Hydrogen Desorption in Mg(BH4)2-Ca(BH4)2 System
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
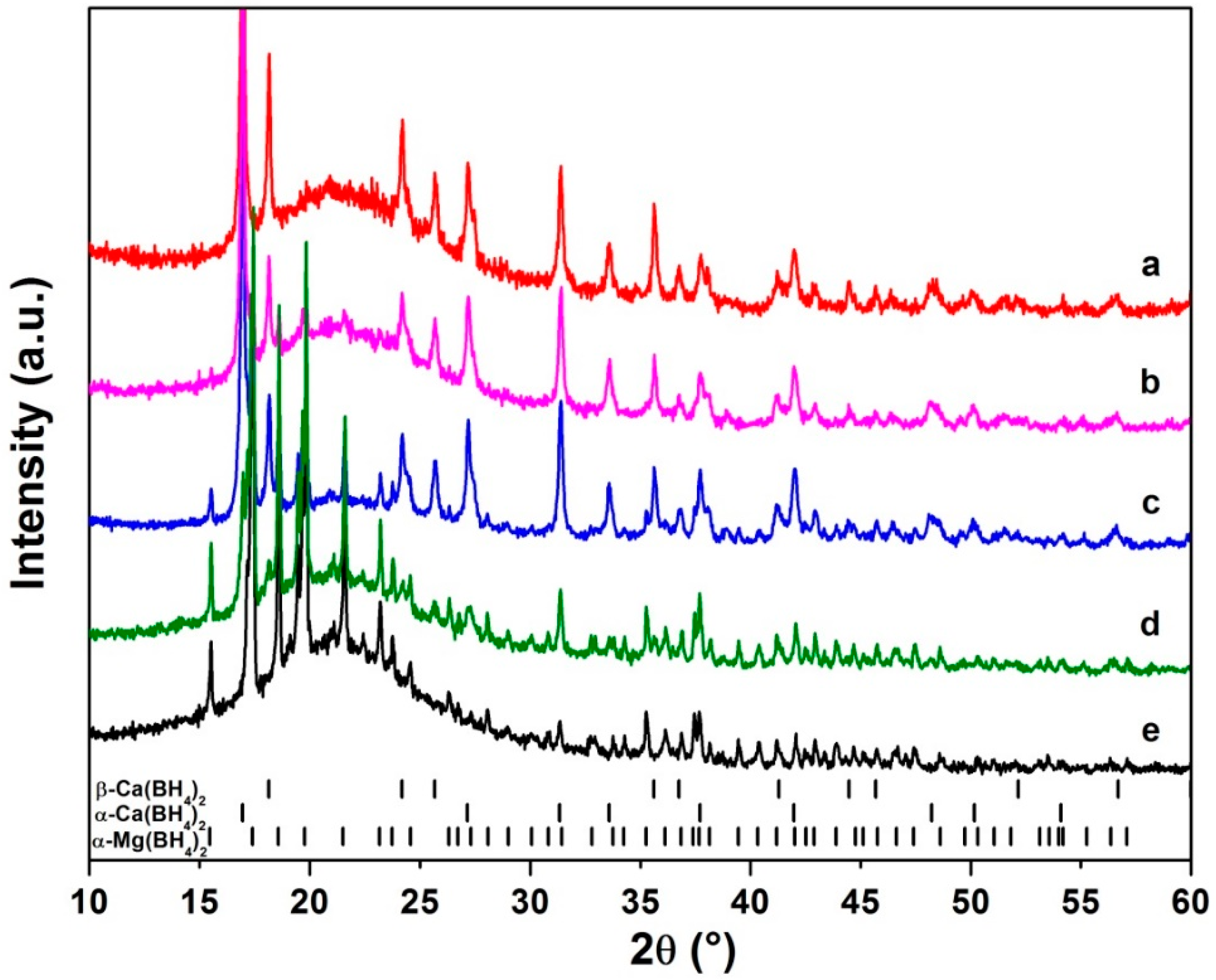
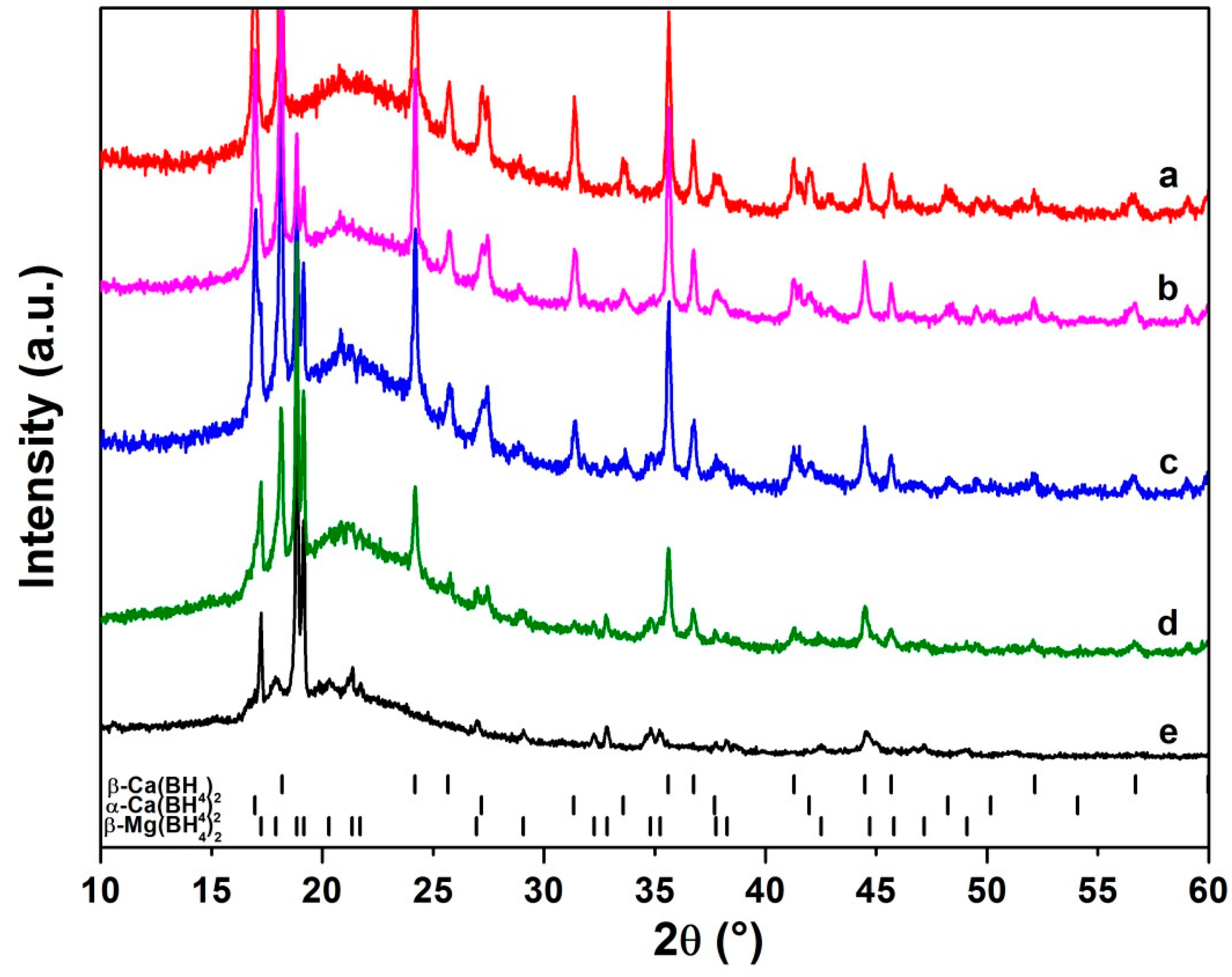
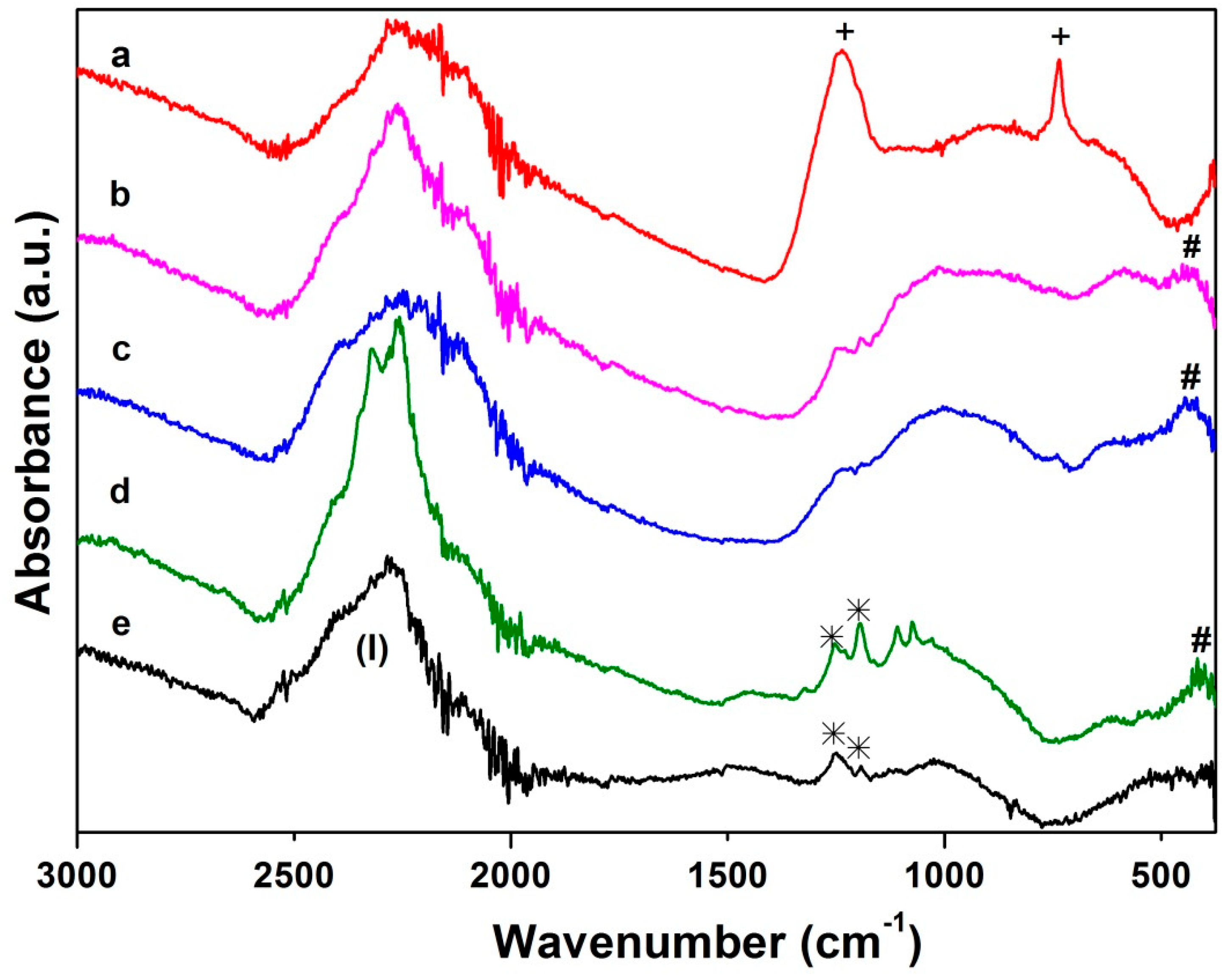
3.1. Synthesis, Structural and Thermal Characterization
3.2. Thermal Decomposition
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Milanese, C.; Jensen, T.R.; Hauback, B.C.; Pistidda, C.; Dornheim, M.; Yang, H.; Lombardo, L.; Zuettel, A.; Filinchuk, Y.; Ngene, P.; et al. Complex hydrides for energy storage. Int. J. Hydrog. Energy 2019, 44, 7860–7874. [Google Scholar] [CrossRef]
- Ozolins, V.; Majzoub, E.H.; Wolverton, C. First-Principles Prediction of Thermodynamically Reversible Hydrogen Storage Reactions in the Li-Mg-Ca-B-H System. J. Am. Chem. Soc. 2009, 131, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Paskevicius, M.; Jepsen, L.H.; Schouwink, P.; Černý, R.; Ravnsbæk, D.B.; Filinchuk, Y.; Dornheim, M.; Besenbacher, F.; Jensen, T.R. Metal borohydrides and derivatives—Synthesis, structure and properties. Chem. Soc. Rev. 2017, 46, 1565–1634. [Google Scholar] [CrossRef] [PubMed]
- Dematteis, E.M.; Santoru, A.; Poletti, M.G.; Pistidda, C.; Klassen, T.; Dornheim, M.; Baricco, M. Phase stability and hydrogen desorption in a quinary equimolar mixture of light-metals borohydrides. Int. J. Hydrog. Energy 2018, 43, 16793–16803. [Google Scholar] [CrossRef]
