Abstract
An increased awareness of the impacts of synthetic refrigerants on the environment has prompted the refrigeration industry and researchers worldwide to seek better alternatives in terms of technical, economic and environmental performance. CO2 refrigerant, also known as R744, has re-emerged as a potential alternative to existing refrigerants with its zero ozone depletion potential (ODP) and impressively low global warming potential (GWP). A refrigeration system utilising this refrigerant, however, suffers performance degradation when it operates in warm or hot climatic regions due to its inevitable operation in the supercritical region. In addition, the CO2 refrigerant properties necessitate the need for components designed to withstand very high operating pressures. These challenges have not been let unnoticed; related industries and researchers are actively involved in research and development of various components and systems which in turn encourages increased applications of these systems. In this paper, a comprehensive review of CO2 refrigeration systems and the state of the art of the technology and its applications in various industries is presented. In particular, the paper reviews recent research and developments on various aspects of CO2 systems including cycle modifications, exergy analysis of the systems, system modelling, transcritical operation consideration and various existing and potential applications.
1. Introduction
The refrigeration and air conditioning (RAC) industry is an inseparable part of economic activities. The industry provides services such as food and medicine preservation, climate control for human comfort, data centres and agricultural production, and any other services that require temperature and humidity control. It is therefore no surprise that this technology segment is amongst the highest electricity consuming sectors. In Australia, RAC electrical energy consumption represented 23.6% of the total electricity production of 258,000 GWh in 2016 [1] with consequent indirect greenhouse gas emissions. Carbon dioxide, also known as R744, is a refrigerant that has recently surged in attractiveness in the refrigeration industry after being overlooked for a relatively long time [2,3,4,5]. The resurgence of popularity of this refrigerant comes after it was realised that most synthetic refrigerants pose dire threats to the environment. The global push to phase out various synthetic refrigerants through international treaties has triggered industries and researchers alike to find more environmentally friendly substitutes and developed the corresponding systems. One such substance is carbon dioxide, CO2, discovered by Dr James Black who called the substance “fixed air” [3]. In terms of global warming potential (GWP) and ozone depletion potential (ODP), CO2 is one of the most environmentally friendly natural refrigerants. It has a GWP of 1 and zero ODP [1]. Another cited reason for adopting the use of CO2 refrigerant—at least in the supermarket and food retailer sector—is “the rising cost of synthetic refrigerants. In the last five years alone, the cost of HFC-404A, a commonly used synthetic refrigerant, has risen substantially—raising not only the cost of initial installation, but also operation and maintenance.” [6]. According to this NCI report, CO2 was priced at around USD 2.2/kg whilst the HFC-blends (R404A and R407A) cost around USD 8.8/kg [6]. Other cited benefits for the use of CO2 as a refrigerant are: (a) reduced size of compressor and piping due to its high volumetric capacity, (b) higher compression isentropic efficiency due to lower compression ratio, and (c) low risk for decomposition within the system because of its stable molecule [7].
The use of CO2 as the primary refrigerant poses a challenge in terms of the system performance due to the potential of the system operating in a supercritical state. As such, heat rejection capacity is degraded, reducing the coefficient of performance (COP) of cooling. Although the critical temperature of the CO2 gas is relatively low, a system utilising CO2 as the refrigerant must operate at very high pressures [8]. Therefore, the CO2 system components, piping and equipment need to be designed to conform to the high pressure requirements [7].
The revival of the CO2 systems has only started recently. For instance, in the US, the first system using this technology was introduced in 2013 [6]. In Australia, Coles supermarket recently installed a CO2 system in Coburg North, Melbourne, claimed to be a world-first CO2 transcritical system combining 100% of the store air conditioning and refrigeration requirements in the one plant design [9].
The historical background of the rise, fall and the re-emergence of CO2 refrigerant in various applications has been well covered by many researchers with relatively detailed accounts reported [2,3,5]. In short, the rise and fall of the R744 refrigeration industry are attributed mainly to the competing technologies, technological advancement in the field and the people’s awareness of and attitude towards environmental issues. Modern industries and other sectors relying on refrigeration processes and researchers alike are challenged to search for an ideal refrigerant that is technically reliable, economically attractive and environmentally friendly. In engineering terms, these are translated into the following ideal refrigerant criteria: it (the refrigerant) “should have good thermophysical properties, higher than ambient condensing temperature, low freezing temperature, high critical temperature, low critical pressure, good heat transfer properties, no ODP or GWP, should be non-toxic, non-flammable, non-corrosive, non-explosive, should have low system cost and should be easily obtainable.” [8]. In terms of the environmental impacts and safety, R744 is among the best natural refrigerants. R744 has zero ODP, its GWP is unity, it is non-toxic, inflammable and non-explosive [5].
The phase down and phase out of ozone depletion causing substances through the Montreal Protocol has narrowed refrigerant options for refrigeration industries to a handful, including ammonia and hydrocarbons. However, these two options have serious drawbacks including toxicity (ammonia) and flammability (both substances) and can be considered only for very specific circumstances [10].
It has been nearly 15 years since a first comprehensive treatment on the subject was published [2] and since then there has been progress in many aspects of the system and technology including in the system performance improvement, cycle analysis, design, applications and other aspects. A new comprehensive review therefore is needed to cover the recent advances in the field and to present all necessary information useful to various audience groups. To the readers who are new to CO2 refrigeration, this review paper covers the necessary information to familiarise them with this topic. To the readers who currently work or are involved in the research on the topic, this review paper can be employed as a quick reference of the state of the art of current CO2 refrigeration systems. Finally, to the readers who are interested in the potential applications of the system in various sectors, the paper provides considerable information from various industries.
This paper focusses on refrigeration and heat pump cycles for CO2 systems; recent developments in CO2 power cycles technology and applications can be found in other review papers, some of which were published relatively recently [11,12,13]. A review specifically for CO2 refrigeration subcooling can be found in [14].
The paper is structured in such a way that it provides a natural flow of knowledge/information on the subject, starting from the basic thermodynamic overview of CO2 refrigeration/heat pump basic cycles in Section 2. This section lays the foundation for understanding the basic CO2 cycles and its uniqueness compared to cycles using other refrigerants. Despite the low (practically zero) direct impact of CO2 refrigerant on the environment as discussed earlier, the indirect impact from low system efficiency can outweigh the former. Therefore, it is imperative to identify the sources of inefficiency through an exergy analysis. This is discussed in Section 3. Once the sources of inefficiency are identified, several ways to address each of them through cycle modifications is discussed in Section 4. This is followed by a short presentation in Section 5 on the several basic configurations of CO2-based refrigeration systems. For design, research and optimisation purposes, system modelling and various modelling tools are presented in Section 6. Transcritical operation of CO2 systems is the one that distinguishes them from systems using other refrigerants; it is also the main source of inefficiency and where potential improvements can be made. This is presented in Section 7 accompanied by a brief discussion on cooling techniques and control mechanisms being investigated to optimise the system performance during transcritical operation. Section 8 discusses applications of CO2 systems and provides brief information on market acceptance and penetration. Section 9 briefly discusses the proposed future direction of research on transcritical systems operating in warm and hot climates. The paper concludes with a brief summary of all topics covered in the paper.
2. CO2 Thermodynamic Basic Cycles Overview
Like systems using other refrigerants, the refrigeration process using CO2 can be depicted using various thermodynamic diagrams such as a T-s, p-h or T-p diagram. Using these diagrams, one will be able to identify various processes and conditions undergone by the refrigerant and to develop a thermodynamically sound approach to make the system perform efficiently and effectively.
A simple CO2 refrigeration system working in the subcritical region is shown in Figure 1. As shown, the process starts at point 1 where low pressure CO2 refrigerant vapour is pressurised in a compressor to a pressure p2. The high pressure vapour at p2 then passes through a condenser where its enthalpy is reduced, crosses the saturated vapour line and appears in the form of a mixture of liquid and vapour. Depending on the condensation process (segment 2′-3), the CO2 refrigerant can stay as a mixture (under the saturation curve) or be totally liquefied or subcooled (i.e., it crosses the saturated liquid line to the left) before it enters the evaporator at point 4 through an expansion valve.
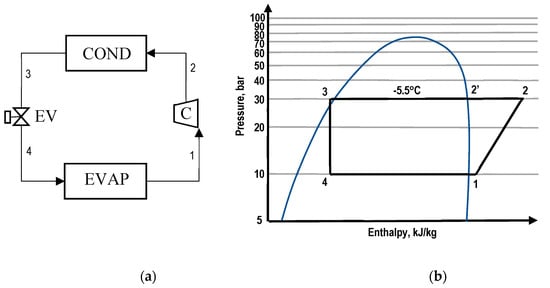
Figure 1.
(a) Schematic, and (b) p-h diagram for a subcritical process of a CO2 refrigeration system.
Transcritical operation occurs beyond the critical point of CO2, which is 73.8 bar and 31.1 °C. In the supercritical state (i.e., above the critical point where liquid and gas phases are indistinguishable), the heat accumulated in the condenser cannot be removed through condensation of the CO2 gas. Instead, the CO2 gas is cooled by a gas cooler at a constant pressure but at slowly reducing (gliding) gas temperatures, as shown in Figure 2.

Figure 2.
A p-h diagram of a transcritical process of a CO2 refrigeration system.
Since the cooling capacity is severely limited without condensation, the performance of the system running in a supercritical state is much degraded. In this state, the system COP is very much dependent on the gas cooler pressure and can vary greatly [15].
Figure 3 shows a CO2 system running at three transcritical pressure levels at the same evaporating temperature. From the diagram it is clear that the COP of the system operating on three different cycles (1-2-3-4-1, 1-2a-3a-4a-1, 1-2b-3b-4b-1) does not increase linearly with a rise in gas pressure. The “s-curve” shape of isotherms in the transcritical region—where states 3, 3a and 3b lie—gives rise to this phenomenon.

Figure 3.
A CO2 system running at three subcritical pressures at the same evaporating temperature.
In other words, for a constant CO2 gas exit temperature, there is an optimum value of gas pressure that maximises the COP of the system operating at the same evaporating temperature [15,16].
The impact of gas cooler pressure on the COP is shown in a plot similar to the one generated by Sawalha [17] (Figure 4). As seen, for a given gas cooler exit temperature, the pressure range from the optimum point to the left initially plateaus, where the span of plateau is narrower as the gas cooler exit temperature decreases. After this range further to the left, the change in discharge pressure is more sensitive to COP degradation than the one on the right side of the optimum point. This sensitivity is also more pronounced at the lower exit temperature. For example, a system operating in supercritical state at 80.5 bar at gas exit temperature of 32 °C has a COP of 2.4. However, if the system operates at 75 bar at the same gas exit temperature, the system COP will drop to around 1.7.

