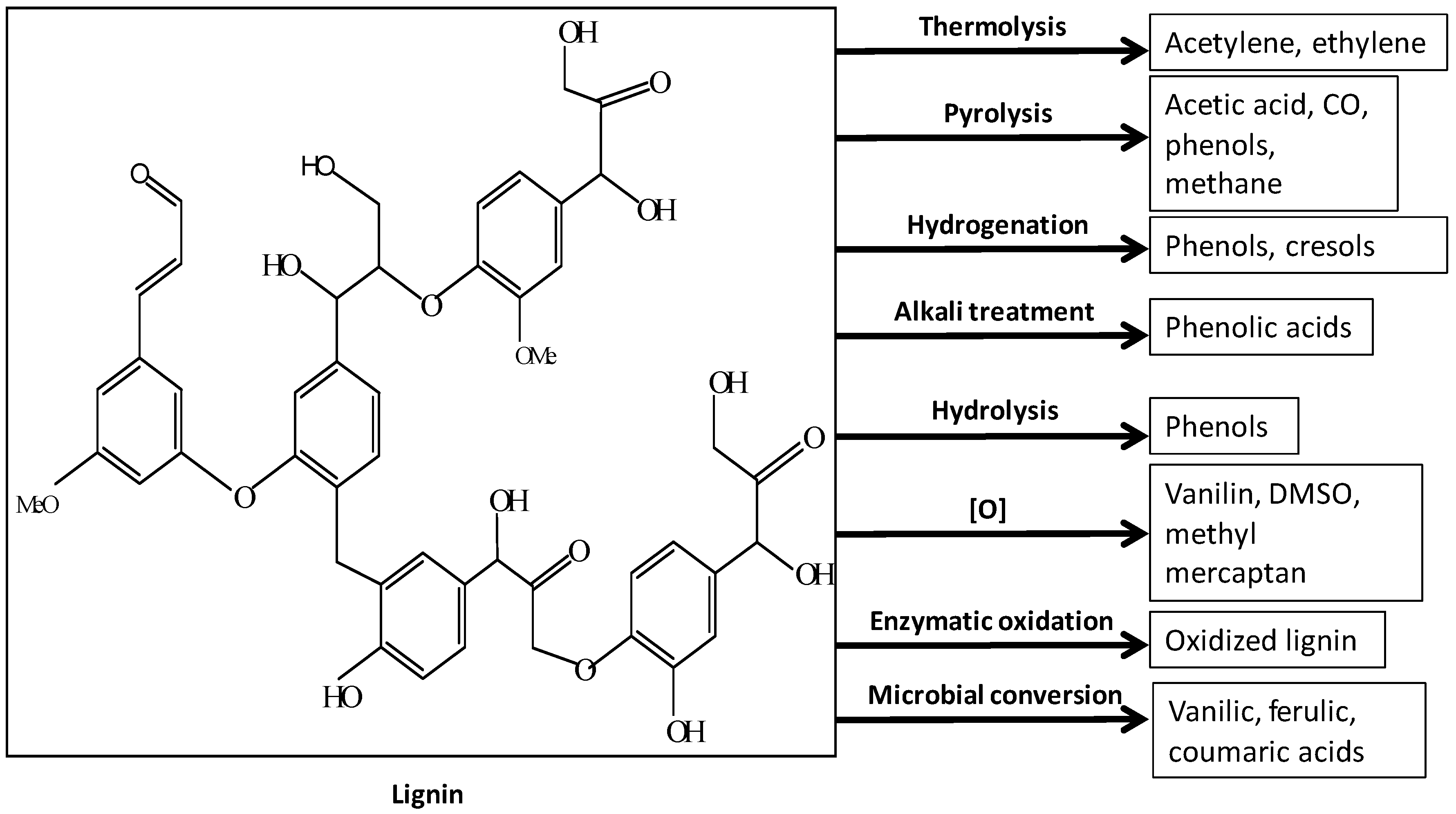
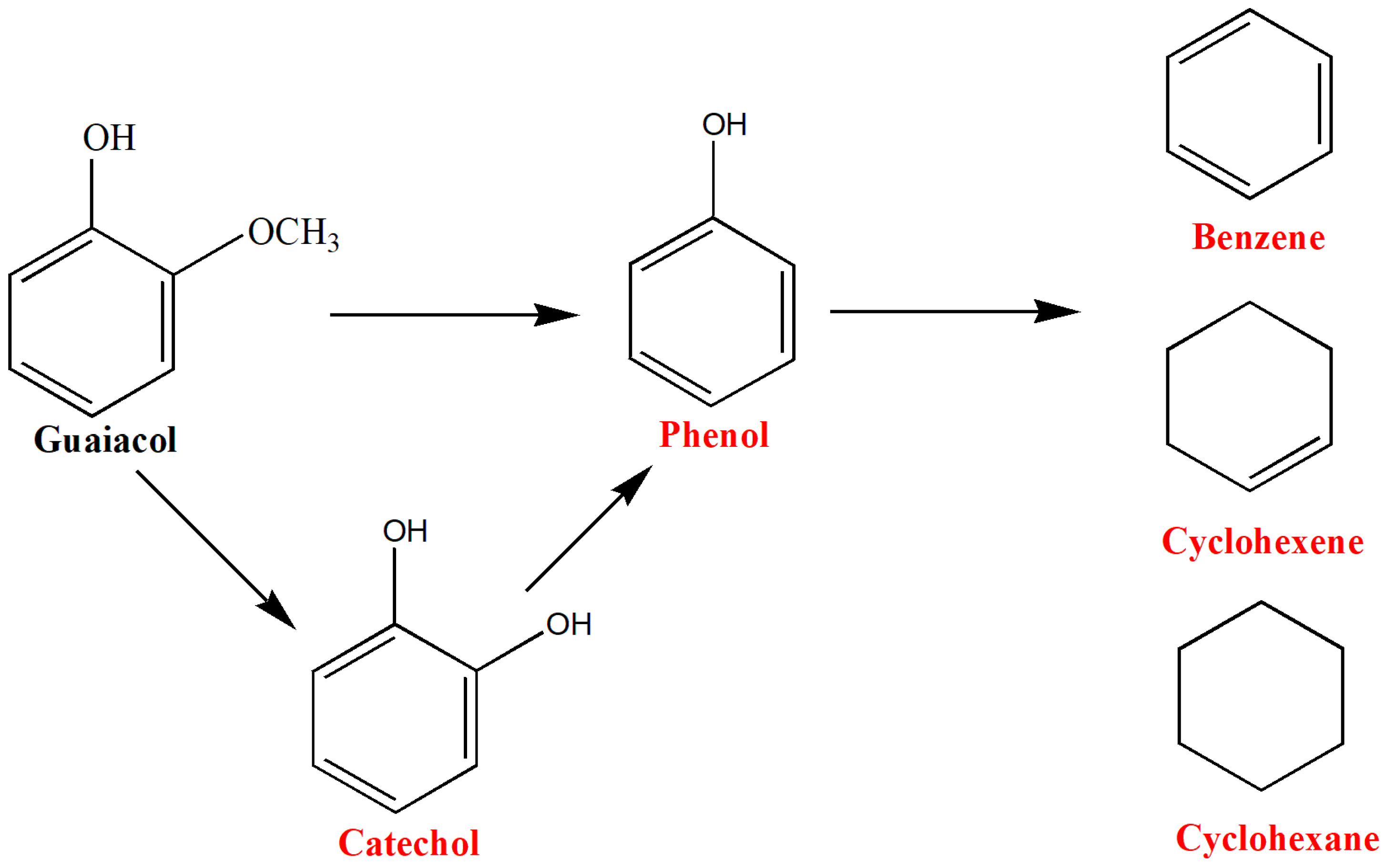
4. Lignin as Feedstock
Lignin-Derived Polymers
5. Conclusion and Future Perspectives
- ▪
- Search for novel reaction media;
- ▪
- Catalyst development and optimization;
- ▪
- Mechanism of the conversion reactions and the structure-property relationship of catalysts;
- ▪
- Multi-functional catalysts and suitable solvent systems;
- ▪
- Efficient processes for purification and separation processes; and
- ▪
- Process composition and large-scale production.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ragauskas, A.J.; Williams, C.K.; Davison, B.H.; Britovsek, G.; Cairney, J.; Eckert, C.A.; Frederick, W.J.; Hallett, J.P.; Leak, D.J.; Liotta, C.L. The path forward for biofuels and biomaterials. Science 2006, 311, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Huber, G.W.; Iborra, S.; Corma, A. Synthesis of transportation fuels from biomass: Chemistry, catalysts, and engineering. Chem. Rev. 2006, 106, 4044–4098. [Google Scholar] [CrossRef] [PubMed]
- Stephanopoulos, G. Challenges in engineering microbes for biofuel production. Science 2007, 5, 801–804. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Yamakoshi, Y.; Hosaka, Y.; Yabushita, M.; Fukuoka, A. Production of sugar alcohols from real biomass by supported platinum catalyst. Catal. Today 2014, 226, 204–209. [Google Scholar] [CrossRef]
- Werpy, T.; Petersen, G. Top value added chemicals from biomass. NATO Adv. Sci. Inst. 2004, 1, 263–275. [Google Scholar]
- Agarwal, B.; Kailasa, K.; Sangwan, R.S.; Elumalai, S. Traversing the history of solid catalysts for heterogeneous synthesis of 5-hydroxymethylfurfural from carbohydrate sugars: A review. Renew. Sustain. Energy Rev. 2018, 82, 2408–2425. [Google Scholar] [CrossRef]
- Somerville, C.; Youngs, H.; Taylor, C.; Davis, S.C.; Long, S.P. Feedstock for lignocellulosic biofuels. Science 2010, 329, 790–791. [Google Scholar] [CrossRef]
- Taarning, E.; Osmundsen, C.M.; Yang, X.B.; Voss, B.; Andersen, S.I.; Christensen, C.H. Zeolite-catalyzed biomass conversion to fuels and chemicals. Energy Environ. Sci. 2011, 4, 793–804. [Google Scholar] [CrossRef]
- Shuai, L.; Pan, X.J. Biohydrocarbons: Next generation liquid fuels from lignocellulosic biomass. Res. Progress Pap. Ind. Biorefin. 4th Isetpp 2010, 1, 1293–1297. [Google Scholar]
- Sun, Y.S.; Lu, X.B.; Zhang, S.T.; Zhang, R.; Wang, X.Y. Kinetic study for Fe(NO3)3 catalyzed hemicellulose hydrolysis of different corn stover silages. Bioresour. Technol. 2011, 102, 2936–2942. [Google Scholar] [CrossRef]
- Pandey, M.P.; Kim, C.S. Lignin depolymerization and conversion: A review of thermochemical methods. Chem. Eng. Technol. 2011, 34, 29–41. [Google Scholar] [CrossRef]
- Rosatella, A.A.; Simeonov, S.P.; Frade, R.F.M.; Afonso, C.A.M. 5-Hydroxymethylfurfural (HMF) as a building block platform: Biological properties, synthesis, and synthetic applications. Green Chem. 2011, 13, 754–793. [Google Scholar] [CrossRef]
- Li, X.; Jia, P.; Wang, T. Furfural: A promising platform compound for sustainable production of C4 and C5 chemicals. ACS Catal. 2016, 6, 7621–7640. [Google Scholar] [CrossRef]
- Zhou, C.; Xia, X.; Lin, C.X.; Tong, D.S.; Beltramini, J. Catalytic conversion of lignocellulosic biomass to fine chemicals and fuels. Chem. Soc. Rev. 2011, 40, 5588–5617. [Google Scholar] [CrossRef] [PubMed]
- Gallezot, P. Conversion of biomass to selected chemical product. Chem. Soc. Rev. 2012, 41, 1538–1558. [Google Scholar] [CrossRef] [PubMed]
- Besson, M.; Gallezot, P.; Pinel, C. Conversion of biomass into chemicals over metal catalysts. Chem. Rev. 2013, 114, 1827–1870. [Google Scholar] [CrossRef] [PubMed]
- Sanders, J.; Scott, E.; Weusthuis, R.; Mooibroek, H. Bio-refinery as the bio-inspired process to bulk chemicals. Macromol. Biosci. 2007, 7, 105–117. [Google Scholar]
- Dornburg, V.; Hermann, B.G.; Patel, M.K. Scenario projections for future market potentials of biobased bulk chemicals. Environ. Sci. Technol. 2008, 42, 2261–2267. [Google Scholar] [CrossRef]
- Sanders, J.P.M.; Clark, J.H.; Harmsen, G.J.; Heeres, H.J.; Heijnen, J.J.; Kersten, S.R.A.; van Swaaij, W.P.M.; Moulijn, J.A. Process intensification in the future production of base chemicals from biomass. Chem. Eng. Process 2012, 51, 117–136. [Google Scholar] [CrossRef]
- Griifn, D.W.; Schultz, M.A. Fuel and chemical products from biomass syngas: A comparison of gas fermentation to thermochemical conversion routes. Environ. Prog. Sustain. Energy 2012, 31, 219–221. [Google Scholar] [CrossRef]
- Kumar, A.; Jones, D.D.; Hanna, M.A. Thermochemical Biomass Gasification: A Review of the Current Status of the Technology. Energies 2009, 2, 556–581. [Google Scholar] [CrossRef]
- Zhang, X.; Solli, C.; Hertwich, E.G.; Tian, X.; Zhang, S. Energy analysis of the process for dimethyl ether production through biomass steam gasification. Ind. Eng. Chem. Res. 2009, 48, 10976–10985. [Google Scholar] [CrossRef]
- Demirbas, M.F.; Balat, M. Recent advances on the production and utilization trends of biofuels: A global perspective. Energy Convers. Manag. 2006, 47, 2371–2381. [Google Scholar] [CrossRef]
- Vispute, T.P.; Zhang, H.; Sanna, A.; Xiao, R.; Huber, G.W. Renewable chemical commodity feedstocks from integrated catalytic processing of pyrolysis oils. Science 2010, 330, 1222–1227. [Google Scholar] [CrossRef]
- Davda, R.R.; Shabaker, J.W.; Huber, G.W.; Cortright, R.D.; Dumesic, J.A. A review of catalytic issues and process conditions for renewable hydrogen and alkanes by aqueous-phase reforming of oxygenated hydrocarbons over supported metal catalysts. Appl. Catal. B 2005, 56, 171–186. [Google Scholar] [CrossRef]
- Huber, G.W.; Cortright, R.D.; Dumesic, J.A. Renewable alkanes by aqueous-phase reforming of biomass-derived oxygenates. Angew. Chem. Int. Ed. 2004, 116, 1575–1577. [Google Scholar] [CrossRef]
- Huber, G.W.; Chheda, J.N.; Barrett, C.J.; Dumesic, J.A. Production of liquid alkanes by aqueous-phase processing of biomass-derived carbohydrates. Science 2005, 308, 1446–1450. [Google Scholar] [CrossRef]
- Simonetti, D.A.; Dumesic, J.A. Catalytic production of liquid fuels from biomass-derived oxygenated hydrocarbons: Catalytic coupling at multiple length scales. Catal. Rev. Sci. Eng. 2009, 51, 441–484. [Google Scholar] [CrossRef]
- Saxena, R.C.; Adhikari, D.K.; Goyal, H.B. Biomass-based energy fuel through biochemical routes: A review. Renew. Sustain. Energy Rev. 2009, 13, 167–178. [Google Scholar] [CrossRef]
- Smith, M.B.J. March’s Advanced Organic Chemistry: Reactions, Mechanisms, and Structure; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2007; pp. 1513–1516. [Google Scholar]
- Li, Z.; Assary, R.S.; Atesin, A.C.; Curtiss, L.A.; Marks, T.J. Rapid ether and alcohol C–O bond hydrogenolysis catalyzed by tandem high-valent metal triflate + supported Pd catalysts. J. Am. Chem. Soc. 2014, 136, 104–107. [Google Scholar] [CrossRef]
- Fenouillot, F.; Rousseau, A.; Colomines, G.; Saint-Loup, R.; Pascault, J.P. Polymers from renewable 1,4:3,6-dianhydrohexitols (isosorbide, isomannide, and isoidide): A review. Prog. Polym. Sci. 2010, 35, 578–622. [Google Scholar] [CrossRef]
- Horiuti, I.; Polanyi, M. Exchange reactions of hydrogen on metallic catalysts. Trans. Faraday Soc. 1934, 30, 1164–1172. [Google Scholar] [CrossRef]
- Schlaf, M. Selective deoxygenation of sugar polyols to α,ω-diols and other oxygen content reduced materials—A new challenge to homogeneous ionic hydrogenation and hydrogenolysis catalysis. Dalton. Trans. 2006, 4645–4653. [Google Scholar] [CrossRef]
- Tuck, C.O.; Perez, E.; Horvath, I.T.; Sheldon, R.A.; Poliakoff, M. Valorization of biomass: Deriving more value from waste. Science 2012, 337, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Jae, J.; Zheng, W.; Karim, A.M.; Guo, W.; Lobo, R.F.; Vlachos, D.G. The role of Ru and RuO2 in the catalytic transfer hydrogenation of 5-hydroxymethylfurfural for the production of 2,5-dimethylfuran. ChemCatChem 2014, 6, 848–856. [Google Scholar] [CrossRef]
- He, Z.; Wang, X. Hydrodeoxygenation of model compounds and catalytic systems for pyrolysis bio-oils upgrading. Catal. Sustain. Energy 2012, 1, 28–52. [Google Scholar] [CrossRef]
- Furimsky, E. Catalytic hydrodeoxygenation. Appl. Catal. A Gen. 2000, 199, 147–190. [Google Scholar] [CrossRef]