- Orimo, S.-I.; Nakamori, Y.; Eliseo, J.R.; Züttel, A.; Jensen, C.M. Complex Hydrides for Hydrogen Storage. Chem. Rev. 2007, 107, 4111–4132. [Google Scholar] [CrossRef] [PubMed]
- Rönnebro, E. Development of group II borohydrides as hydrogen storage materials. Curr. Opin. Solid State Mater. Sci. 2011, 15, 44–51. [Google Scholar] [CrossRef]
- Kulkarni, A.D.; Wang, L.-L.; Johnson, D.D.; Sholl, D.S.; Johnson, J.K. First-Principles Characterization of Amorphous Phases of MB12H12, M = Mg, Ca. J. Phys. Chem. C 2010, 114, 14601–14605. [Google Scholar] [CrossRef]
- Pinatel, E.R.; Albanese, E.; Civalleri, B.; Baricco, M. Thermodynamic modelling of Mg(BH4)2. J. Alloys Compd. 2015, 645, S64–S68. [Google Scholar] [CrossRef]
- Wang, L.; Graham, D.D.; Robertson, I.M.; Johnson, D.D. On the Reversibility of Hydrogen-Storage Reactions in Ca(BH4)2: Characterization via Experiment and Theory. J. Phys. Chem. C 2009, 113, 20088–20096. [Google Scholar] [CrossRef]
- Liu, Y.; Giri, S.; Zhou, J.; Jena, P. Intermediate Phases during Decomposition of Metal Borohydrides, M(BH4)n (M = Na, Mg, Y). J. Phys. Chem. C 2014, 118, 28456–28461. [Google Scholar] [CrossRef]
- Vitillo, J.; Bordiga, S.; Baricco, M. Spectroscopic and Structural Characterization of Thermal Decomposition of γ-Mg(BH4)2: Dynamic Vacuum versus H2 Atmosphere. J. Phys. Chem. C 2015, 119, 25340–25351. [Google Scholar] [CrossRef]
- Zavorotynska, O.; Deledda, S.; Hauback, B.C. Kinetics studies of the reversible partial decomposition reaction in Mg(BH4)2. Int. J. Hydrog. Energy 2016, 41, 9885–9892. [Google Scholar] [CrossRef]