Figure 4.
Effect of gas cooler pressure on COP at various gas exit temperature TGex.
For a given approach temperature difference (ATD), the optimum discharge pressure, Popt, is a function of the ambient temperature, Tamb, and the approach temperature difference in the gas cooler, ΔTgc,app. For an ATD of 5 K, the following correlation was produced [17]:
Popt = 2.7 × (Tamb +ΔTgc,app) − 6.1
A generalised correlation for the optimum pressure is given in [18], claimed to be valid by the authors for the evaporation temperature range from −50 °C to −30 °C and gas cooler exit temperature range from 30 °C to 50 °C.
3. Exergy Analysis of CO2 Systems
While various modelling tools are available to study the performance characteristics of a refrigeration system (see Section 6), it is always fundamental to understand the inherent losses and maximum potential energy that can be extracted from such a system. The traditional energy analysis based on the first law of thermodynamics is a tool to assess how efficient a system utilises a form of energy. For instance, in a refrigeration system, the COP can be evaluated based on the ratio of energy output and energy input. In this case, the energy output is the refrigeration effect or cooling capacity needed from the system to serve a certain load. Once the required cooling capacity is determined, the energy input (i.e., electrical input to run the compressor) is evaluated based on technical specifications of all the components involved. Thus, the use of a very efficient compressor may not necessarily result in high overall system performance if other components such as the condenser or the evaporator are not equally efficient. The traditional first law analysis records all the heat and work transfers but cannot identify the sources and magnitude of energy degradation during its utilisation. To derive the maximum losses and the maximum potential energy extractable, the exergy analysis can be employed. The importance of the exergy analysis lies in the fact that exergy represents the maximum work that a work producing system can deliver [19], or the minimum work that a work consuming system requires [20] thermodynamically. In addition, exergy balance can be regarded as the mathematical statement governing the energy degradation [19]. In other words, exergy analysis is a tool for sustainability. While refrigeration systems do not produce power, the exergy analysis helps identify opportunities for minimising the system power consumption. Furthermore, an exergy analysis is an effective tool to easily identify the source(s) of exergy destruction [21].
In this review, the general formula for the exergy of a system is presented as well as the exergy destruction that occurs in each of the system components. The detailed derivation of the exergy equations can be found in engineering thermodynamics textbooks such as Moran et al. [22], Bejan [23] and Çengel and Boles [20] and books dedicated to exergy methods such as Kotas [19].
The exergy balance of the system can be developed by considering the three types of energy transfer across the system boundary, namely: work transfer, heat transfer and energy transfer associated with mass transfer [19]. In addition, for an irreversible process, the irreversibility term must also be included in the balance. Equation (2) shows an exergy balance for a control volume [19,21,22,23]:
For the system under discussion, the kinetic and potential energies can be neglected; hence the specific steady-flow exergy x in Equation (2) can be calculated as:
Expressed in a simpler form, Equation (2) can be written as:
where and represents the exergy flow rate entering and leaving the control volume, respectively.
Application of the exergy balance of Equation (2) to each of the system components/processes leads to the formulation of irreversibility or exergy losses/destruction of each component. In deriving the exergy balance and exergy destruction of each of the components, the order of process as used in Figure 1 and Figure 2 are adopted, i.e., compression (1–2), condensation or gas cooling (2–3), expansion (3–4) and evaporation (4–1). Also, it is assumed that (1) any process occurs in steady state mode, (2) losses in the piping connecting one component to another are ignored, and (3) each process is adiabatic. The diagrams involved were largely adopted from [24] with Figure 9a introduced to depict the exergy losses when the system operates in subcritical state, a state not covered in Figure 9b by Cavallini [25].
3.1. Exergy Balance of Compression
The exergy balance of a compression process (Process 1–2 of Figure 5) takes into account the exergy transfers associated with mass and , work input and irreversibility, :
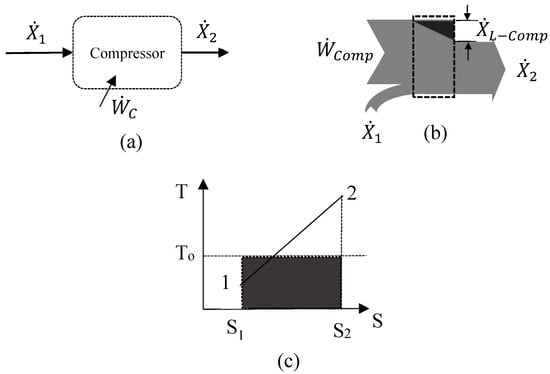
Figure 5.
Exergy balance in compressor. (a) control volume, (b) Grassman diagram, (c) irreversibility on T-s Diagram.
The irreversibility in the compression process, , can be expressed as:
Equation (6) represents adiabatic losses during the compression.
3.2. Exergy Balance of Condensation (Subcritical Operation) and Gas Cooler (Supercritical Operation)
The exergy balance during the condensation and gas cooling (process 2–3 of Figure 6) takes the form:

Figure 6.
Exergy balance in condenser and gas cooler control volume, (a) control volume, (b) Grassman diagram, (c) irreversibility on T-s Diagram.
The exergy transferred to the environment as heat, , is as per the exergy definition zero.
The irreversibility, IRcon, can be calculated from:
While the exergy loss formulation for condenser and gas cooler is identical, for the same evaporator temperature and suction pressure (Figure 3), the system operating in a supercritical mode will suffer much higher losses than that running in a subcritical mode. From Equation (8), additional exergy loss of the system running in supercritical mode can be estimated as:
Any effort to improve the system performance, i.e., minimising the , needs to ensure that the work introduced is less than the saved/recovered exergy. Therefore:
The traditional exergy balance and exergy losses formulation for a condenser/gas cooler is as shown in Equations (7) and (8). The two equations are worth modifying to take into account the impact of heat rejection rate brought about by the cooling medium (air in this case) with the input power required to operate the fan. From the exergy standpoint, the condenser is the reverse of an evaporator; in the condenser the heat is rejected by the condenser whilst the exergy due to cooling medium is flowing into the control volume. Hence, Equation (7) can be modified as follows:
whilst Equation (8) becomes:
3.3. Exergy Balance of Expansion (Throttling)

Figure 7.
Exergy balance during expansion: (a) control volume, (b) Grassman diagram, (c) irreversibility on T-s Diagram.
3.4. Exergy Balance of Evaporation
The exergy balance for evaporation (process 4–1, Figure 8) can be expressed as:

Figure 8.
Exergy balance during evaporation: (a) control volume, (b) Grassman diagram, (c) irreversibility on T-s Diagram.
The exergy transferred to the space, , also called the thermal exergy flow, can be expressed as:
The term in Equation (16) can be interpreted as the minimum work required to maintain the given cooling rate.
Combining Equations (15) and (16) gives the following exergy loss during the evaporation:
Equation (17) can be simplified further to take the form:
The exergy loss during evaporation is shown graphically as a dark-colored area in Figure 8c which can be interpreted from Equation (16) as the difference between the exergy transferred to the load (area 1-b-d-4-1) and the exergy received by the load at load temperature TL (area a-b-c-e-a).
The various exergy losses from all the components detailed above are listed in Table 1 and presented in graphical form in Figure 9 for both subcritical and supercritical operations. As shown, for the same evaporating temperature, the system running in supercritical mode (Figure 9b) will suffer larger exergy losses during the gas cooling process than that operating in a subcritical mode.

Table 1.
Exergy Losses in CO2 Refrigeration System.

Figure 9.
Exergy losses in all the system components (a) subcritical operation, (b) supercritical operation.
The exergy losses occurring during transcritical operation increase the work input from the minimum theoretical requirement to attain the same cooling effect [24]. This is true also for the subcritical operation.
Using the exergy balance derivation by Kotas [19], the exergetic efficiency formulation for a CO2 refrigeration system can be expressed as follows [24]:
The above equation can be slightly modified to include the power required to operate the condenser fan(s) for the case of the air-cooler condenser or gas cooler. This is particularly relevant when the improvement in the system performance is to be realised through the introduction of a cooling system which requires additional fan(s), such as the dew point cooler [26]. In that case, Equation (19) can be modified to become:
It is worth emphasizing that due to inherent exergy destruction or losses, attainment of the optimum (i.e., 100%) exergetic efficiency is not practicable and should not be the main objective of any design improvement.
Findings by Yumrutaş et al. [27] suggest that exergy destruction in the evaporator and condenser are significantly affected by evaporating and condensing temperatures. These temperatures, however, do not affect the exergy destruction in the compressor and the expansion valve. In a study using exergy analysis [28] it was found that the exergy loss in the throttling valve represents more than one third of the irreversibility of the cycle. The same study found that gas cooling and compression were the main sources of exergy destruction for the system with expander.
When comparing single stage systems of R290, R404A and R744, Shilliday et al. [29] observed that a single stage CO2 system was found to exhibit the highest total cycle exergy destruction ratio, i.e., ratio of exergy destroyed per unit of work input. The system also exhibits the greatest increase in that ratio with increased condensing and evaporating temperatures.
The various studies cited above have been directed to either (1) study the impact of various parameters (such as evaporating or gas cooling temperature) on the exergy losses, or (2) comparing the exergy losses of the transcritical CO2 systems with that of traditional refrigeration systems. Another direction of study which has not been attempted so far is the comparison of a CO2 system operating in subcritical mode against that operating in supercritical state.
A case study by Cavallini [24] compares the exergy losses of a single stage CO2 refrigeration system and an R22 system. Both systems operate at an ambient temperature of 28 °C, an evaporating temperature of −10 °C, compression isentropic efficiency of 80%, with the degree of superheat at compressor inlet being 0 °C. The CO2 system operates in the supercritical state with a gas cooler pressure of 78 bar and an outlet gas cooler temperature of 31 °C (i.e., a temperature approach of 3 K). The study found that the greatest exergy loss of 16.6 kJ/kg occurred at the expansion process, followed by compression (9.6 kJ/kg), gas cooling (9.5 kJ/kg) and evaporation (3.1 kJ/kg). The same study reveals that the exergetic efficiency of the transcritical system is about 17% less than that of the R22 system (0.31/0.34) implying slightly greater room for exergetic improvement for the CO2 system. The total efficiency defect of 0.69 (1–0.31) spreads across the various components as follows: compressor: 0.171, gas cooler: 0.170, throttling (0.294) and evaporator (0.055). For the R22 system, with total efficiency defect of 0.374, the defect share of each component is as follows: compressor (0.173), condenser (0.224), throttling (0.161) and evaporation (0.068).
Extending Cavallini’s simple case study on CO2 systems to subcritical operation enables one to: (1) assess the extent of the exergy loss magnitude due to supercritical operation compared to subcritical operation, which in turn (2) demonstrates the impact of minimising or totally eliminating the supercritical operation. If the exergy defects created by supercritical operation cannot be ignored, then minimising or totally eliminating the supercritical operation is an effort worth considering. The performance of the CO2 system with such capability has recently be reported [26]. For this exercise, the Cavallini’s case study is slightly modified for subcritical operation where the ambient temperature is changed from 28 °C to a value below 20 °C which enables the system to operate subcritical (i.e., the CO2 gas condenses within the condenser). The selection of 20 °C temperature as a maximum temperature at which the system can operate in the subcritical state is based on an ATD of 8 K [26]. In such a case, the condenser air inlet temperature can now be much lower than the ambient temperature, a situation which can be envisaged during summer months. This implies that an additional air precooling unit exists that enables the high temperature ambient air to be cooled down to 20 °C or less. The DPCO2 system [26] can serve that role in warm to hot climatic zones with not too high humidity. It is also assumed that the CO2 refrigerant leaving the condenser is saturated liquid. Another case for the supercritical operation is also added in the case study that represents hotter weather which is typical for summer in hot climates. It involves a system operating at ambient temperature of 35 °C with an approach temperature of 3 K resulting in the gas cooler outlet temperature of 38 °C (instead of 31 °C in the existing case study). For the cases where the system operates in a subcritical state, the ambient temperature is assumed 28 °C.
Table 2 presents a summary of the exergetic performances of a CO2 system operating in supercritical and subcritical states. For the subcritical case, condenser air inlet temperatures of 18, 19 and 20 °C were chosen, whilst for supercritical operation, gas cooler exit temperatures of 31 °C and 28 °C (from Cavallini’s case study [24]) were chosen with the ATD value and other conditions adopted from the case study.

Table 2.
Comparison of Exergetic Performance of a CO2 Refrigeration system operating at both supercritical and subcritical states.
As seen, the performance of the system operating in subcritical mode is significantly better than that operating in supercritical mode. As expected, as the condensing temperature is reduced, the refrigeration effect increases for the same evaporating temperature. Observing the values of exergy destruction of each component/process, one can conclude that the largest destruction occurs during expansion, which is in agreement with previous findings [25,27]. However, a close observation also reveals that the exergy destruction during supercritical operation is very much dictated by the ambient temperature (which in turn affects the gas cooler exit temperature for a fixed ATD). On the other hand, for the system running in subcritical state the change in condensing temperature will marginally affect the exergy destruction during expansion.
The impact of bringing down the temperature of air entering the condenser to 20 °C or lower is clearly demonstrated through the figures in Table 2. For instance, the COP of the system operating in supercritical state at ambient temperature of 35 °C is 1.83. The system operating in subcritical mode with condensing temperature of 28 °C (i.e., with condenser air inlet temperature of 20 °C) is 2.91. This is a COP improvement of 59%. The system operating at a condensing temperature of 27 °C avoids 32.7% of exergy losses compared to the same system operating at supercritical state with an ambient temperature of 35 °C.
4. Basic Cycle Modifications
The single stage cycles presented in Section 2 have two main limitations: (a) low system COP due to its low critical temperature, and (b) limited capacity. The exergy analysis discussed in the previous section shows the opportunities for system performance improvements through modifications of the existing basic cycles. Cycle modifications of this basic system is normally carried out to serve the following purposes: (1) to improve the system performance, (2) to enable the system to cope with or meet the load conditions or requirements.
4.1. Internal Heat Exchanger
An internal heat exchanger (IHX) is a heat exchanger introduced to utilise the heat available from the outlet of the CO2 gas cooler to superheat the gas in the compressor suction line, Figure 10. The IHX increases subcooling at the entrance of the expansion device and decreases the amount of flash gas production. The p-h diagram of the CO2 refrigeration system with enthalpy loss and enthalpy gain in an IHX is shown in Figure 11.