- Zakrzewska, M.E.; Bogel-Łukasik, E.; Bogel-Łukasik, R. Ionic liquid-mediated formation of 5-hydroxymethylfurfural—A promising biomass-derived building block. Chem. Rev. 2010, 111, 397–417. [Google Scholar] [CrossRef]
- Kobayashi, H.; Fukuoka, A. Synthesis and utilisation of sugar compounds derived from lignocellulosic biomass. Green Chem. 2013, 15, 1740–1753. [Google Scholar] [CrossRef]
- Chang, R.H.-Y.; Jang, J.; Wu, K.C.W. Cellulase immobilized mesoporous silica nanocatalysts for efficient cellulose-to-glucose conversion. Green Chem. 2011, 13, 2844–2850. [Google Scholar] [CrossRef]
- Takimoto, A.; Shiomi, T.; Ino, K.; Tsunoda, T.; Kawai, A.; Mizukami, F.; Sakaguchi, K. Encapsulation of cellulase with mesoporous silica (SBA-15). Microporous Mesoporous Mater. 2008, 116, 601–606. [Google Scholar] [CrossRef]
- Tuteja, J.; Choudhary, H.; Nishimura, S.; Ebitani, K. Direct synthesis of 1,6-hexanediol from HMF over a heterogeneous Pd/ZrP catalyst using formic acid as a hydrogen source. ChemSusChem 2014, 7, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.H.; Liu, B.; Lv, K.L.; Sun, J.; Deng, K.J. Aerobic oxidation of biomass-derived 5-hydroxymethylfurfural into 5-hydroxymethyl-2-furancarboxylic acid catalyzed by a montmorillonite K-10 clay immobilized molybdenum acetylacetonate complex. Green Chem 2014, 16, 2762–2770. [Google Scholar] [CrossRef]
- Tong, X.; Ma, Y.; Li, Y. Biomass into chemicals: Conversion of sugars to furan derivatives by catalytic processes. Appl. Catal. A Gen. 2010, 385, 1–13. [Google Scholar] [CrossRef]
- Gandini, A.; Belgacem, M.N. Recent contributions to the preparation of polymers derived from renewable resources. J. Polym. Environ. 2002, 10, 105–114. [Google Scholar] [CrossRef]
- Moreaua, C.; Belgacemb, M.N.; Gandini, A. Recent catalytic advances in the chemistry of substituted furans from carbohydrates and in the ensuing polymers. Top. Catal. 2004, 27, 11–30. [Google Scholar] [CrossRef]
- Bicker, M.; Hirth, J.; Vogel, H. Dehydration of fructose to 5-hydroxymethylfurfural in sub- and supercritical acetone. Green Chem. 2003, 5, 280–284. [Google Scholar] [CrossRef]
- Kiermayer, J. A derivative of furfuraldehyde from laevulose. Chem. Ztg 1895, 19, 1003–1006. [Google Scholar]
- Fenton, H.J.H.; Gostling, M.I. The oxidation of polyhydric alcohols in presence of iron. J. Chem. Soc. 1899, 75, 423–425. [Google Scholar] [CrossRef]
- Van Enenstein, W.A.; Blanksma, J.J. Derivatives of furfural and of honey. Chem. Weekbl. 1909, 6, 717. [Google Scholar]
- Haworth, W.N.; Jones, W.G.M. 183. The conversion of sucrose into furan compounds. Part I. 5-hydroxymethylfurfuraldehyde and some derivatives. J. Chem. Soc. 1944, 667–670. [Google Scholar] [CrossRef]
- Zheng, X.; Gu, X.; Ren, Y.; Zhi, Z.; Lu, X. Production of 5-hydroxymethylfurfural and levulinic acid from lignocellulose in aqueous solution and different solvents. Biofuels Bioprod. Biorefin. 2016, 10, 917–931. [Google Scholar] [CrossRef]
- Vigier, K.D.O.; Benguerba, A.; Barrault, J.; Jerome, F. Conversion of fructose and inulin to 5-hydroxymethylfurfural in sustainable betaine hydrochloride-based media. Green Chem. 2012, 14, 285–289. [Google Scholar] [CrossRef]
- Lee, Y.C.; Chen, C.T.; Chiu, Y.T.; Wu, K.C.W. An effective cellulose-to-glucose-to fructose conversion sequence by using enzyme immobilized Fe3O4-loaded mesoporous silica nanoparticles as recyclable biocatalysts. ChemCatChem 2013, 5, 2153–2157. [Google Scholar] [CrossRef]
- Ruby, M.P.; Schuth, F. Synthesis of N-alkyl-4-vinylpyridinium-based cross-linked polymers and their catalytic performance for the conversion of fructose into 5-hydroxymethylfurfural. Green Chem. 2016, 18, 3422–3429. [Google Scholar] [CrossRef]
- Zhao, J.; Zhou, C.; He, C.; Dai, Y.; Jia, X.; Yang, Y. Efficient dehydration of fructose to 5-hydroxymethylfurfural over sulfonated carbon sphere solid acid catalysts. Catal. Today 2016, 264, 123–130. [Google Scholar] [CrossRef]
- Binder, J.B.; Raines, R.T. Simple chemical transformation of lignocellulosic biomass into furanics for fuels and chemicals. J. Am. Chem. Soc. 2009, 13, 1979–1985. [Google Scholar] [CrossRef]
- Zhao, S.; Cheng, M.; Li, J.; Tian, J.; Wang, X. One pot production of 5-hydroxymethylfurfural with high yield from cellulose by a Brønsted–Lewis–surfactant-combined heteropolyacid catalyst. Chem. Commun. 2011, 47, 2176–2178. [Google Scholar] [CrossRef]
- Su, Y.; Brown, H.M.; Huang, X.; Zhou, X.D.; Amonette, J.E.; Zhang, Z.C. Single-step conversion of cellulose to 5-hydroxymethylfurfural (HMF), a versatile platform chemical. Appl. Catal. A Gen. 2009, 361, 117–122. [Google Scholar] [CrossRef]
- Zhao, H.; Holladay, J.E.; Brown, H.; Zhang, Z.C. Metal chlorides in ionic liquid solvents convert sugars to 5-hydroxymethylfurfural. Science 2007, 316, 1597–1600. [Google Scholar] [CrossRef]
- Yong, G.; Zhang, Y.; Ying, J.Y. Efficient catalytic system for the selective production of 5-hydroxymethylfurfural from glucose and fructose. Angew. Chem. 2008, 120, 9485–9488. [Google Scholar] [CrossRef]
- Young, J.; Chan, G.; Zhang, Y. Selective conversion of fructose to 5-hydroxymethylfurfural catalyzed by tungsten salts at low temperatures. ChemSusChem 2009, 2, 731–734. [Google Scholar]
- Kim, B.; Jeong, J.; Lee, D.; Kim, S.; Yoon, H.J.; Lee, Y.S.; Cho, J.K. Direct transformation of cellulose into 5-hydroxymethyl-2-furfural using a combination of metal chlorides in imidazolium ionic liquid. Green Chem. 2011, 13, 1503–1506. [Google Scholar] [CrossRef]
- Tan, M.; Zhao, L.; Zhang, Y. Production of 5-hydroxymethyl furfural from cellulose in CrCl2/Zeolite/BMIMCl system. Biomass Bioenergy 2011, 35, 1367–1370. [Google Scholar] [CrossRef]
- Ståhlberg, T.; Søensen, M.G.; Riisager, A. Direct conversion of glucose to 5-(hydroxymethyl)furfural in ionic liquids with lanthanide catalysts. Green Chem. 2010, 12, 321–325. [Google Scholar] [CrossRef]
- Zhang, Z.; Du, H.; Qian, X.; Chen, E.Y.X. Ionic liquid−water mixtures: Enhanced kw for efficient cellulosic biomass conversion. Energy Fuels 2010, 24, 2410–2417. [Google Scholar] [CrossRef]
- Dee, S.J.; Bell, A.T. A study of the acid-catalyzed hydrolysis of cellulose dissolved in ionic liquids and the factors influencing the dehydration of glucose and the formation of humins. ChemSusChem 2011, 4, 1166–1173. [Google Scholar] [CrossRef]
- Nikolla, E.; Roman-Leshkov, Y.; Moliner, M.; Davis, M.E. One-pot synthesis of 5- (hydroxymethyl)furfural from carbohydrates using tin-beta zeolite. ACS Catal. 2011, 1, 408–410. [Google Scholar] [CrossRef]
- Moliner, M.; Roman-Leshkov, Y.; Davis, M.E. Tin-containing zeolites are highly active catalysts for the isomerization of glucose in water. Proc. Natl. Acad. Sci. USA 2010, 107, 6164–6168. [Google Scholar] [CrossRef]
- Wang, J.; Ren, J.; Liu, X.; Xi, J.; Xia, Q.; Zu, Y.; Lu, G.; Wang, Y. Direct conversion of carbohydrates to 5-hydroxymethylfurfural using Sn-Mont catalyst. Green Chem. 2012, 14, 2506–2512. [Google Scholar] [CrossRef]
- Alam, M.I.; De, S.; Singh, B.; Saha, B.; Abu-Omar, M.M. Titanium hydrogen phosphate: An efficient dual acidic catalyst for 5-hydroxymethylfurfural (HMF) production. Appl. Catal. A Gen. 2014, 486, 42–48. [Google Scholar] [CrossRef]
- Huang, R.; Qi, W.; Su, R.; He, Z. Integrating enzymatic and acid catalysis to convert glucose into 5-hydroxymethylfurfural. Chem. Commun. 2010, 46, 1115–1117. [Google Scholar] [CrossRef] [PubMed]
- Simeonov, S.P.; Coelho, J.A.; Afonso, C.A. Integrated chemo-enzymatic production of 5-hydroxymethylfurfural from glucose. ChemSusChem 2013, 6, 997–1000. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.C.; Dutta, S.; Wu, K.C.W. Integrated, cascading enzyme-/chemocatalytic cellulose conversion using catalysts based on mesoporous silica nanoparticles. ChemSusChem 2014, 7, 3241–3246. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Li, L.; Wei, Q.; Huang, C.; Oleskowicz-Popiel, P.; Xu, J. One-pot conversion of disaccharide into 5-hydroxymethylfurfural catalyzed by imidazole ionic liquid. Sci. Rep. 2016, 6, 26067. [Google Scholar] [CrossRef] [PubMed]
- Ramli, N.A.S.; Amin, A.S. Thermo-kinetic assessment of glucose decomposition to 5-hydroxymethyl furfural and levulinic acid over acidic functionalized ionic liquid. Chem. Eng. J. 2018, 335, 221–230. [Google Scholar] [CrossRef]
- Dutta, S.; De, S.; Alam, I.; Abu-Omar, M.M.; Saha, B. Direct conversion of cellulose and lignocellulosic biomass into chemicals and biofuel with metal chloride catalysts. J. Catal. 2012, 288, 8–15. [Google Scholar] [CrossRef]