- Soloveichik, G.L.; Gao, Y.; Rijssenbeek, J.; Andrus, M.; Kniajanski, S.; Bowman, R.C., Jr; Hwang, S.; Zhao, J. Magnesium borohydride as a hydrogen storage material: Properties and dehydrogenation pathway of unsolvated Mg(BH4)2. Int. J. Hydrog. Energy 2009, 34, 916–928. [Google Scholar] [CrossRef]
- Riktor, M.D.; Sørby, M.H.; Muller, J.; Bardají, E.G.; Fichtner, M.; Hauback, B.C. On the rehydrogenation of decomposed Ca(BH4)2. J. Alloys Compd. 2015, 632, 800–804. [Google Scholar] [CrossRef]
- Bonatto Minella, C.; Garroni, S.; Olid, D.; Teixidor, F.; Pistidda, C.; Lindemann, I.; Gutfleisch, O.; Baró, M.D.; Bormann, R.; Klassen, T.; et al. Experimental Evidence of Ca[B12H12] Formation During Decomposition of a Ca(BH4)2 + MgH2 Based Reactive Hydride Composite. J. Phys. Chem. C 2011, 115, 18010–18014. [Google Scholar] [CrossRef]
- Saldan, I.; Hino, S.; Humphries, T.D.; Zavorotynska, O.; Chong, M.; Jensen, C.M.; Deledda, S.; Hauback, B.C. Structural Changes Observed during the Reversible Hydrogenation of Mg(BH4)2 with Ni-Based Additives. J. Phys. Chem. C 2014, 118, 23376–23384. [Google Scholar] [CrossRef]
- Rueda, M.; Sanz-Moral, L.M.; Girella, A.; Cofrancesco, P.; Milanese, C.; Martín, Á. Reversible hydrogen sorption in the composite made of magnesium borohydride and silica aerogel. Int. J. Hydrog. Energy 2016, 41, 15245–15253. [Google Scholar] [CrossRef]
- Kim, J.; Shim, J.; Cho, Y.W. On the reversibility of hydrogen storage in Ti- and Nb-catalyzed Ca(BH4)2. J. Power Sources 2008, 181, 140–143. [Google Scholar] [CrossRef]