Figure 10.
Schematic of a counter flow double pipe IHX.

Figure 11.
A p-h diagram of a CO2 refrigeration system showing enthalpy loss and gain on both sides of an internal heat exchanger. Processes: 1–2: compression, 2–3: cooling in gas cooler, 3–4: gas cooling in IHX, 4–5: expansion, 5–6: expansion, 6–1: superheating in IHX.
The installation of an IHX is normally aimed to increase the system cooling capacity and improve the COP. The IHX typically increases the system efficiency [8]. However, the benefit of installing an IHX to improve the system performance is affected by the working fluid and the system operating conditions [15]. According to experimental results conducted on a transcritical CO2 mobile air conditioning system, the installation of an IHX improved the cycle performance by up to 25% [30]. An increase in the degree of sub-cooling due to an IHX increases the refrigerating effect; however, this also results in an increase of compressor work [31].
A numerical study of a transcritical CO2 heat pump system [32] reveals that the impact of the IHX on the system performance and discharge pressure was found to be insignificant for a system running low and medium temperature CO2 refrigerant at the outlet of the gas cooler.
Through numerical modelling, Ituna-Yudonago et al. [33] investigated the transient behaviour of the CO2 flowing in an IHX similar to that shown in Figure 10. The research found that the CO2 inlet temperature and mass flow rate affect the CO2 thermophysical properties. The transient behaviour of the IHX was also found to have more influence on the supercritical CO2 gas coming from the gas cooler. The system COP was found to be inversely proportional to the effectiveness of the IHX and highly influenced by the inlet temperature of the CO2 transcritical gas.
The numerical simulation work by [34] which was supported by experimental validation investigated the dependence of the COP of a CO2 system running in transcritical mode on the length of the IHX at varying gas cooler pressure. The COP of a system with a 2 m length IHX was found to increase by about 23% compared to that without an IHX at an ambient temperature of 35 °C. A further increase in IHX length, however, resulted in an insignificant increase in COP. The COP of the same system (i.e., with 2 m IHX) exposed to a higher ambient temperature of 43 °C increases by 35% compared to that without an IHX. This implies that the higher the ambient temperature, the higher the potential of the COP improvement using the IHX. This study also indicates that based on the CO2 thermophysical properties, the improvement in the COP is realised through the increase of cooling capacity which is larger than the increase in power consumption.
The impact of the IHX on the system COP and optimum discharge pressure was also noticed by Sarkar et al. [15]. According to this study, a cooler exit temperature affects the improvement in the COP and the optimum discharge pressure. At a cooler temperature of 30 °C, the COP improves by merely 1% and the optimum discharge pressure decreases only by 2%. However, when the cooler exit temperature is raised to 60 °C, the COP improves by 15% and the optimum discharge pressure decreases by 13%. In studying the system, the authors set the evaporator temperature to 0 °C and assumed the compressor isentropic efficiency to be 70%. They concluded that the use of the IHX can be beneficial if the gas cooler outlet temperature is high.
Findings from a handful of the previous investigations mentioned above suggest that a generalised statement about the impact of the IHX on the system performance cannot be made as it depends of various influencing factors such as working fluid and operating conditions. An earlier theoretical study of Domanski et al. [35] summarises the factors that dictates the benefits of an IHX as “a combination of operating conditions and fluid properties—heat capacity, latent heat, and coefficient of thermal expansion with heat capacity being the most influential property”. This study further unveils that if the refrigerant used already gives a good performance of the basic system then the impact of the IHX is marginal or even “negative”. The assumptions made in their study includes isentropic compression, no-pressure-drop infinite heat exchangers, and no-pressure-drop liquid line/suction line heat exchanger.
4.2. Mechanical Expander
A significant loss due to expansion [28,36,37,38] is one of the main drawbacks of the CO2 cycle. Throttling is an irreversible process which imposes a double penalty, i.e., decrease in refrigerating effect and increase in work input to the compression [39]. A mechanical expander can be introduced to minimise the throttling loss suffered by a transcritical CO2 system and recover this loss for mechanical power generation. Thermodynamically, introduction of a work-generating component, in this case an expander, to the system will improve the system performance by transforming the isenthalphic process to the one closer to isentropic process. A p-h diagram of a CO2 refrigeration system with (and without) a mechanical expander is shown in Figure 12. As shown, the replacement of an expansion valve with a mechanical expander results in an expansion process closer to an isentropic process and therefore increases the cooling effect.

Figure 12.
A p-h diagram of a CO2 system with a mechanical expander. Processes: 1–2: compression, 2–3: gas cooling, 3–4: expansion without mechanical expander, 3–4: expansion with mechanical expander.
Theoretical investigations [24,36,40] present an interesting insight on the introduction of the expander to replace the expansion valve. According to [24,36], an expander can increase the COP of a basic CO2 system due to reduced exergy loss during expansion. However, the benefit of introducing an expander will be cancelled out, in fact it results in reduced COP, by an IHX (Section 4.1) added to the system. This is because an IHX, which cools the fluid leaving the gas cooler, reduces the potential work that would have been extracted by the expander. A similar effect was observed by Huff and Radermacher [40]. Due to the IHX’s role in protecting the compressor from flooding, the installation of this component cannot simply be overlooked.
The work recovered from the expansion loss is normally utilised to drive the compressor resulting in increased compressor efficiency. However, this normally involves a high initial cost and resolving technical issues related to the recovery [31]. The types of energy recovery expanders that have been proposed/employed for CO2 systems include: piston expander, vane expander, scroll expander, screw expander and turbo expander [39,41,42].
In many systems, the compressor and the expander are highly integrated and share the same drive shaft. This can restrict the volumetric flow rate to the volume displacement rate for the case of a positive-displacement-type compressor and expander. Conduction heat loss through the condenser-expander housing can also reduce the recoverable work. The cost of expanders which is comparable to that of compressors is another factor hindering their applications in refrigeration and HVAC systems—for smaller systems in particular [39].
Another type of expansion device is the ejector, Figure 13. An ejector utilises momentum transfer between two streams of fluid, i.e., the primary (motive) high pressure fluid and the suction (secondary) fluid. The primary fluid flows through the primary nozzle converting its pressure energy to kinetic energy drawing in the secondary fluid where both fluids mix in the mixing chamber. In the diffuser section the mixed fluid gains back their pressure energy in the form of static pressure in the diffuser section.

Figure 13.
Schematic of an ejector.
During supercritical operation of a CO2 refrigeration system, the high pressure CO2 gas exiting the gas cooler is employed to increase the pressure of the refrigerant at the exit section of the evaporator which in turn increases the compressor suction pressure and reduces the compressor power input, Figure 14.

Figure 14.
Schematic and p-h diagrams of a CO2 refrigeration system with ejector during transcritical operation. (a) system schematic, (b) p-h diagram.
A derivation of thermo-fluid relationships governing the operation of an ejector is given in [43]. Liu and Groll [44] carried out a study and developed a method for calculating the efficiencies of ejector internal components (i.e., motive nozzle, suction nozzle and mixing zone). They found that these efficiencies are influenced by geometry and operating conditions as opposed to constant efficiencies assumed in previous studies (see e.g., [45,46,47]).
The various constraints of a single ejector system can be overcome by the multi-ejector concept discussed by Hafner et al. [48] and later developed by Banasiak et al. [49], experimentally analysed by Haida et al., [50] and numerically studied by Body et al. [51]. The multi-ejector system discussed in these studies consists of a pack of ejectors placed in parallel as shown in Figure 15. In such an arrangement, the ejectors substitute the role of the expansion valves. The three vapour ejectors E1, E2 and E3 have different mass flow rates, designed in such a way that the mass flow rate through E3 is twice that which flows through E2 and four times that which flows through E1.

Figure 15.
Sketch of a multi-ejector system [51].
From an experimental analysis [50], it was found that the system COP improved by 7% and the exergy efficiency by 13.7% compared to that of the parallel compression system. It was also observed that the compression efficiency is affected by the cooling load and heat rejection. Danfoss [52], which is supported by SINTEF Energy research [51], has developed various multi-[vapor and liquid] ejector products which it claims to improve the performance of CO2 systems.
4.3. Compression Staging
The concept of two or multi-stage compression aims to reduce the work input to the compressor. This is realised through intercooling, flash gas intercooling or flash gas bypass. In the two-stage compression with intercooling, Figure 16, an external fluid cools the CO2 refrigerant coming from the low pressure compressor (LP) as it passes through the intercooler. This cooled CO2 gas is compressed and heated in the high pressure compressor reaching the supercritical state at 4. The gas is then cooled in the gas cooler to state 5 and its pressure is reduced to state 6 before it enters the evaporator. To ensure the gas enters the suction line of the low pressure compressor in the dry state, the gas exiting the evaporator is usually superheated by absorbing some heat from the outlet of the gas cooler through an IHX (see Section 4.1).

Figure 16.
Two-stage compression with intercooling. (a) system schematic, (b) p-h diagram.
In the two-stage compression with flash gas intercooling, Figure 17, he gas entering the high pressure compressor is cooled down (de-superheated) by the gas flashing from the expansion valve located at the outlet of the gas cooler. This results in reduced work input to the compressor. Introducing two-stage compression with an intercooler, Shilliday et al. [29] observed an increased performance (COP) of the CO2 system by up to 12% compared with the single stage system.

Figure 17.
Two-stage compression with flash gas intercooling. (a) system schematic, (b) p-h diagram.
Figure 18 hows the two-stage compression cycle utilising flash gas bypass. In this configuration, the saturated vapour coming from the evaporator at 1 is compressed to state 2 by the low pressure compressor and mixed with saturated vapour 3 from the flash chamber to state 4. This scheme increases the refrigerating effect and reduces the work input to the compressor [31].

Figure 18.
Two-stage compression with flash gas bypass. (a): Schematic, and (b): p-h diagram.
In optimising two-stage CO2 heat pump cycles, Agrawal et al. [18] found that for the two-stage system, in addition to the gas cooler pressure, the intermediate pressure also has an impact on the value of system maximum COP.
4.4. Parallel Compression
In parallel compression, Figure 19, the gas exiting the gas cooler expands through the expansion valve EV1 (1 → 2) and enters the vapor-liquid separator. The liquid portion is further expanded through EV2 valve (3 → 4) before entering the evaporator. The gas portion leaving the separator enter compressor C1 and at point 9 meets with the gas leaving compressor 2.