- Abbott, A.P.; Boothby, D.; Capper, G.; Davies, D.L.; Rasheed, R.K. Deep eutectic solvents formed between choline chloride and carboxylic acids: Versatile alternatives to ionic liquids. J. Am. Chem. Soc. 2004, 126, 9142–9147. [Google Scholar] [CrossRef]
- De Oliveira Vigier, K.; Chatel, G.; Jerome, F. Contribution of deep eutectic solvents for biomass processing: Opportunities, challenges, and limitations. ChemCatChem 2015, 7, 1250–1260. [Google Scholar] [CrossRef]
- Hu, S.; Zhang, Z.; Zhou, Y.; Han, B.; Fan, H.; Li, W.; Song, J.; Xie, Y. Conversion of fructose to 5-hydroxymethylfurfural using ionic liquids prepared from renewable materials. Green Chem. 2008, 10, 1280–1283. [Google Scholar] [CrossRef]
- Ilgen, F.; Ott, D.; Kralish, D.; Reil, C.; Palmberger, A.; Kçnig, B. Conversion of carbohydrates to 5-hydroxymethylfurfural in highly concentrated low melting mixtures. Green Chem. 2009, 11, 1948–1954. [Google Scholar] [CrossRef]
- Assanosi, A.; Farah, M.M.; Wood, J.; Al-Duri, B. A facile acidic choline chloride-p-TSA DES-catalysed dehydration of fructose to 5-hydroxymethylfurfural. RSC Adv. 2014, 4, 39359–39364. [Google Scholar] [CrossRef]
- Zhao, Q.; Sun, Z.; Wang, S.; Huang, G.; Wang, X.; Jiang, Z. Conversion of highly concentrated fructose into 5-hydroxymethylfurfural by acid-base bifunctional HPA nanocatalysts induced by choline chloride. RSC Adv. 2014, 4, 63055–63061. [Google Scholar] [CrossRef]
- Navarro, O.C.; Canós, A.C.; Chornet, S.I. Chemicals from biomass: Aerobic oxidation of 5-hydroxymethyl-2-furaldehyde into diformylfurane catalyzed by immobilized vanadyl-pyridine complexes on polymeric and organofunctionalized mesoporous supports. Top. Catal. 2009, 52, 304–314. [Google Scholar] [CrossRef]
- Halliday, G.A.; Young, R.Y.; Grushin, V.V. One-pot, two-step, practical catalytic synthesis of 2,5-diformylfuran from fructose. Org. Lett. 2003, 5, 2003–2005. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Dai, J.-J.; Xu, H.-J.; Guo, Q.-X.; Fu, Y. Iron-catalyzed selective oxidation of 5-hydroxylfurfural in air: A facile synthesis of 2,5-diformylfuran at room temperature. Chin. Chem. Lett. 2015, 26, 1265–1268. [Google Scholar] [CrossRef]
- Mittal, N.; Nisola, G.M.; Seo, J.G.; Kim, H.; Lee, S.P.; Chung, W.J. Organic radical functionalized SBA-15 as a heterogeneous catalyst for facile oxidation of 5-hydroxymethylfurfural to 2,5-diformylfuran. J. Mol. Catal. 2015, 404, 106–114. [Google Scholar] [CrossRef]
- Mittal, N.; Nisola, G.M.; Malihan, L.B.; Seo, J.G.; Kim, H.; Lee, S.P.; Chung, W.J. One-pot synthesis of 2,5-diformylfuran from fructose using a magnetic bi-functional catalyst. RSC Adv. 2016, 6, 25678–25688. [Google Scholar] [CrossRef]
- Cui, M.; Huang, R.; Qi, W.; Su, R.; He, Z. Cascade catalysis via dehydration and oxidation: One-pot synthesis of 2,5-diformylfuran from fructose using acid and V2O5/ceramic catalysts. RSC Adv. 2017, 7, 7560–7566. [Google Scholar] [CrossRef]
- Lewkowski, J. Synthesis, chemistry and applications of 5-hydroxymethyl-furfural and its derivatives. Pol. J. Chem. 2001, 75, 1943–1946. [Google Scholar] [CrossRef]
- Merat, N.; Verdeguer, P.; Rigal, L.; Gaset, A.; Delmas, M. Process for the manufacture of furan-2,5-dicarboxylic acid. FR 2 1992, 2, 634. [Google Scholar]
- Michael, L.; Richard, H.; Hu, J.; White, J.; Gray, M.J. WO Patent Application. 2008; 54, 804. [Google Scholar]
- Ribeiro, M.L.; Schuchardt, U. Cooperative effect of cobalt acetylacetonate and silica in the catalytic cyclization and oxidation of fructose to 2,5-furan dicarboxylic acid. Catal. Commun. 2003, 4, 83–86. [Google Scholar] [CrossRef]
- Hughes, M.D.; Xu, Y.J.; Jenkins, P.; McMorn, P.; Landon, P.; Enache, D.I.; Carley, A.F.; Attard, G.A.; Hutchings, G.J.; King, F.; et al. Tunable gold catalysts for selective hydrocarbon oxidation under mild conditions. Nature 2005, 437, 1132–1135. [Google Scholar] [CrossRef] [PubMed]
- Gorbanev, Y.Y.; Klitgaard, S.K.; Woodley, J.M.; Christensen, C.H.; Riisager, A. Gold-catalyzed aerobic oxidation of 5-hydroxymethylfurfural in water at ambient temperature. ChemSusChem 2009, 2, 672–675. [Google Scholar] [CrossRef] [PubMed]
- Casanova, O.; Iborra, S.; Corma, A. Biomass into chemicals: Aerobic oxidation of 5-hydroxymethyl-2-furfural into 2,5-furan dicarboxylic acid with gold nanoparticle catalysts. ChemSusChem 2009, 2, 1138–1144. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Shen, J.; Chen, K.; Qin, Y.; Lu, X.; Ouyang, P.; Fu, J. Atomic layer deposition of Pt nanoparticles on low surface area zirconium oxide for the efficient base-free oxidation of 5-hydroxymethylfurfural to 2, 5- furan dicarboxylic acid. Appl. Catal. A 2018, 555, 98–107. [Google Scholar] [CrossRef]
- Kamm, B.; Kamm, M.; Schmidt, M.; Hirth, T.; Schulze, M.; Kamm, B.; Gruber, P.R.; Kamm, M. (Eds.) Biorefineries: Industrial Processes and Products; Wiley: Weinheim, Germany, 2006; pp. 97–149. [Google Scholar]
- Chheda, J.N.; Huber, G.W.; Dumesic, J.A. Liquid-phase catalytic processing of biomass-derived oxygenated hydrocarbons to fuels and chemicals. Angew. Chem. Int. Ed. 2007, 46, 7164–7183. [Google Scholar] [CrossRef]
- Verevkin, S.P.; Emel’yanenko, V.N.; Stepurko, E.N.; Ralys, R.V.; Zaitsau, D.H. Biomass-derived platform chemicals: Thermodynamic studies on the conversion of 5-hydroxymethylfurfural into bulk intermediates. Ind. Eng. Chem. Res. 2009, 48, 10087–10093. [Google Scholar] [CrossRef]
- Schiavo, V.; Descotes, G.; Mentech, J. Catalytic-hydrogenation of 5-hydroxymethylfurfural in aqueous medium. Bull. Soc. Chim. Fr. 1991, 128, 704–711. [Google Scholar]
- Lilga, M.A.; Hallen, R.T.; Werpy, T.A.; White, J.F.; Holladay, J.E.; Frye, G.J.; Zacher, A.H. Hydroxymethylfurfural reduction methods and methods of producing furandimethanol. Battelle Memorial Institute. U.S. Patent 20,070,287,845, 9 June 2006. [Google Scholar]
- Roman-Leshkov, Y.; Barrett, C.J.; Liu, Z.Y.; Dumesic, J.A. Production of dimethylfuran for liquid fuels from biomass-derived carbohydrates. Nature 2007, 447, 982–985. [Google Scholar] [CrossRef]
- Hulsey, M.J.; Yang, H.; Yan, N. Sustainable routes for the synthesis of renewable heteroatom-containing chemicals. ACS Sustain. Chem. Eng. 2018, 6, 5694–5707. [Google Scholar] [CrossRef]
- Bozell, J.J.; Petersen, G.R. Technology development for the production of biobased products from biorefinery carbohydrates the US Department of Energy’s ‘Top 10′ revisited. Green Chem 2010, 12, 539–554. [Google Scholar] [CrossRef]
- Timokhin, B.V.; Baransky, V.A.; Eliseeva, G.D. Levulinic acid in organic synthesis. Russ. Chem. Rev. 1999, 68, 73–84. [Google Scholar] [CrossRef]
- Hayes, D.J.; Fitzpatick, S.; Hayes, M.H.B.; Ross, J.R.H. Biorefineries-Industrial Processes and Products; Kamm, B., Gruber, P., Kamm, M., Eds.; Wiley-VCH Verlag: Weinheim, Germany, 2006. [Google Scholar]
- Bozell, J.J.; Moens, L.; Elliott, D.C.; Wang, Y.; Neuenscwander, G.G.; Fitzpatrick, S.W.; Bilski, R.J.; Jarnefeld, J.L. Production of levulinic acid and use as a platform chemical for derived products. Resour. Conserv. Recycl. 2010, 28, 227–239. [Google Scholar] [CrossRef]
- Harmsen, P.; Hackmann, M. Green building blocks for biobased plastics. Biofuel Bioprod. Biorefin. 2014, 8, 306–324. [Google Scholar] [CrossRef]
- Xin, L.; Zhang, Z.; Qi, J.; Chadderdon, D.J.; Qiu, Y.; Warsko, K.M.; Li, W. Electricity storage in biofuels: Selective electrocatalytic reduction of levulinic acid to valeric acid or gammavalerolactone. ChemSusChem 2013, 6, 674–686. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Ruiz, J.C.; Wang, D.; Dumesic, J.A. Catalytic upgrading of levulinic acid to 5-nonanone. Green Chem 2010, 12, 574–577. [Google Scholar] [CrossRef]
- Horvat, J.; Klaic, B.; Metelko, B.; Sunjic, V. Mechanism of levulinic acid formation. Tetrahedron Lett. 1985, 26, 2111–2114. [Google Scholar] [CrossRef]
- Fitzpatrick, S.W. Feedstocks for the Future. ACS Symp. Ser. 2006, 921, 271–287. [Google Scholar]
- Yang, Z.; Kang, H.; Guo, Y.; Zhuang, G.; Bai, Z.; Zhang, H.; Feng, C.; Dong, Y. Dilute acid conversion of cotton straw to sugars and levulinic acid via 2-stage hydrolysis. Ind. Crop Prod. 2013, 46, 205–209. [Google Scholar] [CrossRef]