- Hino, S.; Fonneløp, J.E.; Corno, M.; Zavorotynska, O.; Damin, A.; Richter, B.; Baricco, M.; Jensen, T.R.; Sørby, M.H.; Hauback, B.C. Halide substitution in magnesium borohydride. J. Phys. Chem. C 2012, 116, 12482–12488. [Google Scholar] [CrossRef]
- Ibikunle, A.A.; Goudy, A.J. Kinetics and modeling study of a Mg(BH4)2/Ca(BH4)2 destabilized system. Int. J. Hydrog. Energy 2012, 37, 12420–12424. [Google Scholar] [CrossRef]
- Dematteis, E.M.; Vaunois, S.; Pistidda, C.; Dornheim, M.; Baricco, M. Reactive Hydride Composite of Mg2NiH4 with Borohydrides Eutectic Mixtures. Crystals 2018, 8, 90. [Google Scholar] [CrossRef]
- Durojaiye, T.; Ibikunle, A.; Goudy, A.J. Hydrogen storage in destabilized borohydride materials. Int. J. Hydrog. Energy 2010, 35, 10329–10333. [Google Scholar] [CrossRef]
- Dematteis, E.M.; Pistidda, C.; Dornheim, M.; Baricco, M. Exploring Ternary and Quaternary Mixtures in the LiBH4-NaBH4-KBH4-Mg(BH4)2-Ca(BH4)2 System. ChemPhysChem 2019, 20, 1348–1359. [Google Scholar] [CrossRef] [PubMed]
- Paskevicius, M.; Ley, M.B.; Sheppard, D.A.; Jensen, T.R.; Buckley, C.E. Eutectic melting in metal borohydrides. Phys. Chem. Chem. Phys. 2013, 15, 19774–19789. [Google Scholar] [CrossRef] [PubMed]
- Dematteis, E.M.; Pinatel, E.R.; Corno, M.; Jensen, T.R.; Baricco, M.; Sturari, S.; Pistidda, C.; Baricco, M. Phase diagrams of the LiBH4–NaBH4–KBH4 system. Phys. Chem. Chem. Phys. 2017, 19, 25071–25079. [Google Scholar] [CrossRef] [PubMed]