Figure 19.
Parallel compression of a CO2 refrigeration cycle. (a) system schematic; (b) p-h diagram.
In a numerical study carried out by Sarkar and Agrawal [53], it was found that parallel compression has more impact when the system operates at lower evaporating temperatures. In this study they also developed the equation for an optimum discharge pressure as a function of evaporating and gas cooler exit temperature for a system involving parallel compression. In addition to an improved optimum COP of up to 47%, parallel compression also reduces the optimum discharge pressure. The study was performed with the evaporator temperature range of −45 °C to 5 °C and the gas cooler exit temperature range from 30 °C up to 60 °C.
For moderate and warm climates, parallel compression elevates the CO2 system status to be the most efficient system among systems being investigated (cascade, conventional booster, cascade with gas bypass, and booster system with parallel compression). Performance improvements of 5% and 3.6% for warmer climate (Athens, Greece) and moderate climate (London, UK), respectively, were found compared to the baseline conventional CO2 booster system [54].
The impact of parallel compression on the performance of a commercial CO2 booster system (see Section 5.1) was carried out by Gullo et al. [55] employing the exergy analysis. The analysis indicated that the highest irreversibilities were found in the gas cooler/condenser, the high stage compressor and the medium temperature (MT) display cabinet. In the study, the outdoor temperature range of 25 °C to 35 °C was considered.
An important issue with parallel compression is that it only compresses the bypassed flash gas which delivers a minor increase in capacity and a small COP improvement at high ambient temperatures.
4.5. Summary of Cycle Modification Improvement Outcomes
The summary of the outcomes of the cycle modification improvement attempts discussed above is presented in Table 3. It should be noted that the summary avoids quoting the values of improvement made from particular case studies carried out with specific conditions as discussed in previous sub-sections. This is to avoid unintended consequences of generalisation of such specific findings.

Table 3.
Summary of Cycle Modification Improvement Outcomes.
5. Several Configurations of CO2-based Refrigeration System
5.1. CO2 Booster System
A booster system can consist of two or more pressure (temperature) sections served by two or more compressors. A schematic of a CO2 booster system with low temperature and medium temperature loads is shown in Figure 20 with a corresponding p-h diagram. The medium temperature compressor (MT-C) elevates the CO2 gas pressure from state 1 to 2 before the gas enters the condenser/gas cooler. During the summer periods, when the ambient temperature is high, the CO2 in the condenser can reach the critical point (temperature) of 31 °C, above which, the gas in the condenser/gas cooler will stay as supercritical fluid as it passes through this component. Below this point, the gas will condense before it passes the high-pressure control valve between points 3 and 4. In the liquid receiver (LR), CO2 gas is sent through the by-pass valve and returns back to the MT-C together with the gas from the LT load and MT load which meet at 8. The liquid at state 5 is split into branches, one enters the MT load/evaporator through the MT expansion valve (MTEV) (states 5–6–7 in Figure 16) and the other enters the LT Load through the LT expansion valve (LTEV) (states 5–8–9). The gas from both branches meets again at state 10 after the one that enters the LT load is pressurised by the LT compressor (9–10). According to [10], the design pressure for the high temperature section is typically 90–120 bar, 40 bar for the MT section and 25 bar for the LT section.

Figure 20.
(a) Schematic of a CO2 booster system: BPV—by-pass valve; LR = liquid receiver; HP-VC: high pressure control valve; MTEV—medium temperature expansion valve; LTEV—low temperature expansion valve, MT-C—medium temperature compressor; LT-C—low temperature compressor., (b) p-h diagram.
The following set of steady state thermodynamic equations governs the operation of the system and is normally used to design and assess the system performance.
The refrigeration effect in the LT evaporator which is equal to the amount of heat absorbed by the refrigerant from the refrigerated space, Q8–9, is given by:
The power required by the compressor to elevate the pressure and temperature of the refrigerant from state 9 to state 10 is:
The refrigeration effect in the MT evaporator can be calculated as follows:
The refrigerant from both evaporators meet at point 11
and - together with the refrigerant coming through the bypass-valve, will enter the MT compressor:
to be pressurised to state 2.
The COP of both the LT loop and MT loop and the total system can be calculated as follows:
LT loop COP:
MT loop COP:
System COP:
Heat rejected in the condenser/gas cooler:
When the ambient temperature becomes higher forcing the system to reject heat beyond the critical point, it follows that the value of each of the above COPs will be lower when the evaporating temperatures of both the LT and MT loops are kept constant.
Such a system was modelled in [56] for supervisory control in the smart grid and in [57] for a supermarket application. According to [57], booster systems for supermarkets normally have four pressure sections (high, intermediate, medium and low). The high-pressure section can operate in either subcritical or transcritical modes depending on the prevailing ambient conditions. The existence of two or more pressure sections makes it possible for a booster system to serve loads operating at difference temperature levels.
5.2. Cascade System
In a cascade system (Figure 21) the HT loop and the LT loop is connected by a cascade HX which acts as an evaporator for the HT loop and condenser for the LT loop. In a simple CO2 cascade system, the LT loop uses CO2 as the refrigerant and operates in the subcritical state. The HT loop—which uses HFC or ammonia as the refrigerant—absorbs heat (cooling load) from the cascade HX. This system is well suited for warm climates. Cascade systems are suitable for conditions where heat rejection temperature is very high while the cooling effect is needed at much lower temperature making the system difficult to deliver the cooling effect using a single refrigerant [58].

Figure 21.
Schematic of a CO2-HFC/NH3 cascade system.
In a study, Pattingale [59] summarised the findings of previous researchers and listed the benefits of using the CO2-NH3 cascade system over the two-stage NH3 refrigeration system as follows: (1) lower capital cost due to the smaller compressor size of the former, (2) constant positive pressure, (3) reduction in NH3 charge, where a charge below 4.5 tons is possible in many circumstances, and (4) design flexibility of the former to enable the NH3 loop hidden from the public area.
A theoretical study carried out by Dopazo et al. [60] points to the existence of a maximum system COP at an optimum condensing temperature of the LT loop. The same trend was observed when the degrees of subcooling and superheating are varied [58]. The work of Dopazo et al. [60] is worth paying special attention as it provides details on how to attain the highest COP of a CO2-NH3 cascade system. They observe that maximum COP is attained when the evaporating temperature (TE) is as high as possible.
5.3. Secondary System
In a CO2 secondary system, Figure 22, CO2 acts as a secondary fluid that serves the load and is cooled by the primary refrigerant—which is typically R134A or ammonia—through a cascade HX. CO2 circulates through the secondary loop by means of a pump and its cooling capacity is higher than other secondary fluids as it undergoes phase change as it passes through the load section. In this configuration, the use of CO2 as the secondary refrigerant is an attractive option as it has a high volumetric capacity which leads to reduced costs of piping and other components. The system operates at constant pressure avoiding pressure pulsation. Since CO2 is a nontoxic refrigerant, its use as a secondary fluid is favourable from the safety standpoint. There is also benefit in the form of low pump power due to the high capacity of latent heat. Using CO2 as the secondary refrigerant allows the use of a high-stage chiller with easily available components. On the downside, the efficiency of secondary systems is somewhat reduced due to additional heat exchange and temperature difference. Pumps needed for the secondary loop are not readily available and may be more expensive and pose technical challenges to the engineers [7].

Figure 22.
Schematic of a CO2 Secondary System.
Table 4 lists the main features, advantage and drawback of various configurations of the CO2 refrigeration systems discussed above.

Table 4.
Summary of main features, advantages and drawbacks of the various configurations of the CO2 refrigeration systems.
6. System Modelling Tools
The complexity of the system due to the unique characteristics of each component coupled with the unique characteristics of the CO2 thermophysical properties makes the system modelling an unavoidable tool in the design of the system for a given task or in evaluating the performance of existing systems. As the discussion in Section 4 has shown, the impact of modifying the cycle such as with the addition of a component into a system cannot be easily generalised for a main clear reason: such a modification does not always bring an improvement to the system performance without any negative consequence. After all, the system is a collection of interrelated components. System modelling can be used to thoroughly assess the impacts. A good system model can also reveal how a system performs and behaves at a particular set of inputs and therefore enable the designer to predict the system performance (COP), the economic performance (rate of return, payback period etc.) and operating characteristics including safety issues. As such, system modelling usually involves exposing the system to typical weather conditions given the system loads which are assumed constant or varied to some extent. The modelling needs to detail the behaviour of the CO2 refrigerant as it circulates around the system loop through various components and it undergoes various changes of states.
The equation of state of CO2 developed by Span and Wagner [61] is widely used and implemented in a number of software packages such as CoolProp [62] and REFPROP [62,63,64] for calculating CO2 properties at a given condition. CoolProp is an open-source thermophysical property library written in C++ that covers pure and pseudo-pure fluids. It covers more than 120 fluids and more than 40 incompressible fluids and humid air [65,66]. It also provides models for properties of mixture, incompressible fluids and psychrometric calculations. CoolProp routines can be accessed/called by other modelling tools such as TRNSYS [67]. REFPROP has the capabilities and modelling scopes similar to that of CoolProp but it is not open-source.
Some manufacturers of refrigeration and air conditioning such as Bitzer and Danfoss have developed software to assist in choosing the best configuration of a product for a particular application or given specifications [68,69]. Bitzer software [68] is as a design tool for refrigeration system products developed by Bitzer. It contains the database of Bitzer products from which the designer can chose various components to build a system. The component specifications and design conditions entered by the designer are used to simulate the system performance (cooling capacity, COP, power input, etc.). The software also produces polynomial coefficients that enables the calculation of cooling capacity, compressor power consumption and refrigerant mass flow rate at a given compressor operating frequency. These coefficients are applicable only to Bitzer products. The CoolSelector software [69] was developed by Danfoss as a design tool for its products. It contains the database of Danfoss products from which the designer can choose various components to build a system. The component specifications and design conditions entered by the designer are used to simulate the system performance (cooling capacity, COP, power input, etc.). The outputs are available in graphical forms as well as report file form. The log(p)-h diagram can be used to show the values of thermophysical properties of various refrigerant (including CO2) at given (p,h) point.
CoolPack, developed by the Technical University of Denmark (DTU), can be used to carry out cycle analysis, component sizing, energy analysis and optimization [70]. It contains a collection of simulation programs for designing, sizing, analysing and optimising refrigerant systems. The programs include: cycle analysis, design, evaluation, auxiliary and dynamic analysis, as well as the refrigeration utilities. CoolPack incorporates the Engineering Equation Solver (EES) as its calculating engine for cycle analysis, system dimensioning, component calculations etc. The cycle analysis is used to calculate the various quantities such as cooling capacity, COP, etc. from given inputs (i.e., evaporation temperature, degree of superheat, condensing temperature, subcooling, refrigeration capacity, isentropic efficiency etc.). For the CO2 systems, the following configurations can be analysed: one-stage cycles, two-stage cycles, combinations of one-stage cycles, transcritical one-stage cycles and transcritical two-stage cycles. In each of the available configurations, sub-configurations such as flooded evaporator, one-stage compressor, etc. are provided, depending on the system. The refrigeration utilities in the package are used to calculate refrigerant and secondary fluid properties, refrigerant plots and cycles and psychrometric charts. A software package called “Simple CO2 one stage Plant” developed by Department of Mechanical Engineering (MEK), Section of Thermal Energy (TES) at the Technical University of Denmark (DTU) [70] can be used to graphically visualise the effect of varying one system parameter (such as discharge pressure, quality of vapor entering the compressor, efficiency of IHX, evaporator temperature, and gas cooler outlet temperature) on the system performance. This package can help in interpreting research outcomes discussed in previous sections.
Modelica [71] is an object-oriented modelling language to model complex systems, including thermal systems. Modelica facilitates the inclusion of new libraries such as thermal systems library using the connector classes [72,73] to establish the communications between the components. Hu et al. [73] used Modelica to perform a real-time optimisation to minimise power consumption of an air-source transcritical CO2 heat pump water heating system. Using the free ThermoFluid library available in Modelica, Pfafferott and Schmitz [74] carried out modelling and transient simulations of CO2-refrigeration systems for integrated on-board cooling systems for future Airbus aircrafts. Models of components such as compressors are available in some of the above-mentioned software packages and in the TIL Library of TLK-Thermo GmbH [75] which also develops software capable of simulating thermal systems.
The Engineering Equation Solver (EES) of the F-Chart Software [76] has been used to model CO2 for various applications [28,77,78]. EES is an equation-solving program; it comes with routines to solve simultaneous non-linear algebraic and differential equations, to carry out optimisation, etc. It has a database of thermodynamic and transport properties of hundreds of substances including CO2 and many other refrigerants. It also has a library of functions for heat transfer calculations. It can be linked to other software modelling packages, programs or languages such as MATLAB, C/C+, TRNSYS [74,79].
If system modelling involves additional components such as thermal storage and other system’s enhancing components, thermal modelling software packages such as TRNSYS [79] can be employed. TRNSYS has the capability of calling other routines such as CoolProp [67] in addition to its capability to accommodate the user-developed routine into its simulation engine linked through the dynamic link library. TRNSYS can also be used for system performance evaluation purposes utilising monitoring data recorded from a refrigeration plant. This is made possible through the TRNSYS data reader that can be used to read large amount of unformatted text data apparently only restricted by the computer memory. A TRNSYS project can have many data readers to read many data from many files.
Table 5 summarises the main features and capabilities of the software packages mentioned in the previous discussion. From available literature, one can infer that the decision to select one particular modeling software package seems to be based mainly on (1) familiarity of the modeler to the program, and (2) availability of such packages to the modeler. Of course, when a specific modelling purpose needs to be served, other factors such as cost, processing speed, user-friendliness or ease of use, etc. can play role in the final selection.