- Fachri, B.A.; Abdilla, R.; Bovenkamp, H.; Rasrendra, C.; Heeres, H.J. Experimental and kinetic modeling studies on the sulfuric acid catalyzed conversion of D-Fructose to 5-Hydroxymethylfurfural and levulinic acid in water. ACS Sustain. Chem. Eng. 2015, 3, 3024–3034. [Google Scholar] [CrossRef]
- Hegner, J.; Pereira, K.C.; DeBoef, B.; Lucht, B.L. Conversion of cellulose to glucose and levulinic acid via solid-supported acid catalysis. Tetrahedron Lett. 2010, 51, 2356–2358. [Google Scholar] [CrossRef]
- Weingarten, R.; Conner, W.C., Jr.; Huber, G.W. Production of levulinic acid from cellulose by hydrothermal decomposition combined with aqueous phase dehydration with a solid acid catalyst. Energy Environ. Sci. 2012, 5, 7559–7574. [Google Scholar] [CrossRef]
- Zhong, S.; Cheng, M.; Li, H.; Shi, T.; Yuan, M.; Wang, X.; Jiang, Z. One pot depolymerization of cellulose into glucose and levulinic acid by heteropolyacid ionic liquid catalysis. RSC Adv. 2012, 2, 9058–9065. [Google Scholar]
- Ren, H.; Zhou, Y.; Liu, L. Selective conversion of cellulose to levulinic acid via microwave-assisted synthesis in ionic liquids. Bioresour. Technol. 2013, 129, 616–619. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Yu, X.; Ma, H.; He, R.; Saritporn, V. Optimization on the conversion of bamboo shoot shell to levulinic acid with environmentally benign acidic ionic liquid and response surface analysis. Chin. J. Chem. Eng. 2013, 21, 544–550. [Google Scholar] [CrossRef]
- Lange, J.P.; Price, R.; Ayoub, P.M.; Louis, J.; Petrus, L.; Clarke, L.; Gosselink, H. A platform of cellulosic transportation fuels. Angew. Chem. Int. Ed. 2010, 49, 4479–4483. [Google Scholar] [CrossRef] [PubMed]
- Horvath, T.; Mehdi, H.; Fabos, V.; Boda, L.; Mika, L.T. γ-Valerolactone—A sustainable liquid for energy and carbon-based chemicals. Green Chem. 2008, 10, 238–242. [Google Scholar] [CrossRef]
- Fegyverneki, D.; Orha, L.; Lang, G.; Horvath, I.T. Gamma-valerolactone-based solvents. Tetrahedron 2010, 66, 1078–1081. [Google Scholar] [CrossRef]
- Manzer, L.E. Catalytic synthesis of α-methylene-γ-valerolactone: A biomass-derived acrylic monomer. Appl. Catal. A 2004, 272, 249–256. [Google Scholar] [CrossRef]
- Manzer, L.E. Feedstocks for the Future. ACS Symp. Ser. 2006, 921, 40–51. [Google Scholar]
- Alonso, D.M.; Wettstein, S.G.; Bond, J.Q.; Root, T.W.; Dumesic, J.A. Production of biofuels from cellulose and corn stover using alkylphenol solvents. ChemSusChem 2011, 4, 1078–1081. [Google Scholar] [CrossRef] [PubMed]
- Gallo, J.M.R.; Alonso, D.M.; Mellmer, M.A.; Dumesic, J.A. Production and upgrading of 5-hydroxymethylfurfural using heterogeneous catalysts and biomass-derived solvents. Green Chem. 2013, 15, 85–90. [Google Scholar] [CrossRef]
- Bohre, A.; Saha, B.; Abu-Omar, M.M. Catalytic upgrading of 5-hydroxymethylfurfural to drop-in-biofuels by solid base and bifunctional metal-acid catalysts. ChemSuSChem 2015, 8, 4022–4029. [Google Scholar] [CrossRef] [PubMed]
- Geilen, F.M.A.; Engendahl, B.; Hölscher, M.; Klankermayer, J.; Leitner, W. Selective homogeneous hydrogenation of biogenic carboxylic acids with [Ru(TriPhos)H]+: A mechanistic study. J. Am. Chem. Soc. 2011, 133, 14349–14358. [Google Scholar] [CrossRef] [PubMed]
- Nemanashi, M.; Noh, J.H.; Meijboom, R. Hydrogenation of biomass-derived levulinic acid to γ-valerolactone catalyzed by mesoporous supported dendrimer-derived Ru and Pt catalysts: An alternative method for the production of renewable biofuels. Appl. Catal. A Gen. 2018, 550, 77–89. [Google Scholar] [CrossRef]
- Piskun, A.S.; Ftouni, J.; Tang, Z.; Weckhuysen, B.M.; Bruijnincx, P.C.A.; Heeres, H.J. Hydrogenation of levulinic acid to γ-valerolactone over anatase-supported Ru catalysts: Effect of catalyst synthesis protocols on activity. Appl. Catal. A Gen. 2018, 549, 197–206. [Google Scholar] [CrossRef]
- Osawa, T.; Tanabe, Y. Facile Synthesis of Optically-Active γ-Valerolactone from Levulinic Acid and Its Esters Using a Heterogeneous Enantio-Selective Catalyst. Catal. Lett. 2018, 148, 824–830. [Google Scholar] [CrossRef]
- Palkovits, R. Pentenoic acid pathways for cellulosic biofuels. Angew. Chem. Int. Ed. 2010, 49, 4336–4338. [Google Scholar] [CrossRef]
- Bond, J.Q.; Alonso, D.M.; Wang, D.; West, R.M.; Dumesic, J.A. Integrated catalytic conversion of γ-valerolactone to liquid alkenes for transportation fuels. Science 2010, 327, 1110–1114. [Google Scholar] [CrossRef]
- Zeitsch, K.J. The Chemistry and Technology of Furfural and its Many By-Products; In Sugar Series; Elsevier Science: Amsterdam, The Netherlands, 2000. [Google Scholar]
- Lichtenthaler, F.W. Unsaturated o- and n-heterocycles from carbohydrate feedstocks. Acc. Chem. Res. 2002, 35, 728–737. [Google Scholar] [CrossRef]
- Peleteiro, S.; Rivas, S.; Alonso, J.L.; Santos, V.; Parajó, J.C. Furfural production using ionic liquids: A review. Bioresour. Technol. 2016, 202, 181–191. [Google Scholar] [CrossRef]
- Brownlee, H.J.; Miner, C.S. Industrial development of furfural. Ind. Eng. Chem. 1948, 40, 201–204. [Google Scholar] [CrossRef]
- Mao, L.Y.; Zhang, L.; Gao, N.B.; Li, A.M. FeCl3 and acetic acid co-catalyzed hydrolysis of corncob for improving furfural production and lignin removal from residue. Bioresour. Technol. 2012, 123, 324–331. [Google Scholar] [CrossRef]
- Win, D.T. Furfural—Old to garbage. AU J. Technol. 2005, 8, 185–190. [Google Scholar]
- Mamman, S.; Lee, J.M.; Kim, Y.C.; Hwang, I.T.; Park, N.J.; Hwang, Y.K.; Chang, J.S.; Hwang, J.S. Furfural: Hemicellulose/xylose derived biochemical. Biofuels Bioprod. Biorefin. 2008, 2, 438–454. [Google Scholar] [CrossRef]
- Wettstein, S.G.; Alonso, D.M.; Gürbüz, E.I.; Dumesic, J.A. A roadmap for conversion of lignocellulosic biomass to chemicals and fuels. Curr. Opin. Chem. Eng. 2012, 1, 218–224. [Google Scholar] [CrossRef]
- Fanta, G.F.; Abbott, T.P.; Herman, A.I.; Burr, R.C.; Doane, W.M. Hydrolysis of wheat straw hemicellulose with trifluoroacetic acid. Fermentation of xylose with Pachysolen tannophilus. Biotechnol. Bioeng. 1984, 26, 1122–1125. [Google Scholar] [CrossRef]
- Van Nguyen, C.; Lewis, D.; Chen, W.-H.; Huang, H.-W.; ALOthman, Z.A.; Yamauchi, Y.; Wu, K.C.-W. Combined treatments for producing 5-hydroxymethylfurfural (HMF) from lignocellulosic biomass. Catal. Today 2016, 278, 344–349. [Google Scholar] [CrossRef]
- Dutta, S.; Bhaumik, A.; Wu, K.C.W. Hierarchically porous carbon derived from polymers and biomass: Effect of interconnected pores on energy applications. Energy Environ. Sci. 2014, 7, 3574–3592. [Google Scholar] [CrossRef]
- Da Costa Sousa, L.; Chundawat, S.P.; Balan, V.; Dale, BE. ‘Cradle-to-grave’assessment of existing lignocellulose pretreatment technologies. Curr. Opin. Biotechnol. 2009, 20, 339–347. [Google Scholar] [CrossRef]
- Sahu, R.; Dhepe, P.L. A one-pot method for the selective conversion of hemicellulose from crop waste into C5 sugars and furfural by using solid acid catalysts. ChemSusChem 2012, 5, 751–761. [Google Scholar] [CrossRef]
- Bhaumik, P.; Dhepe, P.L. Biomass Sugars for Non-Fuel Applications; Royal Society of Chemistry: London, UK, 2016; pp. 1–53. [Google Scholar]
- Heguaburu, V.; Franco, J.; Reina, L.; Tabarez, C.; Moyna, G.; Moyna, P. Dehydration of carbohydrates to 2-furaldehydes in ionic liquids by catalysis with ion exchange resins. Catal. Commun. 2012, 5, 88–91. [Google Scholar] [CrossRef]
- Peleteiro, S.; Garrote, G.; Santos, V.; Parajó, J.C. Conversion of hexoses and pentoses into furans in an ionic liquid. Afinidad 2014, 567, 202–206. [Google Scholar]
- Wu, C.; Chen, W.; Zhong, L.; Peng, X.; Sun, R.; Fang, J.; Zheng, S. Conversion of xylose into furfural using lignosulfonic acid as a catalyst in the ionic liquid. J. Agric. Food Chem. 2014, 62, 7430–7435. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yu, H.; Wang, P.; Dong, H.; Peng, X. Conversion of xylan, D-xylose and lignocellulosic biomass into furfural using AlCl3 as a catalyst in the ionic liquid. Bioresour. Technol. 2013, 130, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Du, B.; Quan, Z.J.; Da, Y.X.; Wang, X.C. Dehydration of biomass to furfural catalyzed by reusable polymer bound sulfonic acid (PEG-OSO3H) in ionic liquid. Catal. Sci. Technol. 2014, 4, 633–638. [Google Scholar] [CrossRef]
- Matsagar, B.M.; Hossain, S.A.; Islam, T.; Alamri, H.R.; Alothman, Z.A.; Yamauchi, Y.; Dhepe, P.L.; Wu, K.C.W. Direct production of furfural in one-pot fashion from raw biomass using Brønsted acidic ionic liquids. Sci. Rep. 2017, 7, 13508. [Google Scholar] [CrossRef]