- Jensen, S.R.H.; Jepsen, L.H.; Skibsted, J.; Jensen, T.R. Phase Diagram for the NaBH4-KBH4 System and the Stability of a Na(1−x)K(x)BH4 Solid Solution. J. Phys. Chem. C 2015, 119, 27919–27929. [Google Scholar] [CrossRef]
- Ley, M.B.; Roedern, E.; Jensen, T.R. Eutectic melting of LiBH4–KBH4. Phys. Chem. Chem. Phys. 2014, 16, 24194–24199. [Google Scholar] [CrossRef] [PubMed]
- Dematteis, E.M.; Roedern, E.; Pinatel, E.R.; Corno, M.; Jensen, T.R.; Baricco, M. A thermodynamic investigation of the LiBH4–NaBH4 system. RSC Adv. 2016, 6, 60101–60108. [Google Scholar] [CrossRef]
- Lutterotti, L.; Matthies, S.; Wenk, H.R. MAUD: A friendly Java program for material analysis using diffraction. IUCr Newsl. CPD 1999, 21, 14–15. [Google Scholar]
- D’Anna, V.; Lawson Daku, L.M.; Hagemann, H. Vibrational spectra and structure of borohydrides. J. Alloys Compd. 2013, 580, S122–S124. [Google Scholar] [CrossRef]
- D’Anna, V.; Spyratou, A.; Sharma, M.; Hagemann, H. FT-IR spectra of inorganic borohydrides. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 128, 902–906. [Google Scholar] [CrossRef]
- Fichtner, M.; Chlopek, K.; Longhini, M.; Hagemann, H. Vibrational Spectra of Ca(BH4)2. J. Phys. Chem. C 2008, 112, 11575–11579. [Google Scholar] [CrossRef]
- Borgschulte, A.; Gremaud, R.; Züttel, A.; Martelli, P.; Remhof, A.; Ramirez-Cuesta, A.J.; Refson, K.; Bardaji, E.G.; Lohstroh, W.; Fichtner, M.; et al. Experimental evidence of librational vibrations determining the stability of calcium borohydride. Phys. Rev. B 2011, 83, 024102. [Google Scholar] [CrossRef]