Table 5.
Summary of main features and capabilities of several software packages or system modelling tools used for CO2 refrigeration systems.
7. Transcritical Operation, Cooling System and Control
When the system operates in the transcritical region there are a number of aspects that need close attention. Firstly, since transcritical operation involves higher pressure at gliding temperatures, the amount of heat rejected can be very significant and this can be utilised internally using an IHX (Section 4.1) or converted to work (Section 4.2) or used externally for space or water heating (Section 8.2). When such heat recovery is not possible for any reason, significant reduction in the approach temperature can lead to enhanced performance of the system [2]. Such a reduction can be attained using a well-designed gas cooler [80]. For economic reasons, finned-tube heat exchangers are normally used as gas coolers [81] whilst possible use of mini-channel heat exchangers is still at the research stage [82]. Research on integrated fin and micro-channel CO2 gas cooler used for a vehicle air conditioning system has been initiated [83]. However, attempts to improve the finned-tube gas cooler’s air side heat transfer coefficient has gained little success so far. This is worsened by the presence of a pseudo-critical region above the CO2 critical point. In this region CO2 gas experiences large variations of properties [84] where the specific heat also reaches its maximum value [85]. Employing a Computational Fluid Dynamic (CFD) model, Santosa et al. [86] developed the air side heat transfer coefficient correlations for the finned-tube gas cooler. As part of this research work, slit-fins was added to the gas cooler, resulting in increased heat rejection rate between 6–8%.
Secondly, the transcritical operation may only occur during a limited number of hours in a year to satisfy the load during this short period. This can result in selecting a compressor with much larger capacity only to satisfy this load. This leads to the concept of controlling the minimum gas cooler temperature through wet-bulb, rather than dry-bulb, temperature as in the case of cooling towers [87]. Hence, the water spray technique whereby the fin surface of the gas cooler is sprayed with fine droplets of water using high pressure water nozzles [87]. According to [87], the water spray system should only operate during peak summer days. The water spray technology comes with a drawback: the possible corrosion of (and deposition of solid on the surface) of heat exchangers. This can be overcome by choosing (treating) water with lower solid content of 60 ppm. Special dip coating can also be used against gas cooler corrosion [88]. Due to the reliance on the ambient wet bulb temperature, this technique is more suitable for warm to hot but dry climate.
As mentioned in Section 2, for a given CO2 outlet temperature there exists a gas pressure that maximises the system COP. The system controller needs to be able to achieve this (i.e., to maximise COP) by controlling the gas cooler pressure. This often involves simulating or experimenting with a vast amount of data. Kim et al. [89] questions the reliability of such an approach in cases where the data required falls beyond the available range. Although it is a non-model based real-time optimisation approach, the extremum seeking control (ESC) [90,91] employed to optimise the system performance is impractical since the optimum COP search relies upon the system transient response [89].
As in conventional systems, the high-side pressure in subcritical operation is controlled by the condensing temperature. Due to pressure independence of the gas temperature during the transcritical operation, the gas cooler pressure is dictated not only by the temperature, but also by the mass of the refrigerant and inside volume [2], as governed by the following equation of state:
Thus, the high-side pressure can be controlled using the following mechanisms: (a) varying the refrigerant mass, (b) varying the volume, or (c) varying the temperature which is accompanied by change in mass and/or volume [8,92]. A detailed discussion on these three mechanisms can be found in [2].
A novel dew point cooler (DPC) [93] is able to provide cooling during transcritical operation when humidity is not extremely high (up to 17 g/kg) and the ambient air temperature is moderate to high [26]. This normally occurs during late spring to early summer in Mediterranean climates. An intensive experimental investigation has revealed that dew point cooling technology can significantly improve the overall CO2 system performance through the reduction in condensing temperature during the mild weather conditions and through shortening the duration of the transcritical operation during hot ambient conditions. A theoretical case study based on these experimental results has been carried out. At ambient conditions of 45 °C dry bulb temperature and 10 °C dew point temperature, the DPC precooling improves the system COP by 140% compared to the conventional CO2 refrigeration system [26].
8. Applications
The CO2 refrigeration cycles have found its way into applications that require refrigeration, cooling, space and water heating. One of the major sectors with a significant use of refrigeration and air conditioning services is the food retail industry, with supermarkets being the forefront. Operating as heat pumps, CO2 systems have also been utilised to provide space and water heating services. These two applications are briefly discussed in this Section with a closing summary on the CO2 systems’ market acceptance and penetration.
8.1. Supermarket: Refrigeration and Air Conditioning
Refrigeration systems in supermarkets in general serve two loads at two different temperature levels, namely: the low temperature room aimed to freeze foods and the medium temperature room functioning as food cabinets to display chilled foods [57]. The consideration of the application of CO2 systems in supermarkets is based on the fact that supermarket refrigeration requirements represent about half of the supermarket energy consumption. Furthermore, refrigerant leaks are an issue often encountered in the direct expansion (DX) system commonly used in the sector [94]. In terms of food safety, CO2 refrigerant poses no or less contamination in case of system leakage [95].
Sharma et al. [96] carried out a comparative study on the CO2 systems and the R404A system. For ambient temperatures below −8 °C the transcritical booster system with by-pass compressor (TBS-BC) was found to be the system with the lowest energy consumption. Above this temperature, the R404A DX system performs best. Geographically, CO2 TBS-BC is suitable for the northern two-thirds of the US whilst for the southern portion the R404A multiplex DX system is superior to CO2 systems. A CO2 system has been shown to outweigh the performance of other refrigeration systems in terms of energy use and environmental impact for systems exposed to the London climate [97]. In general CO2 systems are as (if not more) efficient as other systems in cooler climates.
Many other studies on the applications of CO2 system for supermarkets, e.g., [77,98,99,100] with a recent review on the subject [101] point to the increasing popularity of these systems due to various factors discussed earlier.
8.2. Heat Pump Systems: Heat Utilisation During Transcritical Operation
When operating in transcritical condition, the heat accumulated in the condenser/gas cooler can be utilized as a heat source for water heating or space heating [94]. High temperature glide during transcritical operation makes this possible [102]. Figure 23 shows such a concept. As shown, the water heating unit is typically placed in the upstream of the gas cooler where the temperature is relatively high to enable effective water heating whilst the space heating is placed in the downstream of the gas cooler. Since transcritical operation occurs during warm to hot ambient conditions, this type of heat utilisation needs to take into account the annual duration of transcritical operation and hot water and space heating requirements of a particular application.