- Hoydonckx, H.; Van Rhijn, W.; Van Rhijn, W.; De Vos, D.; Jacobs, P. Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2007. [Google Scholar]
- Liu, D.; Zemlyanov, D.; Wu, T.; Lobo-Lapidus, R.J.; Dumesic, J.A.; Miller, J.T.; Marshall, C.L. Deactivation mechanistic studies of copper chromite catalyst for selective hydrogenation of 2-furfuraldehyde. J. Catal. 2013, 299, 336–345. [Google Scholar] [CrossRef]
- Baijun, L.; Lianhai, L.; Bingchun, W.; Tianxi, C.; Iwatani, K. Liquid phase selective hydrogenation of furfural on Raney nickel modified by impregnation of salts of heteropolyacids. Appl. Catal. A 1998, 171, 117–122. [Google Scholar] [CrossRef]
- Zhang, H.; Canlas, C.; Jeremy Kropf, A.; Elam, J.W.; Dumesic, J.A.; Marshall, C.L. Enhancing the stability of copper chromite catalysts for the selective hydrogenation of furfural with ALD overcoating (II)—Comparison between TiO2 and Al2O3 overcoatings. J. Catal. 2015, 326, 172–181. [Google Scholar] [CrossRef]
- O’Neill, B.J.; Jackson, D.H.K.; Crisci, A.J.; Farberow, C.A.; Shi, F.; Alba-Rubio, A.C.; Lu, J.; Dietrich, P.J.; Gu, X.; Marshall, C.L.; et al. Catalyst design with atomic layer deposition. Angew. Chem. 2013, 125, 14053–14057. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Tamura, M.; Tomishige, K. Catalytic reduction of biomass-derived furanic compounds with hydrogen. ACS Catal. 2013, 3, 2655–2668. [Google Scholar] [CrossRef]
- Zheng, H.-Y.; Zhu, Y.-L.; Teng, B.-T.; Bai, Z.-Q.; Zhang, C.-H.; Xiang, H.-W.; Li, Y.-W. Towards understanding the reaction pathway in vapor phase hydrogenation of furfural to 2-methylfuran. J. Mol. Catal. A: Chem. 2006, 246, 18–23. [Google Scholar] [CrossRef]
- Koso, S.; Furikado, I.; Shimao, A.; Miyazawa, T.; Kunimori, K.; Tomishige, K. Chemoselective hydrogenolysis of tetrahydrofurfuryl alcohol to 1,5-pentanediol. Chem. Commun. 2009, 2035–2037. [Google Scholar] [CrossRef] [PubMed]
- Koso, S.; Ueda, N.; Shinmi, Y.; Okumura, K.; Kizuka, T.; Tomishige, K. Promoting effect of Mo on the hydrogenolysis of tetrahydrofurfuryl alcohol to 1,5-pentanediol over Rh/SiO2. J. Catal. 2009, 267, 89–92. [Google Scholar] [CrossRef]
- Hronec, M.; Fulajtarova, K.; Liptaj, T.; Pronayova, N.; Sotak, T. Bio-derived fuel additives from furfural and cyclopentanone. Fuel Process. Technol. 2015, 138, 564–569. [Google Scholar] [CrossRef]
- Hronec, M.; Fulajtarova, K.; Vavra, I.; Sotak, T.; Dobročka, E.; Mičušik, M. Carbon supported Pd–Cu catalysts for highly selective rearrangement of furfural to cyclopentanone. Appl. Catal. B Environ. 2016, 181, 210–219. [Google Scholar] [CrossRef]
- Wang, Y.; Sang, S.; Zhu, W.; Gao, L.; Xiao, G. CuNi@C catalysts with high activity derived from metal-organic frameworks precursor for conversion of furfural to cyclopentanone. Chem. Eng. J. 2016, 299, 104–111. [Google Scholar] [CrossRef]
- Fang, R.; Liu, H.; Luque, R.; Li, Y. Efficient and selective hydrogenation of biomass-derived furfural to cyclopentanone using Ru catalysts. Green Chem. 2015, 17, 4183–4188. [Google Scholar] [CrossRef]
- Zhang, G.-S.; Zhu, M.-M.; Zhang, Q.; Liu, Y.-M.; He, H.-Y.; Cao, Y. Towards quantitative and scalable transformation of furfural to cyclopentanone with supported gold catalysts. Green Chem. 2016, 18, 2155–2164. [Google Scholar] [CrossRef]
- Dawes, G.J.S.; Scott, E.L.; Le Notre, J.; Sanders, J.P.; Bitter, J.H. Deoxygenation of biobased molecules by decarboxylation and decarbonylation—A review on the role of heterogeneous, homogeneous and bio-catalysis. Green Chem. 2015, 17, 3231–3250. [Google Scholar] [CrossRef]
- Zhang, W.; Zhu, Y.; Niu, S.; Li, Y. A study of furfural decarbonylation on K-doped Pd/Al2O3 catalysts. J. Mol. Catal. A Chem. 2011, 335, 71–81. [Google Scholar] [CrossRef]
- Li, K.; Ozer, R. Vapor phase decarbonylation process. WO Patent Application No. 2010080290A1, 15 July 2010. [Google Scholar]
- Dunlop, A.P. Hydrogenated Furfuryl Alcohol Partial Polymers. U.S. Patent 2,407,066, 3 September 1946. [Google Scholar]
- Verdeguer, P.; Merat, N.; Gaset, A. Lead/platinum on charcoal as catalyst for oxidation of furfural. Effect of main parameters. Appl. Catal. A Gen. 1994, 112, 1–11. [Google Scholar] [CrossRef]
- Verdeguer, P.; Merat, N.; Rigal, L.; Gaset, A. Optimization of experimental conditions for the catalytic oxidation of furfural to furoic acid. J. Chem. Technol. Biotechnol. 1994, 61, 97–102. [Google Scholar] [CrossRef]
- Signoretto, M.; Menegazzo, F.; Contessotto, L.; Pinna, F.; Manzoli, M.; Boccuzzi, F. Au/ZrO2: An efficient and reusable catalyst for the oxidative esterification of renewable furfural. Appl. Catal. B Environ. 2013, 129, 287–293. [Google Scholar] [CrossRef]
- Menegazzo, F.; Fantinel, T.; Signoretto, M.; Pinna, F.; Manzoli, M. On the process for furfural and HMF oxidative esterification over Au/ZrO2. J. Catal. 2014, 319, 61–70. [Google Scholar] [CrossRef]
- Menegazzo, F.; Signoretto, M.; Pinna, F.; Manzoli, M.; Aina, V.; Cerrato, G.; Boccuzzi, F. Oxidative esterification of renewable furfural on gold-based catalysts: Which is the best support? J. Catal. 2014, 309, 241–247. [Google Scholar] [CrossRef]
- Alonso-Fagúndez, N.; Granados, M.L.; Mariscal, R.; Ojeda, M. Selective conversion of furfural to maleic anhydride and furan with VOx/Al2O3 catalysts. ChemSusChem 2012, 5, 1984–1990. [Google Scholar] [CrossRef]
- Li, X.; Ho, B.; Zhang, Y. Selective aerobic oxidation of furfural to maleic anhydride with heterogeneous Mo–V–O catalysts. Green Chem. 2016, 18, 2976–2980. [Google Scholar] [CrossRef]
- Shi, S.; Guo, H.; Yin, G. Synthesis of maleic acid from renewable resources: Catalytic oxidation of furfural in liquid media with dioxygen. Catal. Commun. 2011, 12, 731–733. [Google Scholar] [CrossRef]
- Alonso-Fagundez, N.; Agirrezabal-Telleria, I.; Arias, P.; Fierro, J.; Mariscal, R.; Granados, M.L. Aqueous-phase catalytic oxidation of furfural with H2O2: High yield of maleic acid by using titanium silicalite-1. RSC Adv. 2014, 4, 54960–54972. [Google Scholar] [CrossRef]
- Zhang, J.; Li, J.; Wu, S.B.; Liu, Y. Advances in the catalytic production and utilization of sorbitol. Ind. Eng. Chem. Res. 2013, 52, 11799–11815. [Google Scholar] [CrossRef]
- Tathod, A.; Kane, T.; Sanil, E.S.; Dhepe, P.L. Solid base supported metal catalysts for the oxidation and hydrogenation of sugars. J. Mol. Catal. A Chem. 2014, 388, 90–99. [Google Scholar] [CrossRef]
- Van der Klis, F.; Gootjes, L.; van Haveren, J.; van Es, D.S.; Bitter, J.H. Selective terminal C–C scission of C5-carbohydrates. Green Chem. 2015, 17, 3900–3909. [Google Scholar] [CrossRef]
- Zhang, J.; Lin, L.; Zhang, J.; Shi, J. Efficient conversion of d-glucose into d-sorbitol over MCM-41 supported Ru catalyst prepared by a formaldehyde reduction process. Carbohyd. Res. 2011, 346, 1327–1332. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Durndell, L.J.; Isaacs, M.A.; Parlett, C.M.A.; Lee, A.F.; Wilson, K. Platinum-catalyzed aqueous-phase hydrogenation of d-glucose to d-sorbitol. ACS Catal. 2016, 6, 7409–7417. [Google Scholar] [CrossRef]
- Chen, J.; Wang, S.; Huang, J.; Chen, L.; Ma, L.; Huang, X. Conversion of cellulose and cellobiose into sorbitol catalyzed by ruthenium supported on a polyoxometalate/metal–organic framework hybrid. ChemSusChem 2013, 6, 1545–1555. [Google Scholar] [CrossRef]
- Wang, X.; Meng, L.; Wu, F.; Jiang, Y.; Wang, L.; Mu, X. Efficient conversion of microcrystalline cellulose to 1,2-alkanediols over supported Ni catalysts. Green Chem. 2012, 14, 758–765. [Google Scholar] [CrossRef]
- Liang, G.; Cheng, H.; Li, W.; He, L.; Yu, Y.; Zhao, F. Selective conversion of microcrystalline cellulose into hexitols on nickel particles encapsulated within ZSM-5 zeolite. Green Chem. 2012, 14, 2146–2149. [Google Scholar] [CrossRef]
- Yang, P.; Kobayashi, H.; Hara, K.; Fukuoka, A. Phase change of nickel phosphide catalysts in the conversion of cellulose into sorbitol. ChemSusChem 2012, 5, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Pang, J.; Wang, A.; Zheng, M.; Zhang, Y.; Huang, Y.; Chen, X.; Zhang, T. Catalytic conversion of cellulose to hexitols with mesoporous carbon supported Ni-based bimetallic catalysts. Green Chem. 2012, 14, 614–617. [Google Scholar] [CrossRef]
- Almeida, J.M.A.R.; Da Vià, L.; Demma Carà, P.; Carvalho, Y.; Romano, P.N.; Peña, J.A.O.; Smith, L.; Sousa-Aguiar, E.F.; Lopez-Sanchez, J.A. Screening of mono- and bi-functional catalysts for the one-pot conversion of cellobiose into sorbitol. Catal. Today 2017, 279, 187–193. [Google Scholar] [CrossRef]