- Hagemann, H.; D’Anna, V.; Rapin, J.P.; Černý, R.; Filinchuk, Y.; Kim, K.C.; Sholl, D.S.; Parker, S.F. New fundamental experimental studies on α-Mg(BH4)2 and other borohydrides. J. Alloys Compd. 2011, 509, 2010–2012. [Google Scholar] [CrossRef]
- Filinchuk, Y.; Cerny, R.; Hagemann, H.; Černý, R. Insight into Mg (BH4)2 with synchrotron X-ray diffraction: structure revision, crystal chemistry, and anomalous thermal expansion. Chem. Mater. 2009, 21, 925–933. [Google Scholar] [CrossRef]
- Černý, R.; Penin, N.; Hagemann, H.; Filinchuk, Y. The First Crystallographic and Spectroscopic Characterization of a 3 d -Metal Borohydride: Mn(BH4)2. J. Phys. Chem. C 2009, 113, 9003–9007. [Google Scholar] [CrossRef]
- Dimitrievska, M.; White, J.L.; Zhou, W.; Stavila, V.; Klebanoff, L.E.; Udovic, T.J. Structure-dependent vibrational dynamics of Mg(BH4)2 polymorphs probed with neutron vibrational spectroscopy and first-principles calculations. Phys. Chem. Chem. Phys. 2016, 18, 25546–25552. [Google Scholar] [CrossRef]
- Liu, A.; Xie, S.; Dabiran-Zohoory, S.; Song, Y. High-Pressure Structures and Transformations of Calcium Borohydride Probed by Combined Raman and Infrared Spectroscopies. J. Phys. Chem. C 2010, 114, 11635–11642. [Google Scholar] [CrossRef]
- Shannon Database of Ionic Radii. Available online: http://abulafia.mt.ic.ac.uk/shannon/ptable.php (accessed on 20 May 2019).