Figure 23.
Utilisation of rejected heat for space and water heating during transcritical operation.
In relation to this, a theoretical study by Baheta et al. [103] revealed that the CO2 system COP increases with increasing evaporating temperatures suggesting that the system is more suitable for air conditioning (space cooling) applications rather than refrigeration which demands a lower temperature load.
Yang et al. [104] designed and tested a prototype of a combined R134a and transcritical CO2 heat pump system to provide space heating using water as a medium. In the system, R134a and CO2 loops are coupled by the water pipe originating from a three-way valve (TWV) and passes through the evaporator of the R134a loop. The feed water is cooled by the R134a in the evaporator. The feed water than enters the CO2 gas cooler and heated through heat exchange with the CO2 gas and directed back to the mixing tank. Another branch of feed water flowing through the aforementioned TWV enters the R134a loop condenser through which its temperature increases. This heated feed water then enters the mixing tank and mixes with the feedwater coming from the CO2 loop. The system can deliver hot water at a temperature of 70 °C from a feed water temperature of 50 °C. The findings revealed that the system performance is significantly influenced by ambient temperature and the temperature of the heated space. An increase in ambient temperature from −20 °C to 0 °C results in an increase in heating capacity of 32.6% and COP of 18.2%. When the ambient temperature is held constant and feed water temperature is reduced from 50 °C to 40 °C, the COP increases by 15% but the water temperature is only raised by 10 K from 40 °C to 50 °C.
A special review on transcritical CO2 heat pump systems was published recently and covers the various system components and applications such as water heating, drying, heating in cold climate, and food processing [105].
8.3. Market Acceptance and Penetration
Market attraction to any new (or in the case of CO2 re-emerging) technology, without any external driving/compelling force, is normally dictated by economic considerations, i.e., initial and running costs which affect the return on investment (ROI). This is perhaps the only main challenge to the market acceptance and penetration of these systems. These factors can be broken down into the costs of: refrigerant, energy, equipment, system installation, system maintenance and regulation-related costs [106]. A breakdown of initial and operating costs associated with three real CO2 systems for supermarkets [106] shows that the equipment costs represents about 62 to 69% of the total initial cost of the CO2 systems and 59 to 67% for the alternatives. The only items that were against the CO2 systems were the equipment costs which were 11%, 24% and 17% higher for the three systems, compared to the alternatives. Despite this, overall, CO2 systems present an impressive economic attractiveness: the first system (96.7 kW LT load and 308 kW MT load) starts saving at the time of installation, the second system (73 kW LT load and 220 kW MT load) has a ROI in 2.5 years and the third system (57 kW LT load and 190 kW MT load) has a ROI in 5.6 years. While figures from these specific three cases cannot be regarded as the best representation of the potential economic attractiveness of CO2 in general, they show the trend in an optimistic direction for this technology.
Other factors are positive for CO2 refrigeration, including the environmental considerations discussed earlier in particular the phase down or planned phase out of several widely used refrigerants. CO2 systems will rid the owner of the issues related to the compliance of future regulations [106].
The following numbers gives some highlights on the market penetration of CO2 systems worldwide thus far. The world’s reported largest CO2 refrigeration system to date is owned by Staay Food Group in the Dutch city of Dronten [95]. In the all-in-one city farming hall the system serves the vegetable processor and cooling requirement of the facility, including the humidity and temperature control of lettuce with 300,000 kg annual production capacity. The total system capacity is 3.36 MW. According to [106] there are approximately 3000 CO2 systems serving supermarkets/stores worldwide. In 2017 there have been around 700 CO2 heat pump systems installed in China [107].
9. Future Research Directions
From discussions presented in previous sections it is clear that CO2 refrigeration systems have found their way into various applications—notably in supermarkets, despite the rather lower system performance due to the inherent operation in supercritical mode in warm climates. In a nearly decade-old road map for retailers developed by the Carbon Trust [108] in collaboration with the British Refrigeration Association, CO2 refrigeration was listed under technologies which is available for store refitting. The road map envisaged the CO2 performance improvement through heat recovery of the transcritical operation of the system for desiccant cooling or heating. The road map, however, noted that the heating requirement is normally high during the time when heat recovery potential is low. Another option being suggested was the use of CO2 as a secondary fluid in a secondary system with another refrigerant in the primary loop. The installed costs and higher energy consumption of such systems were noted to be still the main challenges.
Based on the various exergy analyses, improvements have been made to minimize the exergy loss in various components such as expansion through the expander and ejector, IHX and parallel compression. However, various cycle modifications discussed earlier have shown mixed results in terms of improved system performance. Outcomes of various exergy analyses, some having been discussed earlier, point to the supercritical operation as one of the main sources of exergy destruction in these systems. Attempts to improve the gas cooler performance by better design has also arrived at a similar outcome. Unless the significant source of the exergy destruction, namely the presence of supercritical operation, is totally eliminated (or at least minimised), then the attempts at improving the system performance may never be effective.
Based on the above observation, for a CO2 system without heat waste recovery unit, minimising or totally eliminating the supercritical operation seems the most prospective way of bringing a great improvement to the performance of CO2 systems. The observation is based on the following reasoning:
- Supercritical operation exhibits large exergy losses which affect the system performance in terms of COP and cooling capacity degradation.
- While gas cooling was found to have the exergy losses in the same order of magnitude as the exergy losses of the compressor, it was also found that throttling losses during supercritical operation can be significantly much higher than that of the system running in subcritical state. The very high exergy losses during the throttling process is obviously because of allowing the refrigerant from very high pressure to expand without performing any work. In other words, the supercritical operation spreads the exergy degradation to the whole components, not only the gas cooling unit.
- It was found that cycle modification through the introduction of more than one component—for instance, simultaneous introduction of a mechanical expander and IHX—can result in no performance improvement. All the cycle modifications discussed in Section 4 entail the introduction of additional internal components to the system, which will certainly affect the overall system balance which, unfortunately, not always in a beneficial way. This is one of the reasons why the expected improvement can never reach an optimum.
- On the other hand, the external manipulation/modification of cooling medium conditions—in this case bringing down the gas cooler air inlet temperature to enable the system running in subcritical state—does not interfere in the internal interaction between the components, it simply brings the system into its natural condition of operation, where the heat rejection occurs through condensation. Furthermore, such external modification minimises or eliminates the need for system internal modification.
- The technology for this climate manipulation already exists in the form of dew point evaporative cooling [26,93] which works effectively in warm to hot climates. The recent experimental results on a full-scale CO2 refrigeration system coupled with a dew point cooler [26] as reported in Section 7 can be considered a breakthrough, in that the solution is directed at attacking one of the main sources of system exergy destruction.
- Performance improvement of such technology can be done externally, separate from the entire system. In addition, internal modification such as adding new components (IHX, ejector, etc.) to the system will not be negatively impacted by the precooling system. Or, more appropriately, the precooling may deem such components unnecessary, unless it is essential to the operation of the system.
The current main technological challenges for the dew point cooling technology for CO2 systems are: (1) minimising the power consumption of the DPC fan which will directly improve the overall system performance, and (2) improving its performance in warm to hot but more humid climates. The first challenge can be resolved through optimum design of the DPC and possibly improving the heat exchanger design of the existing condenser/gas cooler taking into account its coupling with the DPC. This can also lead to total redesign of the existing heat exchanger to eliminate the duplication of fans and to optimize the shape factor of the newly design cooling system. The second challenge may be overcome through the possible introduction of dehumidification technology such as desiccant dehumidifier to extend the DPC operation to more humid climates. It should be noted that the dehumidification level required by the DPC to enable the CO2 system to work more efficiently in hot and humid climates is not as strict as the dehumidification demand for an air conditioning system operating in the tropics. Bringing down the ambient humidity ratio to 17 g/kg has been found adequate to enable the effective operation of the DPC [26] although 14 g/kg is an ideal value required to totally eliminate the supercritical operation.
10. Conclusions
CO2 refrigeration systems have found their way into various applications due to environmental considerations, safety reasons (e.g., food contamination due to and leakage of gas), and its energy performance comparable to systems using other refrigerants. In particular, for applications such as supermarket refrigeration the cost of refrigerants is one of the deciding factors in favour of CO2 based refrigeration systems.
The performance of CO2 refrigeration systems running in the supercritical state is characterised by the existence of optimum points of operation at specific conditions. This includes the existence of a COP optimum value at varying gas cooler pressures at a given gas outlet temperature. Also, an optimum refrigeration capacity exists at a gas cooler pressure higher than that which gives the maximum COP; however, this maximum cooling effect is very marginal, and therefore the pressure that gives the maximum COP should be prioritised. This transcritical operation characteristic of the CO2 systems originates from the fact that in the supercritical state, the pressure and temperature are independent with gas cooling at constant pressure and gliding temperatures. The s-curve profile of isotherms in the transcritical region results in the existence of these optimums. Several correlations that attempt to locate these optimum points at given conditions have been developed.
The exergy analysis shows that the expansion process during the transcritical operation is the process that contributes to the largest exergy destruction in the CO2 cycle. This is mainly due to the high pressure of gas exiting the gas cooler that must be expanded to enable the cooling effect in the evaporator. To recover this loss, a work recovery device such as an expander or ejector can be employed as a substitute for the expansion device. In the case of the expander, the work recovered can be used to reduce the compressor work input. The use of an expander, while being able to recover some work, may not be the best option due to various practical reasons such as the restriction of the volumetric flow rate to the volume displacement, heat loss through the housing and cost. Another alternative expansion device, called the ejector, which works on the principle of fluid momentum transfer, is not constrained by the above drawbacks and has recently attracted research interest.
Other cycle modifications have been proposed to improve the efficiency of CO2 systems. An IHX takes some heat from the gas cooler outlet to completely vaporise the CO2 fluid leaving the evaporator before it enters the compressor. An appropriate degree of superheat is required to protect the compressor from flooding. However, the combined presence of an IHX and an expander can degrade the system performance due to the cooling of fluid leaving the gas cooler. The effectiveness of an IHX is very much affected by the gas cooler exit temperature, i.e., an IHX is recommended only for a system with higher exit temperature.