- Ribeiro, L.S.; Delgado, J.J.; de Melo Órfão, J.J.; Pereira, M.F. A one-pot method for the enhanced production of xylitol directly from hemicellulose (corncob xylan). Catal. Today 2016, 279, 244–251. [Google Scholar] [CrossRef]
- Li, X.; Guo, T.; Xia, Q.; Liu, X.; Wang, Y. One-pot catalytic transformation of lignocellulosic biomass into alkylcyclohexanes and polyols. ACS Sustain. Chem. Eng. 2018, 6, 4390–4399. [Google Scholar] [CrossRef]
- Yamaguchi, A.; Mimura, N.; Shirai, M.; Sato, O. Intramolecular dehydration of biomass-derived sugar alcohols in high temperature water. J. Jpn. Pet. Inst. 2016, 59, 155–156. [Google Scholar] [CrossRef]
- Buck, H.; Fahn, R.; Hofstadt, C.E. Process for Obtaining Xylitol from Natural Products Containing Xylan. U.S. Patent 3,980,719, 14 September 1976. [Google Scholar]
- Yadav, M.; Mishra, D.K.; Hwang, J.S. Catalytic hydrogenation of xylose to xylitol using ruthenium catalyst on NiO modified TiO2 support. Appl. Catal. A Gen. 2012, 425, 110–116. [Google Scholar] [CrossRef]
- Yi, G.; Zhang, Y. One-pot selective conversion of hemicellulose (xylan) to xylitol under mild conditions. ChemSusChem 2012, 5, 1383–1387. [Google Scholar] [CrossRef]
- Liu, S.; Okuyama, Y.; Tamura, M.; Nakagawa, Y.; Imai, A.; Tomishige, K. Selective transformation of hemicellulose (xylan) into n-pentane, pentanols or xylitol over a rhenium-modified iridium catalyst combined with acids. Green Chem. 2016, 18, 165–175. [Google Scholar] [CrossRef]
- Ribeiro, L.S.; Delgado, J.J.; de Melo Órfão, J.J.; Ribeiro Pereira, M.F. RSC Adv. 2016, 6, 95320–95327. [CrossRef]
- Dietrich, K.; Mejia, C.H.; Verschuren, P.; Rothenberg, G.; Shiju, N.R. One-pot selective conversion of hemicellulose to xylitol. Organ. Process Res Dev. 2017, 21, 165–170. [Google Scholar] [CrossRef]
- Zoulias, E.I.; Piknis, S.; Oreopoulou, V. Effect of sugar replacement by polyols and acesulfame-K on properties of low-fat cookies. J. Sci. Food Agric. 2000, 80, 2049–2056. [Google Scholar] [CrossRef]
- Jeya, M.; Lee, K.M.; Tiwari, M.K.; Kim, J.S.; Gunasekaran, P.; Kim, S.Y.; Kim, I.W.; Lee, J.K. Isolation of a novel high erythritol-producing Pseudozyma tsukubaensis and scale-up of erythritol fermentation to industrial level. Appl. Microbiol. Biotechnol. 2009, 83, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Sohounloue, D.K.; Montassier, C.; Barbier, J. Catalytic hydrogenolysis of sorbitol. React. Kinet. Catal. Lett. 1983, 22, 391–397. [Google Scholar] [CrossRef]
- Musolino, M.G.; Scarpino, L.A.; Mauriello, F.; Pietropaolo, R. Glycerol hydrogenolysis promoted by supported palladium catalysts. ChemSusChem 2011, 4, 1143–1150. [Google Scholar] [CrossRef] [PubMed]
- Fabre, L.; Gallezot, P.; Perrard, A. Catalytic hydrogenation of arabinonic acid and arabinonolactones. Catal. Commun. 2001, 2, 249–253. [Google Scholar] [CrossRef]
- Fabre, L.; Gallezot, P.; Perrard, A. Catalytic hydrogenation of arabinonic acid and lactones to arabitol. J. Catal. 2002, 208, 247–254. [Google Scholar] [CrossRef]
- Sun, J.; Liu, H. Selective hydrogenolysis of biomass-derived xylitol to ethylene glycol and propylene glycol on supported Ru catalysts. Green Chem. 2011, 13, 135–142. [Google Scholar] [CrossRef]
- Werpy, T.; Petersen, G.; Aden, A.; Bozell, J.; Holladay, J.; White, J.; Manheim, A. Top Value Added Chemicals From Biomass. Volume 1-Results Of Screening For Potential Candidates From Sugars And Synthesis Gas. 2004. Available online: https://www.nrel.gov/docs/fy04osti/35523.pdf (accessed on 13 December 2017).
- Craver, A.E. Production of Succinic Acid. U.S. Patent 1,491,465A, 22 September 1924. [Google Scholar]
- Svetlakov, N.; Nikitin, V.; Orekhova, A. Oxidation of tetrahydrofuran and 1,4-butanediol with nitric acid. Russ. J. Appl. Chem. 2002, 75, 669–671. [Google Scholar] [CrossRef]
- Pinazo, J.M.; Domine, M.E.; Parvulescu, V.; Petru, F. Sustainability metrics for succinic acid production: A comparison between biomass-based and petrochemical routes. Catal. Today 2015, 239, 17–24. [Google Scholar] [CrossRef]
- Rosi, L.; Frediani, M.; Frediani, P. Isotopomeric diols by “one-pot” Ru-catalyzed homogeneous hydrogenation of dicarboxylic acids. J. Organomet. Chem. 2010, 695, 1314–1322. [Google Scholar] [CrossRef]
- Succinic Acid Factory, Anhui Sunsing Chemicals Co., Ltd., Chizhou, Anhui, China. Available online: http://product.lookchem.com/item/7510/Succinic-Acid-factory.html (accessed on 28 February 2017).
- Dutta, S.; Wu, L.; Mascal, M. Efficient, metal-free production of succinic acid by oxidation of biomass-derived levulinic acid with hydrogen peroxide. Green Chem. 2015, 17, 2335–2338. [Google Scholar] [CrossRef]
- Choudhary, H.; Nishimura, S.; Ebitani, K. Metal-free oxidative synthesis of succinic acid from biomass-derived furan compounds using a solid acid catalyst with hydrogen peroxide. Appl. Catal. A 2013, 458, 55–62. [Google Scholar] [CrossRef]
- Dalli, S.S.; Tilaye, T.J.; Rakshit, S.K. Conversion of wood-based hemicellulose prehydrolysate into succinic acid using a heterogeneous acid catalyst in a biphasic system. Ind. Eng. Chem. Res. 2017, 56, 10582–10590. [Google Scholar] [CrossRef]
- Ly, B.K.; Tapin, B.; Aouine, M.; Delichere, P.; Epron, F.; Pinel, C.; Especel, C.; Besson, M. Insights into the oxidation state and location of rhenium in RePd/Tio2 catalysts for aqueous-phase selective hydrogenation of succinic acid to 1,4-butanediol as a function of palladium and rhenium deposition methods. ChemCatChem 2015, 7, 2161–2178. [Google Scholar] [CrossRef]
- Ly, B.K.; Minh, D.P.; Pinel, C.; Besson, M.; Tapin, B.; Epron, F.; Especel, C. Effect of addition mode of re in bimetallic Pd–Re/TiO2 catalysts upon the selective aqueous-phase hydrogenation of succinic acid to 1,4-butanediol. Top. Catal. 2012, 55, 466–473. [Google Scholar] [CrossRef]
- Takeda, Y.; Tamura, M.; Nakagawa, Y.; Okumura, K.; Tomishige, K. Hydrogenation of dicarboxylic acids to diols over Re–Pd catalysts. Catal. Sci. Technol. 2016, 6, 5668–5683. [Google Scholar] [CrossRef]
- Pritchard, J.; Filonenko, G.A.; van Putten, R.; Hensen, E.J.M.; Pidko, E.A. Heterogeneous and homogeneous catalysis for the hydrogenation of carboxylic acid derivatives: History, advances, and future directions. Chem. Soc. Rev. 2015, 44, 3808–3833. [Google Scholar] [CrossRef]
- Michel, C.; Gallezot, P. Why is ruthenium an efficient catalyst for the aqueous-phase hydrogenation of biosourced carbonyl compounds? ACS Catal. 2015, 5, 4130–4132. [Google Scholar] [CrossRef]
- Hong, U.G.; Park, H.W.; Lee, J.; Hwang, S.; Kwak, J.; Yi, J.; Song, I.K. Hydrogenation of succinic acid to 1,4-butanediol over rhenium catalyst supported on copper-containing mesoporous carbon. J. Nanosci. Nanotechnol. 2013, 13, 7448–7453. [Google Scholar] [CrossRef]
- Vardon, D.R.; Settle, A.E.; Vorotnikov, V.; Menart, M.J.; Eaton, T.R.; Unocic, K.A.; Steirer, K.S.; Wood, K.N.; Cleveland, N.S.; Moyer, K.E.; et al. Ru-Sn/AC for the Aqueous-phase reduction of succinic acid to 1,4-butanediol under continuous process conditions. ACS Catal. 2017, 7, 6207–6219. [Google Scholar] [CrossRef]
- Jenkins, T. Toward a biobased economy: Examples from the UK. Biofuels Bioprod. Biorefine 2008, 2, 133–143. [Google Scholar] [CrossRef]
- Shao, Z.; Li, C.; Di, X.; Xiao, Z.; Liang, C. Aqueous-phase hydrogenation of succinic acid to c-butyrolactone and tetrahydrofuran over Pd/C, Re/C, and Pd–Re/C catalysts. Ind. Eng. Chem. Res. 2014, 53, 9638–9645. [Google Scholar] [CrossRef]
- Patankar, S.C.; Sharma, A.G.; Yadav, G.D. Biobased process intensification in selective synthesis of c-butyrolactone from succinic acid via synergistic palladium-copper bimetallic catalyst supported on alumina xerogel. Clean Technol. Environ. Policy 2018, 20, 683–693. [Google Scholar] [CrossRef]
- Di, X.; Shao, Z.F.; Li, C.; Li, W.Z.; Liang, C.H. Hydrogenation of succinic acid over supported rhenium catalysts prepared by the microwave-assisted thermolytic method. Catal. Sci. Technol. 2015, 5, 2441–2448. [Google Scholar] [CrossRef]
- Kitchen, H.J.; Vallance, S.R.; Kennedy, J.L.; Tapia-Ruiz, N.; Carassiti, L.; Harrison, A.; Whittaker, A.G.; Drysdale, T.D.; Kingman, S.W.; Gregory, D.H. Modern microwave methods in solid-state inorganic materials chemistry: From fundamentals to manufacturing. Chem. Rev. 2014, 114, 1170–1206. [Google Scholar] [CrossRef] [PubMed]