- Stadie, N.P.; Callini, E.; Richter, B.; Jensen, T.R.; Borgschulte, A.; Züttel, A. Supercritical N2 processing as a route to the clean dehydrogenation of porous Mg(BH4)2. J. Am. Chem. Soc. 2014, 136, 8181–8184. [Google Scholar] [CrossRef]
- Chong, M.; Karkamkar, A.; Autrey, T.; Orimo, S.; Jalisatgi, S.; Jensen, C.M. Reversible dehydrogenation of magnesium borohydride to magnesium triborane in the solid state under moderate conditions. Chem. Commun. 2011, 47, 1330–1332. [Google Scholar] [CrossRef]
- Chong, M.; Matsuo, M.; Orimo, S.; Autrey, T.; Jensen, C.M. Selective Reversible Hydrogenation of Mg(B3H8)2/MgH2 to Mg(BH4)2: Pathway to Reversible Borane-Based Hydrogen Storage? Inorg. Chem. 2015, 54, 4120–4125. [Google Scholar] [CrossRef] [PubMed]
- Riktor, M.D.; Sørby, M.H.; Chłopek, K.; Fichtner, M.; Buchter, F.; Züttel, A.; Hauback, B.C. In situ synchrotron diffraction studies of phase transitions and thermal decomposition of Mg(BH4)2 and Ca(BH4)2. J. Mater. Chem. 2007, 17, 4939. [Google Scholar] [CrossRef]
- He, L.; Li, H.-W.; Tumanov, N.; Filinchuk, Y.; Akiba, E. Facile synthesis of anhydrous alkaline earth metal dodecaborates MB12H12 (M = Mg, Ca) from M(BH4)2. Dalt. Trans. 2015, 44, 15882–15887. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Guo, Z.; Poh, C.K.; Ranjbar, A.; Guo, Y.; Yu, X.; Liu, H. Study on the dehydrogenation kinetics and thermodynamics of Ca(BH4)2. J. Alloys Compd. 2010, 500, 200–205. [Google Scholar] [CrossRef]
- Kim, Y.; Reed, D.; Lee, Y.-S.; Lee, J.Y.; Shim, J.; Book, D.; Cho, Y.W. Identification of the Dehydrogenated Product of Ca(BH4)2. J. Phys. Chem. C 2009, 113, 5865–5871. [Google Scholar] [CrossRef]
- Kim, Y.; Hwang, S.; Lee, Y.S.; Suh, J.; Han, H.N.; Cho, Y.W. Hydrogen Back-Pressure Effects on the Dehydrogenation Reactions of Ca(BH4)2. J. Phys. Chem. C 2012, 116, 25715–25720. [Google Scholar] [CrossRef]
- Minella, C.B.; Pistidda, C.; Garroni, S.; Nolis, P.; Baró, M.D.; Gutfleisch, O.; Klassen, T.; Bormann, R.; Dornheim, M. Ca(BH4)2 + MgH2: Desorption Reaction and Role of Mg on Its Reversibility. J. Phys. Chem. C 2013, 117, 3846–3852. [Google Scholar] [CrossRef]
- Muetterties, E.L.; Merrifield, R.E.; Miller, H.C.; Knoth, W.H.; Downing, J.R. Chemistry of Boranes. III. 1 The Infrared and Raman Spectra of B12H12 -and Related Anions. J. Am. Chem. Soc. 1962, 84, 2506–2508. [Google Scholar] [CrossRef]