The outlet temperature of the gas cooler is one of the key parameters that significantly affects the performance of CO2 systems. A well-designed gas cooler is required to ensure that the value of this parameter is as low as possible.
Compression staging is aimed at reducing compressor work input and can be realised through intercooling (using an external fluid for cooling), flash gas cooling or flash-gas bypass cooling. The effectiveness of each option depends very much on the system operating conditions and specifications.
Parallel compression can also be used to improve system performance although it may only result in a small increase in the system capacity and small COP improvement at high ambient temperatures.
For warmer to hot and relatively dry climates, the most effective way of improving the system performance is to reduce the number of hours the system must operate in the supercritical state. This can be realised for instance through precooling the ambient air using dew point evaporative cooling technology which operates very efficiently in such climates. The improved COP from introducing such a system has been shown to outweigh the increased power consumption of the cooling fans. Future research in this area may include developing high performance dew point cooling systems with low power consumption and exploring the possibility of employing this cooling technology in hot and humid climates.
The CO2 systems have started to show economic attractiveness. The equipment cost, which is currently 10 to 25% higher than for conventional systems and represents a significant portion of the system and installation cost (up to about 74% based on limited cases investigated for the purpose of this review) seems to have been largely recouped by other cost items such as for the refrigerant installation etc. which are comparable or even less than the similar costs items for conventional systems. Specifically, CO2 refrigerant installation costs are lower due to the smaller size of copper pipes. In addition, smaller sizes of CO2 lines allow easy installation which reduces labour costs [106]. In conclusion, as the upfront cost of CO2 refrigeration systems is approaching that of conventional refrigerant systems and energy efficiency improvements for operation in all climates are being made through new developments, CO2 refrigeration should become a serious contender in the future mix of refrigeration systems.
Author Contributions
F.B. reviewed the literature on CO2 refrigeration systems, contributed to the content of the review paper and critically reviewed the work. M.B. reviewed the literature in the field and provided advice on the structure and scope of the work. E.H. reviewed the literature in the field, laid out the basic structure and scope, and produced and edited drafts of the paper.
Funding
This work was funded by the South Australian Government through funding for the South Australian Chair in Energy for Professor Frank Bruno.
Conflicts of Interest
The authors declare no conflict of interest.
List of Symbols
| h | specific enthalpy, kJ/kg |
| H | enthalpy, kJ |
| IR | irreversibility |
| m | mass flow rate, kg/hr |
| s | entropy, kJ/kg-K |
| x | specific exergy, kJ/kg |
| X | exergy, kJ |
| X | exergy transfer rate |
| XL | exergy losses, kJ |
| Subscripts | |
| ev | evaporator |
| th | thermal energy |
| x | exergetic |
| Abbreviations | |
| ATD | approach temperature difference |
| COP | coefficient of performance |
| DPC | dew point cooler |
| EV | expansion valve |
| FG | flash gas |
| HP | high pressure |
| HTF | heat transfer fluid |
| IHX | internal heat exchanger |
| KE | kinetic energy |
| LT | low temperature |
| MT | medium temperature |
| PE | potential energy |
| ROI | return on investment |
| SST | saturation suction temperature |
| WB | wet bulb |
References
- Brodribb, P.; McCann, M. Cold Hard Facts 3. Prepared by Expert Group & Thinkwell Australia Pty Ltd. Canberra for the Australian Government, Department of the Environment and Energy, (DoEE) Energy Innovation and Ozone Protection Branch, International Climate Change and Energy Innovation Division. 2018. Available online: http://www.environment.gov.au/protection/ozone/publications/cold-hard-facts-3 (accessed on 5 February 2019).
- Kim, M.-H.; Pettersen, J.; Bullard, C.W. Fundamental process and system design issues in CO2 vapour compression system. Prog. Energy Combust. Sci. 2004, 30, 119–174. [Google Scholar] [CrossRef]
- Pearson, A. Carbon dioxide—New uses for an old refrigerant. Int. J. Refrig. 2005, 28, 1140–1148. [Google Scholar] [CrossRef]
- Nekså, P.; Walnum, H.T.; Hafner, A. CO2—A refrigerant from the past with prospects of being one of the main refrigerants in the future. In Proceedings of the 2010 9th IIR Gustav Lorentzen Conference 2010—Natural refrigerants—Real alternatives, Sydney, Australia, 12–14 April 2010. [Google Scholar]
- Maina, P.; Huan, Z. A review of carbon dioxide as a refrigerant in refrigeration technology. S. Afr. J. Sci. 2015, 111, 1–10. [Google Scholar] [CrossRef]
- NCI (Navigant Consulting, Inc.). Case Study: Transcritical Carbon Dioxide Supermarket Refrigeration Systems—Prepared for Better Buildings Alliance Building Technologies Office, Office of Energy Efficiency and Renewable Energy—U.S. Department of Energy. 2015. Available online: https://betterbuildingssolutioncenter.energy.gov/sites/default/files/attachments/Transcritical_CO2_Supermarket_Refrigeration_Systems.pdf (accessed on 4 May 2018).
- Emerson Climate Technologies—Commercial CO2 Refrigeration Systems Guide for Subcritical and Transcritical CO2 Applications. Available online: http://www.r744.com/files/675_commercial_co2_guide.pdf (accessed on 30 August 2018).
- Fourie, M. A Subcritical and Transcritical Carbon Dioxide Refrigeration System Utilizing Multiple Expansion Devices. Master of Science in Engineering Thesis, Faculty of Engineering at Stellenbosch University, Stellenbosch, South Africa, 2014. [Google Scholar]
- Ranson, J.; Dusek, J. Coles Putting CO2 on the Map, Accelerate Australia & NZ. 2016. Available online: https://www.arneg.com.au/sites/default/files/communication-review/accelerate-05-2016/attachments/rassegnastampaaccelerateweb.pdf (accessed on 9 July 2018).
- Danfoss—Transcritical CO2 System in a Small Supermarket. 2008. Available online: http://www.r744.com/files/pdf_559.pdf (accessed on 13 July 2018).
- Sarkar, J. Review and future trends of supercritical CO2 Rankine cycle for low-grade heat conversion. Renew. Sustain. Energy Rev. 2015, 48, 434–451. [Google Scholar] [CrossRef]
- Ahn, Y.; Bae, S.J.; Kim, M.; Cho, S.K.; Baik, S.; Lee, J.I.; Cha, J.E. Review of Supercritical CO2 Power Cycle Technology and Current Status of Research and development. Nucl. Eng. Technol. 2015, 47, 647–661. [Google Scholar] [CrossRef]
- Li, M.-J.; Zhu, H.-H.; Guo, J.-Q.; Wang, K.; Tao, W.-Q. The development technology and applications of supercritical CO2 power cycle in nuclear energy, solar energy and other energy industries. Appl. Therm. Eng. 2017, 126, 255–275. [Google Scholar] [CrossRef]
- Llopis, R.; Nebot-Andrés, L.; Sánchez, D.; Catalán-Gil, J.; Cabello, R. Subcooling methods for CO2 refrigeration cycles: A review. Int. J. Refrig. 2018, 93, 85–107. [Google Scholar] [CrossRef]
- Sarkar, J. Review of cycle modifications of transcritical CO2 refrigeration and heat pump systems. J. Adv. Res. Mech. Eng. 2010, 1, 22–29. [Google Scholar]
- Zhang, Z.; Tong, L.; Wang, X. Thermodynamic Analysis of Double-Stage Compression Transcritical CO2 Refrigeration Cycles with an Expande. Entropy 2015, 17, 2544–2555. [Google Scholar] [CrossRef]
- Sawalha, S. Carbon Dioxide in Supermarket Refrigeration. Doctoral Thesis, Royal Institute of Technology (KTH), Stockholm, Sweden, 2008. [Google Scholar]
- Agrawal, N.; Bhattacharyya, S.; Sarkar, J. Optimization of two-stage transcritical carbon dioxide heat pump cycles. Int. J. Therm. Sci. 2007, 46, 180–187. [Google Scholar] [CrossRef]
- Kotas, T.J. The Exergy Method of Thermal Plant Analysis, Reprint ed.; Krieger Publishing Co.: Malabar, FL, USA, 1995; pp. 37–51. [Google Scholar]
- Çengel, Y.A.; Boles, M.A. Thermodynamics—An Engineering Approach, 7th ed.; McGraw Hill: Singapore, 2008. [Google Scholar]
- Ahamed, J.U.; Saidur, R.; Masjuki, H.H. A review on exergy analysis of vapor compression refrigeration system. Renew. Sustain. Energy Rev. 2011, 15, 1593–1600. [Google Scholar] [CrossRef]
- Moran, M.J.; Shapiro, H.N.; Boettner, D.D.; Bailey, M.B. Principles of Engineering Thermodynamics, 7th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012; pp. 329–369. [Google Scholar]
- Bejan, A. Advanced Engineering Thermodynamics, 3rd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2006; pp. 204–221. [Google Scholar]
- Adriansyah, W. Combined Air-conditioning and Tap Water Heating Plant, Using CO2 as Refrigerant for Indonesian Climate Condition. Ph.D. Thesis, Norwegian University of Science and Technology, Trondheim, Norway, 2001. [Google Scholar]
- Cavallini, A.; Zilio, C. Carbon dioxide as a natural refrigerant. Int. J. Low Carbon Technol. 2007, 2, 226–249. [Google Scholar] [CrossRef]
- Belusko, M.; Bruno, F.; Liddle, R.; Alemu, A.; Halawa, E. Performance evaluation of a CO2 refrigeration system enhanced with a dew point cooler. Energies 2019, 12, 1079. [Google Scholar] [CrossRef]
- Yumrutaş, R.; Kunduz, M.; Kanoğlu, M. Exergy analysis of vapor compression refrigeration systems. Exergy Int. J. 2002, 2, 266–272. [Google Scholar] [CrossRef]
- Yang, J.L.; Ma, Y.T.; Li, M.X.; Guan, H.Q. Exergy analysis of transcritical carbon dioxide refrigeration cycle with an expander. Energy 2005, 30, 162–1175. [Google Scholar] [CrossRef]
- Shilliday, J.A.; Tassou, S.A.; Shilliday, N. Comparative energy and exergy analysis of R744, R404A and R290 refrigeration cycles. Int. J. Low-Carbon Technol. 2009, 4, 104–111. [Google Scholar] [CrossRef]
- Boewe, D.E.; Bullard, C.W.; Yin, J.M.; Hrnjak, P.S. Contribution of internal heat to transcritical R744 cycle performance. HVAC R Res. 2001, 7, 155–168. [Google Scholar]
- Singh, S.; Purohit, N.; Dasgupta, M.S. Comparative study of cycle modifications strategies for trans-critical CO2 refrigeration cycle for warm climatic conditions. Case Stud. Therm. Eng. 2016, 7, 78–91. [Google Scholar] [CrossRef]
- Sarkar, J.; Bhattacharyya, S.; Gopal, M.R. Optimization of a transcritical CO2 heat pump cycle for simultaneous cooling and heating applications. Int. J. Refrig. 2004, 27, 830–838. [Google Scholar] [CrossRef]
- Ituna-Yudonago, J.F.; Belman-Flores, J.M.; Elizalde-Blancas, F.; García-Valladares, O. Numerical investigation of CO2 behavior in the internal heat exchanger under variable boundary conditions of the transcritical refrigeration system. Appl. Therm. Eng. 2017, 115, 1063–1078. [Google Scholar] [CrossRef]
- Rigola, J.R.; Ablanque, N.; Pérez-Segarra; Oliva, A. Numerical simulation and experimental validation of internal heat exchanger influence on CO2 trans-critical cycle performance. Int. J. Refrig. 2010, 33, 664–674. [Google Scholar] [CrossRef]
- Domanski, P.A.; Didion, D.A.; Doyle, J.P. Evaluation of Suction Line-Liquid Line Heat Exchange in the Refrigeration Cycle. International Refrigeration and Air Conditioning Conference—Paper 149. 1992. Available online: http://docs.lib.purdue.edu/iracc/149 (accessed on 30 August 2018).
- Robinson, D.M.; Groll, E.A. Efficiencies of transcritical CO2 cycles with and without an expansion turbine. Int. J. Refrig. 1998, 21, 577–589. [Google Scholar] [CrossRef]
- Sarkar, J.; Bhattacharyya, S.; Ramgopal, M. Transcritical CO2 Heat Pump systems: Exergy analysis including heat transfer and fluid flow effects. Energy Convers. Manag. 2005, 46, 2053–2067. [Google Scholar] [CrossRef]
- Fukuta, M.; Yanagisawa, T.; Kosuda, O.; Ogi, Y. Performance of Scroll Expander for CO2 Refrigeration Cycle. International Compressor Engineering Conference. Paper 1768. 2006. Available online: http://docs.lib.purdue.edu/icec/1768 (accessed on 15 August 2017).
- Elbel, S.; Lawrence, N. Review of recent developments in advanced ejector technology. Int. J. Refrig. 2016, 62, 1–18. [Google Scholar] [CrossRef]
- Huff, H.-J.; Radermacher, R. CO2 Compressor-Expander Analysis—Final Report, Prepared for the Air-Conditioning and Refrigeration Technology Institute 4100 N. Fairfax Drive, Suite 200, Arlington, Virginia 22203—CEEE Department of Mechanical Engineering University of Maryland. 2003. Available online: http://organicrankine.com/orc_documents/theory/10060-final.pdf (accessed on 16 August 2018).
- Bo, Z.; Xueyuan, P.; Bei, G.; Ziwen, X.; Pengcheng, S. Design and Experimental Validation of the Slider-Based Free Piston Expander for Transcritical CO2 Refrigeration Cycle. In Proceedings of the 7th IIR Gustav Lorentzen Conference on Natural Working Fluids, Trondheim, Norway, 28–31 May 2006. [Google Scholar]
- Zhang, Z.; Ma, Y.; Li, M.; Zhao, L. Recent advances of energy recovery expanders in the transcritical CO2 refrigeration cycle. HvacR Res. 2013, 19, 376–384. [Google Scholar]
- Kornhauser, A.A. The Use of an Ejector as a Refrigerant Expander. International Refrigeration and Air Conditioning—Conference. Paper 82. 1990. Available online: http://docs.lib.purdue.edu/iracc/82 (accessed on 23 August 2018).
- Liu, F.; Groll, E.A. Study of ejector efficiencies in refrigeration cycles. Appl. Therm. Eng. 2013, 52, 360–370. [Google Scholar] [CrossRef]
- Vereda, C.; Ventas, R.; Lecuona, A.; Venegas, M. Study of an ejector-absorption refrigeration cycle with an adaptable ejector nozzle for different working conditions. Appl. Energy 2012, 97, 305–312. [Google Scholar] [CrossRef]