- Di, X.; Li, C.; Zhang, B.; Qi, J.; Li, W.; Su, D.; Liang, C. Role of Re and Ru in Re−Ru/C bimetallic catalysts for the aqueous hydrogenation of succinic acid. Ind. Eng. Chem. Res. 2017, 56, 4672–4683. [Google Scholar] [CrossRef]
- Datta, R.; Henry, M.J. Lactic acid: Recent advances in products, processes, and technologies—A review. Chem. Technol. Biotechnol. 2006, 81, 1119–1129. [Google Scholar] [CrossRef]
- Hayashi, Y.; Sasaki, Y. Tin-catalyzed conversion of trioses to alkyl lactates in alcohol solution. Chem. Commun. 2005, 2716–2718. [Google Scholar] [CrossRef]
- Wee, Y.-J.; Kim, J.-N.; Ryu, H.-W. Biological production of lactic acid and its applications. Food Technol. Biotechnol. 2006, 44, 163–172. [Google Scholar]
- John, R.P.; Nampoothiri, K.M.; Pandey, A. Fermentative production of lactic acid from biomass: An overview on process developments and future perspectives. Appl. Microbiol. Biotechnol. 2007, 74, 524–534. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, N.; Roychoudhury, P.K.; Srivastava, A. L (+) lactic acid fermentation and its product polymerization. Electron. J. Biotechnol. 2004, 7, 167–179. [Google Scholar]
- Corma, A.; Iborra, S.; Velty, A. Chemical routes for the transformation of biomass into chemicals. Chem. Rev. 2007, 107, 2411–2502. [Google Scholar] [CrossRef] [PubMed]
- Roman-Leshkov, Y.; Davis, M.E. Activation of carbonyl-containing molecules with solid Lewis acids in aqueous media. ACS Catal. 2011, 1, 1566–1580. [Google Scholar] [CrossRef]
- Kong, L.; Li, G.; Wang, H.; He, W.; Ling, F.J. Hydrothermal catalytic conversion of biomass for lactic acid production. Chem. Technol. Biotechnol. 2008, 83, 383–388. [Google Scholar] [CrossRef]
- Rasrendra, C.B.; Makertihartha, I.G.B.N.; Adisasmito, S.; Heeres, H.J. Green chemicals from d-glucose: Systematic studies on the catalytic effects of inorganic salts on the chemo-selectivity and yield in aqueous solutions. Top. Catal. 2010, 53, 1241–1247. [Google Scholar] [CrossRef]
- Holm, M.S.; Saravanamurugan, S.; Taarning, E. Conversion of sugars to lactic acid derivatives using heterogeneous zeotype catalysts. Science 2010, 328, 602–605. [Google Scholar] [CrossRef] [PubMed]
- Taarning, E.; Saravamurugan, S.; Spansberg, M.; Xiong, J.; West, R.M.; Christenssen, C.H. Zeolite-catalyzed isomerization of triose sugars. ChemSusChem 2009, 2, 625–627. [Google Scholar] [CrossRef]
- West, R.M.; Spansberg Holm, M.; Saravanamurugan, S.; Xiong, J.; Beversdorf, Z.; Taarning, E.; Christensen, C.H. Zeolite H-USY for the production of lactic acid and methyl lactate from C3-sugars. J. Catal. 2010, 269, 122–130. [Google Scholar] [CrossRef]
- Chambon, F.; Rataboul, F.; Pinel, C.; Cabiac, A.; Guillon, E.; Essayem, N. Cellulose hydrothermal conversion promoted by heterogeneous Brønsted and Lewis acids: Remarkable efficiency of solid Lewis acids to produce lactic acid. Appl. Catal. B 2011, 105, 171–181. [Google Scholar] [CrossRef]
- Zhang, Z.; Jackson, J.E.; Miller, D.J. Kinetics of aqueous-phase hydrogenation of lactic acid to propylene glycol. Ind. Eng. Chem. Res. 2002, 41, 691–696. [Google Scholar] [CrossRef]
- Zeng, W.; Cheng, D.-G.; Chen, F.; Zhan, X. Catalytic conversion of glucose on Al–Zr mixed oxides in hot compressed water. Catal. Lett. 2009, 133, 221. [Google Scholar] [CrossRef]
- Katryniok, B.; Paul, S.; Dumeignil, F. Highly efficient catalyst for the decarbonylation of lactic acid to acetaldehyde. Green Chem. 2010, 12, 1910–1913. [Google Scholar] [CrossRef]
- Maris, E.P.; Davis, R.J. Hydrogenolysis of glycerol over carbon-supported Ru and Pt catalysts. J. Catal. 2007, 249, 328–337. [Google Scholar] [CrossRef]
- Kishida, H.; Jin, F.; Yan, X.; Moriya, T.; Enomoto, H. Formation of lactic acid from glycolaldehyde by alkaline hydrothermal reaction. Carbohydr. Res. 2006, 341, 2619–2623. [Google Scholar] [CrossRef] [PubMed]
- Ten Dam, J.; Kaptejn, F.; Djanashvili, K.; Hanefeld, U. Tuning selectivity of Pt/CaCO3 in glycerol hydrogenolysis—A design of experiments approach. Catal. Commun. 2011, 13, 1–5. [Google Scholar] [CrossRef]
- Fan, G.-Y.; Zhang, Y.; Zhou, Y.-F.; Li, R.-X.; Chen, H.; Li, H.-J. Hydrogenation of ethyl lactate to 1-propanediol over Ru-B/TiO2 in aqueous solution. Chem. Lett. 2008, 8, 852. [Google Scholar] [CrossRef]
- Dechy-Cabaret, O.; Martin-Vaca, B.; Bourissou, D. Controlled ring-opening polymerization of lactide and glycolide. Chem. Rev. 2004, 104, 6147–6176. [Google Scholar] [CrossRef]
- Tsukegi, T.; Motoyama, T.; Shirai, Y.; Nishida, H.; Endo, T. Racemization behavior of L,L-lactide during heating. Polym. Degrad. Stab. 2007, 92, 552–559. [Google Scholar] [CrossRef]
- Yoo, D.K.; Kim, D.; Lee, D.S. Synthesis of lactide from oligomeric PLA: Effect of temperature, pressure and catalyst. Macromol. Res. 2006, 14, 510–516. [Google Scholar] [CrossRef]
- Noda, M.; Okuyama, H. Thermal catalytic depolymerization of poly(L-lactic acid) oligomer into L, L-lactide: Effects of Al, Ti, Zn and Zr compounds as catalysts. Chem. Pharm. Bull. 1999, 47, 467–471. [Google Scholar] [CrossRef]
- Kricheldorf, H.R.; Kreiser-Saunders, I.; Boettcher, C. Polylactones: 31. Sn(II)octoate-initiated polymerization of L-lactide: A mechanistic study. Polymer 1995, 36, 1253–1259. [Google Scholar] [CrossRef]
- Degee, P.; Dubois, P.; Jerome, R. Bulk polymerization of lactides initiated by aluminum isopropoxide, 2. Beneficial effect of Lewis bases and transfer agentS. Macromol. Chem. Phys. 1997, 198, 1973–1984. [Google Scholar] [CrossRef]
- Zhai, Z.; Li, X.; Tang, C.; Peng, J.; Jiang, N.; Bai, W.; Gao, H.; Liao, Y. Decarbonylation of lactic acid to acetaldehyde over aluminum sulfate catalyst. Ind. Eng. Chem. Res. 2014, 53, 10318–10327. [Google Scholar] [CrossRef]
- Tang, C.M.; Peng, J.S.; Li, X.L.; Zhai, Z.J.; Bai, W.; Jiang, N.; Gao, H.; Liao, Y. Efficient and selective conversion of lactic acid into acetaldehyde using a mesoporous aluminum phosphate catalyst. Green Chem. 2015, 17, 1159–1166. [Google Scholar] [CrossRef]
- Tang, C.; Zhai, Z.; Li, X.; Sun, L.; Bai, W. Highly efficient and robust Mg0.388Al2.408O4 catalyst for gas-phase decarbonylation of lactic acid to acetaldehyde. J. Catal. 2015, 329, 206–217. [Google Scholar] [CrossRef]
- Sad, M.E.; González Pena, L.F.; Padró, C.L.; Apesteguía, C.R. Selective synthesis of acetaldehyde from lactic acid on acid zeolites. Catal. Today 2018, 302, 203–209. [Google Scholar] [CrossRef]
- Huo, Z.; Xiao, J.; Ren, D.; Jin, F.; Wang, T.; Yao, G. Chemoselective synthesis of propionic acid from biomass and lactic acid over a cobalt catalyst in aqueous media. Green Chem. 2017, 19, 1308–1314. [Google Scholar] [CrossRef]
- Xue, J.; Cui, F.; Huang, Z.; Zuo, J.; Chen, J.; Xia, C. Liquid phase hydrogenolysis of biomass-derived lactate to 1,2-propanediol over silica supported cobalt nanocatalyst. Chin. J. Chem. 2011, 29, 1319–1325. [Google Scholar] [CrossRef]
- Xue, J.; Cui, F.; Huang, Z.; Zuo, J.; Chen, J.; Xia, C. Chin. Effect of metal additives on structure and properties of a Co/SiO2 hydrogenation catalyst. J. Catal. 2012, 33, 1642. [Google Scholar]
- Luo, G.; Yan, S.; Qiao, M.; Zhuang, J.; Fan, K. Effect of tin on Ru-B/γ-Al2O3 catalyst for the hydrogenation of ethyl lactate to 1,2-propanediol. Appl. Catal. A 2004, 275, 95–102. [Google Scholar] [CrossRef]
- Feng, J.; Xiong, W.; Jia, Y.; Wang, J.; Liu, D.; Qin, R. Hydrogenation of ethyl lactate over ruthenium catalysts in an additive-free catalytic system. React. Kinet. Mech. Catal. 2011, 104, 89–97. [Google Scholar] [CrossRef]
- Feng, J.; Xiong, W.; Jia, Y.; Wang, J.; Liu, D.; Qin, R. Effect of support on the hydrogenation of ethyl lactate over Ruthenium catalysts. Adv. Mater. Res. 2012, 455–456, 796. [Google Scholar] [CrossRef]
- Zhang, S.; Huo, Z.; Ren, D.; Luo, J.; Fu, J.; Li, L.; Jin, F. Catalytic conversion of ethyl lactate to 1,2-propanediol over CuO. Chin. J. Chem. Eng. 2016, 24, 126–131. [Google Scholar] [CrossRef]
- Brunow, G.; Lundquist, K. Functional Groups and Bonding Patterns in Lignin (Including the Lignin-Carbohydrate Complexes); CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Holladay, J.E.; White, J.F.; Bozell, J.J.; Johnson, D. Top Value Added Chemicals from Biomass. Vol. II: Results of Screening for Potential Candidates from Biorefinery Lignin; U.S. Department of Commerce: Washington, DC, USA, 2007; p. 1.