| Sample | Tonset (°C) Ca(BH4)2 α-β PT | Tpeak (°C) Ca(BH4)2 α-β PT | Tonset (°C) Mg(BH4)2 α-β PT | Tpeak (°C) Mg(BH4)2 α-β PT | Tpeak (°C) Dec. | Tonset (°C); Tpeak (°C) Re-H2 |
|---|---|---|---|---|---|---|
| Mg(BH4)2 | 181 | 191 | 299, 340, 355, 386 | 278; 254 | ||
| 2:1 | 149 | 160 | 191 | 272, 326, 346, 398 | 288; 273 | |
| 1:1 | 149 | 163 | 191 | 282, 390 | ||
| 1:2 | 149 | 162 | 196 | 280, 387 | ||
| Ca(BH4)2 | 157 | 168 | 355, 376 |
| Sample | α-Mg(BH4)2 | β-Mg(BH4)2 | ||||||
|---|---|---|---|---|---|---|---|---|
| a (Å) | c (Å) | V/z (Å3) | a (Å) | b (Å) | c (Å) | V/z (Å3) | ||
| Mg(BH4)2 | BM | 10.347 | 37.115 | 475 | ||||
| DSC | 37.098 | 18.626 | 10.917 | 118 | ||||
| 2:1 | BM | 10.344 | 37.102 | 475 | ||||
| DSC | 37.105 | 18.640 | 10.921 | 118 | ||||
| 1:1 | BM | 10.345 | 37.103 | 475 | ||||
| DSC | 37.107 | 18.647 | 10.921 | 118 | ||||
| 1:2 | BM | 10.342 | 37.088 | 474 | ||||
| DSC | 37.089 | 18.667 | 10.921 | 118 | ||||
| Sample | α-Ca(BH4)2 | β-Ca(BH4)2 | ||||||
|---|---|---|---|---|---|---|---|---|
| a (Å) | b (Å) | c (Å) | V/z (Å3) | a (Å) | c (Å) | V/z (Å3) | ||
| 2:1 | BM | 8.776 | 13.127 | 7.497 | 108 | 6.911 | 4.350 | 65 |
| DSC | 8.744 | 13.104 | 7.480 | 107 | 6.918 | 4.348 | 65 | |
| 1:1 | BM | 8.777 | 13.125 | 7.496 | 108 | 6.914 | 4.346 | 65 |
| DSC | 8.754 | 13.105 | 7.497 | 108 | 6.917 | 4.348 | 65 | |
| 1:2 | BM | 8.775 | 13.125 | 7.499 | 108 | 6.915 | 4.347 | 65 |
| DSC | 8.760 | 13.116 | 7.503 | 108 | 6.917 | 4.347 | 65 | |
| Ca(BH4)2 | BM | 8.775 | 13.125 | 7.499 | 108 | 6.915 | 4.346 | 65 |
| DSC | 8.762 | 13.119 | 7.496 | 108 | 6.917 | 4.348 | 65 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dematteis, E.M.; Baricco, M. Hydrogen Desorption in Mg(BH4)2-Ca(BH4)2 System. Energies 2019, 12, 3230. https://doi.org/10.3390/en12173230
Dematteis EM, Baricco M. Hydrogen Desorption in Mg(BH4)2-Ca(BH4)2 System. Energies. 2019; 12(17):3230. https://doi.org/10.3390/en12173230
Chicago/Turabian StyleDematteis, Erika M., and Marcello Baricco. 2019. "Hydrogen Desorption in Mg(BH4)2-Ca(BH4)2 System" Energies 12, no. 17: 3230. https://doi.org/10.3390/en12173230
APA StyleDematteis, E. M., & Baricco, M. (2019). Hydrogen Desorption in Mg(BH4)2-Ca(BH4)2 System. Energies, 12(17), 3230. https://doi.org/10.3390/en12173230