- Li, D.; Groll, E.A. Transcritical CO2 refrigeration cycle with ejector-expansion device. Int. J. Refrig. 2005, 28, 766–773. [Google Scholar] [CrossRef]
- Ksayer, E.B.; Clodic, D. Enhancement of CO2 refrigeration cycle using an ejector: 1D analysis. In Proceedings of the International Refrigeration and Air Conditioning Conference at Purdue; Purdue University: West Lafayette, IN, USA, 2006. [Google Scholar]
- Hafner, A.; Forsterling, S.; Banasiak, K. Multi-ejector concept for R-744 supermarket Refrigeration. Int. J. Refrig. 2014, 43, 1–13. [Google Scholar] [CrossRef]
- Banasiak, K.; Hafner, A.; Kriezi, E.E.; Madsen, K.B.; Birkelund, M.; Fredslund, K.; Olsson, R. Development and performance mapping of a multiejector expansion work recovery pack for R744 vapour compression units. Int. J. Refrig. 2015, 57, 265–278. [Google Scholar] [CrossRef]
- Haida, M.; Banasiak, K.; Smolka, J.; Hafner, A.; Eikevik, T.M. Experimental analysis of the R744 vapour compression rack equipped with the multi-ejector expansion work recovery module. Int. J. Refrig. 2016, 64, 93–107. [Google Scholar] [CrossRef]
- Bodys, J.; Palacz, M.; Haida, M.; Smolka, J.; Nowak, A.J.; Banasiak, K.; Hafner, A. Full-scale multi-ejector module for a carbon dioxide supermarket refrigeration system: Numerical study of performance evaluation. Energy Convers. Manag. 2017, 138, 312–326. [Google Scholar] [CrossRef]
- How to Choose the Right Multi Ejector Solution™—3 Main Factors to Consider When Designing Your Transcritical CO2 System with a Multi Ejector SolutionTM. Available online: https://danfoss.ipapercms.dk/refrigerationandairconditioning/RA/Infographic/how-to-choose-the-right-multi-ejector-solution-infographics/?page=1 (accessed on 11 March 2019).
- Sarkar, J.; Agrawal, N. Performance optimization of transcritical CO2 cycle with parallel compression economization. Int. J. Therm. Sci. 2010, 49, 838–843. [Google Scholar] [CrossRef]
- Tsamos, K.M.; Ge, Y.T.; Santosa, I.D.; Tassou, S.A.; Bianchi, G.; Mylona, Z. Energy analysis of alternative CO2 refrigeration system configurations for retail food applications in moderate and warm climates. Energy Conv. Mgmt. 2017, 150, 822–829. [Google Scholar] [CrossRef]
- Gullo, P.; Elmegaard, B.; Cortella, G. Advanced exergy analysis of a R744 booster refrigeration system with parallel compression. Energy 2016, 107, 562–571. [Google Scholar] [CrossRef]
- Shafiei, E.S.; Rasmussen, H.; Stoustrup, J. Modeling Supermarket Refrigeration Systems for Demand-Side Management. Energies 2013, 6, 900–920. [Google Scholar] [CrossRef]
- Ge, Y.T.; Tassou, S.A. Thermodynamic analysis of transcritical CO2 booster refrigeration systems in supermarket. Energy Convers. Manag. 2011, 52, 1868–1875. [Google Scholar] [CrossRef]
- Messineo, A. R744-R717 Cascade Refrigeration System: Performance Evaluation compared with a HFC Two-Stage System. Energy Procedia 2012, 14, 56–65. [Google Scholar] [CrossRef]
- Pattingale, A. An Integrated CO2 Production and CO2-NH3 Cascade Refrigeration System; Prepared by Cold Logic Pty Ltd.—Commissioned by Australian Meat Processor Corporation Ltd. (AMPC); Australian Meat Processor Corporation: Port Adelaide SA, Australia, 2016; Available online: https://www.ampc.com.au/uploads/cgblog/id390/2016.1038_Final_Report.pdf (accessed on 30 August 2019).
- Dopazo, J.A.A.; Fernández-Seara, J.; Sieres, J.; Uhía, F.J. Theoretical analysis of a CO2–NH3 cascade refrigeration system for cooling applications at low temperatures. Appl. Therm. Eng. 2009, 29, 1577–1583. [Google Scholar] [CrossRef]
- Span, R.; Wagner, W. A New Equation of State for Carbon Dioxide Covering the Fluid Region from the Triple-Point Temperature to 1100 K at Pressures up to 800 MPa. J. Phys. Chern. Ref. Data 1996, 25, 1509–1596. [Google Scholar] [CrossRef]
- Fang, Y.; De Lorenzo, M.; Lafon, P.; Poncet, S.; Bartosiewicz, Y. An Accurate and Efficient Look-up Table Equation of State for Two-Phase Compressible Flow Simulations of Carbon Dioxide. Ind. Eng. Chem. Res. 2018, 57, 7676–7691. [Google Scholar] [CrossRef]
- NIST. Available online: https://www.nist.gov/srd/refprop (accessed on 26 April 2019).
- McLinden, M.O.; Lemmon, E.W.; Huber, M.L. The REFPROP Database For the Thermophysical Properties of Refrigerants, International Congress of Refrigeration 2003. Washington, DC, USA. Available online: https://ws680.nist.gov/publication/get_pdf.cfm?pub_id=831869 (accessed on 14 August 2018).
- CoolProp. Available online: http://www.coolprop.org/ (accessed on 26 April 2019).
- Bell, I.H.; Wronski, J.; Quoilin, S.; Lemort, V. Pure and Pseudo-pure Fluid Thermophysical Property Evaluation and the Open-Source Thermophysical Property Library CoolProp. IEc Res. 2014, 53, 2498–2508. [Google Scholar] [CrossRef] [PubMed]
- McDowell, T.P.; Bradley, D.E.; Hiller, M.; Lam, J.; Merk, J.; Keilholz, W. TRNSYS 18: The Continued Evolution of the Software. In Proceedings of the IBPSA Building Simulation 2017, San Francisco, CA, USA, 7–9 August 2017. [Google Scholar]
- Bitzer Software. Available online: https://www.bitzer.de/websoftware/ (accessed on 24 August 2018).
- Danfoss Software. Available online: https://www.danfoss.com/en/service-and-support/downloads/?sort=title_asc&filter=segments%3Ddcs (accessed on 24 August 2018).
- CoolPack Software. Available online: http://www.en.ipu.dk/Indhold/refrigeration-and-energy-technology/coolpack.aspx (accessed on 20 August 2018).
- Modelica. Modelica®—Unified Object—Oriented Language for Systems Modeling Language Specification Version 3.4. 2017. Available online: https://www.modelica.org/documents/ModelicaSpec34.pdf (accessed on 20 August 2018).
- Tiller, M.; Modelica Thermal Library. TillerDevelopment of a Modelica Library for Thermal Systems The Modelica Association—Modelica 2000 Workshop. 23–24 October. Available online: https://www.modelica.org/events/workshop2000/proceedings/old/Tiller2.pdf (accessed on 27 April 2019).
- Hu, B.; Li, Y.; Wang, R.Z.; Cao, F.; Xing, Z. Real-time minimization of power consumption for air-source transcritical CO2 heat pump water heater system. Int. J. Refrig. 2018, 85, 395–408. [Google Scholar] [CrossRef]
- Pfafferott, T.; Schmitz, G. Modelling and transient simulation of CO2-refrigerationsystems with Modelica. Int. J. Refrig. 2004, 27, 42–52. [Google Scholar] [CrossRef]
- TLK-Thermo GMBH—TIL Suite—Simulates Thermal Systems. Available online: https://www.tlk-thermo.com/index.php/en/software-products/til-suite (accessed on 20 August 2018).
- F-Chart Software: EES Overview. Available online: http://www.fchart.com/ees/ (accessed on 20 August 2018).
- Suamir, I.; Tassou, S.A. Performance evaluation of integrated trigeneration and CO2 refrigeration systems. Appl. Ther. Eng. 2013, 50, 1487–1495. [Google Scholar] [CrossRef]
- Tsamos, G. Performance investigation of the CO2 gas cooler designs and its integration with the refrigeration system. In Proceedings of the 1st International Conference on Sustainable Energy and Resource Use in Food Chains, ICSEF 2017, Berkshire, UK, 19–20 April 2017. [Google Scholar]
- TRNSYS. TRNSYS Website. Available online: http://www.trnsys.com/ (accessed on 26 April 2019).
- Polzot, A.; D’Agaro, P.; Cortella, G. Energy analysis of a transcritical CO2 supermarket refrigeration with heat recovery. Energy Procedia 2017, 111, 648–657. [Google Scholar] [CrossRef]
- Ge, Y.T.; Cropper, R.T. Simulation and performance evaluation of finned-tube CO2 gas coolers for refrigeration systems. Appl. Therm. Eng. 2009, 29, 957–965. [Google Scholar] [CrossRef]
- Pettersen, J.; Hafner, A.; Skaugen, G. Development of compact heat exchangers for CO2 air-conditioning systems. Int. J. Refrig. 1998, 21, 180–193. [Google Scholar] [CrossRef]
- Li, J.; Jia, J.; Huang, L.; Wang, S. Experimental and numerical study of an integrated fin and micro-channel gas cooler for a CO2 automotive air-conditioning. Appl. Therm. Eng. 2017, 116, 636–647. [Google Scholar] [CrossRef]
- Pitla, S.S.; Robinson, D.M.; Groll, E.A.; Ramadhyani, S. Heat transfer from supercritical carbon dioxide in tube Flow: A Critical Review. Int. J. HVAC R Res. 1998, 4, 281–301. [Google Scholar] [CrossRef]
- Pitla, S.S.; Groll, E.A.; Ramadhyani, S. New correlation to predict the heat transfer coefficient during in-tube cooling of turbulent supercritical CO2. Int. J. Refrig. 2002, 25, 887–895. [Google Scholar] [CrossRef]
- Santosa, I.M.; Gowreesunker, B.L.; Tassou, S.A.; Tsamos, K.M.; Ge, Y. Investigations into air and refrigerant side heat transfer coefficients of finned-tube CO2 gas coolers. Int. J. Heat Mass Transf. 2017, 107, 168–180. [Google Scholar] [CrossRef]
- Lozza, G.; Filippini, S.; Zoggia, F. Using “Water-Spray” Techniques For CO2 Gas Coolers. In Proceedings of the XII European Conference on Technological Innovations in Air Conditioning and Refrigeration Industry, Milano, Italy, 8–9 June 2007. [Google Scholar]
- Güntner—Gas Cooler/Comdenser for CO2. Available online: http://www.r744.com/files/589_2010_09_23_guentner_co2_gas_coolers_info_en.pdf (accessed on 24 August 2018).
- Kim, M.S.; Kang, D.H.; Kim, M.S.; Kim, M. Investigation on the optimal control of gas cooler pressure for a CO2 refrigeration system with an internal heat exchanger. Int. J. Refrig. 2017, 77, 48–59. [Google Scholar] [CrossRef]
- Hu, B.; Li, Y.; Cao, F.; Xing, Z. Extremum seeking control of COP optimization for air-source transcritical CO2 heat pump water heater system. Appl. Energy 2015, 147, 361–372. [Google Scholar] [CrossRef]
- Koeln, J.P.; Alleyne, A.G. Optimal subcooling in vapour compression systems via extremum seeking control: Theory and experiments. Int. J. Refrig. 2014, 43, 14–25. [Google Scholar] [CrossRef]
- Wobst, E.; Flacke, N.; Grohmann, S. Kohlendioxid: Besonderheiten und Einsatzchancen als Ka¨ltemittel; German Refrigeration and Air Conditioning Association: Würzburg, Germany, 1998; pp. 64–74. [Google Scholar]
- Bruno, F. On-site experimental testing of a novel dew point evaporative cooler. Energy Build. 2011, 43, 3475–3483. [Google Scholar] [CrossRef]
- Fricke, B.; Zha, S.; Sharma, V.; Newel, J. Laboratory Evaluation of a Commercial CO2 Booster Refrigeration System. In Proceedings of the 16th International Refrigeration and Air Conditioning Conference, West Lafayette, IN, USA, 11–14 July 2016. [Google Scholar]
- Kallesøe, J. Transcritical CO2 Challenges Traditional NH3/CO2 Cascade Systems. ATMOsphere Europe: Berlin, Germany, 25–27 September 2017. Available online: http://www.atmo.org/presentations/files/59ca7d42163451506442562Qj7YO.pdf (accessed on 28 August 2018).
- Sharma, V.; Fricke, B.; Bansal, P. Comparative analysis of various CO2 configurations in supermarket refrigeration systems. Int. J. Refrig. 2014, 46, 86–99. [Google Scholar] [CrossRef]
- Mylona, Z.; Kolokotroni, M.; Tsamos, K.M.; Tassou, S.A. Comparative analysis on the energy use and environmental impact of different refrigeration systems for frozen food supermarket application. Energy Procedia 2017, 123, 121–130. [Google Scholar] [CrossRef]
- Girotto, S.; Minetto, S.; Neksa, P. Commercial refrigeration system using CO2 as the refrigerant. Int. J. Refrig. 2004, 27, 717–723. [Google Scholar] [CrossRef]
- Braun, M.R.; Beck, S.B.M.; Altan, H. Comparing COP Optimization with Maximizing the Coefficient of System Performance for Refrigeration Systems in Supermarkets. In Proceedings of the 15th International Refrigeration and Air Conditioning Conference, West Lafayette, IN, USA, 14–17 July 2014. [Google Scholar]
- Karampour, M.; Sawalha, S. State-of-the-art integrated CO2 refrigeration system for supermarkets: A comparative analysis. Int. J. Refrig. 2018, 86, 239–257. [Google Scholar] [CrossRef]
- Gullo, P.; Hafner, A.; Banasiak, K. Transcritical R744 refrigeration systems for supermarket applications: Current status and future perspectives. Int. J. Refrig. 2018, 93, 269–310. [Google Scholar] [CrossRef]
- Pitarch, M.; Navarro-Peris, E.; Gonzálvez-Maciá, J.; Corberán, J.M. Evaluation of different heat pump systems for sanitary hot water production using natural refrigerants. Appl. Energy 2017, 190, 911–919. [Google Scholar] [CrossRef]
- Baheta, A.; Hassan, S.; Reduan, A.R.B.; Woldeyohannes, A.D. Performance investigation of transcritical caorbon dioxide refrigeration cycle. Procedia Cirp 2015, 26, 482–485. [Google Scholar] [CrossRef]
- Yang, D.; Song, Y.; Cao, F.; Jin, L.; Wang, X. Theoretical and experimental investigation of a combined R134a and transcritical CO2 heat pump for space heating. Int. J. Refrig. 2016, 72, 156–170. [Google Scholar] [CrossRef]
- Rony, R.U.; Yang, H.; Krishnan, S.; Song, J. Recent Advances in Transcritical CO2 (R744) Heat Pump System: A Review. Energies 2019, 12, 457. [Google Scholar] [CrossRef]
- Hillphoenix—DeCO2ded: Understanding ROI on CO2 Refrigeration Systems. Available online: http://www.r744.com/files/Hillphoenix_CO2_ROI_WhitePaper_v10_Oct24_2014.pdf (accessed on 30 August 2018).
- R744.com. Available online: http://www.r744.com/articles/8469/dongqi_reports_more_than_700_co2_heat_pumps_in_china (accessed on 31 August 2018).
- Carbon Trust Website. Refrigeration Road Map. Available online: https://www.carbontrust.com/media/147175/j7924_ctg021_refrigeration_road_map_aw.pdf (accessed on 30 April 2019).
- Goetzler, W.; Sutherland, T.; Rassi, M.; Burgos, J. Research and Development Roadmap for Next-Generation Low-Global Warming Potential Refrigerants—Navigant Consulting, Inc. Prepared for: U.S. Department of Energy Office of Energy Efficiency and Renewable Energy Building Technologies Office. 2014. Available online: https://www.energy.gov/sites/prod/files/2014/12/f19/Refrigerants%20Roadmap%20Final%20Report%202014.pdf (accessed on 17 April 2019).
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).