- Calvo-Flores, F.G.; Dobado, J.A. Lignin as Renewable Raw Material. ChemSusChem 2010, 3, 1227–1235. [Google Scholar] [CrossRef] [PubMed]
- Stewart, D. Lignin as a base material for materials applications: Chemistry, application, and economics. Ind. Crops Prod. 2008, 27, 202–207. [Google Scholar] [CrossRef]
- Gasser, C.A.; Hommes, G.; Schäffer, A.; Corvini, P.F.X. Multicatalysis reactions: New prospects and challenges of biotechnology to valorize lignin. Appl. Microbiol. Biotechnol. 2012, 95, 1115–1134. [Google Scholar] [CrossRef]
- Li, C.; Zhao, X.; Wang, A.; Huber, G.W.; Zhang, T. Catalytic transformation of lignin for the production of chemicals and fuels. Chem. Rev. 2015, 115, 11559–11624. [Google Scholar] [CrossRef]
- Kang, S.; Li, X.; Fan, J.; Chang, J. Hydrothermal conversion of lignin: A review. Renew. Sustain. Energy Rev. 2013, 27, 546–558. [Google Scholar] [CrossRef]
- Kim, J.S. Production, separation and applications of phenolic-rich bio-oil-A review. Bioresour. Technol. 2015, 178, 90–98. [Google Scholar] [CrossRef]
- Bai, X.L.; Kim, K.H.; Brown, R.C.; Dalluge, E.; Hutchinson, C.; Lee, Y.J.; Dalluge, D. Formation of phenolic oligomers during fast pyrolysis of lignin. Fuel 2014, 128, 170–179. [Google Scholar] [CrossRef]
- Song, Q.; Wang, F.; Cai, J.; Wang, Y.; Zhang, J.; Yu, W.; Xu, J. Lignin depolymerization (LDP) in alcohol over nickel-based catalysts via a fragmentation−hydrogenolysis process. Energy Environ. Sci. 2013, 6, 994–1007. [Google Scholar] [CrossRef]
- Yoshikawa, T.; Yagi, T.; Shinohara, S.; Fukunaga, T.; Nakasaka, Y.; Tago, T.; Masuda, T. Production of phenols from lignin via depolymerization and catalytic cracking. Fuel Process. Technol. 2013, 108, 69–75. [Google Scholar] [CrossRef]
- Zakzeski, J.; Jongerius, A.L.; Bruijnincx, P.C.; Weckhuysen, B.M. Catalytic lignin valorization process for the production of aromatic chemicals and hydrogen. ChemSusChem 2012, 5, 1602–1609. [Google Scholar] [CrossRef] [PubMed]
- Shen, D.; Liu, N.; Dong, C.; Xiao, R.; Gu, S. Catalytic solvolysis of lignin with the modified HUSYs in formic acid assisted by microwave heating. Chem. Eng. J. 2015, 270, 641–647. [Google Scholar] [CrossRef]
- Jung, K.A.; Woo, S.H.; Lim, S.-R.; Park, J.M. Pyrolytic production of phenolic compounds from the lignin residues of bioethanol processes. Chem. Eng. J. 2015, 259, 107–116. [Google Scholar] [CrossRef]
- Das, J.; Halgeri, A.B. Selective synthesis of para-ethylphenol over pore size tailored zeolite. Appl. Catal. A 2000, 194, 359–363. [Google Scholar] [CrossRef]
- Liu, A.; Park, Y.; Huang, Z.L.; Wang, B.W.; Ankumah, R.O.; Biswas, P.K. Product identification and distribution from hydrothermal conversion of walnut shells. Energy Fuels 2006, 20, 446–454. [Google Scholar] [CrossRef]
- Tymchyshyn, M.; Xu, C.B. Liquefaction of biomass in hot compressed water for the production of phenolic compounds. Bioresour. Technol. 2010, 101, 2483–2490. [Google Scholar] [CrossRef]
- Roberts, V.M.; Stein, V.; Reiner, T.; Lemonidou, A.; Li, X.; Lercher, J.A. Towards quantitative catalytic lignin depolymerization. Chemistry 2011, 17, 5939–5948. [Google Scholar] [CrossRef]
- Kim, J.Y.; Park, J.; Kim, U.J.; Choi, J.W. Conversion of lignin to phenol-rich oil fraction under supercritical alcohols in the presence of metal catalysts. Energy Fuels 2015, 29, 5154–5163. [Google Scholar] [CrossRef]
- Mukarakate, J.D.; McBrayer, T.J.; Evans, S.; Budhi, D.J.; Robichaud, K.; Lisa, J.T.; Dam, M.J.; Watson, R.M.; Baldwin, R.M.; Nimlos, M.R. Catalytic fast pyrolysis of biomass: The reactions of water and aromatic intermediates produces phenols. Green Chem. 2015, 17, 4217–4227. [Google Scholar] [CrossRef]
- Omoriyekomwan, J.E.; Tahmasebi, A.; Yu, J.L. Production of phenol-rich bio-oil during catalytic fixed-bed and microwave pyrolysis of palm kernel shell. Bioresour. Technol. 2016, 207, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.B.; Lu, Q.; Ye, X.N.; Li, W.T.; Hu, B.; Dong, C.Q. Production of phenolic-rich bio-oil from catalytic fast pyrolysis of biomass using a magnetic solid base catalyst. Energy Convers. Manag. 2015, 106, 1309–1317. [Google Scholar] [CrossRef]
- Wang, W.; Li, X.; Ye, D.; Cai, L.; Shi, S.Q. Catalytic pyrolysis of larch sawdust for phenol-rich bio-oil using different catalysts. Renew. Energy 2018, 121, 146–152. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, T.; Ma, L.; Zhang, Q.; Yu, Y.; Liu, Q. Characterization and catalytic properties of Ni and NiCu catalysts supported on ZrO2–SiO2 for guaiacol hydrodeoxygenation. Catal. Commun. 2013, 33, 15–19. [Google Scholar] [CrossRef]
- Huynh, T.M.; Armbruster, U.; Pohl, M.M.; Schneider, M.; Radnik, J.; Hoang, D.L.; Phan, B.M.Q.; Nguyen, D.A.; Martin, A. Hydrodeoxygenation of phenol as a model compound for bio-oil on non-noble bimetallic nickel-based catalysts. ChemCatChem 2014, 6, 1940–1951. [Google Scholar] [CrossRef]
- Khromova, S.A.; Smirnov, A.A.; Bulavchenko, O.A.; Saraev, A.A.; Kaichev, V.V.; Reshetnikov, S.I.; Yakovlev, V.A. Anisole hydrodeoxygenation over Ni–Cu bimetallic catalysts: The effect of Ni/Cu ratio on selectivity. Appl. Catal. A 2014, 470, 261–270. [Google Scholar] [CrossRef]
- Nie, L.; De Souza, P.M.; Noronha, F.B.; An, W.; Sooknoi, T.; Resasco, D.E. Selective conversion of m-cresol to toluene over bimetallic Ni–Fe catalysts. J. Mol. Catal. A 2014, 388, 47–55. [Google Scholar] [CrossRef]
- Sun, J.; Karim, A.M.; Zhang, H.; Kovarik, L.; Li, X.S.; Hensley, A.J.; McEwen, J.S.; Wang, Y. Carbon-supported bimetallic Pd–Fe catalysts for vapor-phase hydrodeoxygenation of guaiacol. J. Catal. 2013, 306, 47–57. [Google Scholar] [CrossRef]
- Ohta, H.; Feng, B.; Kobayashi, H.; Hara, K.; Fukuoka, A. Selective hydrodeoxygenation of lignin-related 4-propyl phenol into n-propylbenzene in water by Pt-Re/ZrO2 catalysts. Catal. Today 2014, 234, 139–144. [Google Scholar] [CrossRef]
- Do, P.T.M.; Foster, A.J.; Chen, J.; Lobo, R.F. Bimetallic effects in the hydrodeoxygenation of meta-cresol on γ-Al2O3 supported Pt-Ni and Pt-Co catalysts. Green Chem. 2012, 14, 1388–1397. [Google Scholar] [CrossRef]
- Pepper, J.M.; Hibbert, H. Studies on lignin and related compounds. LXXXVII. High-pressure hydrogenation of maple wood. J. Am. Chem. Soc. 1948, 70, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Cai, J.Y.; Zhang, J.J.; Yu, W.Q.; Wang, F.; Xu, J. Hydrogenation and cleavage of the C-O bonds in the lignin model compound phenethyl phenyl ether over a nickel-based catalyst. Chin. J. Catal. 2013, 34, 651–658. [Google Scholar] [CrossRef]
- Song, Q.; Wang, F.; Xu, J. Hydrogenolysis of lignosulfonate into phenols over heterogeneous nickel catalysts. Chem. Commun. 2012, 48, 7019–7021. [Google Scholar] [CrossRef] [PubMed]
- Li, C.Z.; Zheng, M.Y.; Wang, A.Q.; Zhang, T. One-pot catalytic hydrocracking of raw woody biomass into chemicals over supported carbide catalysts: Simultaneous conversion of cellulose, hemicellulose, and lignin. Energy Environ. Sci. 2012, 5, 6383–6390. [Google Scholar] [CrossRef]
- Parsell, T.H.; Owen, B.C.; Klein, I.; Jarrell, T.M.; Marcum, C.L.; Haupert, L.J.; Amundson, L.M.; Kenttamaa, H.I.; Ribeiro, F.; Miller, J.T.; et al. Cleavage and hydrodeoxygenation (HDO) of C-O bonds relevant to lignin conversion using Pd/Zn synergistic catalysis. Chem. Sci. 2013, 4, 806–813. [Google Scholar] [CrossRef]
- Yan, N.; Zhao, C.; Dyson, P.J.; Wang, C.; Liu, L.T.; Kou, Y. Selective degradation of wood lignin over noble-metal catalysts in a two-step process. ChemSusChem 2008, 1, 626–629. [Google Scholar] [CrossRef]
- Liguori, L.; Barth, T. Palladium-Nafion SAC-13 catalyzed depolymerization of lignin to phenols in formic acid and water. J. Anal. Appl. Pyrolysis 2011, 92, 477–484. [Google Scholar] [CrossRef]
- Stark, K.; Taccardi, N.; Bosmann, A.; Wasserscheid, P. Oxidative depolymerization of lignin in ionic liquids. ChemSusChem 2010, 3, 719–723. [Google Scholar] [CrossRef]
- Crestini, C.; Crucianelli, M.; Orlandi, M.; Saladino, R. Oxidative strategies in lignin chemistry: A new environmentally friendly approach for the functionalization of lignin and lignocellulosic fibers. Catal. Today 2010, 156, 8–22. [Google Scholar] [CrossRef]
- Crestini, C.; Caponi, M.C.; Argyropoulos, D.S.; Saladino, R. Immobilized methyltrioxo rhenium (MTO)/H2O2 systems for the oxidation of lignin and lignin model compounds. Bioorg. Med. Chem. 2006, 14, 5292–5302. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.F. Selective oxidation of Kraft lignin over zeolite- encapsulated Co(II) [H4]salen and [H2]salen complexes. J. Appl. Polym. Sci. 2014, 131, 40809. [Google Scholar] [CrossRef]
- Lu, X.J.; Zhou, X.F. Co(Salen)-catalyzed oxidation of synthetic lignin-like polymer: H2O2 effects. Oxid. Commun. 2014, 37, 572–582. [Google Scholar]
- Zhang, G.; Scott, B.L.; Wu, R.; Silks, L.A.P.; Hanson, S.K. Aerobic oxidation reactions catalyzed by vanadium complexes of Bis(Phenolate) ligands. Inorg. Chem. 2012, 51, 7354–7361. [Google Scholar] [CrossRef] [PubMed]
- Biannic, B.; Bozell, J.J.; Elder, T. Steric effects in the design of Co-Schiff base complexes for the catalytic oxidation of lignin models to para-benzoquinones. Green Chem. 2014, 16, 3635–3642. [Google Scholar] [CrossRef]
- Badamali, S.K.; Luque, R.; Clark, J.H.; Breeden, S.W. Microwave-assisted oxidation of a lignin model phenolic monomer using Co(salen)/SBA-15. Catal. Commun. 2009, 10, 1010–1013. [Google Scholar] [CrossRef]
- Ma, P.; Zhai, H. Selective TEMPO-mediated oxidation of thermomechanical pulp. BioResources 2013, 8, 4396–4405. [Google Scholar] [CrossRef]
- Rahimi, A.; Azarpira, A.; Kim, H.; Ralph, J.; Stahl, S.S. Chemoselective metal-free aerobic alcohol oxidation in lignin. J. Am. Chem. Soc. 2013, 135, 6415–6418. [Google Scholar] [CrossRef]
- Nguyen, J.D.; Matsuura, B.S.; Stephenson, C.R.J. A photochemical strategy for lignin degradation at room temperature. J. Am. Chem. Soc. 2014, 136, 1218–1221. [Google Scholar] [CrossRef]
- Azarpira, A.; Ralph, J.; Lu, F.C. Catalytic alkaline oxidation of lignin and its model compounds: A pathway to aromatic biochemicals. BioEnergy Res. 2014, 7, 78–86. [Google Scholar] [CrossRef]
- Wu, A.; Lauzon, J.M.; Andriani, I.; James, B.R. Breakdown of lignins, lignin model compounds, and hydroxy-aromatics, to C1 and C2 chemicals via metal-free oxidation with peroxide or persulfate under mild conditions. RSC Adv. 2014, 4, 17931–17934. [Google Scholar] [CrossRef]
- Voitl, T.; Rudolf von Rohr, P. Demonstration of a process for the conversion of Kraft lignin into vanillin and methyl vanillate by acidic oxidation in aqueous methanol. Ind. Eng. Chem. Res. 2010, 49, 520–525. [Google Scholar] [CrossRef]
- Cavdar, A.D.; Kalaycioglu, H.; Hiziroglu, S.J. Some of the properties of oriented strandboard manufactured using kraft lignin phenolic resin. Mater. Process. Technol. 2008, 202, 559. [Google Scholar] [CrossRef]
- Lora, J.H.; Glasser, W.G. Recent industrial applications of lignin: A sustainable alternative to nonrenewable materials. J. Polym. Environ. 2002, 10, 39–48. [Google Scholar] [CrossRef]
- Wang, M.; Leitch, M.; Xu, C.C. Synthesis of phenolic resol resins using cornstalk-derived bio-oil produced by direct liquefaction in hot-compressed phenol-water. Ind. Eng. Chem. 2009, 15, 870–875. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kohli, K.; Prajapati, R.; Sharma, B.K. Bio-Based Chemicals from Renewable Biomass for Integrated Biorefineries. Energies 2019, 12, 233. https://doi.org/10.3390/en12020233
Kohli K, Prajapati R, Sharma BK. Bio-Based Chemicals from Renewable Biomass for Integrated Biorefineries. Energies. 2019; 12(2):233. https://doi.org/10.3390/en12020233
Chicago/Turabian StyleKohli, Kirtika, Ravindra Prajapati, and Brajendra K. Sharma. 2019. "Bio-Based Chemicals from Renewable Biomass for Integrated Biorefineries" Energies 12, no. 2: 233. https://doi.org/10.3390/en12020233
APA StyleKohli, K., Prajapati, R., & Sharma, B. K. (2019). Bio-Based Chemicals from Renewable Biomass for Integrated Biorefineries. Energies, 12(2), 233. https://doi.org/10.3390/en12020233